Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds (Polyphenols and Phenolic Acids) Quantification
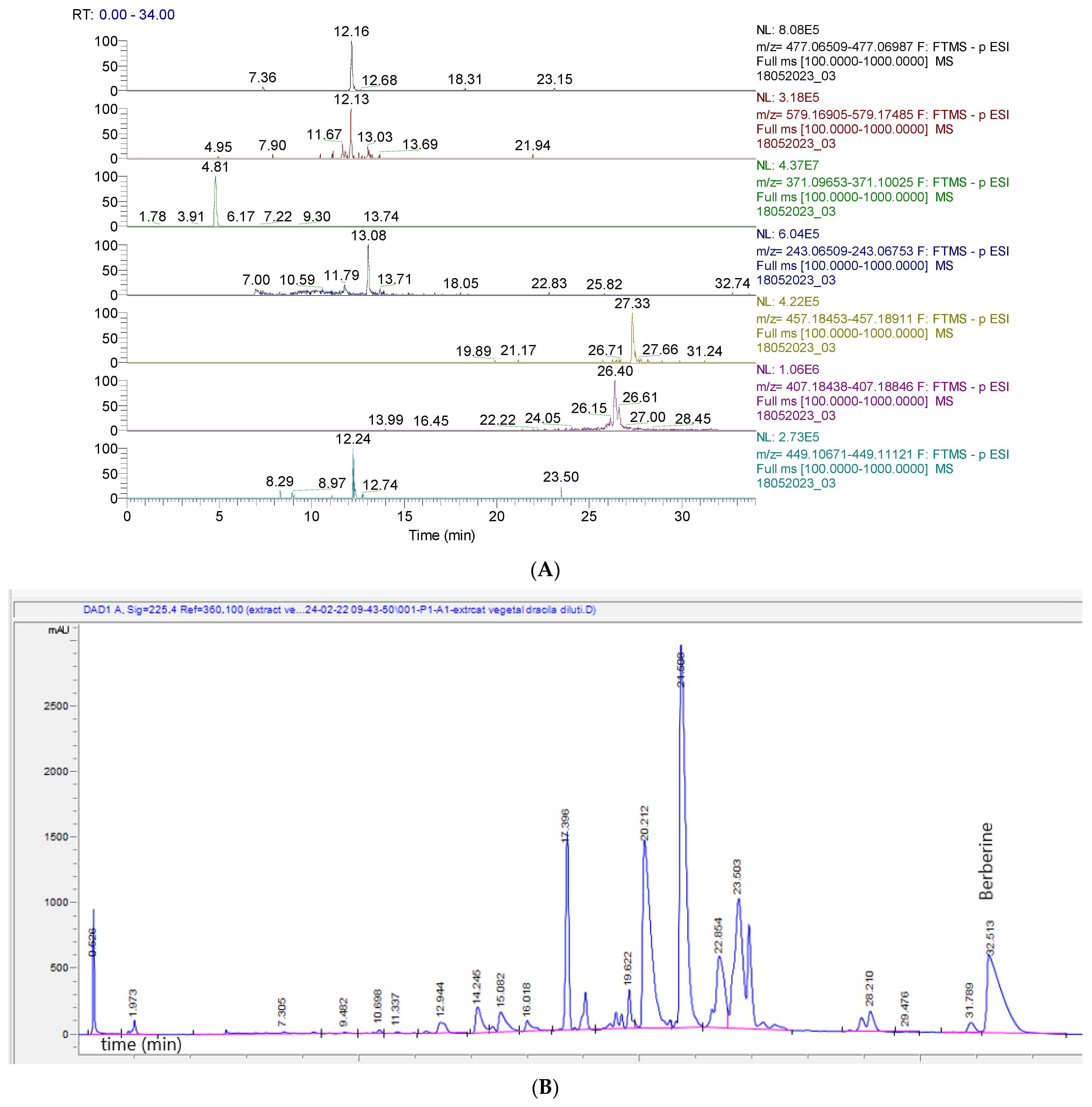
2.2. Identification and Quantification of BVE Phytoconstituents by UHPLC–HRMS/MS and HPLC-DAD
2.3. Antioxidant Activity
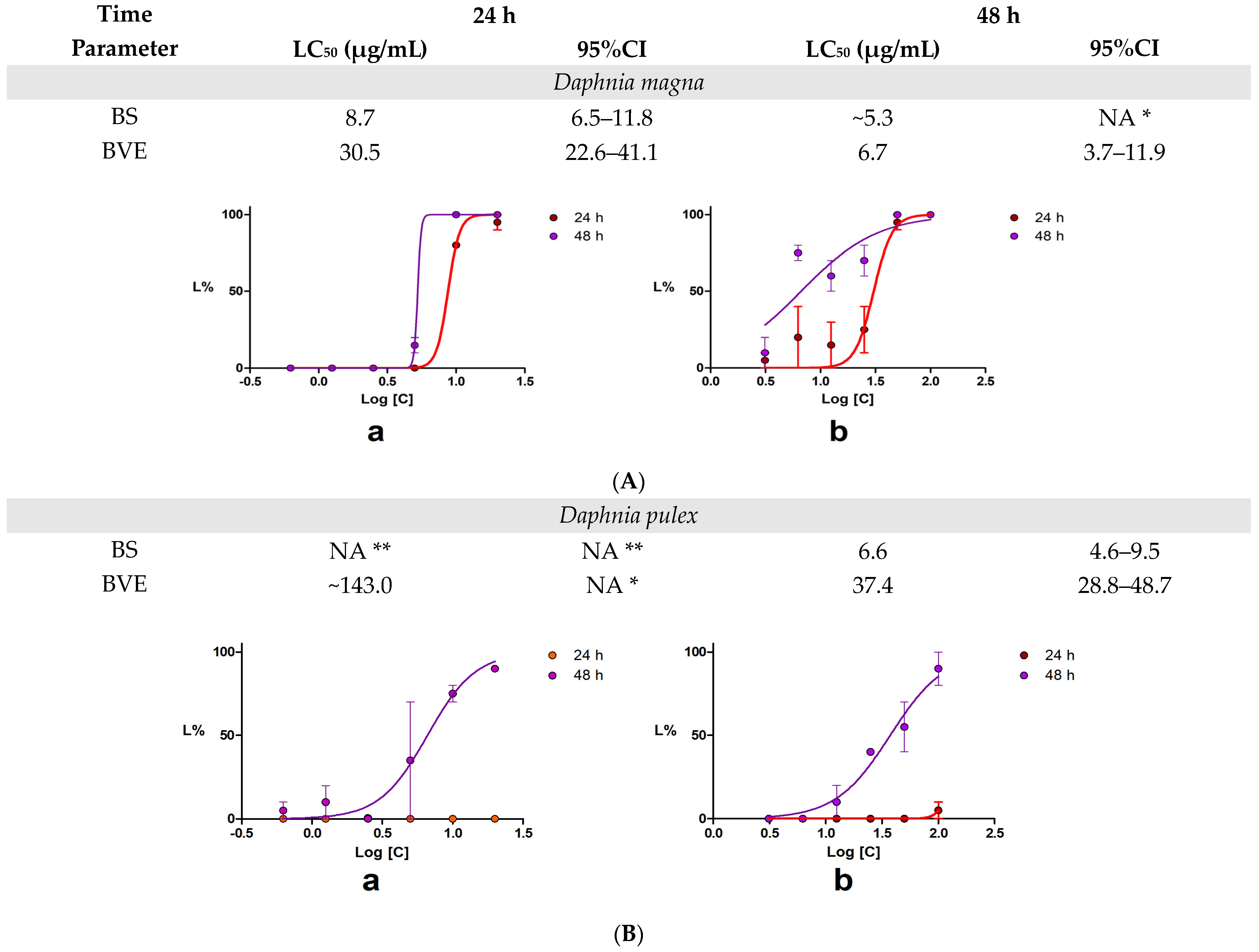
2.4. 48-h Acute Toxicity Test Using Daphnia Magna and Daphnia Pulex
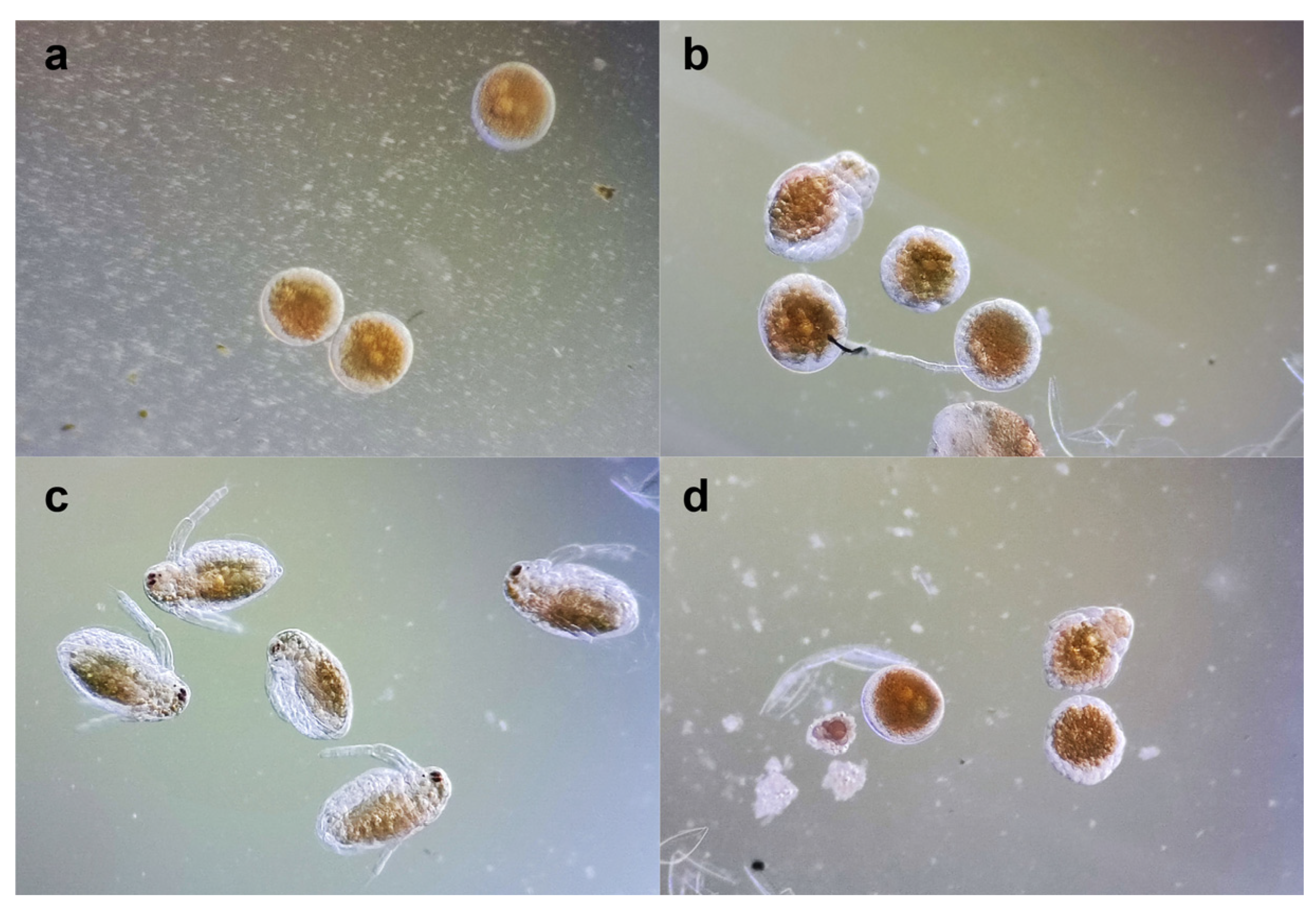
2.5. Daphnia Magna Embryonic Development Assay
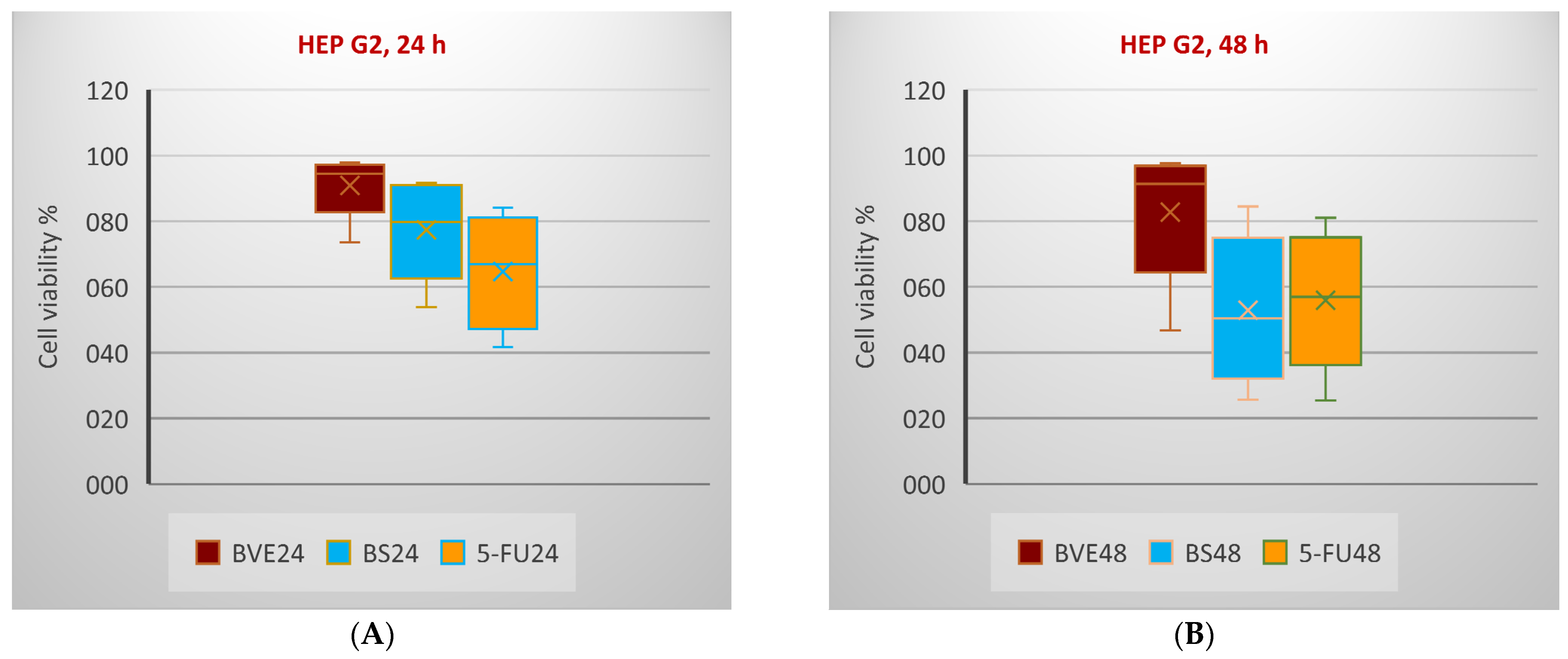
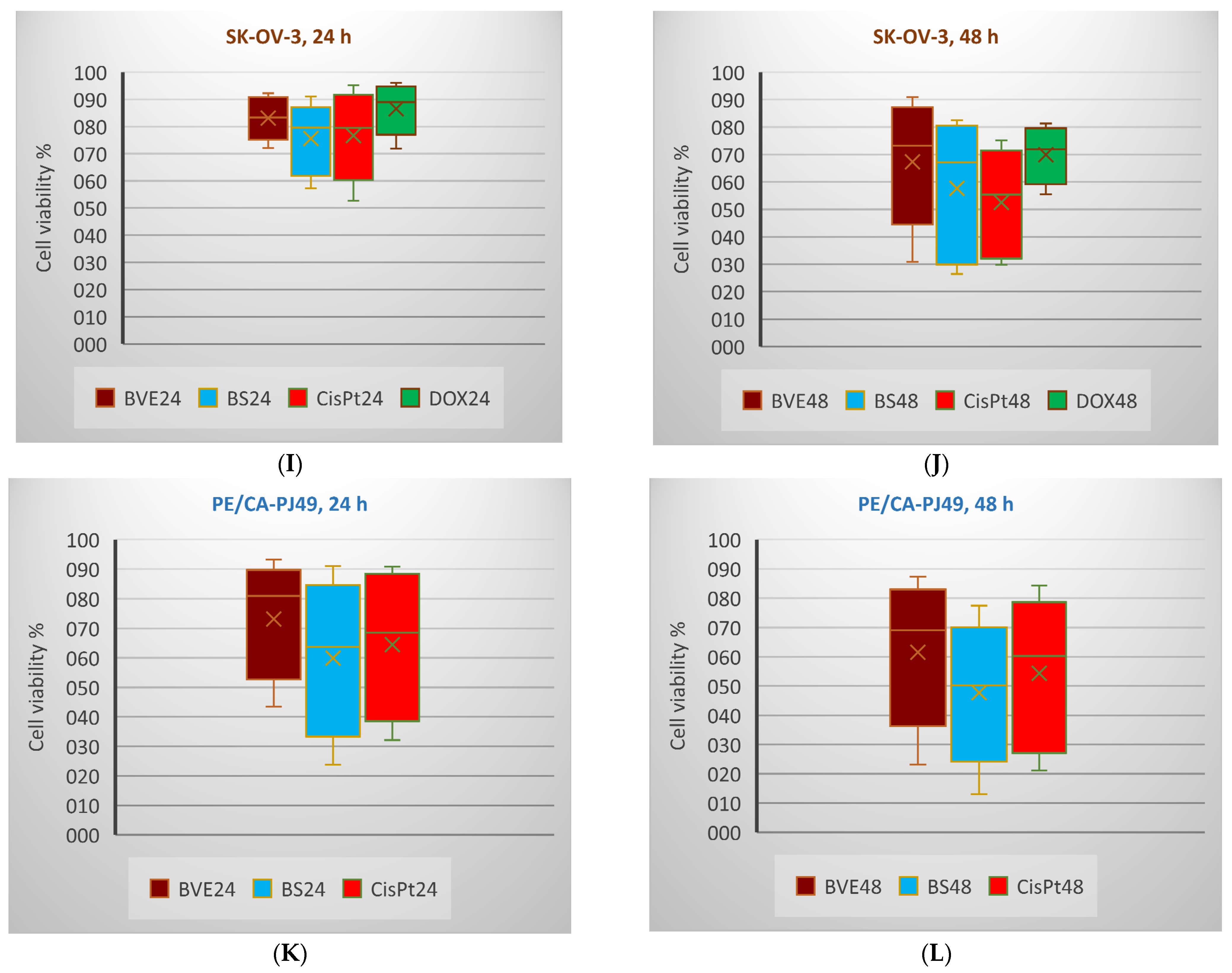
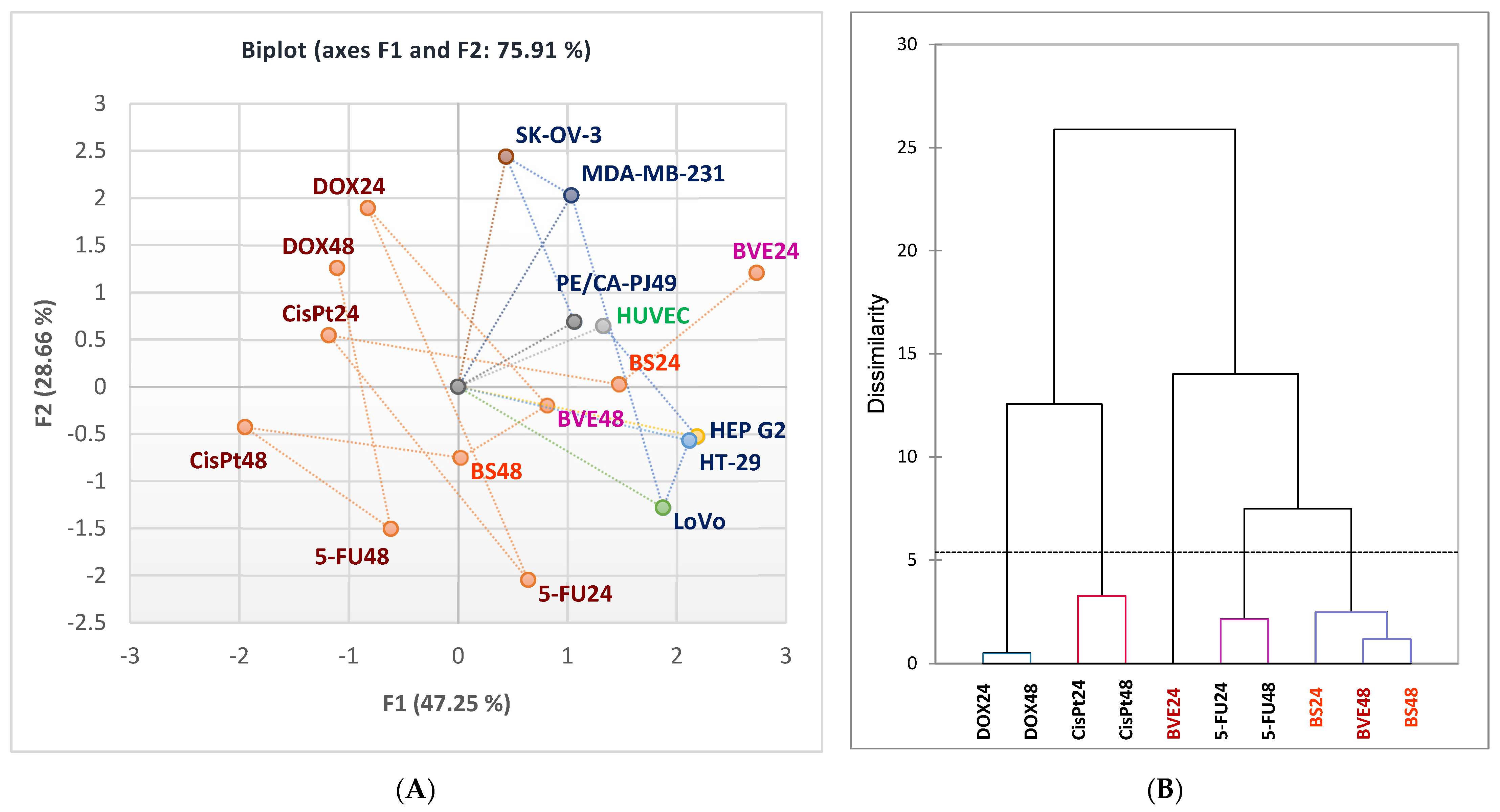
2.6. In Vitro Anticancer Activity
2.7. Statistical Analysis
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
3.1.2. B. vulgaris Extract Preparation
3.2. Total Polyphenol Content (TPC)
3.3. Total Phenolic Acid (TPA)
3.4. Identification and Quantification of Phenolic Constituents and Berberine
3.4.1. Ultra-High-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry (UHPLC–HRMS/MS)
3.4.2. High-Performance Liquid Chromatography
3.5. Antioxidant Activity
3.5.1. Diphenyl-1-Picrylhydrazyl Free Radical Scavenging Assay (DPPH)
3.5.2. Azinobis-3-Ethylbenzotiazoline-6-Sulfonic Acid Assay (ABTS)
3.5.3. Ferric-Reducing Antioxidant Power Assay (FRAP)
3.6. 48-h Acute Toxicity Test Using Daphnia Magna and Daphnia Pulex
3.7. Daphnia Magna Embryonic Development Assay
3.8. In Vitro Anticancer Activity
3.8.1. Cell Cultures and Treatments
3.8.2. MTS Assay
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mana, T.; Devi, O.B.; Singh, Y.D. Therapeutic Application of Berberine: A Consolidated Review. Curr. Pharmacol. Rep. 2023, 9, 329–340. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana, B.; et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef]
- Homśopathic Materia Medica, Berberis Vulgaris (Barberry). Available online: http://homeoint.org/books/boericmm/b/berb.htm (accessed on 26 April 2024).
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and Pharmacological Activities of Berberine in Diabetes Mellitus Treatment. Evid.-Based Complement. Altern. Med. 2021, 2021, 9987097. [Google Scholar] [CrossRef]
- Grădinariu, L.; Dediu, L.; Crețu, M.; Grecu, I.R.; Docan, A.; Istrati, D.I.; Dima, F.M.; Stroe, M.D.; Vizireanu, C. The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L. Animals 2024, 14, 373. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules 2022, 27, 5889. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Al-Jamaan, M.E.; Al-Mutairi, F.R.; Al-Hudiab, A.M.; Al-Einzi, M.S.; Mohamed, A.A.-Z. Antioxidant, Antibacterial, and Antifungal Activities of the Ethanolic Extract Obtained from Berberis vulgaris Roots and Leaves. Molecules 2022, 27, 6114. [Google Scholar] [CrossRef]
- Och, A.; Olech, M.; Bąk, K.; Kanak, S.; Cwener, A.; Cieśla, M.; Nowak, R. Evaluation of the Antioxidant and Anti-Lipoxygenase Activity of Berberis vulgaris L. Leaves, Fruits, and Stem and Their LC MS/MS Polyphenolic Profile. Antioxidants 2023, 12, 1467. [Google Scholar] [CrossRef]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Barrio, D.A.; Carrillo, W. Argentine Patagonia Barberry Chemical Composition and Evaluation of Its Antioxidant Capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef]
- Li, Y.; Lv, X.-M.; Tang, C.; Lai, X.-R.; Zhang, Y.; Fan, G. Quality Evaluation of Cortex Berberidis from Different Geographical Origins by Simultaneous High Performance Liquid Chromatography Combined with Statistical Methods. Trop. J. Pharm. Res. 2016, 15, 1973. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef]
- Veselá, Š.; Ondruška, V.; Kuča, K.; Patočka, J. Freshwater Microcrustacean Daphnia Magna Straus as an Early Screen Model to Compare Toxicity of Acetylcholinesterase Inhibitors. J. Appl. Biomed. 2006, 4, 105–110. [Google Scholar] [CrossRef]
- Paudel, B.; Bhattarai, H.D.; Kim, I.C.; Lee, H.; Sofronov, R.; Ivanova, L.; Poryadina, L.; Yim, J.H. Estimation of Antioxidant, Antimicrobial Activity and Brine Shrimp Toxicity of Plants Collected from Oymyakon Region of the Republic of Sakha (Yakutia), Russia. Biol. Res. 2014, 47, 10. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Pucci, C.; Gabellini, C.; Pellegrino, M.; Andreazzoli, M. Exposure to the Natural Alkaloid Berberine Affects Cardiovascular System Morphogenesis and Functionality during Zebrafish Development. Sci. Rep. 2020, 10, 17358. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organisation. WHO Monographs on Selected Medicinal Plants Volume 4. Available online: https://iris.who.int/bitstream/handle/10665/42052/9789241547055_eng.pdf;jsessionid=A4510414DC6FA90BC17E4906B7CC489F?sequence=4 (accessed on 26 April 2024).
- Hidayat, D.; Dwira, S. Phytochemical Analysis and In Vitro Cytotoxicity Test of Black Soybean (Glycine soja L.) Ethanolic Extract as a Growth Inhibitor of the HCT-116 Colon Carcinoma Cell Line. J. Phys. Conf. Ser. 2018, 1073, 032041. [Google Scholar] [CrossRef]
- Ivan, I.M.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Olaru, O.T.; Nițulescu, G.M.; et al. Phytochemical Profile, Antioxidant and Cytotoxic Potential of Capsicum annuum (L.) Dry Hydro-Ethanolic Extract. Pharmaceutics 2024, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Och, A.; Zalewski, D.; Komsta, Ł.; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and Proapoptotic Activity of Sanguinarine, Berberine, and Extracts of Chelidonium majus L. and Berberis thunbergii DC. toward Hematopoietic Cancer Cell Lines. Toxins 2019, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic Anticancer Effects of Galangin and Berberine through Apoptosis Induction and Proliferation Inhibition in Oesophageal Carcinoma Cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory Potential of Natural Products from Herbal Medicines as Immune Checkpoints Inhibitors: Helping to Fight against Cancer via Multiple Targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef]
- Yang, X.; Huang, N. Berberine Induces Selective Apoptosis through the AMPK-Mediated Mitochondrial/Caspase Pathway in Hepatocellular Carcinoma. Mol. Med. Rep. 2013, 8, 505–510. [Google Scholar] [CrossRef]
- Letašiová, S.; Jantová, S.; Miko, M.; Ovádeková, R.; Horváthová, M. Effect of Berberine on Proliferation, Biosynthesis of Macromolecules, Cell Cycle and Induction of Intercalation with DNA, DsDNA Damage and Apoptosis in Ehrlich Ascites Carcinoma Cells. J. Pharm. Pharmacol. 2010, 58, 263–270. [Google Scholar] [CrossRef]
- Anis, K.V.; Kuttan, G.; Kuttan, R. Role of Berberine as an Adjuvant Response Modifier during Tumour Therapy in Mice. Pharm. Pharmacol. Commun. 1999, 5, 697–700. [Google Scholar] [CrossRef][Green Version]
- Refaat, A.; Abdelhamed, S.; Yagita, H.; Inoue, H.; Yokoyama, S.; Hayakawa, Y.; Saiki, I. Berberine Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Mediated Apoptosis in Breast Cancer. Oncol. Lett. 2013, 6, 840–844. [Google Scholar] [CrossRef]
- Chu, S.-C.; Yu, C.-C.; Hsu, L.-S.; Chen, K.-S.; Su, M.-Y.; Chen, P.-N. Berberine Reverses Epithelial-to-Mesenchymal Transition and Inhibits Metastasis and Tumor-Induced Angiogenesis in Human Cervical Cancer Cells. Mol. Pharmacol. 2014, 86, 609–623. [Google Scholar] [CrossRef]
- Choi, M.S.; Oh, J.H.; Kim, S.M.; Jung, H.Y.; Yoo, H.S.; Lee, Y.M.; Moon, D.C.; Han, S.B.; Hong, J.T. Berberine Inhibits P53-Dependent Cell Growth through Induction of Apoptosis of Prostate Cancer Cells. Int. J. Oncol. 2009, 34, 1221–1230. [Google Scholar] [CrossRef]
- Chen, T.C.; Lai, K.C.; Yang, J.S.; Liao, C.L.; Hsia, T.C.; Chen, G.W.; Lin, J.J.; Lin, H.J.; Chiu, T.H.; Tang, Y.J.; et al. Involvement of Reactive Oxygen Species and Caspase-Dependent Pathway in Berberine-Induced Cell Cycle Arrest and Apoptosis in C6 Rat Glioma Cells. Int. J. Oncol. 2009, 34, 1681–1690. [Google Scholar] [CrossRef]
- Zhao, Y.; Roy, S.; Wang, C.; Goel, A. A Combined Treatment with Berberine and Andrographis Exhibits Enhanced Anticancer Activity through Suppression of DNA Replication in Colorectal Cancer. Pharmaceuticals 2022, 15, 262. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Gao, X.; Guo, F. Mitochondrial Protein Cyclophilin-D-Mediated Programmed Necrosis Attributes to Berberine-Induced Cytotoxicity in Cultured Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2014, 450, 697–703. [Google Scholar] [CrossRef]
- Peng, P.; Kuo, W.-H.; Tseng, H.-C.; Chou, F.-P. Synergistic Tumor-Killing Effect of Radiation and Berberine Combined Treatment in Lung Cancer: The Contribution of Autophagic Cell Death. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 529–542. [Google Scholar] [CrossRef]
- Iizuka, N.; Miyamoto, K.; Hazama, S.; Yoshino, S.; Yoshimura, K.; Okita, K.; Fukumoto, T.; Yamamoto, S.; Tangoku, A.; Oka, M. Anticachectic Effects of Coptidis Rhizoma, an Anti-Inflammatory Herb, on Esophageal Cancer Cells That Produce Interleukin 6. Cancer Lett. 2000, 158, 35–41. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The Anti-Inflammatory Potential of Berberine In Vitro and In Vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef]
- Pereira, G.C.; Branco, A.F.; Matos, J.A.C.; Pereira, S.L.; Parke, D.; Perkins, E.L.; Serafim, T.L.; Sardão, V.A.; Santos, M.S.; Moreno, A.J.M.; et al. Mitochondrially Targeted Effects of Berberine [Natural Yellow 18, 5,6-Dihydro-9,10-Dimethoxybenzo(g)-1,3-Benzodioxolo(5,6-a) Quinolizinium] on K1735-M2 Mouse Melanoma Cells: Comparison with Direct Effects on Isolated Mitochondrial Fractions. J. Pharmacol. Exp. Ther. 2007, 323, 636–649. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.E.; Ghareeb, D.A.; Sarhan, E.E.; Abu-Serie, M.M.; El Demellawy, M.A. In Vitro Biological Assessment of Berberis vulgaris and Its Active Constituent, Berberine: Antioxidants, Anti-Acetylcholinesterase, Anti-Diabetic and Anticancer Effects. BMC Complement. Altern. Med. 2013, 13, 218. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The Antioxidant and Chemical Properties of Berberis vulgaris and Its Cytotoxic Effect on Human Breast Carcinoma Cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Mihaila, M.; Hotnog, C.M.; Bostan, M.; Munteanu, A.C.; Vacaroiu, I.A.; Brasoveanu, L.I.; Uivarosi, V. Anticancer Activity of Some Ruthenium(III) Complexes with Quinolone Antibiotics: In Vitro Cytotoxicity, Cell Cycle Modulation, and Apoptosis-Inducing Properties in LoVo Colon Cancer Cell Line. Appl. Sci. 2021, 11, 8594. [Google Scholar] [CrossRef]
- Costea, L.; Chitescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ionita, T.; Dutu, L.E.; Popescu, M.L.; Luta, E.-A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Ungureanu, A.R.; Chițescu, C.L.; Luță, E.A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Costea, L.; Ozon, E.A.; Fița, A.C.; Balaci, T.D.; et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules 2023, 28, 3668. [Google Scholar] [CrossRef]
- Luță, E.-A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. [Google Scholar] [CrossRef]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Nitulescu, G.; Stancov, G.; Radulescu, D.; Trif, C.; Nitulescu, G.M.; Negres, S.; Olaru, O.T. Evaluation of Topical Anti-Inflammatory Effects of a Gel Formulation with Plantago Lanceolata, Achillea Millefolium, Aesculus Hippocastanum and Taxodium Distichum. Sci. Pharm. 2020, 88, 26. [Google Scholar] [CrossRef]
- Ionus, E.; Schröder, V.; Chiţescu, C.L.; Bucur, L.A.; Lupu, C.E.; Dumitrescu, D.-E.; Popescu, L.; Mihai, D.P.; Olaru, O.T.; Nițulescu, G.M.; et al. Phytochemical, In Vitro, In Vivo, and In Silico Research on the Extract of Ajuga chamaepitys (L.) Schreb. Plants 2024, 13, 1192. [Google Scholar] [CrossRef]
- Ivan, B.-C.; Barbuceanu, S.-F.; Hotnog, C.M.; Olaru, O.T.; Anghel, A.I.; Ancuceanu, R.V.; Mihaila, M.A.; Brasoveanu, L.I.; Shova, S.; Draghici, C.; et al. Synthesis, Characterization and Cytotoxic Evaluation of New Pyrrolo[1,2-b]Pyridazines Obtained via Mesoionic Oxazolo-Pyridazinones. Int. J. Mol. Sci. 2023, 24, 11642. [Google Scholar] [CrossRef]
- Munteanu, A.; Badea, M.; Olar, R.; Silvestro, L.; Mihaila, M.; Brasoveanu, L.I.; Musat, M.G.; Andries, A.; Uivarosi, V. Cytotoxicity Studies, DNA Interaction and Protein Binding of New Al (III), Ga (III) and In (III) Complexes with 5-hydroxyflavone. Appl. Organomet. Chem. 2018, 32, e4579. [Google Scholar] [CrossRef]
- Munteanu, A.; Musat, M.G.; Mihaila, M.; Badea, M.; Olar, R.; Nitulescu, G.M.; Rădulescu, F.Ș.; Brasoveanu, L.I.; Uivarosi, V. New Heteroleptic Lanthanide Complexes as Multimodal Drugs: Cytotoxicity Studies, Apoptosis, Cell Cycle Analysis, DNA Interactions, and Protein Binding. Appl. Organomet. Chem. 2021, 35, e6062. [Google Scholar] [CrossRef]
- Ivan, B.-C.; Barbuceanu, S.-F.; Hotnog, C.M.; Anghel, A.I.; Ancuceanu, R.V.; Mihaila, M.A.; Brasoveanu, L.I.; Shova, S.; Draghici, C.; Olaru, O.T.; et al. New Pyrrole Derivatives as Promising Biological Agents: Design, Synthesis, Characterization, In Silico, and Cytotoxicity Evaluation. Int. J. Mol. Sci. 2022, 23, 8854. [Google Scholar] [CrossRef]
- Maciuca, A.-M.; Munteanu, A.-C.; Mihaila, M.; Badea, M.; Olar, R.; Nitulescu, G.M.; Munteanu, C.V.A.; Bostan, M.; Uivarosi, V. Rare-Earth Metal Complexes of the Antibacterial Drug Oxolinic Acid: Synthesis, Characterization, DNA/Protein Binding and Cytotoxicity Studies. Molecules 2020, 25, 5418. [Google Scholar] [CrossRef]
- Ungureanu, A.R.; Popovici, V.; Oprean, C.; Danciu, C.; Schröder, V.; Olaru, O.T.; Mihai, D.P.; Popescu, L.; Luță, E.-A.; Chițescu, C.L.; et al. Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction. Pharmaceutics 2023, 15, 2125. [Google Scholar] [CrossRef]
- Neagu, R.; Popovici, V.; Ionescu, L.E.; Ordeanu, V.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Antibacterial and Antibiofilm Effects of Different Samples of Five Commercially Available Essential Oils. Antibiotics 2023, 12, 1191. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea barbata (L.) Weber ex F.H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef]


| Phenolic Compounds | |||
| Total Polyphenols (mg Eq Tannic Acid/100 g Extract) | Total Phenolic Acids (mg Eq Chlorogenic Acid/100 g Extract) | ||
| 17.6780 ± 3.9320 | 3.3886 ± 0.3481 | ||
| Antioxidant Activity | |||
| IC50DPPH (mg/mL) | IC50ABTS (mg/mL) | EC50FRAP (mg/mL) | |
| 0.2610 | 0.0442 | 0.1398 | |
| Nr. Crt. | Identified Compound | Phytochemical Classification | Chemical Formula | Adduct Ion/ Monitored Negative Ion (m/z) | Retention Time (min) | Content (µg/g) |
|---|---|---|---|---|---|---|
| 1 | Quercetin | Flavonoid | C15H10O7 | 301.0354 | 15.01 | 28.42 |
| 2 | Rutin (quercetin 3-O-rutinoside) | Flavonoid | C27H30O16 | 609.14613 | 12.39 | 72.41 |
| 3 | Apigenin | Flavonoid | C15H10O5 | 269.04502 | 16.71 | 10.45 |
| 4 | Kaempferol | Flavanol | C15H10O6 | 285.04049 | 16.51 | 68.74 |
| 5 | 6-Methoxyluteolin (Nepetin) | Flavonoid | C16H12O7 | 315.05105 | 16.75 | - |
| 6 | Naringenin | Flavanone | C15H12O5 | 271.06122 | 15.46 | 90.41 |
| 7 | Hesperitin | Flavonoid | C16H14O6 | 301.07179 | 13.71 | 44.00 |
| 8 | Galangin | Flavonoid | C15H10O5 | 269.04557 | 16.71 | 67.21 |
| 9 | Genistein | Isoflavone | C15H10O5 | 269.04502 | 16.73 | - |
| 10 | Glycitein | Isoflavone | C16H12O5 | 283.06122 | 11.15 | 19.21 |
| 11 | Gallic acid | Hydroxybenzoic acid | C7H6O5 | 169.01427 | 1.70 | 540.00 |
| 12 | Chlorogenic acid/Neochlorogenic | Cinnamate ester | C16H18O9 | 353.08783 | 6.08 | 10.54 |
| 13 | Ferulic acid | Hydroxycinnamic acid | C10H10O4 | 193.05066 | 9.94 | 39.36 |
| 14 | AbsCisPtic acid | Terpenoid | C15H20O4 | 263.12891 | 14.76 | 8.61 |
| 15 | p-Coumaric acid | Hydroxycinnamic acid | C9H8O3 | 163.03954 | 8.80 | 30.33 |
| 16 | Syringic acid | Hydroxybenzoic acid | C9H10O5 | 197.04555 | 8.73 | 3.35 |
| 17 | Afrormosin | Isoflavone | C17H14O5 | 297.07687 | 17.17 | - |
| 18 | Kaempferol-3-O-rutinoside | Flavonol glycoside | C27H30O15 | 593.15122 | 9.35 | - |
| 19 | Kaempferol (luteolin)-O-glucoside/ isomers | Flavonoid | C21H20O11 | 447.09331 | 13.56 | - |
| 20 | Vitexin (apigenin 8-C-glucoside)/isovitexin | Flavonol glycoside | C21H20O10 | 431.09839 | 11.98 | - |
| 21 | Azelaic acid | Dicarboxylic acid | C9H16O4 | 187.09761 | 13.99 | - |
| 22 | Apigenin 7-O-glucosylglucoside | Flavonoid | C27H30O15 | 593.15122 | 9.45 | - |
| 23 | Rosmarinic acid | Ester of caffeic acid | C18H16O8 | 359.07727 | 13.42 | - |
| 24 | Carnasol | Diterpene | C20H26O4 | 329.17586 | 18.83 | - |
| 25 | Rosmadial/Isomeri | Diterpene lactone | C20H24O5 | 343.15510 | 20.38 | - |
| 26 | Rosmanol methyl ether | Diterpene | C21H28O5 | 359.18640 | 22.19 | - |
| 27 | Quercetin 3-O-glucuronide | Flavonol glucuronide | C21H18O13 | 477.06749 | 12.16 | 23.04 |
| 28 | Narirutin (naringenin-7-O-rutinoside) | Flavonol glycoside | C27H32O14 | 579.17195 | 12.13 | - |
| 29 | Apigenin-7-O-glucuronide | Flavonoid-7-O-glucuronides | C21H18O11 | 445.07763 | 13.29 | - |
| 30 | Procyanidine B1/B2 | Flavonoid | C30H26O12 | 577.13515 | 16.24 | - |
| 31 | Sinapic acid | Hydroxycinnamic acid | C11H12O5 | 223.06122 | 10.33 | - |
| 32 | Hidroxyferulic acid/Isomers | Hydroxycinnamic acid | C16H20O10 | 371.09839 | 4.81 | - |
| 33 | Valerenic acid | Sesquiterpenoid | C15H22O2 | 233.15473 | 21.33 | - |
| 34 | Lehmannin | Flavanone | C25H28O5 | 407.18642 | 26.40 | - |
| 35 | Ginkgetin | Flavone | C32H22O10 | 565.11404 | 7.25 | - |
| 36 | Taxifolin 3-O-rhamnoside | Flavonoid | C21H22O11 | 449.10896 | 12.24 | - |
| 37 | Piceatannol | Stilbenoid | C14H12O4 | 243.06631 | 13.08 | - |
| 38 | Lignan | Polyphenolic compound | C25H30O8 | 457.18682 | 27.33 | - |
| 39 | Cyanidin 3-O-arabinoside | Anthocyanidin-3-O-glycoside | C20H19ClO10 | 453.05942 | 7.17 | - |
| 40 | Berberine | Isoquinoline alkaloid | C20H18NO4 | - | 32.51 | 78.95 |
| Concentration (µg/mL) | 24 h | 48 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BVE | BS | BVE | BS | |||||||||
| V (%) | SD | IC50 (µg/mL) | V (%) | SD | IC50 (µg/mL) | V (%) | SD | IC50 (µg/mL) | V (%) | SD | IC50 (µg/mL) | |
| HUVEC | ||||||||||||
| 6.25 | 109.38 | 5.13 | 100.60 | 4.15 | 104.59 | 5.04 | 107.52 | 5.67 | ||||
| 12.5 | 107.10 | 5.38 | 97.10 | 5.91 | 100.54 | 4.35 | 103.36 | 6.91 | ||||
| 25 | 104.60 | 1.06 | 96.28 | 4.58 | 103.16 | 3.56 | 105.17 | 3.38 | ||||
| 50 | 101.16 | 0.09 | >>400 | 99.53 | 4.33 | >>400 | 98.71 | 5.03 | >>400 | 96.18 | 2.98 | >400 |
| 100 | 102.78 | 3.54 | 95.35 | 2.21 | 96.38 | 3.59 | 82.99 | 6.04 | ||||
| 200 | 99.10 | 7.94 | 96.72 | 1.86 | 89.95 | 4.76 | 77.15 | 4.99 | ||||
| 400 | 86.53 | 4.69 | 84.60 | 2.30 | 75.48 | 0.09 | 56.10 | 0.09 | ||||
| HEP G2 | ||||||||||||
| 6.25 | 99.57 | 6.47 | 97.46 | 6.76 | 98.31 | 0.33 | 96.91 | 5.81 | ||||
| 12.5 | 97.94 | 4.25 | 91.71 | 8.04 | 97.60 | 2.06 | 84.48 | 0.54 | ||||
| 25 | 96.43 | 0.12 | 90.43 | 4.08 | 96.03 | 6.67 | 65.45 | 2.39 | ||||
| 50 | 94.49 | 1.98 | >400 | 79.68 | 5.30 | >200 | 91.33 | 1.90 | >100 | 50.48 | 2.28 | >50 |
| 100 | 91.83 | 2.97 | 71.19 | 7.51 | 81.87 | 3.20 | 38.32 | 0.22 | ||||
| 200 | 73.62 | 0.52 | 53.77 | 5.48 | 46.76 | 1.95 | 25.69 | 2.44 | ||||
| 400 | 52.86 | 0.93 | 42.16 | 2.80 | 22.62 | 3.36 | 21.82 | 6.02 | ||||
| HT-29 | ||||||||||||
| 6.25 | 100.78 | 2.80 | 100.08 | 1.27 | 99.90 | 4.08 | 98.23 | 5.95 | ||||
| 12.5 | 98.86 | 7.60 | 98.63 | 2.90 | 97.39 | 0.00 | 95.90 | 2.72 | ||||
| 25 | 98.62 | 4.80 | 94.72 | 3.54 | 94.81 | 1.53 | 92.29 | 1.93 | ||||
| 50 | 97.18 | 3.59 | >>400 | 83.49 | 4.75 | >400 | 92.31 | 3.34 | >>400 | 86.48 | 5.16 | >400 |
| 100 | 95.08 | 1.16 | 70.77 | 0.60 | 90.62 | 1.25 | 72.18 | 2.44 | ||||
| 200 | 91.06 | 0.69 | 66.59 | 4.75 | 86.62 | 0.28 | 61.43 | 2.21 | ||||
| 400 | 85.04 | 3.69 | 60.80 | 6.76 | 77.31 | 5.45 | 54.02 | 6.56 | ||||
| LoVo | ||||||||||||
| 6.25 | 98.74 | 4.77 | 97.08 | 5.37 | 92.46 | 3.67 | 95.64 | 4.80 | ||||
| 12.5 | 93.91 | 0.74 | 91.98 | 3.52 | 89.06 | 6.32 | 91.86 | 2.73 | ||||
| 25 | 90.85 | 7.54 | 88.50 | 4.96 | 84.68 | 8.47 | 82.13 | 6.31 | ||||
| 50 | 85.13 | 4.94 | >200 | 79.91 | 0.87 | >50 | 72.68 | 4.55 | >50 | 65.02 | 5.69 | >50 |
| 100 | 75.97 | 2.35 | 38.60 | 1.85 | 48.39 | 0.63 | 28.19 | 1.39 | ||||
| 200 | 53.72 | 4.45 | 26.22 | 2.10 | 14.72 | 0.00 | 15.77 | 1.14 | ||||
| 400 | 25.05 | 4.57 | 15.57 | 8.53 | 3.09 | 2.66 | 8.08 | 2.91 | ||||
| MDA-MB-231 | ||||||||||||
| 6.25 | 96.83 | 4.41 | 87.73 | 6.43 | 75.86 | 4.48 | 71.40 | 3.52 | ||||
| 12.5 | 90.33 | 2.57 | 76.14 | 0.55 | 73.74 | 3.67 | 62.02 | 0.95 | ||||
| 25 | 87.71 | 0.37 | 66.43 | 5.51 | 70.81 | 0.15 | 45.93 | 2.57 | ||||
| 50 | 82.02 | 2.20 | >100 | 49.76 | 1.10 | >25 | 60.88 | 1.84 | >50 | 37.83 | 6.68 | >12.5 |
| 100 | 69.16 | 2.94 | 23.19 | 2.02 | 45.27 | 3.97 | 20.29 | 2.13 | ||||
| 200 | 53.71 | 3.67 | 20.67 | 0.37 | 25.65 | 1.47 | 14.39 | 2.06 | ||||
| 400 | 17.21 | 3.49 | 10.43 | 4.04 | 2.99 | 0.44 | 8.54 | 0.81 | ||||
| PE/CA-PJ49 | ||||||||||||
| 6.25 | 99.85 | 6.60 | 97.37 | 4.32 | 96.00 | 2.18 | 91.19 | 5.00 | ||||
| 12.5 | 93.17 | 6.89 | 91.01 | 8.44 | 87.26 | 0.51 | 77.49 | 6.21 | ||||
| 25 | 86.25 | 7.68 | 78.14 | 5.90 | 78.68 | 7.12 | 62.58 | 0.51 | ||||
| 50 | 80.89 | 2.54 | >100 | 63.65 | 2.81 | >50 | 69.09 | 5.95 | >50 | 50.24 | 4.70 | >50 |
| 100 | 61.92 | 1.17 | 42.84 | 0.34 | 49.39 | 5.35 | 35.37 | 1.91 | ||||
| 200 | 43.51 | 2.40 | 23.86 | 0.34 | 23.20 | 2.06 | 12.96 | 0.88 | ||||
| 400 | 12.25 | 2.54 | 9.75 | 1.71 | 4.70 | 0.51 | 3.95 | 0.66 | ||||
| SK-OV-3 | ||||||||||||
| 6.25 | 99.72 | 4.94 | 99.03 | 4.61 | 93.80 | 6.76 | 91.34 | 5.80 | ||||
| 12.5 | 92.28 | 5.89 | 90.99 | 4.63 | 90.86 | 7.09 | 82.43 | 5.30 | ||||
| 25 | 89.38 | 6.28 | 83.36 | 5.25 | 83.56 | 0.55 | 78.78 | 6.68 | ||||
| 50 | 83.35 | 5.84 | >400 | 79.62 | 6.04 | >200 | 73.17 | 2.56 | >100 | 67.19 | 7.40 | >50 |
| 100 | 78.30 | 2.95 | 66.62 | 1.87 | 58.24 | 3.52 | 33.37 | 6.76 | ||||
| 200 | 72.10 | 7.07 | 57.21 | 4.81 | 30.87 | 3.38 | 26.46 | 2.47 | ||||
| 400 | 55.83 | 3.83 | 39.90 | 1.25 | 16.29 | 0.37 | 10.05 | 0.80 | ||||
| Cancer Cell Line | Cytotoxic Responses | Berberine Concentration | IC50 Value | Reference |
|---|---|---|---|---|
| Liver cancer HEP G2 SMMC-7721 Bel-7402 |
| 3.125, 6.25, 12.5, 25, 50 and 100 µM | HEP G2—34.5 µM, SMMC-7721—25.2 µM Bel-7402—53.6 µM | [21] |
| Ehrlich ascites carcinoma EAC |
| 10, 50 and 100 µg/mL | <1 µg/mL | [22] |
| Dalton’s lymphoma ascites DLA |
| 100–1000 mg/mL | NA | [23] |
| Breast cancer MCF-7 MDA-MB-231 | Dose- and time-dependent inhibitory effects of cancer cell proliferation:
| 10–100 µM 10–100 µg/mL | NA MCF7—15.93 ug/mL | [24] |
| Ovarian cancer CsSki, SiHa, HeLa |
| 20 µM | NA | [25] |
| Prostate cancer LNCaP PC-82 |
| 1–100 µM | NA | [26] |
| Rat glioma C6 | Cytotoxic effects occur in a time- and dose-dependent manner, as follows:
| 100 µM | NA | [27] |
| Colorectal carcinoma HCT116, SW480 LoVo |
| 0–100 µM for 24–72 h | NA | [28] |
| Human prostate cancer LNCaP, PC-3 |
| 0, 5, 10, 20, 50, and 100 µM | LNCap cells: 60 µM PC-3 cells: ≥100 µM | [26,29] |
| Lung cancer A549 |
| 2.5–40 µM | NA | [30] |
| Human esophageal cancer YES-2 |
| 8–32 µM | NA | [31] |
| Oral cancer: OC2 KB |
| 1, 10, and 100 µM (2–12 h) | NA | [32] |
| Human OSCC: HSC-2, HSC-3, HSC-4 Human Promyelocytic Leukemia: HL-60 |
| 10, 20 and 80 µM | 18–136 µM | [32] |
| Mouse melanoma K1735-M2 |
| 0, 10, 25, 50, 75, and 100 µM | NA | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivan, I.M.; Olaru, O.T.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Nițulescu, G.M.; et al. Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract. Molecules 2024, 29, 2053. https://doi.org/10.3390/molecules29092053
Ivan IM, Olaru OT, Popovici V, Chițescu CL, Popescu L, Luță EA, Ilie EI, Brașoveanu LI, Hotnog CM, Nițulescu GM, et al. Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract. Molecules. 2024; 29(9):2053. https://doi.org/10.3390/molecules29092053
Chicago/Turabian StyleIvan, Ionuț Mădălin, Octavian Tudorel Olaru, Violeta Popovici, Carmen Lidia Chițescu, Liliana Popescu, Emanuela Alice Luță, Elena Iuliana Ilie, Lorelei Irina Brașoveanu, Camelia Mia Hotnog, George Mihai Nițulescu, and et al. 2024. "Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract" Molecules 29, no. 9: 2053. https://doi.org/10.3390/molecules29092053
APA StyleIvan, I. M., Olaru, O. T., Popovici, V., Chițescu, C. L., Popescu, L., Luță, E. A., Ilie, E. I., Brașoveanu, L. I., Hotnog, C. M., Nițulescu, G. M., Boscencu, R., & Gîrd, C. E. (2024). Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract. Molecules, 29(9), 2053. https://doi.org/10.3390/molecules29092053











