A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer
Abstract
1. Introduction
2. Results and Discussion
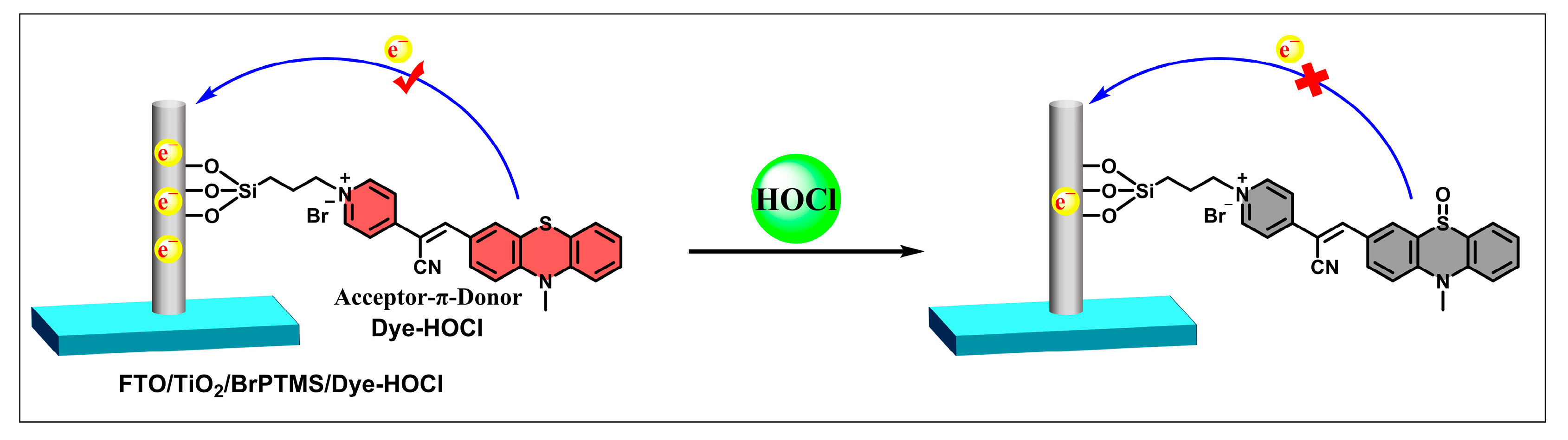
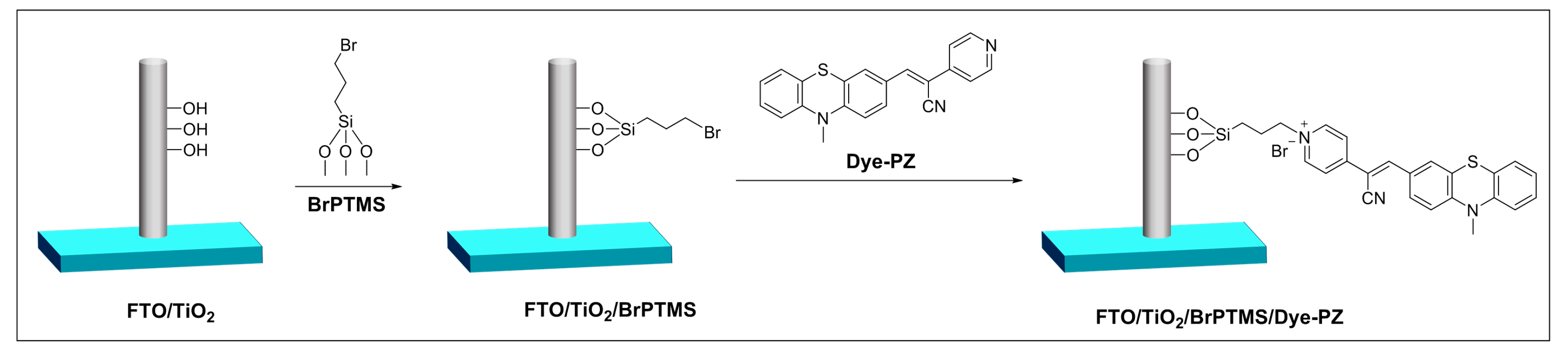
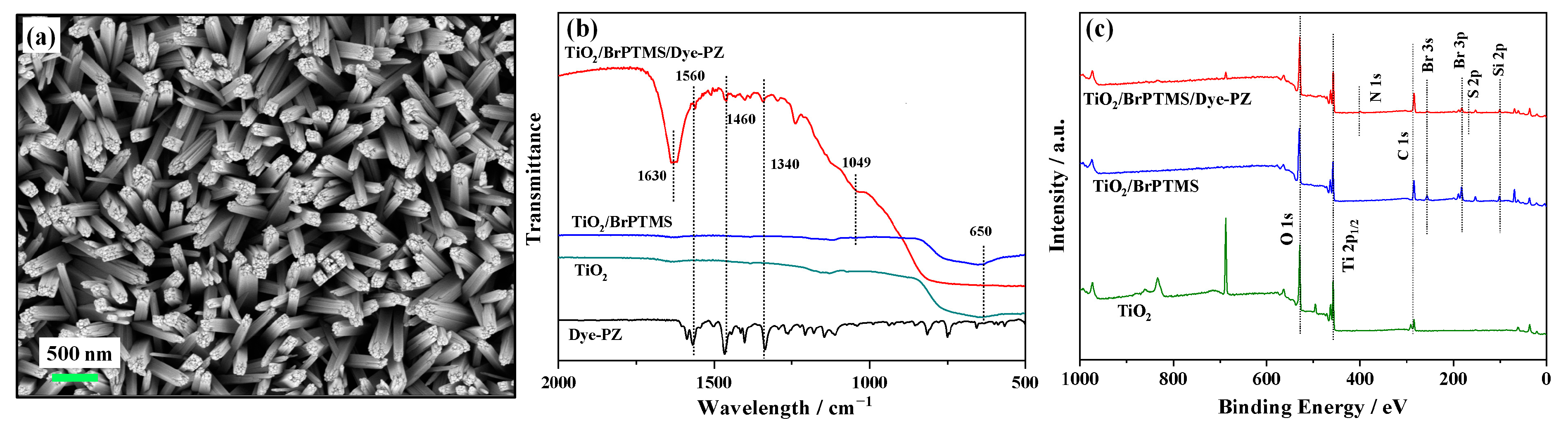
2.1. Preparation and Characterization of the FTO/TiO2/BrPTMS/Dye-PZ Photoanode
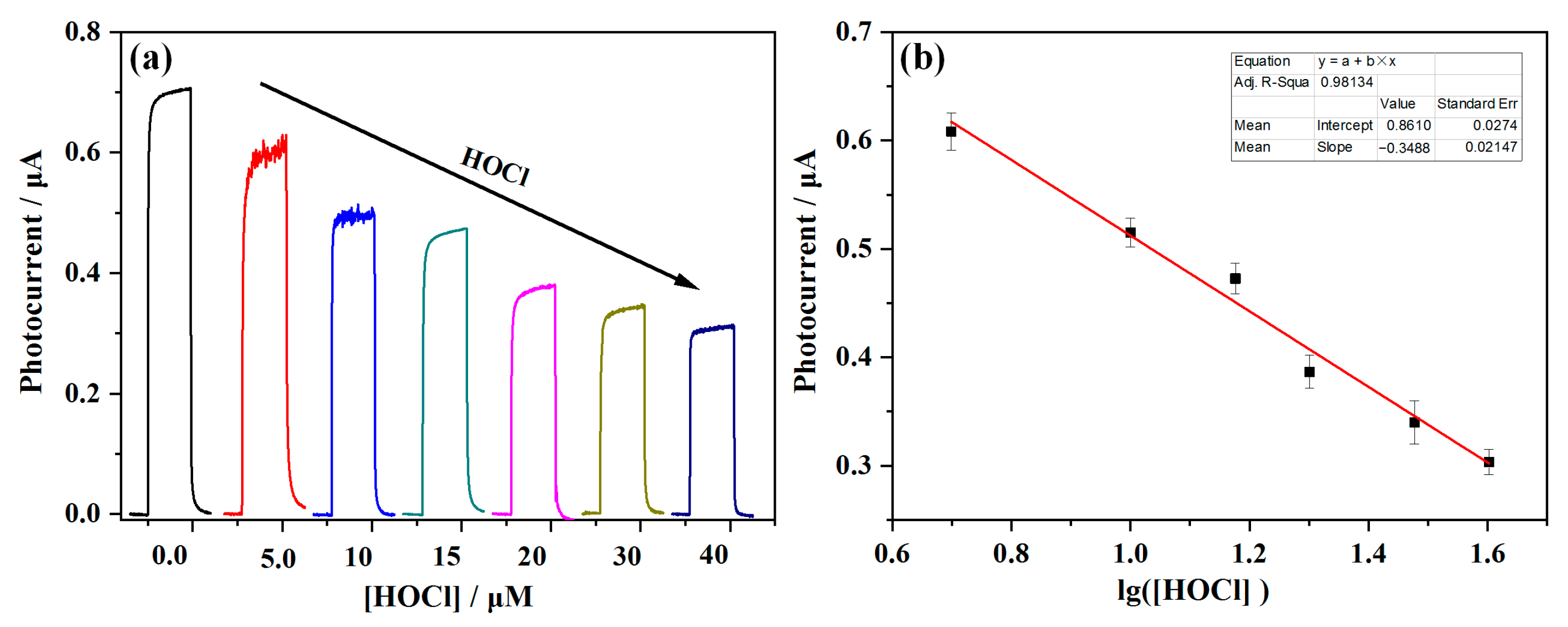
2.2. PEC Response of FTO/TiO2/BrPTMS/Dye-PZ to HOCl
2.3. Analytical Performance
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Synthesis of Dye-PZ
3.3. Preparation of FTO/TiO2/BrPTMS/Dye-PZ
3.4. Characterization and Photoelectrochemical Testing of FTO/TiO2/BrPTMS/Dye-PZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Block, M.S.; Rowan, B.G. Hypochlorous Acid: A Review. J. Oral Maxillofac. Surg. 2020, 78, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Robins, L.I.; Clark, A.; Gafken, P.R.; Alam, S.; Milici, J.; Hassan, R.; Wang, C.Y.; Williams, J.; Meyers, C. Hypochlorous acid as a disinfectant for high-risk HPV: Insight into the mechanism of action. J. Med. Virol. 2022, 94, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, A.D.; Lao, M.; Wang, C.; Abbatt, J.P.D.; Hoffnagle, J.; VandenBoer, T.C.; Kahan, T.F. Near-source hypochlorous acid emissions from indoor bleach cleaning. Environ. Sci.-Process. Impacts 2023, 25, 56–65. [Google Scholar] [CrossRef]
- Duclos, F.; Abell, L.M.; Harden, D.G.; Pike, K.; Nowak, K.; Locke, G.A.; Duke, G.J.; Liu, X.Q.; Fernando, G.; Shaw, S.A.; et al. Triazolopyrimidines identified as reversible myeloperoxidase inhibitors. Medchemcomm 2017, 8, 2093–2099. [Google Scholar] [CrossRef]
- Flemmig, J.; Remmler, J.; Zschaler, J.; Arnhold, J. Detection of the halogenating activity of heme peroxidases in leukocytes by aminophenyl fluorescein. Free Radic. Res. 2015, 49, 768–776. [Google Scholar] [CrossRef]
- Liu, Q.C.; Liu, C.; He, S.; Zhao, L.C.; Zeng, X.S.; Zhou, J.; Gong, J. A New Phenylazo-Based Fluorescent Probe for Sensitive Detection of Hypochlorous Acid in Aqueous Solution. Molecules 2022, 27, 2978. [Google Scholar] [CrossRef]
- Schieven, G.L.; De Fex, H.; Stephenson, L. Hypochlorous acid activates tyrosine phosphorylation signal pathways leading to calcium signaling and TNFα production. Antioxid. Redox Signal. 2002, 4, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M.; Jerlich, A.; Panasenko, O.M.; Arnhold, J.; Pitt, A.R.; Stelmaszynska, T.; Schaur, R.J. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim. Pol. 2000, 47, 889–899. [Google Scholar] [CrossRef]
- Tang, V.; Fu, S.L.; Rayner, B.S.; Hawkins, C.L. 8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response. Redox Biol. 2019, 26, 101274. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Koyani, C.N.; Scheruebel, S.; Jin, G.; Kolesnik, E.; Zorn-Pauly, K.; Mächler, H.; Hoefler, G.; von Lewinski, D.; Heinzel, F.R.; Pelzmann, B.; et al. Hypochlorite-Modified LDL Induces Arrhythmia and Contractile Dysfunction in Cardiomyocytes. Antioxidants 2022, 11, 25. [Google Scholar] [CrossRef]
- Pravalika, K.; Sarmah, D.; Kaur, H.; Wanve, M.; Saraf, J.; Kalia, K.; Borah, A.; Yavagal, D.R.; Dave, K.R.; Bhattacharya, P. Myeloperoxidase and Neurological Disorder: A Crosstalk. ACS Chem. Neurosci. 2018, 9, 421–430. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, Y.L.; Zhao, L.; Hao, Y.Q.; Zhang, Y.T.; Ye, B.X.; Xu, M.T. Dual-Response Ratiometric Electrochemical Microsensor for Effective Simultaneous Monitoring of Hypochlorous Acid and Ascorbic Acid in Human Body Fluids. Anal. Chem. 2020, 92, 15079–15086. [Google Scholar] [CrossRef]
- Hajnsek, M.; Schiffer, D.; Harrich, D.; Koller, D.; Verient, V.; van der Palen, J.; Heinzle, A.; Binder, B.; Sigl, E.; Sinner, F.; et al. An electrochemical sensor for fast detection of wound infection based on myeloperoxidase activity. Sens. Actuators B-Chem. 2015, 209, 265–274. [Google Scholar] [CrossRef]
- Zhang, R.; Song, B.; Yuan, J.L. Bioanalytical methods for hypochlorous acid detection: Recent advances and challenges. Trac-Trends Anal. Chem. 2018, 99, 1–33. [Google Scholar] [CrossRef]
- Okamoto, N.; Bito, T.; Hiura, N.; Yamamoto, A.; Iida, M.; Baba, Y.; Fujita, T.; Ishihara, A.; Yabuta, Y.; Watanabe, F. Food Additives (Hypochlorous Acid Water, Sodium Metabisulfite, and Sodium Sulfite) Strongly Affect the Chemical and Biological Properties of Vitamin B<sub>12</sub> in Aqueous Solution. ACS Omega 2020, 5, 6207–6214. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, F.; Li, D.P.; Qi, J.; Liu, X.M. A novel strategy for evaluation of natural products acting on the myeloperoxidase/hypochlorous acid system by combining high-performance liquid chromatography-photodiode array detection-chemiluminescence and ultrafiltration-mass spectrometry techniques. J. Sep. Sci. 2018, 41, 194–1201. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.C.; Zhang, K.X.; Yuan, X.H.; Xie, X.B.; Zhan, Z.X.; Lv, Y. Novel Near-Infrared Iridium(III) Complex for Chemiluminescence Imaging of Hypochlorous Acid. Anal. Chem. 2023, 95, 8310–8317. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.; Estela, J.M.; Cerdà, V. Automatic in vitro determination of hypochlorous acid scavenging capacity exploiting multisyringe flow injection analysis and chemiluminescence. Anal. Chem. 2007, 79, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.F.; Wang, T.; Zhang, M.Y.; Li, N.; Fan, M.T.; Cui, X.Y. Chemiluminescence molecular sensor for endogenous HOCl in vivo. Sens. Actuators B-Chem. 2021, 339, 129927. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, F.; Wang, Y.; Feng, H.; Meng, Q.T.; Zhang, Z.Q.; Zhang, R. A Redox-Switchable Colorimetric Probe for "Naked-Eye" Detection of Hypochlorous Acid and Glutathione. Molecules 2019, 24, 2455. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, X.L.; Liu, H.; Zhang, Q.L. Recent Progress in Colorimetric and Fluorimetric Probes for the Detection of Hypochlorous Acid. Chin. J. Org. Chem. 2020, 40, 1206–1231. [Google Scholar] [CrossRef]
- He, S.; Dong, H.; Hao, Y.Q.; Zhang, Y.T.; Zhou, Y.L.; Zhang, F.Y.; Li, J.; Jia, Y.Y.; Xiao, G.Q.; Xu, M.T. Quantifying Hypochlorous Acid Concentration in Environmental Water Using Smartphone Colorimetry. J. Anal. Test. 2021, 5, 360–369. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Chen, S.; Zhou, Y.L.; Zhang, Y.T.; Xu, M.T. Recent Progress in Metal-Organic Framework (MOF) Based Luminescent Chemodosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, L.Y.; Xu, Q.L.; Chen, X.Q.; Yoon, J.Y. Design Principles, Sensing Mechanisms, and Applications of Highly Specific Fluorescent Probes for HOCl/OCl−. Acc. Chem. Res. 2019, 52, 2158–2168. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Dehaen, W. Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives. Molecules 2021, 26, 363. [Google Scholar] [CrossRef]
- Dai, M.C.; Yang, Y.J.; Sarkar, S.; Ahn, K.H. Strategies to convert organic fluorophores into red/near-infrared emitting analogues and their utilization in bioimaging probes. Chem. Soc. Rev. 2023, 52, 6344–6358. [Google Scholar] [CrossRef]
- Hou, J.T.; Kwon, N.; Wang, S.; Wang, B.Y.; He, X.J.; Yoon, J.; Shen, J.L. Sulfur-based fluorescent probes for HOCl: Mechanisms, design, and applications. Coord. Chem. Rev. 2022, 450, 214232. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Liu, Y.; Feng, X.; Zhao, B.-X. Recent progress in the development of fluorescent probes for the detection of hypochlorous acid. Sens. Actuators B Chem. 2017, 240, 18–36. [Google Scholar] [CrossRef]
- Kwon, N.; Chen, Y.; Chen, X.; Kim, M.H.; Yoon, J. Recent progress on small molecule-based fluorescent imaging probes for hypochlorous acid (HOCl)/hypochlorite (OCl−). Dye. Pigment. 2022, 200, 110132. [Google Scholar] [CrossRef]
- Ren, C.P.; Nie, W.; Leng, J.Q.; Liu, Z.B. Reactive Fluorescent Probe for Hypochlorite. Prog. Chem. 2021, 33, 942–957. [Google Scholar] [CrossRef]
- Liu, D.; Yue, X.X.; Zhang, H.K.; Li, K.; Yang, Z.H.; Wang, B.H.; Song, X.Z. Probing drug-mediated fluctuations of HClO levels in the endoplasmic reticulum by a ratiometric fluorescent probe with a large emission shift. Dye. Pigment. 2023, 215, 111257. [Google Scholar] [CrossRef]
- Wang, B.Y.; Guo, X.M.; Liu, Z.D.; Wu, Y.Q.; Hou, J.T. A Long-wavelength Emissive Phenothiazine Derived Fluorescent Probe for Detecting HOCl Upregulation in 5-FU Stimulated Living Cells. Chem. Res. Chin. Univ. 2022, 38, 609–615. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Zhang, Y.T.; Sun, Q.L.; Chen, S.; Tang, Z.L.; Zeng, R.J.; Xu, M.T. Phenothiazine-coumarin-pyridine hybrid as an efficient fluorescent probe for ratiometric sensing hypochlorous acid. Microchem. J. 2021, 171, 106851. [Google Scholar] [CrossRef]
- Jiao, X.J.; Huang, K.; He, S.; Liu, C.; Zhao, L.C.; Zeng, X.S. A mitochondria-targeted near-infrared fluorescent probe with a large Stokes shift for real-time detection of hypochlorous acid. Org. Biomol. Chem. 2019, 17, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.S.; Qiu, X.Y.; Liu, C.; Jiao, X.J.; He, S.; Zhao, L.C.; Zeng, X.S. Synthesis and bioapplication of a highly selective and sensitive fluorescent probe for HOCl based on a phenothiazine-dicyanoisophorone conjugate with large Stokes shift. New J. Chem. 2018, 42, 5135–5141. [Google Scholar] [CrossRef]
- Zhi, X.; Qian, Y. A novel red-emission phenothiazine fluorescent protein chromophore based on oxygen–chlorine bond (O-Cl) formation for real-time detection of hypochlorous acid in cells. Talanta 2021, 222, 121503. [Google Scholar] [CrossRef]
- Han, J.; Yang, S.; Wang, B.; Song, X. Tackling the Selectivity Dilemma of Benzopyrylium–Coumarin Dyes in Fluorescence Sensing of HClO and SO2. Anal. Chem. 2021, 93, 5194–5200. [Google Scholar] [CrossRef]
- Onoabedje, E.A.; Egu, S.A.; Ezeokonkwo, M.A.; Okoro, U.C. Highlights of molecular structures and applications of phenothiazine & phenoxazine polycycles. J. Mol. Struct. 2019, 1175, 956–962. [Google Scholar] [CrossRef]
- Simon, A.; Alexandru, T.; Boni, M.; Damian, V.; Stoicu, A.; Dutschk, V.; Pascu, M.L. Interaction of solutions containing phenothiazines exposed to laser radiation with materials surfaces, in view of biomedical applications. Int. J. Pharm. 2014, 475, 270–281. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S.; Chandrabose, R.S.; Karazhanov, S. Recent developments in metal-free organic sensitizers derived from carbazole, triphenylamine, and phenothiazine for dye-sensitized solar cells. Int. J. Energy Res. 2021, 45, 6584–6643. [Google Scholar] [CrossRef]
- Buene, A.F.; Almenningen, D.M. Phenothiazine and phenoxazine sensitizers for dye-sensitized solar cells—an investigative review of two complete dye classes. J. Mater. Chem. C 2021, 9, 11974–11994. [Google Scholar] [CrossRef]
- Luo, J.S.; Wan, Z.Q.; Jia, C.Y. Recent advances in phenothiazine-based dyes for dye-sensitized solar cells. Chin. Chem. Lett. 2016, 27, 1304–1318. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhan, Y.; He, W. Recent development of small-molecule fluorescent probes based on phenothiazine and its derivates. J. Photochem. Photobiol. B-Biol. 2022, 234, 112528. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Yan, S.R.; Yu, Y.B.; Xue, Y.S.; Yu, Y.Y.; Han, C.P. Dual-Responsive Ratiometric Fluorescent Probe for Hypochlorite and Peroxynitrite Detection and Imaging In Vitro and In Vivo. Anal. Chem. 2022, 94, 1415–1424. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Liu, S.J.; Hao, Y.Q.; Sun, J.W.; Chen, S. Phenothiazine-based fluorescence probe for ratiometric imaging of hydrazine in living cells with remarkable Stokes shift. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2020, 227, 117675. [Google Scholar] [CrossRef]
- Rout, Y.; Montanari, C.; Pasciucco, E.; Misra, R.; Carlotti, B. Tuning the Fluorescence and the Intramolecular Charge Transfer of Phenothiazine Dipolar and Quadrupolar Derivatives by Oxygen Functionalization. J. Am. Chem. Soc. 2021, 143, 9933–9943. [Google Scholar] [CrossRef]
- Manusha, P.; Yadav, S.; Satija, J.; Senthilkumar, S. Designing electrochemical NADH sensor using silver nanoparticles/phenothiazine nanohybrid and investigation on the shape dependent sensing behavior. Sens. Actuators B-Chem. 2021, 347, 130649. [Google Scholar] [CrossRef]
- Manusha, P.; Senthilkumar, S. Design and synthesis of phenothiazine based imidazolium ionic liquid for electrochemical nonenzymatic detection of sulfite in food samples. J. Mol. Liq. 2020, 301, 112412. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Wang, J. Chemical adsorption of phenothiazine dyes onto carbon nanotubes: Toward the low potential detection of NADH. Electrochem. Commun. 2006, 8, 71–76. [Google Scholar] [CrossRef]
- Abdi, G.; Alluhaibi, L.; Kowalewska, E.; Mazur, T.; Mech, K.; Podborska, A.; Sławek, A.; Tanaka, H.; Szaciłowski, K. Reservoir computing and photoelectrochemical sensors: A marriage of convenience. Coord. Chem. Rev. 2023, 487, 215155. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, H.; Yang, P.; Luo, L.; Niu, Q.; You, T. Photoactivities regulating of inorganic semiconductors and their applications in photoelectrochemical sensors for antibiotics analysis: A systematic review. Biosens. Bioelectron. 2022, 216, 114634. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, H.; Hao, Y.; Zhang, Y.; Chen, S.; Xu, M.; Zhou, Y. Near-infrared Responsive Photoelectrochemical Biosensors. Electroanalysis 2022, 34, 956–965. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, H.; Ai, S. Applications of two-dimensional layered nanomaterials in photoelectrochemical sensors: A comprehensive review. Coord. Chem. Rev. 2021, 447, 214156. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, Y.; Hao, Y.; Han, H.; Hu, X.; Yang, Y.; Long, X.; He, J.; Zhang, P.; Zeng, R.; et al. A responsive organic probe based photoelectrochemical sensor for hydrazine detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123463. [Google Scholar] [CrossRef]
- Li, T.; Hao, Y.; Dong, H.; Li, C.; Liu, J.; Zhang, Y.; Tang, Z.; Zeng, R.; Xu, M.; Chen, S. Target-Induced In Situ Formation of Organic Photosensitizer: A New Strategy for Photoelectrochemical Sensing. ACS Sens. 2022, 7, 415–422. [Google Scholar] [CrossRef]
- Hao, Y.; Li, T.; Luo, L.; Fan, S.; Chen, S.; Zhang, Y.; Tang, Z.; Xu, M.; Zeng, R.; Chen, S. A reaction based dual-modal probe for fluorescent and photoelectrochemical determination of thiophenol. Sens. Actuators B Chem. 2022, 369, 132405. [Google Scholar] [CrossRef]
- Xiang, Y.; Kong, Y.; Feng, W.; Ye, X.; Liu, Z. A ratiometric photoelectrochemical microsensor based on a small-molecule organic semiconductor for reliable in vivo analysis. Chem. Sci. 2021, 12, 12977–12984. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tu, W.; Zhao, Y.; Wang, X.; Song, J.; Yang, X. Phosphonate-Substituted Ruthenium(II) Bipyridyl Derivative as a Photoelectrochemical Probe for Sensitive and Selective Detection of Mercury(II) in Biofluids. Anal. Chem. 2018, 90, 14423–14432. [Google Scholar] [CrossRef]
- Chu, J.-W.; Trout, B.L. On the Mechanisms of Oxidation of Organic Sulfides by H2O2 in Aqueous Solutions. J. Am. Chem. Soc. 2004, 126, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Zeida, A.; Trujillo, M.; Ferrer-Sueta, G.; Denicola, A.; Estrin, D.A.; Radi, R. Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols. Chem. Rev. 2019, 119, 10829–10855. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhu, H.; Xie, C.; Zhang, L.; Chen, P.; Fan, Q.; Huang, W.; Pu, K. Organic Nanoprobe Cocktails for Multilocal and Multicolor Fluorescence Imaging of Reactive Oxygen Species. Adv. Funct. Mater. 2017, 27, 1700493. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yu, L.; Yang, Z.; Ding, J.; Wang, K.-N.; Zhang, Y. Monocomponent Nanodots with Dichromatic Output Regulated by Synergistic Dual-Stimuli for Cervical Cancer Tissue Imaging and Photodynamic Tumor Therapy. Anal. Chem. 2022, 94, 811–819. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Wang, W.; Feng, G.; Yuan, D.; Zhang, X. Elucidating the Structure-Reactivity Correlations of Phenothiazine-Based Fluorescent Probes toward ClO−. Chem.-A Eur. J. 2018, 24, 8157–8166. [Google Scholar] [CrossRef]
- Xiao, H.; Xin, K.; Dou, H.; Yin, G.; Quan, Y.; Wang, R. A fast-responsive mitochondria-targeted fluorescent probe detecting endogenous hypochlorite in living RAW 264.7 cells and nude mouse. Chem. Commun. 2015, 51, 1442–1445. [Google Scholar] [CrossRef]
- Liang, L.; Liu, C.; Jiao, X.; Zhao, L.; Zeng, X. A highly selective and sensitive photoinduced electron transfer (PET) based HOCl fluorescent probe in water and its endogenous imaging in living cells. Chem. Commun. 2016, 52, 7982–7985. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Yang, Y.; Chen, S.; Zhang, P.; Zeng, R. A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer. Molecules 2024, 29, 614. https://doi.org/10.3390/molecules29030614
Luo L, Yang Y, Chen S, Zhang P, Zeng R. A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer. Molecules. 2024; 29(3):614. https://doi.org/10.3390/molecules29030614
Chicago/Turabian StyleLuo, Lijie, Yewen Yang, Shu Chen, Peisheng Zhang, and Rongjin Zeng. 2024. "A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer" Molecules 29, no. 3: 614. https://doi.org/10.3390/molecules29030614
APA StyleLuo, L., Yang, Y., Chen, S., Zhang, P., & Zeng, R. (2024). A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer. Molecules, 29(3), 614. https://doi.org/10.3390/molecules29030614




