Application of Ordered Porous Silica Materials in Drug Delivery: A Review
Abstract
1. Introduction
2. Ordered Porous Silica Materials
2.1. Zeolites
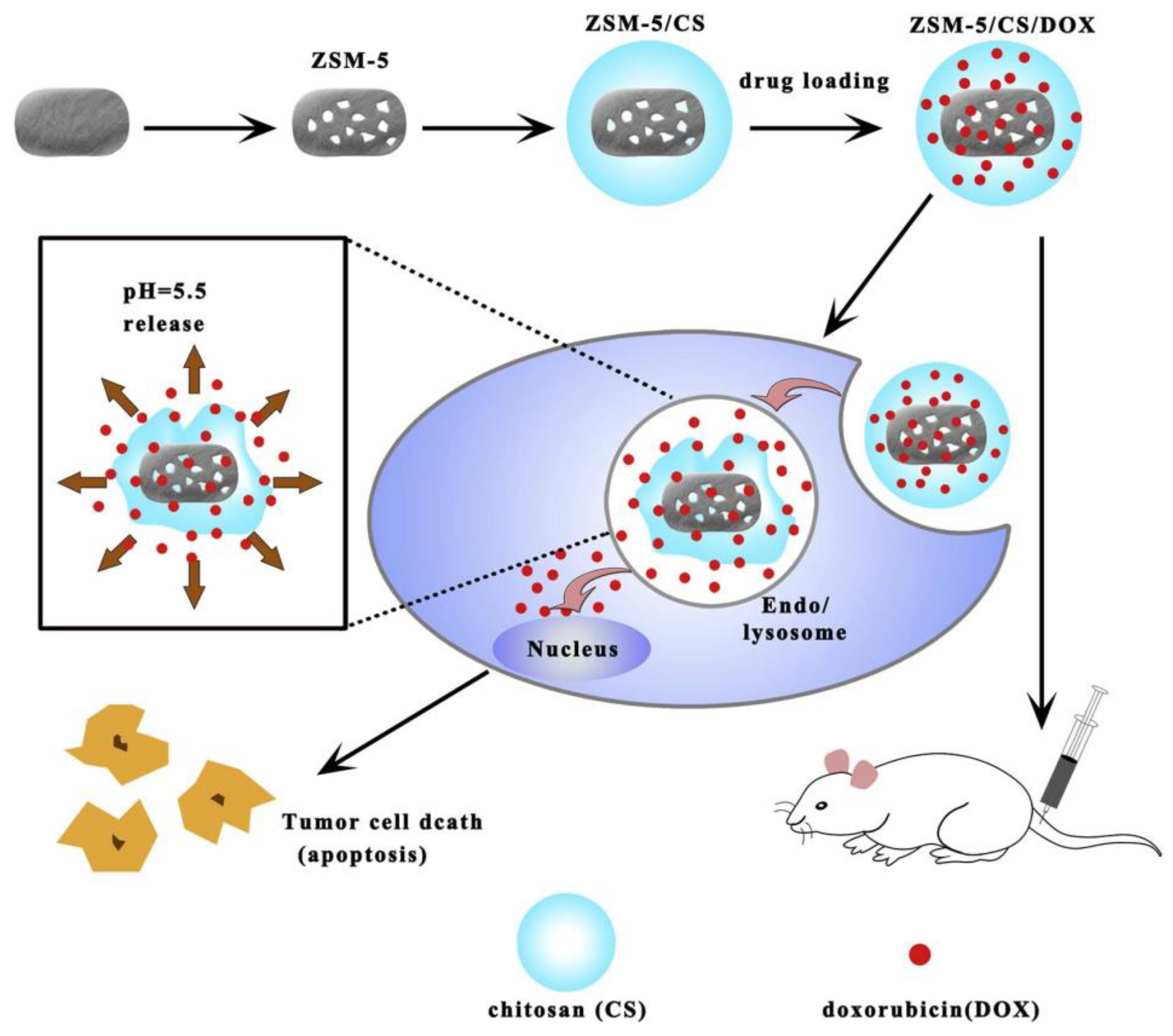
2.1.1. MFI-Type Zeolites
2.1.2. FAU-Type Zeolite
Zeolite Y
Zeolite X
2.1.3. LTA-Type Zeolite
2.1.4. Beta-Type Zeolite
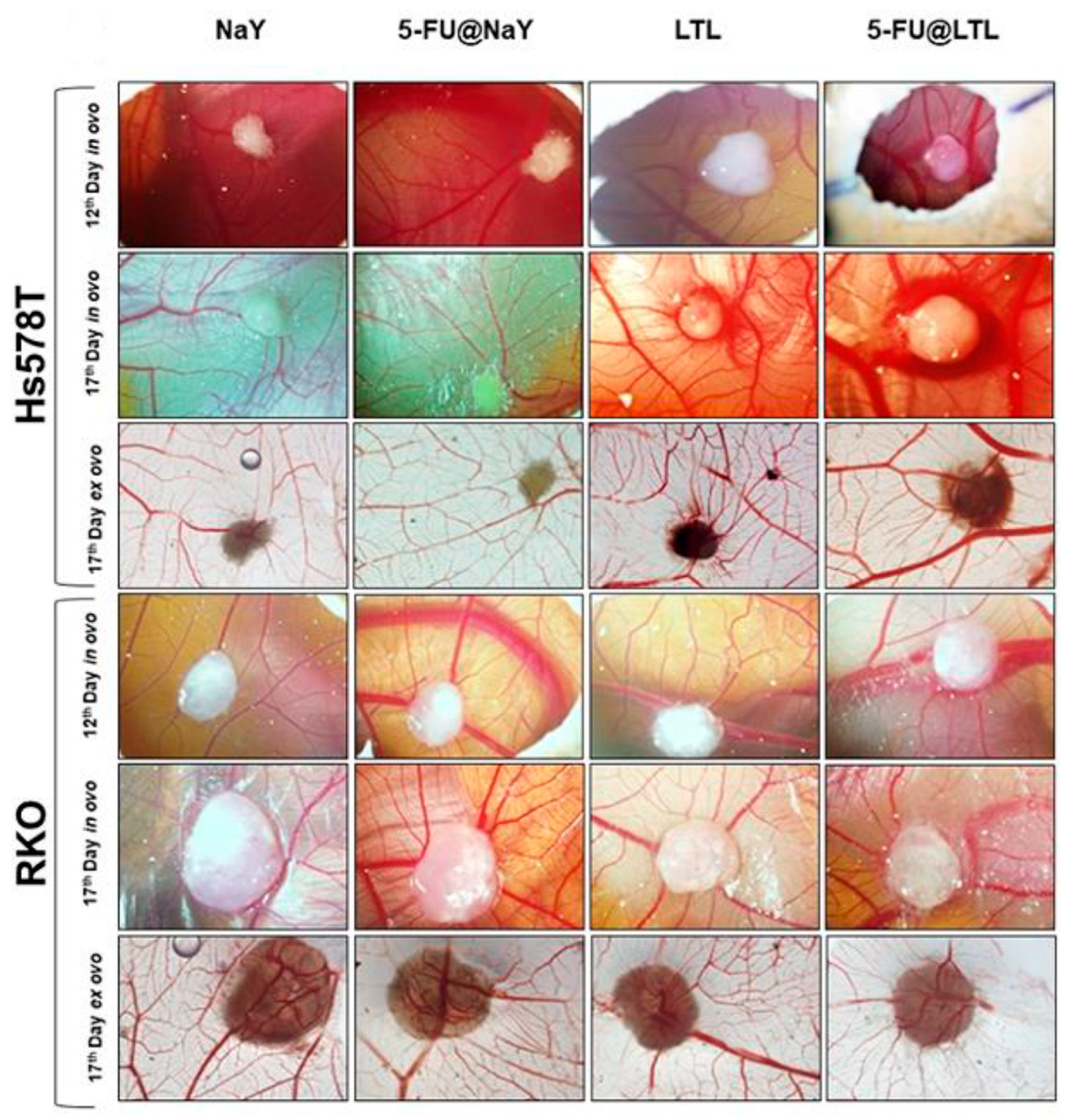
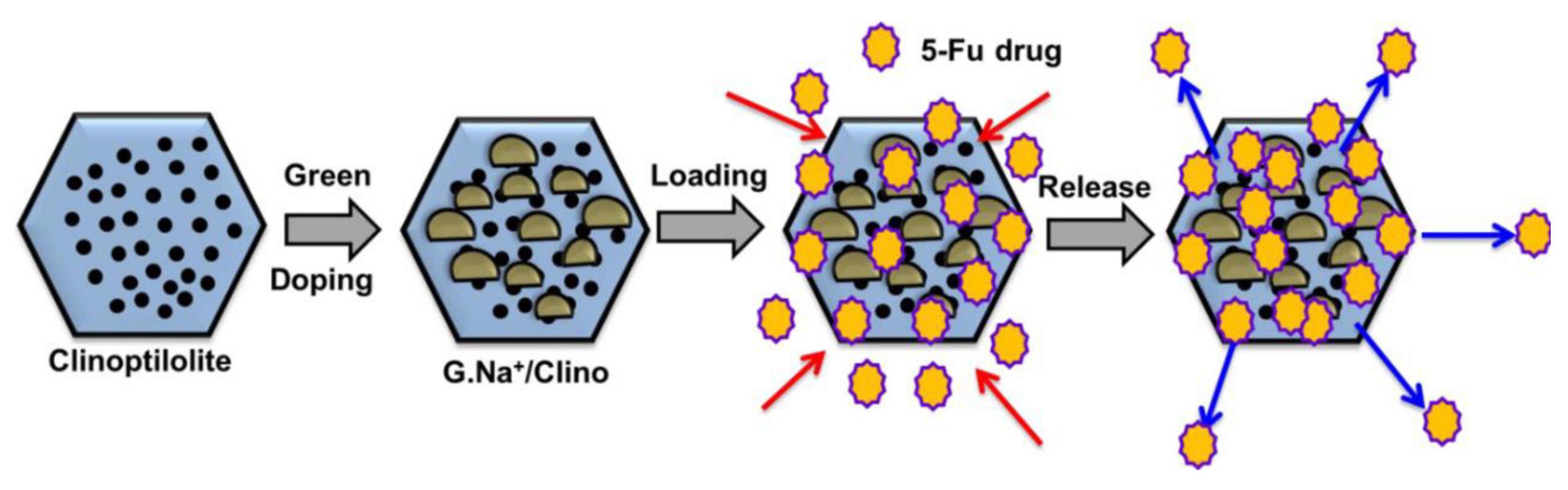
2.1.5. HEU-Type Zeolite
2.1.6. MOR-Type Zeolite
2.2. Ordered Mesoporous Silica Materials
2.2.1. MCM-41
2.2.2. SBA-15
2.2.3. MCM-48
2.2.4. SBA-16
3. Concluding Remarks: Challenges and Future Directions
3.1. Challenges
3.2. Advancements and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.L.-W.; Gao, Y.; Feng, Z.; Mooney, D.J.; Mitragotri, S. Designing drug delivery systems for cell therapy. Nat. Rev. Bioeng. 2024, 2, 944–959. [Google Scholar] [CrossRef]
- Yim, W.; Jin, Z.; Chang, Y.-C.; Brambila, C.; Creyer, M.N.; Ling, C.; He, T.; Li, Y.; Retout, M.; Penny, W.F.; et al. Polyphenol-stabilized coacervates for enzyme-triggered drug delivery. Nat. Commun. 2024, 15, 7295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Li, Z.; Wan, D.; Pan, J. Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma. Molecules 2024, 29, 4405. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P. Regulating Drug Release Performance of Acid-Triggered Dimeric Prodrug-Based Drug Self-Delivery System by Altering Its Aggregation Structure. Molecules 2024, 29, 3619. [Google Scholar] [CrossRef]
- Khoz, R.; Yazdian, F.; Pourmadadi, M.; Rahdar, A.; Fathi-karkan, S.; Pandey, S. Current trends in silica based drug delivery systems. Eur. J. Med. Chem. Rep. 2024, 12, 100206. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Miao, K.; Xia, X.; Zou, Y.; Shi, B. Small Scale, Big Impact: Nanotechnology-Enhanced Drug Delivery for Brain Diseases. Mol. Pharm. 2024, 21, 3777–3799. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Culsum, N.T.U.; Putri, R.M. Recent advances in the utilization of zeolite-based materials for controlled drug delivery. Results Chem. 2023, 5, 100910. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Chen, J.; Zhang, J.; Yang, Q.; Cui, J.; Shi, A.; Wu, J. Environmental stimulus-responsive mesoporous silica nanoparticles as anticancer drug delivery platforms. Colloids Surf. B 2024, 234, 113758. [Google Scholar] [CrossRef]
- Pandya, T.; Patel, S.; Kulkarni, M.; Singh, Y.R.; Khodakiya, A.; Bhattacharya, S.; Prajapati, B.G. Zeolite-based nanoparticles drug delivery systems in modern pharmaceutical research and environmental remediation. Heliyon 2024, 10, e36417. [Google Scholar] [CrossRef] [PubMed]
- Mallette, A.J.; Shilpa, K.; Rimer, J.D. The Current Understanding of Mechanistic Pathways in Zeolite Crystallization. Chem. Rev. 2024, 124, 3416–3493. [Google Scholar] [CrossRef] [PubMed]
- Yuan, E.-H.; Han, R.; Deng, J.-Y.; Zhou, W.; Zhou, A. Acceleration of Zeolite Crystallization: Current Status, Mechanisms, and Perspectives. ACS Appl. Mater. Interfaces 2024, 16, 29521–29546. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ling, Y.; Li, X.; Shi, X.; Tang, B. Preparation of Bi2O3 modified ZSM-5 with photo-deposition method and its application in pyridine base synthesis. Microporous Mesoporous Mater. 2023, 348, 112386. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, J.; Wang, F.; Fan, B.; Hao, W.; Li, R. Migration of Framework Aluminum and Tunable Acidity in Y Zeolite by Post-treatment. Ind. Eng. Chem. Res 2023, 62, 9951–9960. [Google Scholar] [CrossRef]
- Liaquat, I.; Munir, R.; Abbasi, N.A.; Sadia, B.; Muneer, A.; Younas, F.; Sardar, M.F.; Zahid, M.; Noreen, S. Exploring zeolite-based composites in adsorption and photocatalysis for toxic wastewater treatment: Preparation, mechanisms, and future perspectives. Environ. Pollut. 2024, 349, 123922. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Feng, J.; Sun, M. Recent advances of ordered mesoporous silica materials for solid-phase extraction. J. Chromatogr. A 2022, 1675, 463157. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Campos, A.; Martins, J.; Ambrósio, A.F.; Campos, E.J. Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses. ACS Biomater. Sci. Eng. 2020, 6, 6587–6597. [Google Scholar] [CrossRef]
- Xu, H.; Cui, Y.; Tian, Y.; Dou, M.; Sun, S.; Wang, J.; Wu, D. Nanoparticle-Based Drug Delivery Systems for Enhancing Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 1302–1322. [Google Scholar] [CrossRef]
- Rad, M.E.; Soylukan, C.; Kulabhusan, P.K.; Günaydın, B.N.; Yüce, M. Material and Design Toolkit for Drug Delivery: State of the Art, Trends, and Challenges. ACS Appl. Mater. Interfaces 2023, 15, 55201–55231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Quadros, M.; Momin, M. Mesoporous silica nanoparticles: Synthesis and multifaceted functionalization for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2023, 81, 104305. [Google Scholar] [CrossRef]
- Fea, A.M.; Vallino, V.; Cossu, M.; Marica, V.; Novarese, C.; Reibaldi, M.; Petrillo, F. Drug Delivery Systems for Glaucoma: A Narrative Review. Pharmaceuticals 2024, 17, 1163. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Darji, S.; Thompson, D.H. Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024. Pharmaceutics 2024, 16, 1154. [Google Scholar] [CrossRef]
- Dalena, F.; Dib, E.; Onida, B.; Ferrarelli, G.; Daturi, M.; Giordano, G.; Migliori, M.; Mintova, S. Evaluation of Zeolite Composites by IR and NMR Spectroscopy. Molecules 2024, 29, 4450. [Google Scholar] [CrossRef]
- Meng, F.; Wei, K.; Li, Y.; Zhang, L.; Xu, S.; Shi, X.; Tang, B. Efficient synthesis of multifunctional α-Fe2O3 QDs/TS-1 as an artificial nanozyme for simultaneous colorimetric detection and photodegradation of tetracyclines. Chem. Eng. J. 2024, 498, 155225. [Google Scholar] [CrossRef]
- He, Y.; Tang, S.; Yin, S.; Li, S. Research progress on green synthesis of various high-purity zeolites from natural material-kaolin. J. Clean. Prod. 2021, 306, 127248. [Google Scholar] [CrossRef]
- Meng, F.; Ling, Y.; Li, Y.; Guo, M.; Wei, K.; Zhang, M.; Yang, C.; Shi, X.; Tang, B. Photoassisted synthesis of high-crystallinity TS-1 with triblock copolymer F127 and its application for photodegradation of erythromycin. Chem. Eng. J. 2023, 473, 145200. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, E.; Hwang, S.; Liu, P.; Chen, J.G. Confining platinum clusters in indium-modified ZSM-5 zeolite to promote propane dehydrogenation. Nat. Commun. 2024, 15, 6529. [Google Scholar] [CrossRef]
- Kariminezhad, H.; Habibi, M.; Mirzababayi, N. Nanosized ZSM-5 will improve photodynamic therapy using Methylene blue. J. Photochem. Photobiol. B 2015, 148, 107–112. [Google Scholar] [CrossRef]
- Mofarrah, M.; Jafari-Gharabaghlou, D.; Dadashpour, M.; Zarghami, N. Fabricating ZSM-5 zeolite/ polycaprolactone nano-fibers co-loaded with dexamethasone and ascorbic acid: Potential application in osteogenic differentiation of human adipose-derived stem cells. J. Drug Deliv. Sci. Technol. 2023, 79, 103999. [Google Scholar] [CrossRef]
- Al-Sahlawi, F.; Al-Ani, I.; El-Tanani, M.; Farooq, H.A. Preparation and evaluation of biological activity of ZSM-5 nanoparticles loaded with gefitinib for the treatment of non-small cell lung carcinoma. Pharmacia 2024, 71, 1–12. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Y.-X.; Ke, Q.-F.; Zhang, C.-Q.; Guo, Y.-P. Controlled release of core-shell ZSM-5/chitosan ellipsoids loaded with pifithrin-α for enhanced osteoinductivity. Mater. Des. 2017, 122, 118–127. [Google Scholar] [CrossRef]
- Zhou, X.; Moussa, F.M.; Mankoci, S.; Ustriyana, P.; Zhang, N.; Abdelmagid, S.; Molenda, J.; Murphy, W.L.; Safadi, F.F.; Sahai, N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016, 39, 192–202. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Nowak, I.; Woźniak, N.; Chudzińska, J.; Feliczak-Guzik, A. Synthesis and Characterization of Hierarchical Zeolites Modified with Polysaccharides and Its Potential Role as a Platform for Drug Delivery. Pharmaceutics 2023, 15, 535. [Google Scholar] [CrossRef]
- Yang, F.; Wen, X.; Ke, Q.-F.; Xie, X.-T.; Guo, Y.-P. pH-responsive mesoporous ZSM-5 zeolites/chitosan core-shell nanodisks loaded with doxorubicin against osteosarcoma. Mater. Sci. Eng. C 2018, 85, 142–153. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Ding, J.; Yu, J.; Xv, X.; Pan, L.; Lin, P.; Wang, D. Ship-in-a-bottle growth of NaYF4: Yb3+/Tm3+ upconversion nanocrystals in desilicated ZSM-5 zeolite for drug release monitoring. Mater. Res. Bull. 2022, 154, 111926. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Mahmoudnezhad, A.; Bayrami, M.; Sanajou, D.; Tozihi, M.; Roshangar, L.; Pilehvar, Y.; Zarghami, N. Sustained delivery efficiency of curcumin through ZSM-5 nanozeolites/electrospun nanofibers for counteracting senescence of human adipose-derived stem cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102902. [Google Scholar] [CrossRef]
- Basumatary, A.K.; Pant, K.; Priyam Goswami, K.; Raja, V.K.; Pugazhenthi, G. Chromium ion separation from aqueous solution using low-cost FAU zeolite composite ceramic membrane. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Li, S.; Zhang, Y.; Liu, M.; He, B.; Mei, Y.; Zu, Y. Maximizing the utilization of Calcium species in the supercages of CaNa-FAU zeolite for efficient CO2 capture. Chem. Eng. J. 2024, 481, 148661. [Google Scholar] [CrossRef]
- Ranković, M.; Jevremović, A.; Janošević Ležaić, A.; Arsenijević, A.; Rupar, J.; Dobričić, V.; Nedić Vasiljević, B.; Gavrilov, N.; Bajuk-Bogdanović, D.; Milojević-Rakić, M. Can Zeolite-Supporting Acridines Boost Their Anticancer Performance? J. Funct. Biomater. 2023, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Xu, N.; Liu, Q.; Wang, B.; Fan, L.; Zhang, L.; Zhou, R. FAU zeolite membranes synthesized using nanoseeds—Separation mechanism and optimization for the pervaporation dehydration of various organic solvents. J. Membr. Sci. 2024, 696, 122522. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, H.; Liu, X.; Li, R.; Xia, T.; Ma, Y.; Wang, Y.; Chen, K.; Wang, J.; Zeng, P.; et al. The isomorphous substitution of Si(4Al) with P in FAU zeolite and its stabilization effect. Chem. Eng. J. 2024, 486, 150422. [Google Scholar] [CrossRef]
- Joshi, N.; Sivachandiran, L. CO2 adsorption and storage over FAU-zeolites for environmental remediation: Understanding the role of oxygen vacancy and promotor loading. J. Environ. Chem. Eng. 2024, 12, 112828. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, X.; Zhang, Y.; Wang, X.; Li, J.; Yang, J. Grafting of functional organic ligand on zeolite Y for efficient CH4/N2 separation. Sep. Purif. Technol. 2025, 354, 128719. [Google Scholar] [CrossRef]
- Lutz, W. Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef]
- Sakshi, S.; Dey, S.; Chowdhury, S.; Ray, S. Characterization of a Zeolite-Y-Encapsulated Zn(II)Salmphen Complex with Targeted Anticancer Property. ACS Appl. Mater. Interfaces 2023, 15, 55518–55532. [Google Scholar] [CrossRef]
- Bello, M.O.; Abdus-Salam, N.; Adekola, F.A.; Oyewumi-Musa, R.T.; Pal, U. Synthesis and characterization of zeolite Y from agricultural and municipal wastes: A waste management approach. Waste Manag. Bull. 2024, 2, 122–129. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.-W.; Zhang, C.; Jiao, W.-Z.; Liu, Y.-Z. Synthesis of zeolite X from fly ash in an impinging stream reactor and its mechanisms. Chem. Eng. Sci. 2024, 294, 120123. [Google Scholar] [CrossRef]
- Souza, I.M.S.; Borrego-Sánchez, A.; Sainz-Díaz, C.I.; Viseras, C.; Pergher, S.B.C. Study of Faujasite zeolite as a modified delivery carrier for isoniazid. Mater. Sci. Eng. C 2021, 118, 111365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yang, Z.; Shi, H.; Turki Jalil, A.; Mahmood Saleh, M.; Mi, W. Potentiation of Curcumin-loaded zeolite Y nanoparticles/PCL-gelatin electrospun nanofibers for postsurgical glioblastoma treatment. J. Drug Deliv. Sci. Technol. 2023, 80, 104105. [Google Scholar] [CrossRef]
- Bertão, A.R.; Ivasiv, V.; Almeida-Aguiar, C.; Correia, P.R.; Fonseca, A.M.; Bañobre-López, M.; Baltazar, F.; Neves, I.C. Preliminary evaluation of zeolite-based platforms as potential dual drug delivery systems against microbial infections in the tumor microenvironment. Microporous Mesoporous Mater. 2024, 364, 112871. [Google Scholar] [CrossRef]
- Vilaça, N.; Bertão, A.R.; Prasetyanto, E.A.; Granja, S.; Costa, M.; Fernandes, R.; Figueiredo, F.; Fonseca, A.M.; De Cola, L.; Baltazar, F.; et al. Surface functionalization of zeolite-based drug delivery systems enhances their antitumoral activity in vivo. Mater. Sci. Eng. C 2021, 120, 111721. [Google Scholar] [CrossRef]
- Jakubowski, M.; Kucinska, M.; Ratajczak, M.; Pokora, M.; Murias, M.; Voelkel, A.; Sandomierski, M. Zinc forms of faujasite zeolites as a drug delivery system for 6-mercaptopurine. Microporous Mesoporous Mater. 2022, 343, 112194. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Zhang, Y.; Gens, F.; Echalard, A.; Ladam, G.; Hochart, J.; Janicot, T.; Mofaddel, N.; Louis, B. Deciphering the role of faujasite-type zeolites as a cation delivery platform to sustain the functions of MC3T3-E1 pre-osteoblastic cells. Mater. Adv. 2022, 3, 8616–8628. [Google Scholar] [CrossRef]
- Menard, T.; Zarbaliyev, B.; Echalard, A.; Bullier-Marchandin, E.; Gens, F.; Ladam, G.; Guliyeva, N.; Louis, B.; Lutzweiler, G. Synthesis and characterization of carboxylic-and amine-grafted FAU zeolites as inorganic fillers to design biocompatible composites. Microporous Mesoporous Mater. 2024, 363, 112834. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tanaka, S.; Matsuda, N.; Nakayama, N.; Hashimoto, T.; Ishihara, A. Estimation of catalytic cracking of vacuum gas oil by ZSM-5- and β-zeolite-containing two-layered and novel three-layered hierarchical catalysts using Curie point pyrolyzer. J. Anal. Appl. Pyrolysis 2024, 182, 106621. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wang, H.; Liu, C.; Yin, X.; Bai, H.; Xu, M.; Li, Z.; Wang, Y.; Zhu, X.; et al. Hierarchical zeolite coatings featuring a spatial gradient architecture for sequentially-controlled bisphosphonate release in the modulation of osteogenic–osteoclastic balance. Microporous Mesoporous Mater. 2024, 370, 113060. [Google Scholar] [CrossRef]
- Marhoon, A.A.; Hasbullah, S.A.; Asikin-Mijan, N.; Mokhtar, W.N.A.W. Hydrothermal synthesis of high-purity zeolite X from coal fly ash for heavy metal removal: Kinetic and isotherm analysis. Adv. Powder Technol. 2023, 34, 104242. [Google Scholar] [CrossRef]
- Najafi, A.M.; Khorasheh, F.; Soltanali, S.; Ghassabzadeh, H. Equilibrium and Kinetic Insights into the Comprehensive Investigation of CO2, CH4, and N2 Adsorption on Cation-Exchanged X and Y Faujasite Zeolites. Langmuir 2023, 39, 15535–15546. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, L.; Liu, H.; Tian, G.; Peng, G.; Zhuo, L.; Wang, Z. Zeolite-X synthesized from halloysite nanotubes and its application in CO2 capture. J. Taiwan Inst. Chem. Eng. 2022, 133, 104281. [Google Scholar] [CrossRef]
- Han, L.; Wang, X.; Wu, B.; Zhu, S.; Wang, J.; Zhang, Y. In-situ synthesis of zeolite X in foam geopolymer as a CO2 adsorbent. J. Clean. Prod. 2022, 372, 133591. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, X.; Lao, W.; Wang, H.; Chen, S.; Liu, C.; Chen, Z.; Bai, Y.; Zhang, H.; Zhan, X.; et al. Enhancement of the solubility and oral bioavailability of altrenogest through complexation with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2024, 194, 106691. [Google Scholar] [CrossRef]
- Ran Woo, M.; Bak, Y.-W.; Cheon, S.; Suk Kim, J.; Hun Ji, S.; Park, S.; Woo, S.; Oh Kim, J.; Giu Jin, S.; Choi, H.-G. Modification of microenvironmental pH of nanoparticles for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib. Int. J. Pharm. 2024, 659, 124179. [Google Scholar] [CrossRef]
- Domke, A.; Fischer, M.; Jakubowski, M.; Pacholak, A.; Ratajczak, M.; Voelkel, A.; Sandomierski, M. Experimental and computational study on the Ca2+, Mg2+, Zn2+ and Sr2+ exchanged zeolites as a drug delivery system for fluoroquinolone antibiotic–Ciprofloxacin. J. Drug Deliv. Sci. Technol. 2024, 99, 105997. [Google Scholar] [CrossRef]
- Kontogiannidou, E.; Karavasili, C.; Kouskoura, M.G.; Filippousi, M.; Van Tendeloo, G.; Andreadis, I.I.; Eleftheriadis, G.K.; Kontopoulou, I.; Markopoulou, C.K.; Bouropoulos, N.; et al. In vitro and ex vivo assessment of microporous Faujasite zeolite (NaX-FAU) as a carrier for the oral delivery of danazol. J. Drug Deliv. Sci. Technol. 2019, 51, 177–184. [Google Scholar] [CrossRef]
- Binay, M.I.; Kart, D.; Akata, B. Investigating antimicrobial behavior of thymol/Zn encapsulated hierarchically structured zeolite and thymol release kinetics. Microporous Mesoporous Mater. 2024, 376, 113188. [Google Scholar] [CrossRef]
- Olejnik, A.; Panek, R.; Madej, J.; Franus, W.; Goscianska, J. Low-cost zeolitic carriers for delivery of hydroxychloroquine immunomodulatory agent with antiviral activity. Microporous Mesoporous Mater. 2022, 346, 112315. [Google Scholar] [CrossRef]
- Abasian, P.; Radmansouri, M.; Habibi Jouybari, M.; Ghasemi, M.V.; Mohammadi, A.; Irani, M.; Jazi, F.S. Incorporation of magnetic NaX zeolite/DOX into the PLA/chitosan nanofibers for sustained release of doxorubicin against carcinoma cells death in vitro. Int. J. Biol. Macromol. 2019, 121, 398–406. [Google Scholar] [CrossRef]
- Xu, H.; Lu, P.; Fu, G.; Miao, J.; Zhao, J.; Ding, R.; Zhao, L.; Lang, Z.; Yang, X.; Valtchev, V. The strong SDA/framework interactions and acidity study of high-silica LTA-type zeolites. Microporous Mesoporous Mater. 2023, 360, 112724. [Google Scholar] [CrossRef]
- Wruck, K.; Millar, G.J.; Wang, T. Transformation of heulandite type natural zeolites into synthetic zeolite LTA. Environ. Technol. Innov. 2021, 21, 101371. [Google Scholar] [CrossRef]
- Dumbre, D.; Ayoub, E.; Dawaymeh, F.; Abbas, Y.; Elmhamdi, A.; Matouk, Z.; Alazzam, A.; Khaleel, M.; Alamoodi, N. Durable superhydrophobic LTA-zeolite coating on PDMS surface with excellent self-cleaning property. Eur. Polym. J. 2024, 218, 113365. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Marinangeli, L.; Tangari, A.C.; Rosatelli, G.; Ciulla, M.; di Profio, P. Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite. Molecules 2024, 29, 4396. [Google Scholar] [CrossRef]
- Bahraminia, S.; Anbia, M. Carbon dioxide methanation using Ni catalysts supported on low-cost Lynde Type A zeolite synthesized from pretreated Iranian coal gangue. Int. J. Hydrogen Energy 2024, 83, 842–855. [Google Scholar] [CrossRef]
- Si, J.; Guo, R.; Zhang, Y.; Ning, W.; Sun, Y.; Li, W.; Miao, S. Synthesis of Linde A-type zeolite from ball clay with incorporated ruthenium and application in hydrogenation catalysis. Appl. Clay Sci. 2023, 239, 106897. [Google Scholar] [CrossRef]
- Xiang, L.; Guo, Z.; Yang, L.; Qin, Y.; Chen, Z.; Wang, T.; Sun, W.; Wang, C. Modification of crystal growth of NaA zeolite with steric hindrance agents for removing ammonium ion from aqueous solution. J. Ind. Eng. Chem. 2024, 132, 326–334. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K.; Góra-Marek, K. LTA zeolites as catalysts for transesterification of glycerol with dimethyl carbonate. Fuel 2024, 362, 130757. [Google Scholar] [CrossRef]
- Sadeghi, P.; Myers, M.B.; Pareek, V.; Arami-Niya, A. Temperature-regulated gas adsorption on LTA zeolites: Observation of sorption isotherms for methane, hydrogen, nitrogen and carbon dioxide. Appl. Surf. Sci. 2024, 678, 161037. [Google Scholar] [CrossRef]
- Julbe, A.; Drobek, M. Zeolite A Type. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Antúnez-García, J.; Galván, D.H.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Shelyapina, M.G.; Fuentes-Moyado, S. The effect of chemical composition on the properties of LTA zeolite: A theoretical study. Comput. Mater. Sci. 2021, 196, 110557. [Google Scholar] [CrossRef]
- Amorim, R.; Vilaça, N.; Martinho, O.; Reis, R.M.; Sardo, M.; Rocha, J.; Fonseca, A.M.; Baltazar, F.; Neves, I.C. Zeolite Structures Loading with an Anticancer Compound As Drug Delivery Systems. J. Phys. Chem. C 2012, 116, 25642–25650. [Google Scholar] [CrossRef]
- Altoom, N.; Adlii, A.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Al-Otaibi, F.S.; Abukhadra, M.R. Synthesis and characterization of β-cyclodextrin functionalized zeolite-A as biocompatible carrier for Levofloxacin drug; loading, release, cytotoxicity, and anti-inflammatory studies. J. Solid State Chem. 2022, 312, 123280. [Google Scholar] [CrossRef]
- Nakai, M.; Miyake, K.; Inoue, R.; Ono, K.; Al Jabri, H.; Hirota, Y.; Uchida, Y.; Miyamoto, M.; Nishiyama, N. Synthesis of high silica *BEA type ferrisilicate (Fe-Beta) by dry gel conversion method using dealuminated zeolites and its catalytic performance on acetone to olefins (ATO) reaction. Microporous Mesoporous Mater. 2019, 273, 189–195. [Google Scholar] [CrossRef]
- Hould, N.D.; Foster, A.; Lobo, R.F. Zeolite beta mechanisms of nucleation and growth. Microporous Mesoporous Mater. 2011, 142, 104–115. [Google Scholar] [CrossRef]
- Souza, I.M.S.; Borrego-Sánchez, A.; Rigoti, E.; Sainz-Díaz, C.I.; Viseras, C.; Pergher, S.B.C. Experimental and molecular modelling study of beta zeolite as drug delivery system. Microporous Mesoporous Mater. 2021, 321, 111152. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Trendafilova, I.; Trif, L.; Mihály, J.; Makk, J.; Mavrodinova, V. Bicomponent drug formulation for simultaneous release of Ag and sulfadiazine supported on nanosized zeolite Beta. Nano-Struct. Nano-Objects 2020, 24, 100562. [Google Scholar] [CrossRef]
- Karavasili, C.; Kokove, L.; Kontopoulou, I.; Eleftheriadis, G.K.; Bouropoulos, N.; Fatouros, D.G. Dissolution enhancement of the poorly soluble drug nifedipine by co-spray drying with microporous zeolite beta. J. Drug Deliv. Sci. Technol. 2016, 35, 91–97. [Google Scholar] [CrossRef]
- Filippousi, M.; Turner, S.; Katsikini, M.; Pinakidou, F.; Zamboulis, D.; Pavlidou, E.; Van Tendeloo, G. Direct observation and structural characterization of natural and metal ion-exchanged HEU-type zeolites. Microporous Mesoporous Mater. 2015, 210, 185–193. [Google Scholar] [CrossRef]
- Sayed, M.A.; El-Zeiny, H.M.; Khim, J.S.; Ajarem, J.S.; Allam, A.A.; Abukhadra, M.R. Insight into the Loading Properties of Na+ Green-Functionalized Clinoptilolite as a Potential Carrier for the 5-Fluorouracil Drug, its Release Kinetics, and Cytotoxicity. ACS Omega 2022, 7, 6991–7001. [Google Scholar] [CrossRef]
- Wanyonyi, F.S.; Orata, F.; Mutua, G.K.; Odey, M.O.; Zamisa, S.; Ogbodo, S.E.; Maingi, F.; Pembere, A. Application of South African heulandite (HEU) zeolite for the adsorption and removal of Pb2+ and Cd2+ ions from aqueous water solution: Experimental and computational study. Heliyon 2024, 10, e34657. [Google Scholar] [CrossRef]
- Othman, S.I.; Allam, A.A.; Al Fassam, H.; Abu-Taweel, G.M.; Altoom, N.; Abukhadra, M.R. Sonoco Green Decoration of Clinoptilolite with MgO Nanoparticles as a Potential Carrier for 5-Fluorouracil Drug: Loading Behavior, Release Profile, and Cytotoxicity. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4608–4622. [Google Scholar] [CrossRef]
- Kukobat, R.; Škrbić, R.; Vallejos-Burgos, F.; Mercadelli, E.; Gardini, D.; Silvestroni, L.; Zanelli, C.; Esposito, L.; Stević, D.; Atlagić, S.G.; et al. Enhanced dissolution of anticancer drug letrozole from mesoporous zeolite clinoptilolite. J. Colloid Interface Sci. 2024, 653, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Liu, W.; Yu, Q.; Liu, Z.; Xu, S.; An, J.; Li, X.; Zhu, X. An efficient synthesis strategy for MOR zeolite. Microporous Mesoporous Mater. 2022, 346, 112282. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Shelyapina, M.G.; Fuentes-Moyado, S. Investigating patterns in aluminum distribution and stability in mordenite zeolite structure: Analysis and theoretical conclusions from existing experimental data. J. Solid State Chem. 2024, 331, 124548. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Darmokoesoemo, H.; Adu, A.A.; Lawa, Y.; Naat, J.; Riwu, A.A.P.; Bui, M.F.; Wila, E.C.; Fahirah, M.A.; Budiastant, T.A.; et al. Study of mordenite natural zeolite type modified by Cu(II) cation as an oral safe drug carrier for ibuprofen and meloxicam. J. Mol. Liq. 2022, 352, 118734. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Riwu, M.; Darmokoesoemo, H.; Setyawati, H.; Naat, J.; Ayu Widyaningrum, B.; Osagie Aigbe, U.; Eghonghon Ukhurebor, K.; Birundu Onyancha, R.; et al. Synthesis of Zinc(II)-natural zeolite mordenite type as a drug carrier for ibuprofen: Drug release kinetic modeling and cytotoxicity study. Results Chem. 2022, 4, 100578. [Google Scholar] [CrossRef]
- Beurer, A.-K.; Dieterich, S.; Solodenko, H.; Kaya, E.; Merdanoǧlu, N.; Schmitz, G.; Traa, Y.; Bruckner, J.R. Comparative study of lattice parameter and pore size of ordered mesoporous silica materials using physisorption, SAXS measurements and transmission electron microscopy. Microporous Mesoporous Mater. 2023, 354, 112508. [Google Scholar] [CrossRef]
- Lu, T.; Yan, W.; Feng, G.; Luo, X.; Hu, Y.; Guo, J.; Yu, Z.; Zhao, Z.; Ding, S. Singlet oxygen-promoted one-pot synthesis of highly ordered mesoporous silica materials via the radical route. Green Chem. 2022, 24, 4778–4782. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Chen, F.; Qian, S.; Hu, X.; Zhang, B.; Liu, Q. Research progress in MCM family: Focus on the tumor treatment resistance. Biomed. Pharmacother. 2024, 173, 116408. [Google Scholar] [CrossRef]
- Molaei, S.; Ghadermazi, M. Comparative study of two mesoporous magnetic and MCM-41 supported Cu complex: High-performance heterogeneous catalysts for aqueous synthesis of tetrazole. Inorg. Chem. Commun. 2024, 169, 113022. [Google Scholar] [CrossRef]
- Aquino, G.D.; Moreno, M.S.; Benedictto, G.P.; Pereyra, A.M. Industrially compatible synthesis of MCM-41 with spatial organization at the macro-mesoscale. Microporous Mesoporous Mater. 2024, 377, 113225. [Google Scholar] [CrossRef]
- Pires, I.C.B.; Shuchi, S.I.; Tostes, B.d.V.A.; Santos, D.K.D.d.N.; Burnett, W.L.; Leonce, B.C.; Harvey, O.R.; Coffer, J.L.; de Sousa Filho, I.A.; de Athayde-Filho, P.F.; et al. Theranostics Using MCM-41-Based Mesoporous Silica Nanoparticles: Integrating Magnetic Resonance Imaging and Novel Chemotherapy for Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 8097. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Tzankov, B.; Voycheva, C.; Spassova, I.; Kovacheva, D.; Tzankov, S.; Aluani, D.; Tzankova, V.; Lambov, N. Mesoporous silica MCM-41 and HMS as advanced drug delivery carriers for bicalutamide. J. Drug Deliv. Sci. Technol. 2021, 62, 102340. [Google Scholar] [CrossRef]
- Galhano, J.; Marcelo, G.A.; Duarte, M.P.; Oliveira, E. Ofloxacin@Doxorubicin-Epirubicin functionalized MCM-41 mesoporous silica–based nanocarriers as synergistic drug delivery tools for cancer related bacterial infections. Bioorg. Chem. 2022, 118, 105470. [Google Scholar] [CrossRef]
- Septian Dwitya, S.; Lin, K.-S.; Weng, M.-T.; Vukile Mdlovu, N.; Yang, M.-T.; Wu, C.-M. Synthesis and characterization of oleic acid-stabilized cobalt ferrite @MCM-41/nanocomposites for pH-responsive drug delivery. J. Ind. Eng. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Foulady-Dehaghi, R.; Sohrabnezhad, S.; Hadavi, M. Drug delivery with solvent-free synthesized polyimide-COF/amino-functionalized MCM-41 nanohybrid. J. Drug Deliv. Sci. Technol. 2023, 81, 104283. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Pourzadi, N.; Darvishi, S.M.R. tert-Butylamine functionalized MCM-41 mesoporous nanoparticles as drug carriers for the controlled release of cyclophosphamide anticancer drug. Surf. Interfaces 2021, 22, 100842. [Google Scholar] [CrossRef]
- Iga, G.D.; Vieira, J.L.; Santos, É.A.; Albuquerque, J.S.; Urquieta-González, E.A.; Bueno, J.M.C.; Gallo, J.M.R. Should we still be using HCl in the synthesis of SBA-15 and Al-SBA-15? Microporous Mesoporous Mater. 2024, 378, 113222. [Google Scholar] [CrossRef]
- Luján Ferreira, M.; Pedernera, M.; Esperanza Adrover, M. Enhanced CO2 capture by functionalization of SBA-15 with APTES and l-lysine. Chem. Eng. J. 2024, 498, 155431. [Google Scholar] [CrossRef]
- Ulagesan, S.; Santhamoorthy, M.; Tuong Vy Phan, T.; Alagumalai, K.; Thirupathi, K.; Kim, S.-C.; Nam, T.-J.; Choi, Y.-H. Mesoporous silica (SBA-15) with enriched amidoxime functionalities for pH-controlled anticancer drug delivery. Inorg. Chem. Commun. 2022, 146, 110132. [Google Scholar] [CrossRef]
- Albayati, T.M.; Salih, I.K.; Alazzawi, H.F. Synthesis and characterization of a modified surface of SBA-15 mesoporous silica for a chloramphenicol drug delivery system. Heliyon 2019, 5, e02539. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Prokopowicz, M. Amino-modified mesoporous silica SBA-15 as bifunctional drug delivery system for cefazolin: Release profile and mineralization potential. Mater. Lett. 2018, 227, 136–140. [Google Scholar] [CrossRef]
- Pithadia, D.; Patel, A. Mono lacunary silicotungstate anchored to nano-porous MCM-48: Synthesis, characterization and esterification of bioplatform molecules to fuel additives. J. Ind. Eng. Chem. 2023, 119, 236–251. [Google Scholar] [CrossRef]
- Güçbilmez, Y.; Yavuz, Y.; Çalış, İ.; Yargıç, A.Ş.; Koparal, A.S. Low temperature synthesis of MCM-48 and its adsorbent capacity for the removal of basic red 29 dye from model solutions. Heliyon 2023, 9, e15659. [Google Scholar] [CrossRef]
- Kumar, D.; Schumacher, K.; du Fresne von Hohenesche, C.; Grün, M.; Unger, K.K. MCM-41, MCM-48 and related mesoporous adsorbents: Their synthesis and characterisation. Colloids Surf. A 2001, 187–188, 109–116. [Google Scholar] [CrossRef]
- Dasgupta, D.; Das, M.; Thakore, S.; Patel, A.; Kumar, S.; Seshadri, S. Development of a controlled sustainable anticancer drug delivery nanosystem comprising doxorubicin and functionalized MCM-48. J. Drug Deliv. Sci. Technol. 2022, 72, 103419. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Ibarra-Vallejo, E.; García-Uriostegui, L.; Ortega, A. Polyacrylamide-coated MCM-48 mesoporous silica spheres: Synthesis, characterization and drug delivery study. J. Porous Mater. 2018, 25, 649–656. [Google Scholar] [CrossRef]
- Zeleňák, V.; Halamová, D.; Almáši, M.; Žid, L.; Zeleňáková, A.; Kapusta, O. Ordered cubic nanoporous silica support MCM-48 for delivery of poorly soluble drug indomethacin. Appl. Surf. Sci. 2018, 443, 525–534. [Google Scholar] [CrossRef]
- Pajchel, L.; Kolodziejski, W. Synthesis and characterization of MCM-48/hydroxyapatite composites for drug delivery: Ibuprofen incorporation, location and release studies. Mater. Sci. Eng. C 2018, 91, 734–742. [Google Scholar] [CrossRef]
- El-Shafey, S.S.; Sayed Ahmed, S.A.; Aboelenin, R.M.; Fathy, N.A. Synthesis of SBA-16 mesoporous silica from rice husk ash for removal of Rhodamine B cationic dye: Effect of hydrothermal treatment time. Desalination Water Treat. 2024, 317, 100179. [Google Scholar] [CrossRef]
- Rivera-Muñoz, E.M.; Huirache-Acuña, R. Sol Gel-Derived SBA-16 Mesoporous Material. Int. J. Mol. Sci. 2010, 11, 3069–3086. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Gonzalez, G.; Sagarzazu, A.; Cordova, A.; Gomes, M.E.; Salas, J.; Contreras, L.; Noris-Suarez, K.; Lascano, L. Comparative study of two silica mesoporous materials (SBA-16 and SBA-15) modified with a hydroxyapatite layer for clindamycin controlled delivery. Microporous Mesoporous Mater. 2018, 256, 251–265. [Google Scholar] [CrossRef]
- Prihatiningsih, M.C.; Pratama, C.; Kundari, N.A.; Megasari, K.; Ariyanti, D.; Saputra, A.; Kusuma, H.D.; Astuti, P. Rifampicin adsorption and release study using Santa Barbara amorphous-16 modified Al (SBA-16-Al) for a drug delivery system. RSC Adv. 2024, 14, 7371–7382. [Google Scholar] [CrossRef]
- Fekri, M.H.; Soleymani, S.; Mehr, M.R.; Akbari-adergani, B. Synthesis and characterization of mesoporous ZnO/SBA-16 nanocomposite: Its efficiency as drug delivery system. J. Non-Cryst. Solids 2022, 591, 121512. [Google Scholar] [CrossRef]
- Karnopp, J.; Cardoso, T.; Gonçalves, D.; Carollo, A.; Castro, G.; Duarte, A.; Martines, M. Preparation of the Rutin-SBA-16 Drug Delivery System. J. Biomater. Nanobiotechnol. 2020, 11, 1–13. [Google Scholar] [CrossRef]

| Codes | Connection Mode | Channels | Tiling Arrangement | Idealized Cell Data |
|---|---|---|---|---|
| MFI |  | 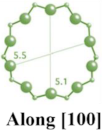  | 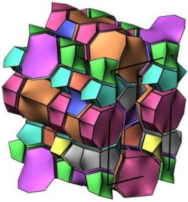 | orthorhombic, Pnma, a = 20.1 Å, b = 19.7 Å, c = 13.1 Å |
| FAU | 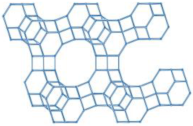 | 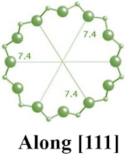 | 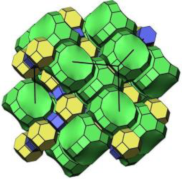 | cubic, Fdm, a = 24.3 Å |
| LTA | 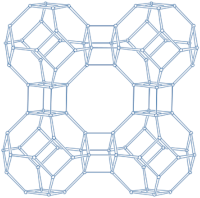 | 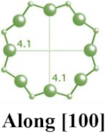 | 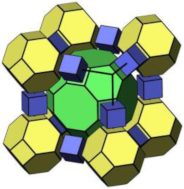 | cubic, Pmm, a = 11.9 Å |
| HEU |  |    | 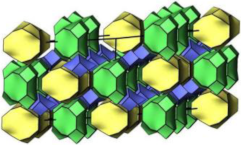 | monoclinic, C2/m, a = 17.5 Å, b = 17.6 Å, c = 7.4 Å, β = 116.1° |
| MOR | 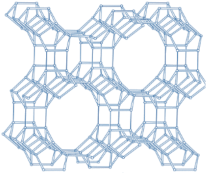 | 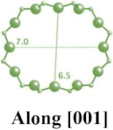  | 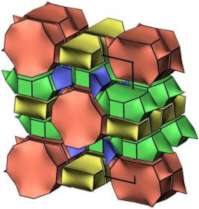 | orthorhombic, Cmcm, a = 18.3 Å, b = 20.5 Å, c = 7.5 Å |
| CHA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wu, J.; Jiang, Z.; Zhang, X.; Wang, Z.; Meng, F.; Liu, Z.; Zhang, T. Application of Ordered Porous Silica Materials in Drug Delivery: A Review. Molecules 2024, 29, 5713. https://doi.org/10.3390/molecules29235713
Liu W, Wu J, Jiang Z, Zhang X, Wang Z, Meng F, Liu Z, Zhang T. Application of Ordered Porous Silica Materials in Drug Delivery: A Review. Molecules. 2024; 29(23):5713. https://doi.org/10.3390/molecules29235713
Chicago/Turabian StyleLiu, Wenwen, Junlin Wu, Zehao Jiang, Xinyu Zhang, Zhenxiang Wang, Fanjun Meng, Zidi Liu, and Teng Zhang. 2024. "Application of Ordered Porous Silica Materials in Drug Delivery: A Review" Molecules 29, no. 23: 5713. https://doi.org/10.3390/molecules29235713
APA StyleLiu, W., Wu, J., Jiang, Z., Zhang, X., Wang, Z., Meng, F., Liu, Z., & Zhang, T. (2024). Application of Ordered Porous Silica Materials in Drug Delivery: A Review. Molecules, 29(23), 5713. https://doi.org/10.3390/molecules29235713





