Abstract
Photodynamic inactivation (PDI) is a highly effective treatment that can eliminate harmful microorganisms in a variety of settings. This study explored the efficacy of a curcumin-rich extract, Curcuma L., (Cur)- and essential oil component, trans-cinnamaldehyde, (Ca)-mediated PDI against Listeria monocytogenes ATCC 15313 (Lm) including planktonic cells and established biofilms on silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET). Applying Ca- and Cur-mediated PDI resulted in planktonic cell reductions of 2.7 and 6.4 log CFU/cm2, respectively. Flow cytometric measurements (FCMs) coupled with CFDA/PI and TOTO®-1 staining evidenced that Ca- doubled and Cur-mediated PDI quadrupled the cell damage. Moreover, the enzymatic activity of Lm cells was considerably reduced by Cur-mediated PDI, indicating its superior efficacy. Photosensitization also affected Lm biofilms, but their reduction did not exceed 3.7 log CFU/cm2. Cur-mediated PDI effectively impaired cells on PET and PTFE, while Ca-mediated PDI caused no (TOTO®-1) or only slight (PI) cell damage, sparing the activity of cells. In turn, applying Ca-mediate PDI to Si largely diminished the enzymatic activity in Lm. SS contained 20% dead cells, suggesting that SS itself impacts Lm viability. In addition, the efficacy of Ca-mediated PDI was enhanced on the SS, leading to increased damage to the cells. The weakened viability of Lm on Si and SS could be linked to unfavorable interactions with the surfaces, resulting in a better effect of Ca against Lm. In conclusion, Cur demonstrated excellent photosensitizing properties against Lm in both planktonic and biofilm states. The efficacy of Ca was lower than that of Cur. However, Ca bears potent antibiofilm effects, which vary depending on the surface on which Lm resides. Therefore, this study may help identify more effective plant-based compounds to combat L. monocytogenes in an environmentally sustainable manner.
1. Introduction
Listeria monocytogenes (Lm) is known to contaminate food during production processes, leading to foodborne illnesses. The food-processing environment (FPE) may be a source of contamination due to the ability of Lm to persist in FPE for an extended time [1]. This persistence is attributed to the difficulty in removing cells from hard-to-clean sites and/or biofilm formation, where the cells are more resistant to cleaning and disinfection and other treatment conditions [2]. Therefore, it is crucial to understand the response of Lm to specific treatments to develop better mitigation strategies.
Photodynamic inactivation (PDI) is a rising antimicrobial strategy that relies on a photochemical reaction that requires light and photosensitizers (PSs) in the presence of oxygen. The treatment works by activating PSs with specific wavelengths of light, which generate reactive oxygen species (ROS) with strong oxidation [3]. Unlike other treatments, PDI does not produce toxic chemicals, and the only energy required is the light source. Moreover, the chance of building up microbial resistance is low because of its multi-target nature [4]. PDI has been successfully used in medicine, dentistry, and the environment [5]. In food science, the use of PDI is gaining importance as a potential solution for eliminating foodborne pathogens. There is an interest in investigating the effects of PDI on these pathogens and the factors that can influence its effectiveness. It is crucial to evaluate the efficacy of PDI under different photosensitizing conditions. However, a comprehensive comparison of the susceptibility of various pathogens to PDI using different photosensitizers is yet to be completed.
Some PSs from natural sources have been suggested. Curcuma L. is an example of a source with various bioactive properties, such as anti-inflammatory, antioxidant, antimicrobial, and anticancer actions [6]. The raw material contains about 2% curcuminoids, including curcumin (diferuloylmethane), bisdemethoxycurcumin, and demethoxycurcumin. Nevertheless, curcumin is Curcuma’s vital fraction, responsible for all its biological activities [7]. Additionally, curcumin acts as a photosensitizer, absorbing photons and inducing reactions that can lead to the formation of ROS [6]. These ROS can cause cell toxicity by interacting with cell membranes or proteins [8]. Under light irradiation at 400–500 nm, the photosensitizing potential of curcumin can be effectively determined. Lately, there has been a growing interest in researching the potential applications of curcumin and PDI in enhancing food safety [9,10,11,12].
Trans-cinnamaldehyde is a phenylpropanoid that is present in edible essential oils like cinnamon bark. In PDI, trans-cinnamaldehyde can act as a pro-photosensitizer, magnifying ROS production, referred to as an oxidative stress-amplified agent [13]. Its photosensitizing properties to combat foodborne pathogens have not been thoroughly studied yet.
This study evaluated the antibacterial and antibiofilm effects of Curcuma L. and trans-cinnamaldehyde via the photodynamic action induced by blue light in Listeria monocytogenes. The bactericidal efficiency of edible photosensitizers was tested against planktonic cells and biofilms established on various surfaces, such as silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET). We investigated the treatment mechanism by analyzing enzymatic activity and membrane damage to Lm through flow cytometry using CFDA/PI staining. To better understand the cell membrane damage status, we also employed TOTO®-1 staining. We then used PCA analysis to resolve how the culture and treatment conditions impact Lm viability. The interactions between Lm and surfaces and the hydrophobicity of Lm on surfaces were analyzed to see if the surface on which Lm resides affects its behavior.
2. Results
2.1. Antibacterial and Antibiofilm Activity
Figure 1 shows the results of the photodynamic treatments at 234 J/cm2 against both planktonic cells and biofilms established on silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET), as measured by plate counting. Blue light alone (BL+) reduced planktonic cells by 0.6 log CFU/cm2, and applying Ca and Cur resulted in an additional reduction of 2.1 and 5.8 log CFU/cm2, respectively (p < 0.05). BL+ also affected biofilms, but the counts were not decreased by more than 1 log CFU/cm2 on SS, PTFE, and Si and 1.7 log CFU/cm2 on PET (p > 0.05). Ca and Cur significantly enhanced the BL effect (p < 0.05). However, we could not achieve the reductions comparable to planktonic cells after BL+ Cur. BL+ Cur was found to be more effective in reducing Lm on PET and PTFE surfaces compared to BL+ Ca. Reduction levels of 1.9 and 2.9 (BL+ Ca) and 3.7 and 3.4 (BL+ Cur) were recorded for PET and PTFE, respectively. We did not find the two statistically different for PTFE. We observed no significant difference in Lm reduction between BL+ Ca and BL+ Cur on Si. The reduction was 2.9 log CFU/cm2 for both. BL+ Ca seemed more effective than BL+ Cur in reducing Lm on SS. BL+ Ca and BL+ Cur reduced Lm by 3.7 and 3.0 log CFU/cm2, respectively. Of note, Ca alone (BL−) was able to reduce biofilms by c.a. 1.4 log CFU/cm2, and in the case of SS, the reduction reached 2 log CFU/cm2 (p < 0.05). This was not observed with Cur alone (BL−) (Supplementary Figure S1).
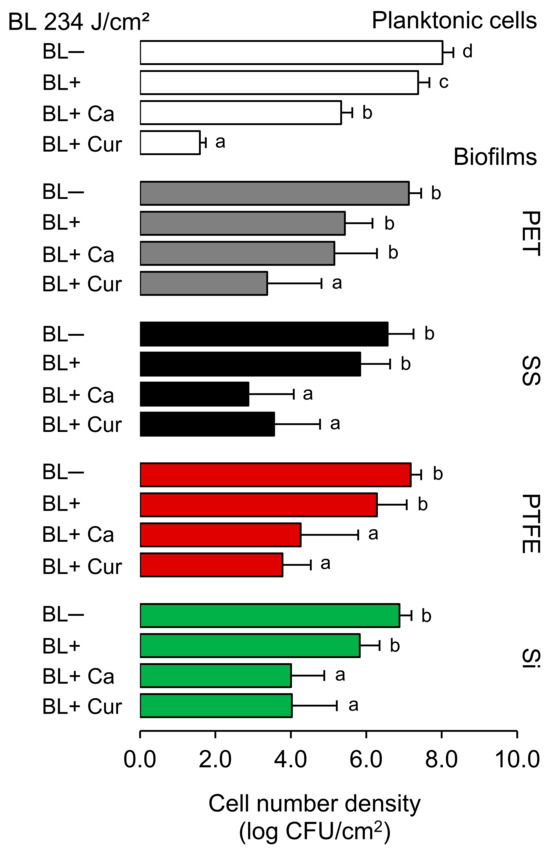
Figure 1.
Effect of photodynamic treatments at 234 J/cm2 against Listeria monocytogenes ATCC 15313 planktonic cells and established biofilms on different surfaces, including silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET). Different letters indicate a significant difference within each culture variant (p < 0.05). BL− and BL+ represent the absence and the presence of blue light illumination. Ca and Cur stand for t-cinnamaldehyde and Curcuma L., respectively.
2.2. Planktonic Cells—FCM Analysis
We analyzed planktonic cells using FCM measurements with CFDA/PI staining. Figure 2 shows that BL+ did not harm Lm cells but reduced the number of active cells by 12% and increased non-active cells by 21%. When we applied photosensitizers, Ca and Cur, they caused a 7% and 21% increase in cell damage, respectively. Cur was more effective than Ca in reducing the activity of Lm cells. We further assessed cell damage using TOTO®-1 (Figure 2, a membrane-impermeable dye commonly used for this purpose. TOTO®-1 staining revealed that BL+ did not cause significant damage, while Ca and Cur increased cell damage by 4% and 17%, respectively. Hence, Ca doubled and Cur quadrupled the damage compared to their BL− counterparts (Supplementary Table S1).
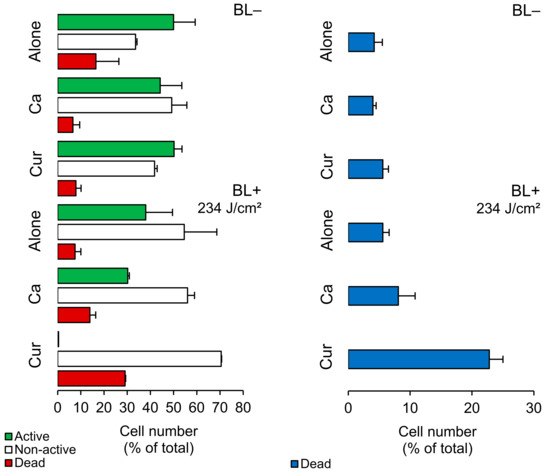
Figure 2.
Cell number of Listeria monocytogenes ATCC 15313 planktonic cells (% of total; mean ± SD) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based (non-)photosensitization experiments (BL−/BL+). Different bar colors indicate different cell subpopulations, as measured by FCM with CFDA/PI (left) and TOTO®-1 staining (right). See Section 4.8 in Materials and Methods for details.
2.3. Biofilms—FCM Analysis
Figure 3 illustrates that when seeded on PET and PTFE, Lm responded similarly to BL+. Both contained fewer dead cells, and the active to non-active cell ratio was close to 60% to 40%. Si emerged with the most non-active cells (46%), while SS had almost 20% dead cells. The same was observed on SS without blue-light exposure (Supplementary Table S2), suggesting SS largely affects Lm fitness. Applying Ca and Cur to PET/PTFE contained, on average, 16% and 50% dead cells, respectively (Figure 3). In the case of Ca, the active cells still constituted the majority. Applying Cur to Si resulted in an almost four-fold increase in cell damage; however, it was inconsistent. With Ca, the percentage of non-active cells was the highest (66%), and active cells were marginal. On SS, Lm responded differently. Ca resulted in 60% dead cells, while Cur produced 45%. At the same time, Cur demonstrated non-active cells at the expense of active cells.
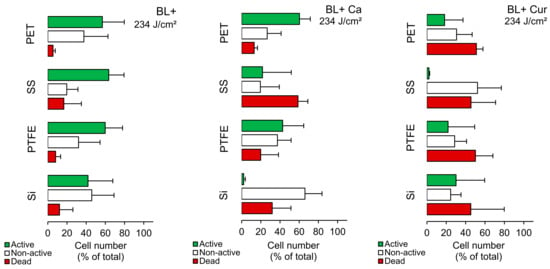
Figure 3.
Cell number of Listeria monocytogenes ATCC 15313 cells (% of total; mean ± SD) on different surfaces, including silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based photosensitization experiments (BL+). CF−PI+ cells were labeled as experiencing damage (red bar) and CF+PI− as retaining activity (green bar). CF−PI− cells were labeled as non-active (white bar).
We included dot plots of TOTO®-1 staining to further investigate cell damage to Lm (Figure 4). Supplementary Figure S2 displays the same but without exposure to blue light (BL−). A line across the plots separates dead cells (lower right) from cells exhibiting increased dye permeability (upper right), also considered dead. Photosensitization increased the dead cell population as Cur caused the most cell damage (Figure 4). BL+ Ca and Cur resulted in greater cell damage on SS than on other surfaces. We could also see considerable damage to the cells on the SS in BL− experiments (Supplementary Figure S2). There was no difference in cell damage between BL+ and BL+ Ca on PET and PTFE. BL+ Ca did not impair cells on Si. Although cells may have been impacted, we failed to classify 11.5% of the population (Figure 4).
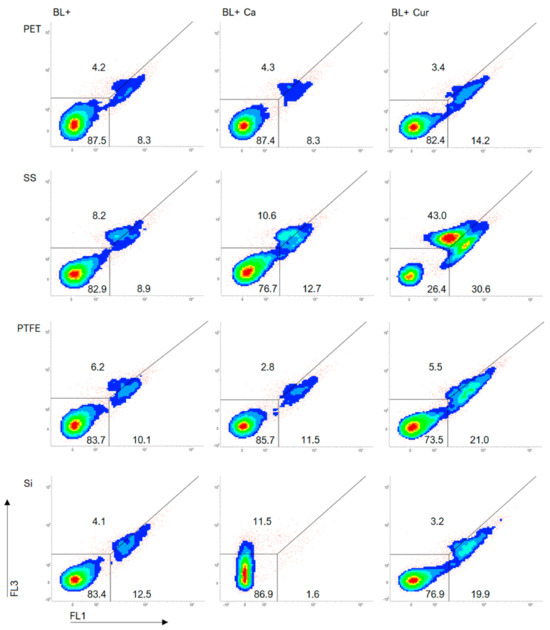
Figure 4.
FCM dot plots of Listeria monocytogenes ATCC 15313 biofilms established on silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based photosensitization (BL+) experiments as measured by FCM with TOTO®-1 staining.
2.4. Adhesion Potential and Hydrophobicity
Both attractive and repulsive forces influence bacterial adhesion to surfaces. Short-range interactions such as polar and apolar interactions are particularly important. Bacterial adhesion becomes more favorable when attractive forces are stronger than repulsive forces [14]. This occurs when the interactions decrease the free energy of adhesion (∆Gadhesion < 0). Our results indicate that the free energy of adhesion between the studied Lm strain and Si/SS is positive (∆Gadhesion > 0 mJ/m2) (Table 1), rendering it thermodynamically unfavorable. The adhesion between the Lm and PET was close to 0 mJ/m2. On the other hand, adhesion to PTFE is more likely to occur due to its negative total free energy of adhesion (∆Gadhesion < 0 mJ/m2).

Table 1.
Free energy of adhesion (ΔGadhesion) between L. monocytogenes ATCC 15313 (b) and surfaces (s): silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET) in aqueous liquid media (l) and their apolar (ΔGblsLW) and polar (ΔGblsAB) components; supplemented with contact angles, surface tension components, and total free energy of interaction (ΔGswsTOT) of the Lm surfaces.
Considering the values of the water contact angle, Si colonized by the Lm was the most hydrophobic (θW > 65°). PTFE was close enough to be considered hydrophobic. The last two, SS and PET, were hydrophilic. Variations in θW occurred more on Si and PTFE because of the random distribution of water drops in areas with varying concentrations of adhered cells. Nonetheless, all surfaces colonized by the Lm were considered hydrophilic because the ∆GTOT values were > 0. The same is suggested by a semi-quantitative measure of hydrophobicity, γS− > 35, and the apolar component γSLW ≤ 45 mJ/m2. It is worth noting that the PTFE had the lowest values, while the SS had the highest values.
2.5. Principal Component Analysis (PCA)
PCA was conducted to further resolve how the culture and treatment conditions impact Lm viability. Figure 5a illustrates the dissimilarity measure among the assayed cells. The culturable and active cells are the farthest away from the other cells. Next are the non-active cells, followed by dead cells labeled with PI and TOTO®-1. The biplot in Figure 5b shows the separation of Ca and Cur variables with respect to PC1 and PC2. It separates all BL+ Cur treatments, with BL+ Cur SS and BL+ Cur P plotting further away. It also scatters two Ca treatments, BL+ Ca SS and BL+ Ca Si, suggesting cell membrane damage and a loss of activity, respectively. We also examined the loading factor scatterplot and observed significant variable clustering (Figure 5c). Variables placed close to each other influence the PCA model similarly and indicate they are correlated. Dead cells-PI and dead cells-TOTO®-1 are variables with a substantial degree of correlation accompanied by BL+ Cur. The next closest variable to them is biofilms SS. There is a strong correlation between planktonic cells and non-active cells, and biofilms Si is the next closest of all biofilms. Other variables were also correlated, e.g., BL+, BL−, and biofilms PET, but they were least influential in determining the PCA model. Finally, we observed a positive correlation between dead cells-TOTO®-1 and dead cells-PI (r = 0.50) and active and culturable cells (r = 0.66). On the other hand, we found a negative correlation between the dead cells-TOTO®-1 and active/culturable cells (r = −0.49/−0.46), active and non-active cells (r = −0.55), and dead-PI and active/culturable cells (r = −0.70/−0.76) (Figure 5d).
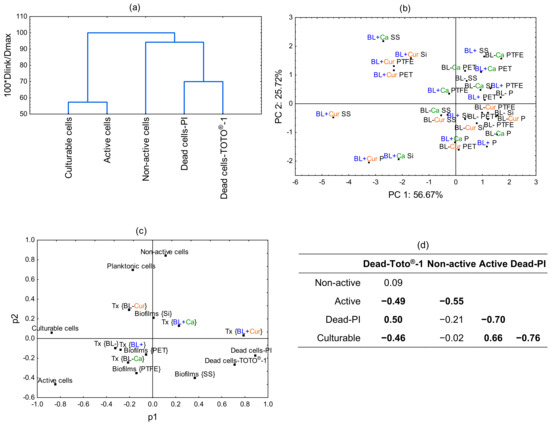
Figure 5.
The dissimilarity dendrogram among Lm assayed cells (a). The biplot of blue light (BL−/BL+), culture (P−planktonic cells and biofilms on PET, SS, PTFE, and Si), and photosensitization (Cur—Curcuma and Ca—t-cinnamaldehyde) variables related to PC1 and PC2 (b). The loading scatterplot (p1 vs. p2) illustrates clustering among the analyzed variables (c). The tested continuous variables include dead cells-PI, dead cells-TOTO®-1, active, non-active, and culturable cells. The selected categorical variables were the culture (planktonic cells and biofilms on PET, SS, PTFE, and Si) and the treatment (BL+/BL−; Cur/Ca). The correlation coefficient (r) between differentially assayed cells measures their relationship strength (d). The correlation coefficients in bold are significant at a p < 0.05. 0—no linear relationship. 0.3—A weak relationship. 0.5—A moderate relationship. 0.7—A strong linear relationship.
3. Discussion
Photodynamic inactivation (PDI) is a highly promising and effective technology that uses light to inactivate harmful microorganisms. This study explored antibacterial and antibiofilm PDI with edible photosensitizers against Lm and focused greatly on evaluating the enzymatic activity and cell damage as part of the antibacterial mechanism. We showed that Ca- and Cur-mediated PDI significantly reduced planktonic cells, reaching 3 and >6 log CFU/cm2, respectively. FCM analysis, coupled with CFDA/PI and TOTO®-1 staining, indicated that Ca doubled and Cur quadrupled cell damage. In addition, Cur diminished the enzymatic activity of planktonic cells.
Cur has been shown to inhibit Gram-negative and Gram-positive bacteria, with studies primarily focusing on growth and biofilm formation inhibition [15]. This has opened a new avenue for designing environmentally friendly PDI technology. Our results are consistent with the previous reports on curcumin in enhancing PDI against various microorganisms, including those of the Listeria genus. Huang et al. [16] achieved almost complete planktonic cell reductions in Listeria at 1.0 μM in 5 min. Bonifácio et al. [17] used 3.7 mg/L and 30 min exposure with a total energy of 270 J/cm2 to achieve the same effect. This may be due to differences in the solubility of curcumin in various solvents, such as water, acetone, DMSO, and ethanol, as used by different research groups. Furthermore, these variations could be attributed to the differences in the methodology employed, the way the vehicle interacts with the bacterial outer membrane, and the purity level of the curcumin that was utilized in the study.
There is much interest in evaluating the bactericidal efficiency of the curcumin-mediated PDI for food safety purposes. Al-Asmari et al. [9] investigated curcumin photosensitization’s impact on sanitizing fresh date fruit. They found that refrigerated storage had the longest shelf life for fruit treated with curcumin photosensitization (up to 98 days). Liu et al. [18] found that Cur-mediated PDI extended oysters’ shelf life by four days, improving sensory quality without affecting texture and flavor. Tao et al. [19] treated the surface of fresh-cut apple slices with Cur-PDI, which improved reductions in Escherichia coli. de Oliveira et al. [10] evaluated the bactericidal activity of Cur at 5 mg/L with PDI against E. coli O157:H7 and L. innocua in the spinach wash water. After a 5 min exposure, high bacterial reduction was achieved even in high chemical oxygen demand/dirty conditions. de Oliveira et al. [11] conducted a study where they treated the surface of spinach, lettuce, and tomatoes with Cur at a 10 mg/L concentration. The PDI treatment resulted in a 3-log reduction of E. coli O157:H7 and L. innocua while having no significant effect on the color and texture of the fresh produce. The Cur-PDI technology could also replace fungicides and pesticides as it is effective against various microorganisms, e.g., Aspergillus flavus [20]. The use of Cur-mediated PDI has shown promising results in controlling biofilms. Bonifácio et al. [17] tested PDI using Cur against L. innocua biofilms and achieved a 4.9-log reduction, which was still less than that of planktonic cells.
In our study, PDI with photosensitizers affected Lm biofilms, but we did not achieve a complete reduction, as the highest was 3.7 log CFU/cm2. Cells treated with Ca showed no damage and preserved activity, while Cur-mediated PDI impaired cells on PET and PTFE. In contrast, Ca-mediated PDI diminished the enzymatic activity of cells on Si. SS contained a great number of dead cells, indicating that SS itself impacts Lm viability. In addition, the efficacy of Ca-mediated PDI was enhanced on SS, leading to increased cell damage to Lm. Our recent study found that exposing Lm on SS for 4 h at 668 J/cm2 resulted in a less than 1-log CFU/cm2 reduction in a dried biofilm model [21]. Gallic acid, used as a photosensitizer, led to an additional 0.5-log CFU/cm2 reduction. This demonstrates that Lm is somewhat responsive to photosensitizers.
Reports suggest that PDI of L. monocytogenes can occur with different PSs. For instance, Lin et al. [22] obtained almost complete inactivation of planktonic cells with 0.6 mg/L methylene blue and a light dose of 120 J/cm2. Given a high inactivation, the use of methylene blue may result in neurotoxicity. Unlike Cur, it is not approved by the US Food and Drug Administration or the European Food Safety Authority as a food additive. Thus, it cannot be considered a suitable PS for food-related applications. The main advantages of using Cur in PDI include its high efficacy as a photosensitizer, as well as its low toxicity. Instead, curcumin can damage the permeability and integrity of bacterial cell membranes, leading to cell death. In addition, curcumin’s lipophilic structure allows it to be directly inserted into the bilayer of liposomes, increasing the permeability of the bilayer [15]. The structure of Cur is likely responsible for increased ROS diffusion across the extracellular matrix and improved efficacy against biofilms of L. innocua [17]. In addition, curcumin’s antibiofilm effect may be due to a reduction in the expression of virulence genes, including inlA, hlyA, and plcA, which may have the effect of reducing the adhesion and invasive capacity of L. monocytogenes [23].
To date, Cur has not been reported as an efficient PS for L. monocytogenes on different surfaces under visible light. This opens the perspective of employing this strategy in antimicrobial polymers. For instance, nanoparticles consisting of hyper-crosslinked polymers enable their use in drug delivery [24]. A biodegradable polymer called poly(lactic-co-glycolic acid) (PLGA) has recently been used in combination with curcumin to kill Staphylococcus bovis and Escherichia coli [25]. This could be further extended to L. monocytogenes and employed in the antimicrobial photoactivity of cur-loaded materials, including those recommended for direct food contact or food packaging.
There is no doubt that medicinal plants demonstrate antimicrobial activity, including against foodborne pathogens [26,27,28]. Various effects are expected to occur, including antibiofilm. Phytochemicals affect biofilm formation and reduce listerial biofilms on surfaces commonly used in antibiofilm assays, which are stainless steel or polystyrene. For instance, Upadhyay et al. [29] reported that cinnamaldehyde, at 5 and 10 mM concentrations, can effectively reduce Listeria biofilms on SS. Liu et al. [30] found that cinnamaldehyde more effectively inhibited Listeria biofilm formation on polystyrene plates than sodium hypochlorite. Although many studies have been on phytochemical efficacy, comparing efficacy on different surfaces has received little attention. One recent study revealed that trans-cinnamaldehyde reduced L. innocua biofilms by 4.6, 4.0, 3.6, and 2.5 log CFU/cm2 from PET, silicone rubber, PTFE, and SS [31]. In contrast to our results, the highest viability of L. innocua was on Si and SS, suggesting that each individual Listeria interacts with surfaces differently.
We revealed that Lm and Si/SS adhesion is thermodynamically unfavorable. This finding is similar to studies conducted by Bernardes et al. [14] and Teixeira et al. [32], which tested Bacillus cereus and Staphylococcus sciuri, respectively. Cells were hydrophilic, the material surface was hydrophobic, and the free energy of adhesion was positive. Van Oss [33] explained that bacterial adhesion is facilitated when hydrophobic surfaces come into contact and remove the water between them. However, it is important to note that adhesion can also occur between hydrophobic and hydrophilic surfaces or two hydrophilic surfaces. Since colonization occurred, the Lm could have produced polymers or specific cellular structures called adhesins, contributing to adhesion. Nonetheless, the thermodynamic aspect of adhesion did not favor the interaction between Lm and the two surfaces.
When considering bacterial adhesion and biofilm formation, it is important to consider other/various properties, including the water contact angle of the materials. Our previous study found that Si and PTFE are hydrophobic, while SS and PET are hydrophilic when using a 90° cut-off angle [31]. According to the quantitative criterion (∆GTOT), they were all considered hydrophobic. This study classified Si and PTFE as hydrophobic and SS and PET as hydrophilic, but eventually, they were considered hydrophilic because the ∆GTOT values for all were >0 mJ/m2. It is because they were colonized by the Lm, and contact angle measurements were related to the hydrophilicity of the cells. θW and ∆GTOT values suggest SS and PET displayed more contact points for the Lm than Si and PTFE did. Given that SS provided more contact points for the Lm, unfavorable interactions between Lm and SS may have contributed to the increased damage to Lm.
Both antimicrobial blue light and essential oils have broad antimicrobial spectra and a multi-target mode of action. Blue light from 400 to 495 nm kills pathogens by generating ROS that damage cellular components. Essential oils have the ability to affect multiple targets in bacteria, specifically targeting their envelopes [34]. Many are Generally Recognized as Safe (GRAS) and widely used in food preservation, flavoring, fragrance, and cosmetic industries. An innovative strategy for developing more effective interventions against pathogens involves seeking bacteria-specific pro-photosensitizers. Lu et al. [35] recently discovered the potent antimicrobial activity of thymol, which showed strong synergy with blue light against Staphylococcus aureus and Pseudomonas aeruginosa. The photochemical reaction in bacteria is likely triggered by blue-light excitation of endogenous proporphyrin-like compounds. Likewise, trans-cinnamaldehyde exhibits antimicrobial properties and targets multiple bacterial structures. While it showed weaker synergy with blue light than Cur, this study highlights the need for further research and exploration of new phytochemicals that may exhibit higher synergy against Listeria monocytogenes.
4. Materials and Methods
4.1. Bacterium and Culture Conditions
Listeria monocytogenes ATCC 15313 originated from the American Type Culture Collection (ATCC™), which is a well-regarded strain in antimicrobial testing experiments [36,37,38]. This strain was cultured overnight at 37 °C on tryptic soy agar (TSA; Merck, Darmstadt, Germany) and an additional 20 h culture at 37 °C in tryptic soy broth (TSB) to obtain stationary-growth-phase cultures.
4.2. Planktonic Cells and Biofilms
A stationary-growth-phase culture, with a concentration of 1 × 108 CFU/mL in PBS, was added to a 24-well flat-bottom polystyrene plate (Costar, Corning, NY, USA) in 1000 µL volume along with photosensitizers at final concentrations. The biofilm inocula were prepared at a concentration of 1 × 106 CFU/mL in TSB. The surfaces of the target coupons in the 24-well polystyrene plate were seeded with 1000 µL/well of the inoculum and incubated for one day at 37 °C. The formed biofilms were washed with PBS and then immersed in 1000 µL of photosensitizer solutions.
4.3. Material Surfaces
Solid coupon surfaces, each with a diameter of 12.7 cm and a thickness of 3 mm, were used in this study. The coupons were made from different materials, including silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel type 316L (SS), and polyethylene terephthalate (PET). These coupons were purchased from BioSurface Technologies, based in Bozeman, MT, USA.
4.4. Hydrophobicity and Free Energy of Adhesion
The OCA 15 Plus goniometer (DATAPHYSICS, Filderstadt, Germany) determined the contact angles between Lm surfaces and water, formamide, and α-bromonaphthalene, respectively. In two independent trials, contact angle measurements were taken three times for each. The van Oss approach [39] was used to calculate the hydrophobicity, which is represented by the total free energy of interaction (ΔGsws) of the surface when immersed in water (w) (mJ/m2). Surface tension elements of the interacting entities can be utilized to conclude ΔGsws as follows (Equation (1)):
where constitutes the Lifshitz–van der Waals component of the surface free energy; and are the electron acceptor and electron donor parameters of the Lewis acid–base component (), with .
Then, three equations of the type below were solved (Equation (2)):
where is the contact angle and . In the end, if the ΔGTOT < 0 mJ/m2, the surface is hydrophobic, and if the ΔGTOT > 0 mJ/m2, it is hydrophilic.
When studying the interaction between two surfaces, the bacterial cells (b) and food-processing surfaces (s) in aqueous liquid media (l), the total free energy of adhesion (ΔGadhesion) can be calculated by solving Equations (3)–(5).
where γbs is the interfacial tension between the bacterial surfaces and the adhesion surface, γbl is the interfacial tension between the bacterial surfaces and the liquid, and γsl is the interfacial tension between the adhesion surfaces and the liquid, which can be found in [14]. These equations help determine the energy required to separate the two surfaces, taking into consideration their polarity () and apolarity (). The ΔGadhesion provides a way to measure the thermodynamic aspects of the adhesion process: if ΔGadhesion < 0 mJ/m2, the process is favorable, but if ΔGadhesion > 0 mJ/m2, the process is unfavorable.
4.5. Phytochemicals
Curcuma L. root powder was obtained from Herbapol (Kraków, Poland), and trans-cinnamaldehyde was obtained from Sigma-Aldrich (St. Louise, MO, USA). The photosensitizers were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich). The curcuminoid-rich samples were collected and sonicated in the dark for approx. 5 min. Control experiments were conducted to ascertain the growth inhibitory effects in DMSO (5–10%, v/v−1), and the concentration used in the inactivation experiments was kept below 5%.
4.6. Photodynamic Treatments
A 60 min blue-light exposure was conducted using an 18 × 3W commercially available LED array (Epistar Corp.; Hsinchu, Taiwan) with wavelengths of 410, 430, and 460 nm placed 10 cm away from the bottom of the 24-well polystyrene plate containing planktonic cells or biofilms on the target coupons. To measure the light intensity, a power meter with a 400–1050 nm power sensor (ACSE, Kraków, Poland) was used. The light intensity was multiplied by the exposure time in seconds to calculate the emission dose in J/cm2. The irradiation was fixed to obtain 234 J/cm2 (BL+). We used sub- and inhibitory concentrations of Curcuma L. (40 µg/mL) and trans-cinnamaldehyde (3 mM), respectively, to test photosensitization efficacy. These concentrations were found in our previous experiment, comprising the minimal inhibitory concentration assay. The photosensitizer solutions were added to the wells of the polystyrene plate, either with planktonic cells or coupons (biofilms), which was then immediately placed under the blue-light source. The bactericidal effects on planktonic and biofilm cells were similarly assessed with BL alone (BL+) and photosensitizers alone. Cells not exposed to light or photosensitizers were used as controls (BL−). Coupons were then transferred onto a new Costar plate. Afterward, they were treated with D/E neutralizing broth (Becton Dickinson, Sparks, MD, USA) for 5 min at room temperature. The coupons were washed using PBS, and then the biofilm-enclosed cells were removed using 1000 µL of PBS through 3 min sonication (Elmasonic SB-120DN; Abchem, Warsaw, Poland). The same neutralization procedure was applied to the planktonic cells after centrifuging at 11,000× g for 2 min (MPW 65R, Medical instruments, Warsaw, Poland) and resuspended in PBS. All of them were kept ready for CFU and FCM assays.
4.7. CFU Assay
CFU counts of planktonic and biofilm cells were determined via 10-fold serial dilutions on TSA plates and enumerated after 24 h incubation at 37 °C.
4.8. FCM Assay
Cells were initially incubated with 50 µM CFDA (5-[and-6-]-carboxyfluorescein diacetate; Sigma-Aldrich), a green cell enzymatic activity marker, delivered in anhydrous dimethyl sulphoxide (Sigma-Aldrich) at 37 °C for 30 min and then 30 µM PI (propidium iodide; Sigma-Aldrich) delivered in double-distilled water, a red nucleic acid probe. The cells were incubated in an ice bath for 10 min. CFDA evaluates intracellular enzymatic activity, as it is hydrolyzed by the cellular esterase into carboxyfluorescein (CF), a membrane-impermeable fluorescent compound. In contrast, PI cannot penetrate cells with intact membranes, indicating cell damage. Consequently, we distinguished cells with diminished activity and compromised membranes (CF−/PI+), referred to as dead, cells that showed intact cells without detectable esterase activity (CF−/PI−), referred to as non-active, and, finally, intact cells preserving enzymatic activity (CF+/PI−), referred to as active. A subset of cells was also incubated with dimeric cyanine nucleic acid stain (TOTO®-1) (1 mM solution in DMSO; Invitrogen™ Thermo Fisher, Waltham, MA, USA) at 2 µM. This particular dye has a high affinity for double-stranded DNA and exhibits a significant increase in fluorescence when it binds to nucleic acid. It is a useful indicator for detecting dead cells. TOTO®-1 has been shown to produce DNA histograms that offer better results than those obtained with propidium iodide (PI) due to having lower coefficients of variation. The cells were analyzed using a BD FACSLyric™ flow cytometer (Becton Dickinson, San Jose CA, USA), which had two lasers—blue (488 nm, air-cooled, 20 mW solid state) and red (633 nm, 17 mW HeNe). The green fluorescence from the CFDA- and TOTO-1-labeled cells was detected at the FL1 channel (530 ± 30 nm), while the red fluorescence of the PI-labeled cells was detected at the FL3 channel (630 ± 22 nm). To monitor the consistency of the instrument optical alignment, BD™ CS&T Beads (Becton Dickinson) were used, and BD FACSFlow™ solution (Becton Dickinson) was used as the sheath fluid. All bacterial analyses were performed at low rate settings, and a total event count of 50,000 was acquired. The data were analyzed using dot plots, which are bivariate displays where each dot represents one measured event, by the BD FACSSuite V1.3 software (Becton Dickinson).
4.9. Statistical Analysis
Mean densities of planktonic cells and biofilms after each photodynamic treatment using Curcuma L. and trans-cinnamaldehyde were recorded as log CFU/cm2. The experiment was repeated three times to ensure accuracy. To analyze the impact of photosensitization among planktonic cells and biofilms, a one-way analysis of variance was performed with Tukey’s test (p < 0.05). A Principal Components Analysis (PCA) was conducted to investigate the impact of photosensitization on Lm cells. Hierarchical cluster analysis was performed on cells to determine their dissimilarity extent. The Pearson correlation coefficient (r) was used to evaluate the direction and strength of correlations between assayed cells, with a significance level of p < 0.05. All statistical analyses were performed using TIBCO® Statistica™ ver. 13.3 (TIBCO Software Inc., Tulsa, OK, USA).
5. Conclusions
Curcumin-rich extracts have demonstrated excellent photosensitizing properties against both Listeria monocytogenes planktonic cells and biofilms. The effectiveness of Ca was comparatively lower than that of Cur. However, Ca has potent antibiofilm effects that vary depending on the surface on which Lm resides. This study may help in identifying more effective plant-based compounds to combat L. monocytogenes in an environmentally sustainable way.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29030685/s1, Figure S1: Cell numbers of Listeria monocytogenes ATCC 15313 planktonic cells and biofilms established on silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based non-photosensitization (BL−) experiments; Table S1: Percent of Listeria monocytogenes ATCC 15313 planktonic cells (% of total) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based (non-)photosensitization experiments as measured by FCM with CFDA/PI and TOTO®-1 staining; Table S2: Percent of Listeria monocytogenes ATCC 15313 biofilms established on silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based non-photosensitization (BL−) experiments as measured by FCM with CFDA/PI; Figure S2: FCM dot plots of Listeria monocytogenes ATCC 15313 biofilms established on silicone rubber (Si), polytetrafluoroethylene (PTFE), stainless steel 316 (SS), and polyethylene terephthalate (PET) in Curcuma L. (Cur)- and t-cinnamaldehyde (Ca)-based non-photosensitization (BL−) experiments as measured by FCM with TOTO®-1 staining.
Author Contributions
Conceptualization, M.A.O.; methodology, A.Z., I.L., J.G., A.D., M.S. and M.A.O.; formal analysis, A.Z., I.L. and M.A.O.; investigation, A.Z., I.L., J.G., A.D., M.S. and M.A.O.; data curation, M.A.O.; writing—original draft preparation, A.Z., I.L. and M.A.O.; writing—review and editing, M.S. and M.A.O.; funding acquisition, M.A.O. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the National Science Centre, Poland; Grant number 2022/45/B/NZ9/00299 (OPUS-23). Moreover, the publication was written as a result of the authors’ internship (A.D. and M.A.O.) at the University of Porto, co-financed by the European Union under the European Social Fund (Operational Program Knowledge Education Development), carried out in the project Development Program at the University of Warmia and Mazury in Olsztyn (POWR.03.05.00-00-Z310/17). This work was also supported by Base Funding (LA/P/0045/2020 (ALiCE), UIDB/00511/2020, and UIDP/00511/2020 (LEPABE)), funded by national funds through [FCT/MCTES (PIDDAC)].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rodríguez-Campos, D.; Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Persistent Listeria monocytogenes isolates from a poultry-processing facility form more biofilm but do not have a greater resistance to disinfectants than sporadic strains. Pathogens 2019, 8, 250. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic action of LED-activated curcumin against Staphylococcus aureus involving intracellular ROS increase and membrane damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Costa, L.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Faustino, M.A.; Cunha, Â.; Gomes, N.C.; Almeida, A. Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT. Antiviral Res. 2011, 91, 278–282. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Pei, J.; Xue, F.; Cui, X.; Xiong, X.; Li, C. The application of photodynamic inactivation to microorganisms in food. Food Chem. X 2021, 12, 100150. [Google Scholar] [CrossRef]
- Maisch, T. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B Biol. 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Draszanowska, A.; Karpińska-Tymoszczyk, M.; Simões, M.; Olszewska, M.A. The effect of turmeric rhizome on the inhibition of lipid oxidation and the quality of vacuum-packed pork meatloaf. Appl. Sci. 2022, 12, 10641. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; De Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. The effect of photosensitization mediated by curcumin on storage life of fresh date (Phoenix dactylifera L.) fruit. Food Control 2018, 93, 305–309. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Tikekar, R.; Nitin, N. Combination of aerosolized curcumin and UV-A light for the inactivation of bacteria on fresh produce surfaces. Food Res. Int. 2018, 114, 133–139. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Tosati, J.V.; Tikekar, R.V.; Monteiro, A.R.; Nitin, N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157:H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, J.; Li, S.; Hamzah, S.S.; Jiang, H.; Zhou, A.; Zeng, S.; Lin, S. Curcumin-based photodynamic sterilization for preservation of fresh-cut hami melon. Molecules 2019, 24, 2374. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, R.; Zhang, H.; Jin, G.-Q.; Wang, B.-W.; Zhu, M.; Zhang, J.; Gao, S.; Zhang, J.-L. Porpholactam-cinnamaldehyde conjugates for promoting ROS generation in photodynamic therapy. J. Porphyr. Phthalocyanines 2021, 25, 1111. [Google Scholar] [CrossRef]
- Bernardes, P.C.; de Andrade, N.J.; Ferreira, S.O.; de Sá, J.P.; Araújo, E.A.; Delatorre, D.M.; Luiz, L.M. Assessment of hydrophobicity and roughness of stainless steel adhered by an isolate of Bacillus cereus from a dairy plant. Braz. J. Microbiol. 2010, 41, 984–992. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The natural product curcumin as an antibacterial agent: Current achievements and problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, B.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes. Food Control 2020, 108, 106886. [Google Scholar] [CrossRef]
- Bonifácio, D.; Martins, C.; David, B.; Lemos, C.; Neves, M.G.P.M.S.; Almeida, A.; Pinto, D.C.G.A.; Faustino, M.A.F.; Cunha, Â. Photodynamic inactivation of Listeria innocua biofilms with food-grade photosensitizers: A curcumin-rich extract of Curcuma longa vs commercial curcumin. J. Appl. Microbiol. 2018, 125, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; Cao, B.; Wu, J.; Wang, Y.; Xue, Y.; Xu, J.; Xue, C.; Tang, Q.J. The effect of novel photodynamic activation method mediated by curcumin on oyster shelf life and quality. Food Res. Int. 2016, 87, 204–210. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, F.; Tang, Q.-J.; Xu, C.-S.; Ni, Z.-J.; Meng, X.-H. Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.; Okoth, S.A.; Sultanbawa, Y. Curcumin-based photosensitization inactivates Aspergillus flavus and reduces aflatoxin B1 in maize kernels. Food Microbiol. 2019, 82, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Dev Kumar, G.; Hur, M.; Diez-Gonzalez, F. Inactivation of dried cells and biofilms of Listeria monocytogenes by exposure to blue light at different wavelengths and the influence of surface materials. Appl. Environ. Microbiol. 2023, 89, e01147-23. [Google Scholar] [CrossRef]
- Lin, S.L.; Hu, J.M.; Tang, S.S.; Wu, X.Y.; Chen, Z.Q.; Tang, S.Z. Photodynamic inactivation of methylene blue and tungsten-halogen lamp light against food pathogen Listeria monocytogenes. Photochem. Photobiol. 2012, 88, 985–991. [Google Scholar] [CrossRef]
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.-G.; Song, W.; Kim, B.J. Rational design of hyper-crosslinked polymers for biomedical applications. J. Polym. Sci. 2023. early view. [Google Scholar] [CrossRef]
- Agel, M.R.; Baghdan, E.; Pinnapireddy, S.R.; Lehmann, J.; Schäfer, J.; Bakowsky, U. Curcumin loaded nanoparticles as efficient photoactive formulations against gram-positive and gram-negative bacteria. Colloids Surf. 2019, 178, 460–468. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Khan, N.; Abbasi, A.M.; Dastagir, G.; Nazir, A.; Shah, G.M.; Shah, M.M.; Shah, M.H. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as a potential source to cure infectious diseases. BMC Complement. Altern. Med. 2014, 14, 122. [Google Scholar] [CrossRef]
- Lencova, S.; Zdenkova, K.; Demnerova, K.; Stiborova, H. Antibacterial and antibiofilm effect of natural substances and their mixtures over Listeria monocytogenes, Staphylococcus aureus and Escherichia coli. LWT 2022, 154, 112777. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.R.; Ananda Baskaran, S.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, L.; Han, J.; Dong, P.; Luo, X.; Zhang, Y.; Zhu, L. Inhibition of biofilm formation and related gene expression of Listeria monocytogenes in response to four natural antimicrobial compounds and sodium hypochlorite. Front. Microbiol. 2021, 11, 617473. [Google Scholar] [CrossRef] [PubMed]
- Gędas, A.; Draszanowska, A.; den Bakker, H.; Diez-Gonzalez, F.; Simões, M.; Olszewska, M.A. Prevention of surface colonization and anti-biofilm effect of selected phytochemicals against Listeria innocua strain. Colloids Surf. B 2023, 228, 113391. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, Z.; Azeredo, J.; Oliveira, R.; Vieira, M.J. Physico-chemical surface characterization of a bacterial population isolated from a milking machine. Food Microbiol. 2005, 22, 247–251. [Google Scholar] [CrossRef]
- van Oss, C.J. Hydrophobicity and hydrophilicity of biosurfactants. Curr. Opin. Coll. Int. Sci. 1997, 2, 503–512. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Natural plant-derived chemical compounds as Listeria monocytogenes inhibitors in vitro and in food model systems. Pathogens 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, Y.; Wu, M.X. Bacteria-specific pro-photosensitizer kills multidrug-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Commun. Biol. 2021, 4, 408. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Simões, L.C.; Saavedra, M.J.; Simões, M. The action of selected isothiocyanates on bacterial biofilm prevention and control. Int. Biodeterior. Biodegrad. 2014, 86, 25–33. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).