Abstract
The aim of this study was to evaluate the cytotoxicity and genotoxicity of potential biocontrol agents for use against phytopathogens of potato seed (Solanum tuberosum L.). Plant extracts from Allium sativum L., Syzygium aromaticum L. Merr. & Perry, Salvia officinalis L., and Curcuma longa L., as well as metabolites of bacteria Lactiplantibacillus plantarum KB2 LAB 03 and yeast Metschnikowia pulcherrima TK1, were investigated. The chemical characteristics of the plant extracts and the metabolic profiles of the tested microorganisms were evaluated by GC-MS. An insect cell line from Spodoptera frugiperda (Sf-9) and human cervix adenocarcinoma cells (HeLa) were used to evaluate cytotoxicity in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The single-cell electrophoresis assay was used to estimate DNA damage. The cytotoxicity and genotoxicity of the microbial metabolites depended on their chemical profiles and pH. The plant extracts induced stronger DNA damage in the Sf-9 cell line than in HeLa cells. The garlic (Allium sativum L.) extract showed the highest cytotoxicity against Sf-9 insect cells (IC50 41.6 mg/mL). The sage (Salvia officinalis L.) extract showed the highest cytotoxicity against HeLa cells (IC50 49.6 mg/mL). This study is the first to investigate not only the potential of these novel biocontrol agents for plant disease control, but also their safety for humans and biodiversity within the context of sustainable agriculture.
1. Introduction
Phytopathogens pose a serious threat to crop productivity worldwide. Current anti-phytopathogen strategies include the development of disease-resistant crops and the application of chemical pesticides. These approaches have contributed to increased crop output and quality over the past few decades. However, the excessive use of toxic chemical compounds has resulted in environmental pollution and a drastic reduction in biodiversity [1]. Prolonged use of chemical treatments during plant cultivation has also resulted in resistance against fungal pathogens [2]. For these reasons, researchers are exploring the use of beneficial microorganisms as an eco-friendly strategy to control crop diseases. A wide range of bacterial and fungal genera show great potential as biocontrol agents for various plant diseases. Beneficial microbes are abundant sources of various natural compounds with the ability to control plant diseases at various levels [1]. These include primary and secondary metabolites: antibiotics, toxins, ribosomal or non-ribosomal peptides, polyketides, and volatile organic compounds.
Lactic acid bacteria (LAB) have the status of generally recognized as safe (GRAS), and are used as natural preservatives in food and feed to control fungal growth and subsequent mycotoxin production [3,4]. Previous studies by our team confirmed the ability of selected LAB to effectively inhibit the growth of fungal phytopathogens of seed potato (Solanum tuberosum L.). The best results were achieved with the strain Lactiplantibacillus plantarum, resulting in a 40–90% reduction in infestation by eight potato pathogens [5].
The Metschnikowia pulcherrima clade of yeast is considered an effective biocontrol agent against pathogenic moulds, mainly due to its ability to bind iron into a complex called pulcherrimin. The lack of free iron in the environment inhibits the growth of many microorganisms [6]. Pulcherrimin-producing yeasts exhibit strong antagonism against moulds belonging to the genera Alternaria, Botrytis, Fusarium, Rhizopus, and Verticillium [7]. Many studies have shown that the M. pulcherrima clade can effectively inhibit the growth of seed potato phytopathogens [8].
Plants are a valuable source of bioactive compounds, including terpenes, phenolic compounds, essential oils, and alkaloids. Numerous studies have investigated the effects of various plant extracts on plant pathogens. Due to the antimicrobial activity, biodegradability, and low toxicity of plant extracts, they can potentially be used in plant protection against pathogens instead of chemical pesticides [9]. In a recent study, garlic water extract with 5-hydroxymethylfurfural (5-HMF) as the main component proved to be particularly effective as a potential biopesticide against potato phytopathogens [10]. In another study, all the studied microorganisms and plant extracts not only inhibited the growth of the tested phytopathogens, but also had positive effects on the physiological parameters of potatoes and reduced the production of mycotoxins by Fusarium, Alternaria, and Phoma [11].
Microorganisms and natural substances of plant origin are considered to have less impact on ecosystems and human health than conventional chemicals for plant protection. However, to ensure the sustainability of biocontrol solutions, it is necessary to understand their possible negative repercussions. The use of living organisms, capable of growth and metabolite production, as biocontrol agents presents specific challenges compared to conventional solutions, because these microorganisms can survive, multiply, move, and effectively colonize other environments. To date, only a limited number of studies have addressed the presence of biocontrol agents in the environment, their environmental fate, and their effects on biodiversity. However their results suggest that while most have low ecotoxicity, others have toxicity equivalent to or greater than conventional solutions. Overall, knowledge of the unintended effects of biocontrol agents in the environment is very incomplete [12].
The purpose of this study was to investigate the potential ecotoxicological impact of selected biocontrol agents on other useful living organisms in the environment and on biodiversity. The main aim was to evaluate the cytotoxicity and genotoxicity of selected plant extracts: garlic (Allium sativum L.), clove (Syzygium aromaticum L. Merr. & Perry), sage (Salvia officinalis L.), and turmeric (Curcuma longa L.). We also evaluated the toxicity of metabolites of L. plantarum and M. pulcherrima, as potential biocontrol agents for potato seeds (Solanum tuberosum L.). Insect cell lines were used, among other methods, as an effective model to investigate the toxicological mechanisms of the biocontrol agents [13]. This research is part of a new trend for developing innovative strategies using diverse biological agents, such as plant extracts and native microorganisms, as well as examining their impact on the environment.
2. Results and Discussion
2.1. Chemical Composition of Aqueous Plant Extracts
In our previous research, we determined the compositions of garlic (Allium sativum L.) and clove (Syzygium aromaticum L. Merr. & Perry) aqueous extracts [10]. The main components of the clove extract were eugenol and eugenol acetate, amounting to 82.39% and 4.56% of the total composition, respectively. The garlic extract was dominated by 5-HMF (33.24%), followed by furan-2-carboxaldehyde (7.09%), acetaldehyde (5.17%), and 3-methylbutanal (4.27%) [10]. The other aqueous extracts used in the present study were sage (Salvia officinalis L.) and turmeric (Curcuma longa L.). The volatile components of the plant extracts were analysed and identified by gas chromatography–mass spectrometry (GC-MS) combined with Kovats retention index. This is a commonly used method for the characterization of volatile compounds in various plant extracts [14]. The chemical compositions of the tested extracts are presented in Table 1 and Table 2. The chromatograms show great variation in the chemical profiles of the plant extracts tested. The most important chemical compounds are listed int the tables, but the synergistic effects of other compounds should not be excluded.

Table 1.
Composition of volatile compounds in sage (Salvia officinalis L.) aqueous extract.

Table 2.
Composition of volatile compounds in turmeric (Curcuma longa L.) aqueous extract.
Salvia spp. belong to the family of Lamiaceae and are associated with potent antimicrobial activity, primarily due to their chemical constituents [15,16]. According the Drugs and Lactation Database LactMed® [17], sage leaves contain mainly tannins (salviatannin), essential oils (including α-thujone, β-thujone, 1,8 cineole, and camphor), flavones, phenolic acids, phenylpropanoid glycosides, triterpenoids, and diterpenes. The tested sage extract was dominated by camphor (31%), α-thujone (24%), and eucalyptol (18%) (Table 1, Figure 1).
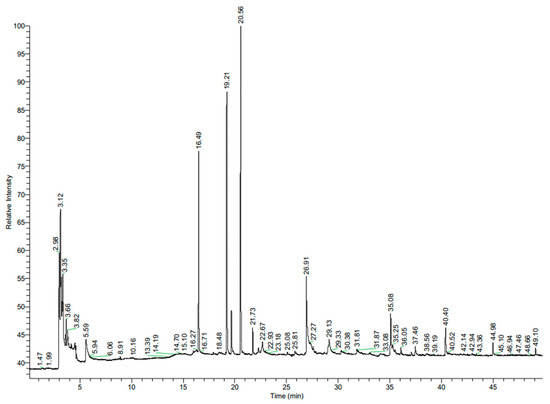
Figure 1.
Chromatogram of sage (Salvia officinalis L.) aqueous extract.
The antimicrobial activity of these compounds is well known [18,19]. Sage essential oil is active against the mycelial growth of the strawberry pathogens Colletotrichum acutatum [20], Alternaria alternata, Botrytis cinerea, and Fusarium oxysporum [16]. It is worth noting the presence in the profile of 2-metoksy-4-vinylphenol (11%). This compound often causes severe skin burns and eye damage [21].
Curcuma longa (turmeric) belongs to the family Zingiberaceae. Turmeric essential oil acts as a natural food preservative and alternative to chemical fungicides. It has been shown to have a significant antifungal effect against Penicillium expansum, Fusarium solani, Rhizopus stolonifera, and Alternaria alternata [22]. In our study, the turmeric extract was composed mainly of 1-hydroxy-2-propanone (acetol) (21%), ar-turmerone (19%), and ethanol (14%) (Table 2, Figure 2).
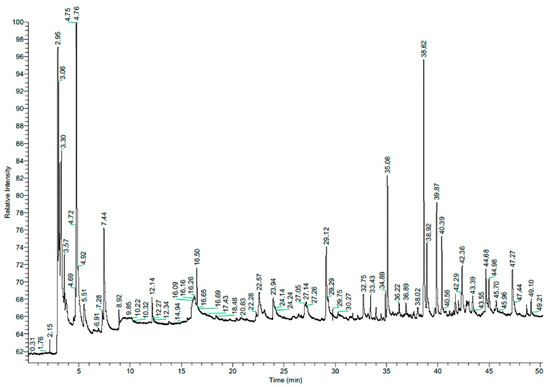
Figure 2.
Chromatogram of turmeric (Curcuma longa L.) aqueous extract.
These compounds have known potential antimicrobial activity [23,24]. However, it is worth noting the significant share (17%) of unidentified compounds in the profile. The effects of these compounds on living organisms are unknown.
2.2. Cytotoxicity of Plant Aqueous Extracts
Sf-9 (Spodoptera frugiperda) and HeLa cells were exposed to the extracts for 24 h. Non-cytotoxic concentrations (IC0) and IC50 were determined for all extracts and both cell lines (except for the garlic extract, for which only the IC20 value was determined due to its weak cytotoxicity towards HeLa cells). Against the Sf-9 insect cell line, the extracts with the highest cytotoxicity were garlic (IC50 41.6 mg/mL), followed by turmeric and sage, and the weakest cytotoxicity was shown by clove (Table 3). Against the model HeLa cell line, sage extract showed the strongest cytotoxicity (IC50 49.6 mg/mL), followed by clove, turmeric, and finally garlic. Overall, the garlic and turmeric extracts showed stronger cytotoxicity against Sf-9 insect cells than against HeLa cells. λ-Cyhalothrin always showed the highest cytotoxicity, which was more than 200 times stronger than the cytotoxicity of the garlic extract against Sf-9 cells.

Table 3.
Cytotoxic (IC50) and non-cytotoxic (IC0) values of plant aqueous extracts against insect Sf-9 and human HeLa cell lines.
S. frugiperda insects are common pests of plants belonging to the Solanaceae Juss. family. Sf-9 cells are the most established insect cells. They are used in agricultural research and toxicity studies. They are particularly valuable for assessing the ecotoxicity of environmental pollutants, studying the control of plant pests and crop protection strategies (especially in mass production systems), evaluating the cytotoxicity of insecticides, and supporting the development of new biopesticides against crop pests [25]. In vitro studies using Sf-9 cells allow for rapid toxicity screening of biopreparations and their components.
The cytotoxicity and mechanism of cytotoxic action of garlic extracts have been investigated for many human cell lines, including tongue squamous carcinoma (SCC-15), normal skin fibroblasts (BJ), epithelial papilloma (KB), leukemic (U-937, Jurkat, E6-1, K-562, TIB-152), cervix adenocarcinoma (HeLa), colorectal cancer (DLD-1), lung squamous carcinoma (SK-MES-1), colon adenocarcinoma (Caco-2), hepatic carcinoma (Hep-G2), prostate cancer (PC-3), breast cancer(MCF-7), and normal human keratinocytes (HaCaT) [26,27,28,29,30,31,32]. The main mechanisms of the cytotoxic action of garlic extracts are the induction of reactive oxygen species (ROS) generation, inhibition of proliferation, and cell death by necrosis or apoptosis [27,28,29,32]. In our study, we used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to evaluate cytotoxicity. The MTT assay is widely used to measure cell metabolic activity, which is an indirect indicator of cell viability. The high cytotoxicity of the garlic extract in our study may be due to the content of acetaldehyde. Although it did not account for a large proportion of the extract’s composition (about 5.2%), acetaldehyde can exhibit strong mitophagy-related cytotoxicity, which involves the removal of damaged mitochondria through lysosomal degradation. The results of the MTT assay may also reflect impaired and reduced mitochondrial function after exposure to this compound [33]. This may be linked to a decrease in mitochondrial membrane potential (MMP), ROS production, and the ATP level in the cell [33,34]. Tigu et al. [32] obtained IC50 values ranging from about 8.8 to 63 mg/mL, depending on the cell line. In our study, the IC50 value for the Sf-9 cells was within this range, at 41.6 mg/mL. As in the study by Tigu et al., normal cells were much more sensitive to garlic extract than cancerous cells.
The cytotoxic potential of clove extracts has likewise been tested on a number of human cell lines, including colon adenocarcinoma (HCT 116, HT-29), breast adenocarcinoma (MCF-7, AU 565), HeLa, Hep-G2, and lung alveolar carcinoma (A-549) [35,36,37,38,39,40,41]. The main mechanism of cytotoxic action of clove extract is the induction of apoptosis. The main component responsible for cytotoxic effect is eugenol [42]. In our study, eugenol and eugenol acetate accounted for more than 86% of all components in the clove extracts. Various extracts from different parts of clove (e.g., leaves, stems, fruits, buds, rhizomes) display cytotoxic effects on cell lines by inhibiting proliferation, inducing ROS, and causing cell cycle arrest [43].
The cytotoxicity of curcumin may be related to its uptake into the cell. As a lipophilic molecule, curcumin interacts with the cell membrane and is then transported into the cell [44]. The more curcumin enters the cell, the greater its cytotoxicity. This mechanism has been demonstrated for cell lines including mouse T lymphoblast EL4, human MCF-7, and murine fibroblasts NIH3T3 [44]. Ar-turmerone accounted for almost 19% of the components in the studied turmeric extract. This compound has been shown to contribute to the uptake of curcumin into the cell in vitro [45]. Many other studies have reported that curcumin inhibits the growth of various cells, including mouse skin melanoma—B16-F1, human colon adenocarcinoma—COLO 205, HCT 116, and Caco-2, human hepatic stellate—LX-2, Hep-G2, and HeLa, in assays based on tetrazolium salts (MTT, MTS, WST-1) [46,47,48,49]. In HeLa cells treated with curcumin, apoptotic changes and cell cycle arrest (in G0/G1 and S phases) were observed in proportion to its concentration [49]. Ar-turmerone may be responsible for this cytotoxic effect and contribute to the induction of apoptosis in cells [50]. Curcumin-based secondary metabolites have well-documented potent cytotoxic activities [51]. Curcumin-based linear diarylheptanoids 35a and 35b have been shown to display antiproliferative activity on human cancer cells (MCF-7 and Hep-G2). Furthermore, 35b increases GADD45B (growth arrest and DNA-damage-inducible, beta) expression, leading to inhibition of MCF-7 proliferation and death. The same study confirmed the importance of the conjugated group for antiproliferative activity [51]. Some linear diarylheptanoids, due to their chemical structure (at least two substituents on aryl rings), demonstrate higher cytotoxic and anticancer properties against cancer cell lines than curcumin [52]. Sage extract may be cytotoxic to Hep-G2 cells, causing morphological changes in cells and inhibiting proliferation, while decreasing the intracellular ATP level, lactate dehydrogenase (LDH) leakage, and apoptosis [53]. Sage extract can also induce apoptosis in human lymphoma and leukaemia cells [54] and show cytotoxicity against other human-derived cell lines, including HeLa and MCF-7 [55]. One of the main components of the tested sage extract was eucalyptol, which can display cytotoxicity in cells by inducing apoptosis [56]. Other components included camphor and α-thujone. All these compounds (camphor, eucalyptol, α-thujone) can be cytotoxic to colon carcinoma cells (MRC-5, HT-29, and HCT 116) [57].
To the authors’ best knowledge, the studied aqueous extracts have not previously been considered as bioinsecticides against Sf-9 cells. Lopes et al. [58] investigated seven different plant extracts (in water, ethanol, or dichloromethane as solvents) against Sf-9 cells, observing that the less polar samples were more cytotoxic. Dichloromethane extract caused a loss of cell viability that exceeded the effect of the commercial insecticide chlorpyrifos. In our study, λ-cyhalothrin always showed more cytotoxicity than the tested extracts. λ-Cyhalothrin belongs to the class of Type II pyrethroid insecticides. This compound has insecticidal properties and is used to control a wide range of insect pests and diseases. It is one of the most powerful pyrethroid insecticides in the world. Due to its acute toxicity, the insecticide is extremely toxic in mice and induces hepatic and renal toxicity in rats. In cells in vitro it induces micronuclei, nucleoplasmic bridges, nuclear buds, necrosis, and apoptosis, which is positively related with carcinogenicity [59]. The main mechanisms of cytotoxicity of λ-cyhalothrin are hepatotoxicity, neurotoxicity, nephrotoxicity, and reproductive toxicity, as well as induction of oxidative stress leading to mitochondrial, lipid, DNA, and protein damage in cells [60]. The overall mechanism of toxic action of λ-cyhalothrin is due to its chemical structure. However, this mechanism is not fully understood and is the subject of systematic research [60].
2.3. Cytotoxicity of Microbial Metabolites
Sf-9 and HeLa cells were exposed to the microbial metabolites for 48 h. L. plantarum KB2 LAB 03 metabolites were more cytotoxic to Sf-9 cells at physiological pH than at neutral pH (Table 4). L. plantarum KB2 LAB 03 metabolites showed greater cytotoxicity after culturing in MRS than after culturing in AM at physiological pH. The cytotoxicity of L. plantarum KB2 LAB 03 fermentation by-products against the HeLa cell line showed less variation. However, the metabolites cultured in MRS also showed greater cytotoxicity than those cultured in AM. Similarly, greater cytotoxicity was observed for yeast metabolites towards Sf-9 cells at physiological pH. The highest cytotoxicity was observed for metabolites cultured on sYPG medium and the lowest after culturing in sYP. In the case of the metabolites cultured on sYPG, no cytotoxicity was observed at pH 6.2, but an enhanced pro-proliferative effect was noted. Yeast cultured in sYP at both pH options had the same effect on HeLa cells (Table 4). The yeast metabolites cultured in sYPG also showed the strongest cytotoxicity towards HeLa cells.

Table 4.
Cytotoxic (IC50) and non-cytotoxic (IC0) values for L. plantarum KB2 LAB 03 and M. pulcherrima TK1 metabolites cultivated in different media against insect Sf-9 and human HeLa cell lines.
In our previous studies, we identified the main metabolites of L. plantarum KB2 LAB 03 after cultivation in MRS medium. The metabolites were mostly lactic, acetic, and isobutyric acids. The main fermentation by-products of the strain cultured in AM (acid whey-based) medium were lactic, acetic, and propionic acids, as well as ethanol [5]. We also studied the main metabolites of M. pulcherrima TK1 after cultivation in YPD medium. The main metabolites were ethanol and glycerol, as well as lactic, succinic, and acetic acids. After fermentation in sYP (acid whey-based) medium, the by-products were lactic acid, ethanol, glycerol, acetic, and succinic acids [8]. Organic acids such as propionic acid can also suppress the viability of HeLa cells, by inducing ROS generation and dysfunction of the mitochondrial membrane, leading to autophagy [61]. Elsewhere, we showed that pure lactic, acetic, and propionic acids inhibit the growth of Caco-2 cells [62]. We screened the cytotoxicity of metabolites of 39 LAB strains against Caco-2 cells (at neutralised pH). The metabolite concentrations ranged from 10 to 200 mg/mL. Cytotoxicity depended on the concentration of metabolites: the higher their concentration, the higher the cytotoxicity.
In the current research, we found LAB metabolite concentrations ranging from 0.001 to 200 mg/mL. Their cytotoxic effect may be related to the acidic pH of some LAB metabolites. In the case of Sf-9 cells, cytotoxicity increased at lower sample pH. Such a relationship was not observed for HeLa cells cultivated with LAB fermentation by-products and not at all for yeast metabolites. This may indicate that a different mechanism is responsible for their cytotoxicity. It has been suggested that the protein nature of secreted metabolites may be responsible for their cytotoxicity [63]. According to the literature, several cell components (cytoplasmic fractions, fermentation by-products including organic acids, cell wall components, exopolysaccharides (EPS), peptidoglycan, conjugated linoleic acids, and S-layer proteins) can have strong cytotoxic effects on various cell lines [64,65,66]. Bacteriocins such as plantaricin A produced by L. plantarum and enzymes such as arginine deiminase produced by some strains of LAB can also inhibit the growth of some cells in vitro [66]. Cell wall components of yeast, β-glucan and chitin, can have the same effect [67,68]. Generally, the cytotoxicity of microbial fermentation by-products depends on the strain being tested, the cell line, and the culture medium. The main mechanisms of their cytotoxic activity are cell cycle arrest, apoptosis, necrosis, increased ROS production, and decreased intracellular ATP and MMP [60,62,69].
2.4. Genotoxicity of Plant Aqueous Extracts
After screening for cytotoxicity, extracts with concentrations close to or below the IC50 values were selected for further testing for genotoxicity in the comet assay (Table 5 and Table 6). Due to their different cytotoxic activity against the two cell lines, different concentrations were used. Concentrations higher than the IC50 values induced severe, unmeasurable DNA damage (apoptotic cells), so were not tested. Due to the wide variety of concentrations tested in the cytotoxicity assays, they could not be standardized in genotoxicity tests.

Table 5.
Genotoxicity of plant aqueous extracts against the insect Sf-9 cell line. Number sign (#) denotes values that are statistically significant relative to the negative control. The same letters denote statistically significant differences between results at the same tested concentration (ANOVA, p ≤ 0.05).

Table 6.
Genotoxicity of plant aqueous extracts against model human HeLa cell line. Number sign (#) denotes values that are statistically significant relative to the negative control; the same letters denote statistically significant differences between results at the same tested concentration (ANOVA, p ≤ 0.05).
The rate of DNA damage in the negative controls was 2.35% ± 5.74% (for HeLa cells) and 4.54% ± 0.48% (for Sf-9 cells). At the tested concentration of 1.3 mg/mL, λ-cyhalothrin was 4-fold more cytotoxic against Sf-9 cells than HeLa cells. It was noticed that λ-cyhalothrin always displayed the strongest genotoxicity, which was also greater against insect Sf-9 cells than human HeLa cells. The genotoxicity of λ-cyhalothrin against Sf-9 cells was 42.32% ± 3.59% at a concentration of 0.63 mg/mL and 42.62% ± 3.31% at a concentration of 1.3 mg/mL. Against HeLa cells, its genotoxicity was 10.46% ± 2.56% at 1.3 mg/mL and 35.20% ± 4.00% for concentrations of 1.3 and 2.5 mg/mL, respectively.
Sf-9 cells were more sensitive to garlic and turmeric extracts than HeLa cells at similar concentrations, and genotoxicity was not proportional to concentration. Garlic extract showed the highest genotoxicity against Sf-9 cells (close to 50%), and clove extract showed the highest genotoxicity against HeLa cells (up to 44.55%) at a concentration of 50 mg/mL (Figure 3).
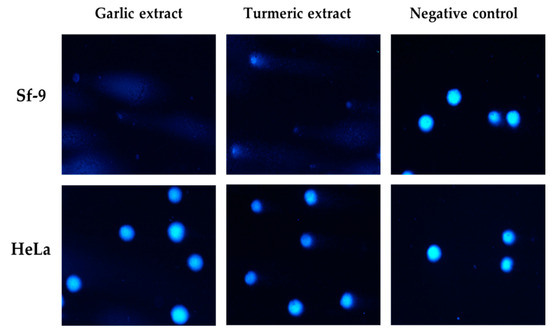
Figure 3.
Representative photographs of comet cells after exposure to garlic and turmeric aqueous extracts (62.5 mg/mL), stained with DAPI, fluorescence microscopy (Nikon, Tokyo, Japan), 20× objective.
The variability of the raw material and extracted components is a significant factor that limits studies on the toxicity of extracts from different plants. Aqueous and alcoholic garlic extracts have been shown to induce genotoxicity in peripheral blood lymphocytes, as evaluated in the comet assay, and cause chromosome aberrations in human leucocytes [70,71]. The main component of our garlic extract was 5-HMF, which can demonstrate genotoxicity at higher concentrations [72]. 5-HMF can induce DNA damage in Hep-G2 cells, suggesting a weak genotoxic effect, probably due to its rapid cell repair capacity [73]. This weak genotoxic effect may explain why in our study HeLa cells were practically resistant to the activity of garlic extract. According to the literature, eugenol—the main component of clove extract—can induce moderate to severe genotoxic effects in hamster lung fibroblasts V79, in a dose-dependent way [74]. In our study, eugenol may have been responsible for the medium genotoxicity of clove extract against the tested cells. Beltzig et al. [75] investigated the genotoxicity of ethanolic solvent and micellar curcumin against several primary and cancerous cell lines. They showed that curcumin had similar genotoxic effects against the cancerous cell lines, but also observed high efficiency of DNA repair after its removal. Crude extract of Curcuma longa L. demonstrates genotoxic activity against genomic DNA in the human tumour cell lines: prostate DU-145 and colon HT-29 [76]. Curcumin at high concentrations displayed a significant increase in MN frequency in the micronucleus (MN) test [77]. The main components of sage extract, camphor, eucalyptol, and α-thujone induced evident DNA breaks in human colon HT-29 and HCT 116 cells, foetal lung fibroblasts MRC-5, and Vero (kidney monkey) cells [57]. These compounds may be responsible for the genotoxicity of sage extract observed in our study.
2.5. Genotoxicity of Microbial Metabolites
After screening for cytotoxicity, extract concentrations close to or below the IC50 value were selected for further testing (Table 7, Table 8, Table 9 and Table 10, Figure 4). Due to their different cytotoxic activity against the two cell lines, the tested concentrations were also different. Concentrations higher than the IC50 values induced very strong unmeasurable DNA damage (including apoptotic cells), so they were not used. In general, the genotoxicity of the bacterial metabolites was not dependent on the pH or concentration (Table 7). The genotoxicity of the metabolites against Sf-9 cells was not dependent on their concentration and remained at similar levels. Metabolites of L. plantarum in AM at physiological pH showed the highest genotoxicity (54.52 ± 4.45%) at a concentration of 50 mg/mL compared to the other bacterial samples (p ≤ 0.05). Yeast metabolites showed generally higher genotoxicity than LAB metabolites. Independently of pH, yeast metabolites in sYPG showed the strongest genotoxicity (Table 8), which was correlated with cytotoxicity. On YPG, greater genotoxicity was observed at physiological (acidic) pH.

Table 7.
Genotoxicity of L. plantarum KB2 LAB 03 metabolites after cultivation in different media against the insect Sf-9 cell line. Number sign (#) denotes values that are statistically different from the negative control; the same letters denote statistically significant differences between results at the same tested concentrations (ANOVA, p ≤ 0.05).

Table 8.
Genotoxicity of M. pulcherrima TK1 metabolites after cultivation in different media against the insect Sf-9 cell line. Number sign (#) denotes values that are statistically different from the negative control; the same letters denote statistically significant differences between results at the same tested concentrations (ANOVA, p ≤ 0.05).

Table 9.
Genotoxicity of L. plantarum KB2 LAB 03 metabolites after cultivation in different media against the human HeLa cell line. Number sign (#) denotes significant differences relative to the negative control.

Table 10.
Genotoxicity of M. pulcherrima TK1 metabolites after cultivation in different media against the human HeLa cell line. Number sign (#) denotes values that are statistically significant relative to the negative control.
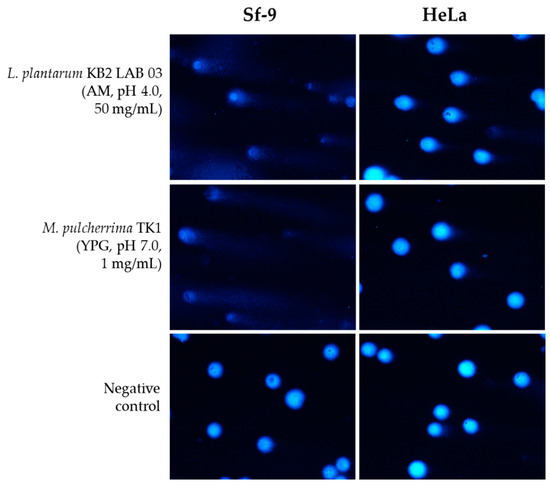
Figure 4.
Representative photographs of comet cells after exposure to L. plantarum KB2 LAB 03 and M. pulcherrima TK1 exemplary metabolites, stained with DAPI, fluorescence microscopy (Nikon, Tokyo, Japan), 20× objective.
The bacterial and yeast metabolites showed no or weak genotoxicity against HeLa cells, compared to the Sf-9 line (Table 9 and Table 10). Genotoxicity was weakly or uncorrelated with metabolite concentration. Metabolites of L. plantarum KB2 LAB 03 in MRS showed the highest but weak genotoxicity regardless of pH, and in AM these metabolites showed the lowest genotoxicity. In the case of HeLa cells, yeast metabolites showed greater genotoxicity than LAB metabolites. Yeast metabolites in sYPG medium showed the weakest genotoxicity at the tested concentrations.
To the best knowledge of the authors, the genotoxicity of by-products of fermentation by LAB and yeast on cell lines has not previously been studied. Since one of the mechanisms for the toxic effects of the tested microbial fermentation by-products may be increased intracellular ROS production, they can induce oxidative damage to DNA, leading to apoptosis [65,78]. Ethanol can also be genotoxic, as was shown in a comet assay using peripheral blood lymphocytes as well as human primary gastric and colon mucosa [79].
3. Materials and Methods
3.1. Biopreparations Design from Lactiplantibacillus Plantarum KB2 LAB 03 and Metschnikowia pulcherrima TK1
Analyses were conducted according to the method described elsewhere [11]. Briefly, Lactiplantibacillus plantarum KB2 LAB 03 and Metschnikowia pulcherrima TK1 strains were used to obtain bacterial biopreparations against potato phytopathogens [5,10]. These strains were selected based on our previous studies, as they produced the most bioactive metabolites that inhibited the growth of potato phytopathogens. The strains were stored in Cryobanks™ at −20 °C. Before the experiments, they were activated, threefold passaged, and cultured. L. plantarum KB2 LAB 03 were cultured in deMan–Rogosa–Sharp (MRS, Merck Life Science, Poznań, Poland) broth for 24 h at 30 °C. M. pulcherrima TK1 were cultured in YPG (Yeast Extract, Peptone, Glucose; BTL, Warsaw, Poland) broth for 72 h at 25 °C on a shaker (Unimax 1010, Schwabach, Germany) at 160 rpm.
L. plantarum KB2 LAB 03 was also activated and cultured on an acid whey-based medium (abbreviated as AM) supplemented with 1.4% peptone K, 0.8% yeast extract (BTL, Poland), 0.2% dipotassium phosphate (Chempur, Poland), 0.2% ammonium citrate (Chempur, Poland), 0.02% magnesium sulphate heptahydrate (Chempur, Poland), 0.5% sodium acetate (Chempur, Poland), and 0.005% magnesium sulphate tetrahydrate (Chempur, Poland). The bacteria were cultured for 48 h at 30 °C.
M. pulcherrima TK1 was also activated and cultured on an acid whey-based medium supplemented with 1% glucose, 0.25% peptone, and 0.13% yeast extract (abbreviated as sYPG) or 0.25% peptone and 0.25% yeast extract (abbreviated as sYP) for 72 h at 25 °C on a shaker at 160 rpm.
After incubation, the samples were centrifuged (10,733× g, 15 min) and decanted. Two supernatant options were used for cyto- and genotoxicity testing—i.e., at physiological pH and after neutralization. The pH was adjusted to 7.0 ± 0.1 (with 0.1 M NaOH and HCl). Finally, the supernatants were filtered with sterile 0.22 μm pore size syringe filters and frozen at −20 °C until analysis.
As a result, several variants of supernatants (post-fermentation fluids) were obtained after microbial cultivation of:
- L. plantarum KB2 LAB 03 in MRS at pH 4.0 and 7.0;
- L. plantarum KB2 LAB 03 in AM at pH 4.0 and 7.0;
- M. pulcherrima TK1 in YPG at pH 5.9 and 7.0;
- M. pulcherrima TK1 in sYP at pH 6.2 and 7.0;
- M. pulcherrima TK1 in sYPG at pH 5.4 and 7.0.
By-products of L. plantarum KB2 LAB 03 and M. pulcherrima TK1 fermentation on the abovementioned media were determined in our previous studies using high-performance liquid chromatography (HPLC) [5,8].
3.2. Design of Biopreparations from Plant Extracts
3.2.1. Plant Material and Extraction Conditions
Plant material was purchased from Dary Natury Sp. z o. o., Grodzisk, Poland, and Herbal Plant KAWON–HURT Nowak sp.j., Gostyń, Poland. The plant material consisted of sage (Salvia officinalis L.) leaves and stems, turmeric (Curcuma longa L.) roots, garlic (Allium sativum L.) bulbs, and clove (Syzygium aromaticum L. Merr. & Perry) flower buds. The aqueous plant extracts were prepared as described elsewhere [11]. First, 50 g of finely ground material was poured into 500 mL of water at 100 °C and left covered without stirring for 1 h. The plant extracts were then sonicated (40 kHz, 25 °C, 30 min) and filtered under reduced pressure.
3.2.2. Gas Chromatography Mass Spectrometry (GC-MS) Analysis of Chemical Composition
The volatile components extracted from the plant extracts were analysed and identified by gas chromatography–mass spectrometry(GC–MS) combined with Kovats retention index. All data were compared to Wiley, Adams, and Nist libraries. Gas chromatography–mass spectrometry (GC–MS) analyses were conducted as described elsewhere [10], using a Thermo Ultra GC Trace with a flame ionization detector, a Thermo DSQ II mass spectrometer (split flow), and Rxi®—1 ms (60 m × 0.25 mm × 0.25 µm film thickness) column from RESTEK. The detector temperature (FID) was 300 °C, the injector temperature was 280 °C. The temperature of the transfer line was 280 °C. The temperature of the ion source was 220 °C. The injection quantity was 1 µL with a 15 mL/min split for aqueous extract samples, and 0.5 µL with a 100 mL/min split for CO2 extract samples. Based on electron spectra from the NIST 2011 library and the Kovats index, the volatile compounds were identified.
3.3. Cell Cultures
The insect cell line from pupa ovarian tissues Spodoptera frugiperda (Sf-9, fall armyworm) was cultured as a monolayer in ready-to-use Sf-900™ III Serum-Free Medium (Thermo Fisher Scientific, Waltham, MA, USA) at 27 °C, in a non-humidified ambient air-regulated incubator (Binder BD 56, GmbH, Tuttlingen, Germany) for 10–14 days until it reached 80% confluence. HeLa (human cervix adenocarcinoma) cells were cultured in high-glucose DMEM (Merck Life Science, Poland), with the addition of 10% fetal bovine serum (FBS), 4 mM GlutaMAXTM (Thermo Fisher Scientific, Waltham, MA, USA), 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) (Merck Life Science, Poland), and 100 µg/mL streptomycin/100 IU/mL penicillin (Merck Life Science, Poland). The Sf-9 cells were purchased from ThermoFisher Scientific, Carlsbad, CA, USA (Lot No. 2177129), while the Caco-2 cells were purchased from Cell Line Services GmbH Eppelheim, Germany (Lot No. 300137-1514SF). Cells were cultured as a monolayer at 37 °C, with 5% CO2, in a humidified incubator (Galaxy 48S, New Brunswick, United Kingdom) for 3–5 days, until they reached 80% confluence. Every 2–3 days, the cells were washed with phosphate buffer saline (PBS, Merck Life Science, Poland) (pH 7.2) without calcium and magnesium, and the medium was renewed. The confluent Sf-9 cells were detached from the substrate by gentle pipetting, centrifuged (200× g, 5 min), and decanted. The pellet was resuspended in a fresh culture medium. The confluent HeLa cells were detached with TrypLETM Express (Thermo Fisher Scientific, Waltham, MA, USA) (37 °C, 8–10 min), centrifuged (307× g, 5 min), and decanted. The pellet was resuspended in a fresh culture medium. After the detachment procedure, a cell count was performed using a hemocytometer and cell viability was determined by trypan blue exclusion. The cells were then ready to use. The viability of the cells used in the experiments was at least 80% (Sf-9) and 90% (HeLa).
3.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
The cytotoxicity of the samples was assessed using the MTT assay. A total of 5000 (HeLa) or 20,000 (Sf-9) cells/well were placed in 96-well plates and incubated for 24 h at 37 °C in 5% CO2 (HeLa) or at 27 °C in non-humidified incubator (Sf-9). The next day, the medium was removed and dilutions of the biopreparations and plant extracts were added on a cell monolayer. The final concentrations of the analysed samples were as follows (mg/mL): 0.39–250 (all plant extracts, Sf-9 cells; garlic and curcuma extracts, HeLa cells); 0.39–100 (sage and clove extracts, HeLa cells); 0.001–200 (metabolites of microorganisms for both cell lines). Each concentration was tested in four replicates. The cells were exposed to the extracts for 24 h and to the metabolites for 48 h. Efforts were made to select the exposure time and concentration in such a way as to determine the IC0 (non-toxic concentration) and IC50 values (the concentration of the test compound that reduces cell viability by 50% compared to the negative control). In the case of low cytotoxicity, the IC20 value was calculated accordingly. Several experiments were performed for the extracts in order to refine the method and determine the IC50 values. The experiment was repeated for comparison using the insecticide λ-cyhalothrin (Merck Life Science, Poland), which is used to control insect pathogens attacking potatoes at concentrations of 0.16–5.0 (Sf-9 cells) and 0.02–5.0 (HeLa cells). Negative controls were non-exposed cells.
After the exposure time, test samples were gently aspirated and MTT (0.5 mg/mL in PBS) was added to each well and incubated for further 3 h. The MTT was then aspirated and dimethyl sulfoxide (DMSO) (Merck Life Science, Poland) was added to dissolve the formazan crystals. Absorbance was measured at 550 nm (using a 620 nm reference filter) in a microplate reader (TriStar2 LB 942, Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). Negative control absorbance represented 100% cell viability. Cell viability (%) was calculated as (sample OD/control OD) × 100%. Cytotoxicity (%) was calculated as 100-cell viability. The results are presented as the mean of four individual readings ± the standard deviation (±SD). The IC0, IC20, and IC50 values were estimated from the resulting curves.
3.5. Single-Cell Electrophoresis Assay (Comet Assay)
Eppendorf tubes were loaded with the appropriate cell culture medium (Sf-9 or HeLa), 1 × 105 cells/sample and the test sample (plant extract or metabolites), so that the final volume was 1 mL. The final volume of the test sample was 20% (v/v). The concentrations of the test samples were selected on the basis of cytotoxicity tests. Samples with cytotoxicity ≤IC50 were selected. The final test concentrations were 1.25–125 mg/mL (Sf-9) and 1.56–250 mg/mL (HeLa) for the extracts and 0.01–100 (Sf-9) and 0.1–200 (HeLa) for metabolites. Cells suspended in the medium without the addition of compounds were used as a negative control. The samples were incubated for 60 min at 27 °C (Sf-9) and 37 °C (HeLa), then centrifuged (15 min, 4 °C, 182× g) and decanted. Low Melting Point (LMP) agarose (Merck Life Science, Poland) was added at 37 °C. The suspension was spotted on warm Normal Melting Point (NMP) agarose double-layered slides and covered with coverslips (hot plate ZF6 Premiere Slide Warmer). Next, the samples were placed on a Chilling Plate for Comet Assay Slides (Cleaver Scientific, Rugby, UK) and allowed to solidify. Alkaline lysis was performed with buffer (2.5 M NaCl, 1% Triton X-100, 100 mM EDTA, 10 mM Tris, pH 10), and the reaction mixture was incubated (60 min, 4 °C). The lysis buffer was decanted, and the slides were flooded with the unwinding buffer (300 mM NaOH, 1 mM EDTA) (20 min, 4 °C). The slides were then placed in an electrophoresis apparatus (CSL-COM20, Cleaver Scientific). Electrophoresis was performed in electrophoretic buffer (300 mM NaOH, 1 mM EDTA, pH > 13) for 20 min at a voltage of 21 V and a current of 29 mA. The slides were neutralized, allowed to dry, and then stained for 60 min at 4 °C with 4′,6-diamidino-2-phenylindole (DAPI) (1 µg/mL). Comet analysis was performed under a fluorescence microscope (Nikon) at a magnification of 200× equipped with a camera (Nikon Digital Sight DS-U3) and with Lucia Comet v.7.0 software (Laboratory Imaging, Prague, Czech Republic). In each trial, 50 randomly selected comets were analysed based on the parameter determining the percentage of DNA in the comet’s “tail”. The results are presented as the mean ± S.E.M.
3.6. Statistical Analysis
Statistical analysis was conducted using one-way ANOVA followed by Tukey’s multiple-comparisons post hoc test, performed using OriginPro 6.1 (Northampton, MA, USA) software at a significance level of p ≤ 0.05. Differences between samples with normal distributions were evaluated using a Student’s t-test.
4. Conclusions
The agricultural sector faces significant challenges due to the rising resistance to chemical pesticides and emerging environmental threats, which also have implications for public health. In this context, one of the most critical strategies in sustainable agriculture is the use of biocontrol microorganisms and natural plant extracts to suppress phytopathogens. Equally important is understanding the dual role of these biocontrol agents—not only in managing plant diseases but also in ensuring safety for humans and contributing to the preservation of biodiversity. In this study, we evaluated the cytotoxicity and genotoxicity of potential biocontrol agents for use against phytopathogens of potato seed (Solanum tuberosum L.). The tested plant extracts with potential biocontrol functions showed cytotoxicity and genotoxicity against both tested cell lines: the insect cell line Sf-9 and the human cell line HeLa. The most cytotoxic extract towards the Sf-9 line was garlic extract. The least cytotoxic was clove extract. Sage extract showed the highest cytotoxicity potential towards the HeLa cell line. Garlic extract showed the lowest cytotoxicity potential towards this cell line. Garlic and turmeric extracts showed stronger cytotoxicity towards Sf-9 insect cells than towards HeLa cells. Garlic extract showed the highest genotoxicity towards Sf-9 cells, and clove extract showed the highest genotoxicity towards HeLa cells. Both extracts and microbial metabolites showed stronger genotoxicity against Sf-9 cells (normal insect cells) than HeLa lines (model human cancer cells). The metabolic profiles of M. pulcherrima TK1 showed stronger cytotoxicity and genotoxicity against both tested cell lines than metabolites of the L. plantarum KB2 strain. More work is needed to understand how the presence of biocontrol agents in the environment can impact biodiversity. It is important not only to examine the effects of individual natural factors but also to explore their interactions with other biocontrol agents. Of course, any analytical approach must take into account the natural high variability of samples, such as plant extracts. Our research focused on Polish plants and their extracts. Any further studies must therefore take into account the geographical and climatic variability of the raw material used. In addition, future studies should aim to expand the use of in vitro cell line models while incorporating in vivo investigations, particularly in insect systems, to provide a more comprehensive understanding of these interactions and their implications. Our in vitro findings provide a foundation for further research. Future studies incorporating in vivo validation will be our next step.
Author Contributions
Conceptualization, B.G., D.K. and A.N.; methodology, A.N. and A.S.; software, A.N. and A.S.; validation, A.N.; investigation, A.N. and A.S.; resources, A.N. and B.G.; data curation, A.N. and A.S.; writing—original draft preparation, A.N. and D.K.; writing—review and editing, A.N. and D.K.; visualization, A.N.; supervision, D.K. and B.G.; project administration, B.G.; funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agency for Restructuring and Modernization of Agriculture, Poland [grant number 00010.DDD.6509.00016.2018.05] and the Polish National Agency for Academic Exchange under the PROM program, co-financed by the European Social Fund under the Knowledge Education Development Operational Program, implemented by the Lodz University of Technology. The APC was funded by the Lodz University of Technology, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Konrad Jastrząbek from the Institute of Natural Products and Cosmetics, Lodz University of Technology for his assistance in the HPLC analysis of the composition from sage (Salvia officinalis L.) and turmeric (Curcuma longa L.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef] [PubMed]
- Deepa, N.; Achar, P.N.; Sreenivasa, M.Y. Current perspectives of biocontrol agents for management of Fusarium verticillioides and its fumonisin in cereals—A review. J. Fungi 2021, 7, 776. [Google Scholar] [CrossRef] [PubMed]
- Perczak, A.; Goliński, P.; Bryła, M.; Waśkiewicz, A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arh Hig Rada Toksikol. 2018, 69, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Steglińska, A.; Kołtuniak, A.; Motyl, I.; Berłowska, J.; Czyżowska, A.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Lactic acid bacteria as biocontrol agents against potato (Solanum tuberosum L.) pathogens. Appl. Sci. 2022, 12, 7763. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: Fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Van Leeuwenhoek 2019, 112, 1425–1445. [Google Scholar] [CrossRef] [PubMed]
- Steglińska, A.; Kołtuniak, A.; Berłowska, J.; Czyżowska, A.; Szulc, J.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Metschnikowia pulcherrima as a biocontrol agent against potato (Solanum tuberosum) pathogens. Agronomy 2022, 12, 2546. [Google Scholar] [CrossRef]
- Šernaitė, L. Plant extracts: Antimicrobial and antifungal activity and appliance in plant protection (Review). Sodinink. Daržinink. 2017, 36, 58–61. [Google Scholar]
- Steglińska, A.; Bekhter, A.; Wawrzyniak, P.; Kunicka-Styczyńska, A.; Jastrząbek, K.; Fidler, M.; Śmigielski, K.; Gutarowska, B. Antimicrobial activities of plant extracts against Solanum tuberosum L. phytopathogens. Molecules 2022, 27, 1579. [Google Scholar] [CrossRef] [PubMed]
- Steglińska, A.; Sulyok, M.; Janas, R.; Grzesik, M.; Liszkowska, W.; Kręgiel, D.; Gutarowska, B. Metabolite formation by fungal pathogens of potatoes (Solanum tuberosum L.) in the presence of bioprotective agents. Int. J. Environ. Res. Public Health 2023, 20, 5221. [Google Scholar] [CrossRef] [PubMed]
- Amichot, M.; Bertrand, C.; Chauvel, B.; Corio-Costet, M.F.; Martin-Laurent, F.; Le Perchec, S.; Mamy, L. Natural products for biocontrol: Review of their fate in the environment and impacts on biodiversity. Environ. Sci. Pollut Res. Int. 2024. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lu, L.; Huang, P.; Yu, B.; Peng, L.; Zou, L.; Ren, Y. Insect cell-based models: Cell line establishment and application in insecticide screening and toxicology research. Insects 2023, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Gallampois, C.M.; Krauss, M.; Meringer, M.; Neumann, S.; Schulze, T.; Wolf, S.; Brack, W. Consensus structure elucidation combining GC/EI-MS, structure generation, and calculated properties. Anal. Chem. 2012, 84, 3287–3295. [Google Scholar] [CrossRef]
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol activity of aromatic and medicinal plants and their bioactive components against soil-borne pathogens. Plants 2023, 12, 706. [Google Scholar] [CrossRef]
- López-Cabeza, R.; Rodríguez-Sabina, S.; Reyes, C.P.; Expósito, D.G.; Giménez, C.; Jiménez, I.A.; Cabrera, R.; Bazzocchi, I.L. Bio-guided isolation of aromatic abietane diterpenoids from Salvia canariensis as biopesticides in the control of phytopathogenic fungi. Pest Manag. Sci. 2024, 80, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, C.J. Drugs and lactation database: LactMed. J. Electron. Resour. Med. Libr. 2012, 9, 272–277. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Camphor and eucalyptol-anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef] [PubMed]
- Fotiadou, E.; Panou, E.; Graikou, K.; Sakellarakis, F.N.; Chinou, I. Volatiles of all native Juniperus species growing in Greece-antimicrobial properties. Foods 2023, 12, 3506. [Google Scholar] [CrossRef] [PubMed]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The use of essential oils from thyme, sage and peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Sigma Aldrich Data Sheet 2-Metoksy-4-vinylphenol. Available online: https://www.sigmaaldrich.com (accessed on 19 November 2024).
- Senouci, H.; Benyelles, N.G.; Dib, M.E.A.; Costa, J.; Muselli, A. Chemical composition and combinatory antifungal activities of Ammoides verticillata, Allium sativum and Curcuma longa essential oils against four fungi responsible for tomato diseases. Comb. Chem. High Throughput Screen. 2020, 23, 196–204. [Google Scholar] [CrossRef]
- Snetkov, P.; Rogacheva, E.; Kremleva, A.; Morozkina, S.; Uspenskaya, M.; Kraeva, L. In-vitro antibacterial activity of curcumin-loaded nanofibers based on hyaluronic acid against multidrug-resistant ESKAPE pathogens. Pharmaceutics 2022, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018, 98, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; Gisder, S.; Hedtke, K.; Hunter, W.B.; Möckel, N.; Müller, U. Standard methods for cell cultures in Apis mellifera research. J. Apic. Res. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Islam, M.S.; Kusumoto, Y.; Al-Mamun, M.A. Cytotoxicity and cancer (HeLa) cell killing efficacy of aqueous garlic (Allium sativum) extract. J. Sci. Res. 2011, 3, 375–382. [Google Scholar] [CrossRef]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude garlic extract inhibits cell proliferation and induces cell cycle arrest and apoptosis of cancer cells in vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Jasamai, M.; Hui, C.S.; Azmi, N.; Kumolosasi, E. Effect of Allium sativum (garlic) methanol extract on viability and apoptosis of human leukemic cell lines. Trop. J. Pharm. Res. 2016, 15, 1479–1485. [Google Scholar] [CrossRef][Green Version]
- Szychowski, K.A.; Binduga, U.E.; Rybczyńska-Tkaczyk, K.; Leja, M.L.; Gmiński, J. Cytotoxic effects of two extracts from garlic (Allium sativum L.) cultivars on the human squamous carcinoma cell line SCC-15. Saudi J. Biol. Sci. 2018, 25, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Rybczyńska-Tkaczyk, K.; Gaweł-Bęben, K.; Swieca, M.; Karas, M.; Jakuczyk, A.; Matysiak, M.; Binduga, U.E.; Gminski, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Babu, B.C.; Sunil, A.; Mukunda, A.; Pynadath, M.K.; Aswathy, A.M. Anti-cancer potency of garlic (Allium sativum) extract in comparison to 5-fluorouracil—An in vitro study. Oral Maxillofac. Patho. J. 2020, 11, 11–15. [Google Scholar]
- Țigu, A.B.; Moldovan, C.S.; Toma, V.-A.; Farcaș, A.D.; Moț, A.C.; Jurj, A.; Fischer-Fodor, E.; Mircea, C.; Pârvu, M. Phytochemical analysis and in vitro effects of Allium fistulosum L. and Allium sativum L. Extracts on human normal and tumor cell lines: A comparative study. Molecules 2021, 26, 574. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhao, Y.; Jiang, Z.; Chen, J. Acetaldehyde induces cytotoxicity via triggering mitochondrial dysfunction and overactive mitophagy. Mol. Neurobiol. 2022, 59, 3933–3946. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhao, Y. Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca(2+) levels. Redox Biol. 2020, 28, 101381. [Google Scholar] [CrossRef]
- Hussain, A.; Sasidharan, S.; Ahmed, T.; Ahmed, M.; Sharma, C. Clove (Syzygium aromaticum) extract potentiates gemcitabine cytotoxic effect on human cervical cancer cell line. Int. J. Cancer Res. 2009, 5, 95–104. [Google Scholar] [CrossRef][Green Version]
- Hussein, N.H.; Maeah, R.K.; Sharba, Z.A.; Aasoon, B.; Sulaiman, G.M.; Ali, A.A.; Taha, A.; Jwad, K.H. Cytotoxic, antioxidant and antibacterial activities of crude extract of Syzygium aromaticum plant. Plant Arch. 2019, 19, 350–355. [Google Scholar]
- Yassin, M.T.; Al-Askar, A.A.; Abdel-Fattah Mostafa, A.; El-Sheikh, M.A. Bioactivity of Syzygium aromaticum (L.) Merr. & L.M. Perry extracts as potential antimicrobial and anticancer agents. J. King Saud Univ.-Sci. 2020, 32, 3273–3278. [Google Scholar] [CrossRef]
- Imade, R.O.; Ayinde, B.A. GC-MS analysis and in vitro cytotoxic activity of Syzygium aromaticum volatile oil and its major constituent–eugenol. J. Sci. Pract. Pharm. 2021, 8, 432–440. [Google Scholar] [CrossRef]
- Nasomyon, T.; Charoonratana, T. Comparative chemical composition and in-vitro cytotoxic activity of Syzygium aromaticum against selected cancer cell lines. Trop. J. Nat. Prod. Res. 2021, 5, 331–335. [Google Scholar] [CrossRef]
- Mekky, A.E.; Emam, A.E.; Selim, M.N.; Abdelmouty, E.S.; Khedr, M. Antibacterial and antineoplastic MCF-7 and Hep-G2 characteristics of the methanolic (80%) clove (Syzygium aromaticum L.) extract. Biomass Conv. Bioref. 2024, 14, 16787–16798. [Google Scholar] [CrossRef]
- Pasri, P.; Mermillod, P.; Khempaka, S. Antioxidant properties and cytotoxic effects of selected edible plants in Southeast Asia for further use as phytogenic antioxidant additives. Saudi J. Biol. Sci. 2023, 30, 103631. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; K, H.; Kannan SK, D.; Raj K, H.; Jayaprakash, B. Evaluation of therapeutic potential of eugenol-a natural derivative of Syzygium aromaticum on cervical cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1977–1985. [Google Scholar] [CrossRef]
- Abdulrahman, M.D.; Hama, H.A. Anticancer of genus Syzygium: A systematic review. Explor. Target Antitumor Ther. 2023, 4, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.; Hon, P.M.; Lee, M.Y.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.E.; Abdul Samad, M.; Bustami Effendi, T.J.; Hazizul Hasan, M. Cytotoxicity studies of aqueous extracts of Curcuma longa and Quercus infectoria. In Proceedings of the International Conference on Science and Social Research 2010, Kuala Lumpur, Malaysia, 5–7 December 2010; pp. 746–751. [Google Scholar] [CrossRef]
- Lawal, R.; James, A.B.; Shaibu, R.; Okoye, O.P.; Odetunde, S.A.; Ajibare, A.C. Cytotoxic effects of Curcuma longa leaves on MCF-7 and HepG2 cells. Fountain J. Natural Appl. Sci. 2022, 11, 31–35. [Google Scholar] [CrossRef]
- Abualhasan, M.; Jaradat, N.; Hawash, M.; Shraim, N.; Asaad, M.; Mousa, A.; Mousa, Z.; Tobeh, R.; Mlitat, B. Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine. Open Life Sci. 2023, 18, 20220767. [Google Scholar] [CrossRef]
- Firoz, H.M.; Nanjundaiah, S.; Sadashiva, C.T.; Neethumol, B.; Rashmi, Y.; Sreedrisya, A.K. Antiproliferative activity and apoptosis-inducing mechanism of Curcuma longa (Turmimax®) on HeLa cell lines. Braz. J. Biol. 2023, 83, e275953. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Choi, J.; Lee, J.; Lee, Y. Induction of apoptosis by Ar-turmerone on various cell lines. Int. J. Mol. Med. 2004, 14, 253–256. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]
- Sudarshan, K.; Perumal, G.; Aidhen, I.S.; Doble, M. Synthesis of Unsymmetrical Linear Diarylheptanoids and their Enantiomers and Antiproliferative Activity Studies. Eur. J. Org. Chem. 2018, 2018, 6379–6387. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Rupasinghe, H.P. Antiproliferative effects of extracts from Salvia officinalis L. and Saliva miltiorrhiza Bunge on hepatocellular carcinoma cells. Biomed. Pharmacother. 2017, 85, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zare Shahneh, F.; Valiyari, S.; Baradaran, B.; Abdolalizadeh, J.; Bandehagh, A.; Azadmehr, A.; Hajiaghaee, R. Inhibitory and cytotoxic activities of Salvia officinalis L. extract on human lymphoma and leukemia cells by induction of apoptosis. Adv. Pharm. Bull. 2013, 3, 51–55. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana aqueous extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed]
- Izham, M.N.M.; Hussin, Y.; Rahim, N.F.C.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Physicochemical characterization, cytotoxic effect and toxicity evaluation of nanostructured lipid carrier loaded with eucalyptol. BMC Complement. Med. Ther. 2021, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.; Vasilijević, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukćević, J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015, 242, 263–271. [Google Scholar] [CrossRef]
- Lopes, A.I.F.; Monteiro, M.; Araújo, A.R.L.; Rodrigues, A.R.O.; Castanheira, E.M.S.; Pereira, D.M.; Olim, P.; Fortes, A.G.; Gonçalves, M.S.T. Cytotoxic plant extracts towards insect cells: Bioactivity and nanoencapsulation studies for application as biopesticides. Molecules 2020, 25, 5855. [Google Scholar] [CrossRef] [PubMed]
- Laborde, M.R.R.; Larramendy, M.L.; Soloneski, S. Cytotoxic and genotoxic profiles of the pyrethroid insecticide lambda-cyhalothrin and its microformulation Karate® in CHO-K1 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2023, 891, 503682. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, Y.; Ling, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Maximiliano, J.E.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; et al. Oxidative stress and mitochondrial damage in lambda-cyhalothrin toxicity: A comprehensive review of antioxidant mechanisms. Environ. Pollut. 2023, 338, 122694. [Google Scholar] [CrossRef]
- Pham, C.H.; Lee, J.-E.; Yu, J.; Lee, S.H.; Yu, K.-R.; Hong, J.; Cho, N.; Kim, S.; Kang, D.; Lee, S.; et al. Anticancer effects of propionic acid inducing cell death in cervical cancer cells. Molecules 2021, 26, 4951. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Rosicka-Kaczmarek, J.; Motyl, I. Anticancer potential of post-fermentation media and cell extracts of probiotic strains: An in vitro study. Cancers 2022, 14, 1853. [Google Scholar] [CrossRef]
- Haghshenas, B.; Abdullah, N.; Nami, Y.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Different effects of two newly-isolated probiotic Lactobacillus plantarum 15HN and Lactococcus lactis subsp. lactis 44Lac strains from traditional dairy products on cancer cell lines. Anaerobe 2014, 30, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer. Biol. 2022, 86 Pt 3, 356–366. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Mahdy, O.M.; Mohamed, H.I.; El-Ansary, A.E. Antioxidants, antimicrobial, and anticancer activities of purified chitinase of Talaromyces funiculosus strain CBS 129594 biosynthesized using crustacean bio-wastes. Agronomy 2022, 12, 2818. [Google Scholar] [CrossRef]
- Upadhyay, T.K.; Trivedi, R.; Khan, F.; Al-Keridis, L.A.; Pandey, P.; Sharangi, A.B.; Alshammari, N.; Abdullah, N.M.; Yadav, D.K.; Saeed, M. In vitro elucidation of antioxidant, antiproliferative, and apoptotic potential of yeast-derived β-1,3-glucan particles against cervical cancer cells. Front. Oncol. 2022, 12, 942075. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Homayouni Rad, A.; Aghebati Maleki, L.; Samadi Kafil, H.; Baghbanzadeh, A. Cytotoxic potentials of cell-free supernatant derived from Lactobacillus casei CRL431 on HCT-116 and HT-29 human colon cancer cell lines. Biointerface Res. Appl. Chem. 2023, 13, 476. [Google Scholar] [CrossRef]
- Sekar, N.; Sundaramoorthy, R.; Majumdar, S.; Bhuyan, K.; Das, J.; Abilash, V.G. Determination of allicin in Allium sativum using high performance liquid chromatography and study of genotoxic effect on human leukocytes. Asian J. Pharm. Clin. Res. 2015, 8, 153–156. [Google Scholar]
- Waqar, Z.; Sajjad, U.R.; Ahsan, N.; Safdar, I.; Qurratulain, A.; Majeed, Q.; Maqbool, A.; Waseem Abbas, M. Genotoxicity assessment in aqueous and alcoholic extracts of Allium sativum, Zingiber officinale and Trachyspermum ammi. Curr. Trends Biomed. Eng. Biosci. 2019, 18, 555978. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Severin, I.; Dumont, C.; Jondeau-Cabaton, A.; Graillot, V.; Chagnon, M.C. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol. Lett. 2010, 192, 189–194. [Google Scholar] [CrossRef]
- Mohammadi Nejad, S.; Özgüneş, H.; Başaran, N. Pharmacological and toxicological properties of eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Beltzig, L.; Frumkina, A.; Schwarzenbach, C.; Kaina, B. Cytotoxic, genotoxic and senolytic potential of native and micellar curcumin. Nutrients 2021, 13, 2385. [Google Scholar] [CrossRef] [PubMed]
- Cosquillo-Rafael, M.F.; Placencia-Medina, M.D.; Miranda-Tomasevich, T.Y.; Moreno-Hinojosa, M.; Retuerto-Figueroa, M.G. In vitro cytotoxic and genotoxic effect of the crude and ethanolic extract from the rhizome of Curcuma longa L. Rev. Peru Med. Exp. Salud Publica 2020, 37, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, L.P.; Liu, Y.; Yang, G.; Yao, X.F.; Zhong, L.F. Curcumin-induced genotoxicity and antigenotoxicity in HepG2 cells. Toxicon 2007, 49, 1219–1222. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.R.; Anadón, A.; Wang, X.; et al. Cancer metabolism: The role of ROS in DNA damage and induction of apoptosis in cancer cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Blasiak, J.; Trzeciak, A.; Malecka-Panas, E.; Drzewoski, J.; Wojewódzka, M. In vitro genotoxicity of ethanol and acetaldehyde in human lymphocytes and the gastrointestinal tract mucosa cells. Toxicol. In Vitro 2000, 14, 287–295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).