Composition, Anti-Diabetic, and Antioxidant Potential of Raphanus sativus Leaves
Abstract
1. Introduction
2. Results
2.1. Proximate Composition of Radish Leaves
2.2. Bioactive Compound Content and Antioxidant Activity of Radish Leaves
2.3. Compsition of Phenolic Compounds of Radish Leaves
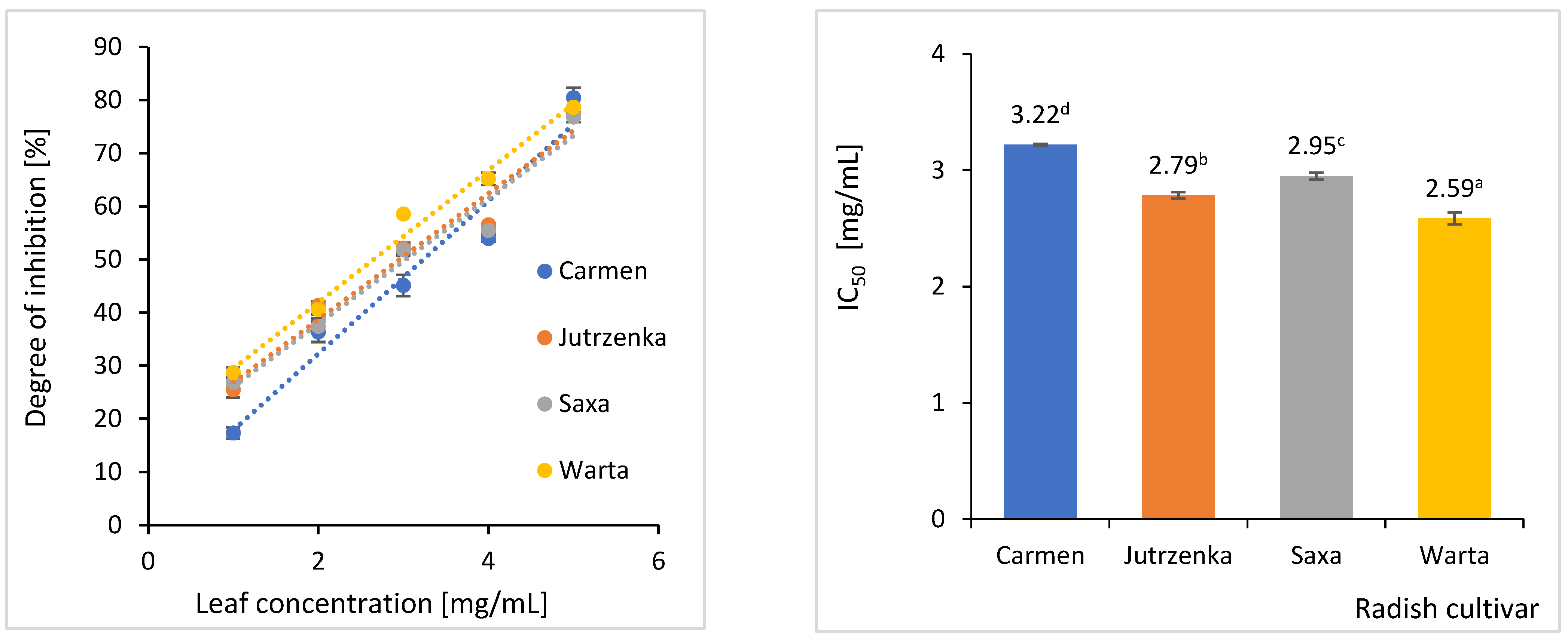
2.4. Effect of Radish Leaves on Starch Hydrolysis
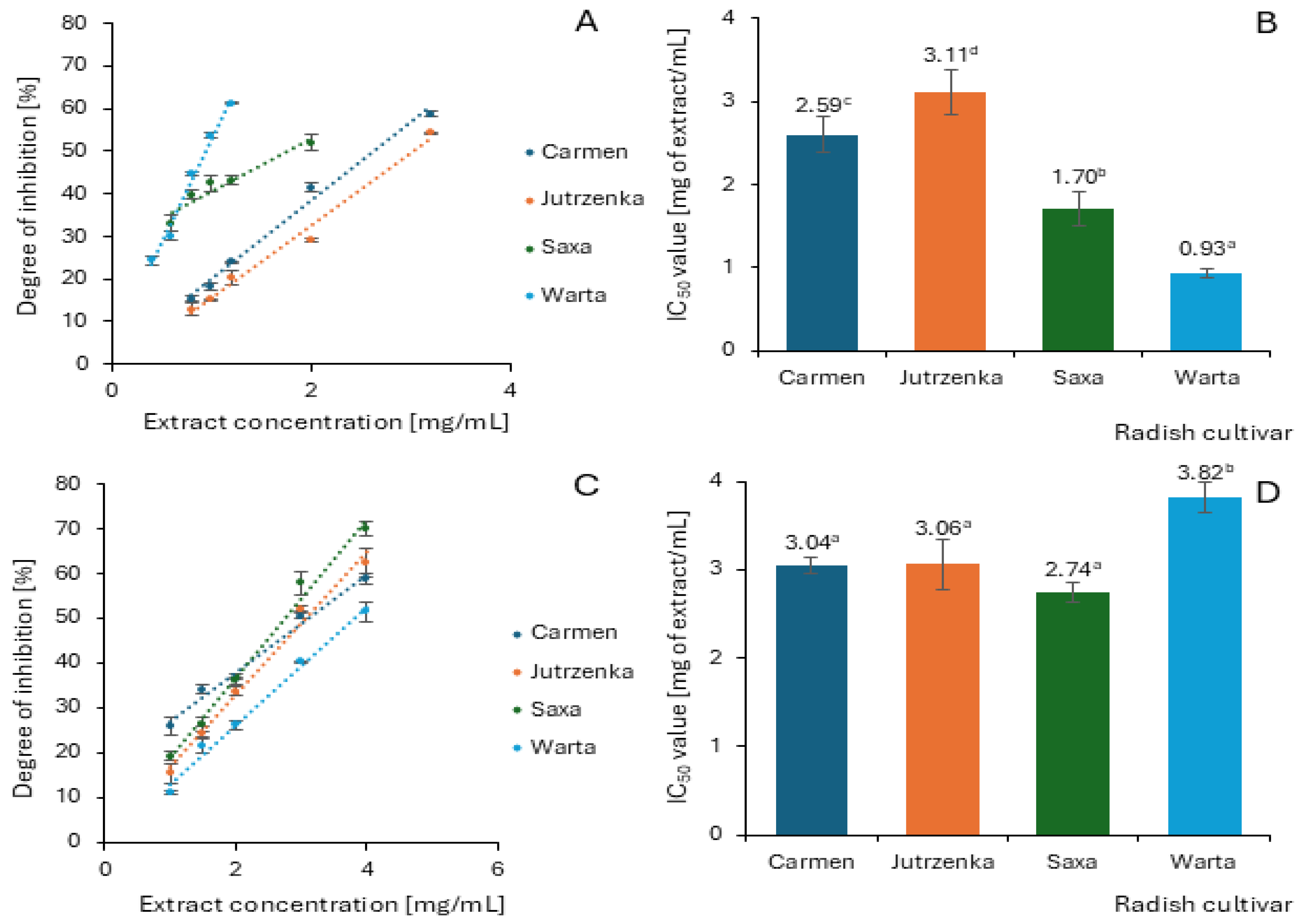
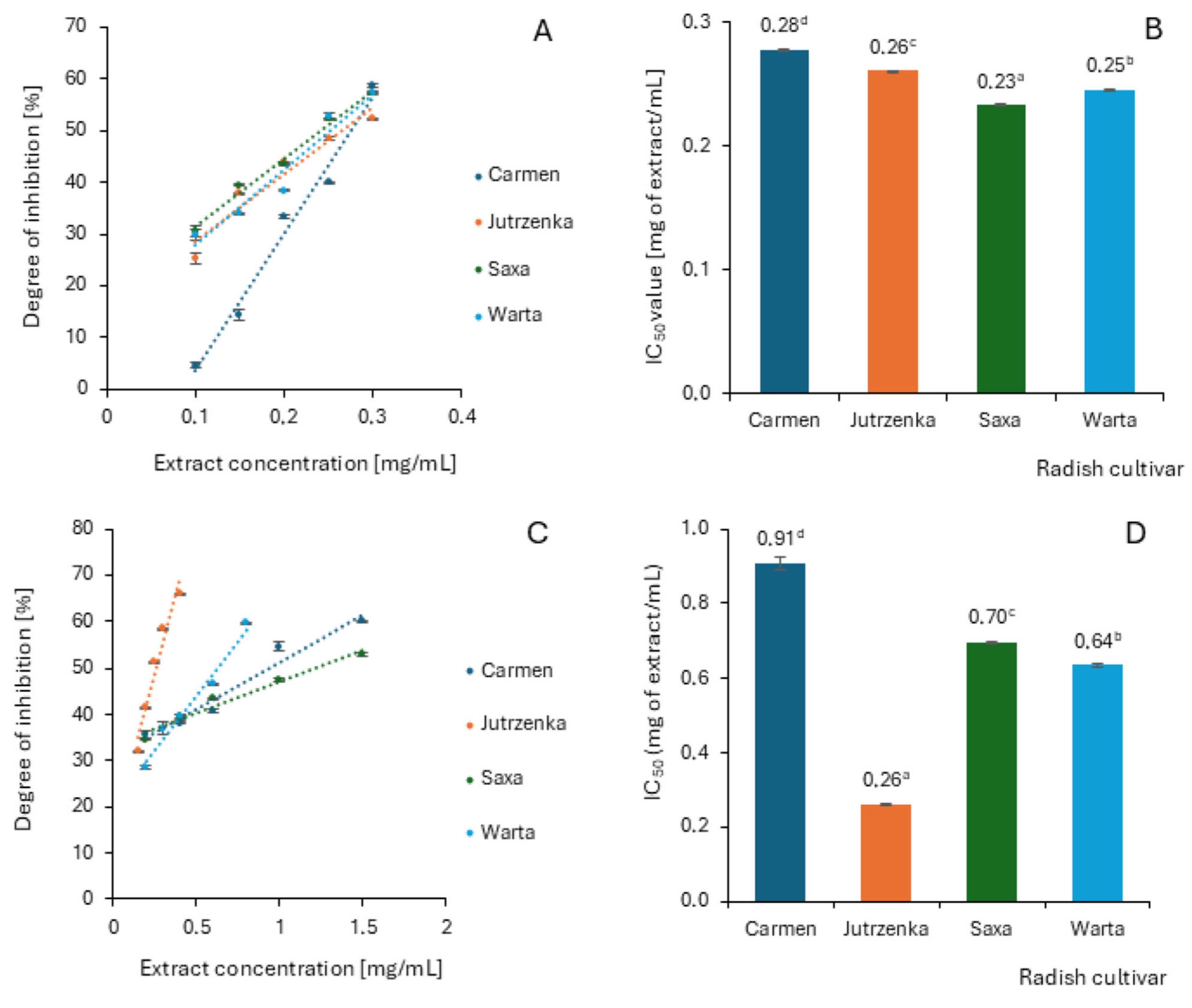
2.5. Effect of Radish Leaf Extracts on α-Amylase and α-Glucosidase Activity
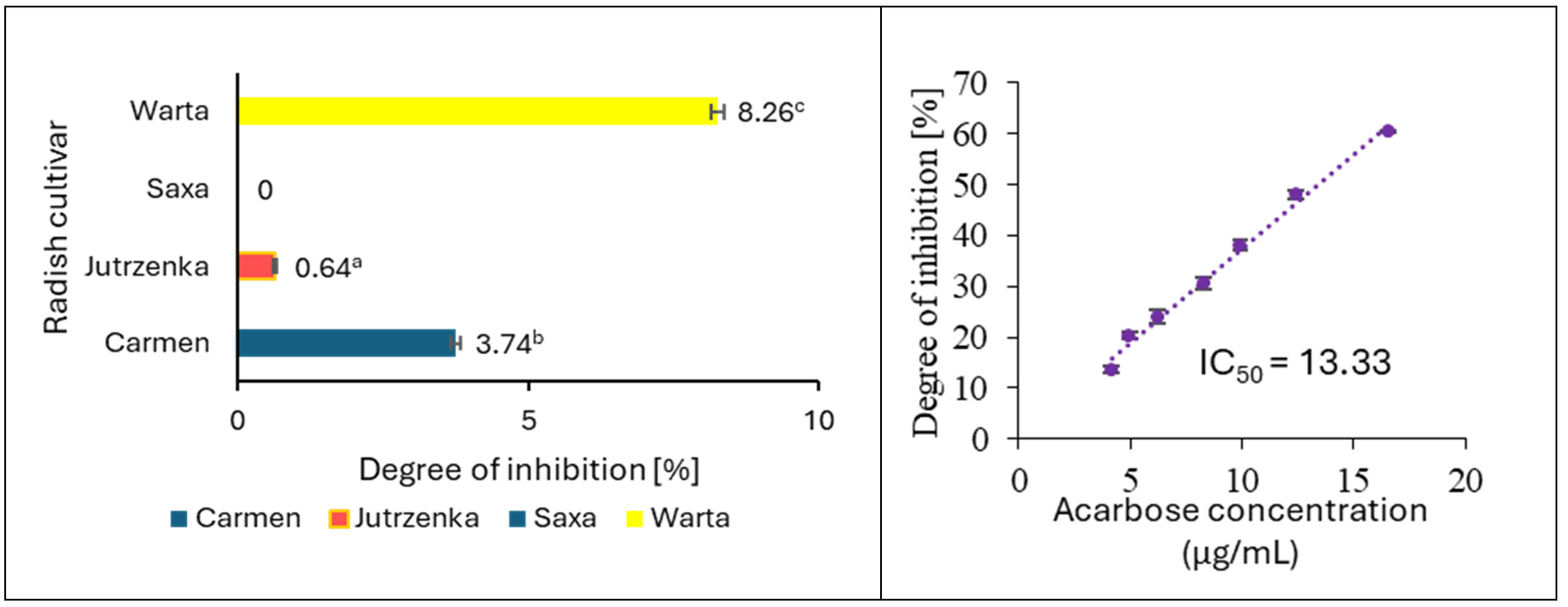
2.6. Effect of Radish Leaf Extracts on AGEs Formation
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Plant Materials
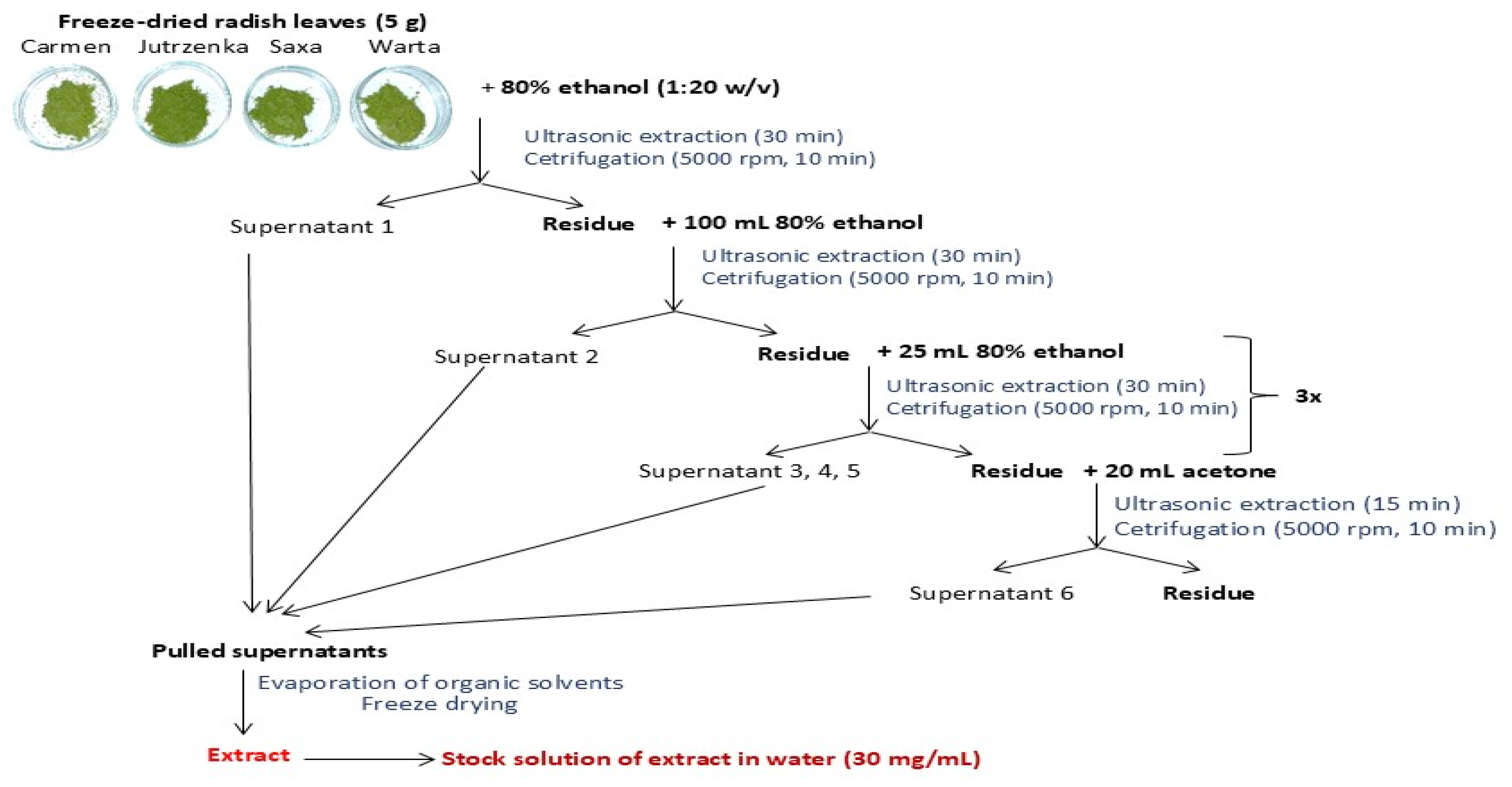
4.3. Preparation of the Extracts
4.4. Identification and Content of Individual Phenolic Compounds
4.5. Determination of Total Phenolics and Total Proanthocyanidins
4.6. Extraction and Analysis of Organic Acids and Vitamin C
4.7. Extraction and Determination of Chlorophyl and Carotenoid Pigments
4.8. Antioxidant Activity
4.9. Proximate Analysis
4.10. In Vitro Digestion of Potato Starch
4.11. Glucose Binding Capacity
4.12. α-Amylase Inhibition Assay
4.13. α-Glucosidase Inhibition Assay
4.14. Protein Glycation Inhibition Assay
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 6 October 2024).
- Medications for Diabetes, Type 2. Available online: https://www.drugs.com/condition/diabetes-mellitus-type-ii.html (accessed on 6 October 2024).
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and plant-derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Blaiti, A.; Ammari, M.; Ciobica, A.; Chelaru, I.A.; Lefter, R.; Nicoara, M. The relevance of some plant extracts in human patients and animal models of diabetes. Acad. Rom. Sci. 2023, 12, 81–90. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dhar, P.S.; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the plant-derived bioactive substances as antidiabetic agent: An extensive review. Biomed. Pharmacother. 2022, 152, 113217. [Google Scholar] [CrossRef] [PubMed]
- Kuc, M.; Cyboran, K.; Machaj, D.; Korzec, T.; Kania, K. Phytotherapy in diabetes: A review of plant. J. Educ. Health Sport 2021, 11, 17–20. [Google Scholar] [CrossRef]
- Thompson, A.S.; Candussi, C.J.; Tresserra-Rimbau, A.; Jennings, A.; Bondonno, N.P.; Hill, C.; Sowah, S.A.; Cassidy, A.; Kühn, T. A healthful plant-based diet is associated with lower type 2 diabetes risk via improved metabolic state and organ function: A prospective cohort study. Diabetes Metab. 2024, 50, 101499. [Google Scholar] [CrossRef]
- Liu, R. Effect of plant-based diet on type 2 diabetes risk: A review of the literature. Int. J. Biol. Life Sci. 2023, 3, 29–30. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Han, H.; Hu, Y.; Zhu, L.; Rimm, E.B.; Hu, F.B.; Sun, Q. Associations between plant-based dietary patterns and risks of type 2 diabetes, cardiovascular disease, cancer, and mortality—A systematic review and meta-analysis. Nutr. J. 2023, 22, 46. [Google Scholar] [CrossRef]
- Aly, T.A.; Fayed Attia Koutb, F.M.; Fayed, S.A.; Ahmed, A.M.; ELRahim, E.A. Biochemical and histopathological evaluation of radish microgreen and clover etiolated sprouts against diabetic mellitus rats. Eur. J. Pharm. Med. Res. 2020, 7, 126–134. [Google Scholar]
- Mohamed, S.M.; Aly, T.A.; Khattab, M.S.; Abdel-Rahim, E.A.; Al-Farga, A. Pathological and biochemical evaluation of radish microgreen on diabetes and aflatoxicosis in rats. Environ. Sci. Pollut. Res. 2023, 30, 98389–98399. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Elnour, R.O.; EzzEldin, O.M.; Mariod, A.A.; Ahmed, R.H.; Eltahir, A.S. Effect of Raphanus sativus on glucose, cholesterol and triglycerides levels in glucose loaded rats. Funct. Foods Health Dis. 2022, 12, 134–141. [Google Scholar] [CrossRef]
- Hendrawati, A.; Djunet, N.A. Raphanus sativus leaves ethanol extract’s effect on heart muscle’s nuclear Factor Kappa B (NFkB) in diabetic rats. MAGNA MEDICA Berk. Ilm. Kedokt. Kesehat. 2024, 11, 198–204. [Google Scholar] [CrossRef]
- Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; Viguera, C.G.; Rimbach, G.; Wagner, A.E. Metabolic activity of radish sprouts derived isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Qadir, R.; Akhtar, M.; Shaukat, U.A.; Almas, T.; Masood, S.; Siddique, F. Screening of phytochemicals and in vitro antidiabetic potential of Raphanus sativus leaves extract. Pak. J. Phytopathol. 2023, 35, 35–41. [Google Scholar] [CrossRef]
- Dhanalakshmi, G. In vitro and In silico study of anti diabetic activity on Raphanus sativus microgreen and mature leaf. Int. J. Phram. Sci. Clin. Res. 2022, 2, 120–126. [Google Scholar]
- Cattivelli, A.; Zannini, M.; Conte, A.; Tagliazucchi, D. Inhibition of starch hydrolysis during in vitro co-digestion of pasta with phenolic compound-rich vegetable foods. Food Biosci. 2024, 61, 104586. [Google Scholar] [CrossRef]
- Gamba, M.; Asllanaj, E.; Raguindin, P.; Glisic, M.; Franco, O.; Minder, B.; Bussler, W.; Metzger, B.; Kera, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Khan, R.S.; Khan, S.S.; Siddique, R. Radish (Raphanus sativus): Potential antioxidant role of bioactive compounds extracted from radish leaves—A review. Pak. J. Med. Health Sci. 2022, 16, 2. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Goyeneche, R.; Rodrigues, C.R.; Quispe-Fuentes, I.; Pellegrini, M.C.; Cumino, A.; Di Scala, K. Radish leaves extracts as functional ingredients: Evaluation of bioactive compounds and health-promoting capacities. Waste Biomass Valorization 2024. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Fayez, S.; Eltanany, B.M.; Abu-Elghait, M.; El-Demerdash, A.; Badawy, M.S.E.; Pont, L.; Benavente, F.; Saber, F.R. Agro-byproduct valorization of radish and turnip leaves and roots as new sources of antibacterial and antivirulence agents through metabolomics and molecular networking. Sci. Hortic. 2024, 328, 112924. [Google Scholar] [CrossRef]
- Sabry, M.M.; El-Halawany, A.M.; Fahmy, W.G.; Eltanany, B.M.; Pont, L.; Benavente, F.; Attia, A.S.; Sherbiny, F.F.; Ibrahim, R.M. Evidence on the inhibitory effect of Brassica plants against Acinetobacter baumannii lipases: Phytochemical analysis, in vitro, and molecular docking studies. BMC Complement. Med. Ther. 2024, 24, 164. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Ibrahim, F.M.; Ragheb, A.Y.; Mohammed, R.S.; Hegazi, N.M.; Shabrawy, M.O.E.L.; Kawashty, S.A.; Marzouk, M.M. Comprehensive phytochemical characterization of Raphanus raphanistrum L.: In vitro antioxidant and antihyperglycemic evaluation. Sci. Afr. 2022, 16, e01154. [Google Scholar] [CrossRef]
- Koley, T.K.; Khan, Z.; Oulkar, D.; Singh, B.K.; Maurya, A.; Singh, B.; Banerjee, K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem. 2020, 13, 1355–1366. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.-J.; Ruan, S.-Y.; Xie, Z.-H.; Dong, Y.; (Lucy) Yu, L. Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chem. 2012, 133, 1569–1576. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Sun, J.; Chen, P.; Harnly, J.A. LC-PDA-ESI/MSn identification of new anthocyanins in purple bordeaux radish (Raphanus sativus L. Variety). J. Agric. Food Chem. 2011, 59, 6616–6627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef]
- Prpa, E.J.; Bajka, B.H.; Ellis, P.R.; Butterworth, P.J.; Corpe, C.P.; Hall, W.L. A systematic review of in vitro studies evaluating the inhibitory effects of polyphenol-rich fruit extracts on carbohydrate digestive enzymes activity: A focus on culinary fruits consumed in Europe. Crit. Rev. Food Sci. Nutr. 2021, 61, 3783–3803. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Deng, H.; Yuan, H.; Fan, X.; Yang, H.; Tan, S. In vitro and in vivo bioaccessibility, antioxidant activity, and color of red radish anthocyanins as influenced by different drying methods. Food Chem. X 2023, 18, 100633. [Google Scholar] [CrossRef] [PubMed]
- Yücetepe, A.; Altin, G.; Özçelik, B. A novel antioxidant source: Evaluation of in vitro bioaccessibility, antioxidant activity and polyphenol profile of phenolic extract from black radish peel wastes (Raphanus sativus L. var. niger) during simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 1376–1384. [Google Scholar] [CrossRef]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Xu, H.; Zhou, Q.; He, J.; Zhu, B.; Li, S.; Wang, M. A review of green methods used in starch–polyphenol interactions: Physicochemical and digestion aspects. Food Funct. 2023, 14, 8071–8100. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Ai, L. Physical barrier effects of dietary fibers on lowering starch digestibility. Curr. Opin. Food Sci. 2022, 48, 100940. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Parada, J.; Santos, J.L. Interactions between starch, lipids, and proteins in foods: Microstructure control for glycemic response modulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 2362–2369. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, F.; Goff, H.D.; Li, Y. Study on starch-protein interactions and their effects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef]
- Lu, Z.H.; Donner, E.; Yada, R.Y.; Liu, Q. Physicochemical properties and in vitro starch digestibility of potato starch/protein blends. Carbohydr. Polym. 2016, 154, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Li, Y.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Unravelling the anthocyanin-binding capacity of native starches from different botanical origins. Food Chem. 2024, 434, 137390. [Google Scholar] [CrossRef] [PubMed]
- Kwaśny, D.; Borczak, B.; Sikora, M.; Kapusta-Duch, J. Preliminary study on the influence of the polyphenols of different groups on the digestibility of wheat starch, measured by the content of resistant starch. Appl. Sci. 2022, 12, 10859. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Morina, F.; Hirota, S.; Takahama, U. Contribution of amylose-procyanidin complexes to slower starch digestion of red-colored rice prepared by cooking with adzuki bean. Int. J. Food Sci. Nutr. 2020, 71, 715–725. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Braojos, C.; Cañas, S.; Gil-Ramírez, A.; Aguilera, Y.; Martín-Cabrejas, M.A. Changes in the cocoa shell dietary fiber and phenolic compounds after extrusion determine its functional and physiological properties. Curr. Res. Food Sci. 2023, 6, 100516. [Google Scholar] [CrossRef]
- Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of berry pomace powders as dietary fiber-rich food ingredients with functional properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Ma, Y.S.; Tsai, Y.H.; Chang, S.K. In vitro hypoglycemic, cholesterol-lowering and fermentation capacities of fiber-rich orange pomace as affected by extrusion. Int. J. Biol. Macromol. 2019, 124, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.S.; Youssef, F.S.; Alahdal, A.M.; Almasri, D.M.; Ashour, M.L. Anti-hyperglycaemic evaluation of Buddleia indica leaves using in vitro, in vivo and in silico studies and its correlation with the major phytoconstituents. Plants 2021, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, D.M.; Justino, A.B.; Sousa, R.M.; Munoz, R.A.; de Aquino, F.J.; Martins, M.M.; Coulart, L.R.; Pivatto, M.; Espindola, F.S.; de Oliveira, A. Antioxidant compounds from Banisteriopsis argyrophylla leaves as α-amylase, α-glucosidase, lipase, and glycation inhibitors. Bioorg. Chem. 2020, 105, 104335. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Kucharska, A.Z. Inhibitory potential of red cabbage against digestive enzymes linked to obesity and type 2 diabetes. J. Agric. Food Chem. 2017, 65, 7192–7199. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A.; Winterhalter, P.; Rimbach, G. Fractionation, enzyme inhibitory and cellular antioxidant activity of bioactives from purple sweet potato (Ipomoea batatas). Food Chem. 2017, 221, 447–456. [Google Scholar] [CrossRef]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 2. α-Glucosidase inhibition by isolated acylated anthocyanins. J. Agric. Food Chem. 2001, 49, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Nyambe-Silavwe, H.; Williamson, G. Chlorogenic and phenolic acids are only very weak inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Res. Int. 2018, 113, 452–455. [Google Scholar] [CrossRef]
- Magaji, U.F.; Sacan, O.; Yanardag, R. Alpha amylase, alpha glucosidase and glycation inhibitory activity of Moringa oleifera extracts. S. Afr. J. Bot. 2020, 128, 225–230. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.W.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as antioxidant/pro-oxidant compounds and donors of reducing species: Relationship with human antioxidant metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Önder, F.C.; Doğrular, N.; Güzdüzalp, E. A comparative study of three Brassicaceae vegetables grown in Canakkale: Determination of total phenolic content and antioxidant activity of pulp and juice samples of radish (Raphanus sativus L.), cabbage (Brassica oleracea L. var capitata L) and cauliflower (Brassica oleracea L.). Çanakkale Onsekiz Mart Üniversitesi Fen Bilim. Enstitüsü Derg. 2020, 6, 30–38. [Google Scholar]
- Kajszczak, D.; Kowalska-Baron, A.; Sosnowska, D.; Podsędek, A. In vitro inhibitory effects of Viburnum opulus bark and flower extracts on digestion of potato starch and carbohydrate hydrolases activity. Molecules 2022, 27, 3118. [Google Scholar] [CrossRef]
- Rӧsch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure—Antioxidant efficiency relationships of phenolic compounds and their contribution to the antioxidant activity of sea buckthorn juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7702–7708. [Google Scholar]
- Podsędek, A.; Frąszczak, B.; Kajszczak, D.; Sosnowska, D. Evaluation of bioactive compounds and antioxidant activity of green and red kale (Brassica oleracea L. var. acephala) microgreens grown under white, red, and blue LED combinations. Agronomy 2024, 14, 2454. [Google Scholar] [CrossRef]
- Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant effects of hemp (Cannabis sativa L.) inflorescence extract in stripped linseed oil. Antioxidants 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Nollet, L.M. Physical characterization and nutrient analysis. In Handbook of Food Analysis, 2nd ed.; Dekker, M., Ed.; CRC Press: New York, NY, USA, 2004; pp. 61–67, 77–78, 173–176. [Google Scholar]
- Kajszczak, D.; Kowalska-Baron, A.; Podsędek, A. Glycoside hydrolases and non-enzymatic glycation inhibitory potential of Viburnum opulus L. fruit—In vitro studies. Antioxidants 2021, 10, 989. [Google Scholar] [CrossRef]
| Compounds | Radish Cultivar | |||
|---|---|---|---|---|
| Carmen | Jutrzenka | Saxa | Warta | |
| Dry matter (g/100 g fw) | 7.94 ± 0.18 a | 8.56 ± 0.12 bc | 8.80 ± 0.16 c | 8.36 ± 0.15 b |
| Ash (g/100 g dw) | 24.19 ± 0.12 c | 21.03 ± 0.41 a | 22.97 ±0.16 bc | 21.98 ± 0.88 ab |
| Protein (g/100 g dw) | 21.47 ± 0.09 a | 22.31 ± 0.10 b | 22.49 ± 0.50 b | 22.60 ± 0.15 b |
| Fat (g/100 g dw) | 5.32 ± 0.07 a | 8.19 ± 0.07 d | 6.14 ± 0.06 c | 5.73 ± 0.10 b |
| Carbohydrates * (g/100 g dw) | 49.02 ± 0.18 ab | 48.18 ± 0.54 a | 48.40 ± 0.63 a | 49.98 ± 0.83 b |
| Dietary fiber (g/100 g dw) | 41.07 ± 1.21 a | 41.64 ± 1.21 ab | 42.98 ± 0.38 ab | 44.39 ± 1.80 b |
| Insoluble dietary fiber (g/100 g dw) | 39.31 ± 1.16 a | 40.06 ± 1.19 ab | 41.56 ± 0.39 ab | 43.08 ± 1.80 b |
| Soluble dietary fiber (g/100 g dw) | 1.76 ± 0.06 b | 1.59 ± 0.05 b | 1.42 ± 0.03 a | 1.31 ± 0.08 a |
| Total organic acids (g/100 g dw) | 0.85 ± 0.02 c | 0.71 ± 0.01 a | 0.77 ± 0.02 b | 0.86 ± 0.03 c |
| Compounds | Radish Cultivar | |||
|---|---|---|---|---|
| Carmen | Jutrzenka | Saxa | Warta | |
| Total phenolics | 955.83 ± 39.68 a | 1386.59 ± 56.41 c | 1124.90 ± 45.58 b | 1367.65 ± 61.75 c |
| Total proanthocyanidins | 226.92 ± 7.51 ab | 207.87 ± 12.24 a | 233.82 ± 8.76 b | 263.94 ± 2.83 c |
| Total chlorophyll | 567.28 ± 1.56 a | 665.40 ± 13.23 b | 642.18 ± 0.41 b | 555.76 ± 12.99 a |
| Chlorophyll a | 387.03 ± 4.95 a | 470.39 ± 18.77 b | 439.90 ± 2.73 b | 382.82 ± 15.24 a |
| Chlorophyll b | 180.26 ± 2.05 ab | 195.01 ± 13.47 b | 202.28 ± 7.82 b | 171.95± 7.41 a |
| Total carotenoids | 55.64 ± 2.94 a | 71.35 ± 5.53 b | 60.58 ± 2.40 ab | 59.16 ± 4.98 a |
| L-ascorbic acid | 635.22 ± 61.18 a | 683.14 ± 36.59 a | 563.32 ± 46.30 a | 630.81 ± 73.02 a |
| Method | Radish Cultivar | |||
|---|---|---|---|---|
| Carmen | Jutrzenka | Saxa | Warta | |
| ABTS | 3.10 ± 0.12 a | 5.31 ± 0.40 c | 4.16 ± 0.27 b | 5.18 ± 0.32 c |
| SARSA | 24.33 ± 2.01 b | 42.55 ± 3.66 c | 19.13 ± 1.81 a | 22.96 ± 1.87 ab |
| FRAP | 3.47 ± 0.11 a | 5.98 ± 0.28 c | 4.48 ± 0.16 b | 5.75 ± 0.33 c |
| FCA | 0.51 ± 0.08 a | 1.86 ± 0.10 c | 0.75 ± 0.09 b | 0.67 ± 0.08 b |
| Phenolic Compound | Radish Cultivar | |||
|---|---|---|---|---|
| Carmen | Jutrzenka | Saxa | Warta | |
| Caffeic acid glucoside | 6.49 ± 0.54 a | 44.46 ± 4.01 c | 20.37 ± 2.51 b | 65.94 ± 8.01 d |
| p-Coumaric acid glucoside | 6.12 ± 0.25 b | 19.92 ± 1.70 c | 2.35 ± 0.10 a | 5.81 ± 0.42 b |
| Sinapic acid glucoside | 1.56 ± 0.14 a | 4.84 ± 0.78 c | 3.09 ± 0.11 b | 3.94 ± 0.40 bc |
| Ferulic acid glucoside | 0.65 ± 0.02 a | 3.52 ± 0.15 d | 1.10 ± 0.06 b | 1.89 ± 0.05 c |
| 1,2-Disinapoylglucoside | - | 24.56 ± 2.79 b | 10.25 ± 0.35 a | 36.80 ± 3.34 c |
| Feruloylmalic acid | - | 40.52 ± 0.86 c | 30.95 ± 1.61 b | 16.79 ± 2.82 a |
| p-Coumaric acid | - | - | - | 72.84 ± 6.29 |
| Sum of hydroxycinnamic acids | 14.82 ± 0.95 a | 137.82 ± 9.18 c | 68.11 ± 4.48 b | 204.01 ± 18.06 d |
| Kaempferol 3-diglucoside | - | 2.29 ± 0.18 a | - | 10.60 ± 0.84 b |
| Kaempferol 3-O-coumaroyl glucoside | 2.75 ± 0.02 a | 4.11 ± 0.40 b | - | 2.61 ± 0.26 a |
| Kaempferol-3-O-glucosyl-rhamnosyl-glucoside | 24.43 ± 0.84 a | 106.78 ± 4.68 c | 28.18 ± 1.36 a | 42.05 ± 2.74 b |
| Kaempferol 3-O-coumaroyl glucoside | 282.62 ± 6.19 a | 430.84 ± 7.80 c | 357.50 ± 9.42 b | 429.84 ± 18.43 c |
| kaempferol-3-O-arabinoside-7-O-rhamnoside | 162.71 ± 0.69 b | 161.44 ± 13.75 b | 130.95 ± 3.49 a | 198.15 ± 18.29 c |
| Kaempferol 3-O-rhamnoside-7-O-rutinoside | 17.54 ± 0.36 b | - | 15.05 ± 0.71 a | 18.68 ± 1.11 b |
| Kaempferol 3-(p-coumaroyl)sophorotrioside | 179.65 ± 4.85 a | 502.53 ± 19.29 d | 317.78 ± 23.15 c | 215.40 ± 19.25 b |
| Kaempferol 3-O-(p-coumaroyl)dirhamnosylhexoside | 190.57 ± 4.43 a | 325.95 ± 16.47 d | 223.23 ± 7.67 a | 283.57 ± 23.15 b |
| Kaempferol 3-O-rutionoside-7-O-rhamnoside | - | 48.93 ± 5.18 | - | - |
| Kaempferol 3-O-p-coumaryl rutinoside-7-O-arabinoside | - | 25.70 ± 1.96 | - | - |
| Sum of flavonols | 860.27 ± 17.39 a | 1608.57 ± 69.71 d | 1072.69 ± 45.00 b | 1200.90 ± 13.20 c |
| Apigenin-C-hexoside-C-pentoside | - | 5.28 ± 0.55 c | 1.74 ± 0.16 a | 3.22 ± 0.52 b |
| Apigenin-7-O-rutinoside | 202.79 ± 4.84 a | 180.16 ± 23.33 a | 186.25 ± 2.96 a | 296.82 ± 15.82 b |
| Sum of flavones | 202.79 ± 4.84 a | 185.44 ± 23.87 a | 187.99 ± 3.12 a | 300.04 ± 16.35 b |
| Pelargonidin -3-(feruloyl)diglucoside-5-(malonyl)glucoside | 5.27 ± 0.23 b | - | 6.61 ± 0.28 c | 4.10 ± 0.12 a |
| Pelargonidin -3-(feruloyl)diglucoside-5-glucoside derivative | 3.52 ± 0.25 a | - | 3.22 ± 0.10 a | 3.40 ± 0.17 a |
| Pelargonidin -3-(p-coumaroyl)diglucoside-5-glucoside derivative | 2.80 ± 0.27 | - | - | - |
| Sum of antocyanins | 11.59 ± 0.75 c | - | 9.83 ± 0.38 b | 7.50 ± 0.05 a |
| Total phenolics | 1089.47 ± 32.13 a | 1931.83 ± 50.29 d | 1338.62 ± 51.74 b | 1712.45 ± 18.11 c |
| Radish Cultivar | Glucose Concentration in the Solution | ||
|---|---|---|---|
| 10 mM | 50 mM | 100 mM | |
| Amount of Glucose Binding [mmol/g of Leaf) | |||
| Carmen | 0.10 ± 0.00 b | 0.18 ± 0.02 bc | 0.31 ± 0.01 ab |
| Jutrzenka | 0.08 ± 0.00 a | 0.11 ± 0.02 a | 0.29 ± 0.01 a |
| Saxa | 0.08 ± 0.00 a | 0.13 ± 0.01 ab | 0.28 ± 0.01 a |
| Warta | 0.10 ± 0.01 b | 0.21 ± 0.02 c | 0.34 ± 0.01 b |
| Sub-Group of Phenolic Compounds | Carmen | Jutrzenka | Saxa | Warta |
|---|---|---|---|---|
| mg/g of Extract | ||||
| Total proanthocyanidins | 0.145 ± 0.003 b | 0.112 ± 0.002 a | 0.216 ± 0.003 c | 0.208 ± 0.002 c |
| Total hydroxycinnamic acids | 1.28 ± 0.06 a | 10.57 ± 0.73 c | 6.67 ± 0.42 b | 17.72 ± 0.96 d |
| Total flavonols | 74.20 ± 1.37 a | 104.96 ± 1.23 c | 84.94 ± 3.61 b | 86.98 ± 0.05 b |
| Total flavones | 17.49 ± 0.42 b | 12.10 ± 1.55 a | 15.95 ± 0.24 b | 21.76 ± 1.18 c |
| Total anthocyanins | 0.65 ± 0.02 a | 0.00 | 0.86 ± 0.03 b | 0.83 ± 0.04b |
| Total phenolics | 93.61 ± 1.78 a | 126.03 ± 3.04 c | 107.56 ± 4.24 b | 124.63 ± 1.89 c |
| Oral digestion; Incubation conditions: 37 °C, 2 min |
| 10–50 mg of freeze-dried radish leaves 0.5 mL of water 0.5 mL of gelatinized potato starch (25 mg/mL) 1.25 mL saliva solution 0.25 mL α-amylase solution (0.1 mg/mL) |
| Gastric digestion; Incubation conditions: 37 °C, 2 h |
| 2.25 mL gastric solution (2 g NaCl in 0.7% HCl in water, pH 1.2) 0.25 mL pepsin solution (3.2 mg/mL) pH correction to a value of 2.0 with 2M NaOH |
| Intestinal digestion; Incubation conditions: 37 °C, 2 h |
| 2.5 mL of water pH correction to a value of 6.0 with 2M NaOH followed to 7.5 with 1M NHCO3 The volume of the sample was adjusted to 8.2 mL with water 0.5 mL of bile salts (100 mg/mL) 1 mL of α-glucosidase solution 0.3 mL of pancreatin solution (0.04 mg/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajszczak, D.; Sosnowska, D.; Frąszczak, B.; Podsędek, A. Composition, Anti-Diabetic, and Antioxidant Potential of Raphanus sativus Leaves. Molecules 2024, 29, 5689. https://doi.org/10.3390/molecules29235689
Kajszczak D, Sosnowska D, Frąszczak B, Podsędek A. Composition, Anti-Diabetic, and Antioxidant Potential of Raphanus sativus Leaves. Molecules. 2024; 29(23):5689. https://doi.org/10.3390/molecules29235689
Chicago/Turabian StyleKajszczak, Dominika, Dorota Sosnowska, Barbara Frąszczak, and Anna Podsędek. 2024. "Composition, Anti-Diabetic, and Antioxidant Potential of Raphanus sativus Leaves" Molecules 29, no. 23: 5689. https://doi.org/10.3390/molecules29235689
APA StyleKajszczak, D., Sosnowska, D., Frąszczak, B., & Podsędek, A. (2024). Composition, Anti-Diabetic, and Antioxidant Potential of Raphanus sativus Leaves. Molecules, 29(23), 5689. https://doi.org/10.3390/molecules29235689







