Penisimplicins A and B: Novel Polyketide–Peptide Hybrid Alkaloids from the Fungus Penicillium simplicissimum JXCC5
Abstract
1. Introduction
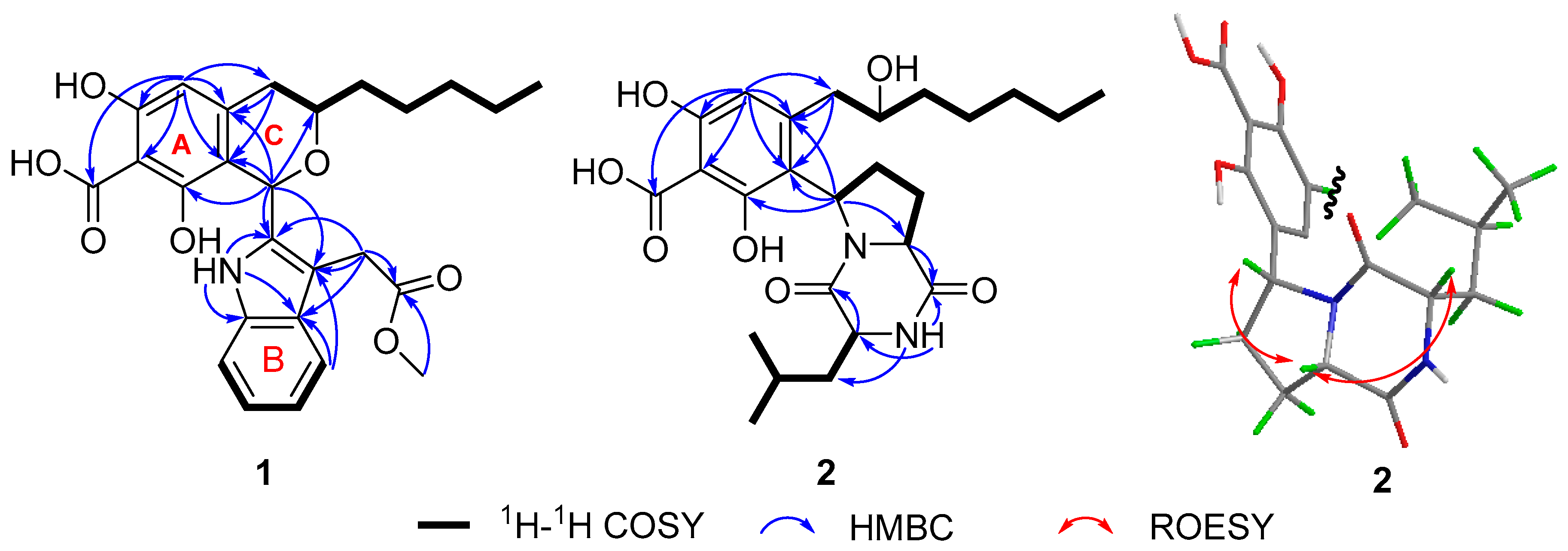
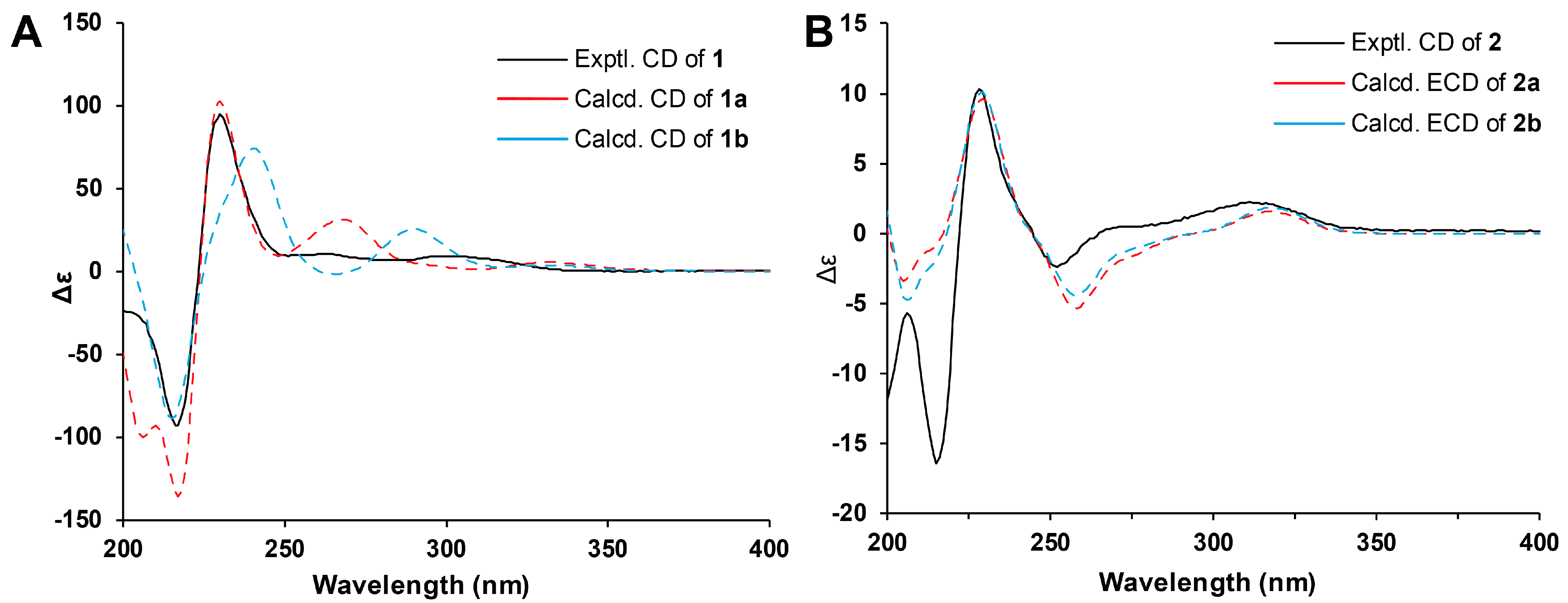
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Genomic DNA Extraction and Identification
3.4. Extraction and Isolation
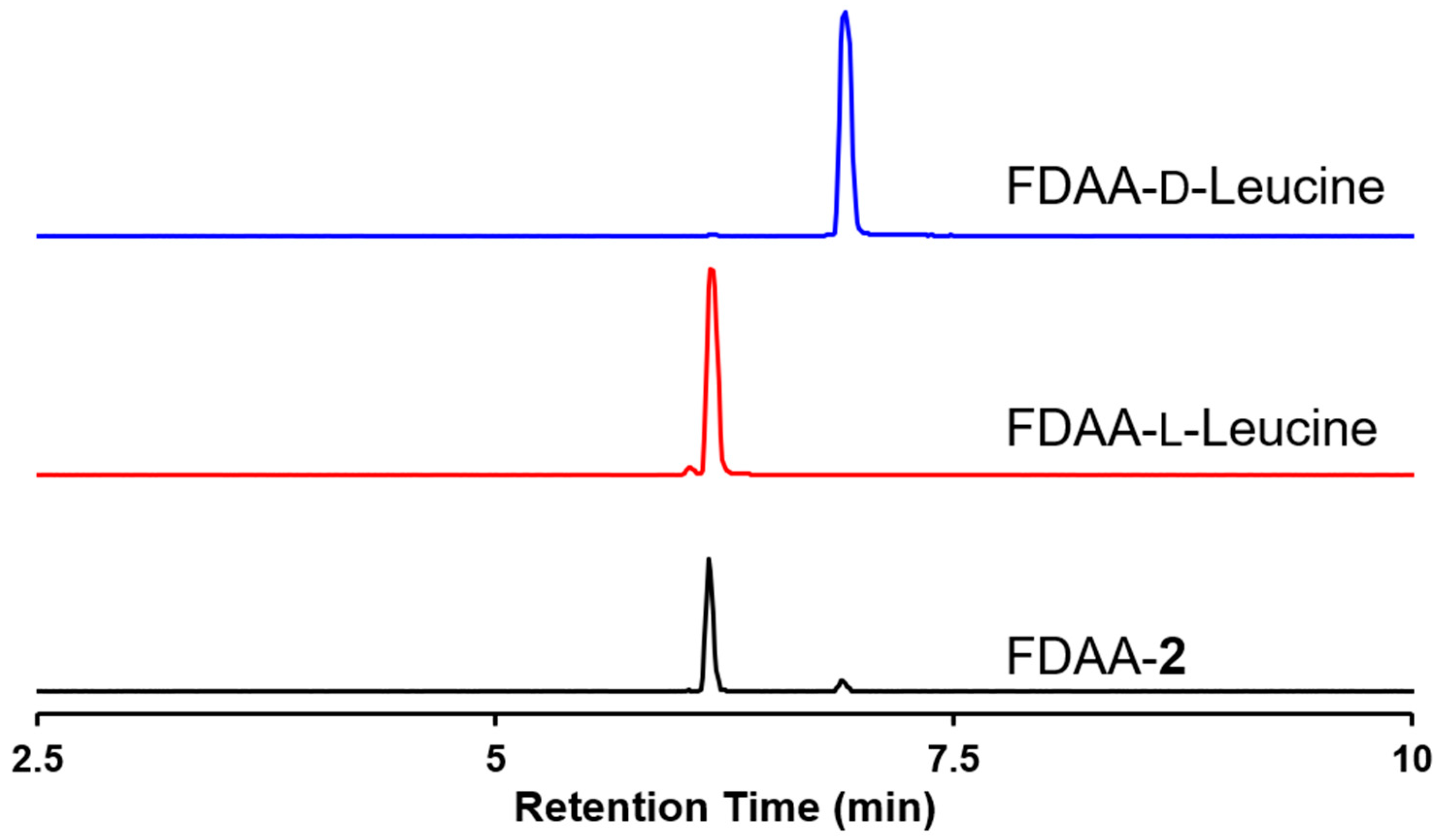
3.5. Marfey’s Method
3.6. Characterization Data
3.6.1. Penisimplicin A (1)
3.6.2. Penisimplicin B (2)
3.7. Computational Details
3.7.1. ECD Calculation
3.7.2. DP4+ Analysis
3.8. Acetylcholinesterase Inhibition Assay
3.9. Anti-inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.S.; Akhtar, S.; Jamal, Q.M.; Rizvi, S.M.; Kamal, M.A.; Khan, M.K.; Siddiqui, M.H. Multiple Targets for the Management of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2016, 15, 1279–1289. [Google Scholar] [CrossRef]
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.A.; Rosa-Neto, P. World Alzheimer Report 2022, Life after Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022. [Google Scholar]
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s Disease: Beyond Cholinesterase Inhibitors. Pharmacol. Ther. 2012, 134, 8–25. [Google Scholar] [CrossRef]
- Winblad, B.; Grossberg, G.; Frölich, L.; Farlow, M.; Zechner, S.; Nagel, J.; Lane, R. Ideal: A 6-Month, Double-Blind, Placebo-Controlled Study of the First Skin Patch for Alzheimer Disease. Neurology 2007, 69, S14–S22. [Google Scholar] [CrossRef] [PubMed]
- Ago, Y.; Koda, K.; Takuma, K.; Matsuda, T. Pharmacological Aspects of the Acetylcholinesterase Inhibitor Galantamine. J. Pharmacol. Sci. 2011, 116, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Ortiz, A.L.; Acosta-Castillo, I.; Prince, M.J. Epidemiology of Dementias and Alzheimer’s Disease. Arch. Med. Res. 2012, 43, 600–608. [Google Scholar] [CrossRef]
- Santoro, A.; Siviero, P.; Minicuci, N.; Bellavista, E.; Mishto, M.; Olivieri, F.; Marchegiani, F.; Chiamenti, A.M.; Benussi, L.; Ghidoni, R.; et al. Effects of Donepezil, Galantamine and Rivastigmine in 938 Italian Patients with Alzheimer’s Disease: A Prospective, Observational Study. CNS Drugs 2010, 24, 163–176. [Google Scholar] [CrossRef]
- Richard, F.; Helbecque, N.; Neuman, E.; Guez, D.; Levy, R.; Amouyel, P. APOE Genotyping and Response to Drug Treatment in Alzheimer’s Disease. Lancet 1997, 349, 539. [Google Scholar] [CrossRef]
- Niu, B.; Zhang, M.; Du, P.; Jiang, L.; Qin, R.; Su, Q.; Chen, F.; Du, D.; Shu, Y.; Chou, K.C. Small Molecular Floribundiquinone B Derived from Medicinal Plants Inhibits Acetylcholinesterase Activity. Oncotarget 2017, 8, 57149–57162. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Ning, Z.; Li, D.-N.; Feng, X.; Yang, X.-P. Primary Investigation for the Mechanism of Biatractylolide from Atractylodis Macrocephalae Rhizoma as an Acetylcholinesterase Inhibitor. Evid. Based Complement. Altern. Med. 2016, 2016, 7481323. [Google Scholar] [CrossRef]
- Youkwan, J.; Sutthivaiyakit, S.; Sutthivaiyakit, P. Citrusosides A–D and Furanocoumarins with Cholinesterase Inhibitory Activity from the Fruit Peels of Citrus hystrix. J. Nat. Prod. 2010, 73, 1879–1883. [Google Scholar] [CrossRef]
- He, F.; Liu, Z.; Yang, J.; Fu, P.; Peng, J.; Zhu, W.-M.; Qi, S.-H. A Novel Antifouling Alkaloid from Halotolerant Fungus Penicillium sp. OUCMDZ-776. Tetrahedron Lett. 2012, 53, 2280–2283. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Xu, W.; Liu, W.; Liu, L.; Zhu, D.; Kang, Y.; Luo, Z.; Li, Q. Three New Cyclopiane-Type Diterpenes from a Deep-sea Derived Fungus Penicillium sp. YPGA11 and Their Effects against Human Esophageal Carcinoma Cells. Bioorganic Chem. 2019, 91, 103129. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Gong, Y.; Chen, X.; Wen, H.; Qi, C.; Chen, C.; Liu, J.; Luo, Z.; Wang, J.; Zhu, H.; et al. Piperazine-2,5-Dione Derivatives and an α-Pyrone Polyketide from Penicillium Griseofulvum and Their Immunosuppression Activity. Phytochemistry 2021, 186, 112708. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Mycophenolic Acid: A One Hundred Year Odyssey from Antibiotic to Immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, R.; Sahai, V. Compactin—A Review. Appl. Microbiol. Biotechnol. 2004, 64, 618–624. [Google Scholar] [CrossRef]
- Dai, C.; Li, X.; Zhang, K.; Li, X.-N.; Wang, W.; Zang, Y.; Chen, X.; Li, Q.; Wei, M.; Chen, C.; et al. Pesimquinolones I–S, Eleven New Quinolone Alkaloids Produced by Penicillium simplicissimum and their Inhibitory Activity on NO Production. Bioorganic Chem. 2021, 108, 104635. [Google Scholar] [CrossRef]
- Dai, C.; Chen, C.; Guan, D.; Chen, H.; Wang, F.; Wang, W.; Zang, Y.; Li, Q.; Wei, M.; Li, X.; et al. Pesimquinolones Produced by Penicillium simplicissimum and their Inhibitory Activity on Nitric Oxide Production. Phytochemistry 2020, 174, 112327. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Zanardi, M.M.; Sarotti, A.M. Sensitivity Analysis of DP4+ with the Probability Distribution Terms: Development of a Universal and Customizable Method. J. Org. Chem. 2021, 86, 8544–8548. [Google Scholar] [CrossRef]
- Ding, T.; Zhou, Y.; Qin, J.-J.; Yang, L.-J.; Zhang, W.-D.; Shen, Y.-H. Chemical Constituents from Wetland Soil Fungus Penicillium Oxalicum GY1. Fitoterapia 2020, 142, 104530. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Yu, Z.; Zhang, J. Cyclo(l-Pro–l-Leu) of Pseudomonas Putida MCCC 1A00316 Isolated from Antarctic Soil: Identification and Characterization of Activity against Meloidogyne Incognita. Molecules 2019, 24, 768. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, R.; Brückner, H. Marfey’s Reagent for Chiral Amino Acid Analysis: A Review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sanchez, J.F.; Chiang, Y.-M.; Oakley, B.R.; Wang, C.C.C. Rational Domain Swaps Reveal Insights about Chain Length Control by Ketosynthase Domains in Fungal Nonreducing Polyketide Synthases. Org. Lett. 2014, 16, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, M.-C.; Tang, Y. Fungal Highly Reducing Polyketide Synthases Biosynthesize Salicylaldehydes That Are Precursors to Epoxycyclohexenol Natural Products. J. Am. Chem. Soc. 2019, 141, 19538–19541. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Feng, J.; Xiang, H.; Cheng, B.; Shao, L.-D.; Li, Y.-P.; Wang, H.; Hu, Q.-F.; Xiao, W.-L.; Matsuda, Y.; et al. Ketoreductase Domain-Catalyzed Polyketide Chain Release in Fungal Alkyl Salicylaldehyde Biosynthesis. J. Am. Chem. Soc. 2023, 145, 11293–11300. [Google Scholar] [CrossRef] [PubMed]
- Biscussi, B.; Sequeira, M.A.; Richmond, V.; Arroyo Mañez, P.; Murray, A.P. New photochromic azoderivatives with potent acetylcholinesterase inhibition. J. Photochem. Photobiol. A 2021, 418, 113375. [Google Scholar] [CrossRef]
- Fahim, A.M.; Farag, A.M.; Mermer, A.; Bayrak, H.; Şirin, Y. Synthesis of novel β-lactams: Antioxidant activity, acetylcholinesterase inhibition and computational studies. J. Mol. Struct. 2021, 1233, 130092. [Google Scholar] [CrossRef]
- Stark, J.R.; Cardon, Z.G.; Peredo, E.L. Extraction of High-Quality, High-Molecular-Weight DNA Depends Heavily on Cell Homogenization Methods in Green Microalgae. Appl. Plant Sci. 2020, 8, e11333. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N] nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.Z.; Yao, J.N.; Feng, T.; Li, Z.H.; Chen, H.P.; Liu, J.K. Irpeksins A−E, 1,10-seco-eburicane-type Triterpenoids from the Medicinal Fungus Irpex lacteus and their Anti-NO Activity. J. Nat. Prod. 2018, 81, 2163–2168. [Google Scholar] [CrossRef]


| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 66.7, CH | 5.97, s | ||
| 2 | 109.9, C | 115.4, C | ||
| 3 | 159.0, C | 160.3, C | ||
| 4 | 102.3, C | 102.7, C | ||
| 5 | 161.1, C | 160.3, C | ||
| 6 | 104.2, CH | 5.96, s | 106.7, CH | 5.82, s |
| 7 | 138.3, C | 141.6, C | ||
| 8 | 34.1, CH2 | 2.44, dd (16.2, 10.3) 2.56, dd (16.2, 3.4) | 42.4, CH2 | 2.55, m |
| 9 | 67.5, CH | 3.58, m | 71.9, CH | 3.63, m |
| 10 | 35.4, CH2 | 1.35, m 1.39, m | 37.0, CH2 | 1.32, m 1.40, m |
| 11 | 24.3, CH2 | 1.16, overlapped 1.22, m | 25.1, CH2 | 1.28, overlapped 1.41, m |
| 12 | 31.1, CH2 | 1.00, m | 31.5, CH2 | 1.25, overlapped |
| 13 | 22.1, CH2 | 1.14, overlapped | 22.2, CH2 | 1.27, overlapped |
| 14 | 13.9, CH3 | 0.69, t (7.4) | 14.0, CH3 | 0.86, t (6.9) |
| 15 | 175.5, C | 175.5, C | ||
| 16 | 136.7, C | 55.8, CH | 5.14, dd (9.7, 3.6) | |
| 17 | 105.1, C | 31.1, CH2 | 1.79, m 2.24, m | |
| 18 | 128.4, C | 26.1, CH2 | 1.98, m 2.63, m | |
| 19 | 118.3, CH | 7.38, d (8.0) | 60.0, CH | 4.29, t-like (8.5) |
| 20 | 118.2, CH | 6.92, dd (8.0, 6.9) | 170.9, C | |
| 21 | 120.7, CH | 7.00, dd (8.1, 6.9) | 52.9, CH | 3.88, dd (8.6, 4.7) |
| 22 | 111.3, CH | 7.27, d (8.1) | 166.2, C | |
| 23 | 134.9, C | 37.8, CH2 | 1.24, overlapped 1.63, ddd (13.6, 8.6, 4.7) | |
| 24 | 29.2, CH2 | 3.51, d (16.5) 3.56, d (16.5) | 24.0, CH | 1.81, m |
| 25 | 171.9, C | 22.9, CH3 | 0.81, d (6.5) | |
| 26 | 21.7, CH3 | 0.80, d (6.4) | ||
| 25-OCH3 | 51.2, CH3 | 3.50, s | ||
| 3-OH | 15.05, s | |||
| 5-OH | 14.40, s | |||
| 16-NH | 10.50, s | |||
| 21-NH | 7.79, s | |||
| Item | 1a (1S,9R) | 1b (1S,9S) |
|---|---|---|
| sDP4+ (H data) | 90.83% | 9.17% |
| sDP4+ (C data) | 100.00% | 0.00% |
| sDP4+ (all data) | 100.00% | 0.00% |
| uDP4+ (H data) | 99.54% | 0.46% |
| uDP4+ (C data) | 99.62% | 0.38% |
| uDP4+ (all data) | 100.00% | 0.00% |
| DP4+ (H data) | 99.95% | 0.05% |
| DP4+ (C data) | 100.00% | 0.00% |
| DP4+ (all data) | 100.00% | 0.00% |
| Item | 2a | 2b |
|---|---|---|
| 9R,16R,19S,21S | 9S,16R,19S,21S | |
| sDP4+ (H data) | 15.86% | 84.14% |
| sDP4+ (C data) | 77.34% | 22.66% |
| sDP4+ (all data) | 39.16% | 60.84% |
| uDP4+ (H data) | 0.28% | 99.72% |
| uDP4+ (C data) | 22.77% | 77.23% |
| uDP4+ (all data) | 0.08% | 99.92% |
| DP4+ (H data) | 0.05% | 99.96% |
| DP4+ (C data) | 50.16% | 49.84% |
| DP4+ (all data) | 0.05% | 99.95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.-Y.; Gao, Y.; Yao, J.-N.; Zhou, L.; Chen, H.-P.; Liu, J.-K. Penisimplicins A and B: Novel Polyketide–Peptide Hybrid Alkaloids from the Fungus Penicillium simplicissimum JXCC5. Molecules 2024, 29, 613. https://doi.org/10.3390/molecules29030613
Wang Q-Y, Gao Y, Yao J-N, Zhou L, Chen H-P, Liu J-K. Penisimplicins A and B: Novel Polyketide–Peptide Hybrid Alkaloids from the Fungus Penicillium simplicissimum JXCC5. Molecules. 2024; 29(3):613. https://doi.org/10.3390/molecules29030613
Chicago/Turabian StyleWang, Qing-Yuan, Yang Gao, Jian-Neng Yao, Li Zhou, He-Ping Chen, and Ji-Kai Liu. 2024. "Penisimplicins A and B: Novel Polyketide–Peptide Hybrid Alkaloids from the Fungus Penicillium simplicissimum JXCC5" Molecules 29, no. 3: 613. https://doi.org/10.3390/molecules29030613
APA StyleWang, Q.-Y., Gao, Y., Yao, J.-N., Zhou, L., Chen, H.-P., & Liu, J.-K. (2024). Penisimplicins A and B: Novel Polyketide–Peptide Hybrid Alkaloids from the Fungus Penicillium simplicissimum JXCC5. Molecules, 29(3), 613. https://doi.org/10.3390/molecules29030613






