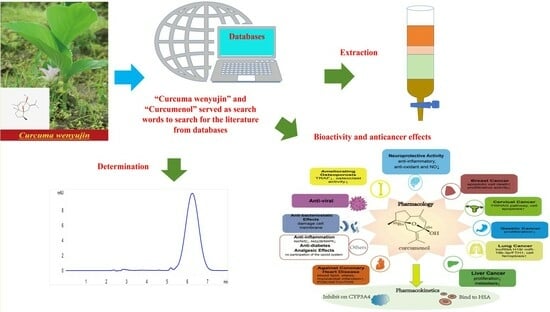
The Extraction, Determination, and Bioactivity of Curcumenol: A Comprehensive Review
Abstract
1. Introduction
| Curcuma wenyujin | Curcumina longa | |
|---|---|---|
| The Content of Unique Chemical Markers to Distinguish the Two Herbs [23] | ||
| Curcumenol | > | Curcumenol |
| Curcumenone | > | Curcumenone |
| Neocurdione | > | Neocurdione |
| Curdione | > | Curdione |
| Curcumin | < | Curcumin |
| Physiological effects | ||
| Anti-inflammatory [24] | Anti-inflammatory [25] | |
| Anti-oxidant [26] | Anti-oxidant [27] | |
| Antimicrobial [28] and antiviral activity [29] | Antimicrobial [30] and antiviral activity [31] | |
| Anti-diabetic [32] | Anti-diabetic [33] | |
| Hepatoprotective [34] | Hepatoprotective [35] | |
| Effects on the cardiovascular system [36] | Effects on the cardiovascular system [37] | |
| Analgesic effect [38] | Analgesic effect [39] | |
| Effect on the nervous system: Alzheimer’s disease [40] | Effect on the nervous system: improve memory impairment [41] | |
| Anti-cancer: glioma [42], breast cancer [43], liver cancer [44], gastric cancer [45], colon cancer [46], leukemia [47], cervical cancer [48], lung cancer [49] | Anti-cancer: liver cancer [50], colon cancer [51], cervical cancer [52], human thyroid cancer [53], human nasopharyngeal carcinoma cells [54], lung cancer [55] | |
| Effects on the respiratory system [56] | ||
| Effect on the digestive system [57] | ||
| Anticoagulant effect [58] | ||
2. Traditional Medicinal Uses of Curcumenol-Related Herbs
| Scientific Name | Traditional Name | Plant Part Used | Effective Compounds and Ref. | Traditional Medicine Use | Monotherapy or an Adjunct and Ref. |
|---|---|---|---|---|---|
| Curcuma zedoaria | Zedoary, Zingiberaceae | Rhizome | Curcumenol [17] | Osteoarthritis | An adjunct to allopathic medication [59] |
| Curcuma zedoaria | White turmeric | Rhizome | Curcumenol [18] | Anti-inflammatory | An adjunct to allopathic medication [60] |
| Curcuma zedoaria | White turmeric | Rhizome | Curcumenol and six other compounds [19] | Psoriasis | An adjunct to allopathic medication [61] |
| Curcuma | Curcuma phaeocaulis, Curcuma kwangsiensis, Curcuma wenyujin | Rhizome | Curcumenol, Curcumol, β-elemene, curdione [21] | Anti-fungal | An adjunct to allopathic medication [62] |
| Curcuma zedoaria | Zingiberaceae | Rhizome | Curcumenol and some principal sesquiterpenes [22] | Hepatoprotective | An adjunct to allopathic medication [63] |
| Curcuma zedoaria | Zingiberaceae | Rhizome | Curcumenol, dihydrocurdione [64,65] | Analgesic | An adjunct to allopathic medication [66] |
| Curcuma | Zingiberaceae | Curcuma aromatica Salisb. rhizome | Curzerene, isoprocurcumenol, and (+)-curcumenol [67] | Coronary heart disease | An adjunct to allopathic medication [68] |
| Curcuma zedoaria | Zingiberaceae | Rhizome | Curcumenol [69] | Osteoporosis | An adjunct to allopathic medication [70] |
| Curcuma zedoary | Rhizome | Curcumenol [71] | Liver cancer | An adjunct to allopathic medication [72] | |
| Curcuma | Rhizome | Curcumenol [73] | Cervical cancer | An adjunct to allopathic medication [74] | |
| Curcuma wenyujin | Wenyujin | Rhizome | Curcumenol [13] | Lung cancer | An adjunct to allopathic medication [75] |
| Curcuma zedoaria rhizome | Zingiberaceae | C. zedoaria rhizomes | Curcumenol, 4,8-dioxo-6β-methoxy-7α,11-epoxycarabrane, and zedoarofuran [15] | Gastric cancer | An adjunct to allopathic medication [76] |
| Curcuma zedoaria | Temu putih | Rhizome | Curcumenone, Curcumenol [16] | Breast cancer | An adjunct to allopathic medication [77] |
3. Sources of Curcumenol
4. Physicochemical Properties of Curcumenol
5. Extraction Methods
| Matrix | Pretreatment/Extraction Approach | Procedure | Recovery (%) | Ref. |
|---|---|---|---|---|
| Biological Samples | ||||
| Crude hemolymph extract | Organ lysis | The exposed hemolymph was taken in an aseptic manner and suspended in a quantity of sterile distilled water. Ten cycles of freezing and thawing were applied to the gut and muscle tissues; then, they were homogenized, sonicated, centrifuged, and analyzed. | _ | [86] |
| Plant | ||||
| Crude turmeric samples | Pressurized liquid water extraction | Curcuma Radix was dried at 60 °C and sieved with a 60-mesh sieve. The dried powder and diatomaceous earth were added to a stainless steel extraction cell. The extraction of the sample was obtained using the following parameters: 40% of the flush volume, methanol at 100 °C, static extraction for 5 min, 1000 psi. | _ | [23] |
| Powdered rhizome | Ultrasonication in methanol | The powdered rhizome was extracted with methanol at 40 kHz, 200 W for 1 h using ultrasound, then filtered through 0.45 μm pores before analysis. | 103.62 | [87] |
| Powdered rhizome | Steam distillation | Powdered rhizome was exposed to vapor at 90 °C, followed by extraction for 30 min. | _ | [88] |
| Powdered rhizome | Extraction with n-hexane | n-Hexane was shaken with samples for 30 min at 40 °C, and centrifuged at 4000 rpm. | _ | [89] |
| Powdered rhizome | Pressurized liquid extraction | The extraction of the sample was achieved with the following conditions: static cycle, 1, filtering through a 0.45 μm icon filter for 40% of the flush volume; pressure, 1000 psi; static extraction period, 5 min; particle size, 0.2–0.3 mm; temperature, 100 °C, with methanol. | 100.5 | [90] |
| Different parts of the plant, powdered | Extraction with dichloromethane | Dichloromethane was added and the sample was macerated for 7 days at 15–20 °C. | _ | [64] |
| Powdered rhizome | Cold immersion with methanol | A 0.5 g sample of powder was placed in a 100 mL triangle bottle, then 50 mL methanol was added, before cold soaking for 12 h, shaking every several minutes, until the supernatant was poured out. | 99.6 | [91] |
| Four sources of Curcuma Radix decoction pieces | Heating reflux with water | Decoction pieces were added in 10-fold amounts (m L/g) of water, soaked for 1 h, heated and reflowed twice, filtered, and concentrated. | 116.8 | [92] |
| Powdered rhizome | Ultrasonication in ethanol | Ultrasound with 70% ethanol for 45 min. | 95.8–104.1 | [93] |
| Powdered rhizome | Pressurized liquid extraction | Methanol; 120 °C; particle size, 0.2–0.3 mm; static extraction time, 5 min; pressure, 1500 psi; static cycle, 1; and 60% flush volume. | _ | [94] |
| Formulation | ||||
| Decoction | Immersed in water and ethanol | Immersed in 50% ethanol, 70% ethanol, 100% ethanol, and water for 1 h each, then 2 h reflux. | _ | [95] |
6. Methods for Qualitative and Quantitative Analysis
| Analytical Method | Research Objectives/Title | Matrix and Sample Preparation Method | Result | Ref. |
|---|---|---|---|---|
| High-performance liquid chromatography (HPLC) | Determination of curcumenol in Yujin. | Powdered rhizome | Recovery value of curcumenol 103.62%. | [87] |
| HPLC | Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and HPLC. | Powdered rhizome | With intra- and inter-day fluctuations of less than 1.57% and 1.98%, respectively, this approach demonstrated good repeatability for the quantification of the abovementioned 11 components in three species of Curcuma rhizomes. | [90] |
| HPLC | Quantitation of Curcumenol extracted from water. | Four sources of Curcuma Radix decoction pieces | For C. kwangsiensis, C. wenyujin, C. longa, and C. phaeocaulis, the contents of curcumenol in the water extract were 0.066, 0.271, 0.058, and 0.310 mg/g, respectively. | [92] |
| Ultra-performance liquid chromatography (UPLC) | Determination of the curcumenol content in Curcuma kwangsiensis, vinegar-boiled Curcuma kwangsiensis, and water extract residues using UPLC. | Powdered rhizome and water residues | The established analysis method is accurate, stable, and sensitive; it can be used for the quality assay of Curcuma kwangsiensis, and it provides a scientific basis for the resource utilization of water extract residues. | [103] |
| High resolution gas chromatography (HRGC) | Quantitation of curcumenol. | Different parts of this plant, powdered | Standard samples of curcumenol within the concentration range of 0.03–0.93 mg/mL. | [64] |
| Liquid chromatography tandem–mass spectrometry (LC–MS) | Fingerprint of Curcuma phaeocaulis using LC–MS. | Powdered rhizome | Peaks 4–6 and 9 were identified as curcumin, demethoxycurcumin, curcumenol, and curcumone, respectively, through the contrast of the retention time and the online UV spectra and the molecular weight of the chemical standards. | [104] |
| Ultra-high-performance liquid chromatography coupled with the triple quadrupole tandem mass spectrometry method (UPLC-QQQ-MS) | Identification of curcumenol. | Decoction, dispensing granule decoction | It was the first time that 17 chemicals in the Astragali Radix–Curcumae Rhizoma (AR–CR) herb pair were simultaneously analyzed using UPLC-QQQ-MS, offering a workable approach in terms of overall quality control. | [95] |
| Ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry | Identification of curcumenol. | Crude turmeric samples, pressurized liquid extractions | This new method led to the discovery of curcumenol and some other components as unique chemical markers for identification. | [23] |
| Gas chromatography–mass spectrometry (GC/GC–MS) | Identification of curcumenol. | Powdered rhizome | Identified curcumenol. | [88] |
| GC–MS | Identification of curcumenol. | Powdered rhizome | Two isomeric forms of curcumenol comprised the GC chromatogram’s major components, taking up 28.68 ± 0.91% and 17.96 ± 0.69% of the control plants’ total volatiles, respectively. | [89] |
| GC–MS | Identification and quantitation of curcumenol in Curcuma rhizomes. | Powdered rhizome | Curcumenol and four other compounds were optimized as markers for the quality control of Ezhu. | [94] |
| GC–MS | Determination of curcumenol in Curcuma rhizomes. | Powdered rhizome | Nine sesquiterpenoids were effectively quantified using the validated method in 18 samples of three Curcuma species, utilized as Ezhu. | [97] |
| GC–MS | Determination of curcumenol in the fractionation of volatile constituents originating from the Curcuma rhizome using GC. | Powdered rhizome | The structures of the compound were identified as curcumenol using a mass spectrometer (MS) and NMR spectra, respectively. | [105] |
7. Other Factors Influencing the Content of Curcumenol
8. Bioactivity
| Curcumenol | Curcumin | ||||||
|---|---|---|---|---|---|---|---|
| Disease/ Curcumenol Activity | Model | Treatment Doses | Mechanism | Ref. | Treatment Doses | Model | Ref. |
| Anti-inflammatory activity | BV-2 microglial cells | 5–20 μM | Inhibiting Akt-dependent nuclear factor kappa-B (NF-κB) activation and the downregulation of Akt and p38 mitogen-activated protein kinase (MAPK) signaling | [18] | 5–20 μM | BV-2 microglial cells | [111] |
| Anti-inflammatory activity | HaCaT cells | 200 μg/mL | Inhibits the overexpression of inflammatory factors | [19] | 20 μM | HaCaT cells | [112] |
| Analgesic effects | Mice | 10 mg/kg | - | [64] | 1000 mg/kg to 2000 mg/kg | Mice | [113] |
| Analgesic effects | Mice | 12, 22, 29 μmol/kg | No involvement with the opioid system | [65] | - | - | - |
| Anti-oxidant ability and neuroprotective activity | NG108-15 cells | 4 μM | - | [5] | 25–100 μM | NG108-15 cells | [114] |
| Against coronary heart disease | Coronary heart disease rats | - | Improving blood lipid level, blood stasis, and myocardial infarction, and controlling the signaling pathway of PI3K/AKT/mTOR | [67] | - | - | - |
| Anti-diabetic activity | Human hepatocellular carcinoma (HepG2) cells | 10 μM | - | [32] | - | Human | [115] |
| Ameliorating osteoporosis | Mice | 100 μM | Impairs the stability of TRAF6 enhanced by IPMK and suppresses excessive osteoclast activity | [69] | 100 mg/kg | Mice | [116] |
| SARS-CoV-2 infection | - | - | High-affinity interaction with proteins involved in coronavirus infection | [117] | 10 µg/mL | DG614 strain and Delta variant | [118] |
| Anti-bacteriostatic effects | Gram-negative and Gram-positive bacteria | 50 μg/mL | - | [20] | - | - | - |
| Anti-bacteriostatic effects | P. capsici | 20 μg/mL | Damages the cell membrane | [21] | - | - | - |
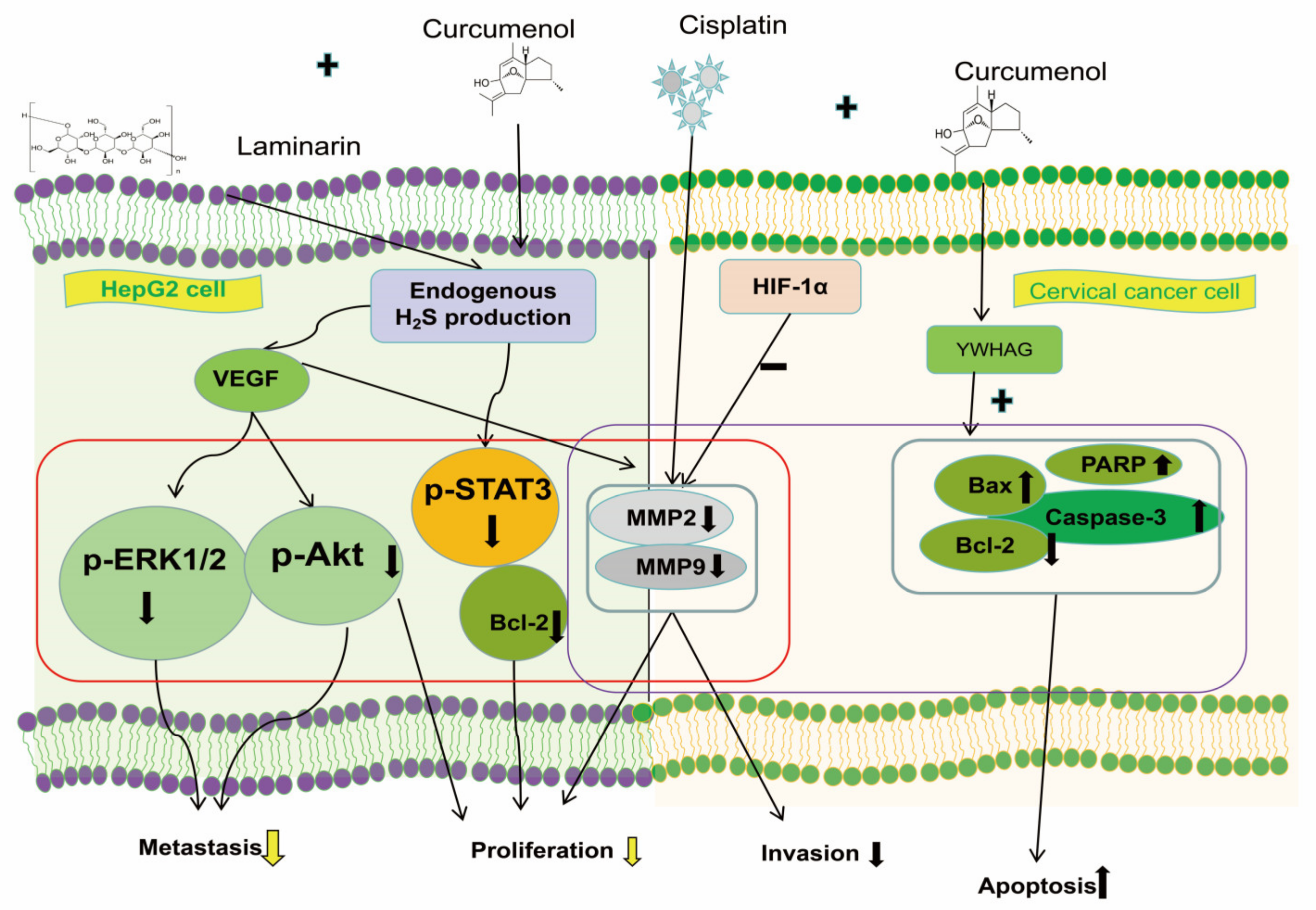
| Breast cancer | MCF-7 cells | 9.3 ± 0.3 μg/mL | Anti-proliferative activity and induces apoptotic cell death | [16] | 20 μM | MCF-7 cells | [119] |
| Gastric cancer | AGS cells | 263.34 ± 2.97 μM | Inhibition of proliferation | [15] | 32 μM | AGS cells | [120] |
| Lung cancer | H1299 and H460 cells | 100–400 μg/mL | Via the lncRNA H19/miR-19b-3p/FTH1 axis for lung cancer cell ferroptosis | [13] | 5–30 μM | H460 cells | [121] |
| Cervical cancer | HeLa and C33A Cells | - | Through the tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein gamma polypeptide (YWHAG) pathway, facilitating cervical cancer cell apoptosis | [73] | 20 μM | HeLa and C33A Cells | [122] |
| Inhibitory to cytochrome P450 (CYP) enzymes | Cytochrome P450 3A4 (CYP3A4) | 12.6 ± 1.3 μM | - | [123] | 11.93 ± 3.49 µM | CYP3A4 | [124] |
| Binding to human serum albumin (HSA) | HSA | 60 μM | - | [125] | - | HSA | [126] |
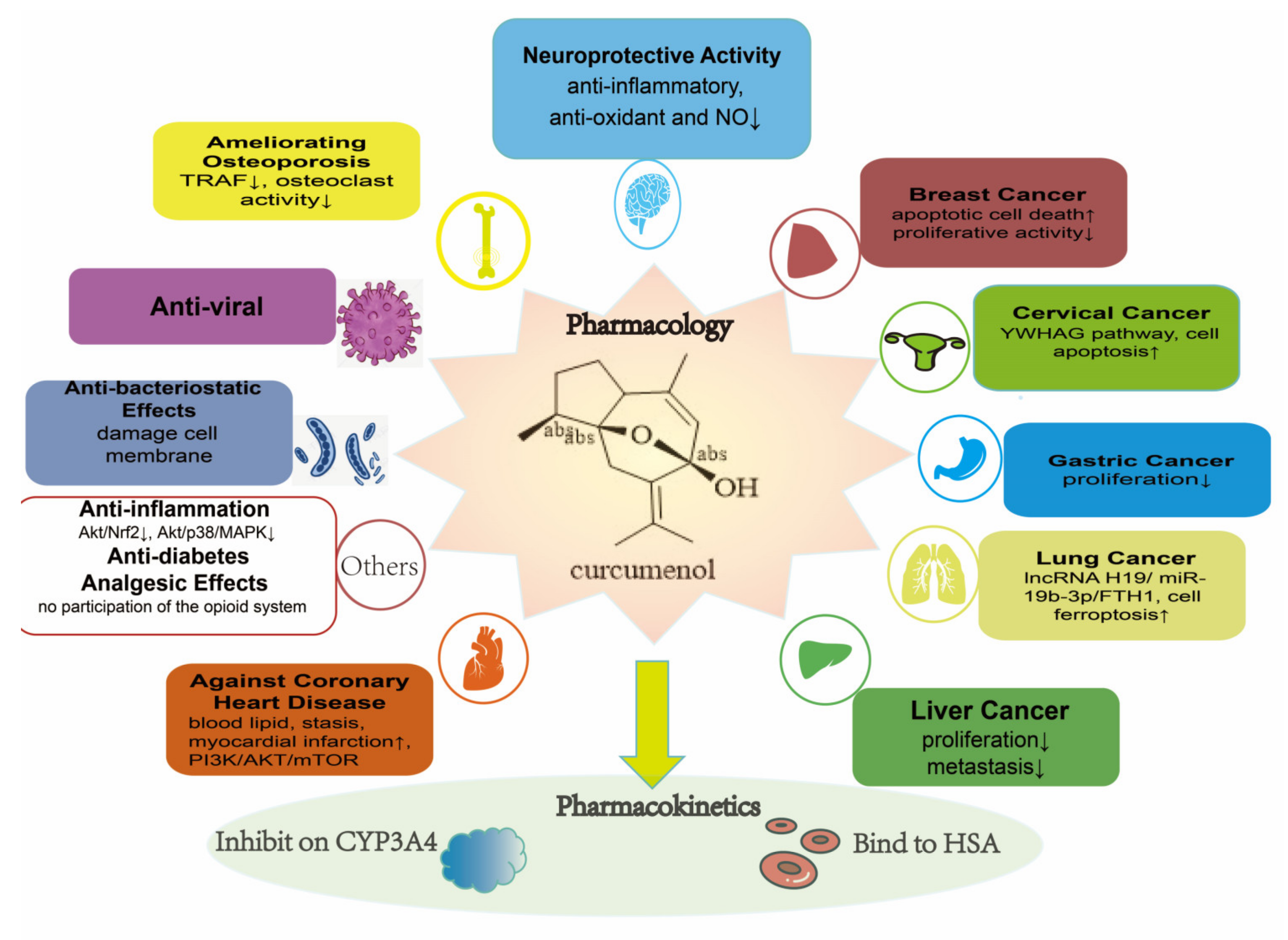
8.1. Anti-Inflammatory Activity
8.2. Analgesic Effects
8.3. Anti-Oxidant Ability and Neuroprotective Activity
8.4. Coronary Heart Disease (CHD)
8.5. Anti-Diabetic Activity
8.6. Ameliorating Osteoporosis
8.7. Anti-Viral and Anti-Bacteriostatic Effects
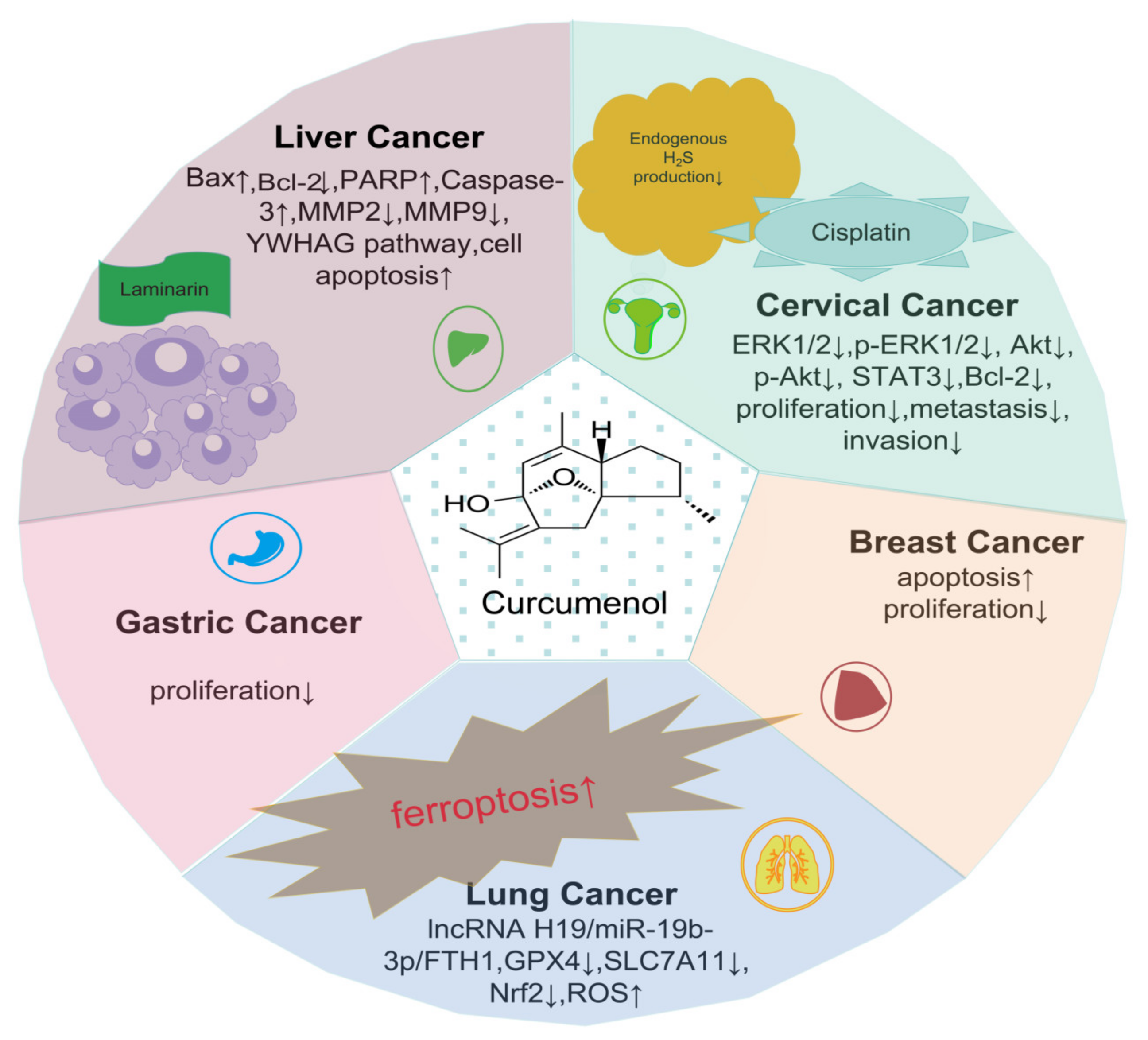
8.8. Diversity of Anti-Cancer Effects
8.8.1. Effects on Breast Cancer
8.8.2. Effects on Gastric Cancer
8.8.3. Effects on Lung Cancer
8.8.4. Synergistic Anti-Cancer Effects
8.9. Pharmacokinetics Study
8.9.1. Inhibitory Effect on Human Liver Cytochrome P450 Enzymes
8.9.2. Binding to Human Serum Albumin (HSA) In Vitro
8.10. Other Effects
9. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGS cells | human gastric adenocarcinoma cells |
| AR | Astragali Radix |
| AR–CR | Astragali Radix–Curcuma Rhizoma |
| ATDC5 chondrocytes | mouse embryonic tumor cells |
| CAS | chemical abstracts service |
| CaSki | human cervical cancer |
| CASR | Curcuma aromatica salisb. rhizome |
| CHD | coronary heart disease |
| CR | Curcuma Rhizoma |
| CYP | cytochrome P450 |
| CYP3A4 | cytochrome P450 3A4 |
| GC | gas chromatography |
| GC–MS | gas chromatography–mass spectrometry |
| MS | mass spectrometry |
| H460 cells | human large cell lung cancer cells |
| HaCaT cells | human immortalized keratinocytes |
| HeLa | human cervical carcinoma cell line |
| HPLC | high-performance liquid chromatography |
| HRGC | high-resolution gas chromatography |
| HSA | human serum albumin |
| HT-29 | human colorectal adenocarcinoma cells |
| IL-1β | Interleukin-1β |
| LC-MS | liquid chromatography tandem mass spectrometry |
| MAPK | mitogen-activated protein kinases |
| MCF-7 cells | human breast cancer cell line |
| MIC | minimal inhibitory concentration |
| MMP | matrix metalloproteinase |
| MTT | methylthiazolyldiphenyl-tetrazolium bromide |
| NF-κB | nuclear factor kappa-B |
| NMR | nuclear magnetic resonance |
| OA | osteoarthritis |
| pSTAT3 | signal transducer and activator of transcription 3 protein phosphorylation |
| PC-3 | human prostate cancer cell |
| RSD | relative standard deviation |
| TCM | traditional Chinese medicine |
| TLC | thin-layer chromatography |
| TNF-α | tumor necrosis factor alpha |
| UPLC | ultra-performance liquid chromatography; |
| UPLC-QQQ-MS | ultra-high-performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry method |
| UV | ultraviolet |
| YWHAG | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein gamma polypeptide |
| ZTO | zedoary turmeric oil |
References
- Fang, F.; Cheng, Z.H.; Guo, Y.L.; Cai, Y.B. Comparative Analysis of the Volatile Components in the Fresh Roots and Rhizomes of Curcuma wenyujin by Static Headspace Gas Chromatography Mass Spectrometry. Chin. J. Chem. 2006, 24, 1346–1351. [Google Scholar] [CrossRef]
- Yin, M.; Chu, S.; Zhao, Y.; Zheng, X.; HS, P. Materia medica illustrations related to the regional names in Zhejiang province in Ben Cao Tu Jing. Zhonghua Yi Shi Za Zhi 2022, 52, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, W.; Tu, H.; Chen, N.; Zhong, Z.; Yan, P.; Dong, J. Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κ B signaling. Bioorg. Med. Chem. Lett. 2017, 27, 1835–1839. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Zhao, B.; Jiang, Z.; Feng, L.; Jia, X. Advance in research on anticancer drug β-elemene and its derivatives. Chin. Tradit. Herb. Drug. 2018, 49, 1184–1191. [Google Scholar] [CrossRef]
- Hamdi, O.A.A.; Ye, L.J.; Kamarudin, M.N.A.; Hazni, H.; Paydar, M.; Looi, C.Y.; Shilpi, J.A.; Kadir, H.A.; Awang, K. Neuroprotective and Antioxidant Constituents from Curcuma zedoaria Rhizomes. Rec. Nat. Prod. 2015, 9, 349–355. [Google Scholar]
- Li, Y.; Wu, Y.; Li, Y.; Guo, F. Review of the traditional uses, phytochemistry, and pharmacology of Curcuma wenyujin Y. H. Chen et C. Ling. J. Ethnopharmacol. 2021, 269, 113689. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Sakurai, Y.; Numabe, S.; Takemoto, T. Structure of curcumenol. Chem. Pharm. Bull. 1968, 16, 39–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, J.; Ji, F.; Kang, J.; Wang, H.; Li, S.; Jin, D.-Q.; Zhang, Q.; Sun, H.; Guo, Y. Absolute Configurations and NO Inhibitory Activities of Terpenoids from Curcuma longa. J. Agric. Food. Chem. 2015, 63, 5805–5812. [Google Scholar] [CrossRef] [PubMed]
- Asem, S.D.; Laitonjam, W.S. A new guaianolide sesquiterpene lactone from Curcuma leucorrhiza Roxb. Nat. Prod. Res. 2014, 28, 477–482. [Google Scholar] [CrossRef]
- Qiu, G.; Yan, P.; Shao, W.; Zhou, J.; Lin, W.; Fang, L.; Zhao, X.; Dong, J. Two new sesquiterpenoids including a sesquiterpenoid lactam from Curcuma wenyujin. Chem. Pharm. Bull. 2013, 61, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Aspollah Sukari, M.; Wah, T.S.; Saad, S.M.; Rashid, N.Y.; Rahmani, M.; Lajis, N.H.; Hin, T.-Y.Y. Bioactive sesquiterpenes from Curcuma ochrorhiza and Curcuma heyneana. Nat. Prod. Res. 2010, 24, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pan, T.; Xiang, Y.; Zhang, M.; Xie, H.; Liang, Z.; Chen, B.; Xu, C.; Wang, J.; Huang, X.; et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact. Mater. 2022, 13, 23–36. [Google Scholar] [CrossRef]
- Peng, B.; Zhou, X.; Shi, J.; Li, Z. Effects of volatile oil and three main components from Curcuma phaeocaulis Valeton on liver cancer and endometrial carcinoma cell lines. West China J. Pharm. Sci. 2007, 2007, 312–313. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, D.; Lee, S.R.; Ko, Y.-J.; Sung Kang, K.; Chung, S.J.; Kim, K.H. Sesquiterpenes from Curcuma zedoaria rhizomes and their cytotoxicity against human gastric cancer AGS cells. Bioorg. Chem. 2019, 87, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Hamdi, O.A.; Syed Abdul Rahman, S.N.; Awang, K.; Abdul Wahab, N.; Looi, C.Y.; Thomas, N.F.; Abd Malek, S.N. Cytotoxic Constituents from the Rhizomes of Curcuma zedoaria. Sci. World J. 2014, 2014, 321943. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Chen, Z.; Chen, C.; Han, C.; Li, X.; Tian, H.; Cheng, X.; Zhang, K.; Zhou, T.; et al. Curcumenol mitigates chondrocyte inflammation by inhibiting the NF-κB and MAPK pathways, and ameliorates DMM-induced OA in mice. Int. J. Mol. Med. 2021, 48, 192. [Google Scholar] [CrossRef]
- Lo, J.Y.; Kamarudin, M.N.A.; Hamdi, O.A.A.; Awang, K.; Kadir, H.A. Curcumenol isolated from Curcuma zedoaria suppresses Akt-mediated NF-κB activation and p38 MAPK signaling pathway in LPS-stimulated BV-2 microglial cells. Food. Funct. 2015, 6, 3550–3559. [Google Scholar] [CrossRef]
- Wang, J. Inhibition of HaCaT Cell Proliferation by Active Constituents of Rhizoma Curcumae and Its Mechanism of Action Based on NF-κB Signaling Pathway. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2019. [Google Scholar]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Khan, N.A. Antibacterial Activities of Selected Pure Compounds Isolated from Gut Bacteria of Animals Living in Polluted Environments. Antibiotics 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, F.; Li, Q.; Xu, S.; Zhao, X.; Xue, P.; Feng, X. Antifungal activity of zedoary turmeric oil against Phytophthora capsici through damaging cell membrane. Pestic. Biochem. Physiol. 2019, 159, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yoshikawa, M. Inhibitory effect and action mechanism of sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/lipopolysaccharide-induced liver injury. Bioorg. Med. Chem. Lett. 1998, 8, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bai, X.; Yang, F.Q.; Zhang, X.J.; Hu, Y.; Li, P.; Wan, J.B. Discriminating from species of Curcumae Radix (Yujin) by a UHPLC/Q-TOFMS-based metabolomics approach. Chin. Med. 2016, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, L.; Zhang, L.; Zhang, T. β-Elemene inhibits 7,12-dimethylbenz(a)anthracene/12-O-tetradecanoylphorbol-13-acetate-induced skin tumorigenesis through suppression of NF-κB-associated signaling events in the mouse skin model. J. Biochem. Mol. Toxicol. 2020, 34, e22550. [Google Scholar] [CrossRef] [PubMed]
- Gouthamchandra, K.; Sudeep, H.V.; Chandrappa, S.; Raj, A.; Naveen, P.; Shyamaprasad, K. Efficacy of a Standardized Turmeric Extract Comprised of 70% Bisdemothoxy-Curcumin (REVERC3) against LPS-Induced Inflammation in RAW264.7 Cells and Carrageenan-Induced Paw Edema. J. Inflamm. Res. 2021, 14, 859–868. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Chung, H.W.; Lee, Y.C.; Kim, H.R.; Kwon, H.J.; Chae, H.J. Curcumin and Curcuma longa L. extract ameliorate lipid accumulation through the regulation of the endoplasmic reticulum redox and ER stress. Sci. Rep. 2017, 7, 6513. [Google Scholar] [CrossRef] [PubMed]
- Mhillaj, E.; Tarozzi, A.; Pruccoli, L.; Cuomo, V.; Trabace, L.; Mancuso, C. Curcumin and Heme Oxygenase: Neuroprotection and Beyond. Int. J. Mol. Sci. 2019, 20, 2419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Z.; Chen, D.; Huang, Z.; Li, Y.; Lan, X.; Su, P.; Pan, W.; Zhou, W.; Zheng, X.; et al. Variation on Composition and Bioactivity of Essential Oils of Four Common Curcuma Herbs. Chem. Biodivers. 2017, 14, e1700280. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, Q.; Zhao, Y.; Ren, X.; Bao, K.; Li, X.; Jiang, C. Exploring mechanism of Curcuma wenyujin against COVID-19. Chin. Tradit. Herb. Drugs 2020, 51, 1977–1983. [Google Scholar] [CrossRef]
- Salehi, B.; Rodrigues, C.F.; Peron, G.; Dall’Acqua, S.; Sharifi-Rad, J.; Azmi, L.; Shukla, I.; Singh Baghel, U.; Prakash Mishra, A.; Elissawy, A.M.; et al. Curcumin nanoformulations for antimicrobial and wound healing purposes. Phytother. Res. 2021, 35, 2487–2499. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Chen, F.; Wu, H.; Mo, J.; Gan, L. Terpenoids from Curcuma wenyujin increased glucose consumption on HepG2 cells. Fitoterapia 2017, 121, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, P.C.; Arimboor, R.; Indulekha, P.S.; Menon, A.N. Turmeric (Curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. Int. J. Food Sci. Nutr. 2012, 63, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Su, D.; Zhang, J.; Ji, D.; Mao, J.; Hao, M.; Wang, Q.; Yu, M.; Mao, C.; Lu, T. Raw and vinegar processed Curcuma wenyujin regulates hepatic fibrosis via bloking TGF-β/Smad signaling pathways and up-regulation of MMP-2/TIMP-1 ratio. J. Ethnopharmacol. 2020, 246, 111768. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Tu, H.; Zhu, Y.; Guan, Y.; Liu, H.; Ling, W.; Yan, P.; Dong, J. Curcumolide, a unique sesquiterpenoid from Curcuma wenyujin displays anti-angiogenic activity and attenuates ischemia-induced retinal neovascularization. Phytomedicine 2019, 64, 152923. [Google Scholar] [CrossRef] [PubMed]
- Abolfazli, S.; Mortazavi, P.; Kheirandish, A.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. Regulatory effects of curcumin on nitric oxide signaling in the cardiovascular system. Nitric Oxide 2023, 143, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.T.; Bai, Y.; Cao, P.; Ren, K.X.; Chen, J.; Zhang, T.; Fan, B.Y.; Qiao, Y.; Yan, H.Y.; Wang, J.J.; et al. The analgesic effects of β-elemene in rats with neuropathic pain by inhibition of spinal astrocytic ERK activation. Mol. Pain. 2022, 18, 17448069221121562. [Google Scholar] [CrossRef]
- Limcharoen, T.; Dasuni Wasana, P.W.; Hasriadi Muangnoi, C.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Curcumin Diglutaric Acid, a Prodrug of Curcumin Reduces Pain Hypersensitivity in Chronic Constriction Injury of Sciatic Nerve Induced-Neuropathy in Mice. Pharmaceuticals 2020, 13, 212. [Google Scholar] [CrossRef]
- Qi, Y.; Qin, W.; Kang, K.; Jiang, H.; Li, Z.; Wang, Y.; Jia, D. Effects of Wenyujin Essential Oil on tau Protein Phosphorylation in Mice with Aβ-induced Alzheimer Disease through PI3k/Akt Pathway. Chin. J. Inf. Tradit. Chin. Med. 2017, 24, 45–48. [Google Scholar] [CrossRef]
- Eun, C.S.; Lim, J.S.; Lee, J.; Lee, S.P.; Yang, S.A. The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement. Altern. Med. 2017, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, H.; You, Y.; Wang, R.; Tan, T.; Wang, W.; Yin, L.; Zeng, Z.; Zeng, Y.; Xie, T. Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J. Nanobiotechnol. 2021, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Tan, W.; Tian, K.; Yu, H.; Qiang, W.A.; Wang, Y.T. Combined effects of furanodiene and doxorubicin on the migration and invasion of MDA-MB-231 breast cancer cells in vitro. Oncol. Rep. 2017, 37, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, X.; Shi, Y.; Ma, C.; Zhang, H.; Zhang, J.; Lu, Y.; Wei, J.; Li, L.; Han, L. Crosstalk of LncRNA HOTAIR and SP1-mediated repression of PDK1 contributes to β-Elemene-inhibited proliferation of hepatocellular carcinoma cells. J. Ethnopharmacol. 2022, 283, 114456. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Liu, B.; Song, H.; Yu, R.; Zou, D.; Chen, Y.; Ma, Y.; Lv, F.; Xu, L.; Zhang, Z.; et al. β-Elemene inhibits the metastasis of multidrug-resistant gastric cancer cells through miR-1323/Cbl-b/EGFR pathway. Phytomedicine 2020, 69, 153184. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Gu, J.; Chang, X.; Liu, F.; Liang, Y.; Yang, X.; Liang, L.; Tang, D. Metabonomics study on orthotopic transplantion mice model of colon cancer treated with Astragalus membranaceus-Curcuma wenyujin in different proportions via UPLC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2021, 193, 113708. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Yang, W.; Xie, C.Y.; Ni, Q.C.; Pan, X.D.; Dong, J.H.; Liu, Z.M.; Wang, X.S. Induction of caspase-3-dependent apoptosis in human leukemia HL-60 cells by δ-elemene. Yakugaku Zasshi 2011, 131, 1383–1394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, C.B.; Ky, N.; Ng, H.M.; Hamza, M.S.; Zhao, Y. Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr. Cancer Ther. 2010, 9, 36–49. [Google Scholar] [CrossRef]
- Song, G.; Lu, H.; Chen, F.; Wang, Y.; Fan, W.; Shao, W.; Lu, H.; Lin, B. Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells. Mol. Med. Rep. 2018, 17, 5964–5969. [Google Scholar] [CrossRef]
- Abdel-Lateef, E.; Mahmoud, F.; Hammam, O.; El-Ahwany, E.; El-Wakil, E.; Kandil, S.; Abu Taleb, H.; El-Sayed, M.; Hassenein, H. Bioactive chemical constituents of Curcuma longa L. rhizomes extract inhibit the growth of human hepatoma cell line (HepG2). Acta Pharm. 2016, 66, 387–398. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, N.Y.; Suh, J.; Kim, D.H.; Kim, W.; Ann, J.; Lee, J.; Baek, J.H.; Na, H.K.; Surh, Y.J. Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer Lett. 2018, 431, 219–229. [Google Scholar] [CrossRef]
- Jiang, J.L.; Jin, X.L.; Zhang, H.; Su, X.; Qiao, B.; Yuan, Y.J. Identification of antitumor constituents in curcuminoids from Curcuma longa L. based on the composition-activity relationship. J. Pharm. Biomed. Anal. 2012, 70, 664–670. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Exp. Cell Res. 2016, 341, 157–165. [Google Scholar] [CrossRef]
- Kuo, C.L.; Wu, S.Y.; Ip, S.W.; Wu, P.P.; Yu, C.S.; Yang, J.S.; Chen, P.Y.; Wu, S.H.; Chung, J.G. Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int. J. Oncol. 2011, 39, 319–328. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hung, C.C.; Wang, C.C.N.; Lin, H.Y.; Huang, S.H.; Sheu, M.J. Demethoxycurcumin sensitizes the response of non-small cell lung cancer to cisplatin through downregulation of TP and ERCC1-related pathways. Phytomedicine 2019, 53, 28–36. [Google Scholar] [CrossRef]
- Emami, B.; Shakeri, F.; Ghorani, V.; Boskabady, M.H. Relaxant effect of Curcuma longa on rat tracheal smooth muscle and its possible mechanisms. Pharm. Biol. 2017, 55, 2248–2258. [Google Scholar] [CrossRef]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Booth, J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: A pilot study. J. Altern. Complement. Med. 2004, 10, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Feng, M.; Liao, T.; Yang, N.; Fang, Y.; Wang, P.; Liu, Z. LC-MS Combined with Network Pharmacology to Explore the Mechanism of “Wenyujin Rhizoma Concisum-Angelicae Sinensis Radix” Essential Oil in the Treatment of Knee Osteoarthritis. Chin. J. Mod. Appl. Pharm. 2023, 40, 2403–2413. [Google Scholar] [CrossRef]
- Wang, K.; Shi, X.; Ke, J.; Ma, Y.; Ye, M. Mechanism of action of Rhizoma Acori graminei-Curcuma aromatic in treatment of Alzheimer’s disease: A study based on network pharmacology. Hum. J. Tradit. Chin. Med. 2023, 39, 163–170. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; He, Z.; Lu, C. Meta-analysis of Efficacy and Safety of Yinxieling Tablet and Its Optimized Formula in the Treatment of Psoriasis Vulgaris. Tradit. Chin. Drug Res. Clin. Pharmacol. 2021, 32, 1048–1054. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y. Clinical study on the vaginal flora of acrobic vaginitis and the effects of the treatments. Prog. Obstet. Gynecol. 2009, 18, 832–835. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, Q.; Zhao, X.; Xie, M.; Guo, P. Combined Sanleng Ezhu Decoction with Western medicine in treating liver fibrosis in chronic hepatitis B of liver spleen deficiency and blood stasis syndrome. Hebei J. TCM. 2022, 44, 1834–1838. [Google Scholar] [CrossRef]
- Pamplona, C.R.; Souza, M.M.d.; Machado, M.d.S.; Filhoa, V.C.; Navarro, D.; Yunes, R.A.; Monache, F.D.; Niero, R. Seasonal Variation and Analgesic Properties of Different Parts from Curcuma zedoaria Roscoe (Zingiberaceae) Grown in Brazil. Z. Naturforsch. C. J. Biosci. 2006, 61, 6–10. [Google Scholar] [CrossRef][Green Version]
- De Fátima Navarro, D.; De Souza, M.M.; Neto, R.A.; Golin, V.; Niero, R.; Yunes, R.A.; Monache, F.D.; Cechinel-Filho, V. Phytochemical analysis and analgesic properties of Curcuma zedoaria grown in Brazil. Phytomedicine 2002, 9, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Bai, M. Preparation and application of Yu Jin powder. J. Chin. Med. Mater. 1994, 7, 46. [Google Scholar] [CrossRef]
- Fei, C.; Ji, D.; Tong, H.; Li, Y.; Su, L.; Qin, Y.; Bian, Z.; Zhang, W.; Mao, C.; Li, L.; et al. Therapeutic mechanism of Curcuma aromatica Salisb. rhizome against coronary heart disease based on integrated network pharmacology, pharmacological evaluation and lipidomics. Front. Pharmacol. 2022, 13, 950749. [Google Scholar] [CrossRef]
- Li, J. Clinical observation on stable angina of coronary heart disease treated with Sanleng Ezhu decoction. Tianjin J. Tradit. Chin. Med. 2007, 24, 470–471. [Google Scholar]
- Wang, S.; Ma, Q.; Xie, Z.; Shen, Y.; Zheng, B.; Jiang, C.; Yuan, P.; An, Q.; Fan, S.; Jie, Z. An Antioxidant Sesquiterpene Inhibits Osteoclastogenesis Via Blocking IPMK/TRAF6 and Counteracts OVX-Induced Osteoporosis in Mice. J. Bone. Miner. Res. 2021, 36, 1850–1865. [Google Scholar] [CrossRef]
- Lu, J.; Li, Z.; Du, G.; Zhang, J.; Pan, F.; Zhang, Z.; Wang, Y.; Shi, J.; Lian, Y.; Zhan, H. Systematic review and Meta-analysis of Gusongbao preparation in treatment of primary osteoporosis. China J. Chin. Mater. Medica 2023, 48, 3086–3096. [Google Scholar] [CrossRef]
- Han, H.; Wang, L.; Liu, Y.; Shi, X.; Zhang, X.; Li, M.; Wang, T. Combination of Curcuma zedoary and kelp inhibits growth and metastasis of liver cancerin vivoandin vitro via reducing endogenous H2S levels. Food. Funct. 2019, 10, 224–234. [Google Scholar] [CrossRef]
- Wang, G. Curative Effect and Clinical Evaluation of the Anticancer of Compound Zedoary Turmeric Oil Hepatic Artery Embolism in Treatment of Liver Cancer. China Foreign Med. Treat. 2016, 35, 110–111. [Google Scholar] [CrossRef]
- Mao, Z.; Zhong, L.; Zhuang, X.; Liu, H.; Peng, Y.; Zhang, H. Curcumenol Targeting YWHAG Inhibits the Pentose Phosphate Pathway and Enhances Antitumor Effects of Cisplatin. Evid. Based. Complement. Alternat. Med. 2022, 2022, 3988916. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhong, G. Effect analysis of Curcuma zedoary oil combined with conventional chemotherapy on ovarian cancer. J. Med. Theory Pract. 2018, 31, 554–555. [Google Scholar] [CrossRef]
- Li, P.; Ke, H. Effect Analysis of Zedoary Turmeric Oil Combined with Cisplatin and Docetaxel in Treatment of Advanced Non-Small Cell Lung Cancer. Chin. Community Dr. 2023, 39, 77–79. [Google Scholar]
- Gao, D.; Zhang, J.; Li, H. Effects of Chanpi Ezhu Decoction on Adjuvant Chemotherapy in the Treatment of Gastric Cancer. World J. Integr. Tradit. West. Med. 2021, 16, 2329–2333. [Google Scholar] [CrossRef]
- Zeng, X.; Cui, J.; Zhou, Q.; Li, N.; Liu, J.; Li, J.; Huang, D.; Cai, P.; Zhou, R.; Yan, J.; et al. Research status of Jinrong granule against breast hyperplasia and breast cancer and its clinical application. Chin. J. Clin. Pharmacol. 2023, 39, 295–299. [Google Scholar] [CrossRef]
- Lobo, R.; Prabhu, K.S.; Shirwaikar, A.; Shirwaikar, A. Curcuma zedoaria Rosc. (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009, 61, 13–21. [Google Scholar] [CrossRef]
- Etoh, H.; Kondoh, T.; Yoshioka, N.; Sugiyama, K.; Ishikawa, H.; Tanaka, H. 9-Oxo-neoprocurcumenol from Curcuma aromatica (Zingiberaceae) as an Attachment Inhibitor against the Blue Mussel, Mytilus edulis galloprovincialis. Biosci. Biotechnol. Biochem. 2003, 67, 911–913. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fujii, E.; Endo, M.; Wada, K.; Tokunaga, Y.; Shiba, N.; Hohsho, H.; Shibuya, H.; Muraki, T. Antiinflammatory potency of dehydrocurdione, a zedoary-derived sesquiterpene. Inflamm. Res. 1998, 47, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Shunying, Z.; Yang, Y.; Huaidong, Y.; Yue, Y.; Guolin, Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Chanotiya, C.S.; Thakuri, B.C.; Mathela, C.S. Germacranolide Rich Essential Oil from Neolitsea pallens. Nat. Prod. Commun. 2006, 2, 291–293. [Google Scholar] [CrossRef]
- Kharkwala, G.C.; Pandea, C.; Tewari, G.; Panwarb, A.; Pandeb, V. Terpenoid composition and antimicrobial activity of essential oil from Torilis japonica (Houtt.) DC. J. India. Chem. Soc. 2017, 94, 191–194. [Google Scholar]
- Zhang, L.; Shen, S.; Gao, Y.; Shi, S.; Zhou, C.; Mo, J.; Xu, Y.; Lin, L.; Gan, L. Tautomerism and bioactivities of curcumenol, a common sesquiterpenoid widely existing in edible plants. Food. Funct. 2019, 10, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G. The importance of method validation in herbal drug research. J. Pharm. Biomed. Anal. 2022, 214, 114735. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, N.A.; Sagathevan, K.; Anwar, A.; Siddiqui, R. Biologically active metabolite(s) from haemolymph of red-headed centipede Scolopendra subspinipes possess broad spectrum antibacterial activity. AMB. Express. 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Liu, X.; Wang, Y.; Ren, X.; Dong, Y.; Song, R.; Ma, J.; Fan, Q.; Wei, J.; et al. Fast discrimination and quantification analysis of Curcumae Radix from four botanical origins using NIR spectroscopy coupled with chemometrics tools. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 254, 119626. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Sasaki, Y.; Tanaka, K.; Kuba, Y.; Fushimi, H.; Cai, S.-Q. Morphological, genetic, and chemical polymorphism of Curcuma kwangsiensis. J. Nat. Med. 2008, 62, 413–422. [Google Scholar] [CrossRef]
- El-Hawaz, R.F.; Grace, M.H.; Janbey, A.; Lila, M.A.; Adelberg, J.W. In vitro mineral nutrition of Curcuma longa L. affects production of volatile compounds in rhizomes after transfer to the greenhouse. BMC. Plant. Biol. 2018, 18, 122. [Google Scholar] [CrossRef]
- Yang, F.Q.; Wang, Y.T.; Li, S.P. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2006, 1134, 226–231. [Google Scholar] [CrossRef]
- Chen, S. Determination of Three Effective Constituents in Rhizoma Curcumae from Different Area by HPLC. Chin. Pharm. J. 2009, 44, 1742–1744. [Google Scholar]
- Shi, D.; Su, B.; Zhang, J.; Dai, Y. Study on Analgesic Effect of 4 Sources of Curcumae Radix Decoction Pieces and Comparison of Curcuminol Content in Its Water Extracts. Chin. Pharm. 2020, 31, 2209–2213. [Google Scholar] [CrossRef]
- Wang, M.; Yu, M.; Peng, M.; Yin, Z.; Mao, C.; Su, L.; Ji, D.; Lu, T. Quality evaluation of Curcumae Radix from different origins based on UPLC characteristic chromatogram, multicomponent content, and chemometrics. China J. Chin. Mater. Med. 2022, 47, 2964–2974. [Google Scholar] [CrossRef]
- Yang, F.Q.; Li, S.P.; Chen, Y.; Lao, S.C.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 552–558. [Google Scholar] [CrossRef]
- Yin, G.; Cheng, X.; Tao, W.; Dong, Y.; Bian, Y.; Zang, W.; Tang, D. Comparative analysis of multiple representative components in the herb pair Astragali Radix-Curcumae Rhizoma and its single herbs by UPLC-QQQ-MS. J. Pharm. Biomed. Anal. 2018, 148, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Xu, L.; Liu, H.; Luan, Y.; Xu, X.; Yu, Y.; Lin, Y. Determination of 8 Main Active Compounds in Curcumae Rhizoma by HPLC Wavelength Switching Method. Chin. J. Mod. Appl. Pharm. 2021, 38, 2227–2233. [Google Scholar] [CrossRef]
- Yang, F.Q.; Li, S.P.; Zhao, J.; Lao, S.C.; Wang, Y.T. Optimization of GC–MS conditions based on resolution and stability of analytes for simultaneous determination of nine sesquiterpenoids in three species of Curcuma rhizomes. J. Pharm. Biomed. Anal. 2007, 43, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Shiea, J.; Lin, H.; Bhat, S.M.; Lee, C.; Huang, M.; Ponnusamy, V.K.; Cheng, S. Thin layer chromatography/desorption flame-induced atmospheric pressure chemical ionization/mass spectrometry for the analysis of volatile and semi-volatile mixtures. Rapid. Commun. Mass. Spectrom. 2022, 36, e9409. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, I.M.; Abdel-Aziz, M.M.; Elhady, S.S.; Bagalagel, A.A.; Malatani, R.T.; Elkady, W.M. Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity. Molecules 2022, 27, 7965. [Google Scholar] [CrossRef] [PubMed]
- Depmeier, T.; Lange, T.; Hanekamp, W.; Strünker, T.; Lehr, M. HPLC fluorescence assay for measuring the activity of diacylglycerol lipases and the action of inhibitors thereof. Anal. Biochem. 2022, 657, 114889. [Google Scholar] [CrossRef] [PubMed]
- Sammut Bartolo, N.; Vella Szijj, J.; Ferrito, V.; Serracino-Inglott, A. HPLC-UV Method Development and Validation to Monitor Difluprednate Synthesis. J. Chromatogr. Sci. 2023, 61, 322–328. [Google Scholar] [CrossRef]
- Gajula, S.N.R.; Khairnar, A.S.; Jock, P.; Kumari, N.; Pratima, K.; Munjal, V.; Kalan, P.; Sonti, R. LC-MS/MS: A sensitive and selective analytical technique to detect COVID-19 protein biomarkers in the early disease stage. Expert. Rev. Proteom. 2023, 20, 5–18. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, L.; Zeng, Z.; Wei, M.; Lei, Y.; Lin, W. Determination of curcumenol content in Curcuma kwangsiensis, Vingar-Boiled Curcuma kwangsiensis, and Water Residues by UPLC. Chem. Bio Eng. 2020, 37, 62–65. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, G.; Zhao, C.; Gong, X.; Li, M. Fingerprint of Curcuma phaeocaulis by LC-MS. China J. Chin. Mater. Med. 2008, 33, 2218–2221. [Google Scholar]
- Yang, F.Q.; Wang, H.K.; Chen, H.; Chen, J.D.; Xia, Z.N. Fractionation of Volatile Constituents from Curcuma Rhizome by Preparative Gas Chromatography. J. Autom. Methods Manag. Chem. 2011, 2011, 942467. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Huang, X.; Zhu, Y.; Li, J.; Miao, Y.; Du, H.; Liu, D. Comparison of Chemical Constituents and Pharmacological Effects of Different Varieties of Chrysanthemum Flos in China. Chem. Biodivers. 2021, 18, e2100206. [Google Scholar] [CrossRef] [PubMed]
- Çakmakçı, R.; Mosber, G.; Milton, A.H.; Alatürk, F.; Ali, B. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr. Microbiol. 2020, 77, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Saffariha, M.; Azarnivand, H.; Zare Chahouki, M.A.; Tavili, A.; Nejad Ebrahimi, S.; Jahani, R.; Potter, D. Changes in the essential oil content and composition of Salvia limbata C.A. Mey at different growth stages and altitudes. Biomed. Chromatogr. 2021, 35, e5127. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Li, L.; Huang, H.; Wang, B.; Zhang, T. Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods. Molecules 2019, 24, 2956. [Google Scholar] [CrossRef]
- Zhen, F.; Sun, X.; Jin, X. Phenological period and quality comparison of different producing areas of Wenyujin in southern Zhejiang. J. Zhejiang Agr. Sci. 2019, 60, 823–824. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Cho, J.W.; Lee, K.S.; Kim, C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar]
- Sengupta, S.; Tripathi, A. Evaluation of analgesic and prophylactic activity of curcumin against chikungunya-infected acute/chronic arthralgic mice. J. Med. Virol. 2023, 95, e28661. [Google Scholar] [CrossRef]
- Mahakunakorn, P.; Tohda, M.; Murakami, Y.; Matsumoto, K.; Watanabe, H.; Vajaragupta, O. Cytoprotective and cytotoxic effects of curcumin: Dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol. Pharm. Bull. 2003, 26, 725–728. [Google Scholar] [CrossRef]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Fan, D.; Lu, J.; Yu, N.; Xie, Y.; Zhen, L. Curcumin Prevents Diabetic Osteoporosis through Promoting Osteogenesis and Angiogenesis Coupling via NF-κB Signaling. Evid. Based Complement. Altern. Med. 2022, 2022, 4974343. [Google Scholar] [CrossRef]
- Dave, G.S.; Rakholiya, K.D.; Kaneria, M.J.; Galvadiya, B.P.; Vyas, S.R.; Kanbi, V.H.; Patel, M.P. High affinity interaction of Solanum tuberosum and Brassica juncea residue smoke water compounds with proteins involved in coronavirus infection. Phytother. Res. 2020, 34, 3400–3410. [Google Scholar] [CrossRef] [PubMed]
- Marín-Palma, D.; Tabares-Guevara, J.H.; Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Yepes, L.M.; Rugeles, M.T.; Zapata-Builes, W.; Hernandez, J.C.; Taborda, N.A. Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms. Molecules 2021, 26, 6900. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Pan, Y.Y.; Chen, O.; Luan, Y.; Xue, X.; Zhao, J.J.; Liu, L.; Jia, H.Y. Curcumin inhibits MCF-7 cells by modulating the NF-κB signaling pathway. Oncol. Lett. 2017, 14, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Deng, Q.; Liu, Y.; Zhao, P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/β-Catenin Signaling Pathway. Med. Sci. Monit. 2017, 23, 163–171. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.S.; Jung, E.J.; Lee, J.Y.; Tsang, B.; Lim, J.M.; Song, Y.S. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol. Carcinog. 2016, 55, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Fang, Z.; Zhang, Y.; Cao, Y.; Yang, L.; Yin, J. Inhibitory effects of curcumenol on human liver cytochrome P450 enzymes. Phytother. Res. 2010, 24, 1213–1216. [Google Scholar] [CrossRef]
- Shamsi, S.; Tran, H.; Tan, R.S.; Tan, Z.J.; Lim, L.Y. Curcumin, Piperine, and Capsaicin: A Comparative Study of Spice-Mediated Inhibition of Human Cytochrome P450 Isozyme Activities. Drug Metab. Dispos. 2017, 45, 49–55. [Google Scholar] [CrossRef]
- Hamdi, O.; Feroz, S.; Shilpi, J.; Anouar, E.; Mukarram, A.; Mohamad, S.; Tayyab, S.; Awang, K. Spectrofluorometric and Molecular Docking Studies on the Binding of Curcumenol and Curcumenone to Human Serum Albumin. Int. J. Mol. Sci. 2015, 16, 5180–5193. [Google Scholar] [CrossRef]
- Dezhampanah, H.; Shabanzade, Z. Investigation of binding interaction between human serum albumin with zirconium complex of curcumin and curcumin. J. Biomol. Struct. Dyn. 2022, 40, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2020, 93, e12970. [Google Scholar] [CrossRef]
- Krstanović, F.; Britt, W.J.; Jonjić, S.; Brizić, I. Cytomegalovirus Infection and Inflammation in Developing Brain. Viruses 2021, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M.; Cauvi, D.M.; Toomey, C.B.; Hultman, P.; Kono, D.H. Mercury-induced inflammation and autoimmunity. Biochim. Biophys. Acta. Gen. Subj. 2019, 1863, 129299. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Chang, Y.-H.; Lin, Y.-C.; Chen, B.; Guevara, B.E.K.; Hsu, C.-K. Inflammation in Wound Healing and Pathological Scarring. Adv. Wound. Care 2023, 12, 288–300. [Google Scholar] [CrossRef]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clin. Rev. Allergy. Immunol. 2022, 64, 222–238. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing. Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef] [PubMed]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; El-Kholy, W. Neuroprotective effect of ginger in the brain of streptozotocin-induced diabetic rats. Ann. Anat. 2014, 196, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Yulug, B.; Kilic, E.; Altunay, S.; Ersavas, C.; Orhan, C.; Dalay, A.; Tuzcu, M.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Exerts Neuroprotective Activity in Traumatic Brain Injury in Male Mice. CNS. Neurol. Disord. Drug Targets 2018, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhao, C.; Lee, S.M.-Y. Neuroprotective Potency of Saffron Against Neuropsychiatric Diseases, Neurodegenerative Diseases, and Other Brain Disorders: From Bench to Bedside. Front. Pharmacol. 2020, 11, 579052. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/newsroom/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 December 2023).
- Tsai, A.G.; Bessesen, D.H. Obesity. Ann. Intern. Med. 2019, 170, ITC33–ITC48. [Google Scholar] [CrossRef]
- Shiriki Kumanyika, W.H.D. Solving Population-wide Obesity-Progress and Future Prospects. N. Engl. J. Med. 2020, 383, 2197–2200. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Subcellular Biochemistry; Springer: New York, NY, USA, 2019; pp. 453–476. [Google Scholar]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Ye, Y.Y.; Guo, B.F.; Liu, S. Effects of platycodin D in combination with different active ingredients of Chinese herbs on proliferation and invasion of 4T1 and MDA-MB-231 breast cancer cell lines. Chin. J. Integr. Med. 2012, 10, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Eltayeb, N.M.; Khairuddean, M.; Salhimi, S.M. Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Nat. Prod. Res. 2019, 35, 3166–3170. [Google Scholar] [CrossRef]
- Jung, E.B.; Trinh, T.A.; Lee, T.K.; Yamabe, N.; Kang, K.S.; Song, J.H.; Choi, S.; Lee, S.; Jang, T.S.; Kim, K.H.; et al. Curcuzedoalide contributes to the cytotoxicity of Curcuma zedoaria rhizomes against human gastric cancer AGS cells through induction of apoptosis. J. Ethnopharmacol. 2018, 213, 48–55. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Zhao, H.; Li, X.; Wei, C.; Wei, R.G. Determ ination of curcumol in plasma by HPLC-MS/MS method and its pharmacokinetics in Beagle dogs. Acta Pharm. Sin. B. 2007, 42, 973–977. [Google Scholar] [CrossRef]
- Center for Disease Control. Breast Cancer Statistics. Available online: https://www.cdc.gov/cancer/breast/statistics/index.htm (accessed on 1 December 2023).
- American Cancer Society. Key Statistics for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 1 December 2023).
- Siddiqui, J.A.; Singh, A.; Chagtoo, M.; Singh, N.; Godbole, M.M.; Chakravarti, B. Phytochemicals for breast cancer therapy: Current status and future implications. Curr. Cancer. Drug Targets 2015, 15, 116–135. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Rêma, A.; Amorim, I.; Vale, N. Drug Combinations: A New Strategy to Extend Drug Repurposing and Epithelial-Mesenchymal Transition in Breast and Colon Cancer Cells. Biomolecules 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Ducrey, E.; Castrogiovanni, C.; Meraldi, P.; Nowak-Sliwinska, P. Forcing dividing cancer cells to die; low-dose drug combinations to prevent spindle pole clustering. Apoptosis 2021, 26, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.U.; Greninger, P.; Zhang, X.; Friedman, A.A.; Amzallag, A.; Cortez, E.; Sahu, A.D.; Lee, J.S.; Dastur, A.; Egan, R.K.; et al. A landscape of response to drug combinations in non-small cell lung cancer. Nat. Commun. 2023, 14, 3830. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Liang, H. Interactive Association of Drugs Binding to Human Serum Albumin. Int. J. Mol. Sci. 2014, 15, 3580–3595. [Google Scholar] [CrossRef]
- Ashraf, G.M.; Gupta, D.D.; Alam, M.Z.; Baeesa, S.S.; Alghamdi, B.S.; Anwar, F.; Alqurashi, T.M.A.; Al Abdulmonem, W.; Alyousef, M.A.; Alhumaydhi, F.A.; et al. Unravelling Binding of Human Serum Albumin with Galantamine: Spectroscopic, Calorimetric, and Computational Approaches. ACS. Omega 2022, 7, 34370–34377. [Google Scholar] [CrossRef]
- Zhong, G.; Cai, X.; Wei, R.; Wei, S.; Cao, X. Curcumenol improves renal function in 5/6 nephrectomy-induced chronic renal failure rats via the SIRT1/NF-κB pathway. Anat. Rec. 2023, 306, 3189–3198. [Google Scholar] [CrossRef]

| Part | Autumn | Winter | Spring | Summer |
|---|---|---|---|---|
| Roots | 15.70 ± 0.14 | 8.90 ± 0.15 | 8.70 ± 0.12 | 1.5 ± 0.10 |
| Mother rhizome | 33.10 ± 0.12 | 9.10 ± 0.08 | 5.90 ± 0.05 | 6.0 ± 0.01 |
| Rugous rhizome | 10.40 ± 0.03 | 3.10 ± 0.03 | 2.00 ± 0.04 | 2.9 ± 0.03 |
| Part | Autumn | Winter | Spring | Summer |
|---|---|---|---|---|
| Roots | 8.50 ± 0.27 | 3.10 ± 0.15 | 4.40 ± 0.06 | 1.60 ± 0.10 |
| Mother rhizome | 25.00 ± 0.25 | 9.40 ± 0.17 | 6.10 ± 0.15 | 7.40 ± 0.14 |
| Rugous rhizome | 6.70 ± 0.03 | 2.90 ± 0.08 | 1.50 ± 0.06 | 1.50 ± 0.03 |
| Producing Areas | Content of Volatile Oils |
|---|---|
| Taoshan Town, Ruian City, Zhejiang Province, China | 2.0% |
| Mayu Town, Ruian City, Zhejiang Province, China | 1.8% |
| Siqian Town, Wenzhou City, Zhejiang Province, China | 1.5% |
| Yongjia County, Wenzhou City, Zhejiang Province, China | 3.1% |
| Leqing City, Zhejiang Province, China | 2.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, Y.; Li, G.; Cheng, C.; Sui, X.; Wu, Q. The Extraction, Determination, and Bioactivity of Curcumenol: A Comprehensive Review. Molecules 2024, 29, 656. https://doi.org/10.3390/molecules29030656
Li J, Sun Y, Li G, Cheng C, Sui X, Wu Q. The Extraction, Determination, and Bioactivity of Curcumenol: A Comprehensive Review. Molecules. 2024; 29(3):656. https://doi.org/10.3390/molecules29030656
Chicago/Turabian StyleLi, Jie, Yitian Sun, Guohua Li, Chunsong Cheng, Xinbing Sui, and Qibiao Wu. 2024. "The Extraction, Determination, and Bioactivity of Curcumenol: A Comprehensive Review" Molecules 29, no. 3: 656. https://doi.org/10.3390/molecules29030656
APA StyleLi, J., Sun, Y., Li, G., Cheng, C., Sui, X., & Wu, Q. (2024). The Extraction, Determination, and Bioactivity of Curcumenol: A Comprehensive Review. Molecules, 29(3), 656. https://doi.org/10.3390/molecules29030656