Increased Antioxidant Performance of Lignin by Biodegradation Obtained from an Extract of the Mushroom Pleurotus eryngii
Abstract
1. Introduction
2. Results and Discussion
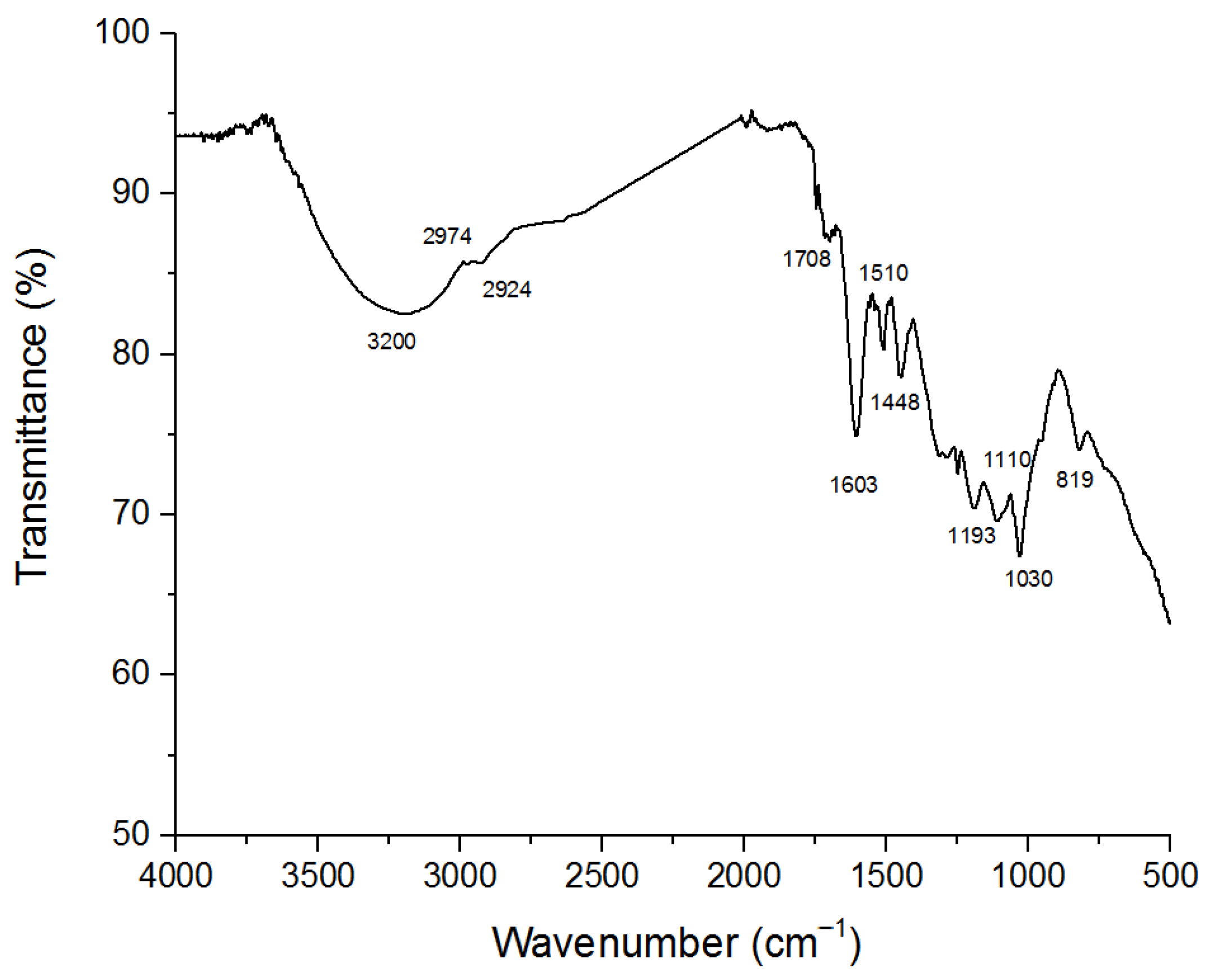
2.1. Lignin Characterization
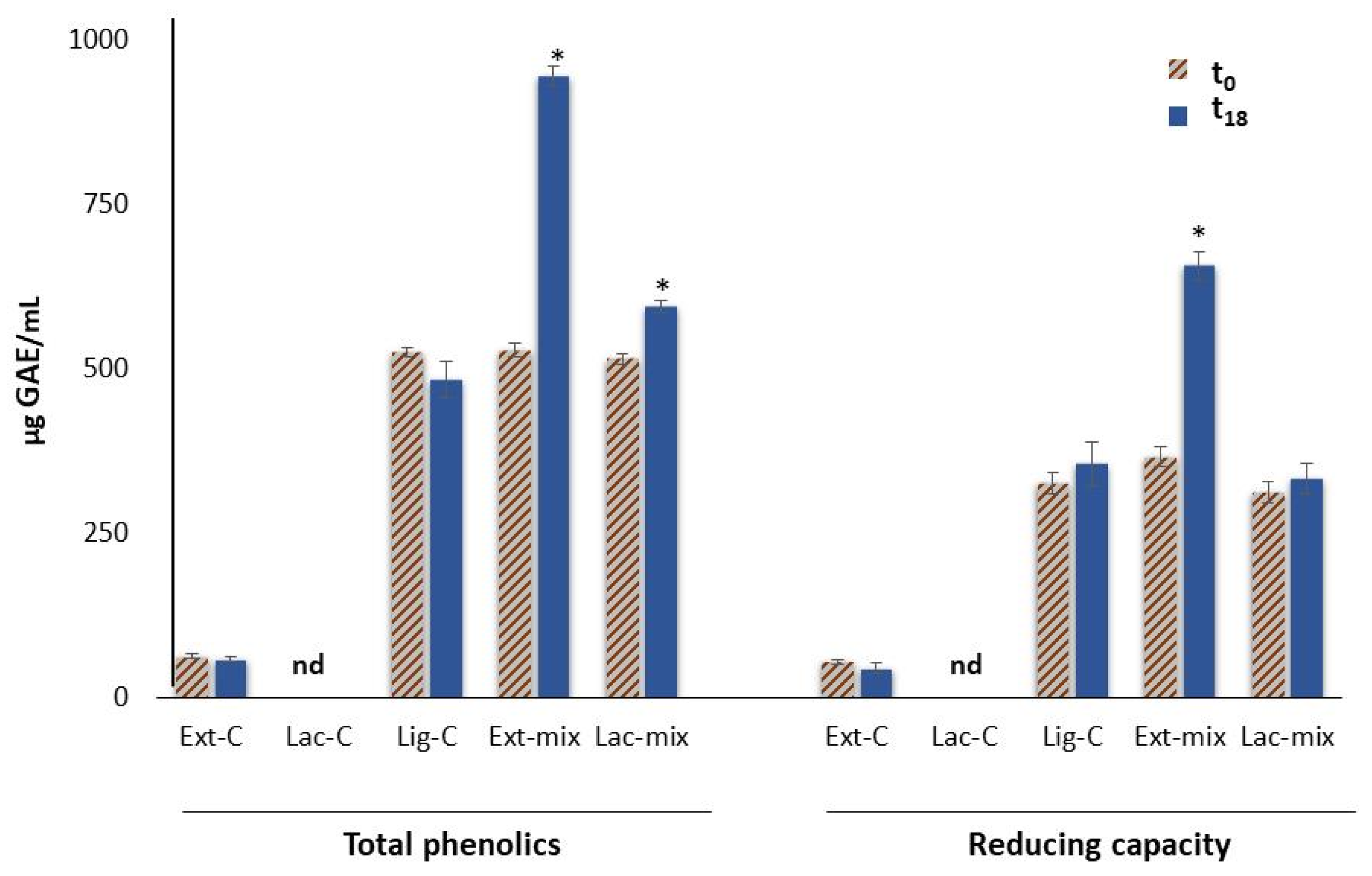
2.2. Total Phenolics and Educing Apacity
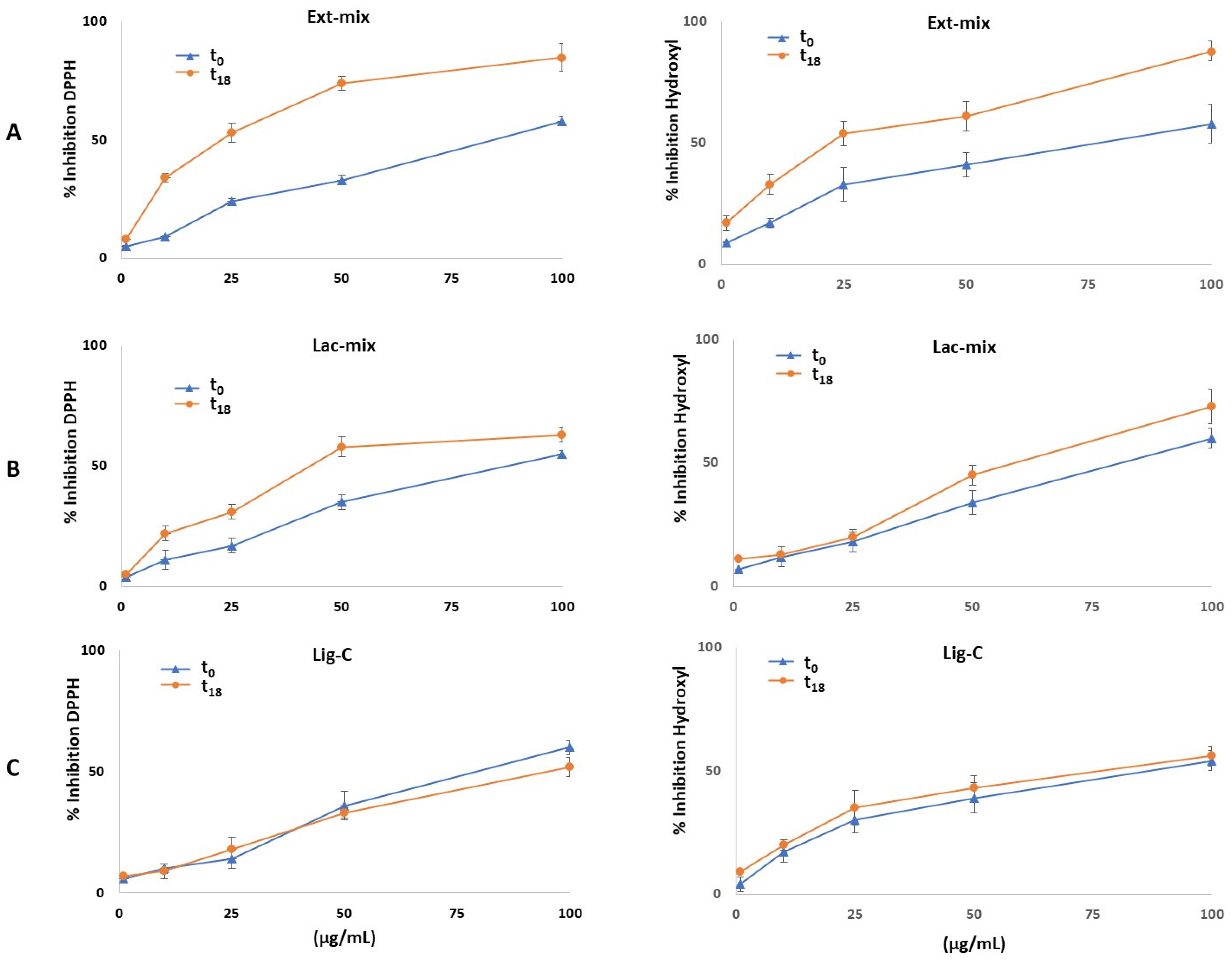
2.3. Antioxidant Activity
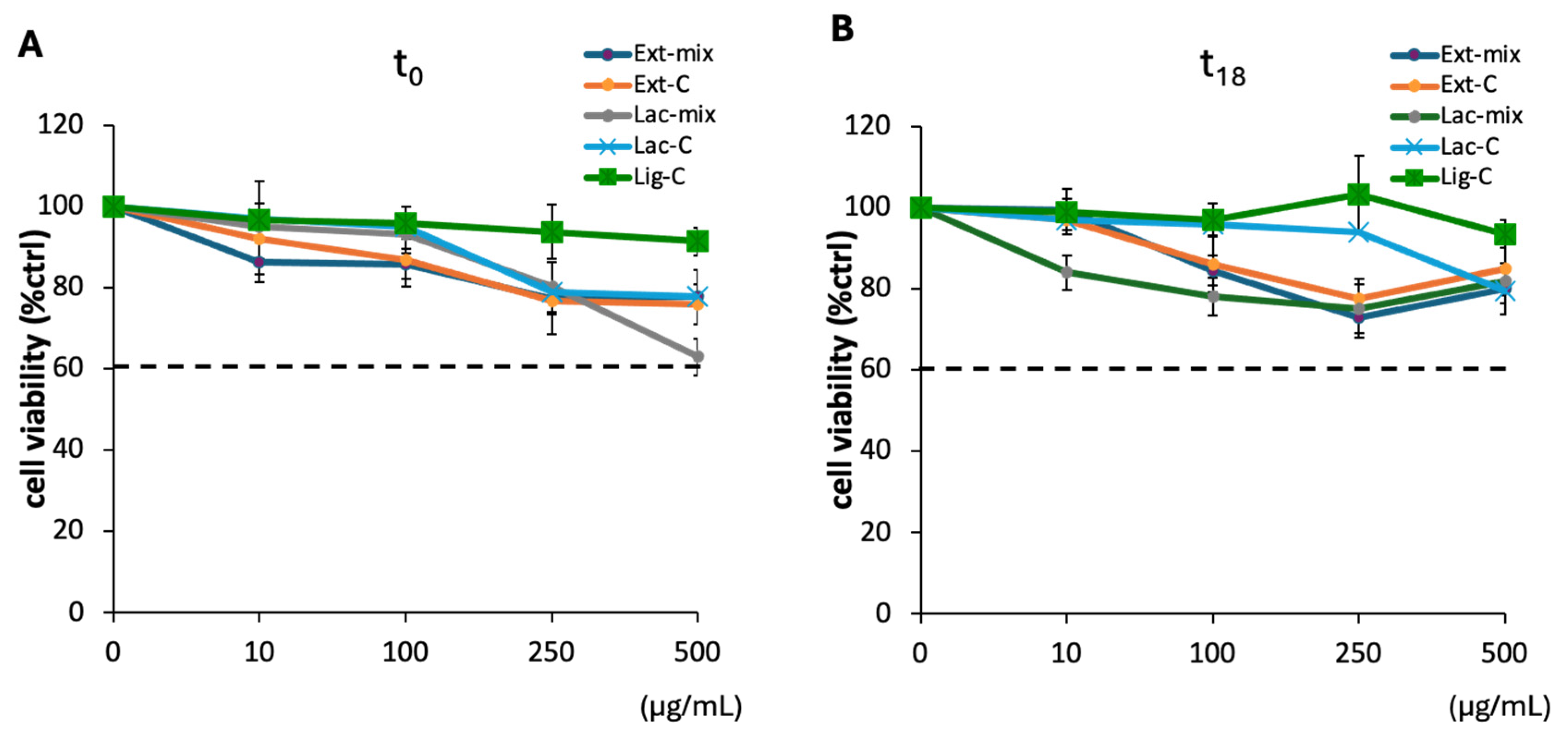
2.4. Effect of Lignin on DI-TNC1 Viability
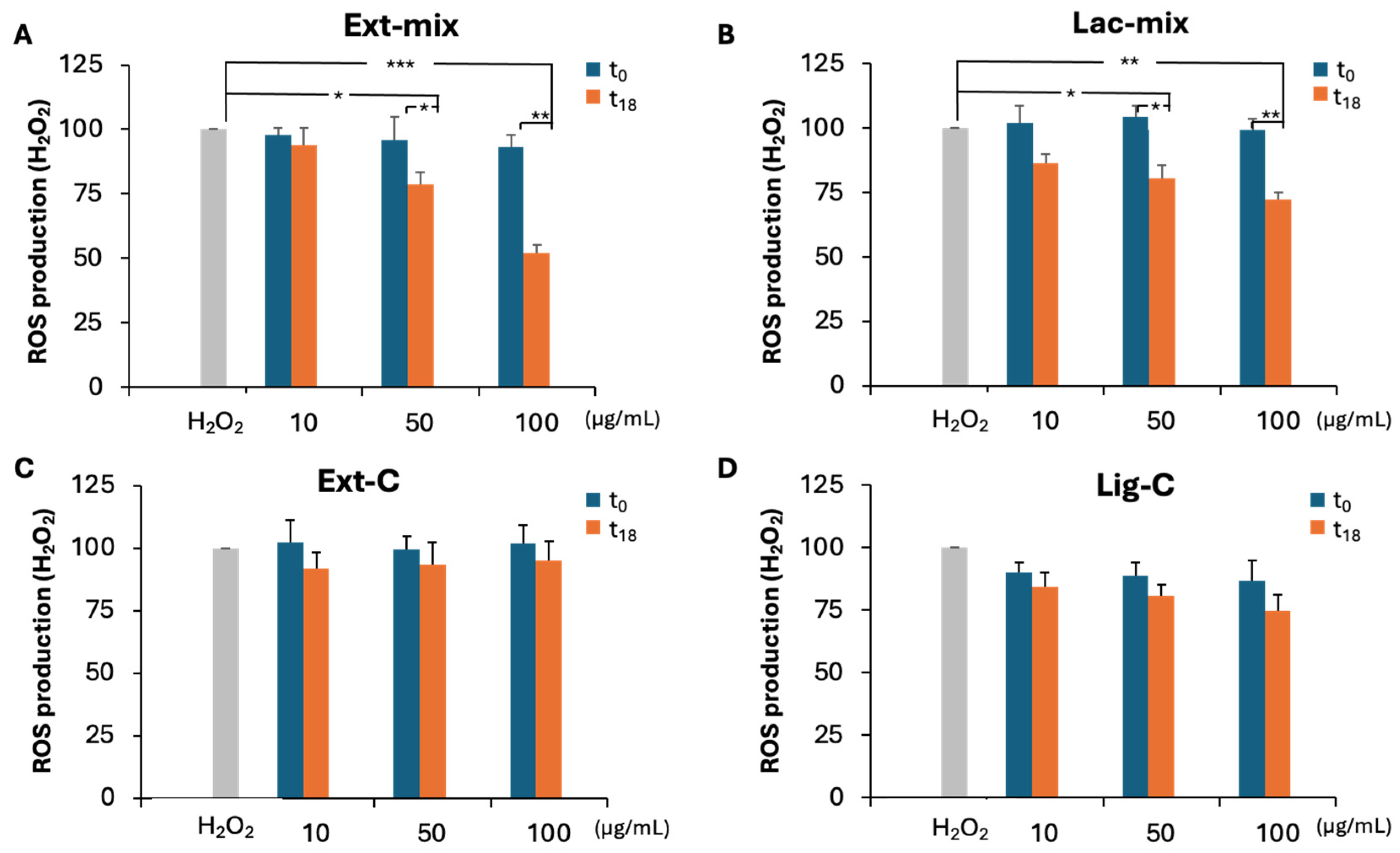
2.5. Effect of Treated Lignin on ROS Production
3. Materials and Methods
3.1. Preparation of Enzymatic Extract
3.2. Enzymatic Laccase Assay
3.3. Enzymatic Peroxidase Assay
3.4. Lignin
3.4.1. Chemical Characterization by Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.4.2. Lignin Solution
3.5. Enzymatic Treatment of Lignin with Extract or Laccase
3.6. Total Phenolics
3.7. Reducing Capacity
3.8. DPPH and Hydroxyl Radical Scavenging Activity
3.9. MTT Viability Assay
3.10. Intracellular Reactive Oxygen Species Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Linger, J.G.; Vardon, D.R.; Guarnieri, M.T.; Karp, E.M.; Hunsinger, G.B.; Franden, M.A.; Johnson, C.W.; Chupka, G.; Strathmann, T.J.; Pienkos, P.T.; et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. USA 2014, 111, 12013–12018. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Hashimoto, K.; Suzuki, F.; Ogiwara, T.; Satoh, K.; Ito, H.; Hatano, T.; Takashi, Y.; Fujisawa, S.I. Molecular requirements of lignin–carbohydrate complexes for expression of unique biological activities. Phytochemistry 2005, 66, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Qin, L.; Ge, Y. Enhancing antioxidant performance of lignin by enzymatic treatment with laccase. ACS Sustain. Chem. Eng. 2018, 6, 2591–2595. [Google Scholar] [CrossRef]
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Recent advances in lignin antioxidant: Antioxidant mechanism, evaluation methods, influence factors and various applications. Int. J. Biol. Macromol. 2023, 251, 125992. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Rusdipoetra, R.A.; Suwito, H.; Puspaningsih, N.N.T.; Haq, K.U. Theoretical insight of reactive oxygen species scavenging mechanism in lignin waste depolymerization products. RSC Adv. 2024, 14, 6310–6323. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. Review A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Shu, F.; Jiang, B.; Yuan, Y.; Li, M.; Wu, W.; Jin, Y.; Xiao, H. Biological activities and emerging roles of lignin and lignin-based products. A Review. Biomacromolecules 2021, 22, 4905–4918. [Google Scholar] [CrossRef]
- Verma, S.R.; Dwivedi, U.N. Lignin genetic engineering for improvement of wood quality: Applications in paper and textile industries, fodder and bioenergy production. S. Afr. J. Bot. 2014, 91, 107–125. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin depolymerization and biotransformation to industrially important chemicals/biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Williamson, J.J.; Rashid, G.M.M. Bacterial enzymes for lignin depolymerisation: New biocatalysts for generation of renewable chemicals from biomass. Curr. Opin. Biotech. 2020, 55, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wan, C.X. Biological valorization strategies for converting lignin into fuels and chemicals. Renew. Sust. Energy Rev. 2017, 73, 610–621. [Google Scholar] [CrossRef]
- Lu, F.J.; Chu, L.; Gau, R.J. Free radical-scavenging properties of lignin. Nutr. Cancer 1998, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Xi, F.; Norris, J.Q. Antioxidant properties of phenolic lignin model compounds. J. Wood Chem. Technol. 1997, 17, 73–90. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Cárcamo-Martínez, A.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for pharmaceutical and biomedical applications–Could this become a reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Reshmy, R.; Athiyaman Balakumaran, P.; Divakar, K.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Sindhu, R. Microbial valorization of lignin: Prospects and challenges. Bioresour. Technol. 2022, 344, 126–240. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Zhao, D.; Jia, L.; Qin, B.; Cao, X.; Zang, L.; Lu, F.; Liu, F. Biological degradation of lignin: A critical review on progress and perspectives. Ind. Crops Prod. 2002, 188, 115715. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Á.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez, A.; Del Río, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar] [PubMed]
- Aza, P.; Camarero, S. Fungal Laccases: Fundamentals, Engineering and Classification Update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Z.; Shi, J.; Yang, C.; Fang, Y.; Chen, G.; Chen, H.; Tian, C. Enzymatic hydrolysis of corn stover lignin by laccase, lignin peroxidase, and manganese peroxidase. Bioresour. Technol. 2022, 361, 127699. [Google Scholar] [CrossRef]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin biodegradation with laccase-mediator systems. Front Energy Res 2014, 2, 12. [Google Scholar] [CrossRef]
- Kumar, V.P.; Sridhar, M.; Rao, R.G. Biological depolymerization of lignin using laccase harvested from the autochthonous fungus Schizophyllum commune employing various production methods and its efficacy in augmenting in vitro digestibility in ruminants. Sci. Rep. 2022, 12, 11170. [Google Scholar] [CrossRef]
- Faix, O. Fourier Transformed Infrared Spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin-Heidelberg, Germany, 1992; pp. 458–464. [Google Scholar]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the physicochemical properties and thermal stability of organosolv and kraft lignins from hardwood and softwood biomass for their potential valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Radany, E.H.; Brenner, M.; Besnard, F.; Bigornia, V.; Bishop, J.M.; Deschepper, C.F. Directed establishment of rat brain cell lines with the phenotypic characteristics of type 1 astrocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 6467–6471. [Google Scholar] [CrossRef]
- Petraglia, T.; Latronico, T.; Fanigliulo, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Antioxidant activity of polysaccharides from the edible mushroom Pleurotus eryngii. Molecules 2023, 28, 2176. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant compounds from edible mushrooms as potential candidates for treating age-related neurodegenerative diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef]
- Setti, L.; Giuliani, S.; Spinozzi, G.; Pifferi, P.G. Laccase catalyzed-oxidative copuling of 3-methyl 2-benzothiazolinone hidrazone and methoxyphenols. Enzym. Microb. Tecnol. 1999, 25, 258–289. [Google Scholar]
- Morkunas, I.; Gmerek, J. The possible involvement of peroxidase in defense of yellow lupine embryo axes against Fusarium oxysporum. J. Plant Physiol. 2007, 164, 185–194. [Google Scholar] [CrossRef]
- Argenziano, R.; Moccia, F.; Esposito, R.; D’Errico, G.; Panzella, L.; Napolitano, A. Recovery of lignins with potent antioxidant properties from shells of edible nuts by a green ball milling/deep eutectic solvent (DES)-based protocol. Antioxidants 2022, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Larocca, M.; Di Marsico, M.; Riccio, P.; Rossano, R. The in vitro antioxidant properties of Muscari comosum bulbs and their inhibitory activity on enzymes involved in inflammation, post-prandial hyperglycemia, and cognitive/neuromuscular functions. J. Food Biochem. 2018, 42, e12580. [Google Scholar] [CrossRef]
- Latronico, T.; Depalo, N.; Valente, G.; Fanizza, E.; Laquintana, V.; Denora, N.; Fasano, A.; Striccoli, M.; Colella, M.; Agostiano, A.; et al. Cytotoxicity study on luminescent nanocrystals containing phospholipid micelles in primary cultures of rat astrocytes. PLoS ONE 2016, 11, e0153451. [Google Scholar] [CrossRef]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, A.; Rossano, R.; Liuzzi, G.M. Neuroprotective potential of isothiocyanates in an in vitro model of neuroinflammation. Inflammopharmacology 2021, 29, 561–571. [Google Scholar] [CrossRef]
| Total Phenolics (μg GAEs/mL) | Reducing Capacity (μg GAEs/mL) | Radical-Scavenging Activity (IC50 μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| DPPH | Hydroxyl | |||||||
| sample | t0 | t18 | t0 | t18 | t0 | t18 | t0 | t18 |
| Ext-C | 62.2 ± 3.7 | 58.4 ± 2.6 | 54.4 ± 4.2 | 47.3 ± 5.1 | nd | nd | nd | nd |
| Lac-C | nd | nd | nd | nd | nd | nd | nd | nd |
| Lig-C | 525.3 ± 7.6 | 483.6 ± 26.9 | 326.5 ± 16.2 | 355.3 ± 34.0 | 88.2 ± 5.9 | 92.8 ± 2.1 | 79.2 ± 3.5 | 77.7 ± 5.1 |
| Ext-mix | 528.2 ± 10.1 | * 945.5 ± 15.7 | 366.1 ± 15.9 | * 656.9 ± 22.8 | 91.2 ± 5.5 | * 27.4 ± 3.7 | 76.3 ± 1.7 | * 35.9 ± 4.4 |
| ttExt-mix | 513.6 ± 9.7 | 527.4 ± 20.6 | 370.2 ± 11.5 | 366.8 ± 14.4 | 94.3 ± 8.1 | 92.22 ± 5.1 | 78.2 ± 5.5 | 77.9 ± 3.7 |
| Lac-mix | 514.9 ± 7.3 | * 594.8 ± 9.5 | 312.7 ± 17.8 | 333.0 ± 23.2 | 87.8 ± 3.4 | * 62.5 ± 2.8 | 81.1 ± 3.3 | * 64.3 ± 2.9 |
| Gallic acid (positive control) | 5.3 ± 0.3 | 3.4 ± 0.2 | ||||||
| Laccase (U/mL) | Peroxidase (U/mL) |
|---|---|
| 213.0 ± 5.8 | 52.2 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petraglia, T.; Latronico, T.; Pepe, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Increased Antioxidant Performance of Lignin by Biodegradation Obtained from an Extract of the Mushroom Pleurotus eryngii. Molecules 2024, 29, 5575. https://doi.org/10.3390/molecules29235575
Petraglia T, Latronico T, Pepe A, Crescenzi A, Liuzzi GM, Rossano R. Increased Antioxidant Performance of Lignin by Biodegradation Obtained from an Extract of the Mushroom Pleurotus eryngii. Molecules. 2024; 29(23):5575. https://doi.org/10.3390/molecules29235575
Chicago/Turabian StylePetraglia, Tania, Tiziana Latronico, Antonietta Pepe, Aniello Crescenzi, Grazia Maria Liuzzi, and Rocco Rossano. 2024. "Increased Antioxidant Performance of Lignin by Biodegradation Obtained from an Extract of the Mushroom Pleurotus eryngii" Molecules 29, no. 23: 5575. https://doi.org/10.3390/molecules29235575
APA StylePetraglia, T., Latronico, T., Pepe, A., Crescenzi, A., Liuzzi, G. M., & Rossano, R. (2024). Increased Antioxidant Performance of Lignin by Biodegradation Obtained from an Extract of the Mushroom Pleurotus eryngii. Molecules, 29(23), 5575. https://doi.org/10.3390/molecules29235575








