Fluorescent Sensor Based on 1H-Pyrazolo[3,4-b]quinoline Derivative for Detecting Zn2+ Cations
Abstract
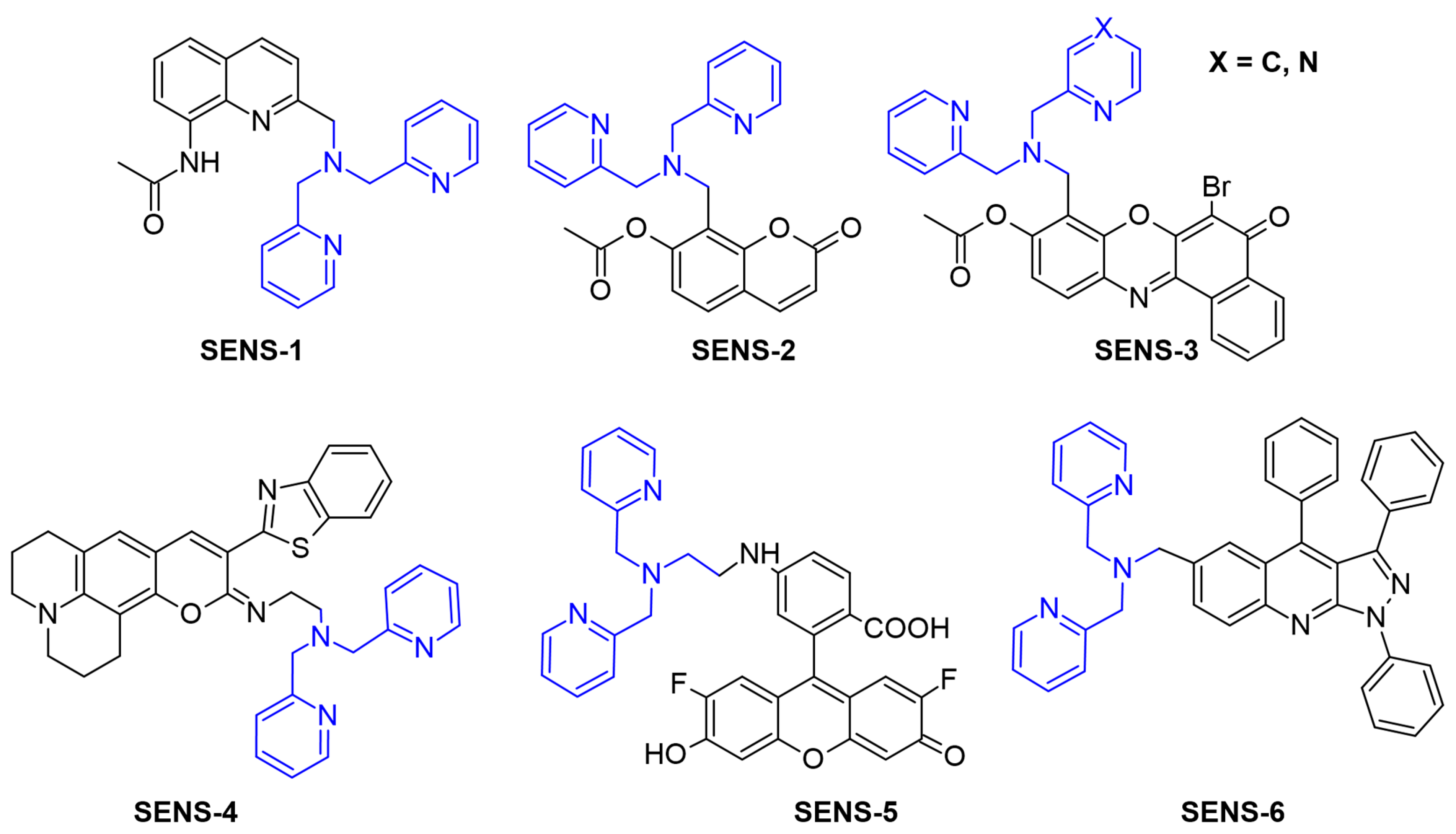
1. Introduction
2. Results and Discussion
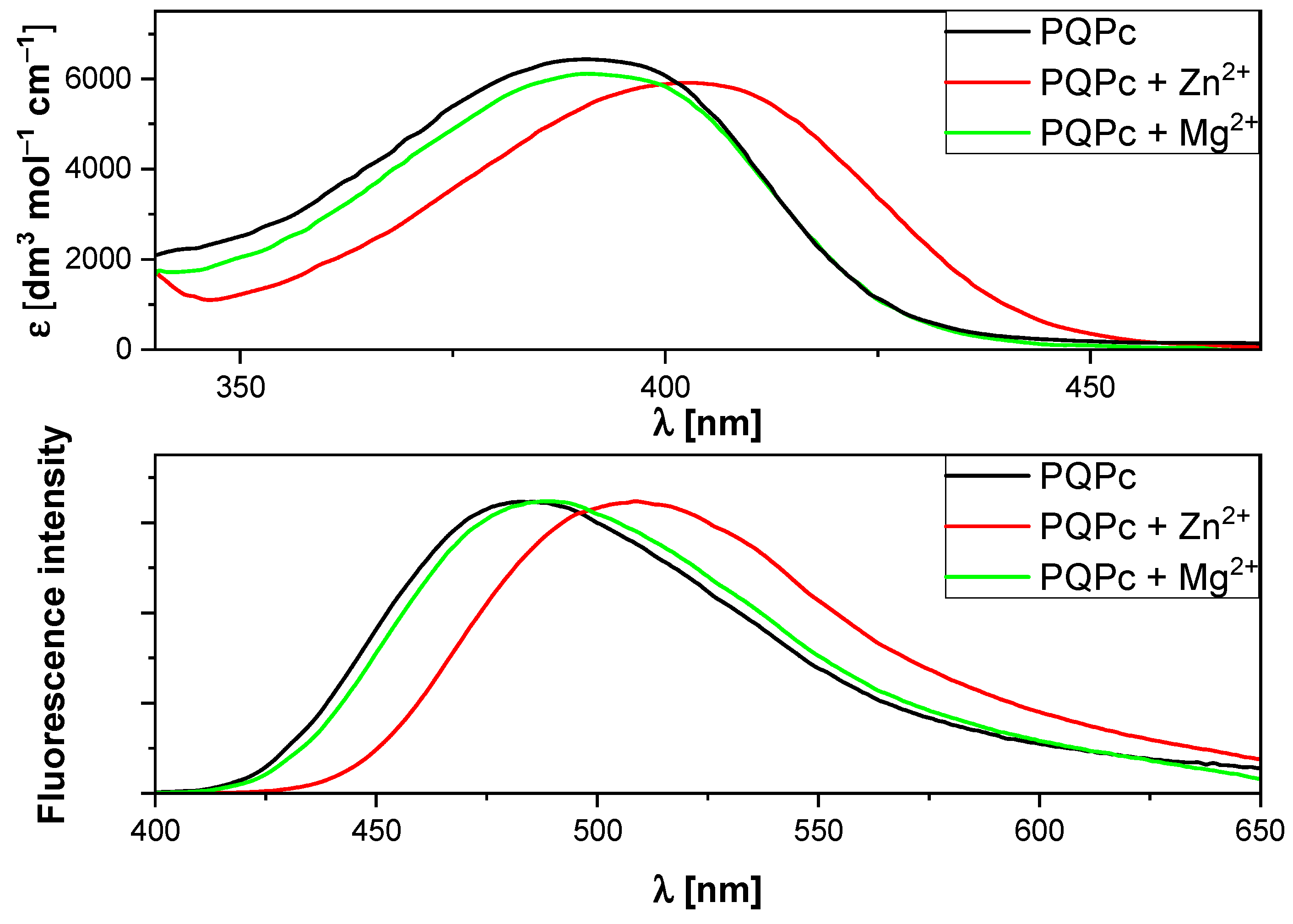
2.1. Spectroscopic Studies
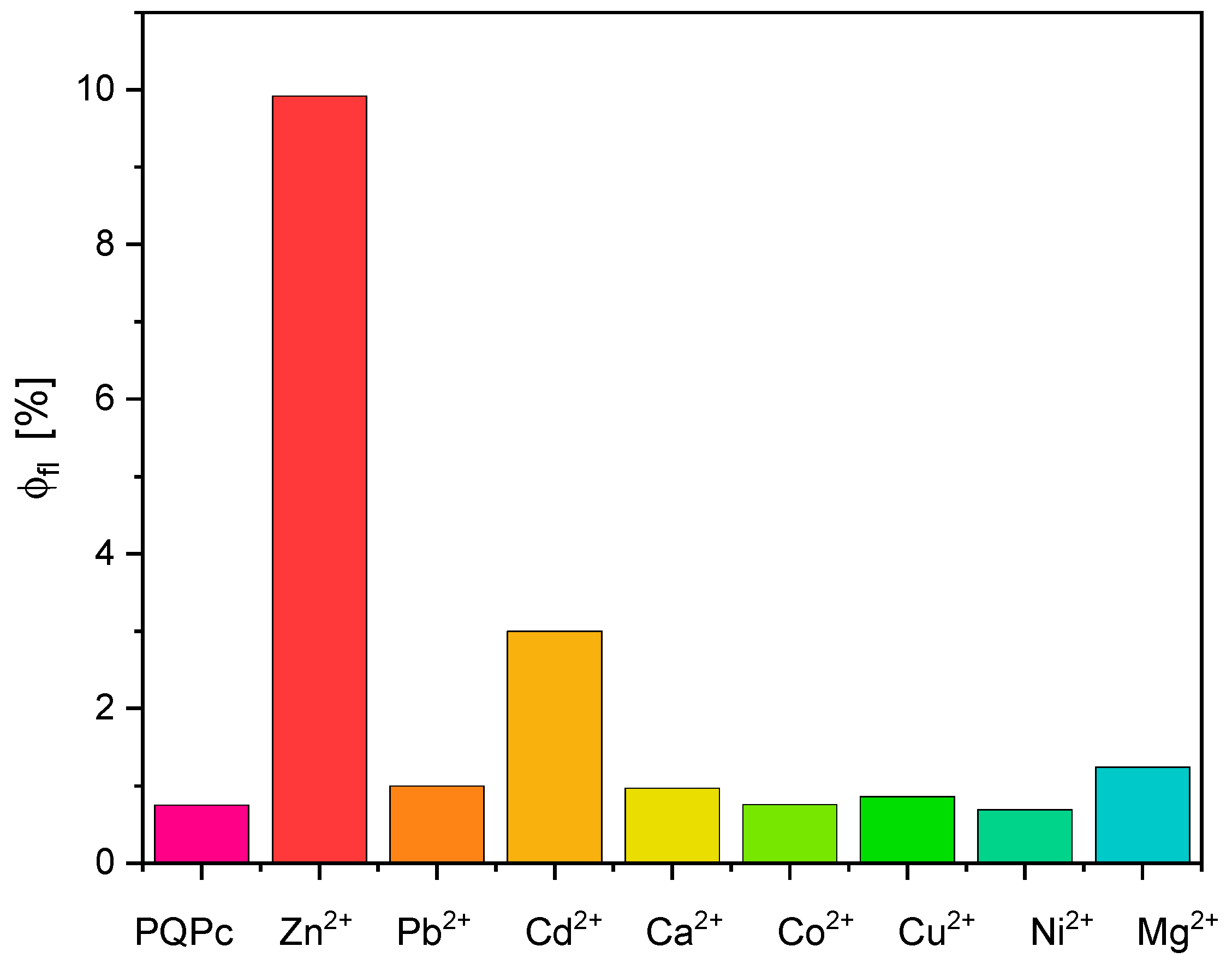
2.2. Bivalent Cation Sensing Studies of PQPc
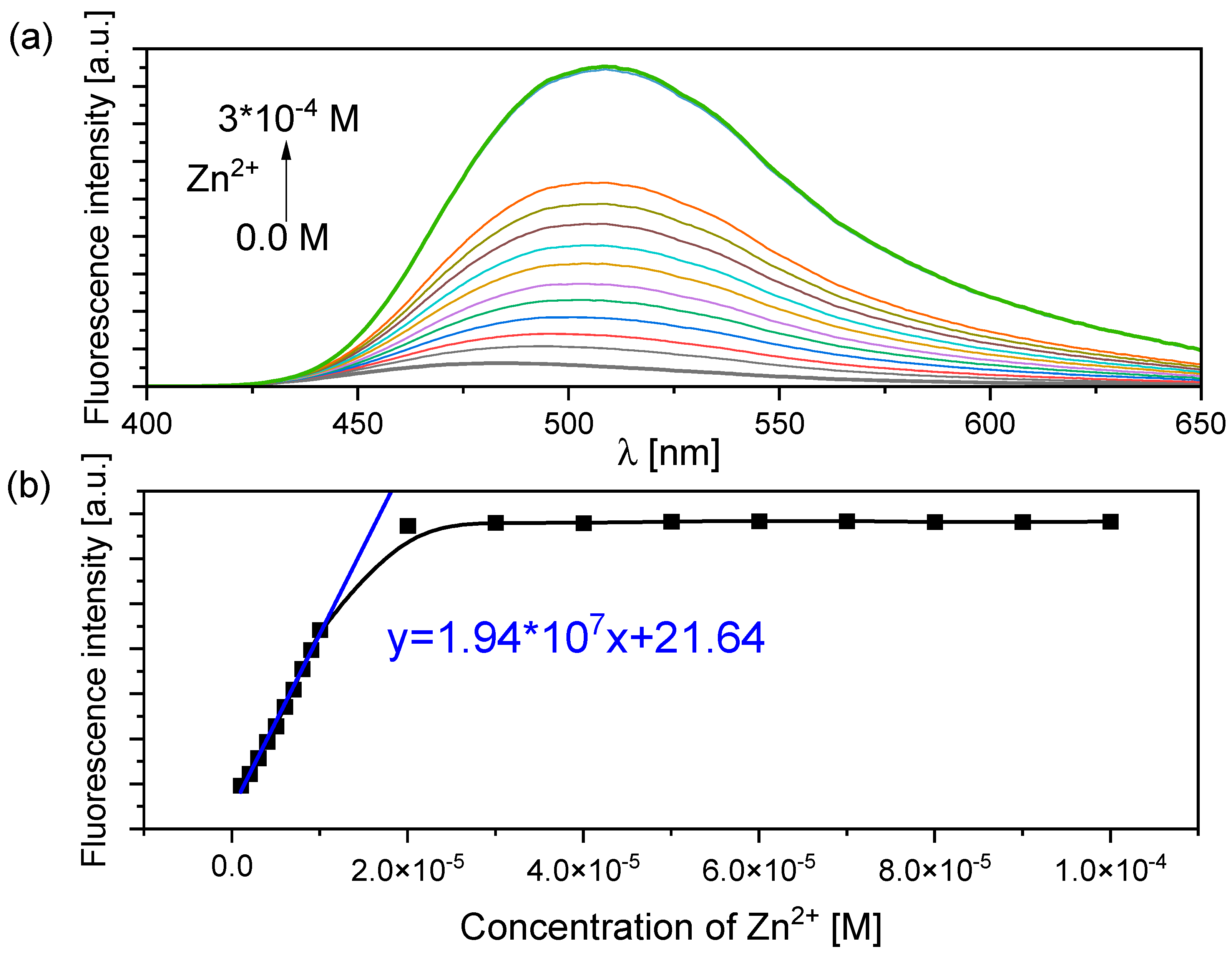
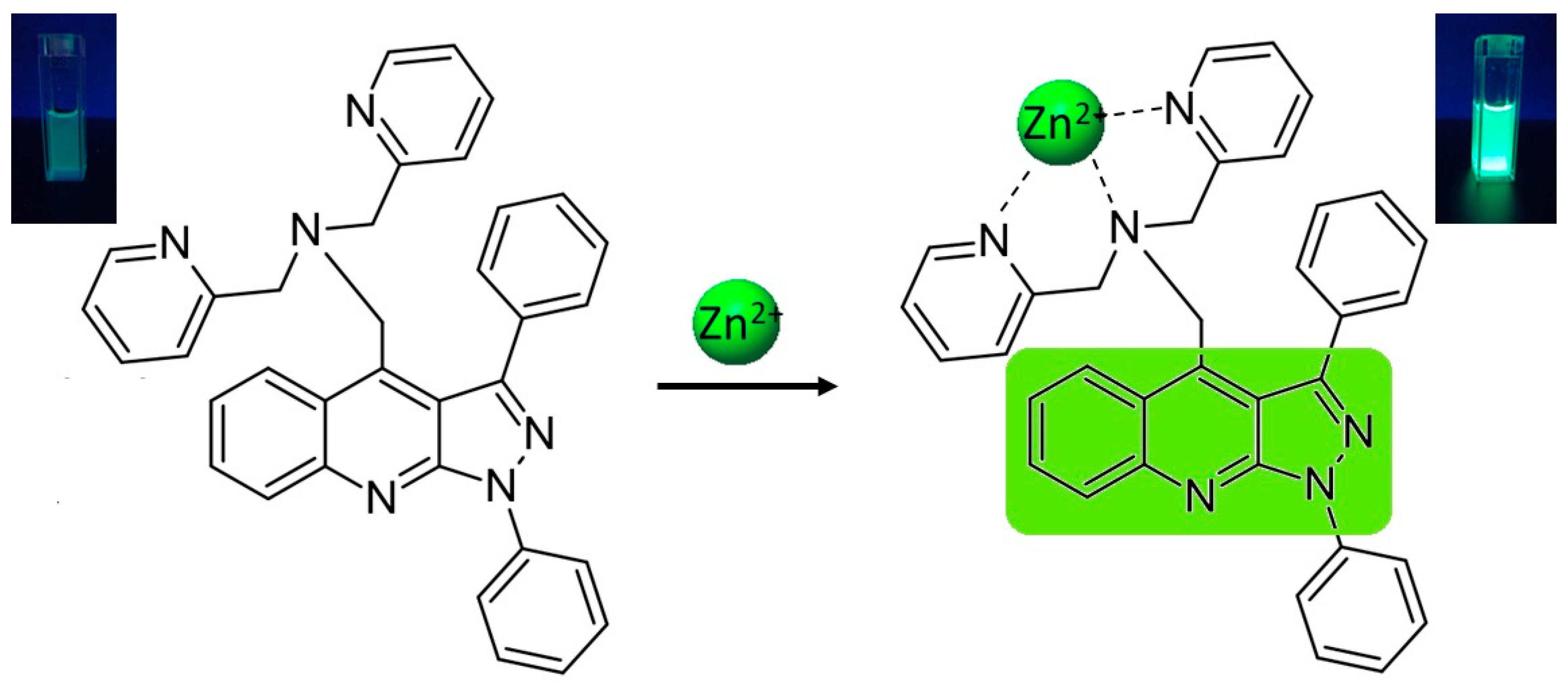
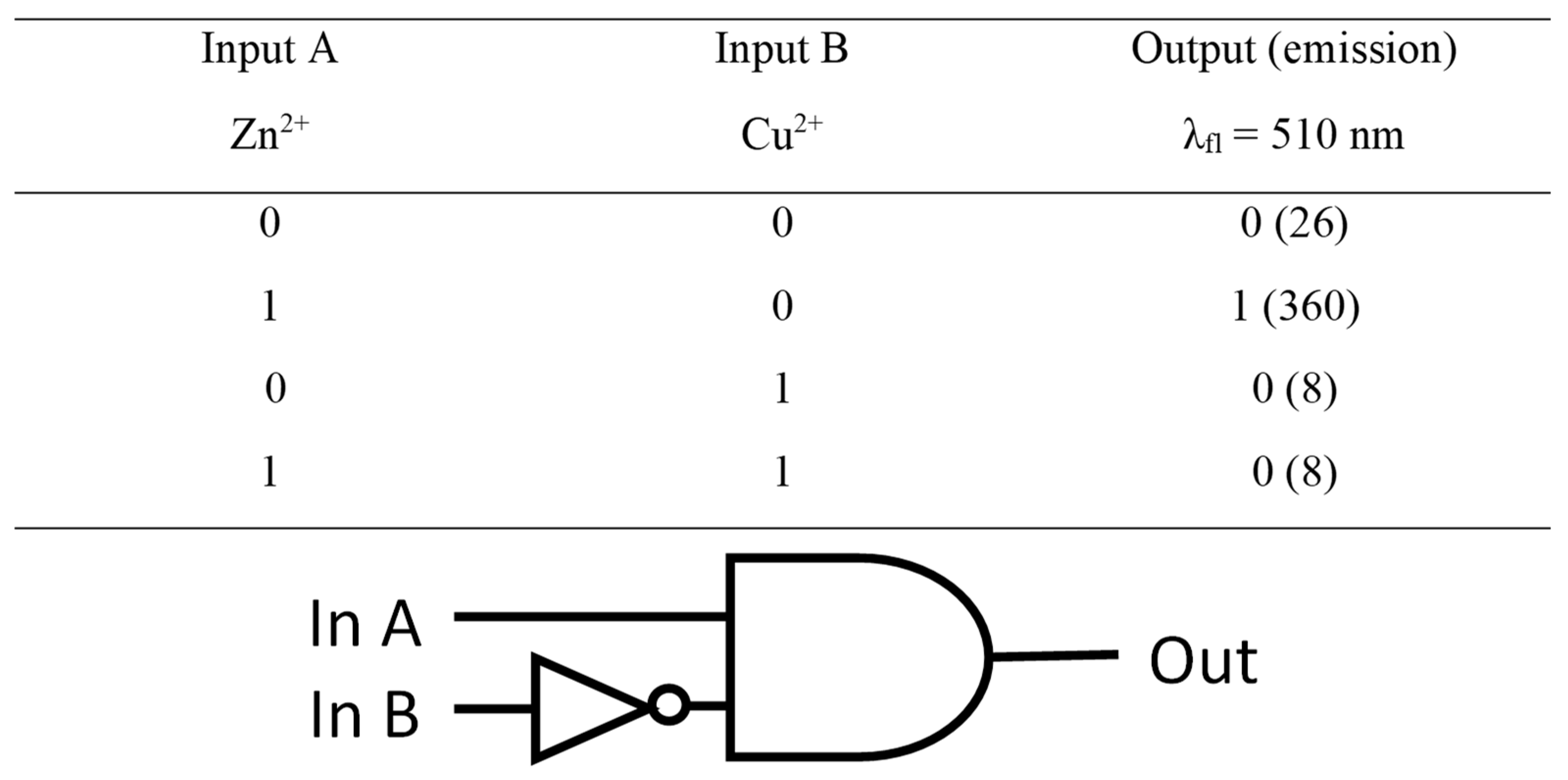
2.3. Sensory Properties of PQPc toward Zn2+ Ions
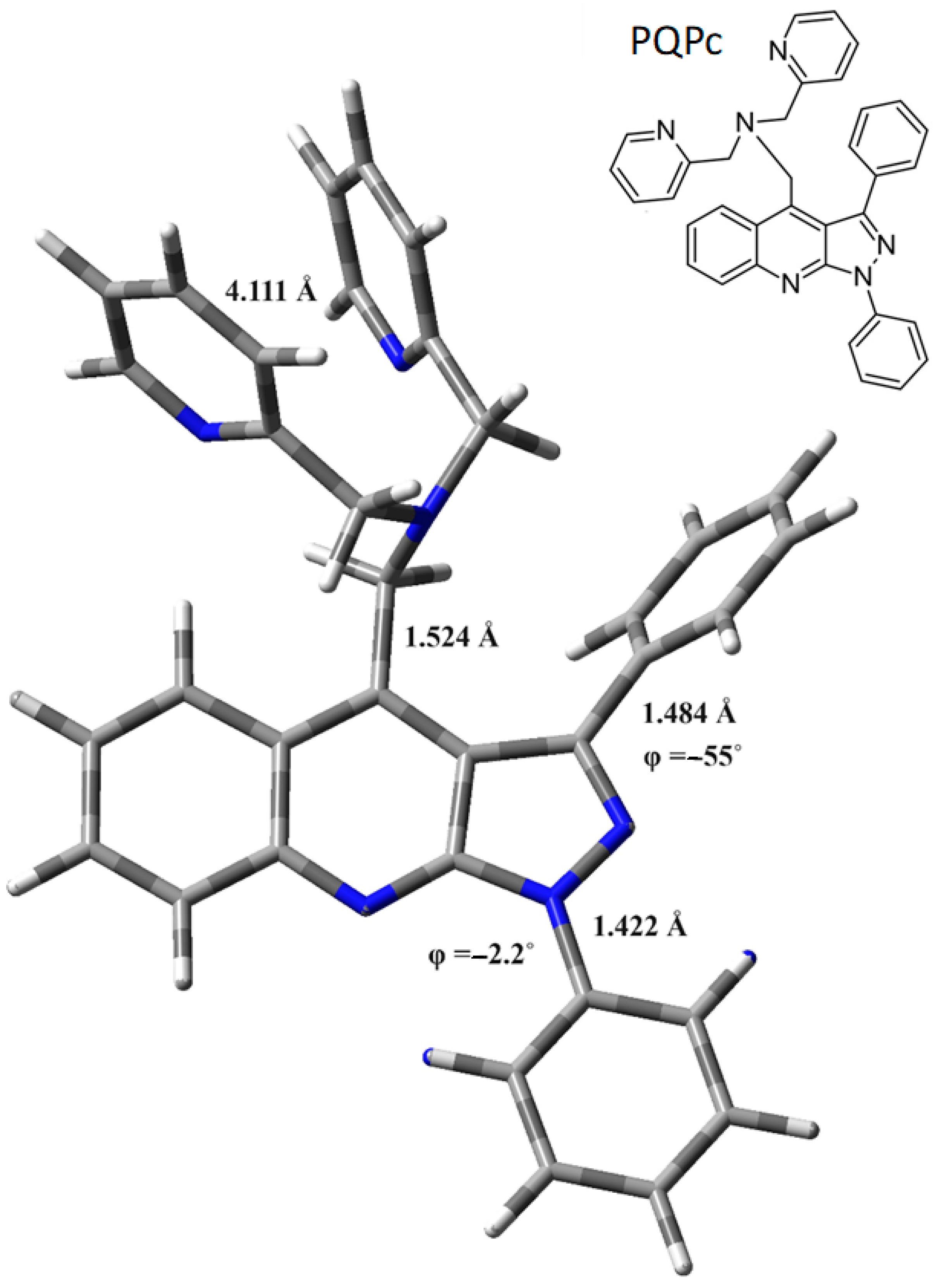
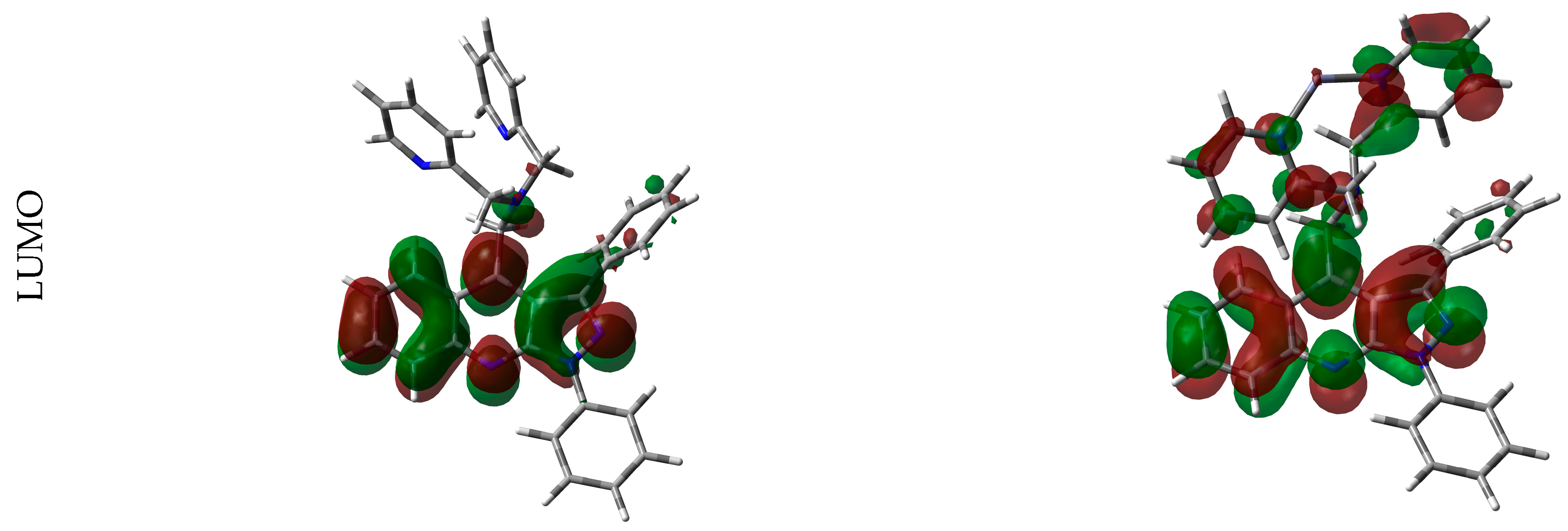
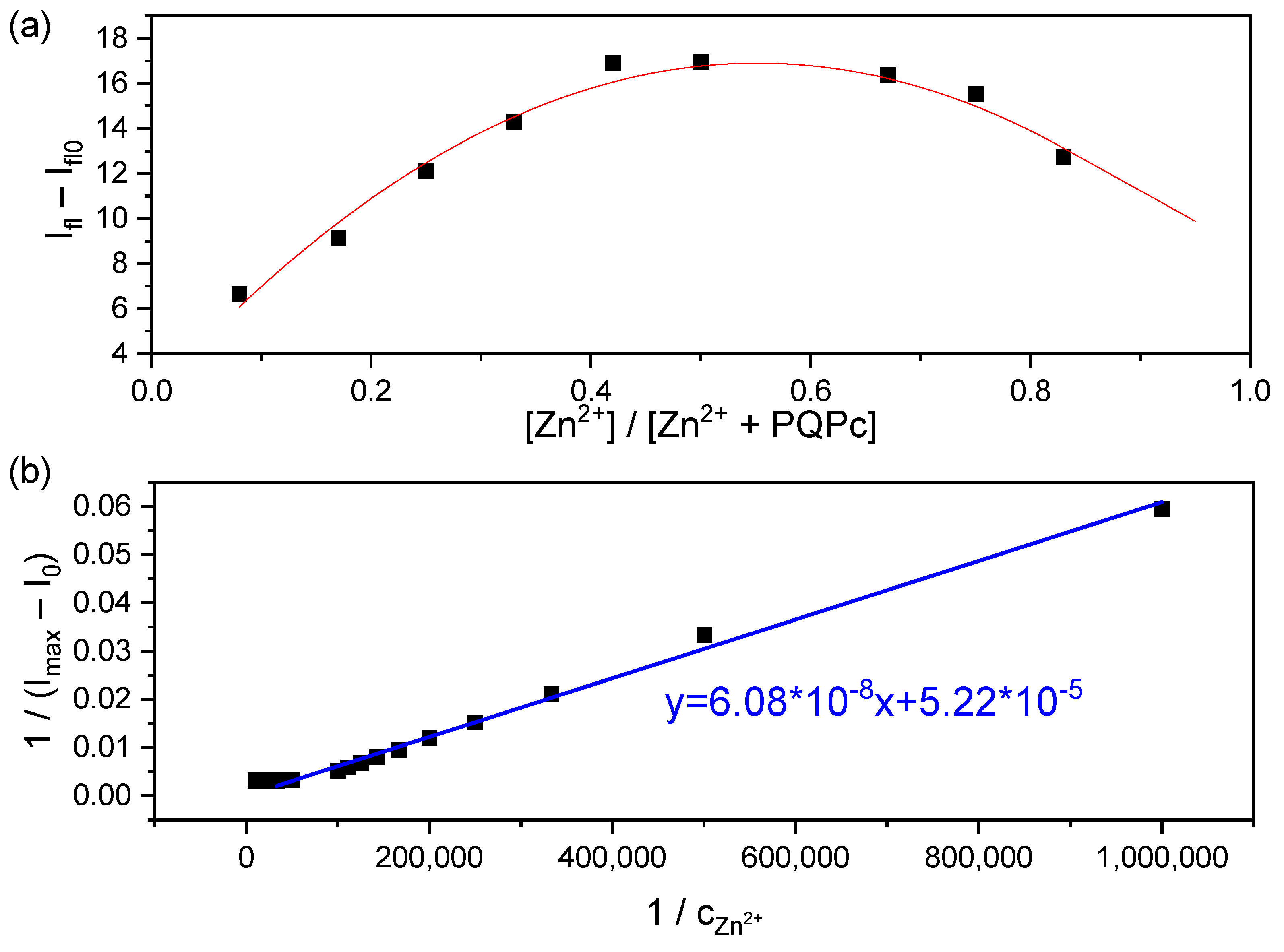
2.4. Binding Stoichiometry and Sensing Mechanism
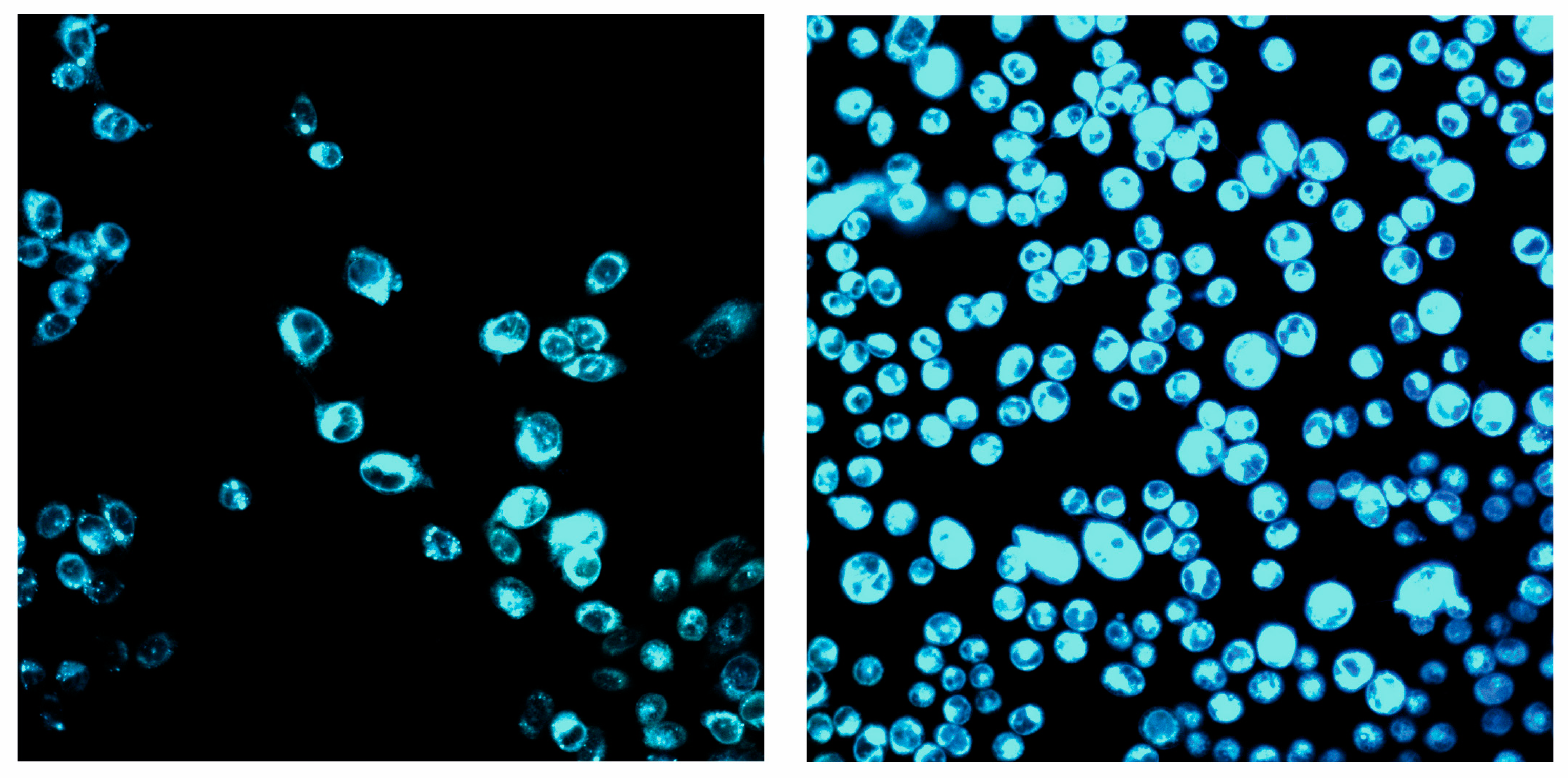
2.5. Biological Application Study
3. Materials and Methods
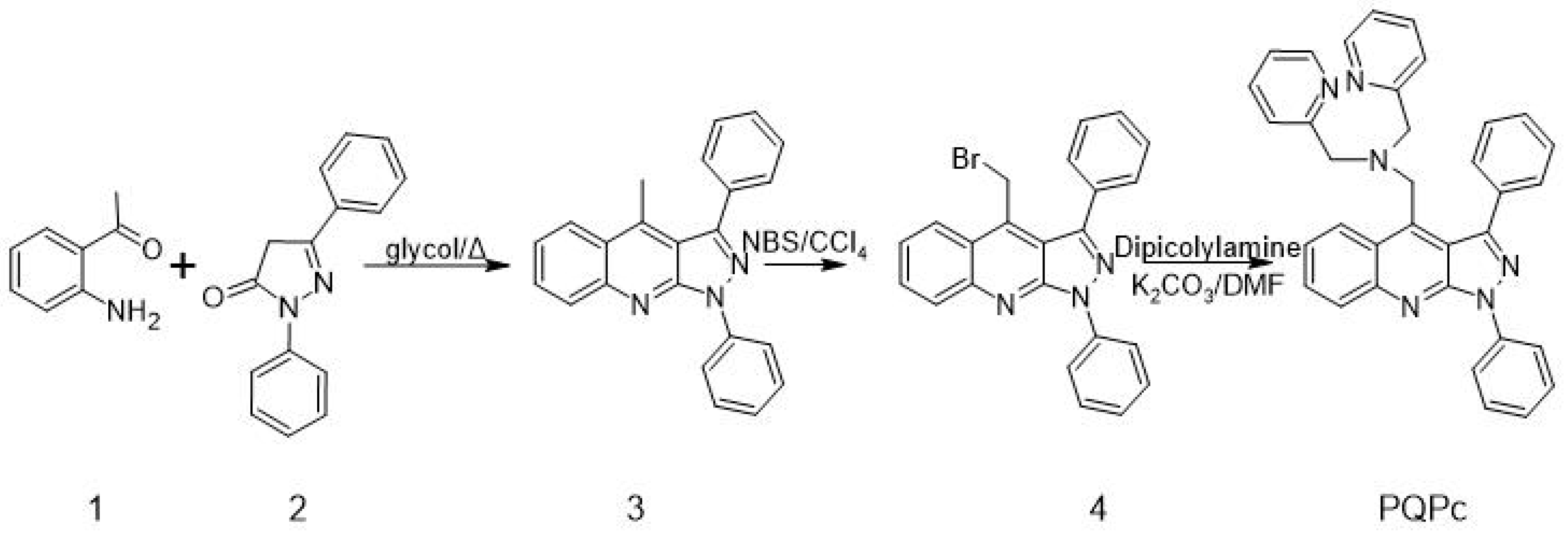
3.1. Synthesis
The Synthesis of Derivative PQPc
3.2. Materials, UV–Vis, and Fluorescence Measurements
3.3. Biological Methods
3.3.1. Cytotoxicity Activity (MTS Assay)
3.3.2. Detection of Zn in Cells Using a Sensor
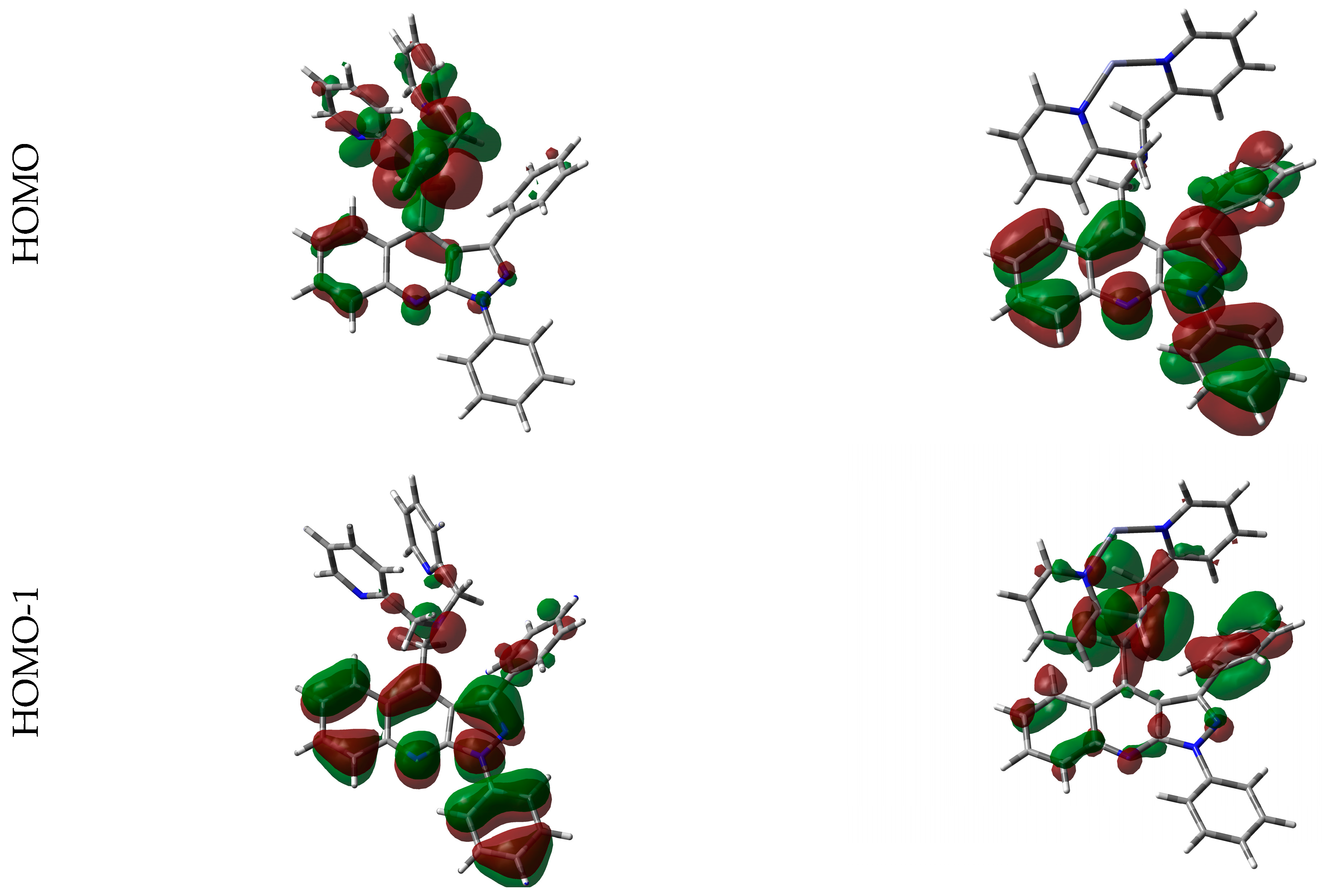
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Admakin, L.A. Microelements and the Organic System of Coal. Coke Chem. 2014, 57, 321–331. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Microelements and Their Role in Human Health. In Soil Components and Human Health; Springer: Berlin/Heidelberg, Germany, 2018; pp. 317–374. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Plants. In Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; pp. 57–65. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- King, J.C.; Cousins, R.J. Zinc. In Modern Nutrition in Health and Disease, 10th ed.; Shils, M.E., Shike, M., Ross, A.C., Caballero, B., Cousins, R.J., Eds.; Lippincott Williams and Witkins: Baltimore, MD, USA, 2006; pp. 271–285. [Google Scholar]
- Mońka, I.; Wiechuła, D. Importance of zinc for the human body in the aspect of zinc supplementation. Ann. Acad. Med. Siles. 2017, 71, 314–325. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Jarosz, M. Normy Żywienia dla Populacji Polskiej-Nowelizacja; Instytut Żywności i Żywienia: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Yanagisawa, H. Zinc deficiency and clinical practice-validity of zinc preparations. Yakuga. Zasshi 2008, 128, 333–339. [Google Scholar] [CrossRef]
- Chaffee, B.W.; King, J.C. Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 118–137. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A Multipurpose Trace Element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Toxicological Review of Zinc and Compounds. 2005. Available online: https://iris.epa.gov/static/pdfs/0426tr.pdf (accessed on 13 November 2023).
- Valeur, B. Molecular Fluorescence. Principles and Applications; Willey-VCH Verlag GmbH: Weinheim, Germany, 2002; pp. 273–357, 428–433. [Google Scholar]
- Balzani, V.; Credi, A.; Venturipp, M. Molecular Devices and Machines. Concepts and Perspectives for the Nanoworld, 2nd ed.; Willey-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 264–265, 259–293. [Google Scholar]
- Prasanna de Silve, A. Molecular Logic-Based Computation; The Royal Society of Chemistry: London, UK, 2013; pp. 38–39. [Google Scholar]
- Chen, X.; Ma, Y.; Zhang, Y.; Chen, Q.; Wang, H.; Wang, Z. A Selective and Reversible Fluorescent Probe for Zn2+ Detection in living Cells. ChemistrySelect 2020, 5, 4017–4027. [Google Scholar] [CrossRef]
- Zastrow, M.L.; Radford, R.J.; Chyan, W.; Anderson, C.T.; Zhang, D.Y.; Loas, A.; Tzounopoulos, T.; Lippard, S.J. Reaction based Probes for Imaging mobile Zinc in Live Cells and Tissues. ACS Sens. 2016, 1, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Komasu, K.; Urano, Y.; Kojima, H.; Nagano, T. Development of an Iminocoumarin-Based Zinc Sensor Suitable for Ratiometric Fluorescence Imaging of Neuronal Zinc. J. Am. Chem. Soc. 2007, 129, 13447–13454. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Kikuchi, K.; Urano, Y.; Nagano, T. Improvement and Biological Applications of Fluorescent Probes for Zinc, ZnAFs. J. Am. Chem. Soc. 2002, 124, 6555–6562. [Google Scholar] [CrossRef]
- Tao, Y.T.; Balasubramaniam, E.; Danel, A.; Jarosz, B.; Tomasik, P. Sharp green electroluminescence from 1H-pyrazolo[3,4-b]quinoline-based light emitting diodes. Appl. Phys. Lett. 2000, 77, 1575–1577. [Google Scholar] [CrossRef]
- Rurack, K.; Danel, A.; Rotkiewicz, K.; Grabka, D.; Spieles, M.; Rettig, W. 1,3-Diphenyl-1H-pyrazolo[3,4-b]quinoline; A versatile Fluorophore for the Design of Brightly Emissive molecular Sensors. Org. Lett. 2002, 4, 4647–4650. [Google Scholar] [CrossRef]
- Mac, M.; Uchacz, T.; Danel, A.; Danel, K.; Kolek, P.; Kulig, E. A new fluorescent sesnor based on 1H-pyrazolo[3,4-b]quinoline skeleton. Part 2. J. Fluoresc. 2011, 21, 375–383. [Google Scholar] [CrossRef][Green Version]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.-P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef]
- Cao, Y.; Shang, C.; Zheng, Z.; Sun, C. Substituent derivatives of benzothiazole-based fluorescence probes for hydrazine with conspicuous luminescence properties: A theoretical study. Spectrochim. Acta A 2022, 279, 121449. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, X.; Sun, C.; Cui, J. Theoretical Investigation on the ESIPT Process and Detection Mechanism for Dual-Proton Type Fluorescent Probe. Int. J. Mol. Sci. 2022, 23, 2132. [Google Scholar] [CrossRef]
- Chen, W.; Han, J.; Wang, X.; Liu, X.; Liu, F.; Wang, F.; Yu, R.-Q.; Jiang, J.-H. Aggregation-Induced Emission-Based Fluorescence Probe for Fast and Sensitive Imaging of Formaldehyde in Living Cells. ACS Omega 2018, 3, 14417–14422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Zhu, S.; Peng, J.; Li, L. Preparation of exciplex-based fluorescent organic nanoparticles and their application in cell imaging. RSC Adv. 2017, 7, 40842–40848. [Google Scholar] [CrossRef]
- Bissell, R.A.; Prasanna de Silva, A.; Gunaratne, H.Q.N.; Lynch, P.L.M.; Maguire, G.E.M.; Sandanayake, K.R.A.S. Molecular Fluorescent Signalling with ’Fluor-Spacer-Receptor’ Systems: Approaches to Sensing and Switching Devices via Supramolecular Photophysics. Chem. Soc. Rev. 1992, 21, 187–195. [Google Scholar] [CrossRef]
- Straight, S.D.; Andréasson, J.; Kodis, G.; Bandyopadhyay, S.; Mitchell, R.H.; Moore, T.; Moore, A.; Gust, D. Molecular AND and INHIBIT gates based on control of porphyrin fluorescence by photochromes. J. Am. Chem. Soc. 2005, 127, 9403–9409. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D.; Yao, C.-Y.; Moody, T.S.; Prasanna de Silva, A. Fluorescent molecular logic gates based on photoinduced electron transfer (PET) driven by a combination of atomic and biomolecular inputs. Chem. Commun. 2020, 56, 6838–6841. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.C. Logical sensing with fluorescent molecular logic gates based on photoinduced electron transfer. Coordin Chem. Rev. 2021, 426, 213598. [Google Scholar] [CrossRef]
- Prasanna de Silva, A.; Gunaratne, H.Q.N.; Maguire, G.E.M. ’Off-On’ Fluorescent Sensors for Physiological levels of Magnesium Ions based on Photoinduced Electron Transfer (PET), which also behave as Photoionic OR Logic Gates. J. Chem. Soc. Chem. Commun. 1994, 1213–1214. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Sakr, A.R.; Bojinov, V.B. Design and synthesis of a novel PET and ICT based 1,8-naphthalimide FRET bichromophore as a fourinput Disabled-Enabled-OR logic gate. Sens. Actuat B-Chem. 2015, 221, 625–634. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Mac Dónail, D.A.; Parker, D. Luminescent molecular logic gates: The two-input inhibit (INH) function. Chem. Commun. 2000, 93–94. [Google Scholar] [CrossRef]
- Li, M.; Guo, Z.; Zhu, W.; Marken, F.; James, T.D. A redox-activated fluorescence switch based on a ferrocene–fluorophore–boronic ester conjugate. Chem. Commun. 2014, 51, 1293–1296. [Google Scholar] [CrossRef]
- Uchacz, T.; Szlachcic, P.; Wojtasik, K.; Mac, M.; Stadnicka, K. Amino derivatives of 1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline–Photophysics and implementation of molecular logic switches. Dyes Pigment. 2016, 124, 277–292. [Google Scholar] [CrossRef]
- Grabka, D.; Danel, A.; Kolbus, A.; Szary, K. Photophysical properties of 6-N,N-dimethylpyrazolo[3,4-b]quinoline substituted with pyridyl in the 3-position. Opt. Mater. 2017, 66, 527–533. [Google Scholar] [CrossRef]
- Huy, B.T.; Thangadurai, D.T.; Sharipov, M.; Nghia, N.N.; Cuong, N.V.; Lee, Y.-I. Recent advances in turn off-on fluorescence sensing strategies for sensitive biochemical analysis—A mechanistic approach. Microchem. J. 2022, 179, 107511. [Google Scholar] [CrossRef]
- Mac, M.; Uchacz, T.; Danel, A.; Musiolik, H. Applications of fluorescent sensor based on 1H-pyrazolo[3,4-b]quinoline in analytical chemistry. J. Fluoresc. 2013, 23, 1207–1215. [Google Scholar] [CrossRef]
- Danel, A.; Kolbus, A.; Grabka, D.; Kucharek, M.; Pokładko-Kowar, M. 1H-Pyrazolo[3,4-b]quinoline derivative with the chelating substituent: Synthesis and spectral properties as fluorescent senor for cation detection. Dyes Pigment. 2021, 195, 109713. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Z. Validity and Reliability of Benesi-Hildebrand Method. Acta Phys. -Chim. Sin. 2007, 23, 1353–1359. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V.; Sozonov, D.I. Off-on fluorescent sensor based on the bis(2,4,7,8,9-pentamethyldipyrrolylmethene-3-yl)methane for detection of Cd2+ and Hg2+ cations. J. Lumin. 2017, 183, 315–321. [Google Scholar] [CrossRef]
- He, X.; Xie, Q.; Fan, J.; Xu, C.; Xu, W.; Li, Y.; Ding, F.; Chen, H.D.H.; Shen, J. Dual-functional chemosensor with colorimetric/ratiometric response to Cu(II)/Zn(II) ions and its applications in bioimaging and molecular logic gates. Dyes Pigment. 2020, 177, 108255. [Google Scholar] [CrossRef]
- Stork, C.J.; Li, Y.V. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J. Mol. Signal. 2010, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35–43. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; p. 54. [Google Scholar]
- Huang, C.Y. [27] Determination of binding stoichiometry by the continuous variation method: The job plot. Method. Enzymol. 1982, 87, 509–525. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016; Available online: https://scholar.google.com/scholar_lookup?title=Gaussian+16+Revision+A.03&author=Frisch,+M.J.&author=Trucks,+G.W.&author=Schlegel,+H.B.&author=Scuseria,+G.E.&author=Robb,+M.A.&author=Cheeseman,+J.R.&author=Scalmani,+G.&author=Barone,+V.&author=Petersson,+G.A.&author=Nakatsuji,+H.&publication_year=2016 (accessed on 13 November 2023).
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A At. Mol. Opt. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Miertuš, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
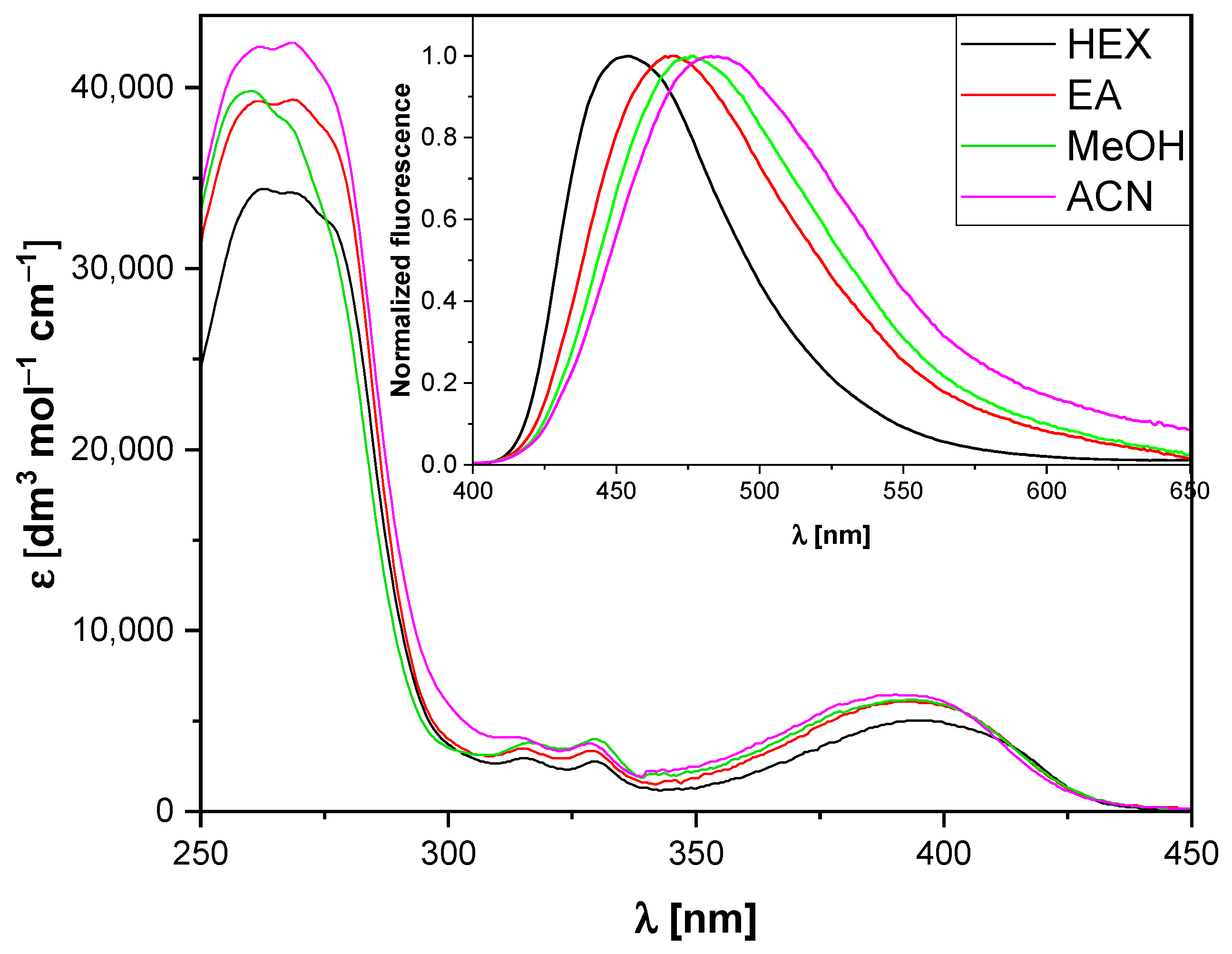

| Solvent | λabs [nm] | λfl [nm] | [%] | |
|---|---|---|---|---|
| HEX | 0 | 395 | 454 | 12.87 |
| DEE | 0.095 | 393 | 466 | 2.03 |
| EA | 0.202 | 392 | 469 | 1.40 |
| THF | 0.209 | 395 | 476 | 1.20 |
| MeOH | 0.309 | 394 | 476 | 0.99 |
| ACN | 0.305 | 396 | 483 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolbus, A.; Uchacz, T.; Danel, A.; Gałczyńska, K.; Moskwa, P.; Kolek, P. Fluorescent Sensor Based on 1H-Pyrazolo[3,4-b]quinoline Derivative for Detecting Zn2+ Cations. Molecules 2024, 29, 823. https://doi.org/10.3390/molecules29040823
Kolbus A, Uchacz T, Danel A, Gałczyńska K, Moskwa P, Kolek P. Fluorescent Sensor Based on 1H-Pyrazolo[3,4-b]quinoline Derivative for Detecting Zn2+ Cations. Molecules. 2024; 29(4):823. https://doi.org/10.3390/molecules29040823
Chicago/Turabian StyleKolbus, Anna, Tomasz Uchacz, Andrzej Danel, Katarzyna Gałczyńska, Paulina Moskwa, and Przemysław Kolek. 2024. "Fluorescent Sensor Based on 1H-Pyrazolo[3,4-b]quinoline Derivative for Detecting Zn2+ Cations" Molecules 29, no. 4: 823. https://doi.org/10.3390/molecules29040823
APA StyleKolbus, A., Uchacz, T., Danel, A., Gałczyńska, K., Moskwa, P., & Kolek, P. (2024). Fluorescent Sensor Based on 1H-Pyrazolo[3,4-b]quinoline Derivative for Detecting Zn2+ Cations. Molecules, 29(4), 823. https://doi.org/10.3390/molecules29040823








