Anti-Melanogenic Effects of Umbelliferone: In Vitro and Clinical Studies
Abstract
1. Introduction
2. Results and Discussion
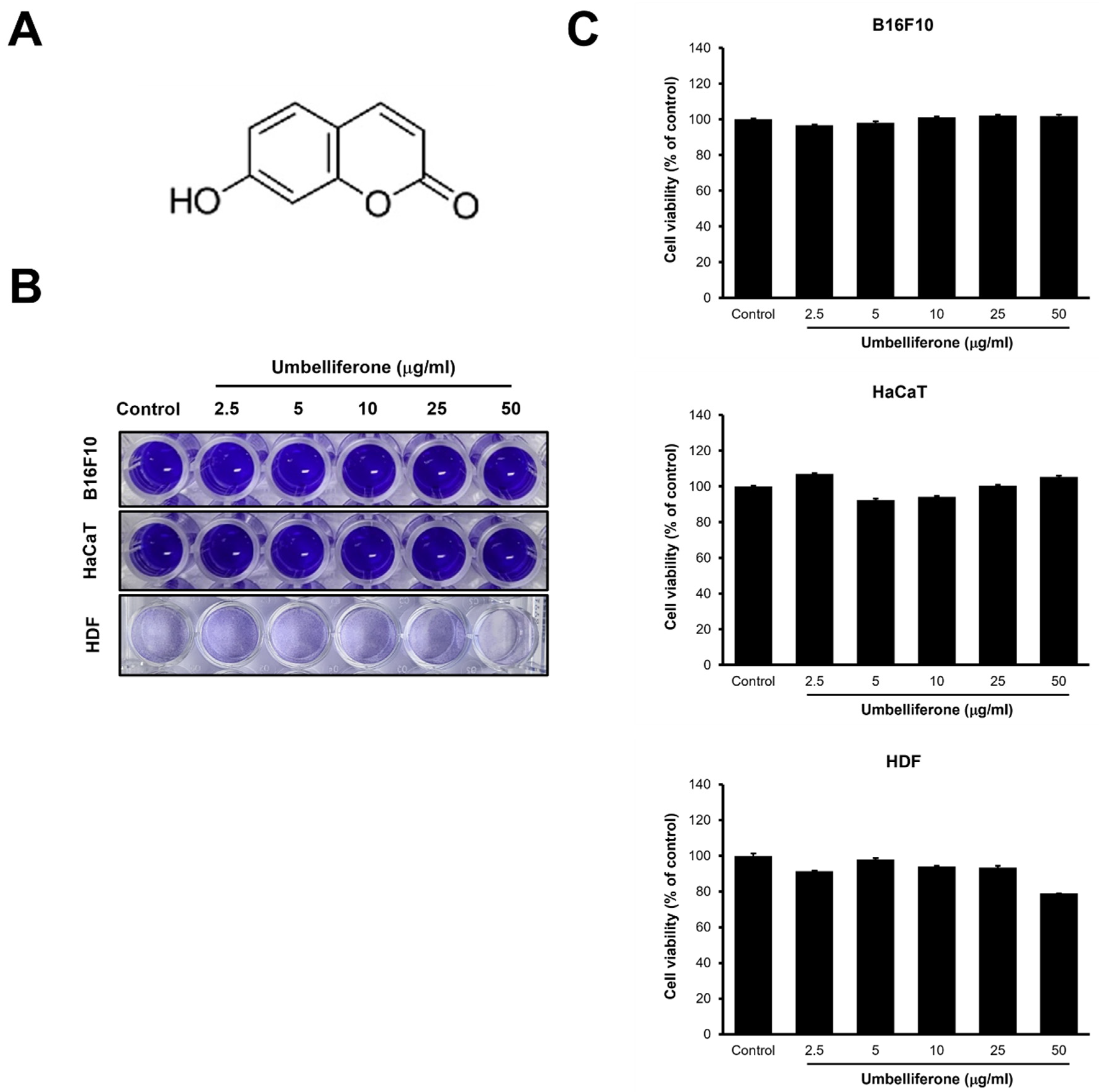
2.1. Cell Viability and Cytotoxicity Effects of Umbelliferone
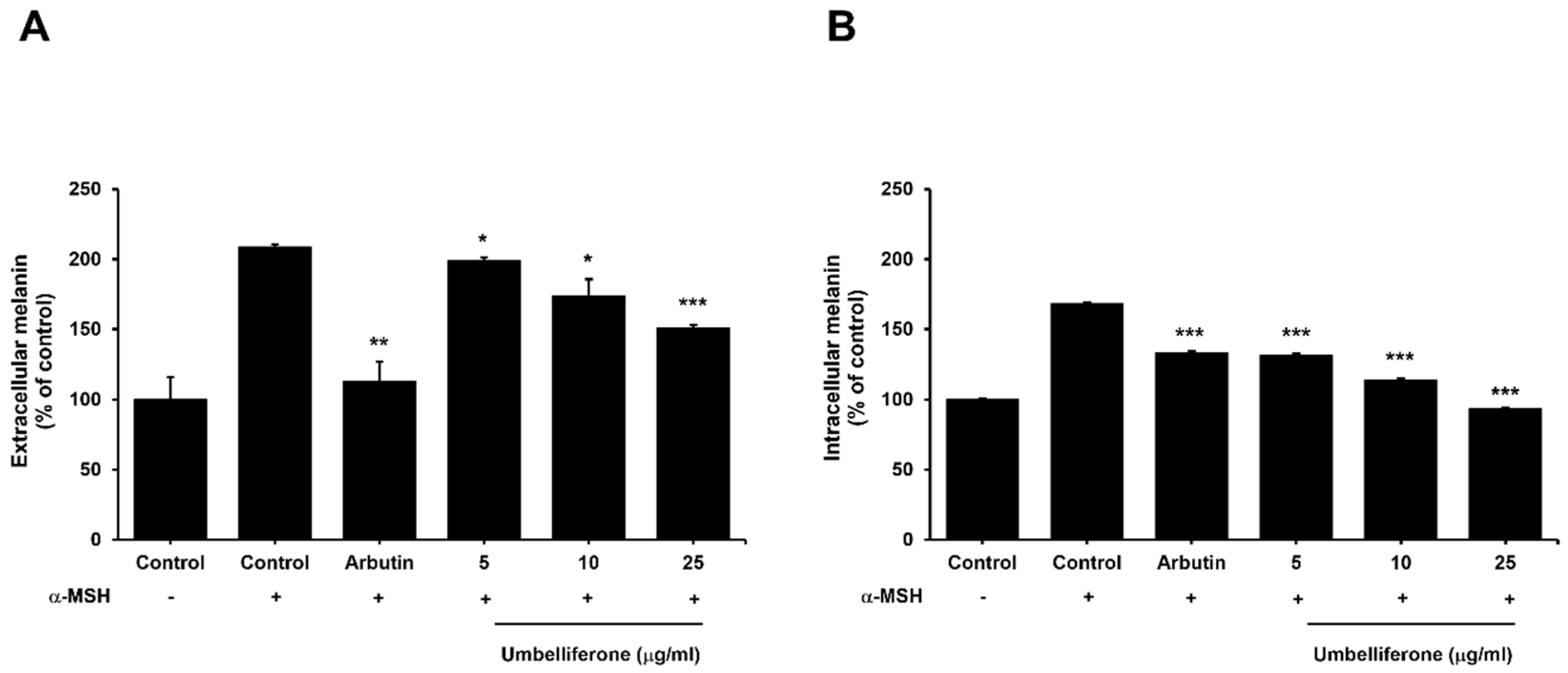
2.2. Effect of Umbelliferone on the Extracellular and Intracellular Melanin Content in B16F10 Melanoma Cells
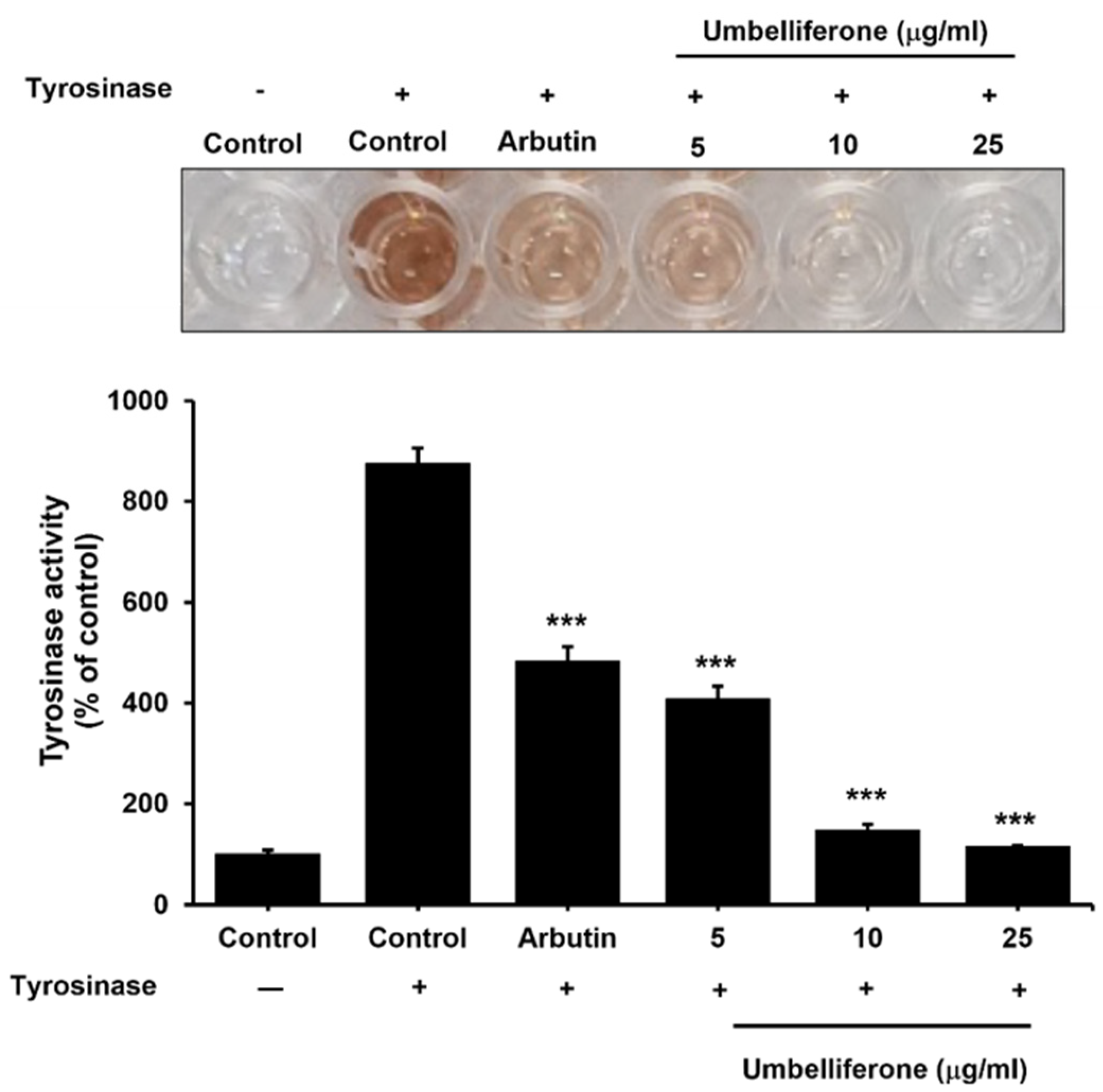
2.3. Effect of Umbelliferone on the Tyrosinase Enzyme in the Mushroom Tyrosinase Assay
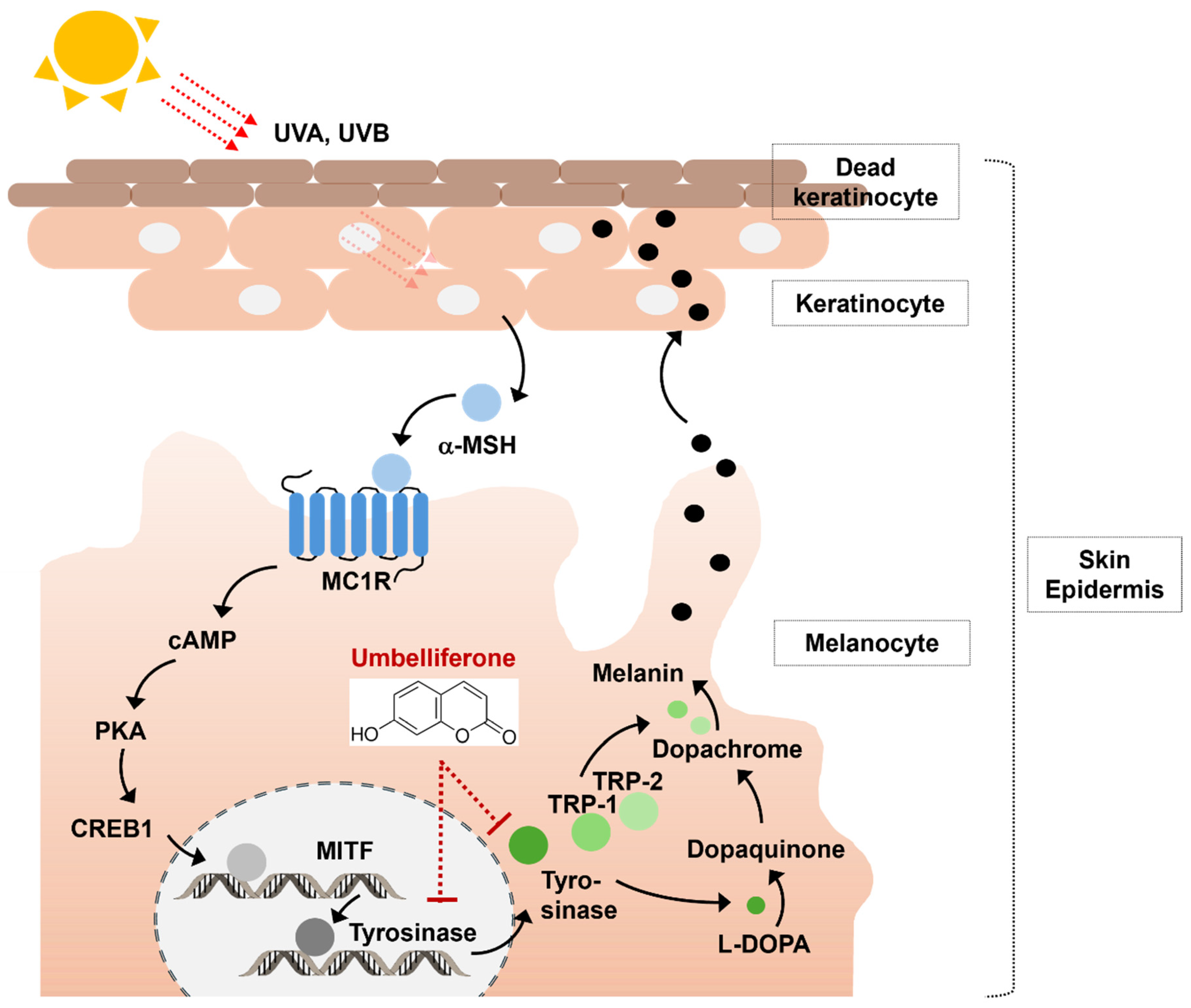
2.4. Effect of Umbelliferone on Tyrosinase and MITF Expression in B16F10 Cells
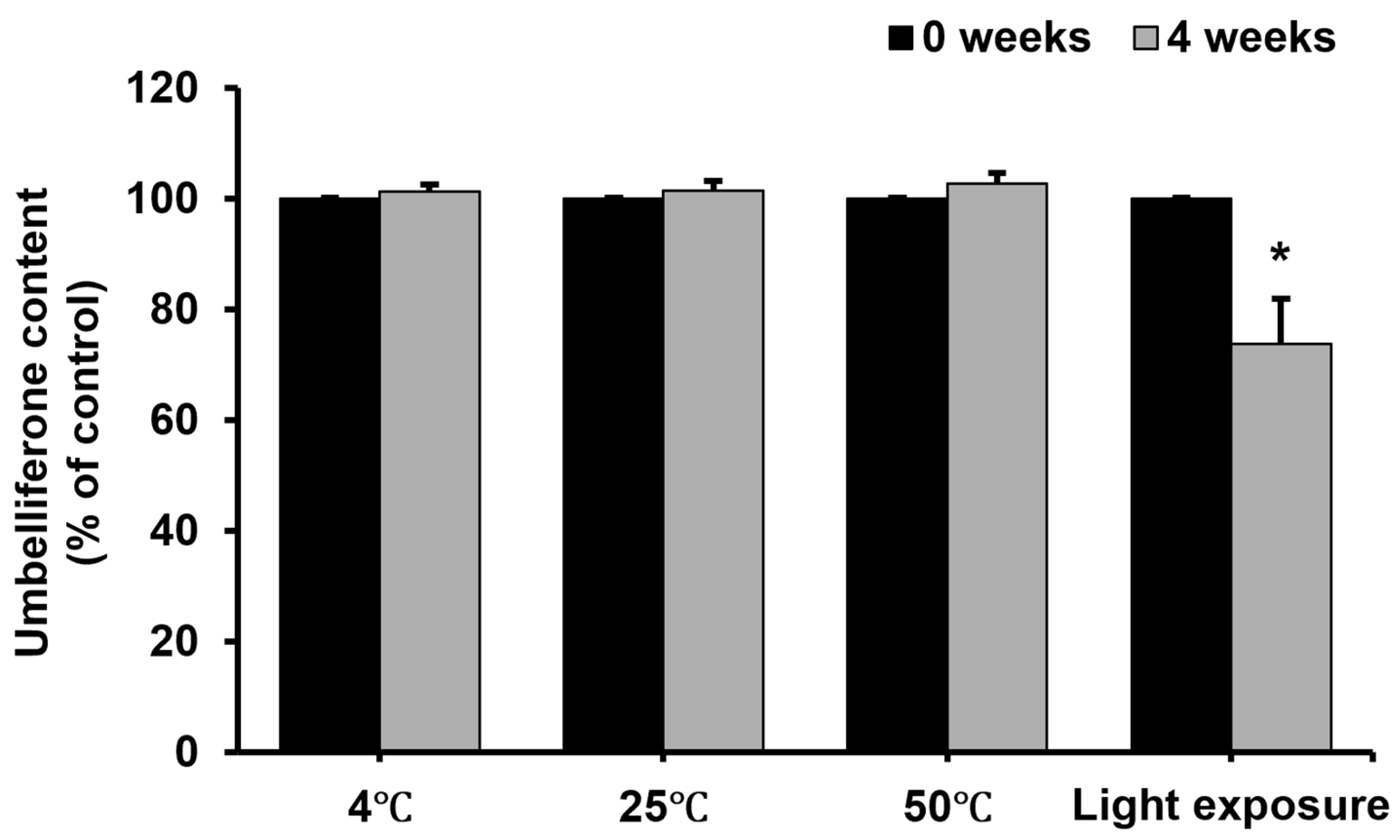
2.5. Stability of Umbelliferone in Formulation Under Different Conditions
2.6. Effect of Umbelliferone on Skin Pigmentation and Lightness in a Clinical Trial
3. Materials and Methods
3.1. Cell Culture and Chemicals
3.2. Cell Viability and Cytotoxicity
3.3. Determination of Melanin Content
3.4. Tyrosinase Activity Assay
3.5. Quantitative Real-Time Polymerase Chain Reaction
3.6. Formulation of the Ampoule Containing Umbelliferone
3.7. Stability Test of Umbelliferone in the Formulation
3.8. Randomized Controlled Clinical Trial
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Szalay, S.; Wertz, P.W. Protective Barriers Provided by the Epidermis. Int. J. Mol. Sci. 2023, 24, 3145. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef]
- Shin, J.A.; Sun, M.; Jeong, J.-M. Borage Oil Treated with Immobilized Lipase Inhibits Melanogenesis. Lipids 2020, 55, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hyun, C. Acenocoumarol, an Anticoagulant Drug, Prevents Melanogenesis in B16F10 Melanoma Cells. Pharmaceuticals 2023, 16, 604. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Hushcha, Y.; Blo, I.; Oton-Gonzalez, L.; Mauro, G.D.; Martini, F.; Tognon, M.; Mattei, M.D. MicroRNAs in the Regulation of Melanogenesis. Int. J. Mol. Sci. 2021, 22, 6104. [Google Scholar] [CrossRef]
- Ha, S.Y.; Jung, J.Y.; Yang, J.K. Camellia japonica Essential Oil Inhibits Alpha-MSH-induced Melanin Production and Tyrosinase activity in B16F10 Melanoma Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 6328767. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Ubeid, A.A.; Do, S.; Nye, C.; Hantash, B.M. Potent Low Toxicity Inhibition of Human Melanogenesis by Novel Indole-Containing Octapeptides. Biochim. Biophys. Acta 2012, 1820, 1481–1489. [Google Scholar] [CrossRef]
- Cheng, S.-L.; Liu, R.H.; Sheu, J.-N.; Chen, S.-T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of A375 Human Malignant Melanoma Cells Treated with Arbutin. J. Biomed. Sci. 2007, 14, 87–105. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Bernacik, K.; Typek, R. Umbelliferone Instability During an Analysis Involving Its Extraction Process. Monatsh. Chem. 2018, 149, 1327–1340. [Google Scholar] [CrossRef]
- Kesharwani, S.; Patel, D.K.; Kesharwani, R. Bioactive-Loaded Nanomedicine for the Management of Health and Disease, 1st ed.; Apple Academic Press: New York, NY, USA, 2022. [Google Scholar]
- Kornicka, A.; Balewski, Ł.; Lahutta, M.; Kokoszka, J. Umbelliferone and Its Synthetic Derivatives as Suitable Molecules for the Development of Agents With Biological Activities: A Review of Their Pharmacological and Therapeutic Potential. Pharmaceuticals 2023, 16, 1732. [Google Scholar] [CrossRef] [PubMed]
- Mazimba, O. Umbelliferone: Sources, Chemistry and Bioactivities Review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Radha, G.V.; Sadhana, B.; Trideva, S.K.; Ganapaty, S. Bioactive Umbelliferone and Its Derivatives: An Update. J. Pharmacogn. Phytochem. 2019, 8, 59–66. [Google Scholar]
- Lin, Z.; Cheng, X.; Zheng, H. Umbelliferon: A Review of Its Pharmacology, Toxicity and Pharmacokinetics. Inflammopharmacology 2023, 31, 1731–1750. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S.; Çelik, T.A. Anticancer Effect of Umbelliferone on MKN-45 and MIA PaCa-2 Cell Lines. Toxicol. Vitr. 2023, 93, 105694. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Lee, J.-H.; Lee, D.-H.; Lee, J.-H.; Kim, D.-K. Umbelliferone Reduces the Expression of Inflammatory Chemokines in HaCaT Cells and DNCB/DFE-Induced Atopic Dermatitis Symptoms in Mice. Int. Immunopharmacol. 2019, 75, 105830. [Google Scholar] [CrossRef]
- PlCruz, L.F.; de Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.A.R.; Gonçalves, T.P.R.; Dos Santos Lima, L.A.R.; et al. Umbelliferone (7-Hydroxycoumarin): A Non-toxic Antidiarrheal and Antiulcerogenic Coumarin. Biomed. Pharmacother. 2020, 129, 110432. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.-N.T. The Complexity of the Nrf2 Pathway: Beyond the Antioxidant Response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Kim, K.; Huh, Y.; Lim, K.-M. Anti-pigmentary Natural Compounds and Their Mode of Action. Int. J. Mol. Sci. 2021, 22, 6206. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.M. The Effect of Emodin on Melanogenesis Through The Modulation of ERK and MITF Signaling Pathway. Nat. Prod. Res. 2022, 36, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D.; Lynch, K.; Langelaan, D.N. The C-terminal Transactivation Domain of MITF Interacts Promiscuously with Co-activator CBP/p300. Sci. Rep. 2023, 13, 16094. [Google Scholar] [CrossRef]
- Kim, E.; Zucconi, B.E.; Wu, M.; Nocco, S.E.; Meyers, D.J.; McGee, J.S.; Venkatesh, S.; Cohen, D.L.; Gonzalez, E.C.; Ryu, B.; et al. MITF Expression Predicts Therapeutic Vulnerability to p300 Inhibition in Human Melanoma. Cancer Res. 2019, 79, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, M.C.; Houtzagers, L.E.; Strub, T.; Krossa, I.; Jager, M.J. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int. J. Mol. Sci. 2022, 23, 6001. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, W.; Yang, X. Coordinated MicroRNA/mRNA Expression Profiles Reveal Unique Skin Color Regulatory Mechanisms in Chinese Giant Salamander (Andrias davidianus). Animals 2023, 13, 1181. [Google Scholar] [CrossRef]
- Abdel-Malek, Z.; Swope, V.B.; Suzuki, I.; Akcali, C.; Harriger, M.D.; Boyce, S.T.; Urabe, K.; Hearing, V.J. Mitogenic and Melanogenic Stimulation of Normal Human Melanocytes by Melanotropic Peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 1789–1793. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, H.-K.; Lee, K.J.; Jeon, H.W.; Cui, S.; Lee, Y.M.; Moon, B.J.; Kim, Y.-H.; Lee, Y.-S. Inhibitory Effect of Glyceollin Isolated from Soybean Against Melanogenesis in B16 Melanoma Cells. BMB Rep. 2010, 43, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Pandey, R.P.; Lim, H.N.; Jung, H.J.; Thuan, N.H.; Kim, T.-S.; Sohng, J.K. Synthesis of Umbelliferone Derivatives in Escherichia coli and Their Biological Activities. J. Biol. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Leal, L.K.; Ferreira, A.A.; Bezerra, G.A.; Matos, F.J.; Viana, G.S. Antinociceptive, Anti-inflammatory and Bronchodilator Activities of Brazilian Medicinal Plants Containing Coumarin: A Comparative Study. J. Ethnopharmacol. 2000, 70, 151–159. [Google Scholar] [CrossRef]
- Lino, C.S.; Taveira, M.L.; Viana, G.S.B.; Matos, F.J.A. Analgesic and Antiinflammatory Activities of Justicia pectoralis Jacq and Its Main Constituents: Coumarin and Umbelliferone. Phytother. Res. 1997, 11, 211–215. [Google Scholar] [CrossRef]
- Matos, M.J. Coumarin and Its Derivatives. Molecules 2021, 26, 6320. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Lalevee, J.; Cheng, D.C. Efficacy Analysis of New Copper Complex for Visible Light (455, 530 nm) Radical/Cationic Photopolymerization: The Synergic Effects and Catalytic Cycle. PLoS ONE 2022, 17, e0270679. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Memarian, H.R.; Sabzyan, H. Electron-transfer Induced Photo-oxidation of 4, 5-Dihydro-1, 2, 4-Oxadiazoles: Experimental and Computational Studies. J. Photochem. Photobiol. A 2022, 432, 114032. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and Photostabilization of Drugs and Drug Products. Int. J. Photoenergy 2016, 2016, 8135608. [Google Scholar] [CrossRef]
- Arsalan, A.; Qadeer, K.; Ali, S.A.; Ahmed, S.; Khan, R.A. The Effect of Albumin in Photostabilization of Riboflavin: A Kinetic Study. J. Photochem. Photobiol. A 2020, 394, 112456. [Google Scholar] [CrossRef]
- Chen, Z.; Tu, Y.; Zhang, D.; Liu, C.; Zhou, Y.; Li, X.; Wu, X.; Liu, R. A Thermosensitive Nanoplatform for Photoacoustic Imaging and NIR Light Triggered Chemo-photothermal Therapy. Biomater. Sci. 2020, 8, 4299–4307. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Rossatto, V.; Gallarate, M.; Trotta, M.; Debernardi, F. Vitamin A Palmitate Photostability and Stability over Time. J. Cosmet. Sci. 2004, 55, 233–252. [Google Scholar] [CrossRef]
- Fadel, M.; Kassab, K. Evaluation of the Photostability and Photodynamic Efficacy of Rose Bengal Loaded in Multivesicular Liposomes. Trop. J. Pharm. Res. 2011, 10, 289–297. [Google Scholar] [CrossRef]
- Connors, K.A.; Amidon, G.L.; Stella, V.J. Chemical Stability of Pharmaceuticals. The Handbook for Pharmacists, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Tonnesen, H.H. Photostability of Drugs and Drug Formulations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Alam, M.B.; Bajpai, V.K.; Lee, J.; Zhao, P.; Byeon, J.-H.; Ra, J.-S.; Majumder, R.; Lee, J.S.; Yoon, J.-I.; Rather, I.A.; et al. Inhibition of Melanogenesis by Jineol from Scolopendra subspinipes mutilans via MAP-Kinase Mediated MITF Downregulation and the Proteasomal Degradation of Tyrosinase. Sci. Rep. 2017, 7, 45858. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′–3′) |
|---|---|
| Tyrosinase | F: 5′-ATA GGT GCA TTG GCT TCT GG-3′ |
| R: 5′-TCT TCA CCA TGC TTT TGT GG-3′ | |
| MITF | F: 5′-TCA AGT TTC CAG AGA CGG GT-3′ |
| R: 5′-CAT CAT CAG CCT GGA ATC AA-3′ | |
| GAPDH | F: 5′-ACC CAC TCC TCC ACC TTT GA-3′ |
| R: 5′-CTG TTG CTG TAG CCA AAT TCG T-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.J.; Jung, M.S.; Jin, H.U.; Kim, M.S.; An, C.E. Anti-Melanogenic Effects of Umbelliferone: In Vitro and Clinical Studies. Molecules 2024, 29, 5571. https://doi.org/10.3390/molecules29235571
Kim DJ, Jung MS, Jin HU, Kim MS, An CE. Anti-Melanogenic Effects of Umbelliferone: In Vitro and Clinical Studies. Molecules. 2024; 29(23):5571. https://doi.org/10.3390/molecules29235571
Chicago/Turabian StyleKim, Da Jung, Min Sook Jung, Hee Un Jin, Mi Sun Kim, and Chae Eun An. 2024. "Anti-Melanogenic Effects of Umbelliferone: In Vitro and Clinical Studies" Molecules 29, no. 23: 5571. https://doi.org/10.3390/molecules29235571
APA StyleKim, D. J., Jung, M. S., Jin, H. U., Kim, M. S., & An, C. E. (2024). Anti-Melanogenic Effects of Umbelliferone: In Vitro and Clinical Studies. Molecules, 29(23), 5571. https://doi.org/10.3390/molecules29235571






