Anticancer Metallocenes and Metal Complexes of Transition Elements from Groups 4 to 7
Abstract
1. Introduction
2. d-Elements of Group 4
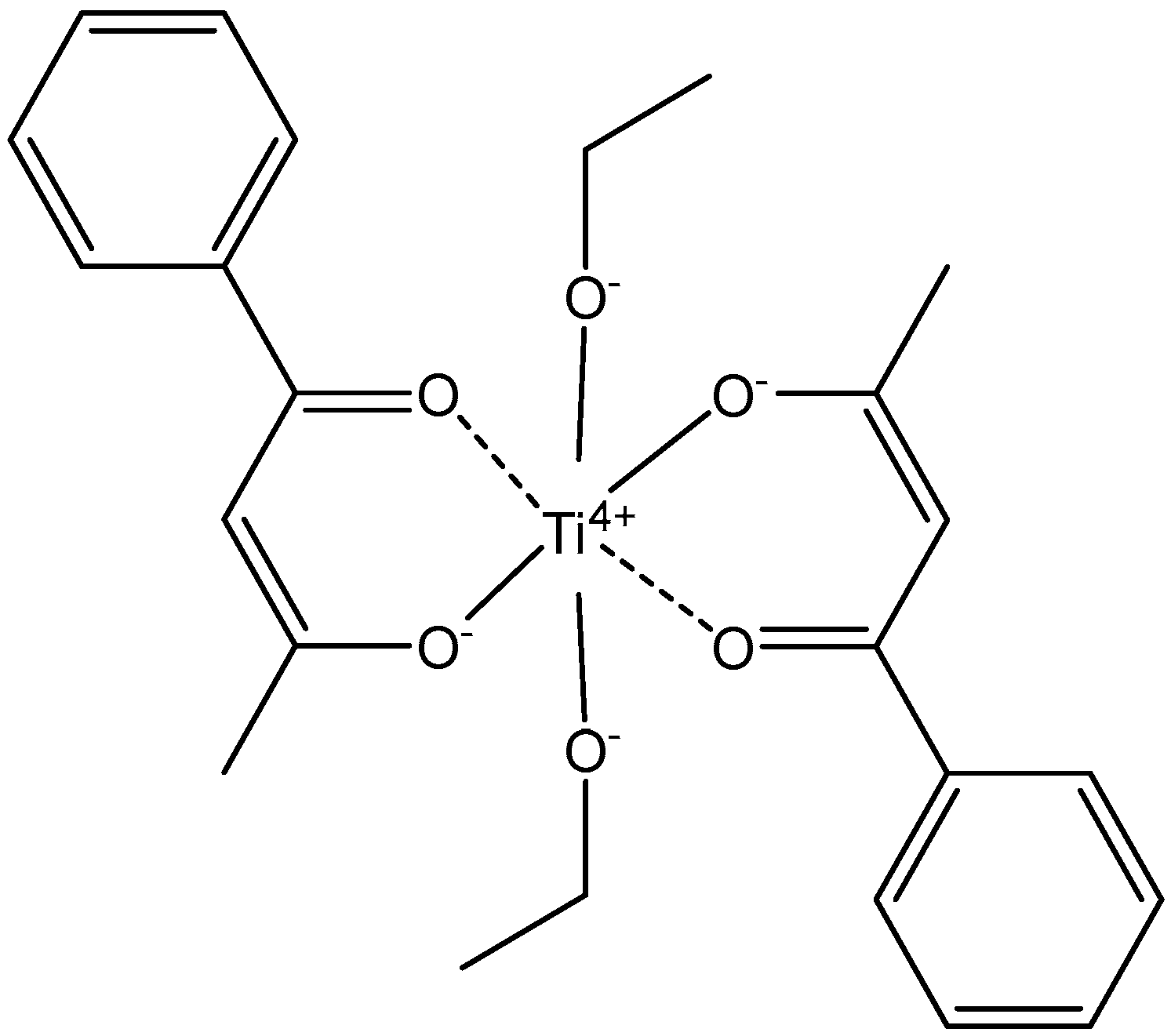
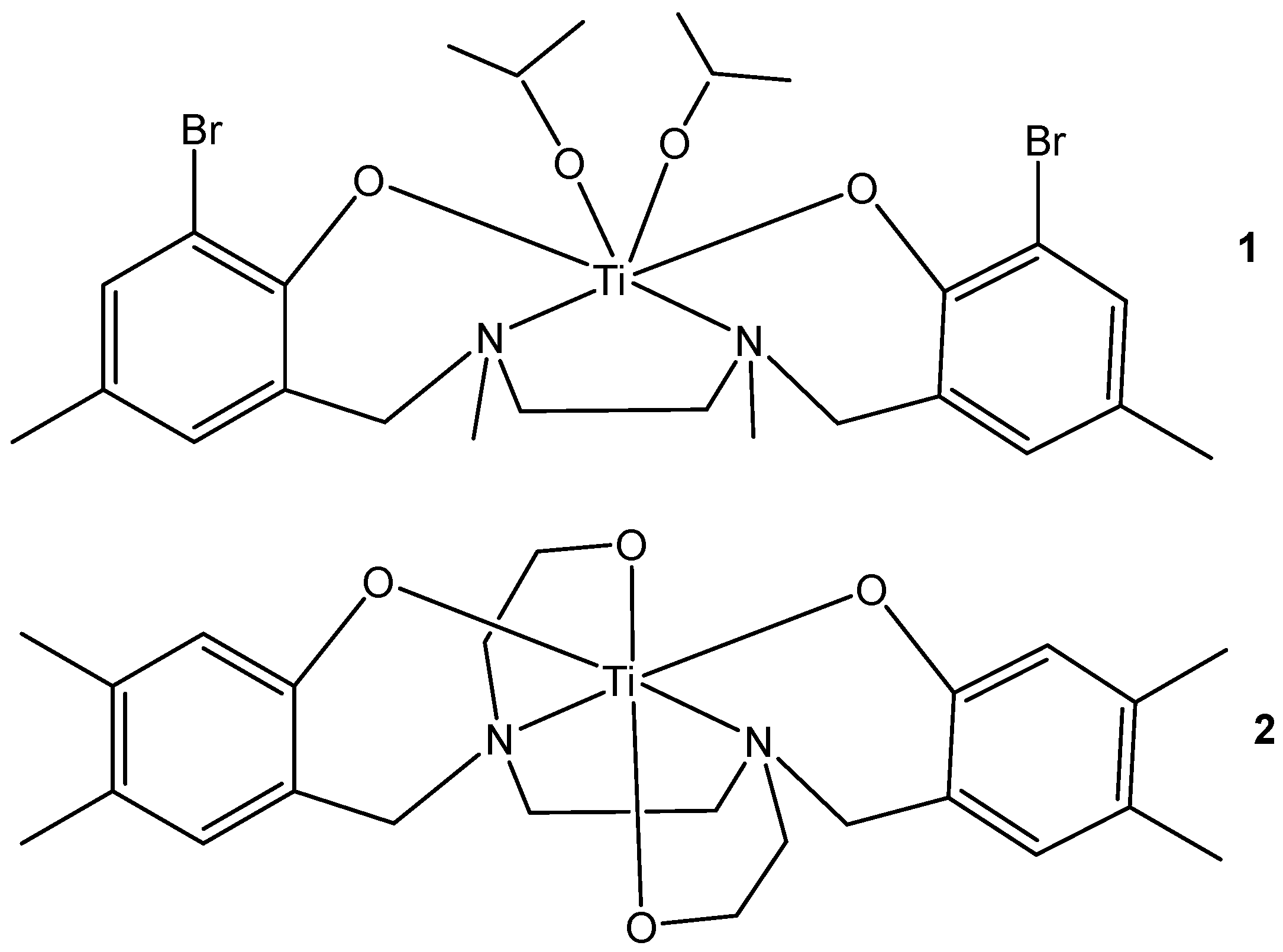
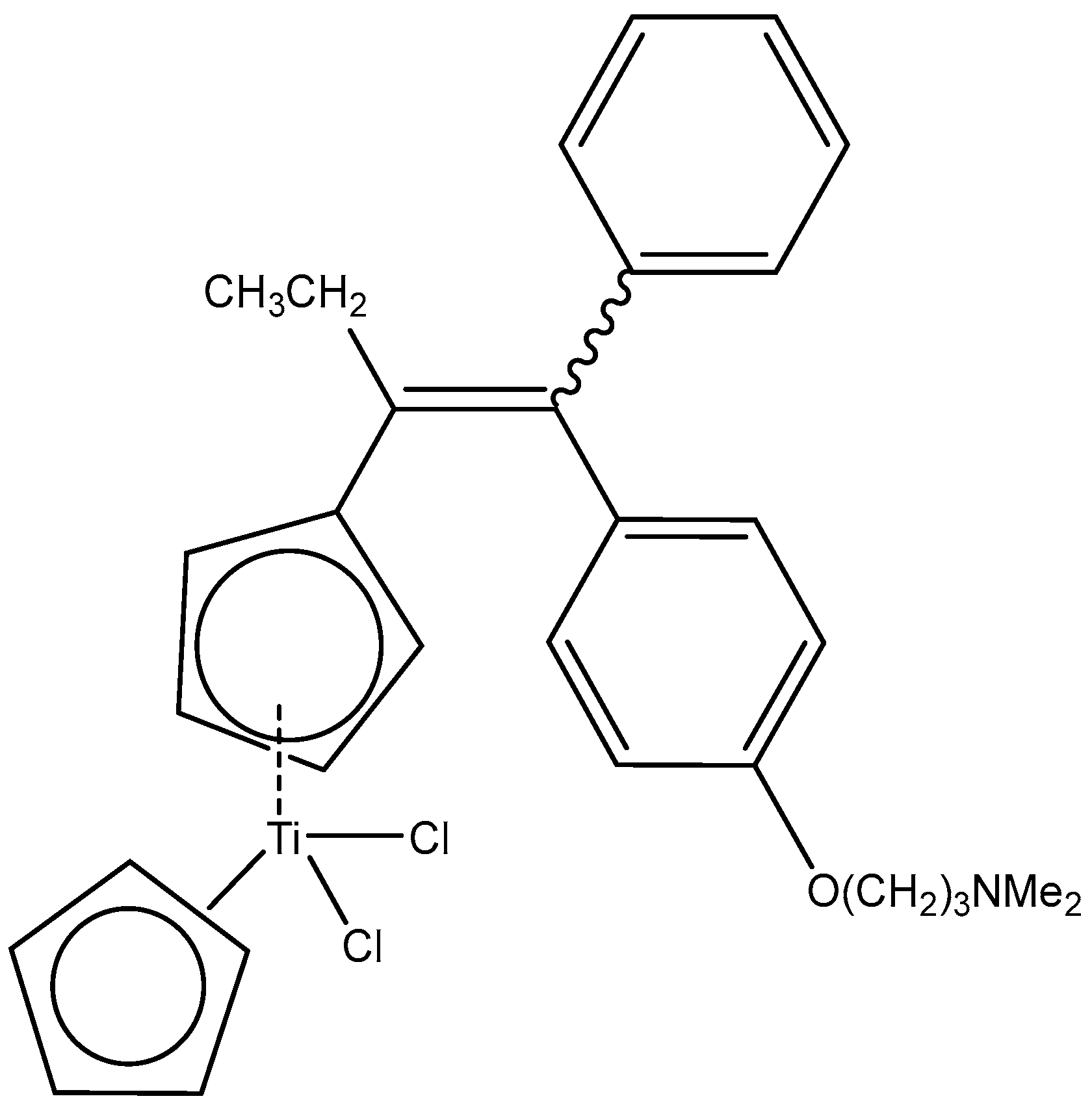
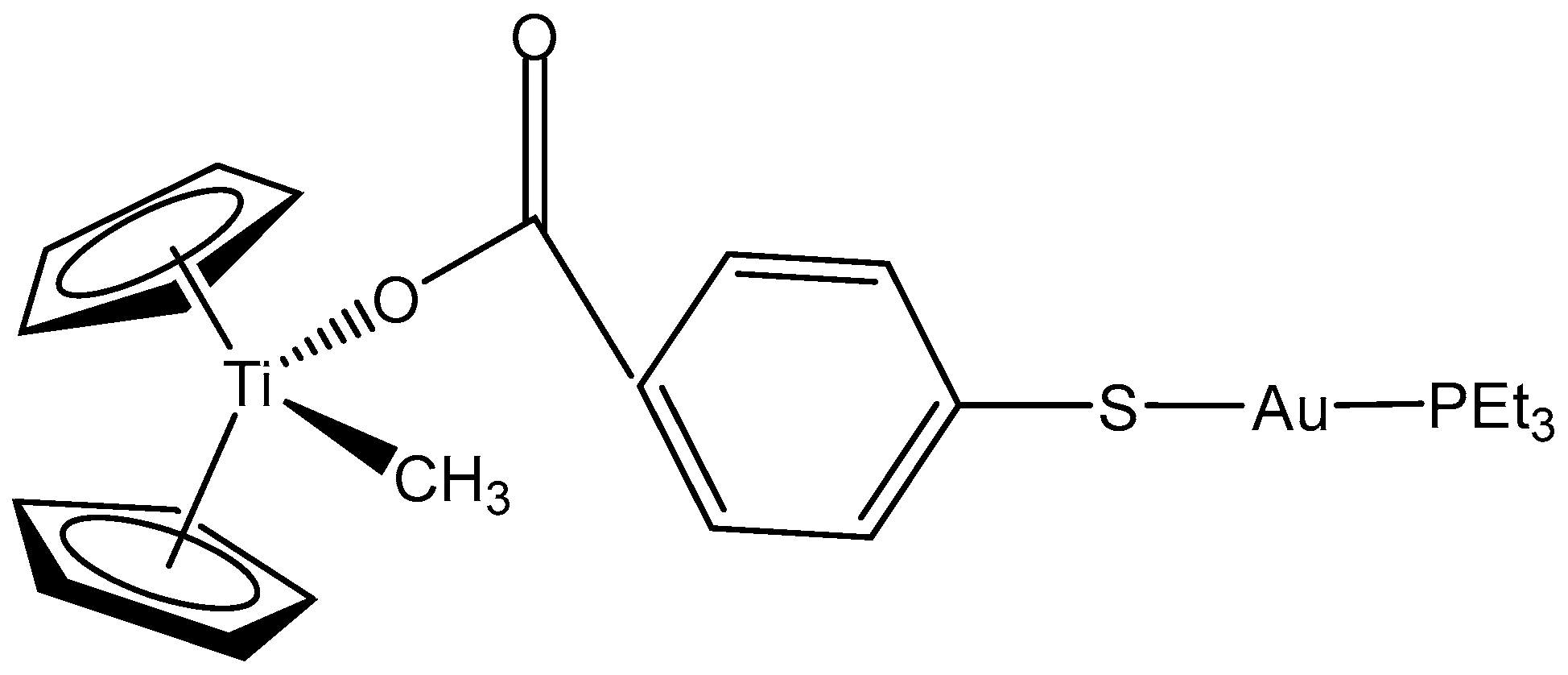
2.1. Titanocenes and Other Ti(IV) Complexes
2.2. Zirconium(IV) Complexes
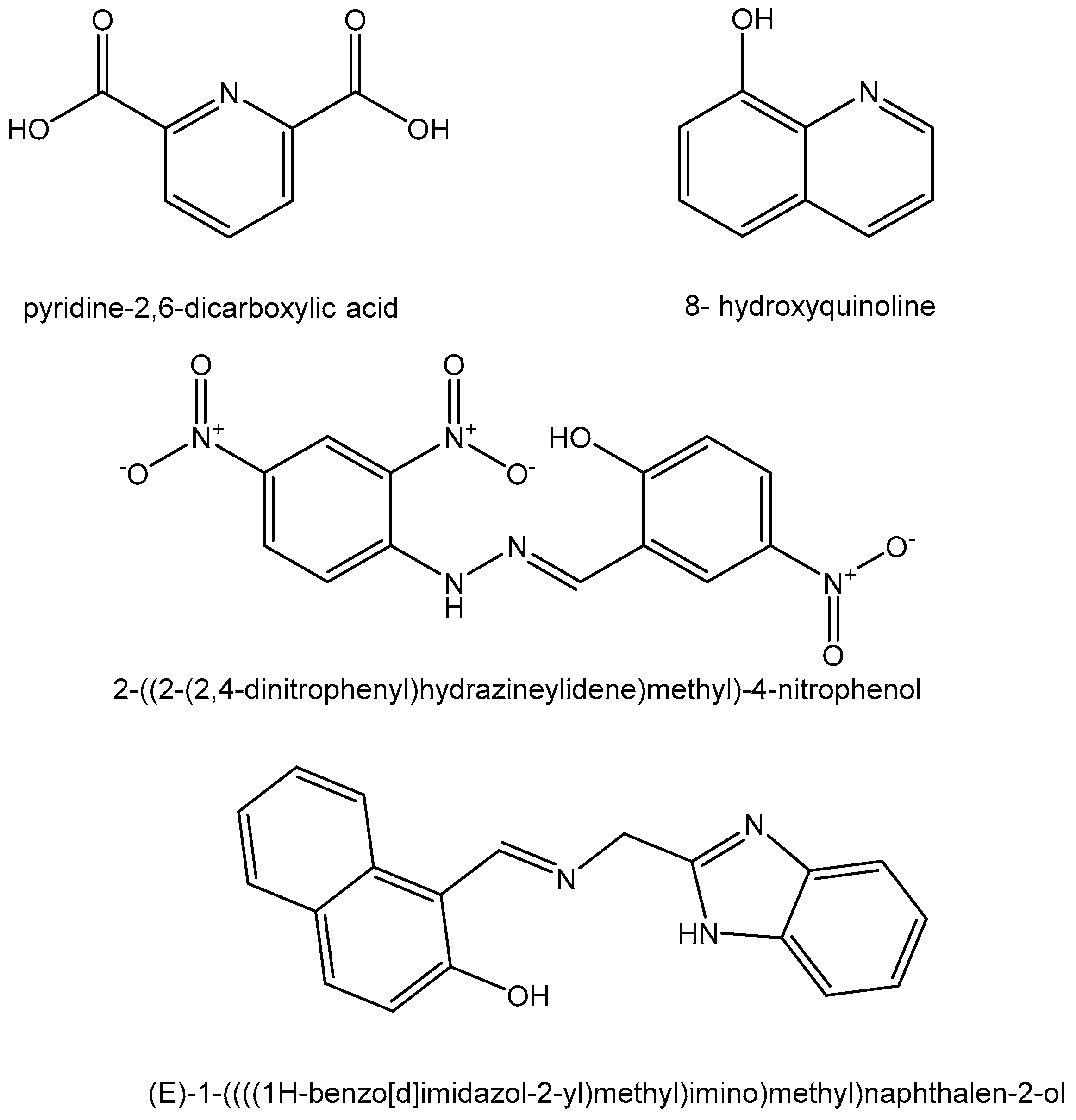
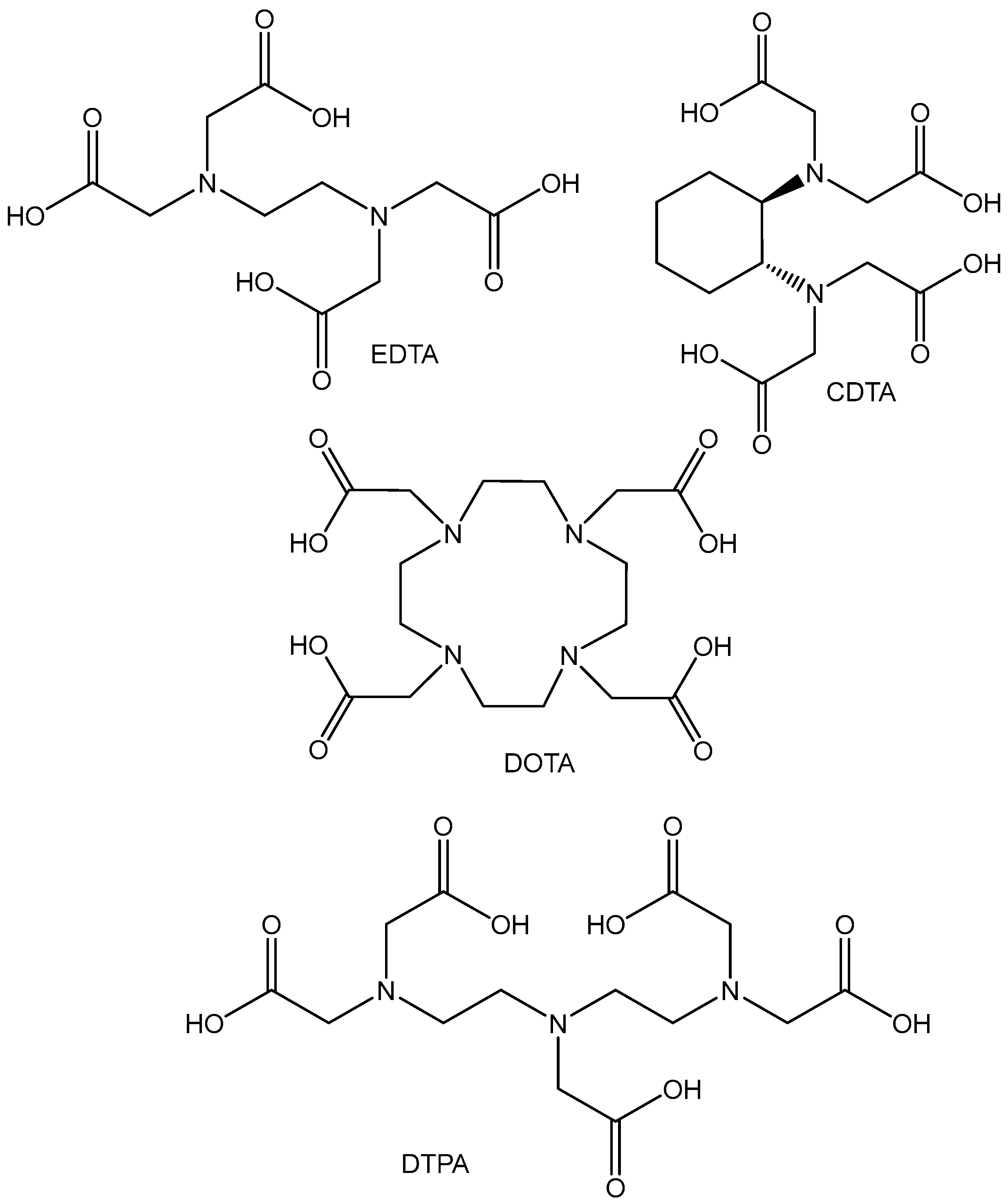
2.3. Hafnium(IV) Complexes
3. d-Elements of Group 5
Vanadocenes and Other Vanadium Complexes
4. d-Elements of Group 6
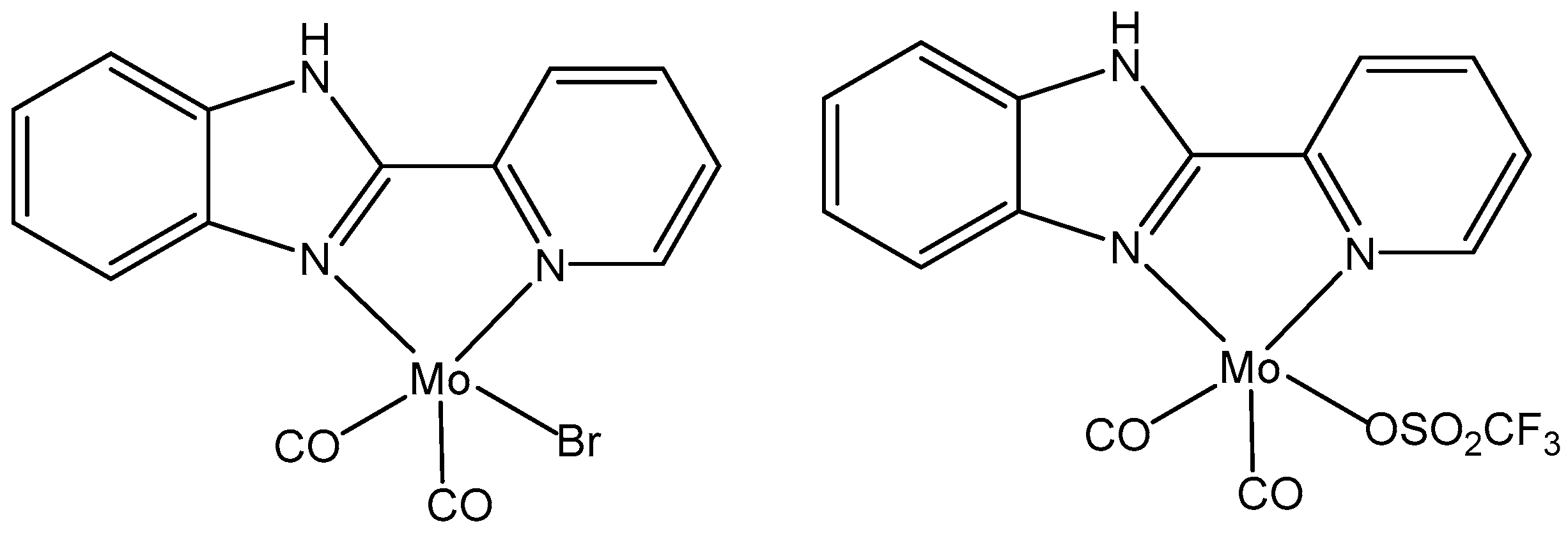
4.1. Molybdenum(II) Complexes
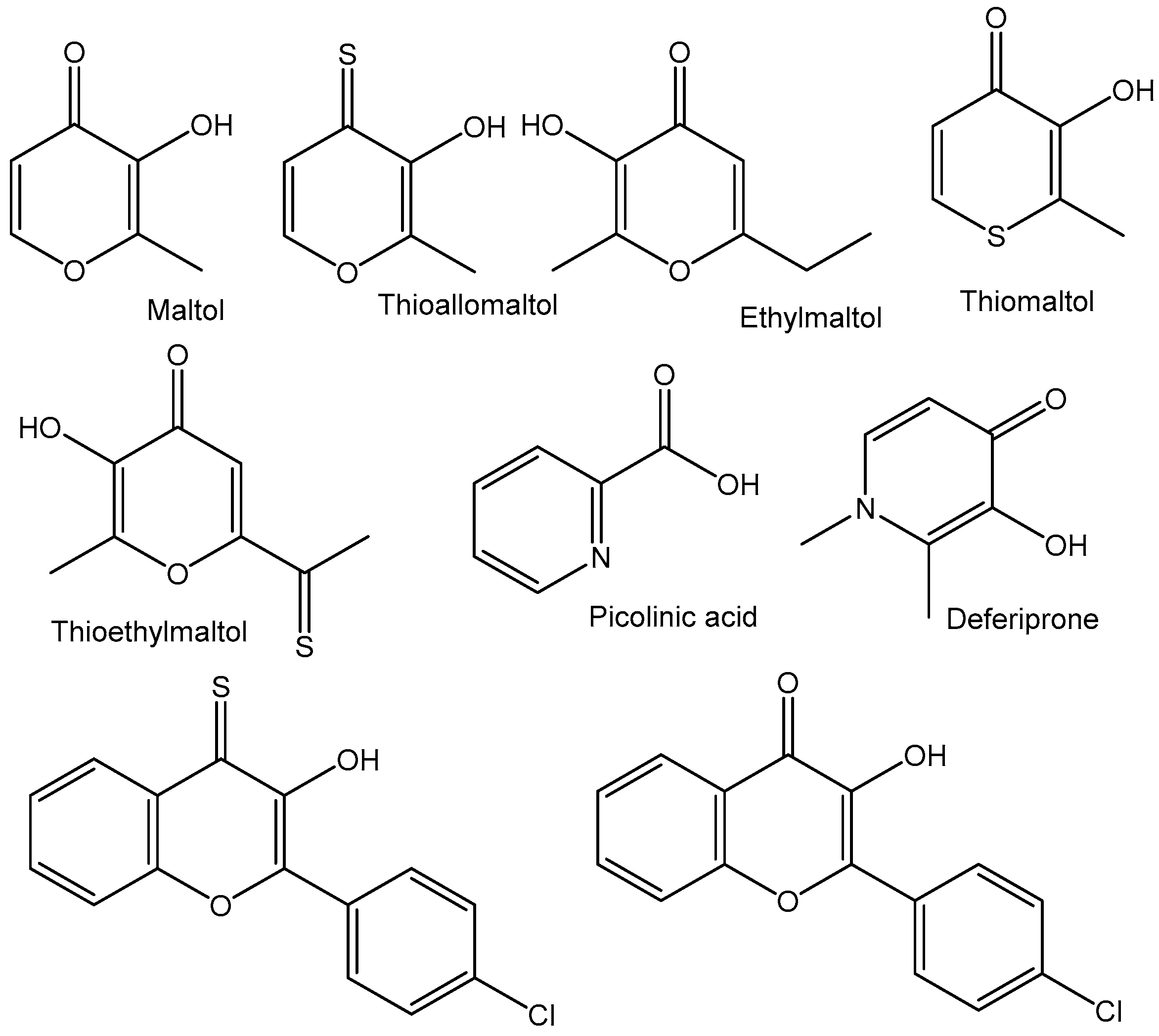
4.2. Tungstenocenes
5. d-Elements of Group 7
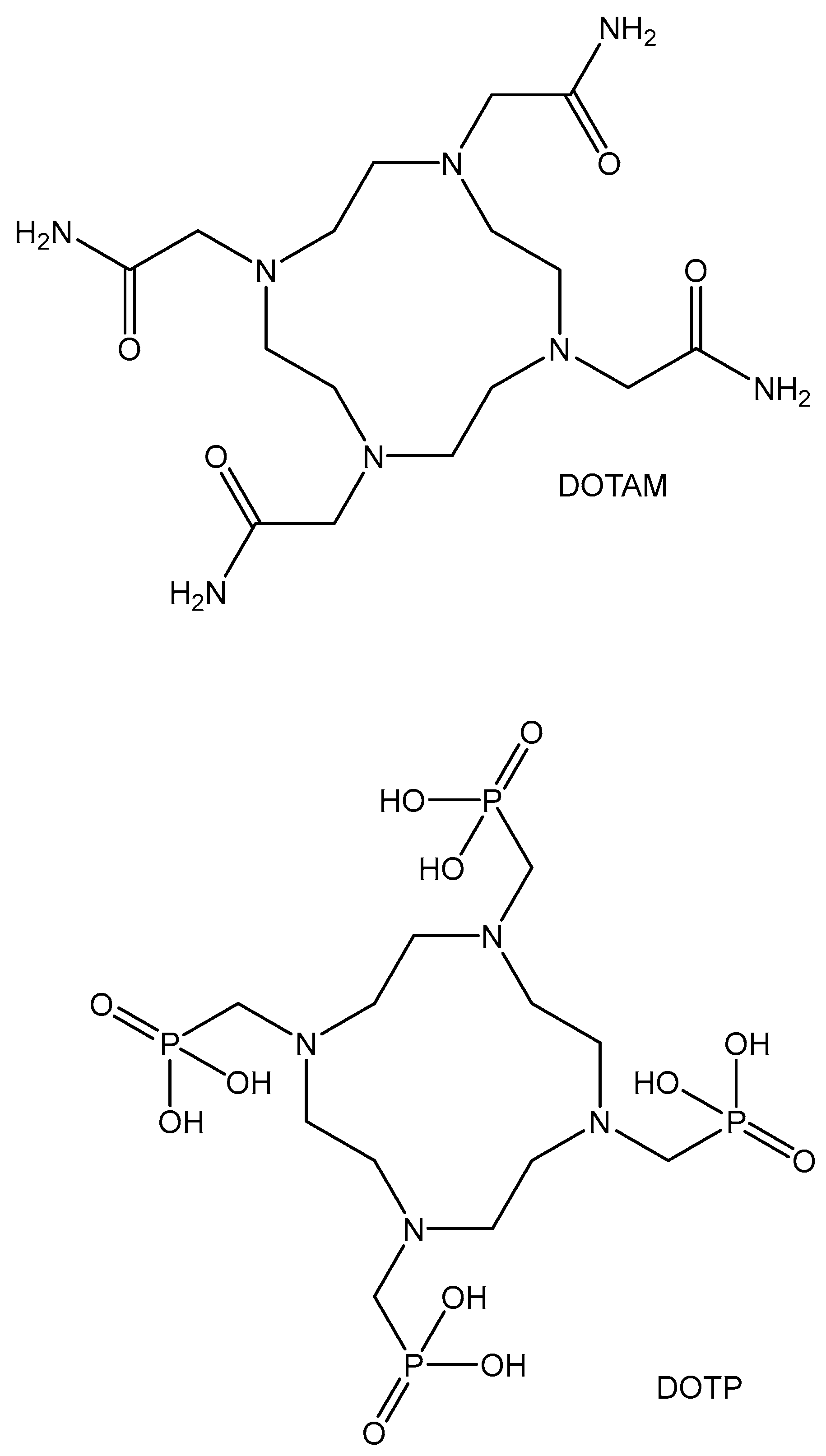
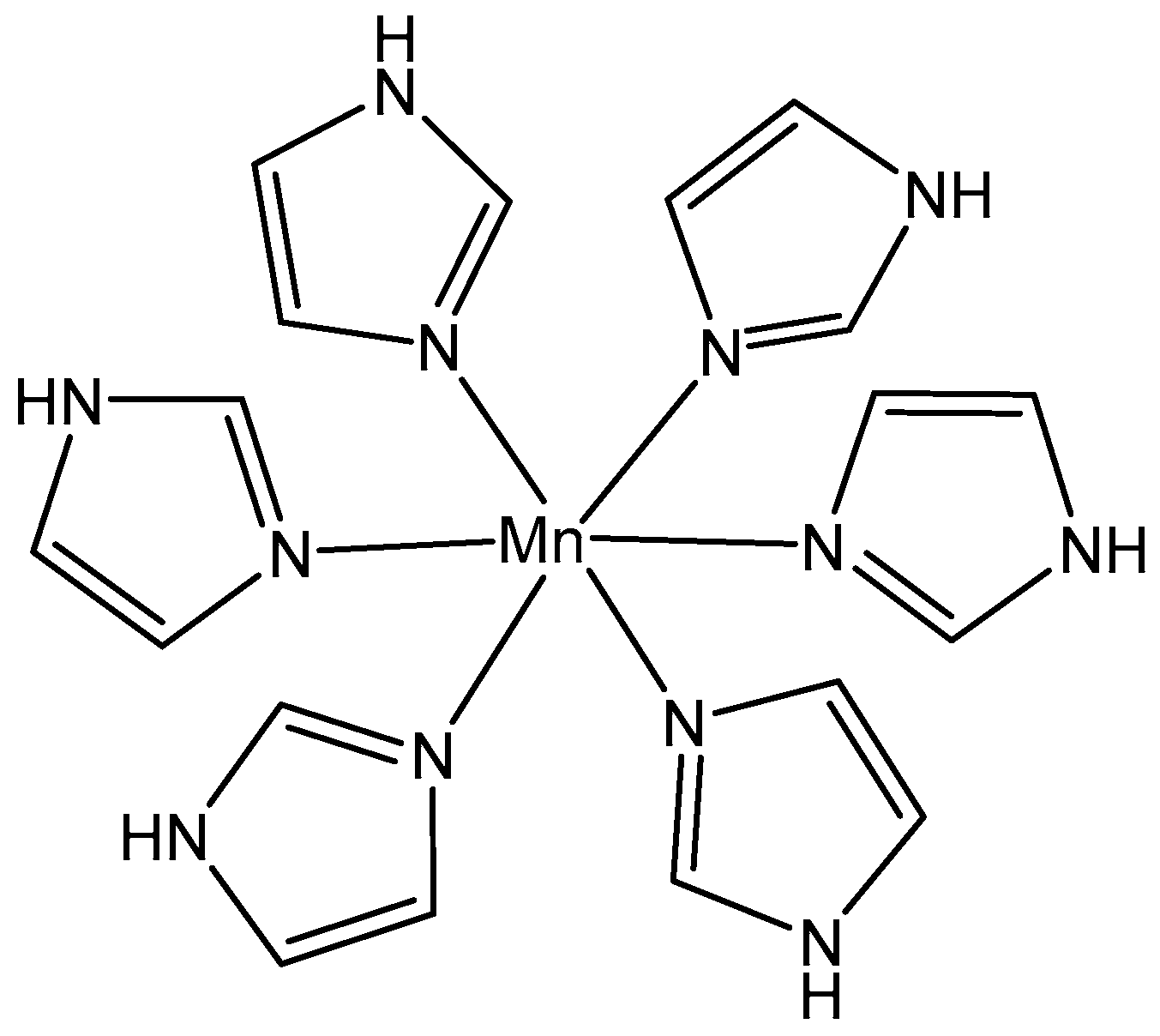
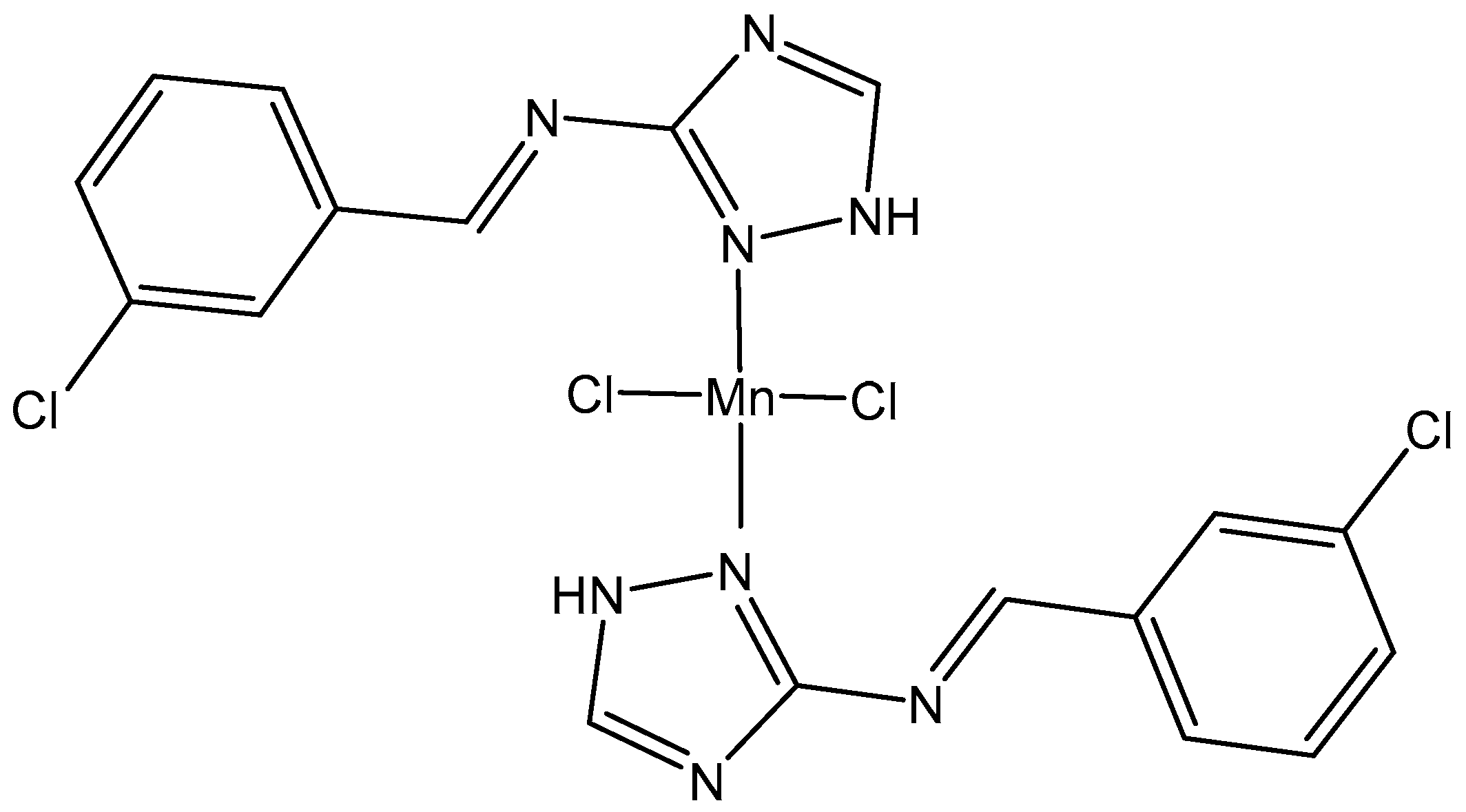
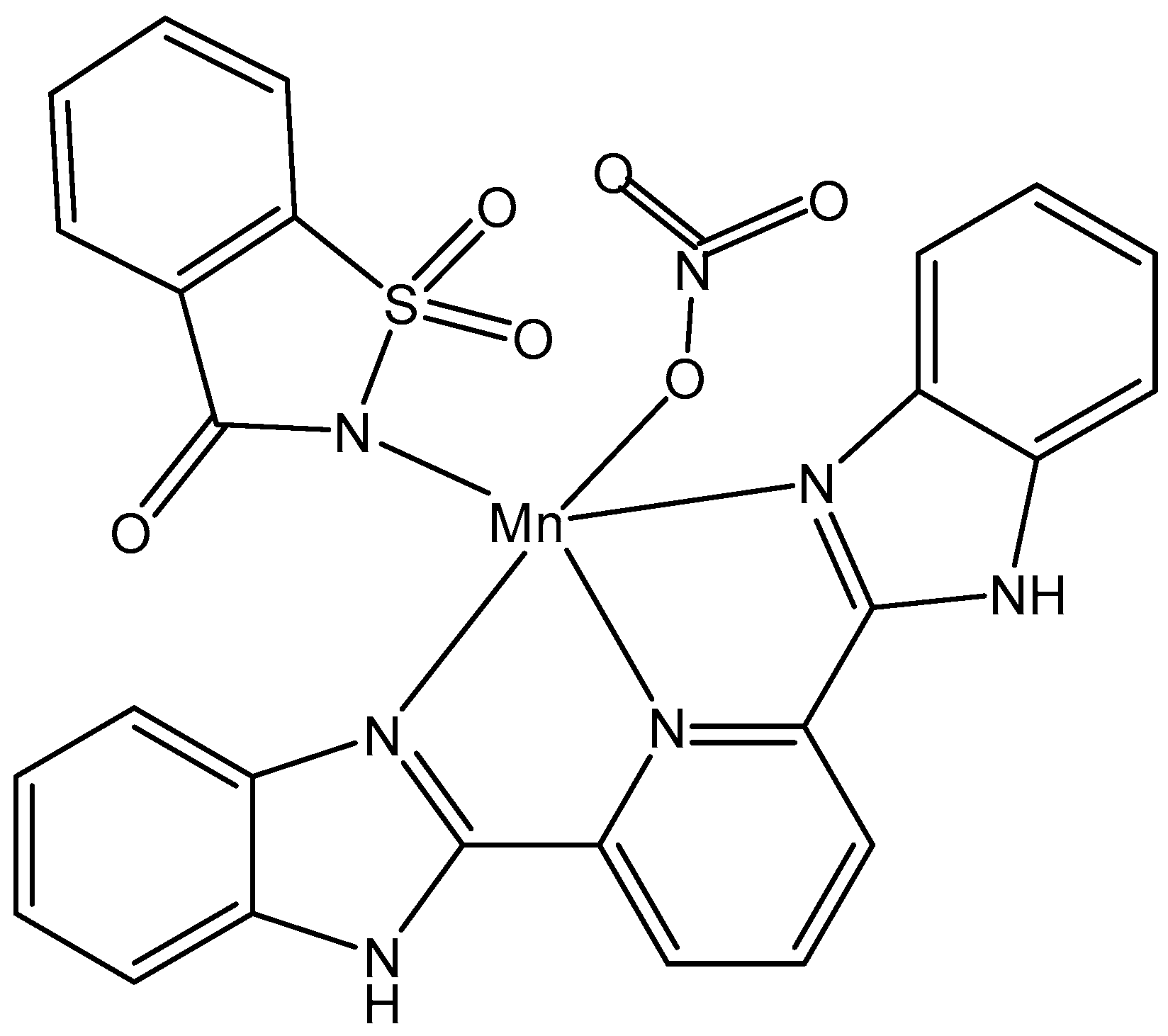
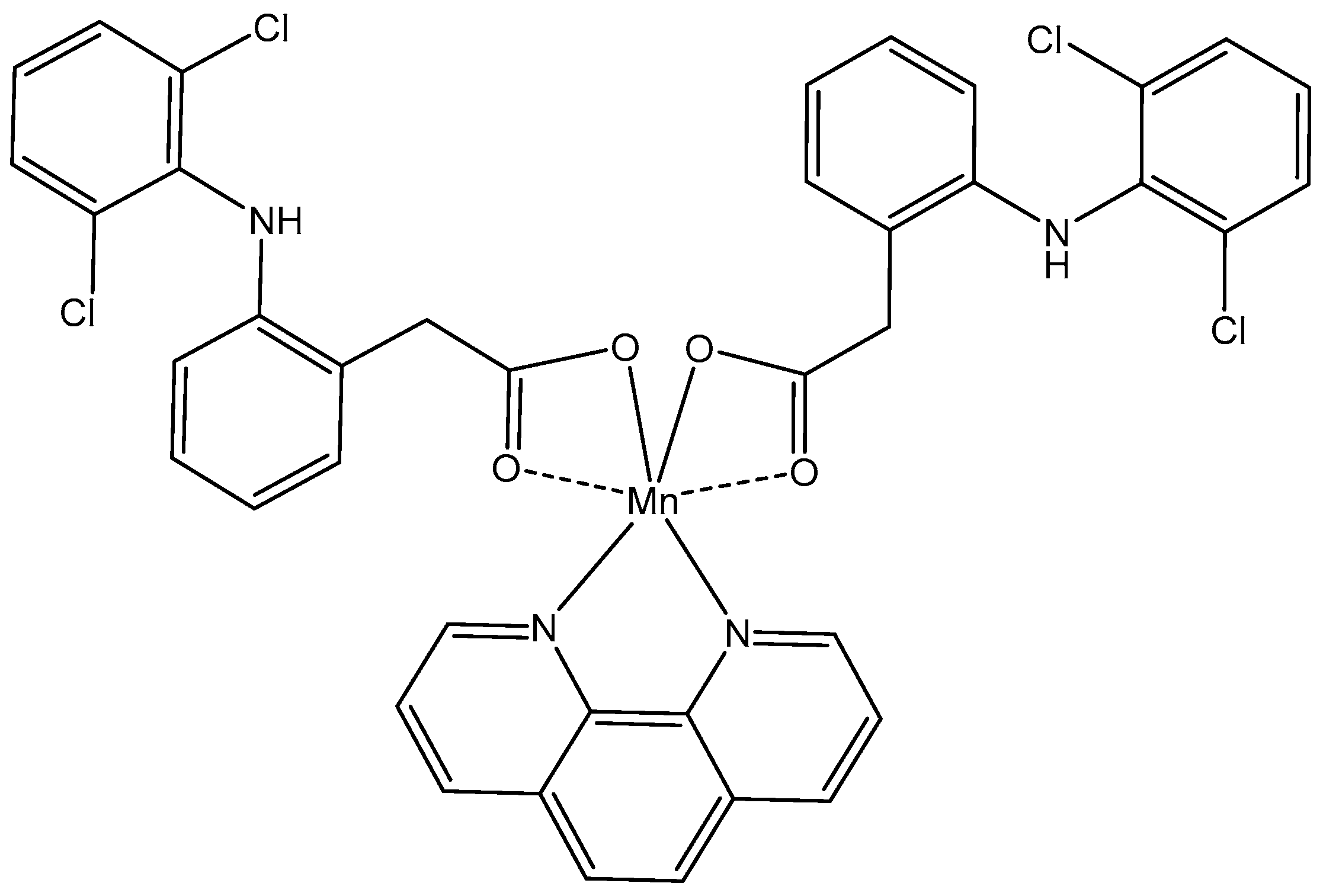
5.1. Manganese(II) Complexes
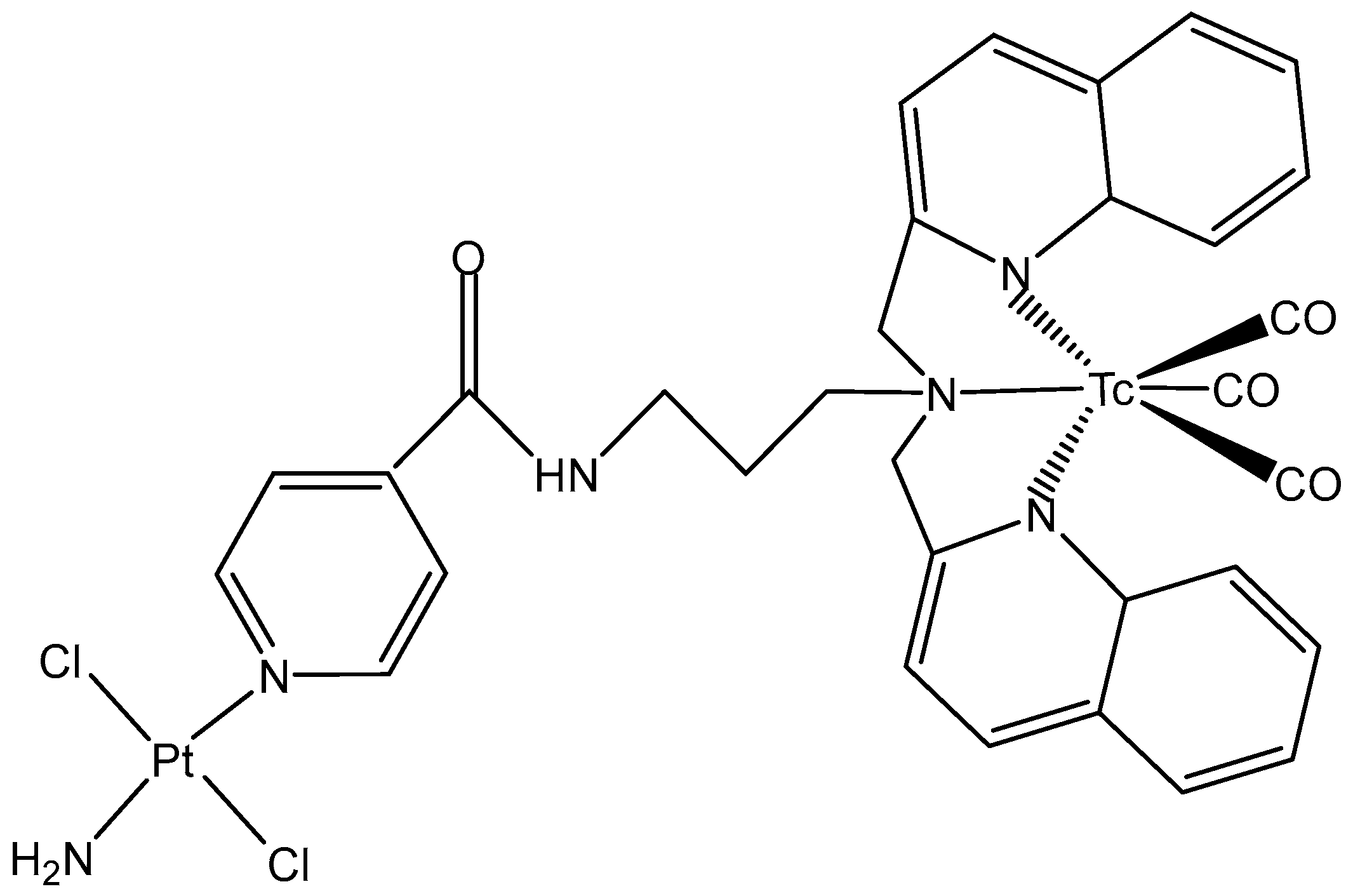
5.2. Technetium Complexes
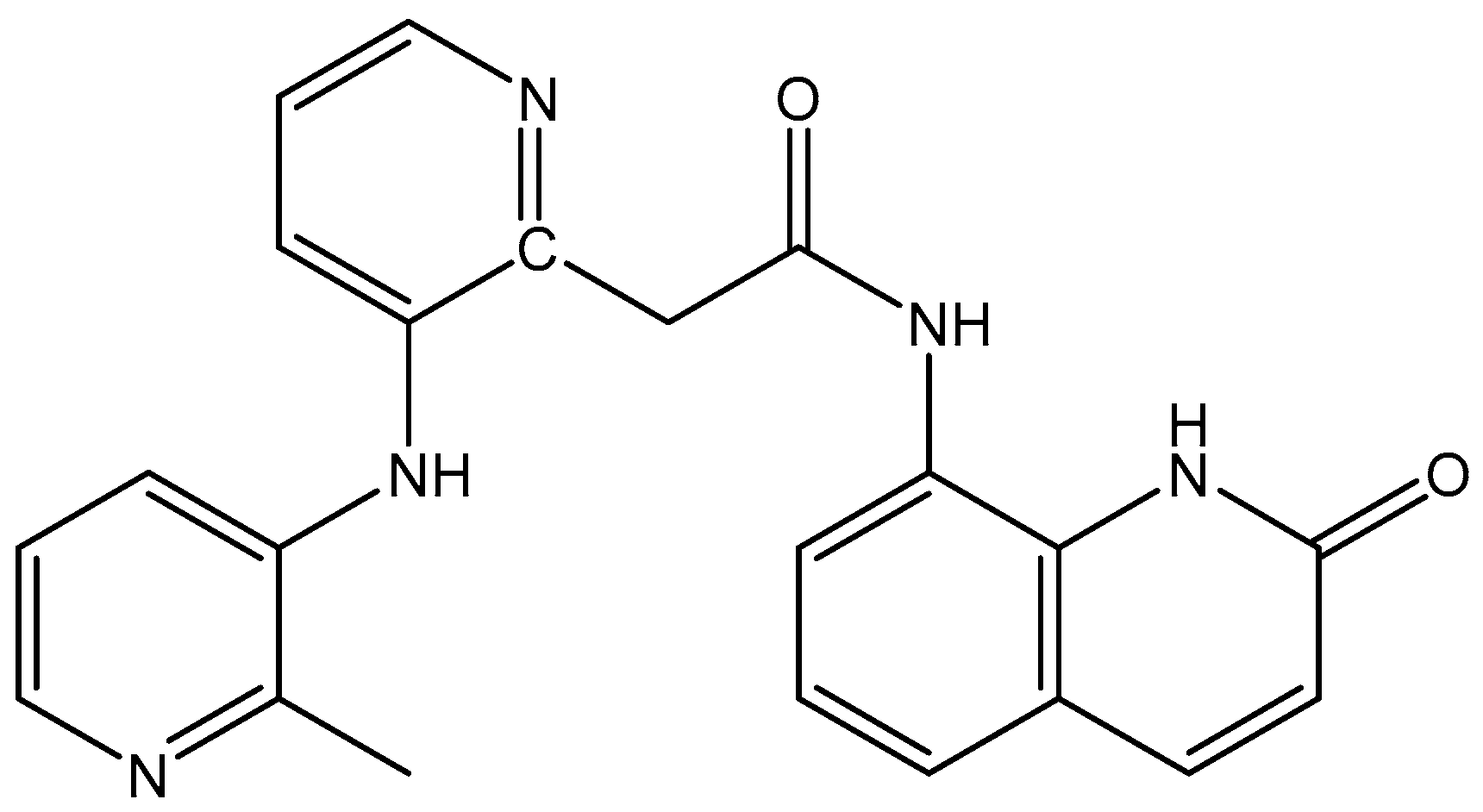
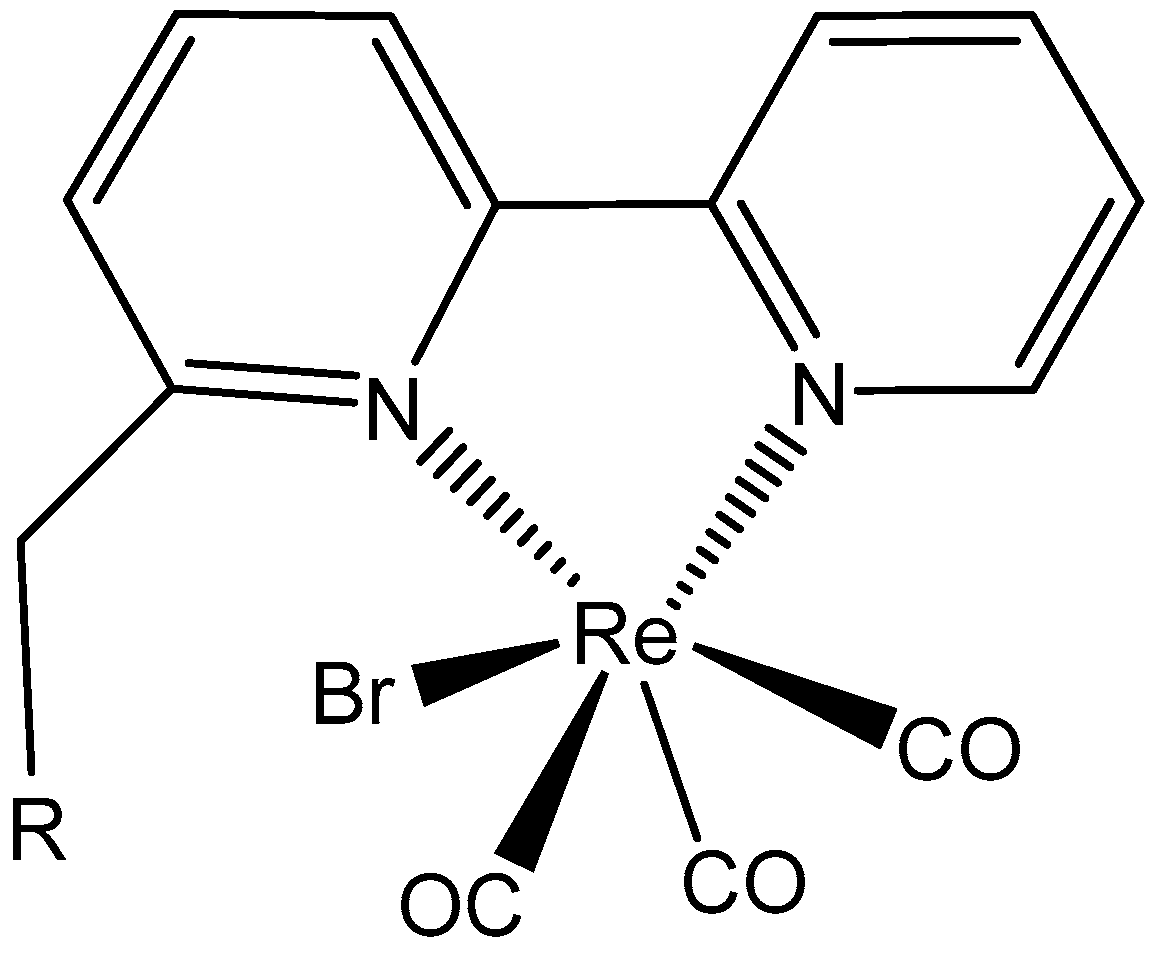
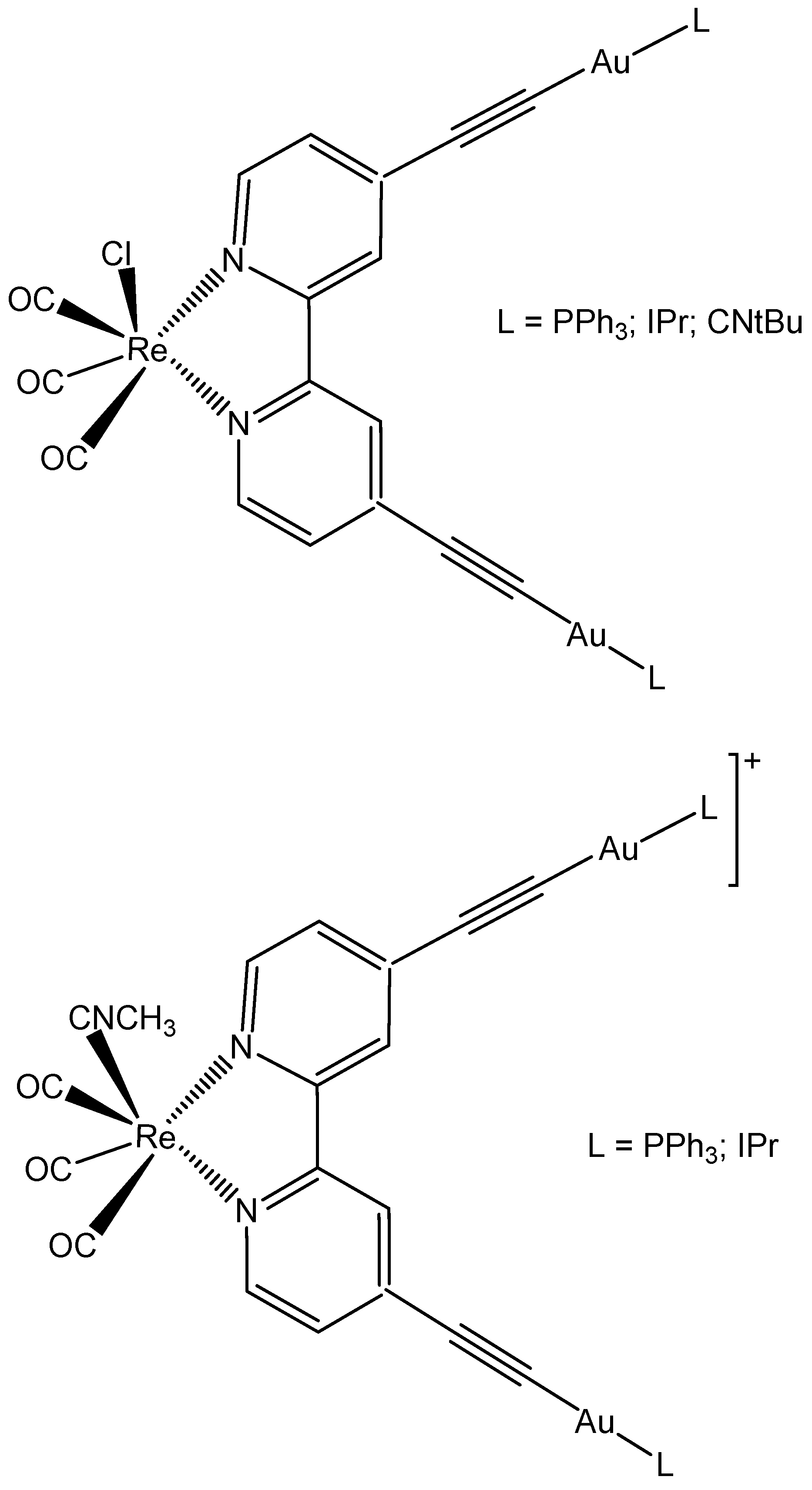
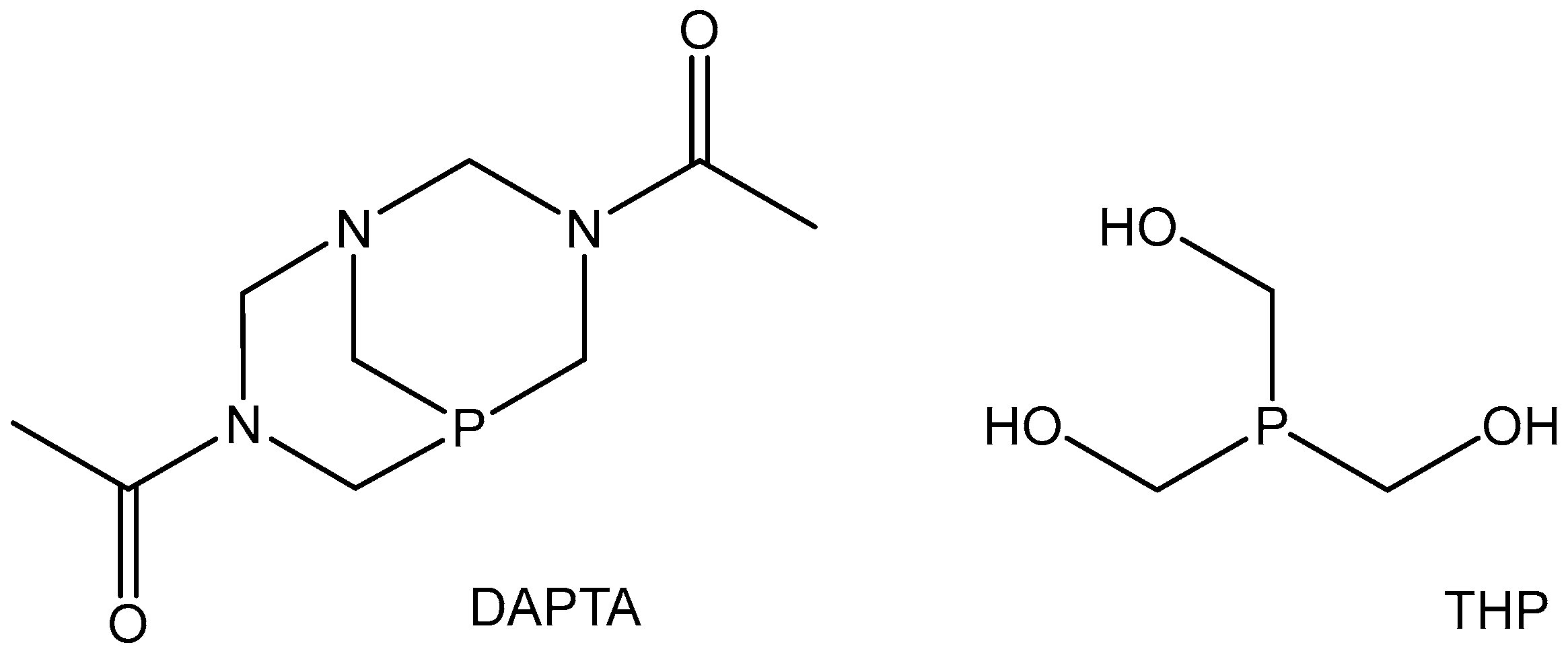
5.3. Rhenium Complexes
6. Conclusions and Prospective
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Coverdale, J.P.; Laroiya-McCarron, T.; Romero-Canelón, I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics 2019, 7, 31. [Google Scholar] [CrossRef]
- Sohrabi, M.; Saeedi, M.; Larijani, B.; Mahdavi, M. Recent advances in biological activities of rhodium complexes: Their applications in drug discovery research. Eur. J. Med. Chem. 2021, 216, 113308. [Google Scholar] [CrossRef]
- Scattolin, T.; Voloshkin, V.A.; Visentin, F.; Nolan, S.P. A critical review of palladium organometallic anticancer agents. Cell Rep. Phys. Sci. 2021, 2, 100446. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, C.; Wu, K.J.; Leung, C.H. Iridium (III) complexes targeting apoptotic cell death in cancer cells. Molecules 2019, 24, 2739. [Google Scholar] [CrossRef]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond platinums: Gold complexes as anticancer agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar]
- Soumya, R.S.; Hela, P.G. Nano silver based targeted drug delivery for treatment of cancer. Der Pharm. Lett. 2013, 5, 189–197. [Google Scholar]
- Kostova, I. Biological and Medical Significance of Chemical Elements; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023. [Google Scholar]
- Kostova, I. The Role of Complexes of Biogenic Metals in Living Organisms. Inorganics 2023, 11, 56. [Google Scholar] [CrossRef]
- Goswami, A.K.; Kostova, I. Medicinal and Biological Inorganic Chemistry; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2022. [Google Scholar]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Impl. Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Noumbissi, S.; Scarano, A.; Gupta, S. A literature review study on atomic ions dissolution of titanium and its alloys in implant dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Emwas, A.H. Therapeutic properties of vanadium complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmaco-toxicological mechanisms and multi-applications with a summary of further research trends. J. Tr. Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Anke, M. Vanadium-an element both essential and toxic to plants, animals and humans. Anal. Real. Acad. Nac. Farm. 2004, 70, 961–999. [Google Scholar]
- Rehder, D. Vanadium: Biological, Environmental, and Engineering Aspects. Adv. Chem. Res. 2020, 2, 002. [Google Scholar] [CrossRef]
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and melanoma: A systematic review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Treviño, S.; Diaz, A. Vanadium and insulin: Partners in metabolic regulation. J. Inorg. Biochem. 2020, 208, 111094. [Google Scholar] [CrossRef]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic or anticancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Nakai, M.; Fukui, H. Comparison of various properties between titanium-tantalum alloy and pure titanium for biomedical applications. Mater. Transact. 2007, 48, 380–384. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.T.; Qiu, J.X. The clinical application of porous tantalum and its new development for bone tissue engineering. Materials 2021, 14, 2647. [Google Scholar] [CrossRef]
- Halmi, M.I.E.; Ahmad, S.A. Chemistry, biochemistry, toxicity and pollution of molybdenum: A mini review. J. Biochem. Microbiol. Biotechnol. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Schwarz, G. Molybdenum cofactor and human disease. Curr. Opin. Chem. Biol. 2016, 31, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R.; Bittner, F. Cell biology of molybdenum. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Hille, R.; Nishino, T.; Bittner, F. Molybdenum enzymes in higher organisms. Coord. Chem. Rev. 2011, 255, 1179–1205. [Google Scholar] [CrossRef]
- Rana, M.; Bhantana, P.; Sun, X.C.; Imran, M.; Shaaban, M.; Moussa, M.; Hu, C.X. Molybdenum as an essential element for crops: An overview. Int. J. Sci. Res. Growth 2020, 24, 18535. [Google Scholar]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 2, 101–139. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, N.; Karimi-Nazari, E.; Yaghoubi, F.; Zarei, S.; Azadmanesh, F.; Reza, J.Z.; Sargazi, S. Molybdenum cofactor biology and disorders related to its deficiency; a review study. J. Nutr. Food Secur. 2019, 4, 206–217. [Google Scholar] [CrossRef]
- Novotny, J.A.; Peterson, C.A. Molybdenum. Adv. Nutr. 2018, 9, 272–273. [Google Scholar] [CrossRef]
- Crawford, A.M.; Cotelesage, J.J.; Prince, R.C.; George, G.N. The catalytic mechanisms of the molybdenum and tungsten enzymes. In Metallocofactors that Activate Small Molecules; Ribbe, M., Ed.; Structure and Bonding; Springer: Cham, Switzerland, 2018; Volume 179. [Google Scholar]
- Iksat, N.N.; Zhangazin, S.B.; Madirov, A.A.; Omarov, R.T. Effect of molybdenum on the activity of molybdoenzymes. Eurasian J. Appl. Biotechnol. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Xing, Y.; Cai, Y.; Cheng, J.; Xu, X. Applications of molybdenum oxide nanomaterials in the synergistic diagnosis and treatment of tumor. Appl. Nanosci. 2020, 10, 2069–2083. [Google Scholar] [CrossRef]
- Bolt, A.M.; Mann, K.K. Tungsten: An emerging toxicant, alone or in combination. Curr. Environ. Health Rep. 2016, 3, 405–415. [Google Scholar] [CrossRef]
- Rieber, M. Cancer pro-oxidant therapy through copper redox cycling: Repurposing disulfiram and tetrathiomolybdate. Curr. Pharm. Des. 2020, 26, 4461–4466. [Google Scholar] [CrossRef]
- Bertinat, R.; Westermeier, F.; Gatica, R.; Nualart, F. Sodium tungstate: Is it a safe option for a chronic disease setting, such as diabetes? J. Cell. Physiol. 2019, 234, 51–60. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between polyoxometalates and biological systems: From drug design to artificial enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, J.F.; Han, Q.; Shangguan, P.; Liu, L.L.; Chen, L.J.; Song, Y.F. Multicomponent Self-Assembly of a Giant Heterometallic Polyoxotungstate Supercluster with Antitumor Activity. Angew. Chem. Int. Ed. Engl. 2021, 60, 11253–11257. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Manganese: Its role in disease and health. Met. Ions Life Sci. 2019, 19, 253–266. [Google Scholar]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Lingappa, U.F.; Monteverde, D.R.; Magyar, J.S.; Valentine, J.S.; Fischer, W.W. How manganese empowered life with dioxygen (and vice versa). Free Rad. Biol. Med. 2019, 140, 113–125. [Google Scholar] [CrossRef]
- Anagianni, S.; Tuschl, K. Genetic disorders of manganese metabolism. Curr. Neurol. Neurosci. Rep. 2019, 19, 33. [Google Scholar] [CrossRef]
- Martins, A.C.; Krum, B.N.; Queirós, L.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Aschner, M. Manganese in the diet: Bioaccessibility, adequate intake, and neurotoxicological effects. J. Agric. Food Chem. 2020, 68, 12893–12903. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Koren, S.A.; Abisambra, J.F. Manganese-enhanced magnetic resonance imaging: Overview and central nervous system applications with a focus on neurodegeneration. Front. Aging Neurosci. 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Doan, B.-T.; Meme, S.; Beloeil, J.-C. General Principles of MRI. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; Merbach, A., Helm, L., Tóth, É., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2013; Volume 1, pp. 1–24. [Google Scholar]
- Brandt, M.; Cardinale, J.; Rausch, I.; Mindt, T.L. Manganese in PET imaging: Opportunities and challenges. J. Label. Comp. Radiopharmac. 2019, 62, 541–551. [Google Scholar] [CrossRef]
- Garda, Z.; Molnár, E.; Kálmán, F.K.; Botár, R.; Nagy, V.; Baranyai, Z.; Brücher, E.; Kovács, Z.; Tóth, I.; Tircsó, G. Effect of the Nature of Donor Atoms on the Thermodynamic, Kinetic and Relaxation Properties of Mn(II) Complexes Formed With Some Trisubstituted 12- Membered Macrocyclic Ligands. Front. Chem. 2018, 6, 232. [Google Scholar] [CrossRef]
- Drahoš, B.; Kotek, J.; Hermann, P.; Lukeš, I.; Tóth, É. Mn2+ Complexes with Pyridine-Containing 15- Membered Macrocycles: Thermodynamic, Kinetic, Crystallographic, and 1H/17O Relaxation Studies. Inorg. Chem. 2010, 49, 3224–3238. [Google Scholar] [CrossRef]
- Drahoš, B.; Lukeš, I.; Tóth, É. Manganese(II) Complexes as Potential Contrast Agents for MRI. Eur. J. Inorg. Chem. 2012, 12, 1975–1986. [Google Scholar] [CrossRef]
- Ruth, T.J. The shortage of technetium-99m and possible solutions. Annu. Rev. Nucl. Part. Sci. 2020, 70, 77–94. [Google Scholar] [CrossRef]
- Zolle, I. Technetium-99m Pharmaceuticals: Preparation and Quality Control in Nuclear Medicine; Springer: Berlin, Germany, 2007. [Google Scholar]
- Mandegaran, R.; Dhillon, S.; Jen, H. Beyond the bones and joints: A review of ligamentous injuries of the foot and ankle on 99mTc-MDP-SPECT/CT. Br. J. Radiol. 2019, 92, 20190506. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J. A Review of 99mTc-labeled Tumor Metabolic Imaging Agents. Mini Rev. Med. Chem. 2022, 22, 1586–1596. [Google Scholar] [CrossRef]
- Haase, A.A.; Bauer, E.B.; Kühn, F.E.; Crans, D.C. Speciation and toxicity of rhenium salts, organometallics and coordination complexes. Coord. Chem. Rev. 2019, 394, 135–161. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Pourhabib, Z.; Ranjbar, H.; Bahrami Samani, A.; Shokri, A.A. Appraisement of 186/188Re-HEDP, a new compositional radiopharmaceutical. J. Radioanal. Nucl. Chem. 2019, 322, 1133–1138. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Cheng, L. Titanium-based nanomaterials for cancer theranostics. Coord. Chem. Rev. 2021, 430, 213662. [Google Scholar] [CrossRef]
- Muhammad, N.; Guo, Z. Metal-based anticancer chemotherapeutic agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef]
- Skoupilova, H.; Hrstka, R.; Bartosik, M. Titanocenes as anticancer agents: Recent insights. Med. Chem. 2017, 13, 334–344. [Google Scholar] [CrossRef]
- Zhao, T.K.; Grützke, M.; Gotz, K.H.; Druzhenko, T.; Huhn, T. Synthesis and X-ray structure analysis of cytotoxic heptacoordinate sulfonamide salan titanium(IV)-bischelates. Dalton Trans. 2015, 44, 16475–16485. [Google Scholar] [CrossRef]
- Glasner, H.; Tshuva, E.Y. A marked synergistic effect in antitumor activity of salan titanium(IV) complexes bearing two differently substituted aromatic rings. J. Am. Chem. Soc. 2011, 133, 16812–16814. [Google Scholar] [CrossRef]
- Shpilt, Z.; Tshuva, E.Y. Binding of the anticancer Ti(IV) complex phenolaTi to serum proteins: Thermodynamic and kinetic aspects. J. Inorg. Biochem. 2022, 232, 111817. [Google Scholar] [CrossRef]
- Miller, M.; Mellul, A.; Braun, M.; Sherill-Rofe, D.; Cohen, E.; Shpilt, Z.; Tabach, Y. Titanium tackles the endoplasmic reticulum: A first genomic study on a titanium anticancer metallodrug. Iscience 2020, 23, 7. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, P.; Liu, N.; Li, S.; Yang, M.; Yang, Z. Facile synthesis of [ONON] type titanium (IV) bis-chelated complexes in alcoholic solvents and evaluation of anti-tumor activity. J. Inorg. Biochem. 2022, 235, 111925. [Google Scholar] [CrossRef]
- Das, R.; Chatterjee, D.R.; Shard, A. Oxidation states in metallocenes: A key mechanistic component in cancer alleviation. Coord. Chem. Rev. 2024, 504, 215666. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Miller, M. Coordination complexes of titanium(IV) for anticancer therapy. Met. Ions Life Sci. 2018, 18, 219–250. [Google Scholar]
- Serrano, R.; Martinez-Argudo, I.; Fernandez-Sanchez, M.; Pacheco-Linan, P.J.; Bravo, I.; Cohen, B.; Calero, R.; Ruiz, M.J. New titanocene derivative with improved stability and binding ability to albumin exhibits high anticancer activity. J. Inorg. Biochem. 2021, 223, 111562. [Google Scholar] [CrossRef]
- Levina, A.; Crans, D.C.; Lay, P.A. Advantageous reactivity of unstable metal complexes: Potential applications of metal-based anticancer drugs for intratumoral injections. Pharmaceutics 2022, 14, 790. [Google Scholar] [CrossRef]
- Meker, S.; Braitbard, O.; Hall, M.D.; Hochman, J.; Tshuva, E.Y. Specific design of titanium(IV) phenolato chelates yields stable and accessible, effective and selective anticancer agents. Chem.-Eur. J. 2016, 22, 9986–9995. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Vazquez-Salgado, A.M.; Rivero, K.I.; Negron, L.J.; Delgado, Y.; Benjamin-Rivera, J.A.; Vazquez-Maldonado, A.L.; Parks, T.B.; Munet-Colon, C.; Tinoco, A.D. Expanding the therapeutic potential of the iron chelator deferasirox in the development of aqueous stable Ti(IV) anticancer complexes. Inorg. Chem. 2017, 56, 7788–7802. [Google Scholar] [CrossRef]
- Guk, D.A.; Gibadullina, K.R.; Burlutskiy, R.O.; Pavlov, K.G.; Moiseeva, A.A.; Tafeenko, V.A.; Beloglazkina, E.K. New Titanocene (IV) Dicarboxylates with Potential Cytotoxicity: Synthesis, Structure, Stability and Electrochemistry. Int. J. Mol. Sci. 2023, 24, 3340. [Google Scholar] [CrossRef]
- Tsave, O.; Iordanidou, C.; Hatzidimitriou, A.; Yavropoulou, M.P.; Kassi, E.N.; Nasiri-Ansari, N.; Salifoglou, A. Structural Speciation of Ti(IV)-(α-Hydroxycarboxylic Acid) Complexes in Metabolism-Related (Patho) Physiology—In Vitro Approaches to (Pre) Adipocyte Differentiation and Mineralization. Int. J. Mol. Sci. 2023, 24, 11865. [Google Scholar] [CrossRef]
- Fernández-Gallardo, J.; Elie, B.T.; Sadhukha, T.; Prabha, S.; Sanaú, M.; Rotenberg, S.A.; Ramos, J.W.; Contel, M. Heterometallic titanium–gold complexes inhibit renal cancer cells in vitro and in vivo. Chem. Sci. 2015, 6, 5269–5283. [Google Scholar] [CrossRef]
- Elie, B.T.; Fernández-Gallardo, J.; Curado, N.; Cornejo, M.A.; Ramos, J.W.; Contel, M. Bimetallic Titanocene-Gold Phosphane Complexes Inhibit Invasion, Metastasis, and Angiogenesis-Associated Signaling Molecules in Renal Cancer. Eur. J. Med. Chem. 2019, 161, 310–322. [Google Scholar] [CrossRef]
- Allen, O.R.; Knox, R.J.; McGowan, P.C. Functionalised cyclopentadienyl zirconium compounds as potential anticancer drugs. Dalton Trans. 2008, 39, 5293–5295. [Google Scholar] [CrossRef]
- Lord, R.M.; Mannion, J.J.; Hebden, A.J.; Nako, A.E.; Crossley, B.D.; McMullon, M.W.; Janeway, F.D.; Phillips, R.M.; McGowan, P.C. Mechanistic and Cytotoxicity Studies of Group IV β-Diketonate Complexes. ChemMedChem 2014, 9, 1136–1139. [Google Scholar] [CrossRef]
- Schneider, F.; Zhao, T.; Huhn, T. Cytotoxic heteroleptic heptacoordinate salan zirconium(IV)-bis-chelates—synthesis, aqueous stability and X-ray structure analysis. Chem. Commun. 2016, 52, 10151–10154. [Google Scholar] [CrossRef]
- Abdolmaleki, S.; Ghadermazi, M.; Ashengroph, M.; Saffari, A.; Sabzkohi, S.M. Cobalt(II), zirconium(IV), calcium(II) complexes with dipicolinic acid and imidazole derivatives: X-ray studies, thermal analyses, evaluation as in vitro antibacterial and cytotoxic agents. Inorg. Chim. Acta 2018, 480, 70–82. [Google Scholar] [CrossRef]
- El-Shwiniy, W.H.; Shehab, W.S.; Mohamed, S.F.; Ibrahium, H.G. Synthesis and cytotoxic evaluation of some substituted pyrazole zirconium(IV) complexes and their biological assay. Appl. Organomet. Chem. 2018, 32, e4503. [Google Scholar] [CrossRef]
- Malghe, Y.S.; Prabhu, R.C.; Raut, R.W. Synthesis, characterization and biological activities of mixed ligand Zr (IV) complexes. Acta Pol. Pharm. 2009, 66, 45–50. [Google Scholar]
- Yang, M.; Liu, N.; Wang, P.; Zhao, T. Synthesis and cytotoxicity study of water soluble 8-hydroxyquinoline stabilized zirconium(IV) complexes. Inorg. Chem. Commun. 2023, 153, 110795. [Google Scholar] [CrossRef]
- Adam, M.S.S.; El-Hady, O.M.; Makhlouf, M.M.; Bayazeed, A.; El-Metwaly, N.M.; Mohamad, A.D.M. Effect of oxy-vanadium (IV) and oxy-zirconium (IV) ions in O, N-bidentate arylhydrazone complexes on their catalytic and biological potentials that supported via computerized usages. J. Taiw. Inst. Chem. Engin. 2022, 132, 104168. [Google Scholar] [CrossRef]
- Al-Hakimi, A.N.; Alminderej, F.; Aroua, L.; Alhag, S.K.; Alfaifi, M.Y.; Samir, M.O.; Mahyoub, J.A.; Elbehairi, S.E.I.; Alnafisah, A.S. Design, synthesis, characterization of zirconium (IV), cadmium (II) and iron (III) complexes derived from Schiff base 2- aminomethylbenzimidazole, 2-hydroxynaphtadehyde and evaluation of their biological activity. Arab. J. Chem. 2020, 13, 7378–7389. [Google Scholar] [CrossRef]
- Bhatt, N.B.; Pandya, D.N.; Wadas, T.J. Recent Advances in Zirconium-89 Chelator Development. Molecules 2018, 23, 638. [Google Scholar] [CrossRef]
- Melendez-Alafort, L.; Ferro-Flores, G.; De Nardo, L.; Ocampo-García, B.; Bolzati, C. Zirconium immune-complexes for PET molecular imaging: Current status and prospects. Coord. Chem. Rev. 2023, 479, 215005. [Google Scholar] [CrossRef]
- Pandya, D.N.; Bhatt, N.; Yuan, H.; Day, C.S.; Ehrmann, B.M.; Wright, M.; Bierbach, U.; Wadas, T.J. Zirconium tetraazamacrocycle complexes display extraordinary stability and provide a new strategy for zirconium-89-based radiopharmaceutical development. Chem. Sci. 2017, 8, 2309–2314. [Google Scholar] [CrossRef]
- Pandya, D.N.; Henry, K.E.; Day, C.S.; Graves, S.A.; Nagle, V.L.; Dilling, T.R.; Sinha, A.; Ehrmann, B.M.; Bhatt, N.B.; Menda, Y.; et al. Polyazamacrocycle ligands facilitate Zr-89 radiochemistry and Yield Zr-89 complexes with remarkable stability. Inorg. Chem. 2020, 59, 17473–17487. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, P.; Liu, N.; Zhao, W.; Yang, M.; Li, S.; Huhn, T. Synthesis and X-ray structure analysis of cytotoxic heptacoordinated Salan hafnium (IV) complexes stabilized with 2, 6-dipicolinic acid. J. Inorg. Biochem. 2023, 240, 112094. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, P.; Zhang, Q.; Liu, N.; Wang, Y.; Wang, P.; Zhao, T. Novel bimetallic oxido-bridged phenolato hafniumIV complex with enhanced anti-tumor activity and aqueous stability. Res. Chem. 2023, 6, 101161. [Google Scholar] [CrossRef]
- Jayaraman, V.; Bhavesh, G.; Chinnathambi, S.; Ganesan, S.; Aruna, P. Synthesis and characterization of hafnium oxide nanoparticles for bio-safety. Mater. Express 2014, 4, 375–383. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Campbell, S.A.; Karthikeyan, S. Finite element analysis of hollow out of-plane HfO2 microneedles for transdermal drug delivery applications. Biomed. Microdevices 2018, 20, 19. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Y.; Zhang, H.; Xia, Z.; Xie, T.; Li, W.; Zhong, D.; Zhu, H.; Zhou, M. Gram-scale synthesis of highly biocompatible and intravenous injectable hafnium oxide nanocrystal with enhanced radiotherapy efficacy for cancer theranostic. Biomaterials 2020, 226, 119538. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal− organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar]
- Marill, J.; Anesary, N.M.; Zhang, P.; Vivet, S.; Borghi, E.; Levy, L.; Pottier, A. Hafnium oxide nanoparticles: Toward an in vitro predictive biological effect? Radiat. Oncol. 2014, 9, 150. [Google Scholar] [CrossRef]
- Pottier, A.; Borghi, E.; Levy, L. New use of metals as nanosized radioenhancers. Anticancer Res. 2014, 34, 443–453. [Google Scholar]
- Prasad, K.S.; Ramachandrappa, S.U. Potential medicinal applications of vanadium and its coordination compounds in current research prospects: A review. Curr. Bioact. Comp. 2020, 16, 201–209. [Google Scholar] [CrossRef]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Ertik, O.; Kalındemirtaş, F.D.; Kaya, B.; Yanardag, R.; Kuruca, S.E.; Şahin, O.; Ülküseven, B. Oxovanadium (IV) complexes with tetradentate thiosemicarbazones. Synthesis, characterization, anticancer enzyme inhibition and in vitro cytotoxicity on breast cancer cells. Polyhedron 2021, 202, 115192. [Google Scholar] [CrossRef]
- Clarke, M.J.; Zhu, F.; Frasca, D.R. Non-platinum chemotherapeutic metallopharmaceuticals. Chem. Rev. 1999, 99, 2511–2533. [Google Scholar] [CrossRef]
- Brandhonneur, N.; Boucaud, Y.; Verger, A.; Dumait, N.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum cluster loaded PLGA nanoparticles as efficient tools against epithelial ovarian cancer. Int. J. Pharmaceut. 2021, 592, 120079. [Google Scholar] [CrossRef]
- Saraiva, M.S.; Quintal, S.; Portugal, F.C.M.; Lopes, T.A.; Félix, V.; Nogueira, J.M.F.; Meireles, M.; Drew, M.G.B.; Calhorda, M.J. Nitrogen donor ligands bearing N-H groups: Effect on catalytic and cytotoxic activity of molybdenum η3-allyldicarbonyl complexes. J. Organomet. Chem. 2008, 693, 3411–3418. [Google Scholar] [CrossRef]
- Köpf-Maier, P.; Leitner, M.; Köpf, H. Tumor Inhibition by Metallocenes: Antitumor Activity of Niobocene and Tungstocene Dichlorides. J. Inorg. Nucl. Chem. 1980, 42, 1789–1791. [Google Scholar] [CrossRef]
- Dominguez-Garcia, M.; Ortega-Zuniga, C.; Meléndez, E. New tungstenocenes containing 3- hydroxy-4-pyrone ligands: Antiproliferative activity on HT-29 and MCF-7 cell lines and binding to human serum albumin studied by fluorescence spectroscopy and molecular modeling methods. J. Biol. Inorg. Chem. 2013, 18, 195–209. [Google Scholar] [CrossRef]
- Cseh, K.; Berasaluce, I.; Fuchs, V.; Banc, A.; Schweikert, A.; Prado-Roller, A.; Keppler, B.K. Anticancer Tungstenocenes with a Diverse Set of (O, O–),(O, S–) and (O, N–) Chelates—A Detailed Biological Study Using an Improved Evaluation via 3D Spheroid Models. Pharmaceutics 2023, 15, 1875. [Google Scholar] [CrossRef]
- Prihantono, I.R.; Raya, I.; Warsinggih, I. Potential anticanceractivity of Mn(II) complexes containing arginine dithiocarba-mate ligand on MCF-7 breast cancer cell lines. Ann. Med. Surg. 2020, 60, 396–402. [Google Scholar] [CrossRef]
- Wang, Z.W.; Chen, Q.Y.; Liu, Q.S. Manganese(II) complexes of quinoline derivatives: Characterization, catalase activity, interaction with mitochondria and anticancer activity. Transit. Met. Chem. 2014, 39, 917–924. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Aboelez, M.O.; Abdel-Mawgoud, A.A.H. DNA interaction, antimicrobial, anticancer activities and molecular docking study of some new VO(II), Cr(III), Mn(II) and Ni(II) mononuclear chelates encompassing quaridentate imine ligand. J. Photochem. Photobiol. B Biol. 2017, 170, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Khan, M.U.; Hayat, M.; Khanam, H.; Saeed, M.; Owais, M.; Khalid, M.; Ahmad, M. Role of biologically important imidazole moiety on the antimicrobial and anticancer activity of Fe(III) and Mn(II) complexes. J. Biomol. Struct. Dyn. 2021, 39, 4037–4050. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, T.V.; Mohanapriy, S.; Bhuvaneswari, N. Synthesis, characterization and biological evaluation of heterocyclic triazole derived schiff base ligands comprising Mn(II) complexes: Implications of their DNA/protein binding docking and anticancer activity studies. Ind. J. Chem. Sect. A 2021, 60, 797–805. [Google Scholar]
- Icsel, C.; Yilmaz, V.T.; Aydinlik, S.; Aygun, M. New manganese(II), iron(II), cobalt (II), nickel(II) and copper(II) saccharinate complexes of 2,6-bis(2-benzimidazolyl)pyridine as potential anticancer agents. Eur. J. Med. Chem. 2020, 202, 112535. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Suntharalingam, K. A reactive oxygen species-generating, cancer stem cell-potent manganese(II) complex and its encapsulation into polymeric nanoparticles. Chem. Sci. 2019, 10, 7792–7800. [Google Scholar] [CrossRef] [PubMed]
- Quental, L.; Raposinho, P.; Mendes, F.; Santos, I.; Navarro-Ranninger, C.; Alvarez-Valdes, A.; Huang, H.; Chao, H.; Rubbiani, R.; Gasser, G. Combining Imaging and Anticancer Properties with New Heterobimetallic Pt(II)/M(i) (M = Re, 99mTc) Complexes. Dalton Trans. 2017, 46, 14523–14536. [Google Scholar] [CrossRef]
- Darshani, T.; Fronczek, F.R.; Priyadarshani, V.V.; Samarakoon, S.R.; Perera, I.C.; Perera, T. Synthesis and characterization of novel naphthalene-derivatized tridentate ligands and their net neutral rhenium tricarbonyl complexes and cytotoxic effects on non-small cell lung cancer cells of interest. Polyhedron 2020, 187, 114652. [Google Scholar] [CrossRef]
- Delasoie, J.; Pavic, A.; Voutier, N.; Vojnovic, S.; Crochet, A.; Nikodinovic-Runic, J.; Zobi, F. Identification of novel potent and non-toxic anticancer, anti-angiogenic and antimetastatic rhenium complexes against colorectal carcinoma. Eur. J. Med. Chem. 2020, 204, 112583. [Google Scholar] [CrossRef]
- Luengo, A.; Redrado, M.; Marzo, I.; Fernández-Moreira, V.; Gimeno, M.C. Luminescent Re(I)/Au(I) Species As Selective Anticancer Agents for HeLa Cells. Inorg. Chem. 2020, 59, 8960–8970. [Google Scholar] [CrossRef]
- Liew, H.S.; Mai, C.-W.; Zulkefeli, M.; Madheswaran, T.; Kiew, L.V.; Delsuc, N.; Low, M.L. Recent emergence of rhenium(I) tricarbonyl complexes as photosensitizers for cancer therapy. Molecules 2020, 25, 4176. [Google Scholar] [CrossRef]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in vitro anticancer activity of rhenium(I) tricarbonyl complexes bearing water-soluble phosphines. Inorg. Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef] [PubMed]





| Element | Location and Biofunctions | Compounds with Anticancer Activity | Toxicity, Antidotes | References |
|---|---|---|---|---|
| Titanium | Bio-stimulant; one of the most biocompatible metal implants | Ti(IV) complexes—for treatment of cancer (budotitane, Ti–salan, and titanocenes) | Ti metal is not toxic; TiO2 is a carcinogen | [11,12] |
| Vanadium | Stabilizes blood sugar levels; protects bones and teeth; insulin mimetic | Vanadocenes—inhibition of cancerous tumor growth; insulin-mimetic agents | Non-serious hazard; V2O5 is more toxic | [13,14,15,16,17,18,19] |
| Tantalum | Biocompatible and non-reactive with bio-tissues | Ta metal in long-term surgical implants and bone defects repairing | Low-soluble Ta compounds are moderately toxic | [20,21] |
| Molybdenum | Part of enzyme xanthine oxidase, in purine metabolism | MoO42− prevents oxidation of lipids; protects antioxidant systems; MoS42−-Cu chelator, in breast cancer and esophageal carcinoma; isotopes—for radio-diagnostics | Excess of Mo disturbs purine metabolism—endemic gout | [22,23,24,25,26,27,28,29,30,31,32] |
| Tungsten | Essential for some anaerobic bacteria | Polyoxotungstates—antiviral, antibacterial, anticancer agents | W quantities in nature are low; W is nontoxic | [33,34,35,36,37] |
| Manganese | Bone, liver, lungs, muscles, pancreas, kidney; Mn-SOD in mitochondria | Mn—part of enzymes catalyzing redox reactions; PET and PET/MR imaging | Excess of Mn causes manganism; in ROS production | [38,39,40,41,42,43,44,45,46,47,48] |
| Technetium | Tc—in radiation imaging as a tracer; 99mTc concentrates in gastrointestinal tract and thyroid gland | 99mTc (γ-emitter)—in SPECT for diagnostic imaging of bone, brain, lungs, thyroid, liver; radiation treatment of cancers with minimal adverse effects | Short half-life and rapid excretion of Tc radioisotopes minimize the toxic effects | [49,50,51,52] |
| Rhenium | β-emitters 186Re and 188Re in radio imaging; therapeutic properties on malignant tumors, bone metastases, rheumatoid arthritis | 188Re-HEDP for bone pain palliation in prostate cancer, 188Re-P2045—for therapy of small cell lung cancer and neuroendocrine carcinomas; Re(I) complexes—anticancer properties and reduce ROS production | No reports on the toxicity of metal and its compounds | [53,54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostova, I. Anticancer Metallocenes and Metal Complexes of Transition Elements from Groups 4 to 7. Molecules 2024, 29, 824. https://doi.org/10.3390/molecules29040824
Kostova I. Anticancer Metallocenes and Metal Complexes of Transition Elements from Groups 4 to 7. Molecules. 2024; 29(4):824. https://doi.org/10.3390/molecules29040824
Chicago/Turabian StyleKostova, Irena. 2024. "Anticancer Metallocenes and Metal Complexes of Transition Elements from Groups 4 to 7" Molecules 29, no. 4: 824. https://doi.org/10.3390/molecules29040824
APA StyleKostova, I. (2024). Anticancer Metallocenes and Metal Complexes of Transition Elements from Groups 4 to 7. Molecules, 29(4), 824. https://doi.org/10.3390/molecules29040824


.jpg)




