The Impact of Selected Lachancea Yeast Strains on the Production Process, Chemical Composition and Aroma Profiles of Beers
Abstract
1. Introduction
2. Results
2.1. The Influence of Selected Lachancea Yeast Strains on the Kinetics of Beer Fermentation
2.2. The Effect of Selected Lachancea Yeast Strains on the Concentration of Sugars, Glycerol and Real Extract in Beers
2.3. The Effect of Selected Lachancea Yeast Strains on the Ethanol Concentration, pH and Caloric Value of the Beers Produced
2.4. The Effect of Selected Lachancea Yeast Strains on the Concentration of Organic Acids in Beers
| Citric Acid | Malic Acid | Succinic Acid | Lactic Acid | Acetic Acid | |
|---|---|---|---|---|---|
| g/L | |||||
| Wort | 0.86 ± 0.12 ab | 2.58 ± 0.41 a | 0.53 ± 0.41 | n/d | n/d |
| MN477031 | 0.68 ± 0.09 bcd | 1.97 ± 0.25 abc | 0.50 ± 0.47 | 0.01 ± 0.01 a | 0.3 ± 0.14 ac |
| PYCC7194 | 0.78 ± 0.08 bcd | 2.14 ± 0.18 a | 0.51 ± 0.40 | 6.97 ± 0.55 b | 0.03 ± 0.02 b |
| PYCC6701 | 0.58 ± 0.12 c | 1.74 ± 0.04 b | 0.45 ± 0.38 | 2.53 ± 0.81 c | n/d |
| PYCC4675 | 0.72 ± 0.01 d | 1.87 ± 0.05 c | 0.46 ± 0.28 | 1.69 ± 0.11 c | 0.04 ± 0.12 abc |
| PYCC5883 | 0.96 ± 0.11 ab | 2.40 ± 0.41 a | 0.57 ± 0.48 | 0.83 ± 0.05 d | 2.83 ± 0.57 d |
| PYCC5195 | 0.82 ± 0.22 abcd | 2.22 ± 0.23 a | 0.52 ± 0.18 | 0.12 ± 0.05 e | 0.53 ± 0.41 bc |
| PYCC4135 | 0.78 ± 0.11 abcd | 2.23 ± 0.21 a | 0.59 ± 0.76 | 0.13 ± 0.02 e | 0.16 ± 0.08 c |
| PYCC2908 | 0.58 ± 0.06 c | 1.68 ± 0.14 bc | 0.41 ± 0.25 | 2.22 ± 0.09 c | n/d |
| PYCC6986 | 0.64 ± 0.03 c | 1.76 ± 0.06 bc | 0.48 ± 0.35 | 0.68 ± 0.08 d | 0.01 ± 0.01 b |
| PYCC6375 | 0.76 ± 0.04 abd | 2.22 ± 0.07 a | 0.53 ± 0.33 | 2.19 ± 0.05 c | 0.22 ± 0.10 ac |
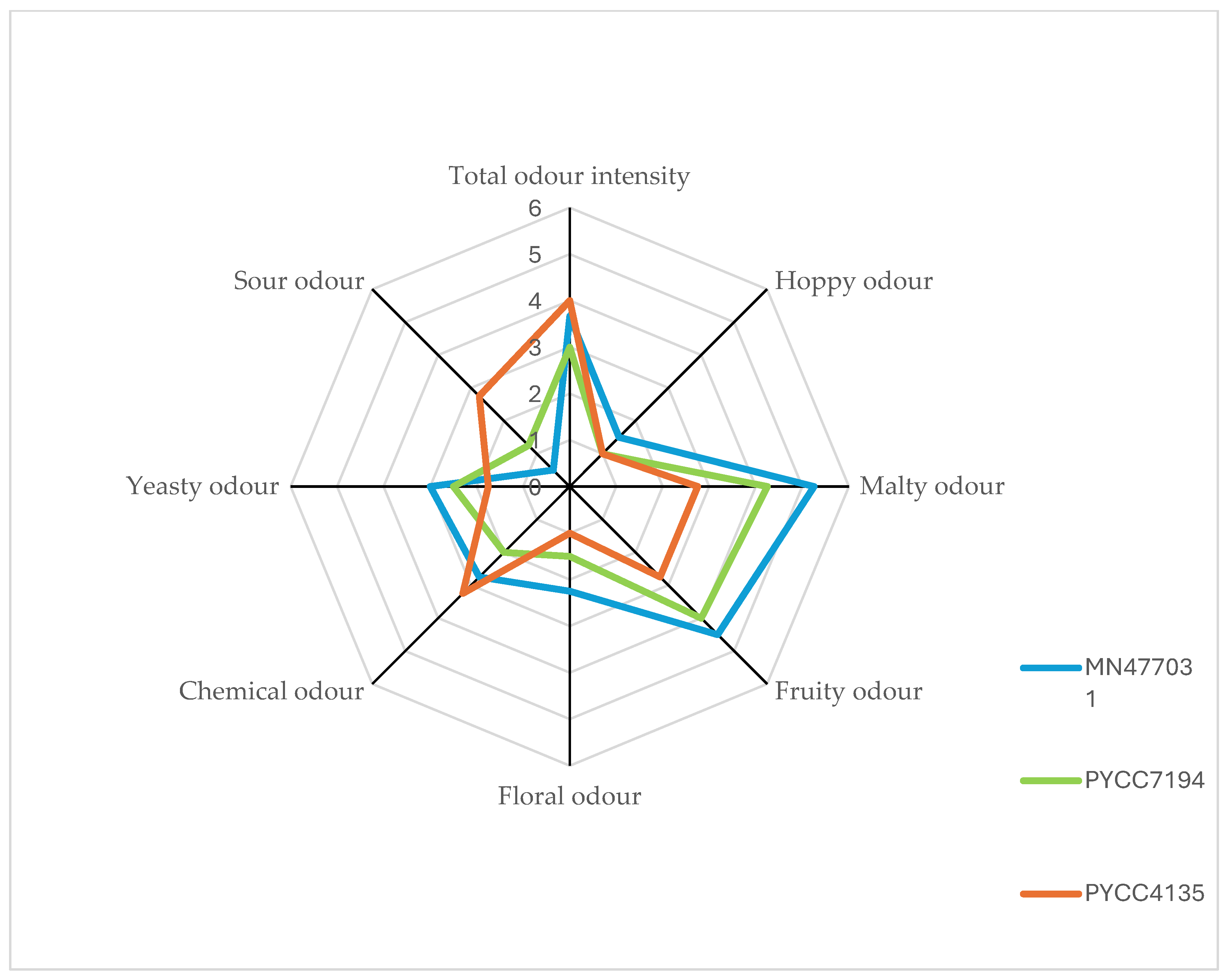
2.5. The Effect of Selected Lachancea Yeast Strains on the Volatile Composition of the Beers Produced
2.6. Potential of the L. thermotolerans and L. fermentati Strains for the Production of Different Types of Beers
3. Materials and Methods
3.1. Materials
3.1.1. Yeast Strains
3.1.2. Hops
3.2. Methods
3.2.1. Wort Production
3.2.2. Yeast Propagation and Fermentation
3.2.3. Analytical Determinations
Daily Weight Loss
Ethanol Content, Real Extract and Calorific Value
The pH Measurement
Volatile Compounds
Organic Acid Analysis
Sugar and Glycerol Contents
Sensory Analysis (QDA)
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Health-Related Aspects of Beer: A Review. Int. J. Food Prop. 2012, 15, 350–373. [Google Scholar] [CrossRef]
- Hampton, A.; Pham, T.; Du, X. Impact of Flavor Factorized by Alcohol Level and Flavor Type on ‘Beer Refreshing Perception’ in a Model Study and the Exploration of Sensory Drivers for ‘Refreshing. J. Am. Soc. Brew. Chem. 2023, 82, 1–14. [Google Scholar] [CrossRef]
- Krebs, G.; Gastl, M.; Becker, T. Chemometric Modeling of Palate Fullness in Lager Beers. Food Chem. 2021, 342, 128253. [Google Scholar] [CrossRef]
- Schreurs, M.; Piampongsant, S.; Roncoroni, M.; Cool, L.; Herrera-Malaver, B.; Vanderaa, C.; Theßeling, F.A.; Kreft, Ł.; Botzki, A.; Malcorps, P.; et al. Predicting and Improving Complex Beer Flavor through Machine Learning. Nat. Commun. 2024, 15, 2368. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Hejna, A. More than Just a Beer—The Potential Applications of by-Products from Beer Manufacturing in Polymer Technology. Emergent. Mater. 2022, 5, 765–783. [Google Scholar] [CrossRef]
- Lei, H.; Zhao, H.; Yu, Z.; Zhao, M. Effects of Wort Gravity and Nitrogen Level on Fermentation Performance of Brewer’s Yeast and the Formation of Flavor Volatiles. Appl. Biochem. Biotechnol. 2012, 166, 1562–1574. [Google Scholar] [CrossRef]
- Postigo, V.; Esteban, S.; Arroyo, T. Lachancea Thermotolerans, an Innovative Alternative for Sour Beer Production. Beverages 2023, 9, 20. [Google Scholar] [CrossRef]
- Langstaff, S.A.; Lewis, M.J. THE MOUTHFEEL OF BEER—A REVIEW. J. Inst. Brew. 1993, 99, 31–37. [Google Scholar] [CrossRef]
- Carlquist, M.; Gibson, B.; Karagul Yuceer, Y.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; de Billerbeck, G.M.; et al. Process Engineering for Bioflavour Production with Metabolically Active Yeasts—A Mini-Review. Yeast 2015, 32, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Missbach, B.; Majchrzak, D.; Sulzner, R.; Wansink, B.; Reichel, M.; Koenig, J. Exploring the Flavor Life Cycle of Beers with Varying Alcohol Content. Food Sci. Nutr. 2017, 5, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Olšovská, J.; Štěrba, K.; Vrzal, T.; Čejka, P. Nutritional Composition and Energy Value of Different Types of Beer and Cider. Kvasny Prumysl. 2019, 65, 32–37. [Google Scholar] [CrossRef]
- Porretta, S.; Donadini, G. A Preference Study for No Alcohol Beer in Italy Using Quantitative Concept Analysis. J. Inst. Brew. 2008, 114, 315–321. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for Brewers: Exploiting Natural Diversity for Naturally Diverse Beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Satora, P.; Pater, A.; Bogacz, S. Low Lactic Acid-Producing Strain of Lachancea Thermotolerans as a New Starter for Beer Production. Biomolecules 2020, 10, 256. [Google Scholar] [CrossRef]
- Larroque, M.N.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and Non-Saccharomyces Native Yeasts on Beer Aroma Compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by Non-Conventional Yeasts in Sequential Beer Fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Li, R.; Sun, Y. Effects of Honey Variety and Non-Saccharomyces Cerevisiae on the Flavor Volatiles of Mead. J. Am. Soc. Brew. Chem. 2019, 77, 40–53. [Google Scholar] [CrossRef]
- Gatto, V.; Binati, R.L.; Lemos Junior, W.J.F.; Basile, A.; Treu, L.; de Almeida, O.G.G.; Innocente, G.; Campanaro, S.; Torriani, S. New Insights into the Variability of Lactic Acid Production in Lachancea Thermotolerans at the Phenotypic and Genomic Level. Microbiol. Res. 2020, 238, 126525. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea Thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S.; Fracassetti, D.; Rustioni, L. An Integrative View of the Role of Lachancea Thermotolerans in Wine Technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.A.; Lahue, C.; Gordy, C.L.; Little, J.L.; Nichols, L.M.; Calvert, M.D.; Dunn, R.R.; Smukowski Heil, C. Sugar-Seeking Insects as a Source of Diverse Bread-Making Yeasts with Enhanced Attributes. Yeast 2022, 39, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Siesto, G.; Pietrafesa, R.; Tufariello, M.; Gerardi, C.; Grieco, F.; Capece, A. Application of Microbial Cross-over for the Production of Italian Grape Ale (IGA), a Fruit Beer Obtained by Grape Must Addition. Food Biosci. 2023, 52, 102487. [Google Scholar] [CrossRef]
- Vicente, J.; Vladic, L.; Navascués, E.; Brezina, S.; Santos, A.; Calderón, F.; Tesfaye, W.; Marquina, D.; Rauhut, D.; Benito, S. A Comparative Study of Lachancea Thermotolerans Fermentative Performance under Standardized Wine Production Conditions. Food Chem. X 2024, 21, 101214. [Google Scholar] [CrossRef]
- Aredes, R.S.; Peixoto, F.C.; Sphaier, L.A.; Silva, V.N.H.; Duarte, L.M.; de Carvalho Marques, F.F. Determination of Carbohydrates in Brewer’s Wort by Capillary Electrophoresis with Indirect UV Detection. J. Food Compos. Anal. 2023, 120, 105321. [Google Scholar] [CrossRef]
- D’Amore, T.; Russell, I.; Stewart, G.G. Sugar Utilization by Yeast during Fermentation. J. Ind. Microbiol. 1989, 4, 315–323. [Google Scholar] [CrossRef]
- Fairbairn, S.; Engelbrecht, L.; Setati, M.E.; du Toit, M.; Bauer, F.F.; Divol, B.; Rossouw, D. Combinatorial Analysis of Population Dynamics, Metabolite Levels and Malolactic Fermentation in Saccharomyces Cerevisiae/ Lachancea Thermotolerans Mixed Fermentations. Food Microbiol. 2021, 96, 103712. [Google Scholar] [CrossRef]
- du Plessis, H.W.; du Toit, M.; Hoff, J.W.; Hart, R.S.; Ndimba, B.K.; Jolly, N.P. Characterisation of Non-Saccharomyces Yeasts Using Different Methodologies and Evaluation of Their Compatibility with Malolactic Fermentation. South Afr. J. Enol. Vitic. 2017, 38, 46–63. [Google Scholar] [CrossRef]
- Sroka, P.; Tuszyński, T. Changes in Organic Acid Contents during Mead Wort Fermentation. Food Chem. 2007, 104, 1250–1257. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Poreda, A.; Tuszyński, T. Rotary Jet Head—A Device for Accelerating the Fermentation Process in Brewing. J. Inst. Brew. 2016, 122, 317–321. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Pérez-Través, L.; Pérez, D.; Roldán López, D.; Briand, L.E.; Pérez-Torrado, R.; Querol, A. Identification and Assessment of Non-Conventional Yeasts in Mixed Fermentations for Brewing Bioflavored Beer. Int. J. Food Microbiol. 2023, 399, 110254. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Mizuno, S.; Hiroshima, T.; Shioya, S. Effect of Carbon and Nitrogen Additions on Consumption Activity of Apparent Extract of Yeast Cells in a Brewing Process. J. Am. Soc. Brew. Chem. 2002, 60, 163–169. [Google Scholar] [CrossRef]
- Magalhães, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and Maltotriose Utilisation by Group I Strains of the Hybrid Lager Yeast Saccharomyces Pastorianus. FEMS Yeast Res 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of the Potential of Commercial Non-Saccharomyces Yeast Strains of Torulaspora Delbrueckii and Lachancea Thermotolerans in Beer Fermentation. Int. J. Food Sci. Technol. 2020, 55, 2049–2059. [Google Scholar] [CrossRef]
- Galaz, V.; Franco, W. Lachancea Quebecensis a Novel Isolate for the Production of Craft Beer. Foods 2023, 12, 3347. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Krystian, K.; Satora, P.; Skoneczny, S.; Zdaniewicz, M.; Pater, A. The Use of Non-Saccharomyces Yeast and Enzymes in Beer Production. Acta Univ. Cibiniensis-Ser. E Food Technol. 2020, 24, 223–246. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing and Malting, 6th ed.; Hendel, O., Ed.; Versuchs- und Lehranstalt für Brauerei in Berlin (VLB): Berlin, Germany, 2019. [Google Scholar]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea Thermotolerans as an Alternative Yeast for the Production of Beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Monošík, R.; Magdolen, P.; Streďanský, M.; Šturdík, E. Monitoring of Monosaccharides, Oligosaccharides, Ethanol and Glycerol during Wort Fermentation by Biosensors, HPLC and Spectrophotometry. Food Chem. 2013, 138, 220–226. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor Impacts of Glycerol in the Processing of Yeast Fermented Beverages: A Review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, S.H.; Mortazavian, A.M.; Rezaei, K. Alcohol-Free Beer: Methods of Production, Sensorial Defects, and Healthful Effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Pater, A.; Knapik, A.; Duliński, R. The Effect of Different Oat (Avena Sativa L) Malt Contents in a Top-Fermented Beer Recipe on the Brewing Process Performance and Product Quality. J. Cereal Sci. 2021, 101, 103301. [Google Scholar] [CrossRef]
- Osburn, K.; Amaral, J.; Metcalf, S.R.; Nickens, D.M.; Rogers, C.M.; Sausen, C.; Caputo, R.; Miller, J.; Li, H.; Tennessen, J.M.; et al. Primary Souring: A Novel Bacteria-Free Method for Sour Beer Production. Food Microbiol. 2018, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Baroň, M.; Fiala, J. Chasing after Minerality, Relationship to Yeast Nutritional Stress and Succinic Acid Production. Czech J. Food Sci. 2012, 30, 188–193. [Google Scholar] [CrossRef]
- Tyrell, T. Strategies for Reducing Succinic Acid Concentrations in Beer. J. Am. Soc. Brew. Chem. 2014, 72, 246–252. [Google Scholar] [CrossRef]
- Li, H.; Liu, F. Changes in Organic Acids during Beer Fermentation. J. Am. Soc. Brew. Chem. 2015, 73, 275–279. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-Active Volatile Compounds in Beer: Production, Regulation and Control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nagahisa, K.; Shimizu, H.; Shioya, S. Simultaneous Control of Apparent Extract and Volatile Compounds Concentrations in Low-Malt Beer Fermentation. Appl. Microbiol. Biotechnol. 2006, 73, 549–558. [Google Scholar] [CrossRef]
- Peddie, H.A.B. Ester Formation in Brewery Fermentations. J. Inst. Brew. 1990, 96, 327–331. [Google Scholar] [CrossRef]
- Williamson, S.A.; Iverson, W.G. Determination of Short-Chain Diols and Selected Fermentation By-Products in Beer. J. Am. Soc. Brew. Chem. 1993, 51, 114–120. [Google Scholar] [CrossRef]
- Olsen, A.; Christensen, B.W.; Madsen, J.I. Onion-Like Off-Flavour in Beer: Isolation and Identification of The Culprits. Carlsberg Res. Commun. 1988, 53, 1–8. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces Cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Strong, G.; England, K. Beer Judge Certification Program 2021 Beer Style Guidelines. 2021. Available online: https://brewstock.com/wp-content/uploads/2022/01/BJCP-Style-Guide-2021.pdf (accessed on 10 November 2024).
- Lermusieau, G.; Bulens, M.; Collin, S. Use of GC-Olfactometry to Identify the Hop Aromatic Compounds in Beer. J. Agric. Food Chem. 2001, 49, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Johnson, J.B.; Batley, R.; Lal, P.; Wakeling, L.; Naiker, M. Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders. Beverages 2021, 7, 28. [Google Scholar] [CrossRef]
- Piry, J.; Prlhla, A.; Ďurčanská, J.; Farkaš, P. Fractionation of Volatiles from Blackcurrant (Ribes nigrum L.) by Different Extractive Methods. Food Chem. 1995, 54, 73–77. [Google Scholar] [CrossRef]
- Alegre, Y.; Sáenz-Navajas, M.P.; Ferreira, V.; García, D.; Razquin, I.; Hernández-Orte, P. Rapid Strategies for the Determination of Sensory and Chemical Differences between a Wealth of Similar Wines. Eur. Food Res. Technol. 2017, 243, 1295–1309. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Two Bavarian Wheat Beers by Means of the Sensomics Approach. J. Agric. Food Chem. 2013, 61, 11303–11311. [Google Scholar] [CrossRef]
- Fritsch, H.T.; Schieberle, P. Identification Based on Quantitative Measurements and Aroma Recombination of the Character Impact Odorants in a Bavarian Pilsner-Type Beer. J. Agric. Food Chem. 2005, 53, 7544–7551. [Google Scholar] [CrossRef]
- Schieberle, P. Primary Odorants of Pale Lager Beer Differences to Other Beers and Changes during Storage. Z. Lebensm. Unters. Forch. 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Tokita, K.; Takazumi, K.; Oshima, T.; Shigyo, T. A New Method for Analyzing the Characteristic Flavor of Beer Using Selectable One-Dimensional or Two-Dimensional Gas Chromatography-Olfactometry/ Mass Spectrometry. J. Am. Soc. Brew. Chem. 2014, 72, 154–161. [Google Scholar] [CrossRef]
- Satora, P.; Semik-Szczurak, D.; Tarko, T.; Bułdys, A. Influence of Selected Saccharomyces and Schizosaccharomyces Strains and Their Mixed Cultures on Chemical Composition of Apple Wines. J. Food Sci. 2018, 83, 424–431. [Google Scholar] [CrossRef]
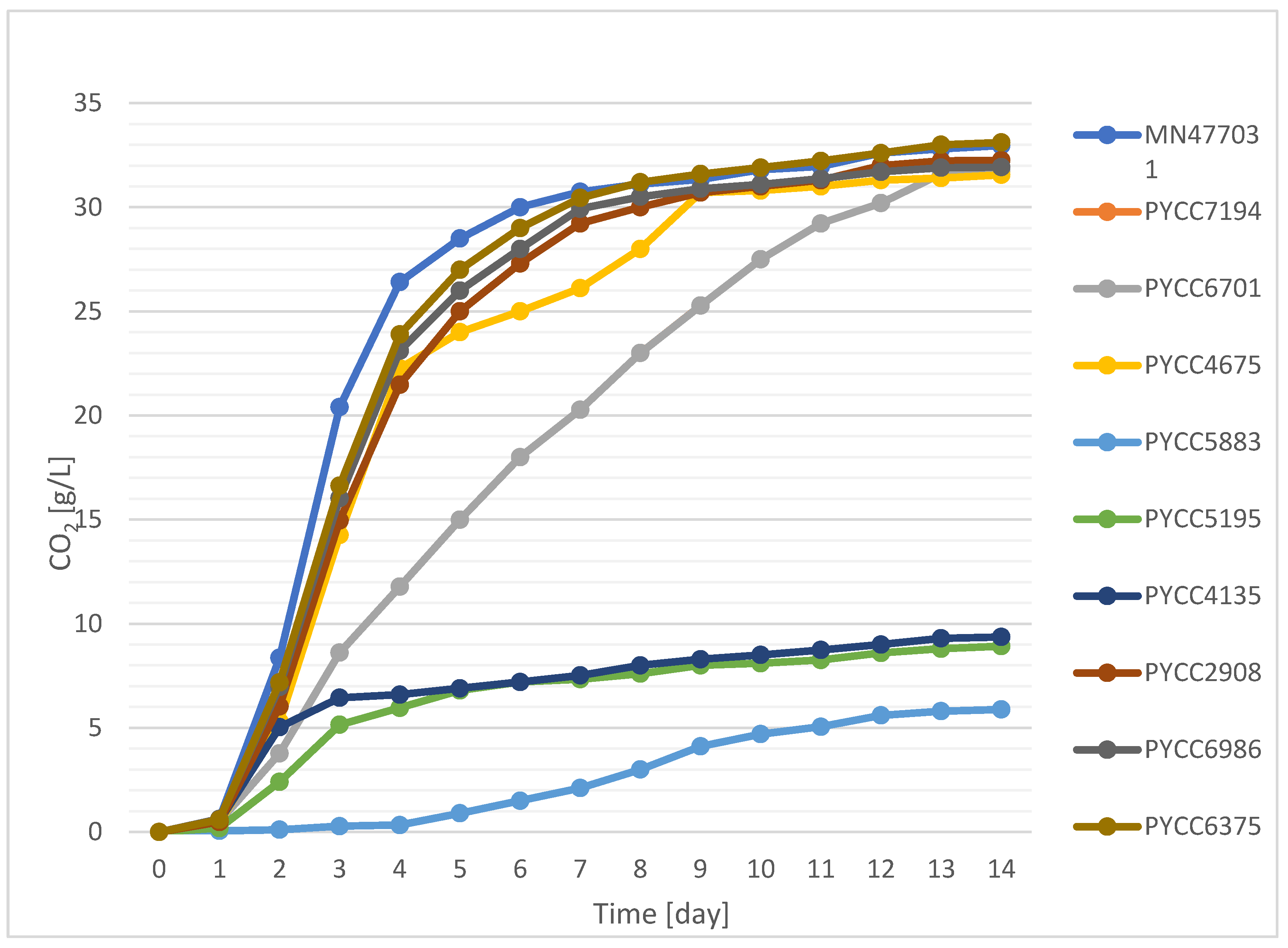
| Fructose | Glucose | Sucrose | Maltose | Glycerol | Real Extract | |
|---|---|---|---|---|---|---|
| g/L | % [w/w] | |||||
| Wort | 6.22 ± 1.05 a | 13.6 ± 2.38 a | 4.19 ± 1.31 a | 58.1 ± 2.26 a | n/d | 11.9 ± 0.09 a |
| MN477031 | 2.52 ± 0.55 b | 2.56 ± 0.46 bf | n/d | 4.96 ± 0.34 b | 6.53 ± 1.31 a | 5.82 ± 0.04 bd |
| PYCC7194 | 2.12 ± 0.27 bd | 1.00 ± 0.22 ce | n/d | 11.3 ± 1.75 c | 3.14 ± 0.35 b | 6.20 ± 0.21 c |
| PYCC6701 | 4.20 ± 0.43 c | 2.52 ± 0.24 b | 2.10 ± 0.62 b | 17.2 ± 3.24 d | 6.44 ± 2.08 ad | 5.87 ± 0.06 bd |
| PYCC4675 | 1.70 ± 0.18 d | 1.35 ± 0.16 cd | n/d | 6.18 ± 1.52 be | 3.1 ± 0.92 bc | 5.76 ± 0.02 b |
| PYCC5883 | 1.54 ± 0.19 d | 1.86 ± 0.45 d | 2.77 ± 0.38 b | 58.1 ± 2.22 a | 1.59 ± 0.59 c | 10.6 ± 0.04 e |
| PYCC5195 | 2.55 ± 0.62 be | 1.31 ± 0.99 bce | 2.50 ± 0.61 b | 52.5 ± 5.14 a | 1.80 ± 0.69 c | 10.1 ± 0.04 f |
| PYCC4135 | 2.23 ± 0.13 be | 2.35 ± 0.35 b | 3.39 ± 0.18 a | 57.6 ± 1.63 a | 3.91 ± 1.14 bd | 10.3 ± 0.03 g |
| PYCC2908 | 1.48 ± 0.43 d | 1.30 ± 0.47 cde | n/d | 8.18 ± 1.47 ce | 2.65 ± 0.79 bc | 5.91 ± 0.05 d |
| PYCC6986 | 1.63 ± 0.82 bd | 0.66 ± 0.29 e | n/d | 4.31 ± 1.73 b | 3.01 ± 0.47 b | 5.85 ± 0.03 bd |
| PYCC6375 | 1.81 ± 0.66 bd | 3.64 ± 0.7 f | n/d | 8.92 ± 2.08 ce | 3.16 ± 0.99 bc | 5.77 ± 0.02 b |
| Ethanol [% v/v] | pH | Caloric Value [kcal/100 mL] | |
|---|---|---|---|
| MN477031 | 4.04 ± 0.06 af | 4.43 ± 0.04 a | 43.0 ± 0.37 abe |
| PYCC7194 | 3.8 ± 0.08 b | 3.60 ± 0.13 b | 43.0 ± 0.35 abe |
| PYCC6701 | 4.06 ± 0.03 a | 3.78 ± 0.07 b | 43.3 ± 0.05 a |
| PYCC4675 | 4.03 ± 0.07 af | 3.94 ± 0.03 c | 42.7 ± 0.31 b |
| PYCC5883 | 0.89 ± 0.02 ce | 4.65 ± 0.05 d | 43.5 ± 0.03 c |
| PYCC5195 | 1.02 ± 0.05 d | 4.55 ± 0.03 e | 42.3 ± 0.11 d |
| PYCC4135 | 0.84 ± 0.05 e | 4.74 ± 0.05 d | 42.1 ± 0.30 d |
| PYCC2908 | 3.96 ± 0.03 f | 3.74 ± 0.03 b | 42.9 ± 0.27 abe |
| PYCC6986 | 4.09 ± 0.06 af | 4.17± 0.04 f | 43.4 ± 0.35 ac |
| PYCC6375 | 3.95 ± 0.01 f | 3.88 ± 0.03 c | 42.3 ± 0.04 d |
| Compound [µg/L] | m/z | LRI | PYCC 5883 | PYCC 6701 | PYCC 4675 | MN 47708 | PYCC 2908 | PYCC 5195 | PYCC 6375 | PYCC 7194 | PYCC 4135 | PYCC 6986 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Propanol (isopropyl alcohol) | 59 | 480 | 171 ± 6.23 a | 116 ± 9.42 b | 141 ± 5.26 c | 59.7 ± 17.5 d | 160 ± 45.1 abcgh | 409 ± 40.2 e | 11.2 ± 1.32 f | 157 ± 4.93 gh | 166 ± 6.23 ah | 36.9 ± 17.2 d |
| 2-Methyl-3-buten-2-ol | 71 | 611 | 22.8 ± 5.06 a | 27 ± 3.52 a | 4.52 ± 4.04 bc | 6.33 ± 0.33 b | 9.86 ± 3.58 b | 5.28 ± 1.91 bc | n/d | 3.34 ± 0.15 c | 31 ± 3.13 a | n/d |
| 2-Methyl-1-propanol (isobutyl alcohol) | 33 | 617 | 150 ± 18.2 a | 535 ± 16.7 b | 558 ± 16.6 b | 538 ± 83.1 b | 373 ± 15.7 c | 447 ± 86.5 bcd | 409 ± 15.7 d | 435 ± 21.2 bd | 371 ± 9.51 ce | 588 ± 77.8 bf |
| Isoamyl alcohol | 42 | 723 | 1370 ± 181 a | 7889 ± 254 b | 5650 ± 422 c | 4318 ± 319 d | 5510 ± 238 c | 2474 ± 234 | 3345 ± 31.9 f | 2177.76 ± 160 e | 3171 ± 68.9 g | 4757 ± 262 |
| 2-Methyl-1-butanol | 56 | 740 | 419 ± 41.0 a | 941 ± 29.2 b | 1157 ± 37.5 c | 13.6 ± 0.67 d | 1170 ± 75.3 c | 651 ± 61.2 e | 11.6 ± 1.00 f | 11.3 ± 0.73 f | 645 ± 5.16 e | 1074 ± 16.7 g |
| L-(+)-2.3-Butanediol | 29 | 802 | n/d | 18.5 ± 0.07 a | n/d | n/d | n/d | n/d | n/d | n/d | n/d | 19.5± 4.04 a |
| 2-Ethyl-1-hexanol | 57 | 1016 | 410 ± 28.5 a | 242 ± 17.5 b | 349 ± 50.5 a | 233 ± 12.4 bc | 281 ± 63.1 bc | 374 ± 20.0 a | 201 ± 30.5 bce | 213 ± 7.64 c | 504 ± 25 d | 165 ± 14.8 e |
| Dihydromyrcenol | 59 | 1067 | 1.63 ± 0.31 abd | 1.35 ± 0.20 abcde | 1.09 ± 0.24 acef | 1.22 ± 0.19 acde | 1.38 ± 0.19 abcde | 1.57 ± 0.05 b | 1.18 ± 0.09 ce | 1.37 ± 0.02 d | 0.98 ± 0.25 ef | 0.97 ± 0.02 f |
| Phenylethanol | 91 | 1084 | 55.8 ± 22.8 a | 1368 ± 60.1 b | 1566 ± 31.1 c | 1720 ± 110 d | 1792 ± 123 d | 815 ± 42.8 e | 1323 ± 13.7 b | 1606 ± 78.8 cd | 793 ± 33.4 e | 1621 ± 36.1 cd |
| 4-Ethylguaiacol | 137 | 1254 | 1218 ± 52 a | 5.04 ± 0.16 b | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| Decanol | 55 | 1272 | n/d | n/d | 2.68 ± 0.16 a | 3.68 ± 0.19 b | n/d | n/d | n/d | n/d | n/d | n/d |
| Nerolidol | 69 | 1575 | n/d | 2.16 ± 0.27 a | 2.44 ± 0.53 a | 1.19 ± 0.18 bd | 1.40 ± 0.03 b | 0.24 ± 0.04 c | 1.32 ± 0.12 b | 0.84 ± 0.22 d | 0.26 ± 0.06 c | 1.72 ± 0.04 e |
| Ethyl Acetate | 61 | 614 | 38.7 ± 0.6 a | 1544 ± 40.7 b | 949 ± 21.3 c | 937 ± 23.3 c | 2061 ± 327 d | 336 ± 19 e | 961 ± 6.1 c | 782 ± 29.7 f | 94.2 ± 7.6 g | 1147 ± 36.6 h |
| Ethyl propanoate | 57 | 699 | 1.12± 0.36 a | 123 ± 15.4 b | 61.3 ± 1.77 c | 45.1 ± 1.31 d | 101 ± 21.1 b | 13.4 ± 1.60 e | 65.3 ± 2.55 c | 50.9 ± 5.61 d | 44.4 ± 6.85 d | 82.3 ± 11.7 e |
| Ethyl butanoate | 71 | 789 | 1.39 ± 0.22 a | 6.02 ± 0.24 b | 2.79 ± 0.31 cf | n/d | 3.74 ± 0.59 df | n/d | 2.32 ± 0.15 e | 1.69 ± 0.48 a | n/d | 3.56 ± 0.46 f |
| Ethyl lactate | 45 | 806 | n/d | 15.4 ± 2.55 a | n/d | n/d | 23.1 ± 2.86 b | 20.3 ± 18.6 abc | n/d | 46.57 ± 11.96 c | n/d | n/d |
| 2-Methyl-1-butyl acetate | 43 | 869 | n/d | 8.67 ± 0.14 | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| Ethyl hexanoate | 88 | 986 | 2.21 ± 0.2 a | 12.3 ± 0.69 b | 4.26 ± 0.82 cd | 4.97 ± 0.71 c | 5.99 ± 1.74 c | n/d | 3.60 ± 0.12 d | 4.31 ± 0.26 c | n/d | 4.09 ± 0.80 c |
| Ethyl octanoate | 88 | 1180 | 13.3 ± 3.15 a | 61.2 ± 6.92 b | 27 ± 2.40 c | 69.3 ± 12.5 b | 28.5 ± 2.73 c | 6.27 ± 0.19 d | 18.2 ± 2.59 af | 17.5 ± 3.03 af | 3.10 ± 0.23 e | 21.7 ± 4.30 cf |
| 2-Phenethyl acetate | 104 | 1228 | 2.12 ± 0.32 a | 5752 ± 1991 b | 14.5 ± 1.50 c | 11.8 ± 1.98 cd | 15.4 ± 3.03 c | 8.21 ± 2.00 d | 8.9 ± 0.37 de | 13.5 ± 1.47 c | 5.60 ± 0.57 f | 6.03 ± 0.67 f |
| Propyl nonanoate | 61 | 1370 | 3.26 ± 0.16 a | 3.81 ± 0.58 a | 2.96 ± 0.33 ab | 2.81 ± 0.05 b | 2.56 ± 0.53 bd | 1.14 ± 0.24 c | 1.93 ± 0.16 d | 1.15 ± 0.17 c | 1.43 ± 0.21 c | 2.22 ± 0.23 d |
| Ethyl decanoate | 88 | 1397 | 0.55 ± 0.05 a | 7.80 ± 0.96 b | 9.54 ± 3.35 b | 2585 ± 43 c | 18.9 ± 4.90 d | 1.05 ± 0.13 e | 2.77 ± 0.08 f | 3.61 ± 0.03 g | 0.92 ± 0.14 e | 212 ± 13.5 h |
| Ethyl dodecanoate | 88 | 1581 | 3.59 ± 0.22 a | 6.60 ± 0.46 b | 3.29 ± 1.12 adf | 19.5 ± 3.17 c | 2.76 ± 0.49 df | 1.38 ± 0.13 e | 1.20 ± 0.25 eg | 3.15 ± 0.13 f | 0.98 ± 0.20 g | 8.48 ± 1.08 h |
| Isoamyl acetate | 43 | 872 | 44.6 ± 5.06 a | 250 ± 16 b | 68.4 ± 5.76 c | 70.9 ± 12.3 c | 74.5 ± 9.10 c | 36.5 ± 6.19 a | 59.6 ± 2.74 d | 79.1 ± 6.01 c | 15.5 ± 3.78 e | 112 ± 11.7 f |
| Beer Type | Recommended Strain | Odour Description | Tentative Identification (On the Basis of Odour and the MS Detector/ Suspected Compound) |
|---|---|---|---|
| Low-alcohol | PYCC4135 | flower fruit tropical fruit flowers, lilies of the valley freshener ice/fresh candy flower sweet, candy candy | 2-Methyl-3-buten-2-ol Isobutyl acetate Ethyl hexanoate Phenylethyl Alcohol Isoamyl alcohol Dihydromyrcenol 1-Nonanol β-Damascenone Ethyl decanoate |
| Sour | PYCC7194 | fruit, floral boiled potatoes, sulphuric sweet, fruity, pineapple red apple roasted coffee sweet fruit mint rose floral notes | 2-Methyl-3-buten-2-ol Dimethyl disulfide Ethyl lactate Ethyl hexanoate (5-Methyl-2-furyl)methanethiol ethyl isovalerate Dihydromyrcenol Phenylethyl Alcohol 1-Nonanol |
| Regular | MN477031 | flower fruity, bubblegum cooked vegetables sweet banana fruity, pineapple fruity, banana sweet wort, berry fruity red apple roasted coffee rose oil plum | 2-Methyl-3-buten-2-ol Ethyl propanoate Dimethyl disulfide Isobutyl acetate Ethyl lactate Isoamyl acetate 3-Hexen-2-one Ethyl hexanoate (5-Methyl-2-furyl)methanethiol Phenylethyl Alcohol 1-Nonanol β-Damascenone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdaniewicz, M.; Satora, P.; Kania, P.; Florkiewicz, A. The Impact of Selected Lachancea Yeast Strains on the Production Process, Chemical Composition and Aroma Profiles of Beers. Molecules 2024, 29, 5674. https://doi.org/10.3390/molecules29235674
Zdaniewicz M, Satora P, Kania P, Florkiewicz A. The Impact of Selected Lachancea Yeast Strains on the Production Process, Chemical Composition and Aroma Profiles of Beers. Molecules. 2024; 29(23):5674. https://doi.org/10.3390/molecules29235674
Chicago/Turabian StyleZdaniewicz, Marek, Paweł Satora, Paulina Kania, and Adam Florkiewicz. 2024. "The Impact of Selected Lachancea Yeast Strains on the Production Process, Chemical Composition and Aroma Profiles of Beers" Molecules 29, no. 23: 5674. https://doi.org/10.3390/molecules29235674
APA StyleZdaniewicz, M., Satora, P., Kania, P., & Florkiewicz, A. (2024). The Impact of Selected Lachancea Yeast Strains on the Production Process, Chemical Composition and Aroma Profiles of Beers. Molecules, 29(23), 5674. https://doi.org/10.3390/molecules29235674







