Cyclization Strategies in Carbonyl–Olefin Metathesis: An Up-to-Date Review
Abstract
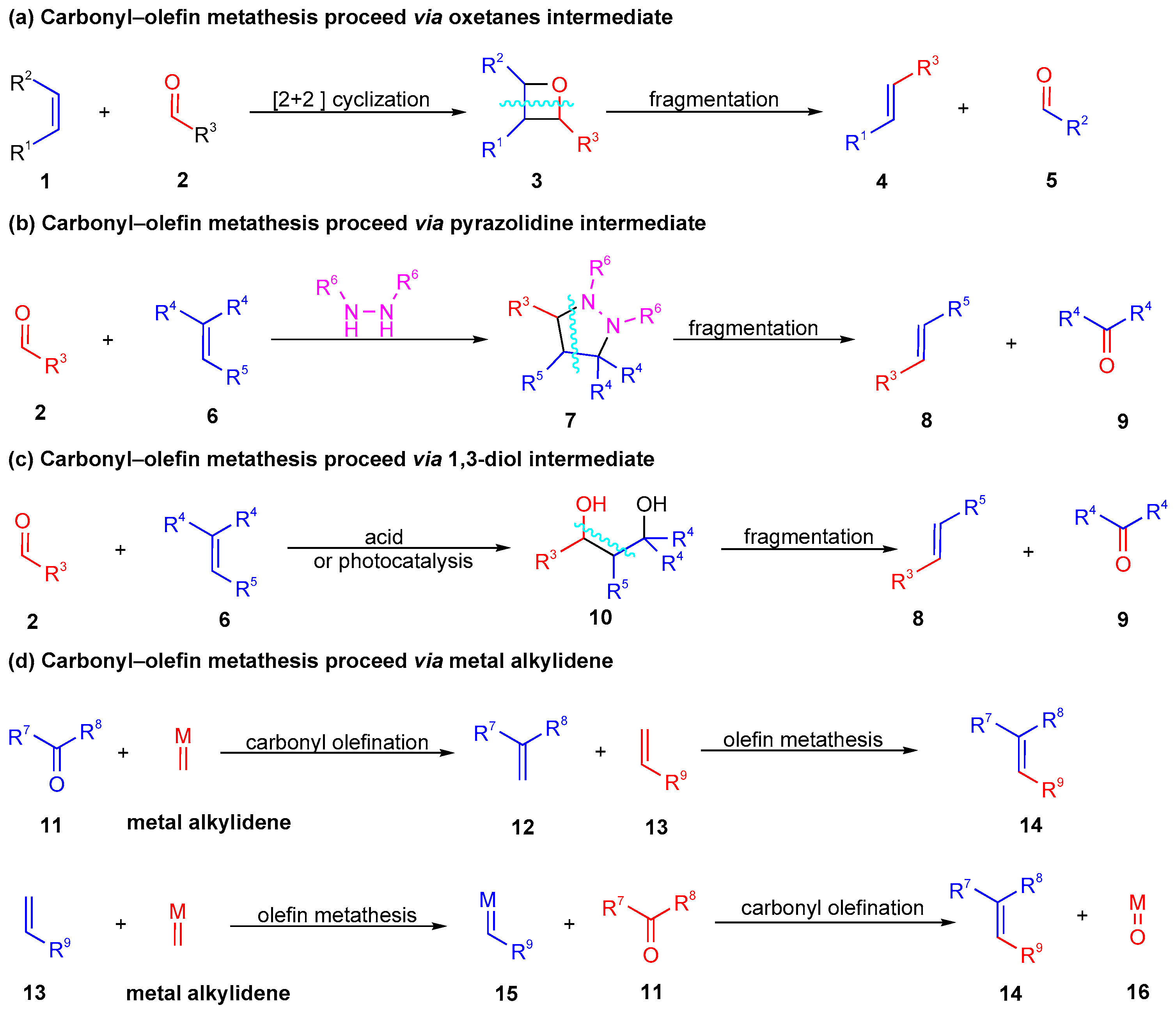
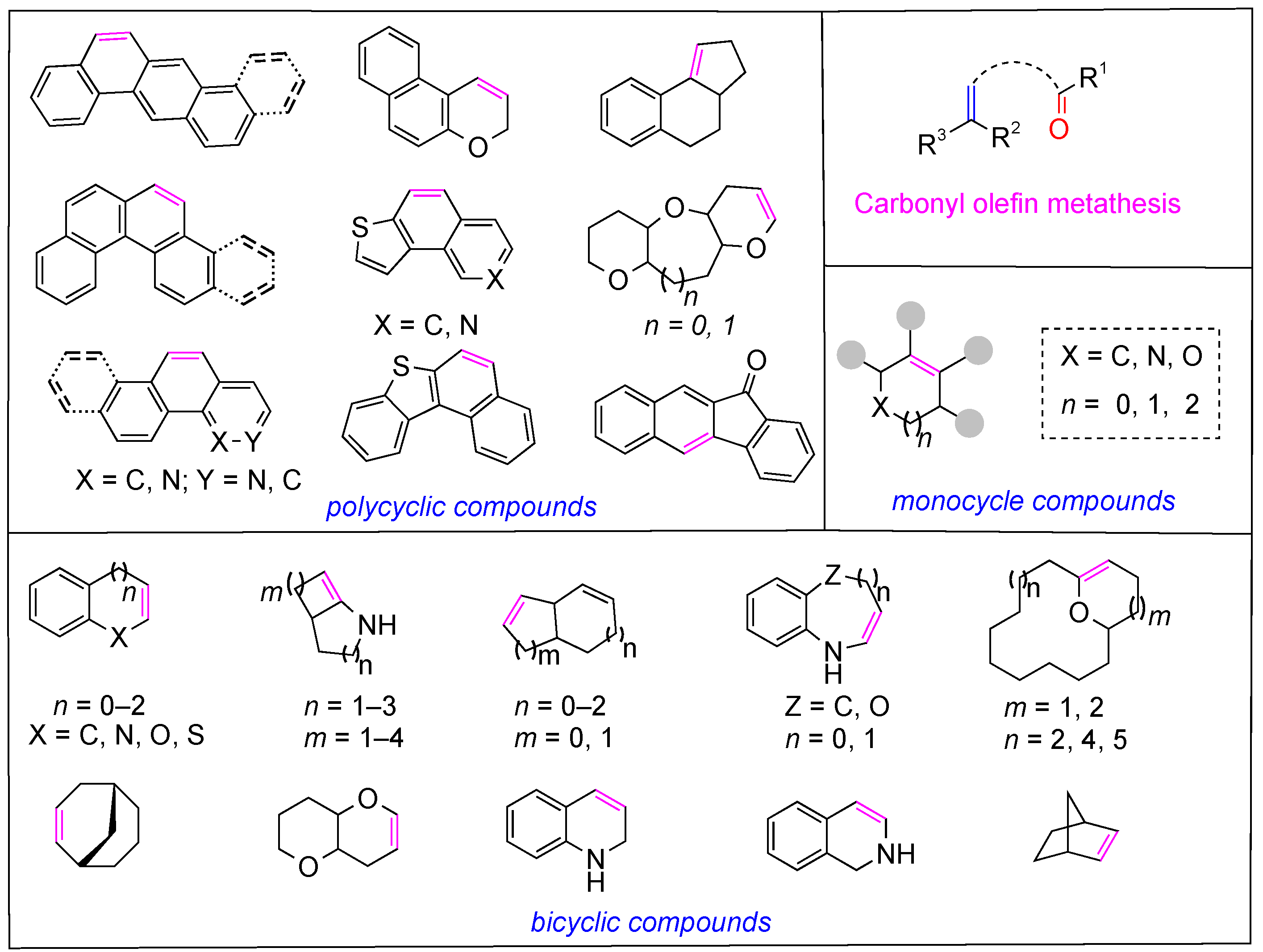
1. Introduction
2. The Formation of Monocyclic Compounds
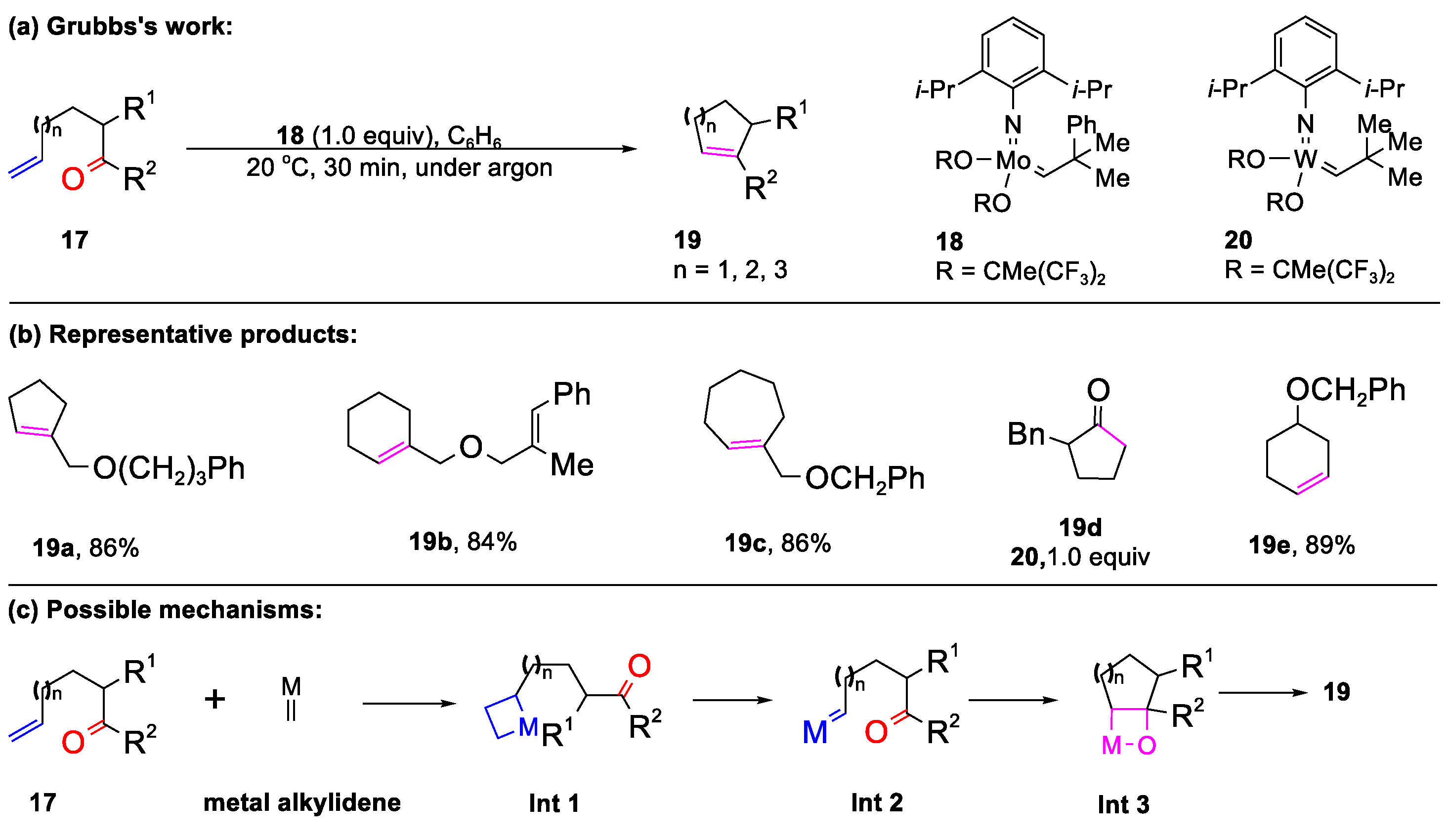
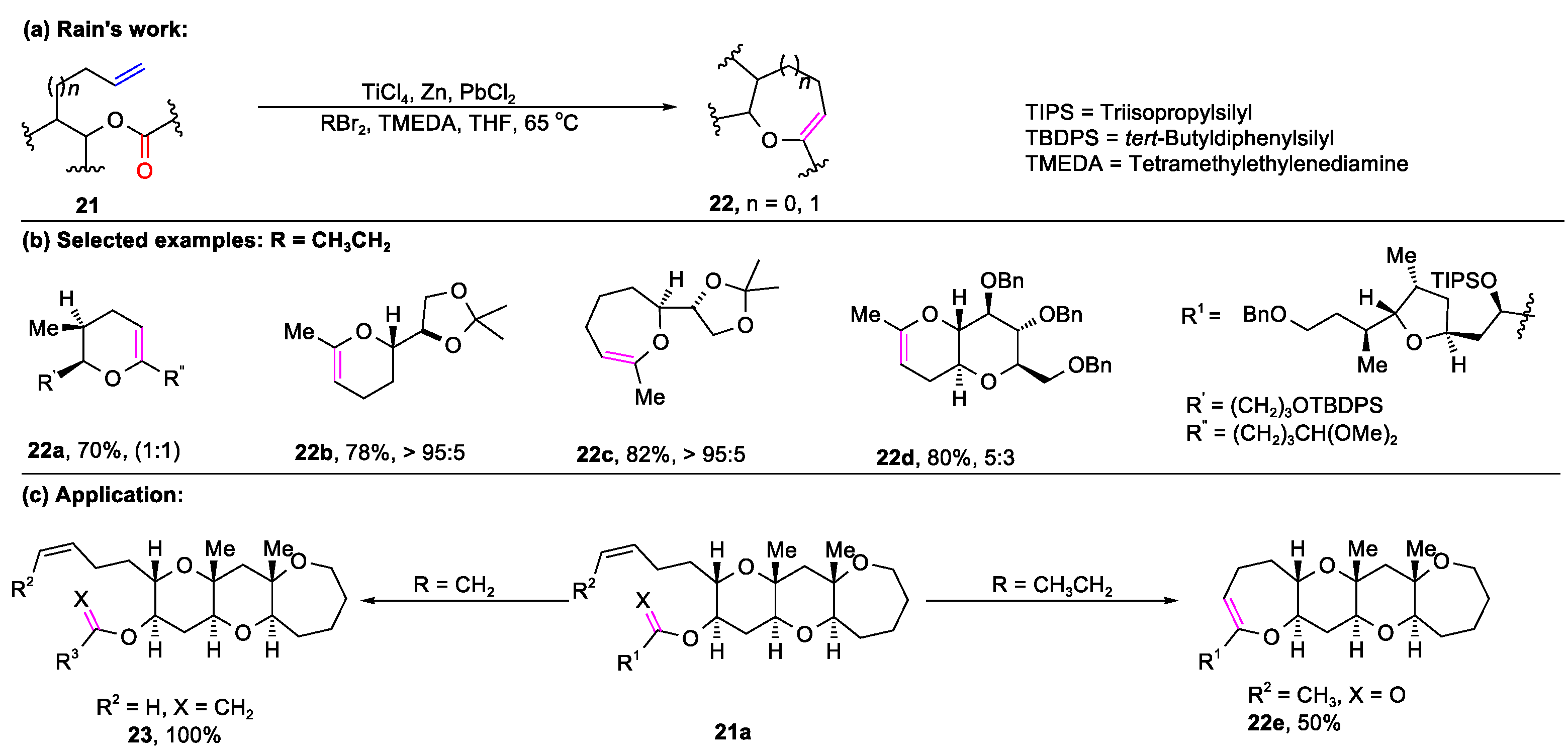
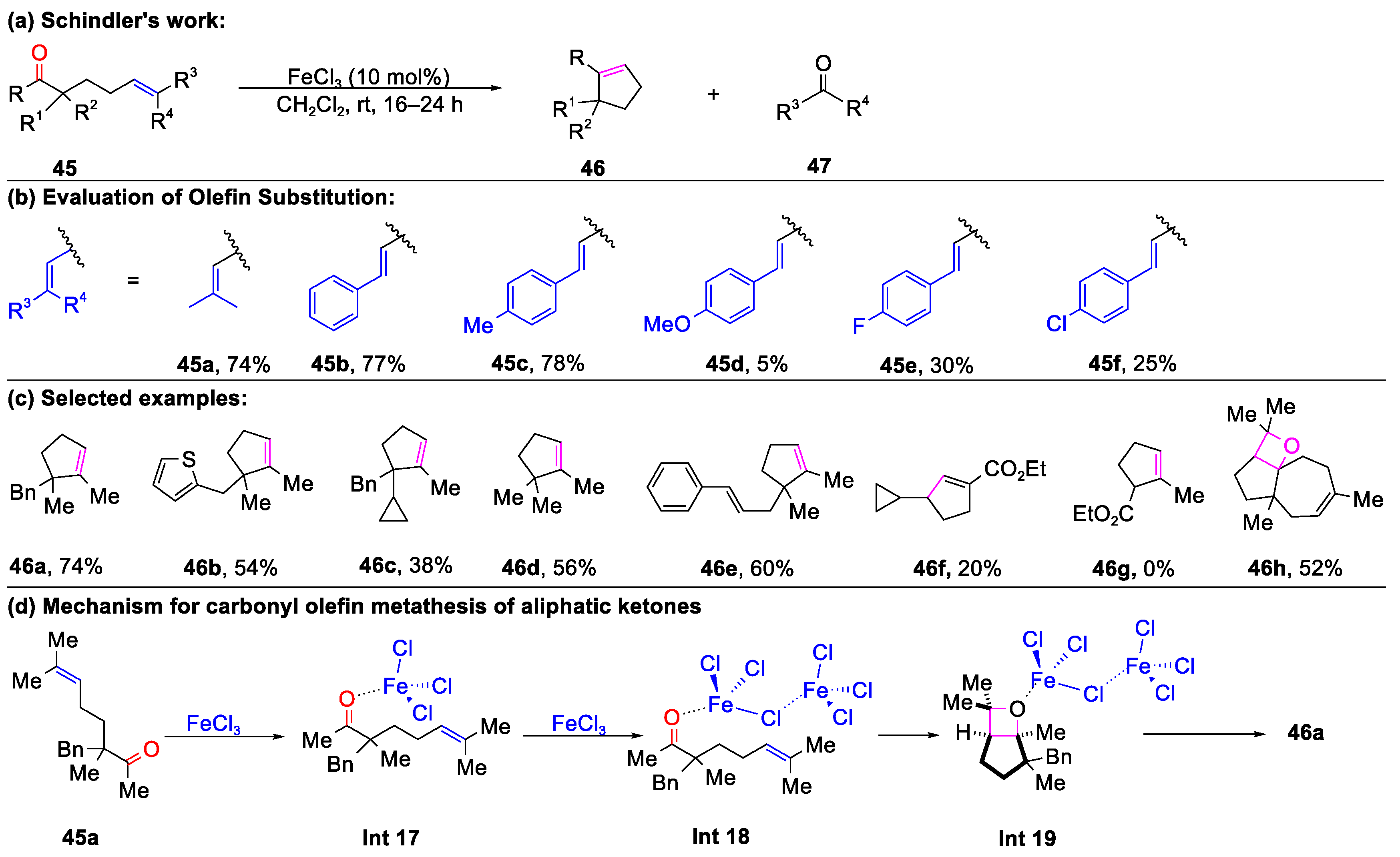
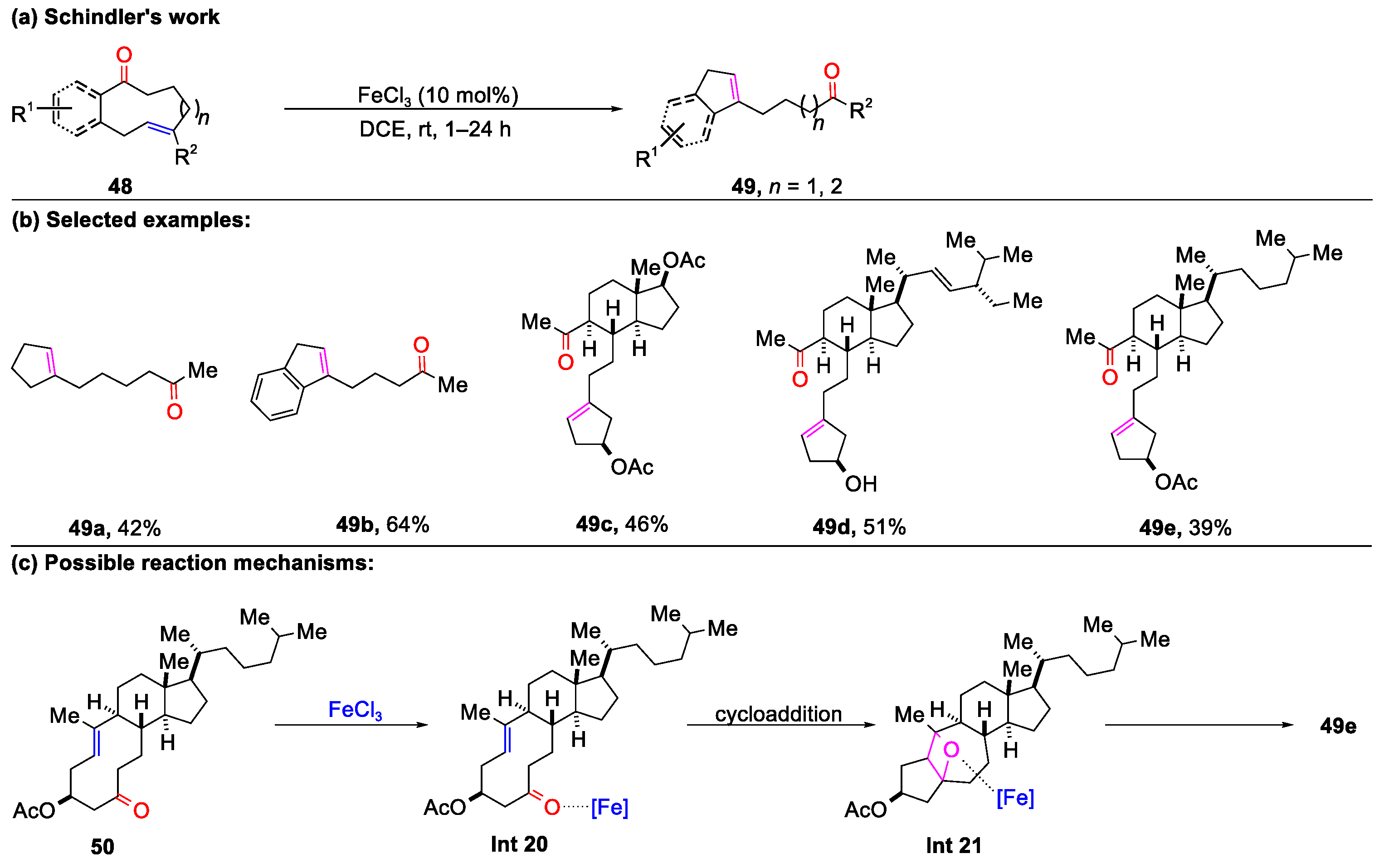
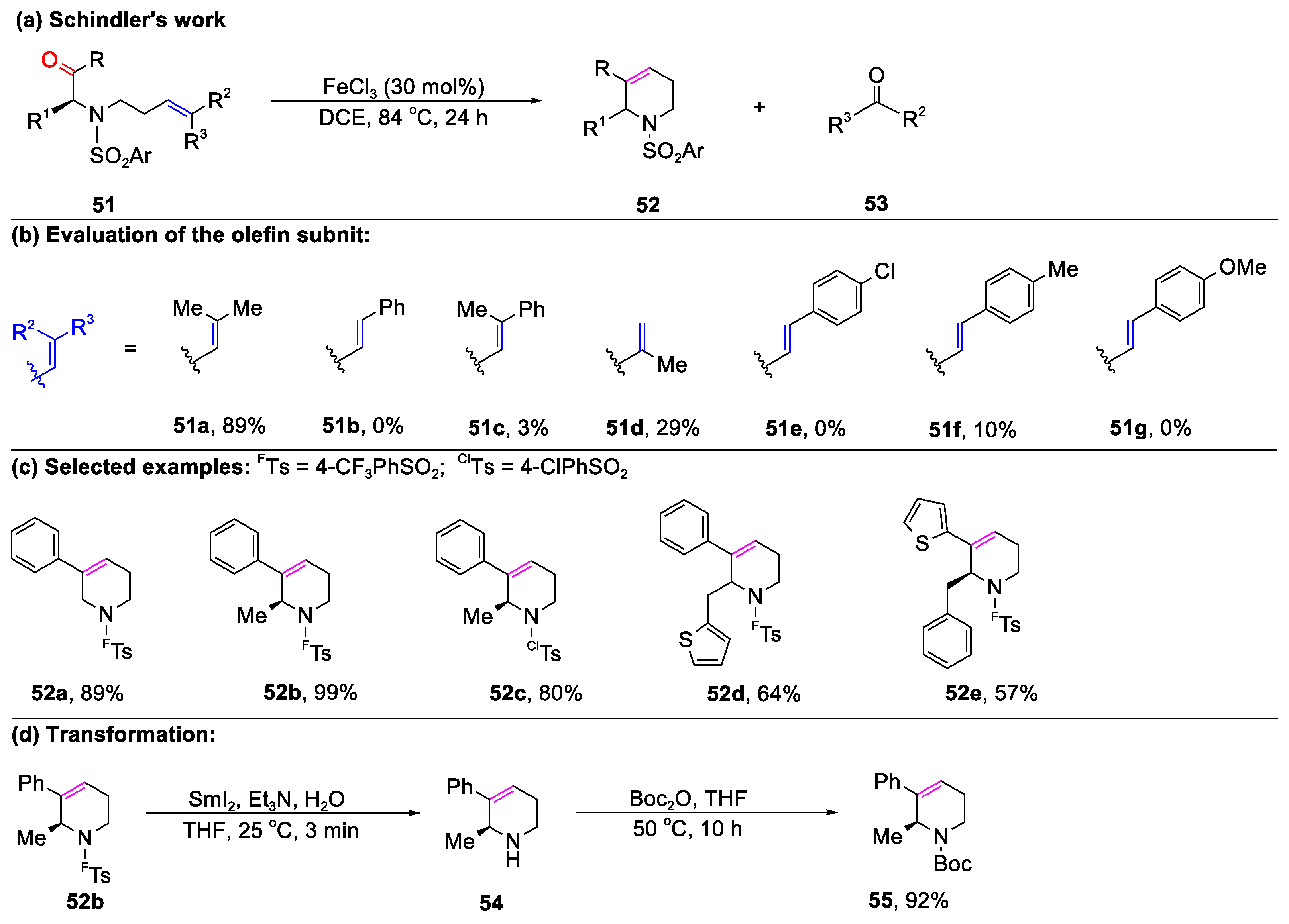
2.1. Carbonyl–Olefin Metathesis Cyclization Reaction Catalyzed by Metal
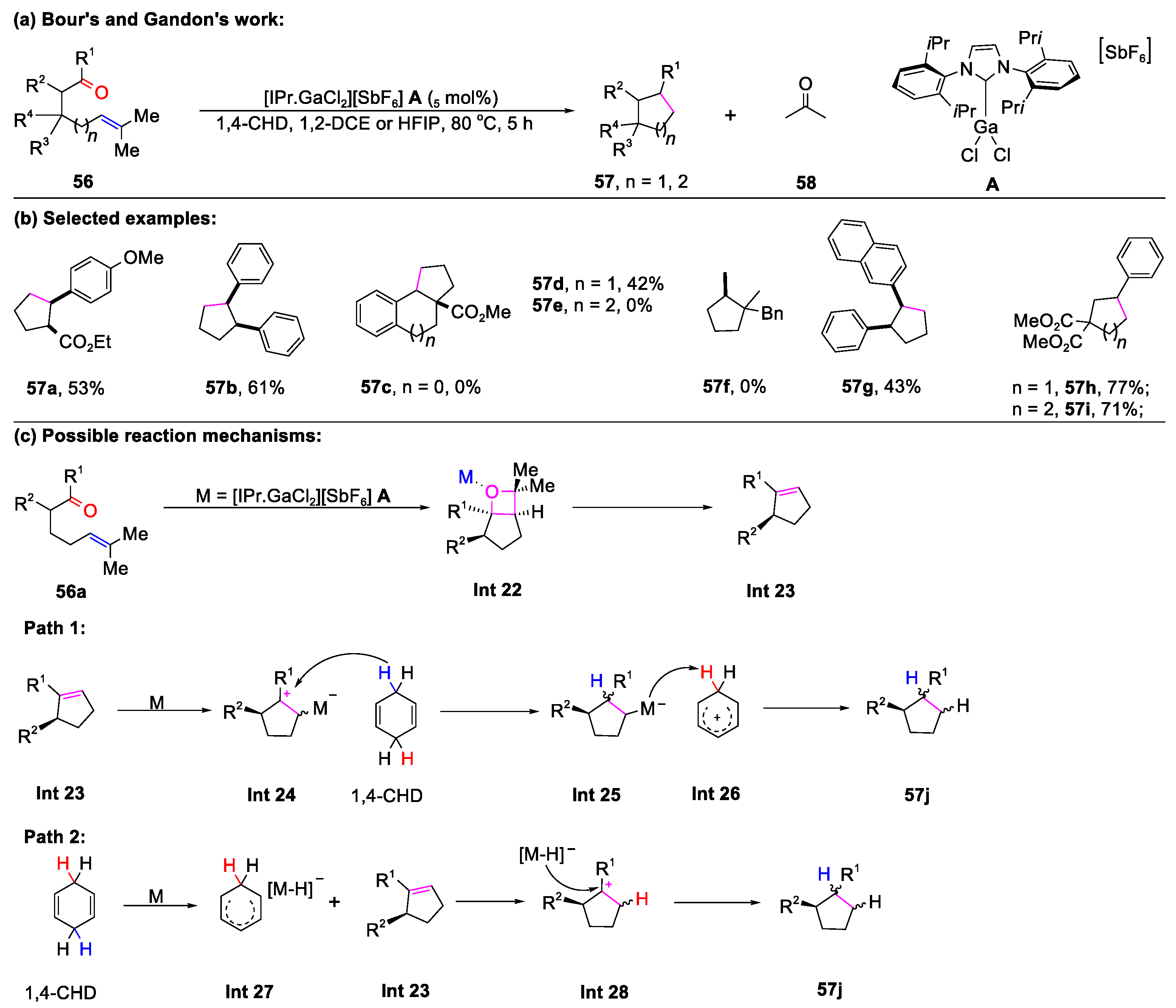
2.2. Carbonyl–Olefin Metathesis Cyclization Reaction Catalyzed by Metal-Free Condition
3. The Formation of Bicyclic Compounds
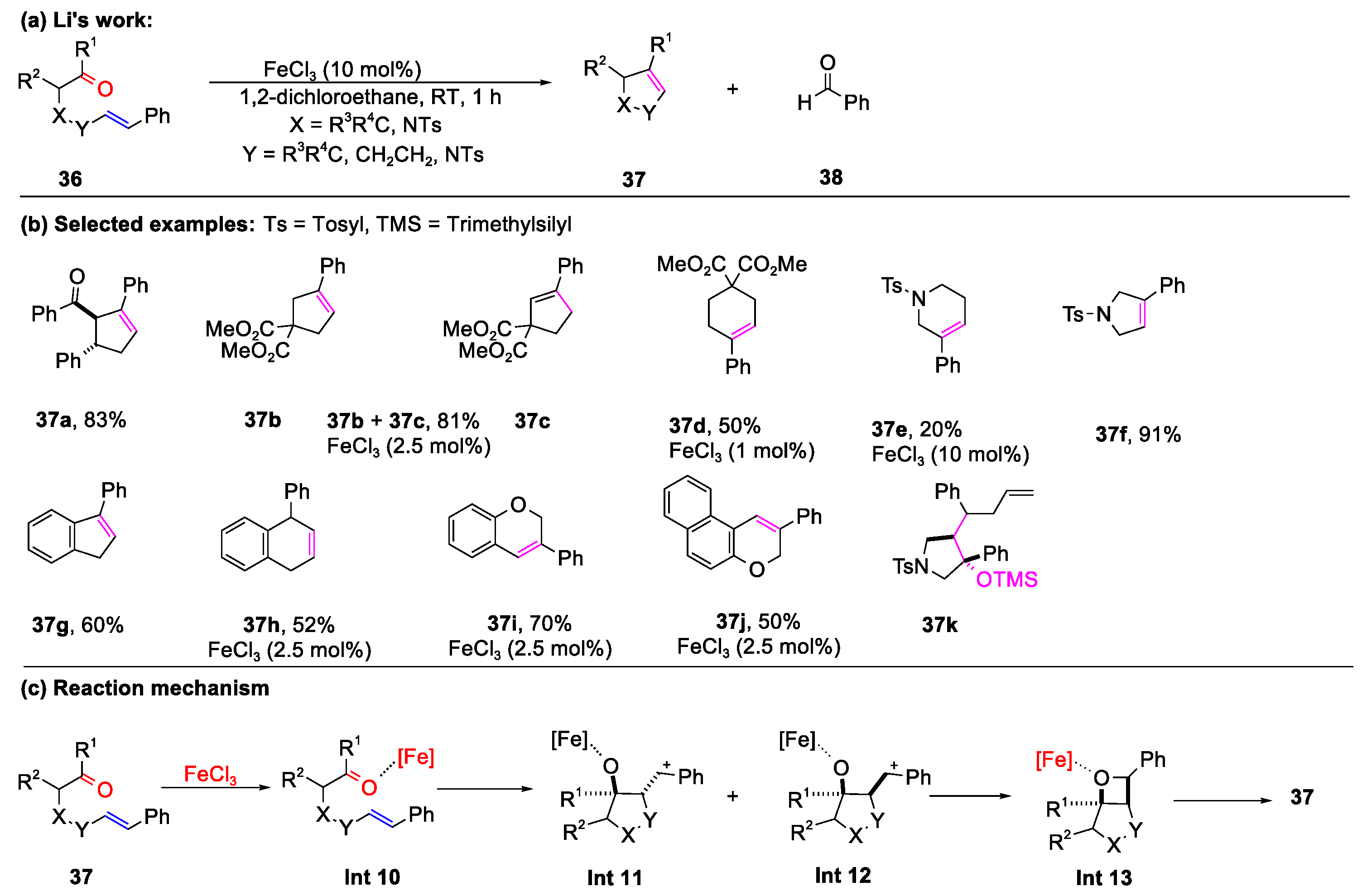
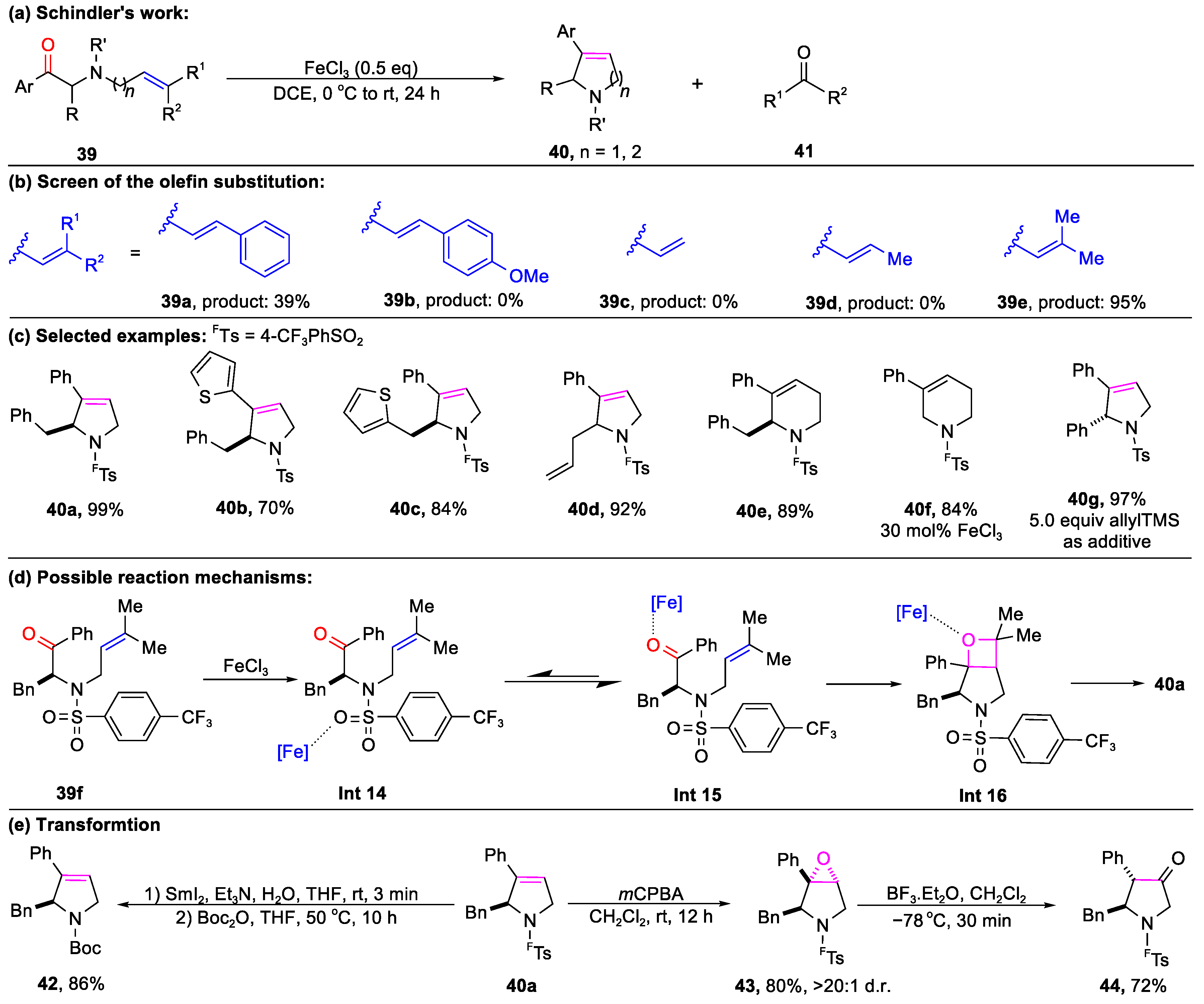
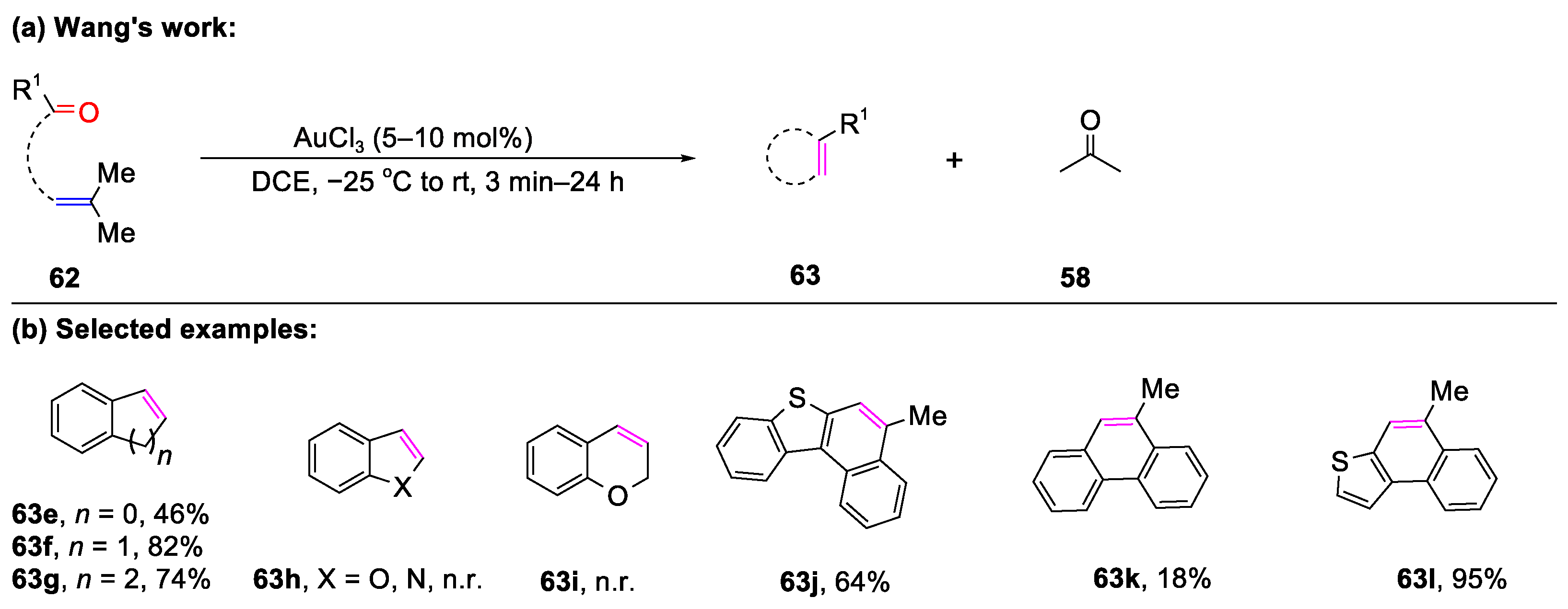
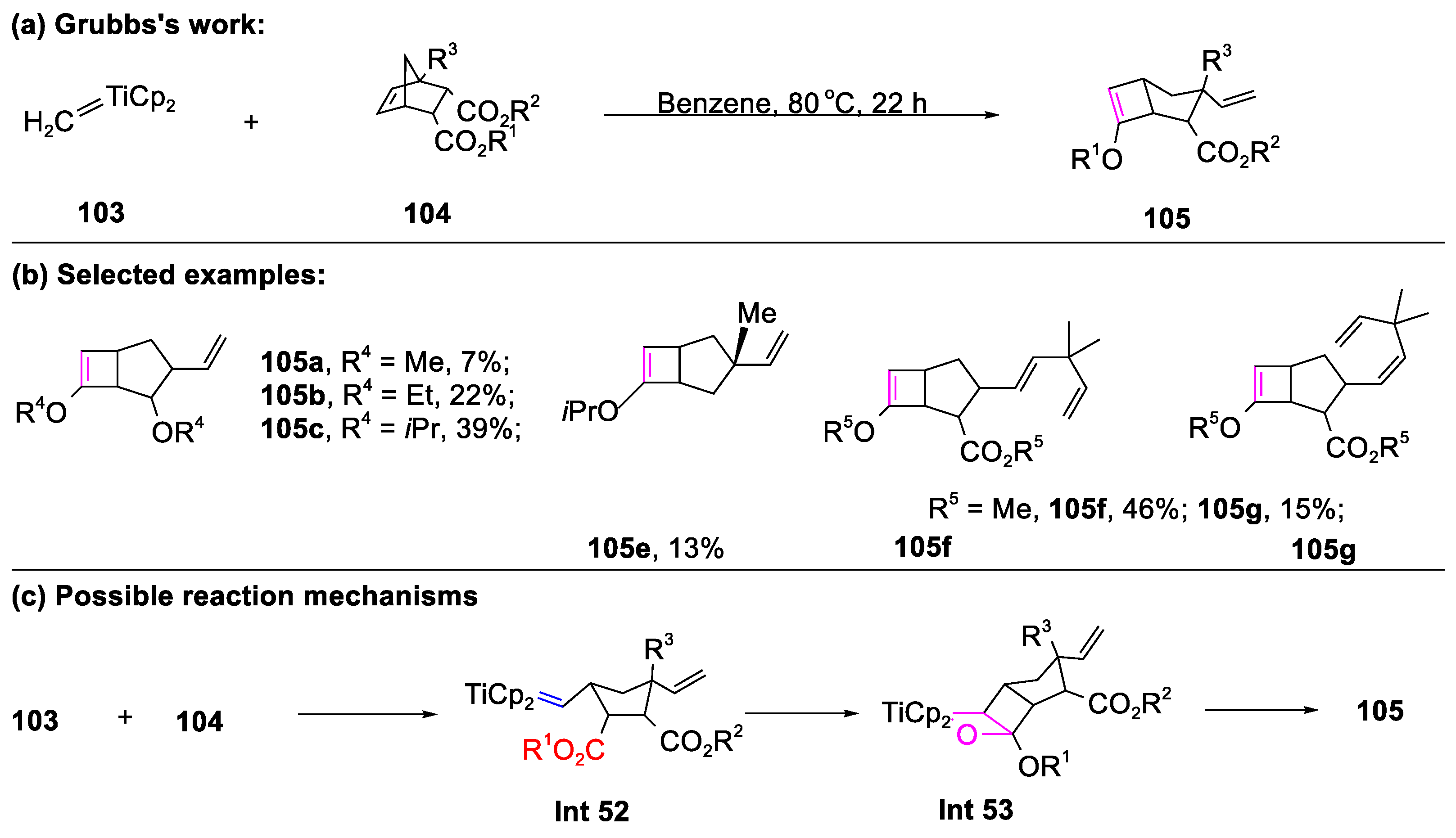
3.1. The Formation of Bicyclic Compounds Catalyzed by Metal
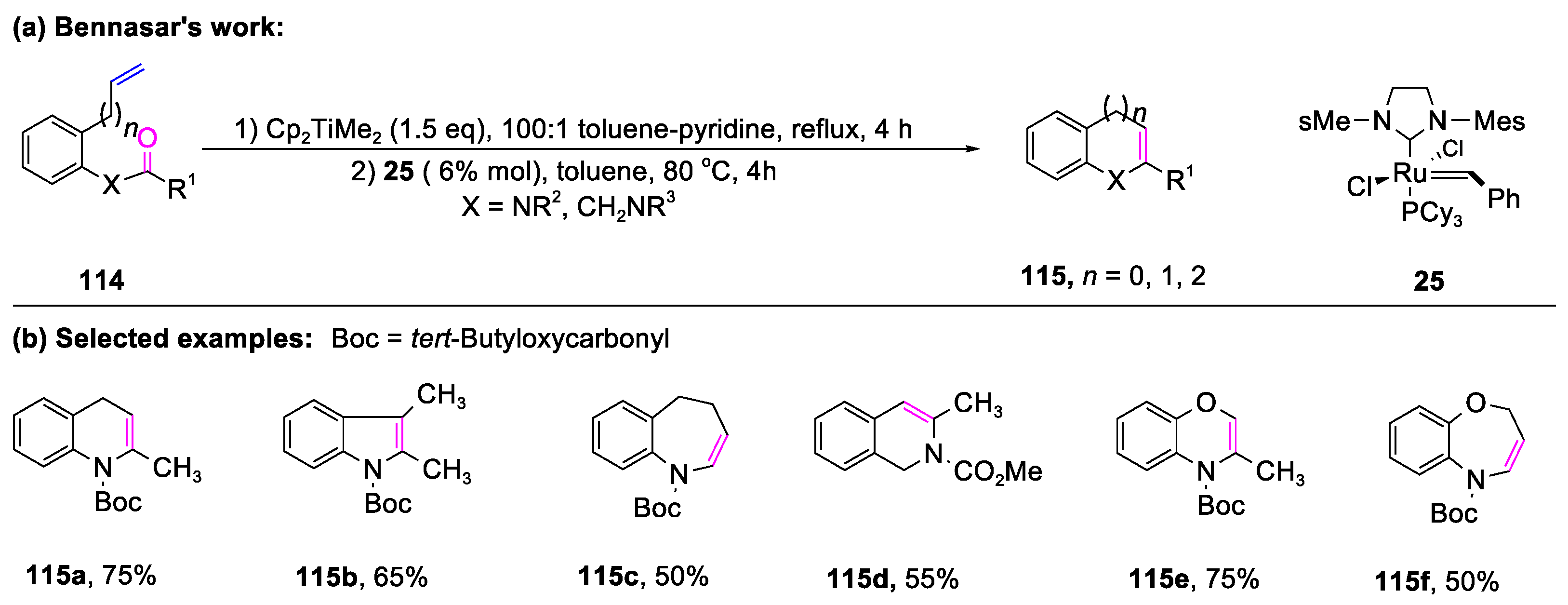
3.2. The Formation of Bicyclic Compounds Catalyzed by Metal-Free Conditions
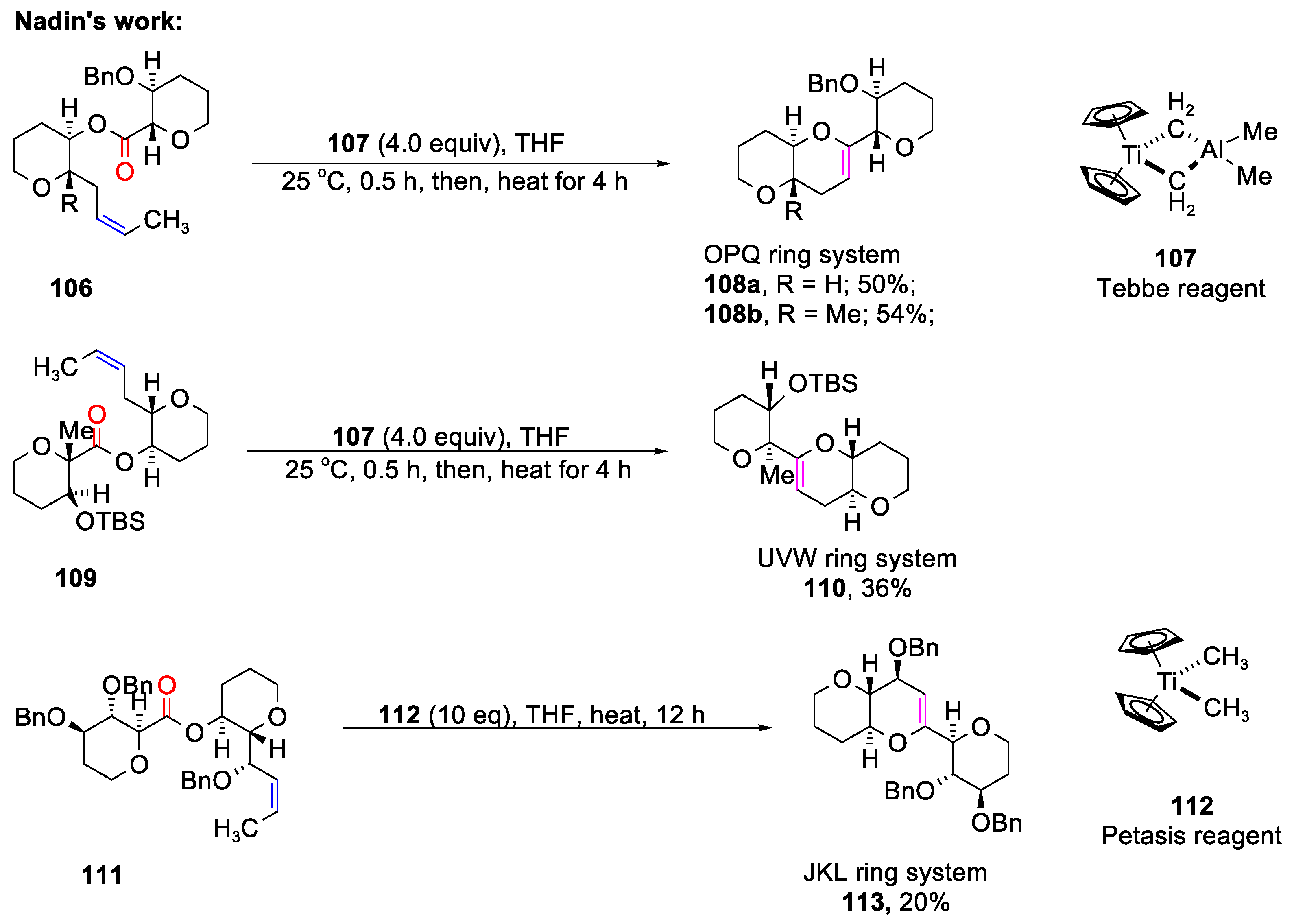
4. The Formation of Polycyclic Compounds
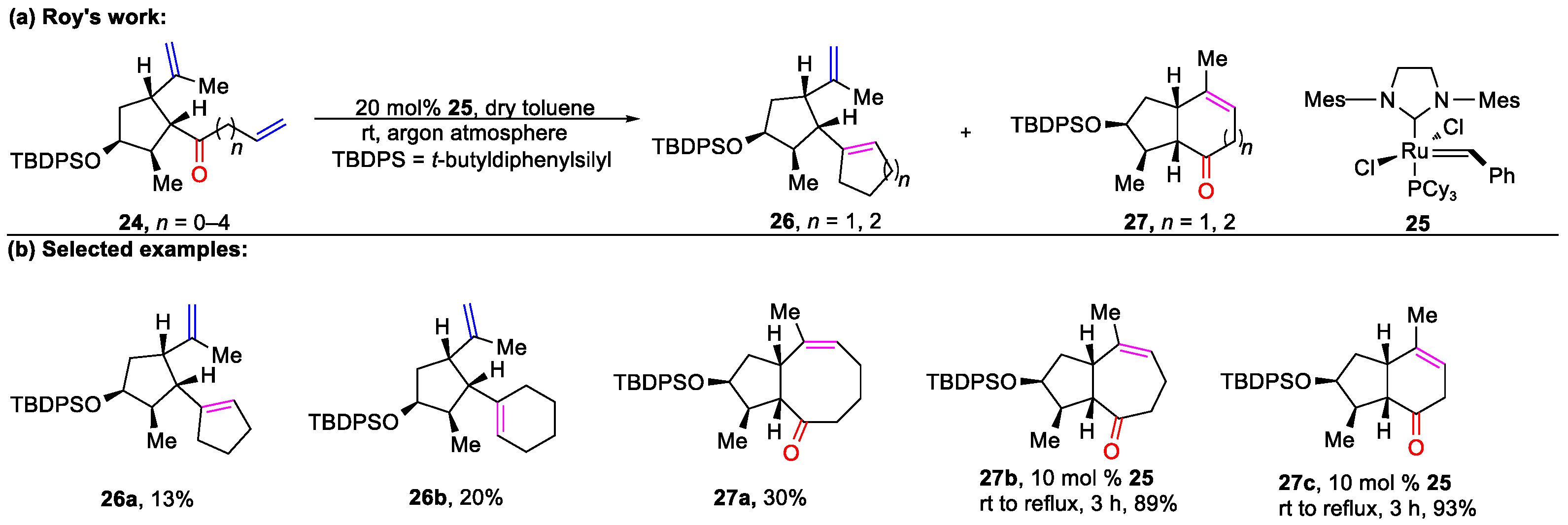
4.1. The Formation of Polycyclic Compounds Catalyzed by Metal
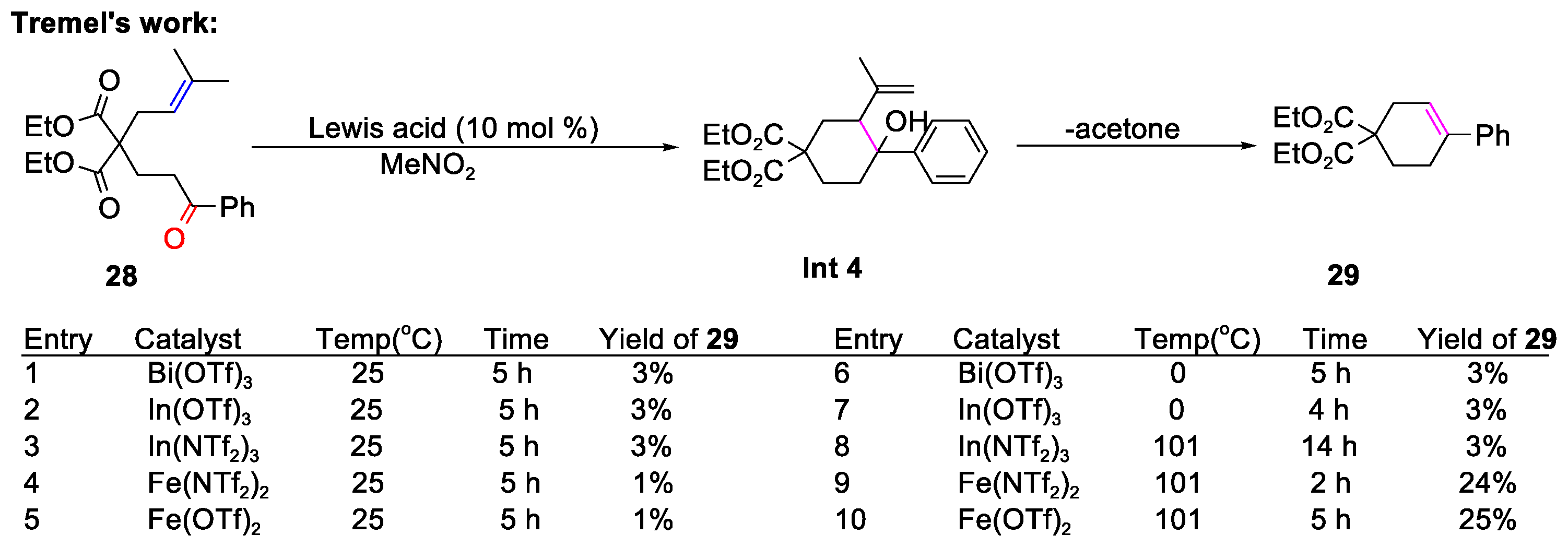
4.2. The Formation of Polycyclic Compounds Catalyzed by Metal-Free Conditions
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.L.; Ba, Y.Y.; Zhang, J.Y.; Sun, D.M. Study and Applications of Stereoselective Olefin Metathesis Reactions. Chin. J. Org. Chem. 2020, 40, 2725–2741. [Google Scholar] [CrossRef]
- Liu, P.; Ai, C.J. Olefin Metathesis Reaction in Rubber Chemistry and Industry and Beyond. Ind. Eng. Chem. Res. 2018, 57, 3807–3820. [Google Scholar] [CrossRef]
- Fürstner, A. Olefin Metathesis and Beyond. Angew. Chem. Int. Ed. 2000, 39, 3012–3043. [Google Scholar] [CrossRef]
- Connon, S.J.; Blechert, S. Recent Developments in Olefin Cross-Metathesis. Angew. Chem. Int. Ed. 2003, 42, 1900–1923. [Google Scholar] [CrossRef]
- Sauer, D.F.; Schiffels, J.; Hayashi, T.; Schwaneberg, U.; Okuda, J. Olefin metathesis catalysts embedded in β-barrel proteins: Creating artificial metalloproteins for olefin metathesis. Beilstein J. Org. Chem. 2018, 14, 2861–2871. [Google Scholar] [CrossRef]
- Goldman, A.S.; Roy, A.H.; Huang, Z.; Ahuja, R.; Schinski, W.; Broolhart, M. Catalytic Alkane Metathesis by Tandem Alkane Dehydrogenation-Olefin Metathesis. Science 2006, 312, 257–261. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Metathesis Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4490–4527. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Zhugralin, A.R. The remarkable metal-catalysed olefin metathesis reaction. Nature 2007, 450, 243–251. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.L.; Sanders, D.P.; Grubbs, R.H.A. General Model for Selectivity in Olefin Cross Metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, C.; Koengeter, T.; Mu, Y.; Hoveyda, A.H. Impact on Ethylene on Efficiency and Stereochemical in Olefin Metathesis: When to Add It, When to Remove It, and When to Avoid It. Angew. Chem. Int. Ed. 2020, 59, 22324–22348. [Google Scholar]
- Nickel, A.; Maruyama, T.; Tang, H.; Murphy, P.D.; Greene, B.; Yusuff, N.; Wood, J.L. Total Synthesis of Ingenol. J. Am. Chem. Soc. 2004, 126, 16300–16301. [Google Scholar] [CrossRef]
- Evans, P.A.; Cui, J.; Gharpure, S.J.; Polosukhin, A.; Zhang, H.R. Enantioselective Total Synthesis of the Potent Antitumor Agent (−)-Mucocin Using a Temporary Silicon-Tethered Ring-Closing Metathesis Cross-Coupling Reaction. J. Am. Chem. Soc. 2003, 125, 14702–14703. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Vassilikogiannakis, G.; Montagnon, T. The Total Synthesis of Coleophomones B and C. Angew. Chem. 2002, 114, 3410–3415, Also published in Angew. Chem. Int. Ed. 2002, 41, 3276–3281. [Google Scholar] [CrossRef]
- Hirama, M.; Oishi, T.; Uehara, H.; Inoue, M.; Maruyama, M.; Oguri, H.; Satake, M. Total synthesis of ciguatoxin CTX3C. Science 2001, 294, 1904–1907. [Google Scholar] [CrossRef]
- Maleczka, R.E.; Terrell, L.R.; Geng, F.; Ward, J.S. Total Synthesis of Proposed Amphidinolide A via a Highly Selective Ring-Closing Metathesis. Org. Lett. 2002, 4, 2841–2844. [Google Scholar] [CrossRef]
- Ogawa, K.A.; Goetz, A.E.; Boydston, A.J. Metal-Free Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc. 2015, 137, 1400–1403. [Google Scholar] [CrossRef]
- Thomas, R.M.; Grubbs, R.H. Synthesis of Telechelic Polyisoprene via Ring-opening Metathesis Polymerization in the Presence of Chain Transfer Agent. Macromolecules 2010, 43, 3705–3709. [Google Scholar] [CrossRef]
- Lucas, F.; Peruch, F.; Carlotti, S.; Deffieux, A.; Leblanc, A.; Boisson, C. Synthesis of Dihydroxy Poly(ethylene-co-butadiene) via Metathetical Depolymerization: Kinetic and Mechanistic Aspects. Polymer 2008, 49, 4935–4941. [Google Scholar] [CrossRef]
- Mouawia, A.; Nourry, A.; Gaumont, A.C.; Pilard, J.F.; Dea, I. Controlled Metathetic Depolymerization of Natural Rubber in Ionic Liquids: From Waste Tires to Telechelic Polyisoprene Oligomers. ACS Sustain. Chem. Eng. 2017, 5, 696–701. [Google Scholar] [CrossRef]
- Camm, K.D.; Castro, N.M.; Liu, Y.W.; Czechura, P.; Snelgrove, J.L.; Fogg, D.E. Tandem ROMP-Hydrogenation with a Third-Generation Grubbs Catalyst. J. Am. Chem. Soc. 2007, 129, 4168–4169. [Google Scholar] [CrossRef]
- Lu, Y.X.; Tournilhac, F.; Leibler, L.; Guan, Z.B. Making Insoluble Polymer Networks Malleable via Olefin Metathesis. J. Am. Chem. Soc. 2012, 134, 8424–8427. [Google Scholar] [CrossRef]
- Valiulin, R.A.; Arisco, T.M.; Kutateladze, A.G. DoubleTandem [4π+2π]·[2π+2π]·[4π+2π]·[2π+2π] Synthetic Sequence with Photoprotolytic Oxametathesis and Photoepoxidation in the Chromone Series. J. Org. Chem. 2011, 76, 1319–1332. [Google Scholar] [CrossRef]
- Valiulin, R.A.; Kutateladze, A.G. Harvesting the Strain Installed by a Paterno–Büchi Step in a Synthetically Useful Way: High-Yielding Photoprotolytic Oxametathesis in Polycyclic Systems. Org. Lett. 2009, 11, 3886–3889. [Google Scholar] [CrossRef]
- Schopov, I.; Jossifov, C. A Carbonyl-Olefin Exchange Reaction–New Route to Polyconjugated Polymers, 1. A New Synthesis of Polyphenylacetylene. Makromol. Chem. Rapid Commun. 1983, 4, 659–662. [Google Scholar] [CrossRef]
- Lambert, T.H. Development of a Hydrazine-Catalyzed Carbonyl-Olefin Metathesis Reaction. Synlett 2019, 30, 1954–1965. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Schindler, C.S. Lewis Acid Catalyzed Carbonyl-Olefin Metathesis. Synlett 2017, 28, 1501–1509. [Google Scholar]
- Ravindar, L.; Lekkala, R.; Rakesh, K.P.; Asiri, A.M.; Marwani, H.M.; Qin, H.L. Carbonyl-Olefin Metathesis: A Key Review. Org. Chem. Front. 2018, 5, 1381–1391. [Google Scholar] [CrossRef]
- Riehl, P.S.; Nasrallah, D.J.; Schindler, C.S. Catalytic, Transannular Carbonyl-Olefin Metathesis Reactions. Chem. Sci. 2019, 10, 10267–10274. [Google Scholar] [CrossRef]
- Becker, M.R.; Watson, R.B.; Schindler, C.S. Beyond olefins: New metathesis directions for synthesis. Chem. Soc. Rev. 2018, 47, 7867–7881. [Google Scholar] [CrossRef]
- Albright, H.; Davis, A.J.; Gomez-Lopez, J.L.; Vonesh, H.L.; Quachm, P.K.; Lambert, T.H.; Schindler, C.S. Carbonyl-Olefin Metathesis. Chem. Rev. 2021, 121, 9359–9406. [Google Scholar] [CrossRef] [PubMed]
- Kohler, E.P.; Richtmyer, N.K. Isoxazoline oxides. IX. The reaction between triphenyl isoxazoline oxide and organic magnesium compounds. J. Am. Chem. Soc. 1930, 52, 2038–2046. [Google Scholar] [CrossRef]
- Adames, G.; Bibby, C.; Grigg, R. Rhodium(I) Catalysed Rearrangements of Vinyl Epoxides and Oxetans. J. Chem. Soc. Chem. Commun. 1972, 491–492. [Google Scholar] [CrossRef]
- Jones, G.; Acquadro, M.A.; Carmody, M.A. Long-Chain Enals via Carbonyl-Olefin Metathesis. An Application in Pheromone Synthesis. J. Chem. Soc. Chem. Commun. 1975, 206–207. [Google Scholar] [CrossRef]
- Barlow, M.G.; Coles, B.; Haszeldine, R.N. Heterocyclic Polyfluoro-Compounds. Part 31. Photochemical Oxetan Formationfrom Fluoroketones and Perfluoroaldehydes and 1,2-Difluoroethylene. J. Chem. Soc. Perkin Trans. 1 1980, 2258–2267. [Google Scholar] [CrossRef]
- Perez–Ruiz, R.; Miranda, M.A.; Alle, R.; Meerholz, K.; Griesbeck, A.G. An Efficient Carbonyl-Alkene Metathesis of Bicyclic Oxetanes: Photoinduced Electron Transfer Reduction of the Paterno–Büchi Adducts from 2,3-Dihydrofuran and Aromatic Aldehydes. Photochem. Photobiol. Sci. 2006, 5, 51–55. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R.; Viggiani, L. Paterno–Büchi Reaction Between Furan and Heterocyclic Aldehydes: Oxetane Formation vs. Metathesis. Photochem. Photobiol. Sci. 2010, 9, 1134–1138. [Google Scholar] [CrossRef]
- Jossifov, C.; Schopov, I. Synthesis of Polymethylacetylene from Mesityl Oxide. Eur. Polym. J. 1993, 29, 621–623. [Google Scholar] [CrossRef]
- Zhang, Y.; Rohanna, J.; Zhou, J.; Iyer, K.; Rainier, J.D. Total Synthesis of Brevenal. J. Am. Chem. Soc. 2011, 133, 3208–3216. [Google Scholar] [CrossRef]
- Jossifov, C. Polymer Formation via Reductive Coupling of a Diketone by Metathesis Catalytic Systems. Eur. Polym. J. 1998, 34, 883–885. [Google Scholar] [CrossRef]
- Penchev, H.; Dimova, S.S.; Zaharieva, K.L.; Ublekov, F.S.; Novakov, C.; Sinigersky, V. Synthesis of polyphenylacetylene by iron(III) chloride catalyzed carbonyl olefin metathesis polymerization of chalcone. Bulg. Chem. Commun. 2018, 50, 169–173. [Google Scholar]
- Xu, X.W.; Yang, F.Y.H.; Zhang, X.F.; Gao, Y.Z.; Su, W.P. Visible-Light-Induced Paternò–Büchi Reaction of Anthraquinones for the Synthesis of Spirocyclic Oxetanes. Asian J. Org. Chem. 2023, 12, e202300069. [Google Scholar] [CrossRef]
- Scharf, D.; Korte, F. Photosensibilisierte Cyclodimerisierung Von Norbornen. Tetrahedron Lett. 1963, 4, 821–823. [Google Scholar] [CrossRef]
- Pitzer, L.; Sandfort, F.; Strieth-Kalthoff, F.; Glorius, F. Carbonyl-Olefin Cross-Metathesis through a Visible-Light-Induced 1,3-Diol Formation and Fragmentation Sequence. Angew. Chem. Int. Ed. 2018, 57, 16219–16223. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Postema, M.H.D.; Claiborne, C.F. Olefin Metathesis in Cyclic Ether Formation. Direct Conversion of Olefinic Esters to Cyclic Enol Ethers with Tebbe-type Reagents. J. Am. Chem. Soc. 1996, 118, 1565–1566. [Google Scholar] [CrossRef]
- Stille, J.R.; Santarsiero, B.D.; Grubbs, R.H. Rearrangement of Bicyclo[2.2.1]Heptane Ring Systems by Titanocene Alkylidene Complexes to Bicyclo[3.2.0]Heptane Enol Ethers–Total Synthesis of (±)-Δ(9,12)-Capnellene. J. Org. Chem. 1990, 55, 843–862. [Google Scholar] [CrossRef]
- Fu, G.C.; Grubbs, R.H. Synthesis of Cycloalkenes via Alkylidene-Mediated Olefin Metathesis and Carbonyl Olefination. J. Am. Chem. Soc. 1993, 115, 3800–3801. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Tumas, W. Polymer Synthesis and Organotransition Metal Chemistry. Science 1989, 243, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.; Schrock, R.R. Recent advances in the chemistry of d alkylidene and metallacycobutane complexes. Prog. Inorg. Chem. 1991, 39, 1–74. [Google Scholar]
- Lyer, K.; Rainier, J.D. Olefinic Ester and Diene Ring-Closing Metathesis Using a Reduced Titanium Alkylidene. J. Am. Chem. Soc. 2007, 129, 12604–12605. [Google Scholar]
- Chakraborty, P.; Roy, S.C. Study towards diversity oriented synthesis of optically active substituted cyclopentane fused carbocyclic and oxacyclic medium-sized rings: Competition between Grubbs-II catalyzed ring closing olefin metathesis and ring closing carbonyl-olefin metathesis. J. Chem. Sci. 2016, 128, 1831–1840. [Google Scholar] [CrossRef]
- Tremel, P.; Lacobucci, C.; Massi, L.; Olivero, S.; Gal, J.F.; Dunach, E. Catalytic intramolecular carbonyl-ene reaction with ketones: Evidence for a retro-ene process. New J. Chem. 2015, 39, 7453–7458. [Google Scholar] [CrossRef]
- Saa, C. Iron(III)-Catalyzed Ring-Closing Carbonyl-Olefin Metathesis. Angew. Chem. Int. Ed. 2016, 55, 10960–10961. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.R.; Zimmerman, P.M.; Gianino, J.B.; Schindler, C.S. Iron(III)-catalysed carbonyl-olefin metathesis. Nature 2016, 533, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.N.; Li, W.J.; Xi, H.; Bai, X.H.; Ma, E.L.; Yan, X.Y.; Li, Z.P. FeCl3-Catalyzed Ring-Closing Carbonyl-Olefin Metathesis. Angew. Chem. Int. Ed. 2016, 55, 10410–10413. [Google Scholar] [CrossRef] [PubMed]
- de Nanteuil, F.; Waser, J. Synthesis of Aminocyclobutanes by Iron-Catalyzed [2+2] Cycloaddition. Angew. Chem. Int. Ed. 2013, 52, 9009–9013. [Google Scholar] [CrossRef]
- Monfette, S.; Fogg, D.E. Equilibrium Ring-Closing Metathesis. Chem. Rev. 2009, 109, 3783–3816. [Google Scholar] [CrossRef]
- Groso, E.J.; Golonka, A.N.; Harding, R.A.; Alexander, B.W.; Sodano, T.M.; Schindler, C.S. 3-Aryl-2,5-Dihydropyrroles via Catalytic Carbonyl-Olefin Metathesis. ACS Catal. 2018, 8, 2006–2011. [Google Scholar] [CrossRef]
- Lugwig, J.R.; Phan, S.; McAtee, C.C.; Zimmerman, P.M.; Devery, J.J.; Schindler, C.S. Mechanistic Investigations of the Iron(III)-Catalyzed Carbonyl-Olefin Metathesis Reaction. J. Am. Chem. Soc. 2017, 139, 10832–10842. [Google Scholar]
- Albright, H.; Riehl, P.S.; McAtee, C.C.; Reid, J.P.; Ludwig, J.R.; Karp, L.A.; Zimmerman, P.M.; Sigman, M.S.; Schindler, C.S. Catalytic Carbonyl-Olefin Metathesis of Aliphatic Ketones: Iron(III) Homo-Dimers as Lewis Acidic Superelectrophiles. J. Am. Chem. Soc. 2019, 141, 1690–1700. [Google Scholar] [CrossRef]
- Ludwig, J.R.; Watson, R.B.; Nasrallah, D.J.; Gianino, J.B.; Zimmerman, P.M.; Wiscons, R.A.; Schindler, C.S. Interrupted carbonyl-olefin metathesis via oxygen atom transfer. Science 2018, 361, 1363–1369. [Google Scholar] [CrossRef]
- Rykaczewski, K.A.; Groso, E.J.; Vonesh, H.L.; Gaviria, M.A.; Richardson, A.D.; Zehnder, T.E.; Schindler, C.S. Tetrahydropyridines via FeCl3-Catalyzed Carbonyl-Olefin Metathesis. Org. Lett. 2020, 22, 2844–2848. [Google Scholar] [CrossRef]
- Djurovic, A.; Vayer, M.; Li, Z.L.; Guilot, R.; Baltaze, J.P.; Gandon, V.; Bour, C. Synthesis of Medium-Sized Carbocycles by Gallium-Catalyzed Tandem Carbonyl-Olefin Metathesis/Transfer Hydrogenation. Org. Lett. 2019, 21, 8132–8137. [Google Scholar] [CrossRef]
- Michelet, B.; Bour, C.; Gandon, V. Gallium-Assisted Transfer Hydrogenation of Alkenes. Chem. Eur. J. 2014, 20, 14488–14492. [Google Scholar] [CrossRef]
- Keess, S.; Oestreich, M. Cyclohexa-1,4-Dienes in Transition Metal-Free Ionic Transfer Processes. Chem. Sci. 2017, 8, 4688–4695. [Google Scholar] [CrossRef]
- Dvis, A.J.; Watson, R.B.; Nasrallah, D.J.; Gomez-Lopez, J.L.; Schindler, C.S. Superelectrophilic aluminium(III)-ion pairs promote a distinct reaction path for carbonyl-olefin ring-closing metathesis. Nat. Catal. 2020, 3, 787–796. [Google Scholar] [CrossRef]
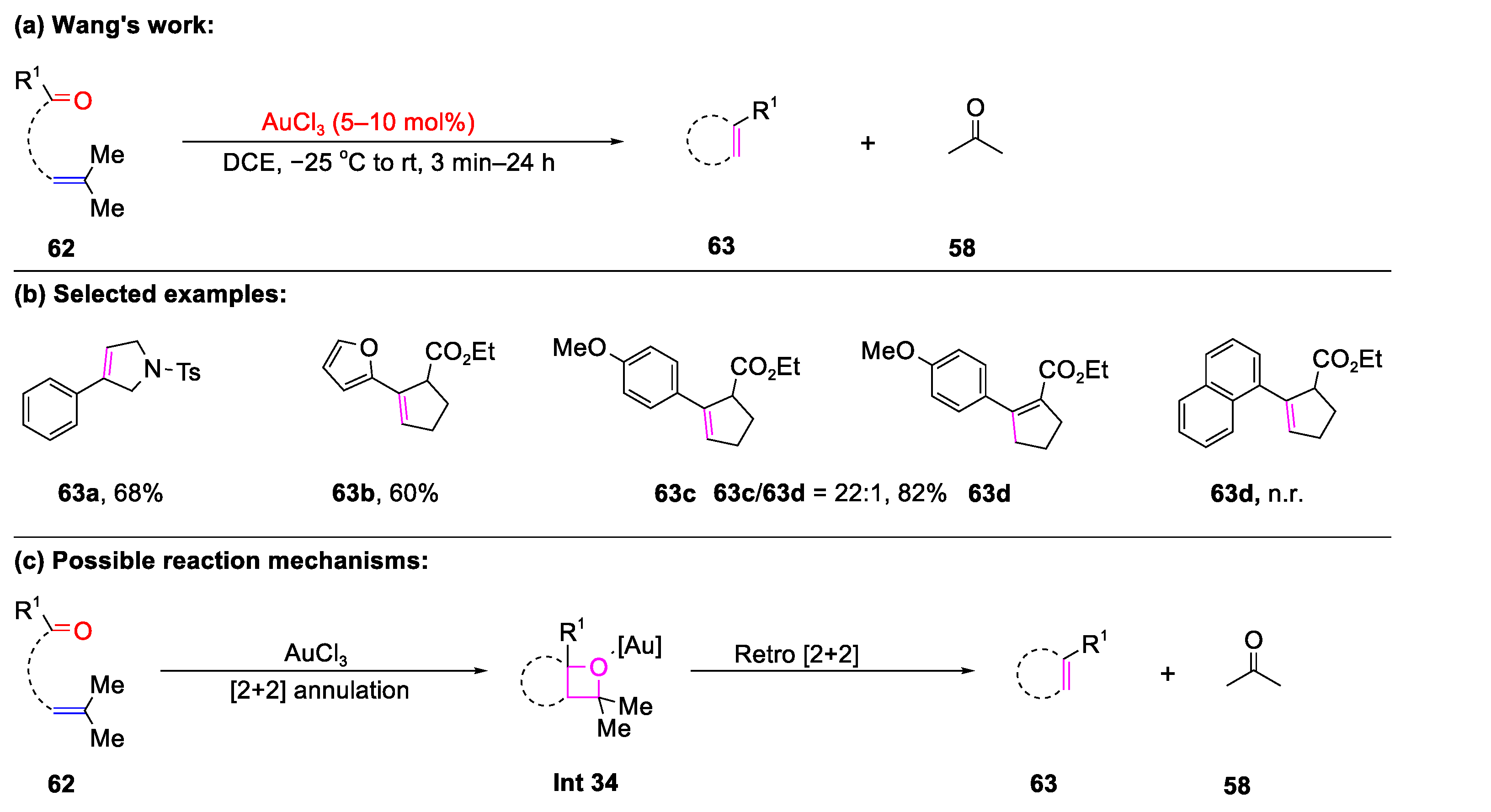
- Wang, R.; Chen, Y.; Shu, M.; Zhao, W.W.; Tao, M.L.; Du, C.; Fu, X.Y.; Li, A.; Lin, Z.H. AuCl3-Catalyzed Ring-Closing Carbonyl-Olefin Metathesis. Chem. Eur. J. 2020, 26, 1941–1946. [Google Scholar] [CrossRef]
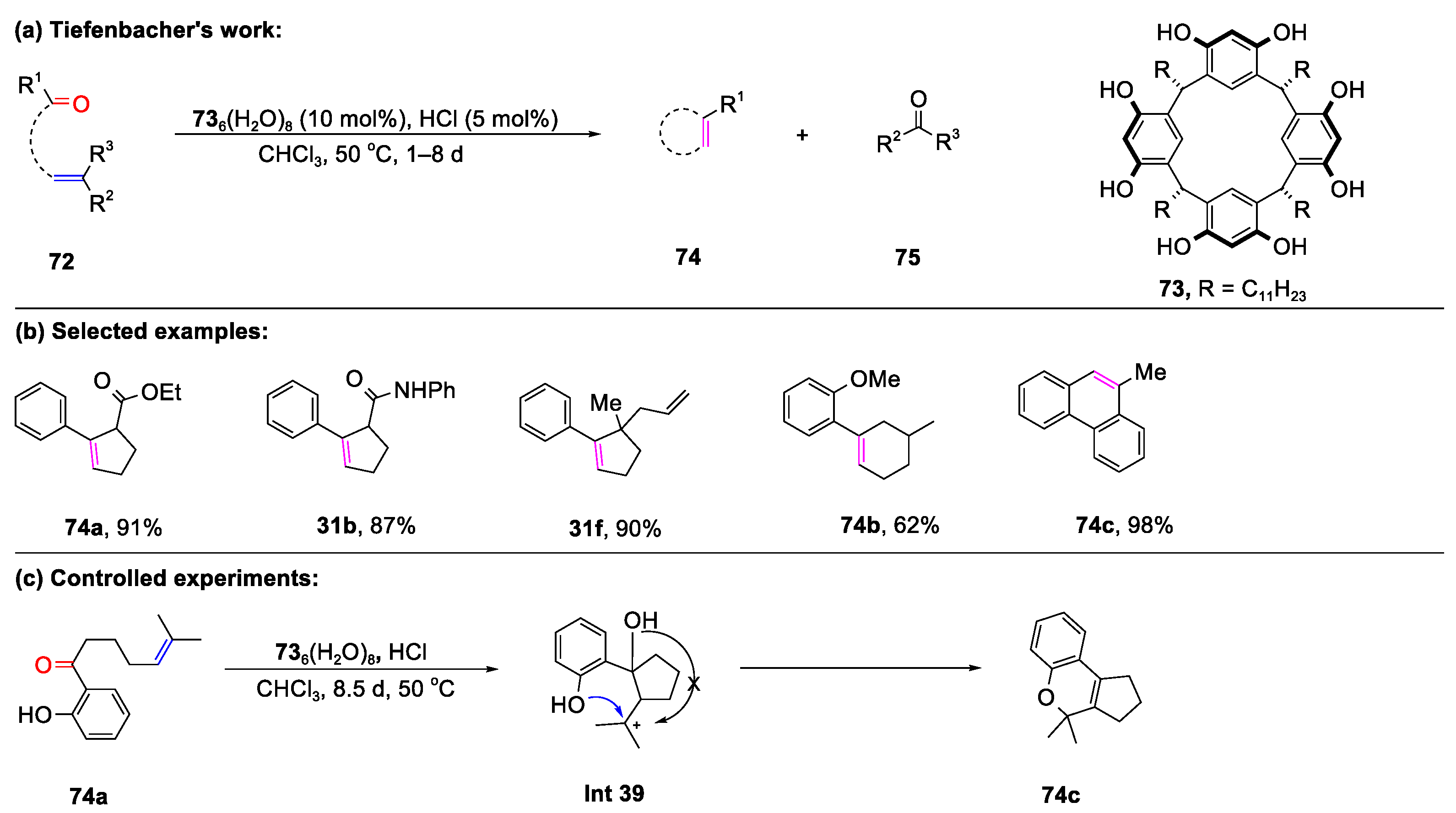
- Catti, L.; Tiefenbacher, K. Bronsted Acid-Catalyzed Carbonyl-Olefin Metathesis Inside a Self-Assembled Supramolecular Host. Angew. Chem. Int. Ed. 2018, 57, 14589–14592. [Google Scholar] [CrossRef]
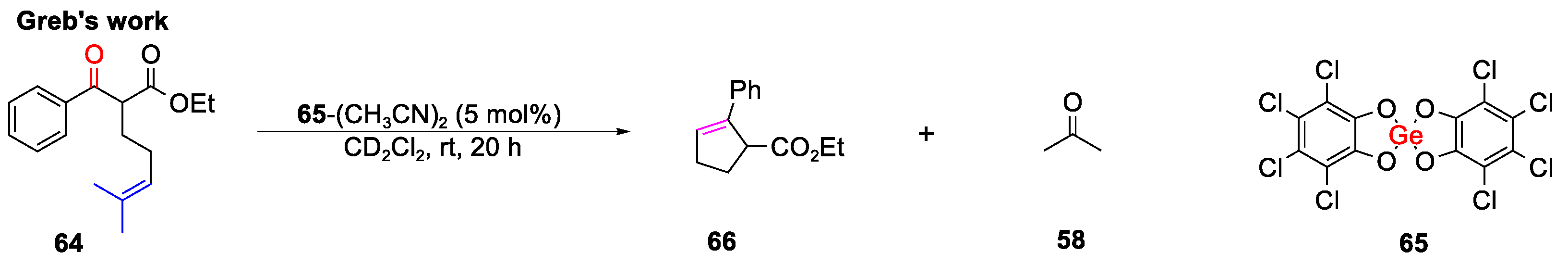
- Rith, D.; Wadepohl, H.; Greb, L. Bis(perchlorocatecholato)germane: Hard and Soft Lewis Superacid with Unlimited Water Stability. Angew. Chem. Int. Ed. 2020, 59, 20930–20934. [Google Scholar] [CrossRef]
- Pizzio, M.G.; Cenizo, Z.B.; Mendez, L.; Sarotti, A.M.; Mata, E.G. InCl3-catalyzed intramolecular carbonyl-olefin metathesis. Org. Biomol. Chem. 2023, 21, 8141–8151. [Google Scholar] [CrossRef]
- Jones, G.; Schwartz, S.B.; Marton, M.T. Regiospecific Thermal Cleavage of Some Oxetan Photoadducts: Carbonyl-Olefin Metathesis in Sequential Photochemical and Thermal Steps. J. Chem. Soc. Chem. Commun. 1973, 374–375. [Google Scholar] [CrossRef]
- Khripach, V.A.; Zhabinskii, V.N.; Kuchto, A.I.; Zhiburtovich, Y.Y.; Gromak, V.V.; Groen, M.B.; Van der Louw, J.; De Groot, A. Intramolecular Cycloaddition/Cycloreversion of (E)-3β,17β-Diacetoxy-5,10-secoandrost-1(10)-en-5-one. Tetrahedron Lett. 2006, 47, 6715–6718. [Google Scholar] [CrossRef]
- Zhang, Q.; Tiefenbacher, K. Terpene cyclization catalysed inside a self-assembled cavity. Nat. Chem. 2015, 7, 197–202. [Google Scholar] [CrossRef]
- Zhang, Q.; Catti, L.; Pleiss, J.; Tiefenbacher, K. Terpene Cyclizations inside a Supramolecular Catalyst: Leaving-Group-Controlled Product Selectivity and Mechanistic Studies. J. Am. Chem. Soc. 2017, 139, 11482–11492. [Google Scholar] [CrossRef]
- Catti, L.; Pçthig, A.; Tiefenbacher, K. Host-Catalyzed Cyclodehydration-Rearrangement Cascade Reaction of Unsaturated Tertiary Alcohols. Adv. Synth. Catal. 2017, 359, 1331–1338. [Google Scholar] [CrossRef]
- Huck, F.; Catti, L.; Reber, G.L.; Tiefenbacher, K. Expanding the Protecting Group Scope for the Carbonyl Olefin Metathesis Approach to 2,5-Dihydropyrroles. J. Org. Chem. 2022, 87, 419–428. [Google Scholar] [CrossRef]
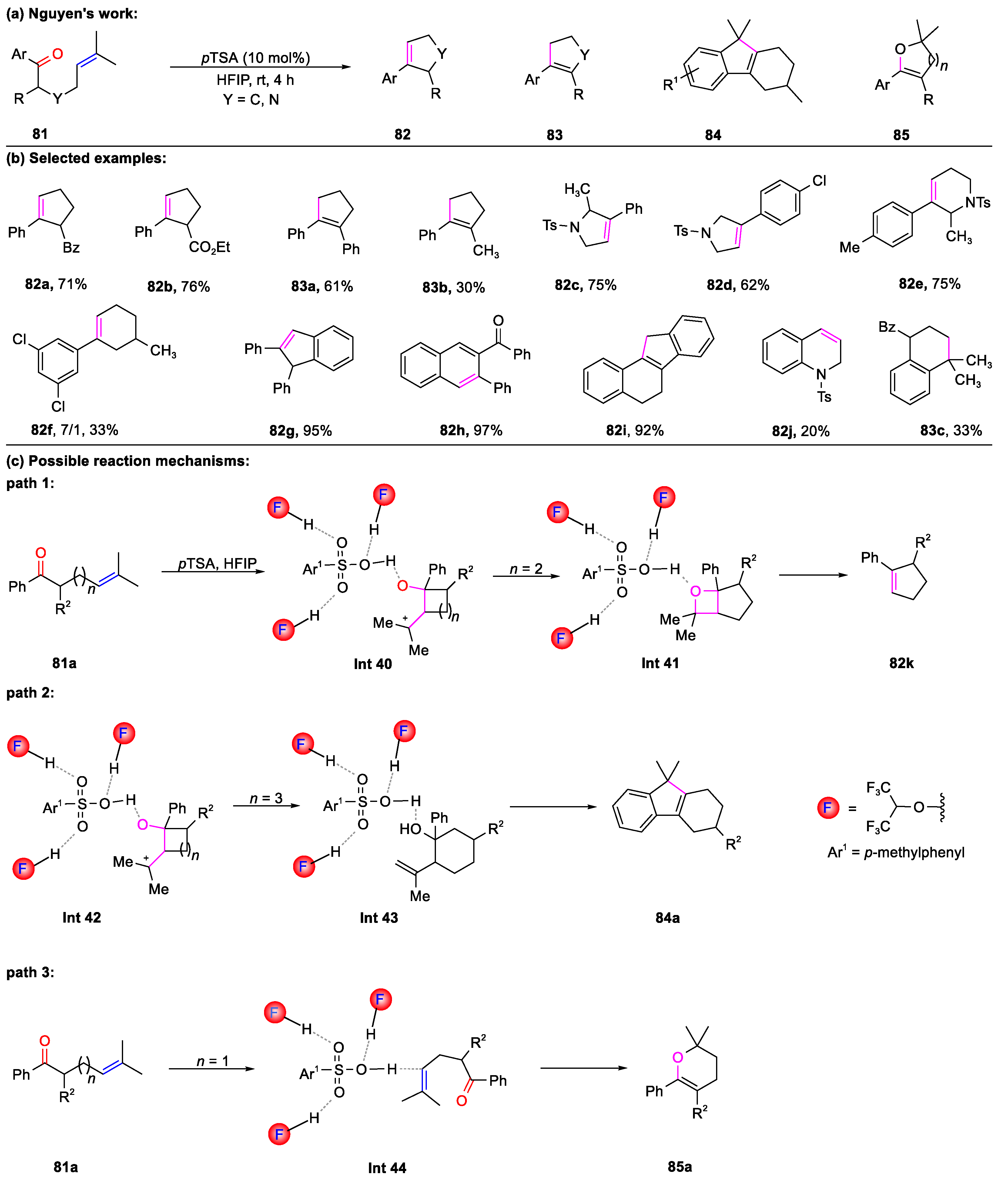
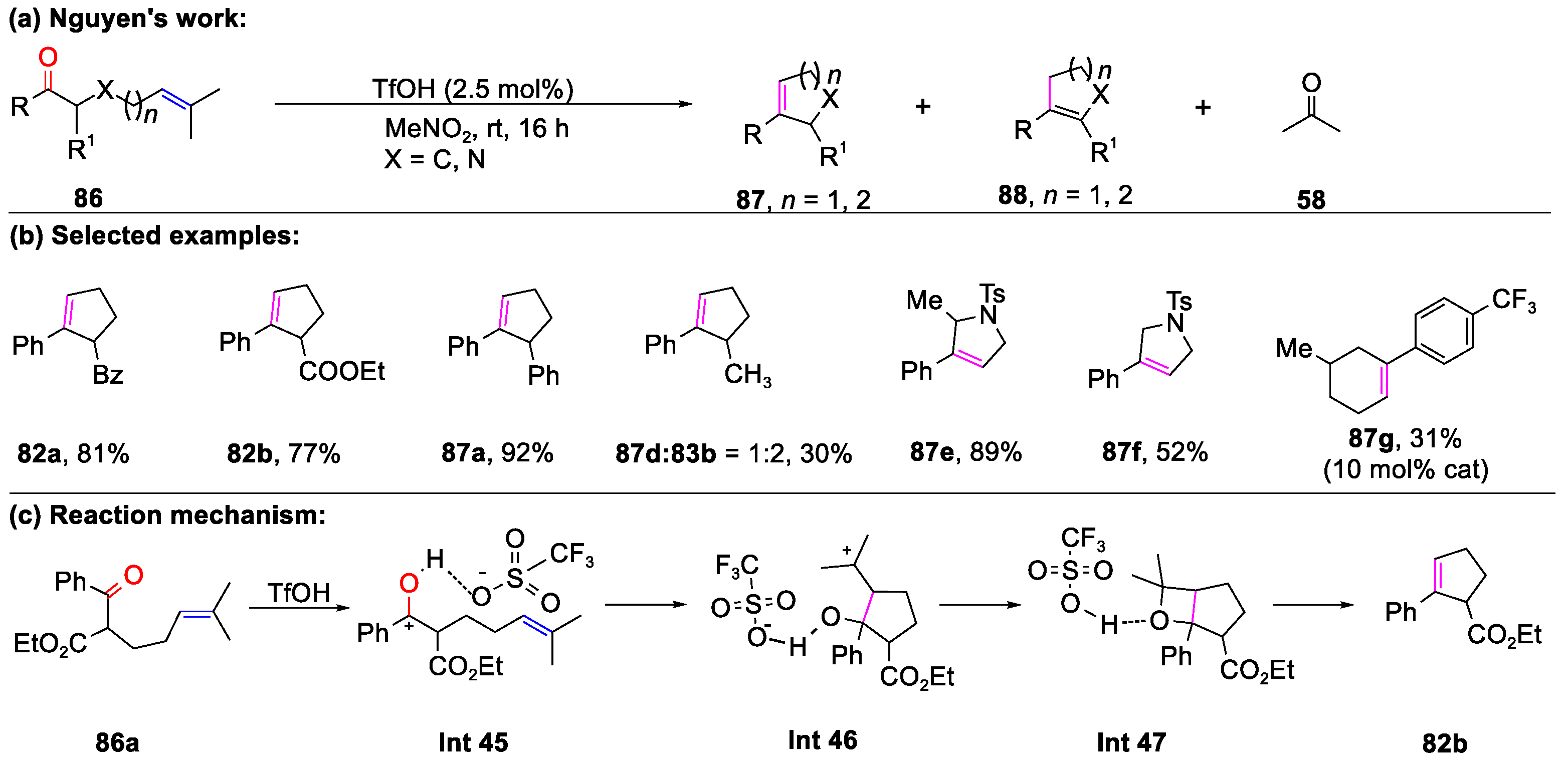
- To, T.A.; Pei, C.; Koenigs, R.M.; Nguyen, T.V. Hydrogen Bonding Networks Enable Brønsted Acid-Catalyzed Carbonyl-Olefin Metathesis. Angew. Chem. Int. Ed. 2022, 61, e202117366. [Google Scholar]
- To, T.A.; Mai, B.K.; Nguyen, T.V. Toward Homogeneous Brønsted-Acid-Catalyzed Intramolecular Carbonyl-Olefin Metathesis Reactions. Org. Lett. 2022, 24, 7237–7241. [Google Scholar] [CrossRef]
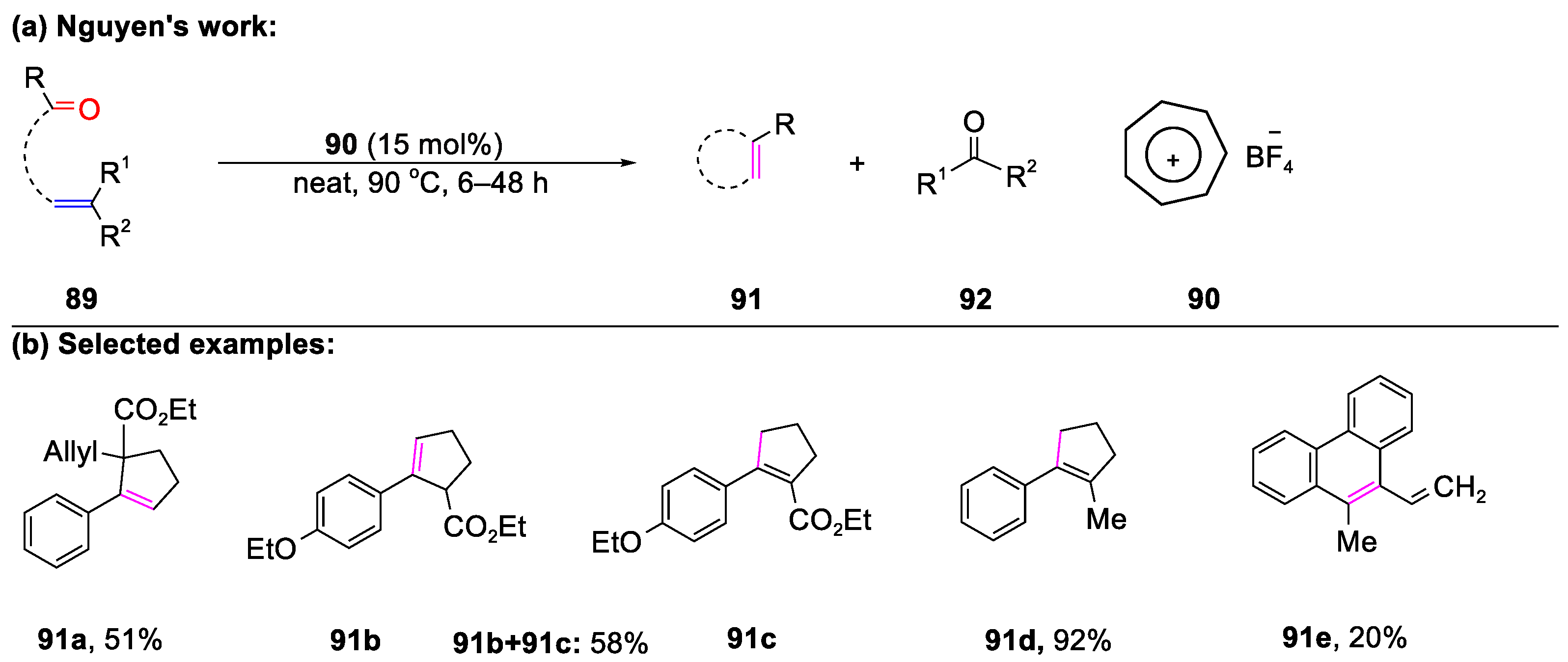
- Tran, U.P.N.; Oss, G.; Pace, D.P.; Ho, J.; Nguyen, T.V. Tropylium-promoted carbonyl-olefin metathesis reactions. Chem. Sci. 2018, 9, 5145–5151. [Google Scholar] [CrossRef]
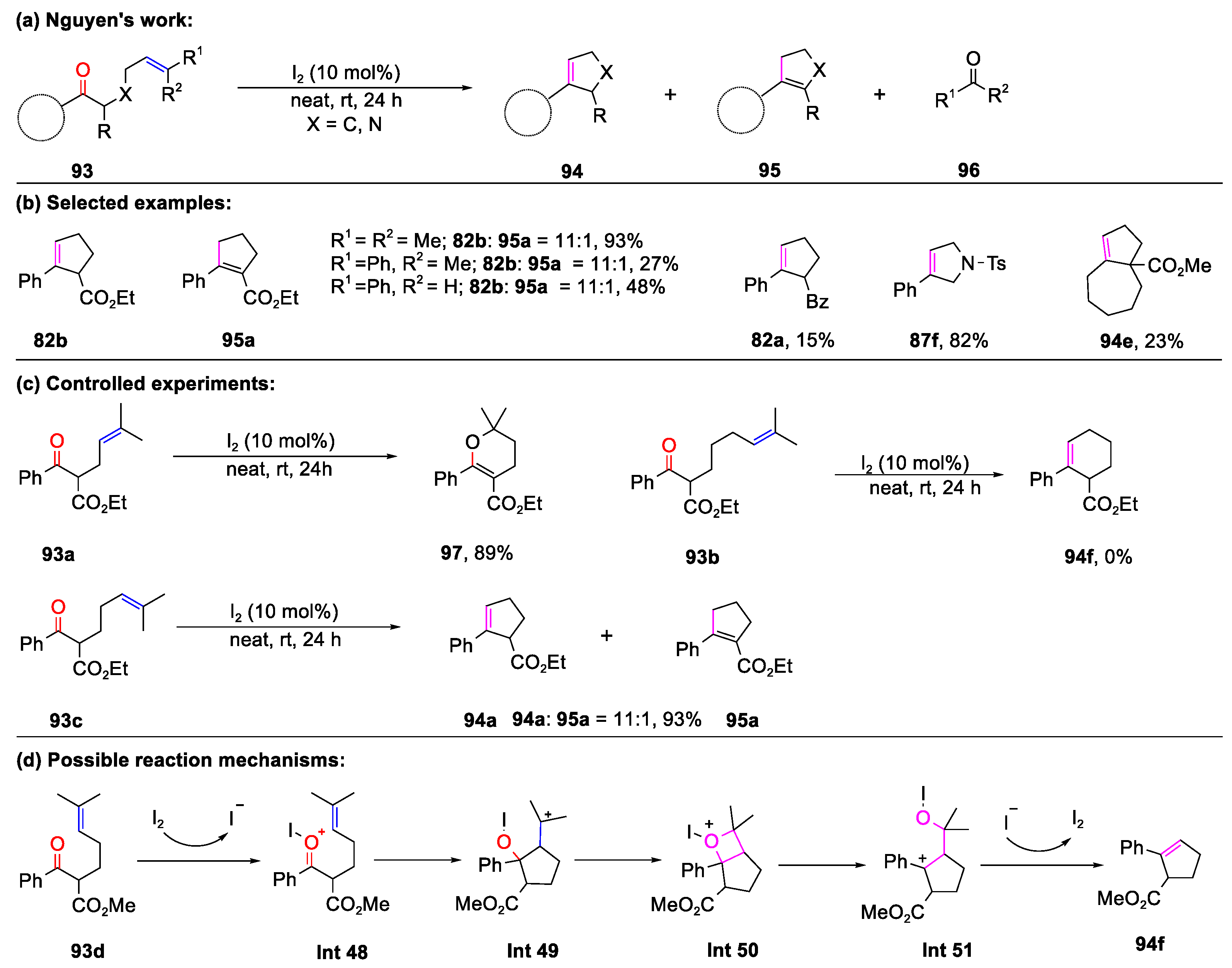
- Tran, U.P.N.; Oss, G.; Breugst, M.; Detmar, E.; Pace, D.P.; Liyanto, K.; Nguyen, T.V. Carbonyl-Olefin Metathesis Catalyzed by Molecular Iodine. ACS Catal. 2019, 9, 912–919. [Google Scholar] [CrossRef]
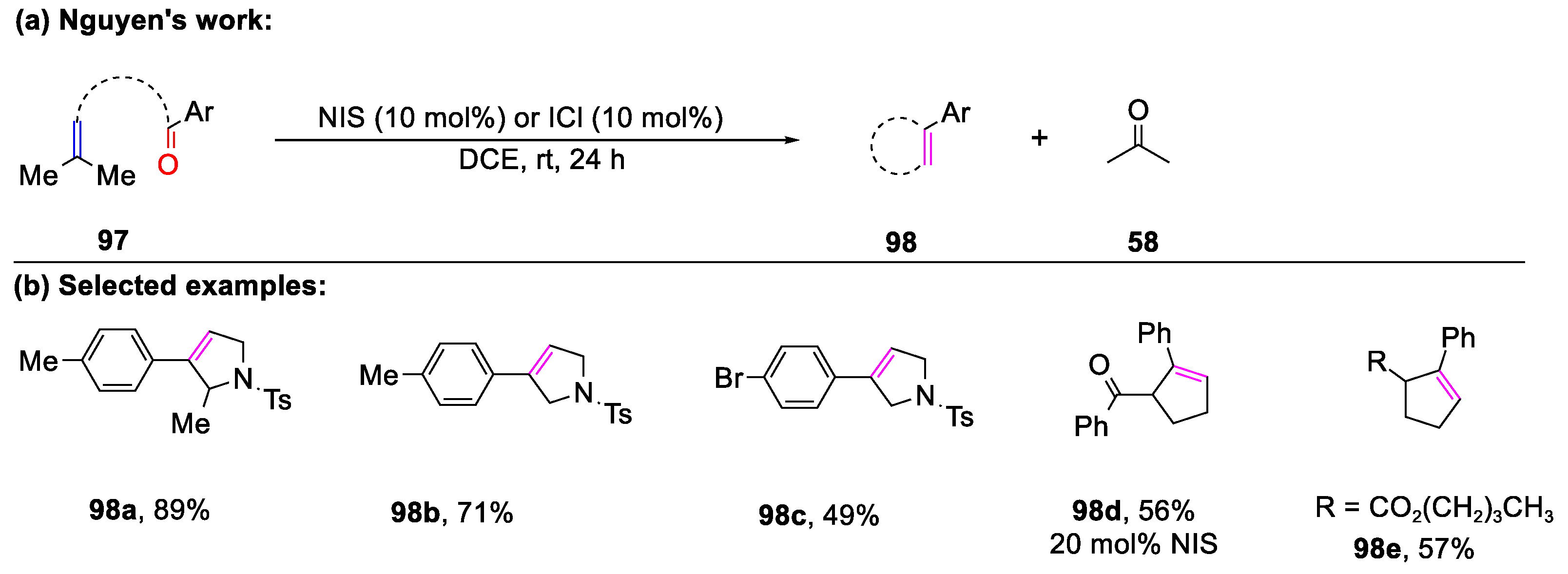
- Nguyen, T.V.; Oss, G. Iodonium-Catalyzed Carbonyl-Olefin Metathesis Reactions. Synlett 2019, 30, 1966–1970. [Google Scholar]
- Jackson, A.C.; Goldman, B.E.; Snider, B.B. Intramolecular and Intermolecular Lewis Acid Catalyzed Ene Reactions Using Ketones as Enophiles. J. Org. Chem. 1984, 49, 3988–3994. [Google Scholar] [CrossRef]
- Demole, E.; Enggist, P.; Borer, M.C. Applications Synthetiques de la Cyclisaton D’alcools Tertiaries γ-ethyleniques en α-Bromote-trahydrofurannes Sous L’action Du N-Bromosuccinimide. Helv. Chim. Acta 1971, 54, 1845–1864. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Postema, M.H.D.; Yue, E.W.; Nadin, A. An Olefin Metathesis Based Strategy for the Construction of the JKL, OPQ, and UVW Ring Systems of Maitotoxin. J. Am. Chem. Soc. 1996, 118, 10335–10336. [Google Scholar] [CrossRef]
- Bennasar, M.L.; Roca, T.; Monerris, M.; García-Díaz, D. Sequential N-Acylamide Methylenation-Enamide Ring-Closing Metathesis: A Synthetic Entry to 1,4-Dihydroquinolines. Tetrahedron Lett. 2005, 46, 4035–4038. [Google Scholar] [CrossRef]
- Bennasar, M.L.; Roca, T.; Monerris, M.; García-Díaz, D. Sequential N-Acylamide Methylenation-Enamide Ring-ClosingMetathesis: Construction of Benzo-Fused Nitrogen Heterocycles. J. Org. Chem. 2006, 71, 7028–7034. [Google Scholar] [CrossRef]
- Clark, J.S.; Kettle, J.G. Synthesis of Brevetoxin Subunits by Sequential Ring-closing Metathesis and Hydroboration. Tetrahedron Lett. 1997, 38, 123–126. [Google Scholar]
- Clark, J.S.; Kettle, J.G. Enantioselective Synthesis of Medium-ring Subunits of Brevetoxin A by Ring-closing Metathesis. Tetrahedron Lett. 1997, 38, 127–130. [Google Scholar] [CrossRef]
- Fujimura, O.; Fu, G.C.; Grubbs, R.H. The Synthesis of Cyclic Enol Ethers via Molybdenum Alkylidene-Catalyzed Ring-Closing Metathesis. J. Org. Chem. 1994, 59, 4029–4031. [Google Scholar] [CrossRef]
- Rainier, J.D.; Allwein, S.P. An Iterative Approach to Fused Ether Ring Systems. J. Org. Chem. 1998, 63, 5310–5311. [Google Scholar] [CrossRef]
- Majumder, U.; Rainier, J.D. Olefinic-ester cyclizations using Takai-Utimoto reduced titanium alkylidenes. Tetrahedron Lett. 2005, 46, 7209–7211. [Google Scholar] [CrossRef]
- Rohanna, J.C.; Rainier, J.D. Olefinic-Lactone Cyclizations to Macrocycles. Org. Lett. 2009, 11, 493–495. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Rainier, J.D. Olefinic-Amide and Olefinic-Lactam Cyclizations. Org. Lett. 2009, 11, 3774–3776. [Google Scholar] [CrossRef]
- Hong, S.H.; Sanders, D.P.; Lee, C.W.; Grubbs, R.H.J. Prevention of Undesirable Isomerization during Olefin Metathesis. J. Am. Chem. Soc. 2005, 127, 17160–17161. [Google Scholar] [CrossRef]
- Valiulin, R.A.; Arisco, T.M.; Kutateladze, A.G. Photoinduced Intramolecular Cyclopentanation vs Photoprotolytic Oxametathesis in Polycyclic Alkenes Outfitted with Conformationally Constrained Aroylmethyl Chromophores. J. Org. Chem. 2013, 78, 2012–2025. [Google Scholar] [CrossRef]
- Soicke, A.; Slavov, N.; Neudorfl, J.M.; Schmalz, H.G. Metal-Free Intramolecular Carbonyl-Olefin Metathesis of ortho-Prenylaryl Ketones. Synlett 2011, 17, 2487–2490. [Google Scholar]
- Ni, S.J.; Franzén, J. Carbocation Catalysed Ring Closing Aldehyde-Olefin Metathesis. Chem. Commun. 2018, 54, 12982–12985. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Jermaks, J.; MacMillan, S.N.; Lambert, T.S. Synthesis of 2H-Chromenes via Hydrazine-Catalyzed Ring-Closing Carbonyl-Olefin Metathesis. ACS Catal. 2019, 9, 9259–9264. [Google Scholar] [CrossRef]
- Collins, K.D.; Glorius, F. Intermolecular Reaction Screening as a Tool for Reaction Evaluation. Acc. Chem. Res. 2015, 48, 619–627. [Google Scholar] [CrossRef]
- Zhang, Y.; Sim, J.H.; MacMillan, S.N.; Lambert, T.H. Synthesis of 1,2-Dihydroquinolines via Hydrazine-Catalyzed Ring Closing Carbonyl-Olefin Metathesis. Org. Lett. 2020, 22, 6026–6030. [Google Scholar] [CrossRef]
- Engler, T.A.; LaTessa, K.O.; Iyengar, R.; Chai, W.; Agrios, K. Stereoselective syntheses of substituted pterocarpans with anti-HIV activity, and 5-aza-/5-thia-pterocarpan and 2-aryl-2,3-dihydrobenzo furan analogues. Bioorg. Med. Chem. 1996, 4, 1755–1769. [Google Scholar] [CrossRef]
- Stille, J.R.; Grubbs, R.H. Synthesis of (±)-Δ(9,12)-Capnellene Using Titanium reagents. J. Am. Chem. Soc. 1986, 108, 855–856. [Google Scholar] [CrossRef]
- Roberts, S.W.; Rainier, J.D. Synthesis of an A–E Gambieric Acid Subunit with Use of a C-Glycoside Centered Strategy. Org. Lett. 2007, 9, 2227–2230. [Google Scholar] [CrossRef]
- Zhang, Y.; Rainier, J.D. Two-Directional Olefinic-Ester Ring Closing Metathesis using Reduced Ti Alkylidenes. A Rapid Entry into Polycyclic Ether Skeletons. Org. Lett. 2009, 11, 237–239. [Google Scholar] [CrossRef]
- Hong, B.K.; Li, H.H.; Wu, J.B.; Zhang, J.; Lei, X.G. Total Syntheses of (−)-Huperzine Q and (+)-Lycopladines B and C. Angew. Chem. Int. Ed. 2015, 54, 1011–1015. [Google Scholar] [CrossRef]
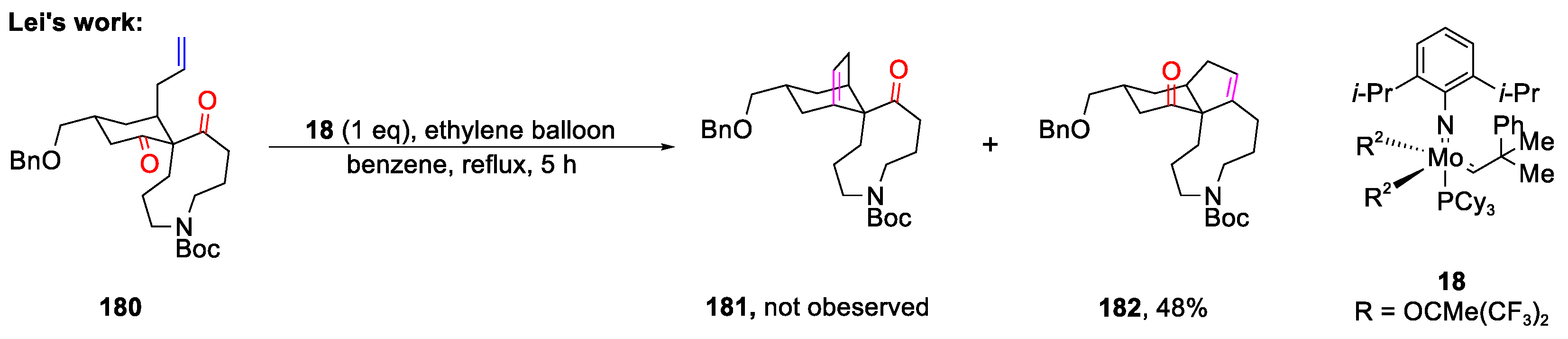
- McAtee, C.C.; Riehl, P.S.; Schindler, C.S. Polycyclic Aromatic Hydrocarbons via Iron(III)-Catalyzed Carbonyl-Olefin Metathesis. J. Am. Chem. Soc. 2017, 139, 2960–2963. [Google Scholar] [CrossRef]
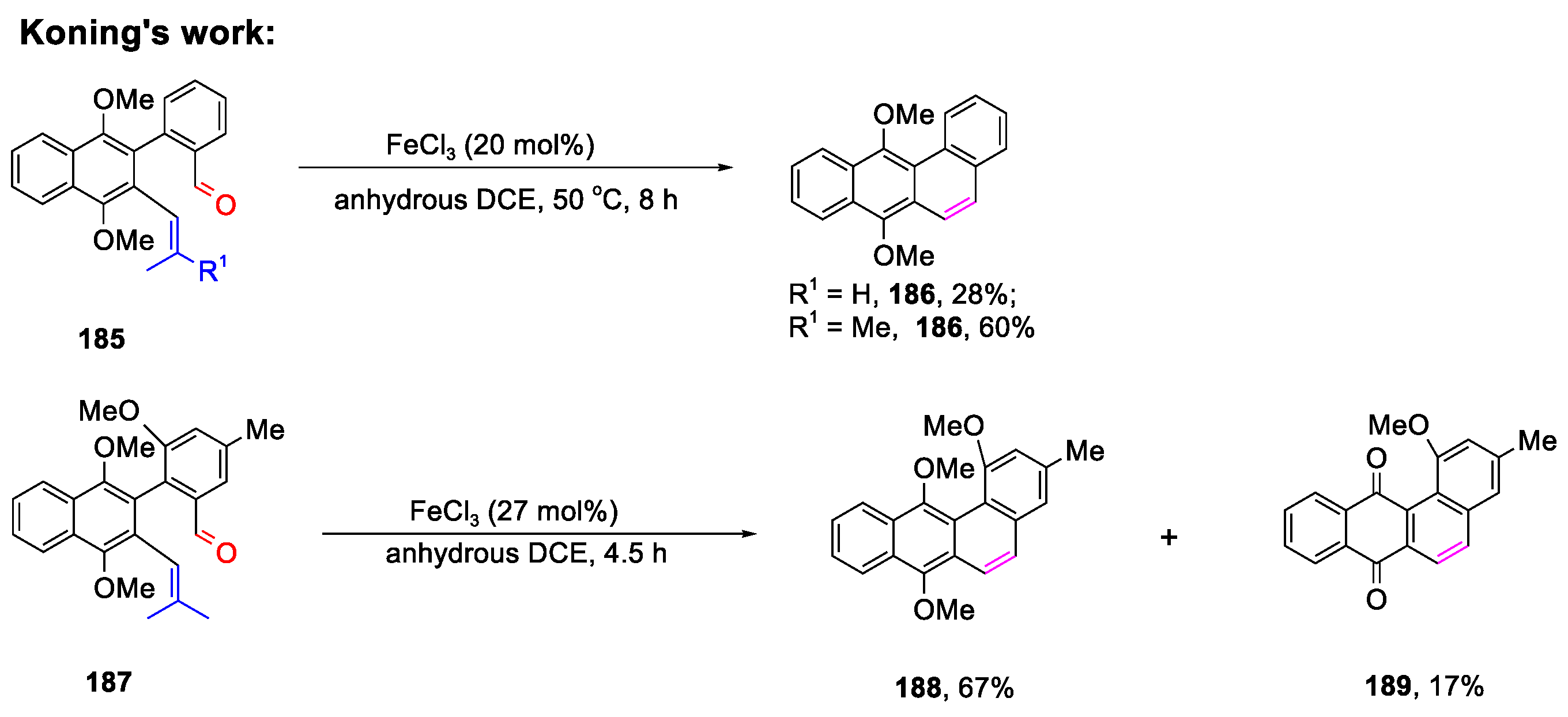
- Jagot, F.; Ntsimango, S.; Ngwira, K.J.; Fernandes, M.A.; Koning, C.B. Synthesis of Angucycline/Tetrangulol Derivatives Using Suzuki–Miyaura Cross-Coupling and Ring-Closing Carbonyl-Olefin Metathesis Reactions. Eur. J. Org. Chem. 2022, 24, e202200348. [Google Scholar] [CrossRef]
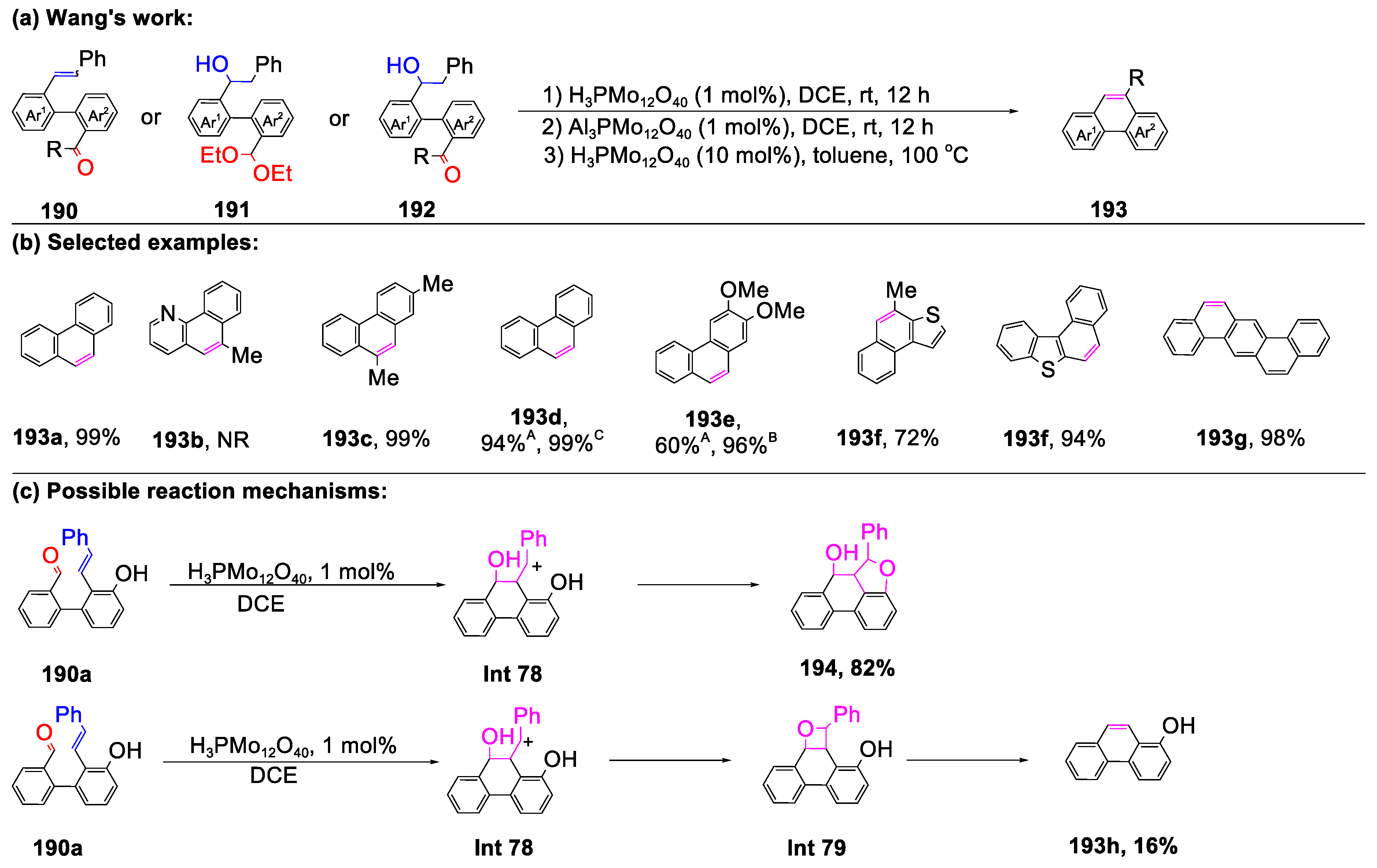
- Chen, Y.; Liu, D.; Wang, R.; Xu, L.; Tan, J.Y.; Shu, M.; Tian, L.F.; Jin, Y.; Zhang, X.K.; Lin, Z.H. Brønsted Acid-Catalyzed Carbonyl-Olefin Metathesis: Synthesis of Phenanthrenes via Phosphomolybdic Acid as a Catalyst. J. Org. Chem. 2022, 87, 351–362. [Google Scholar] [CrossRef]
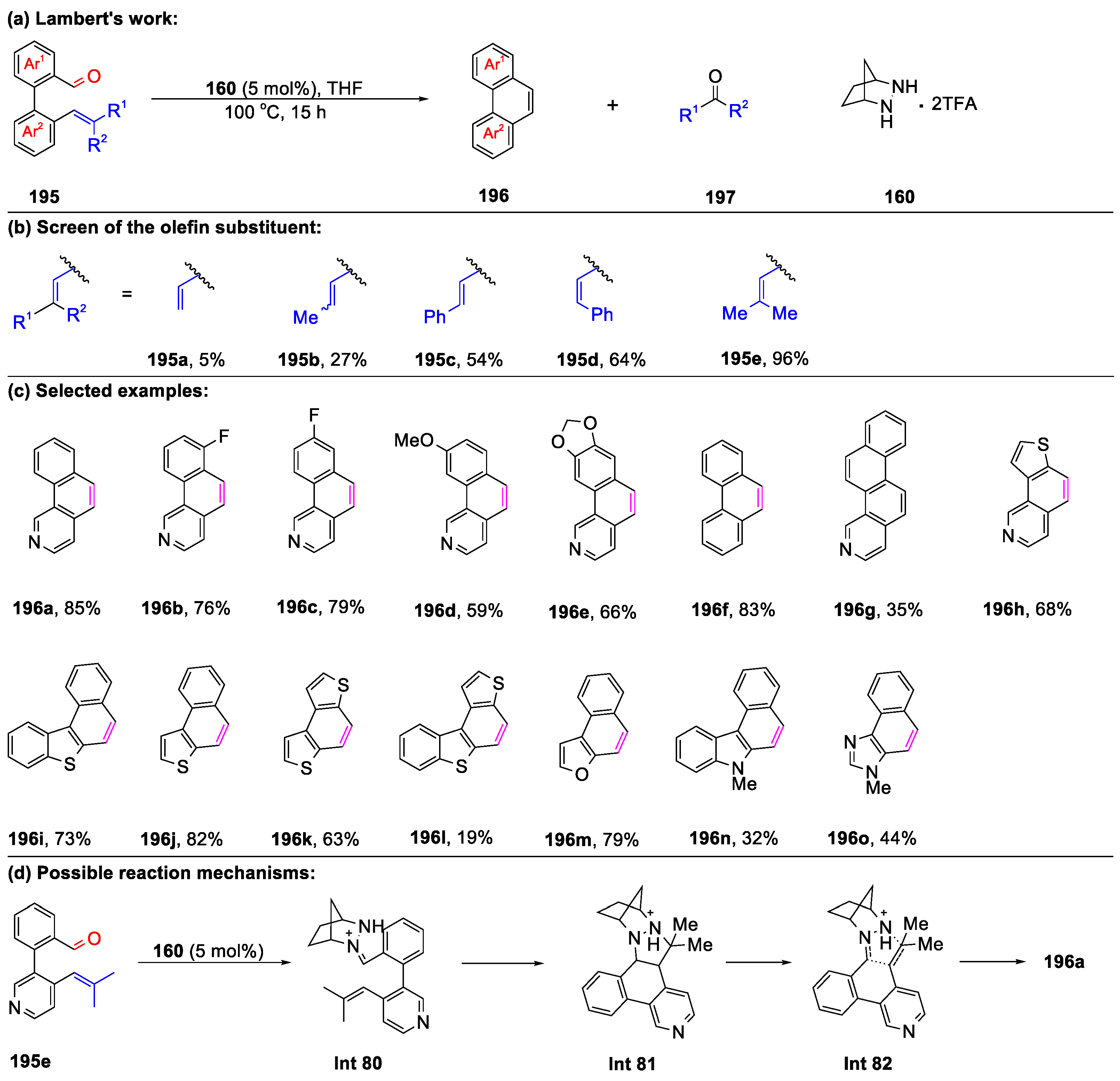
- Cho, E.K.; Quach, P.K.; Zhang, Y.F.; Sim, J.H.; Lambert, T.H. Polycyclic heteroaromatics via hydrazine-catalyzed ring-closing carbonyl-olefin metathesis. Chem. Sci. 2022, 13, 2418–2422. [Google Scholar] [CrossRef]






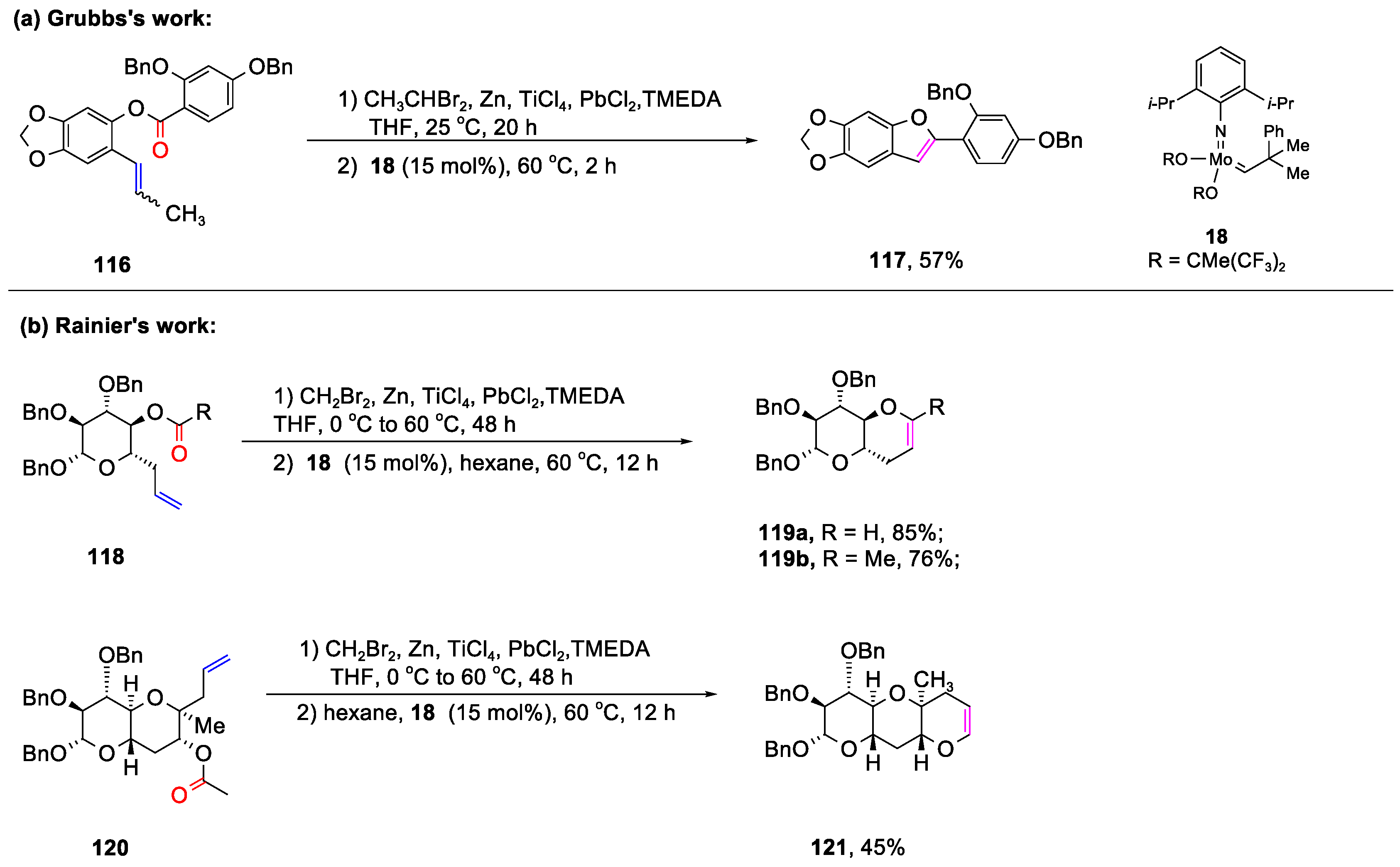
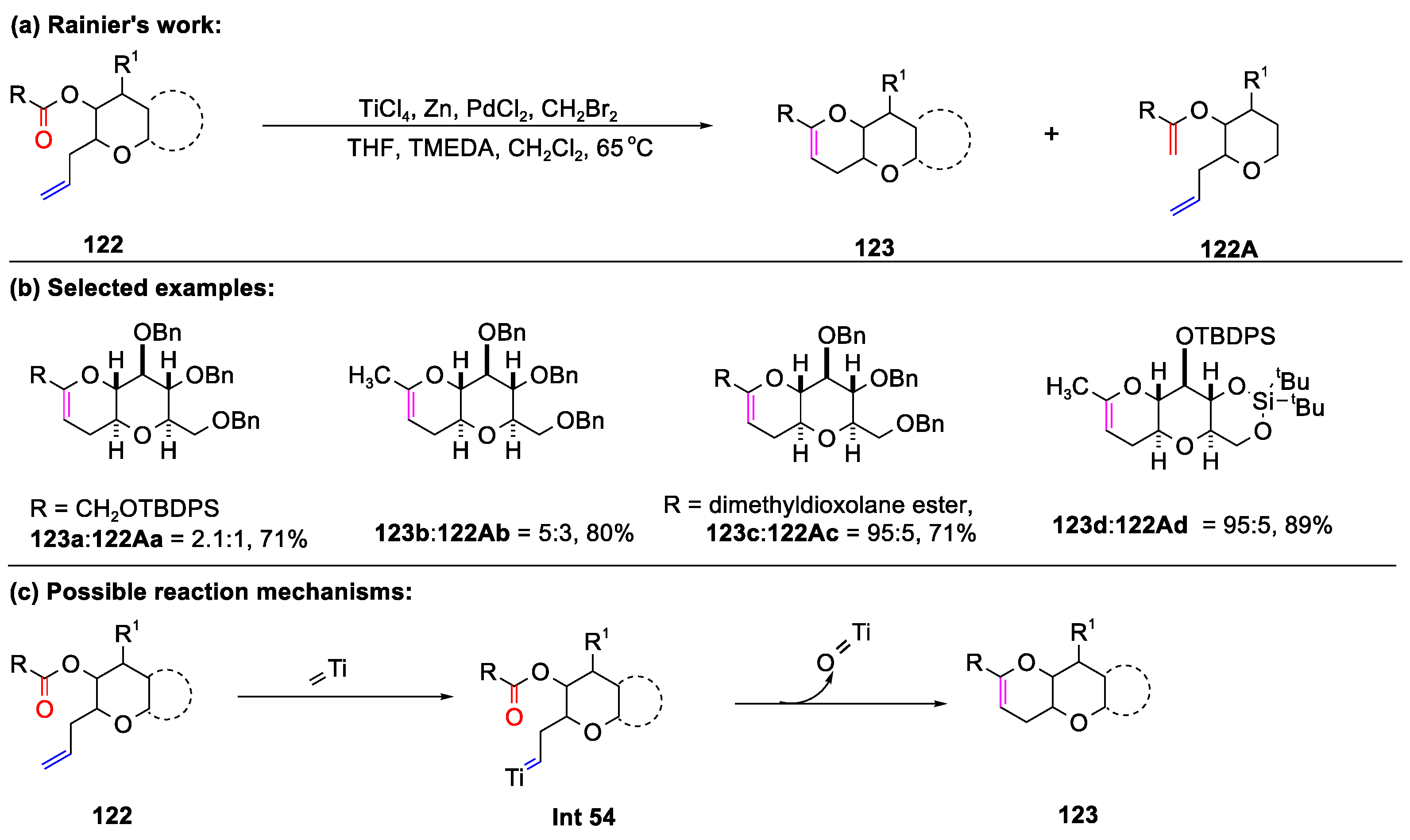
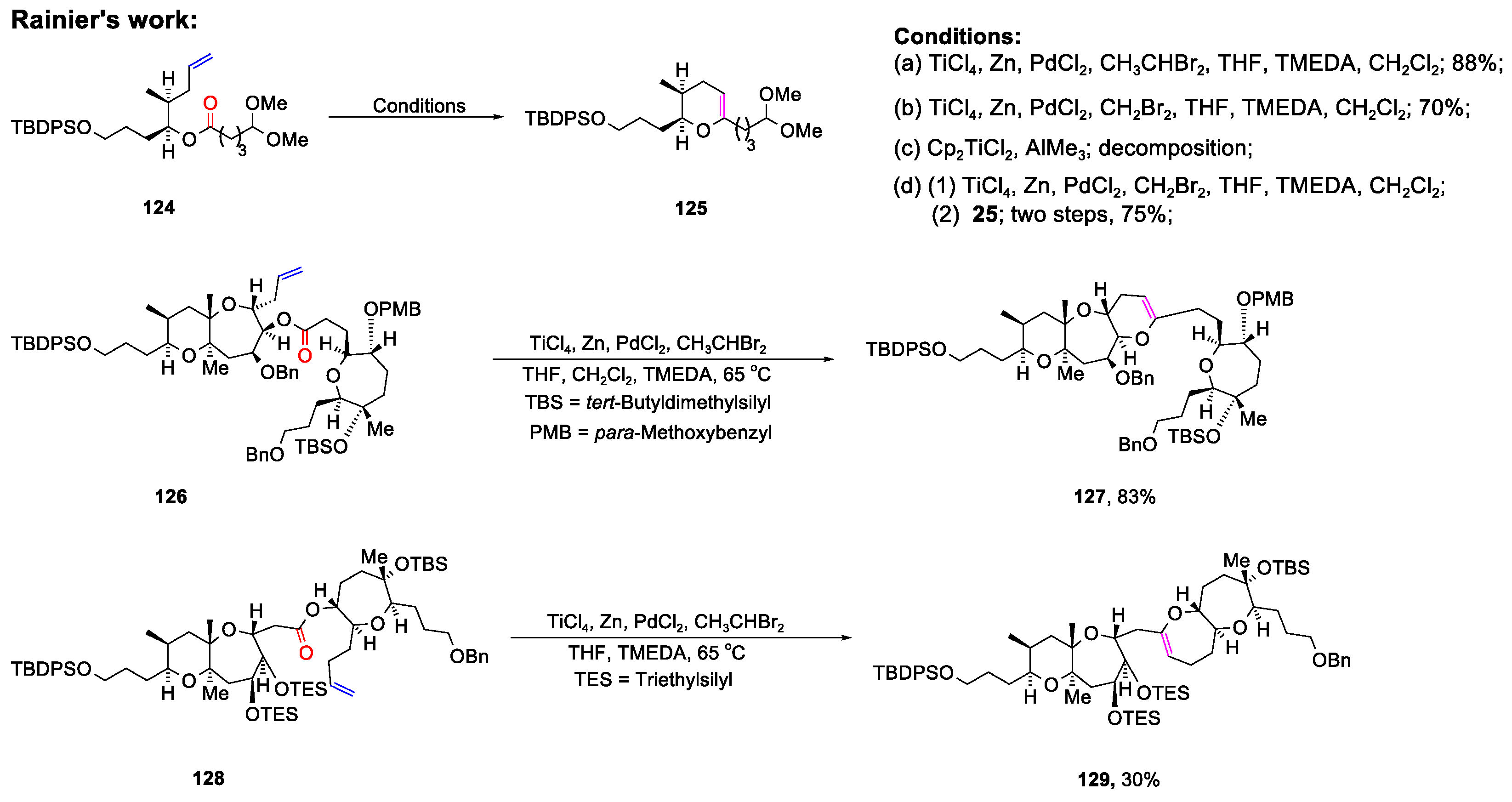

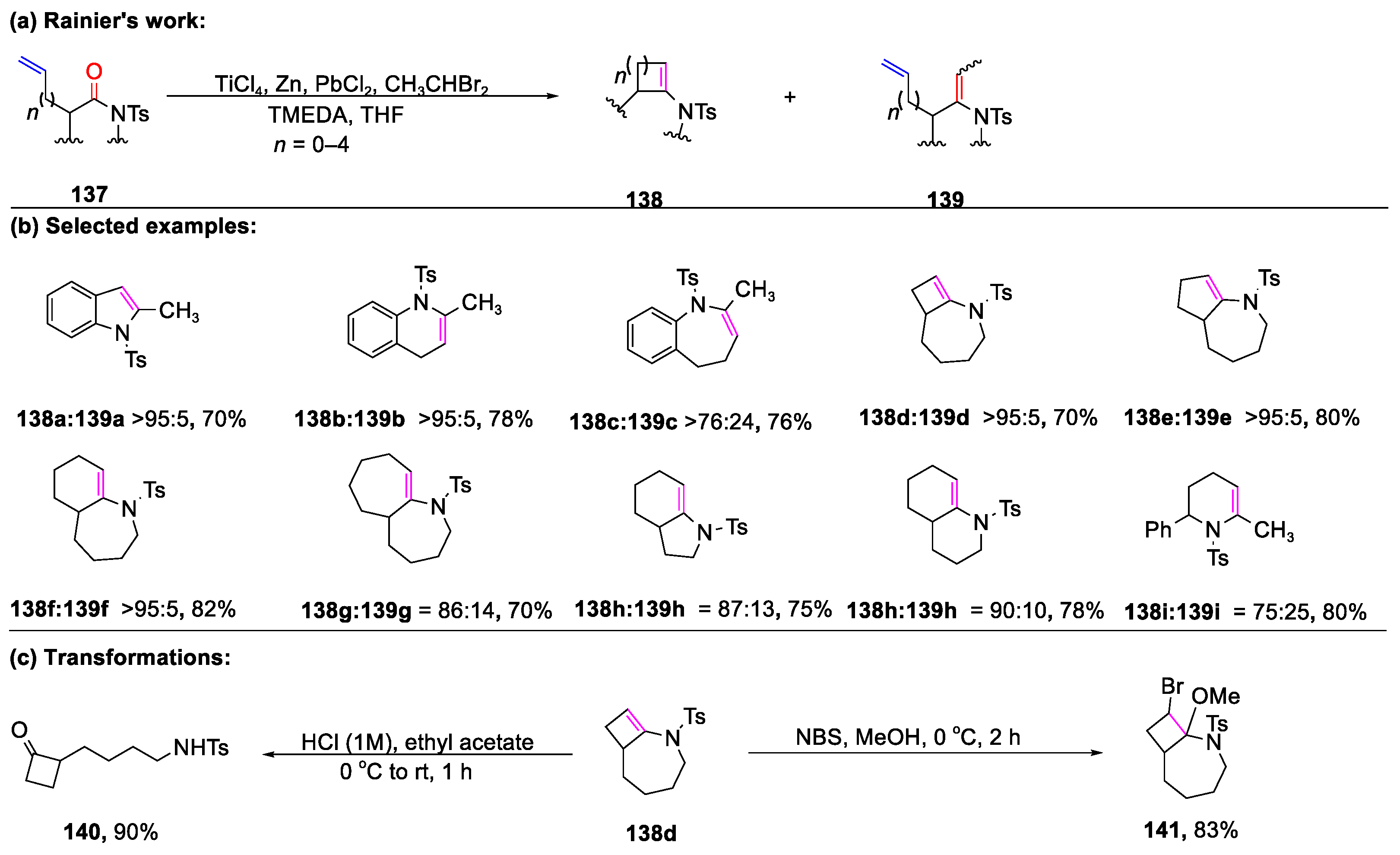
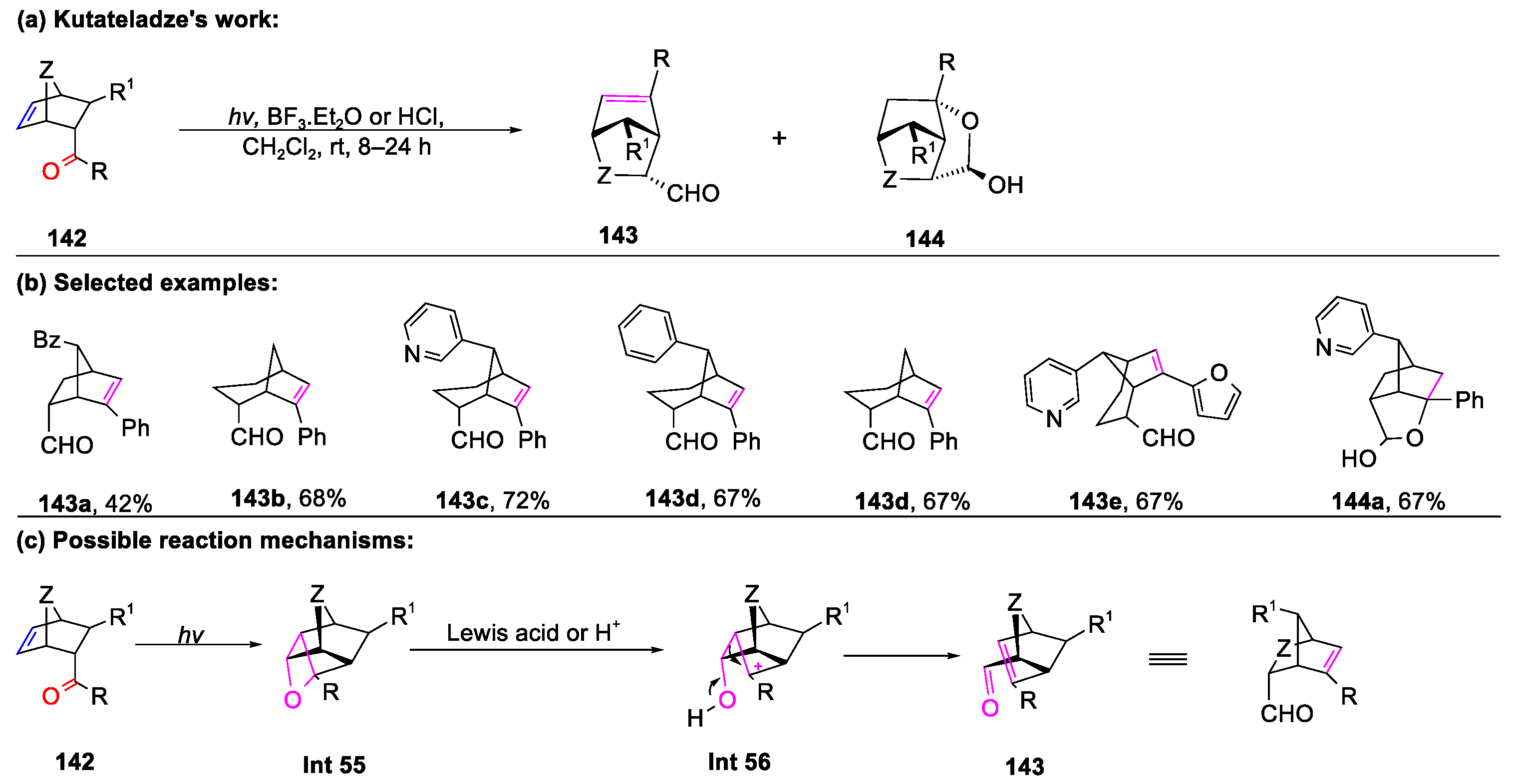
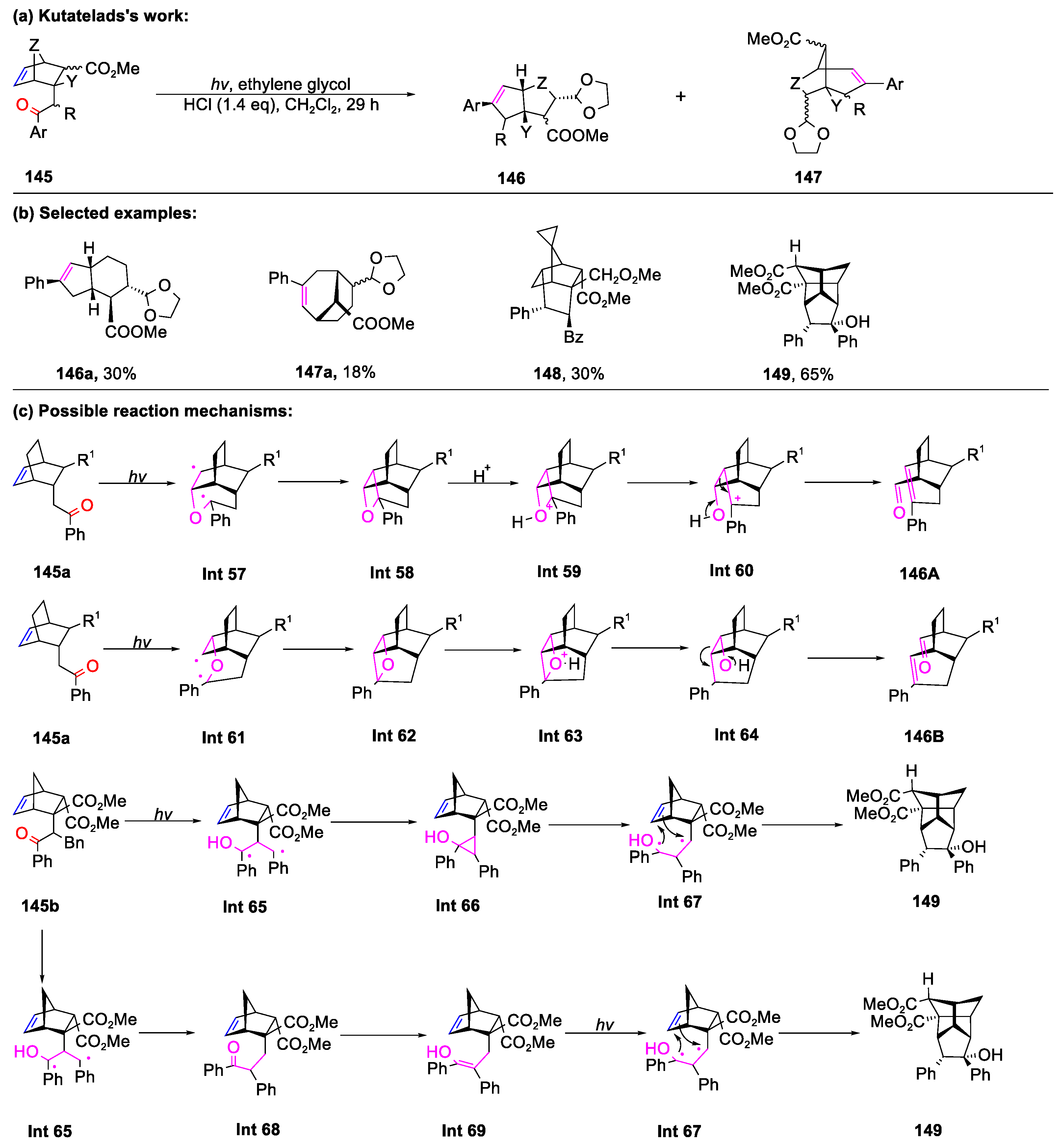
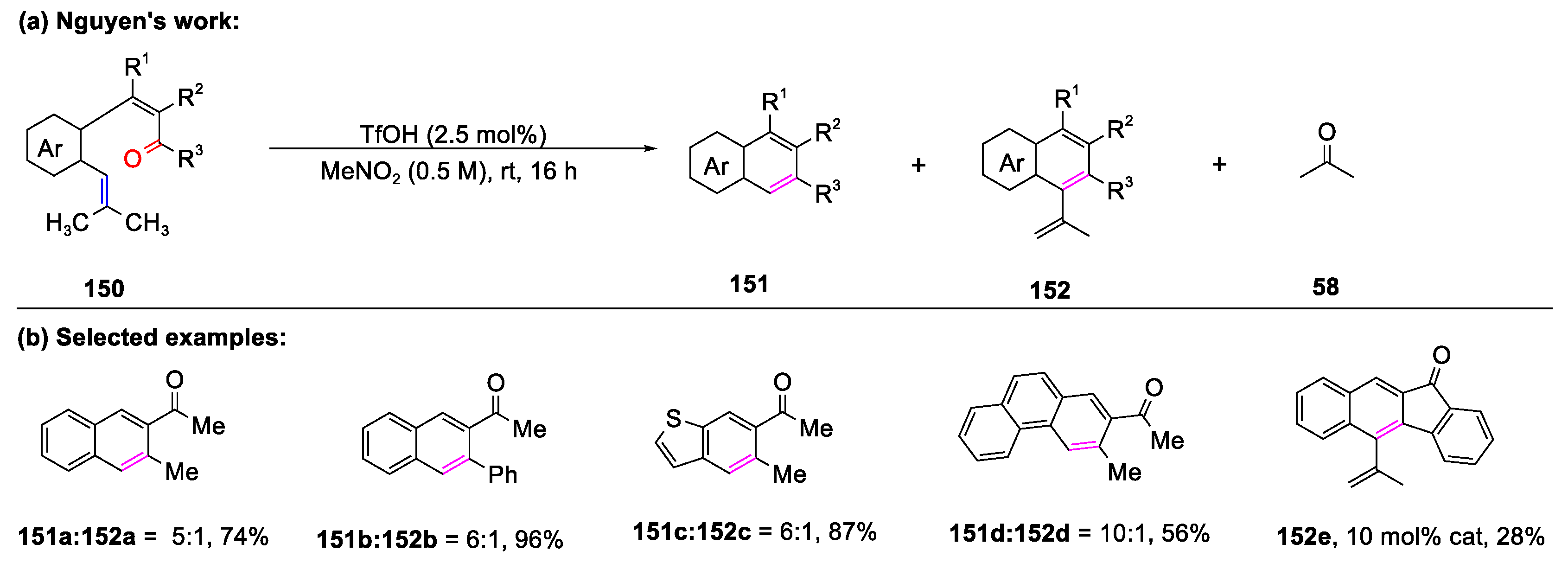
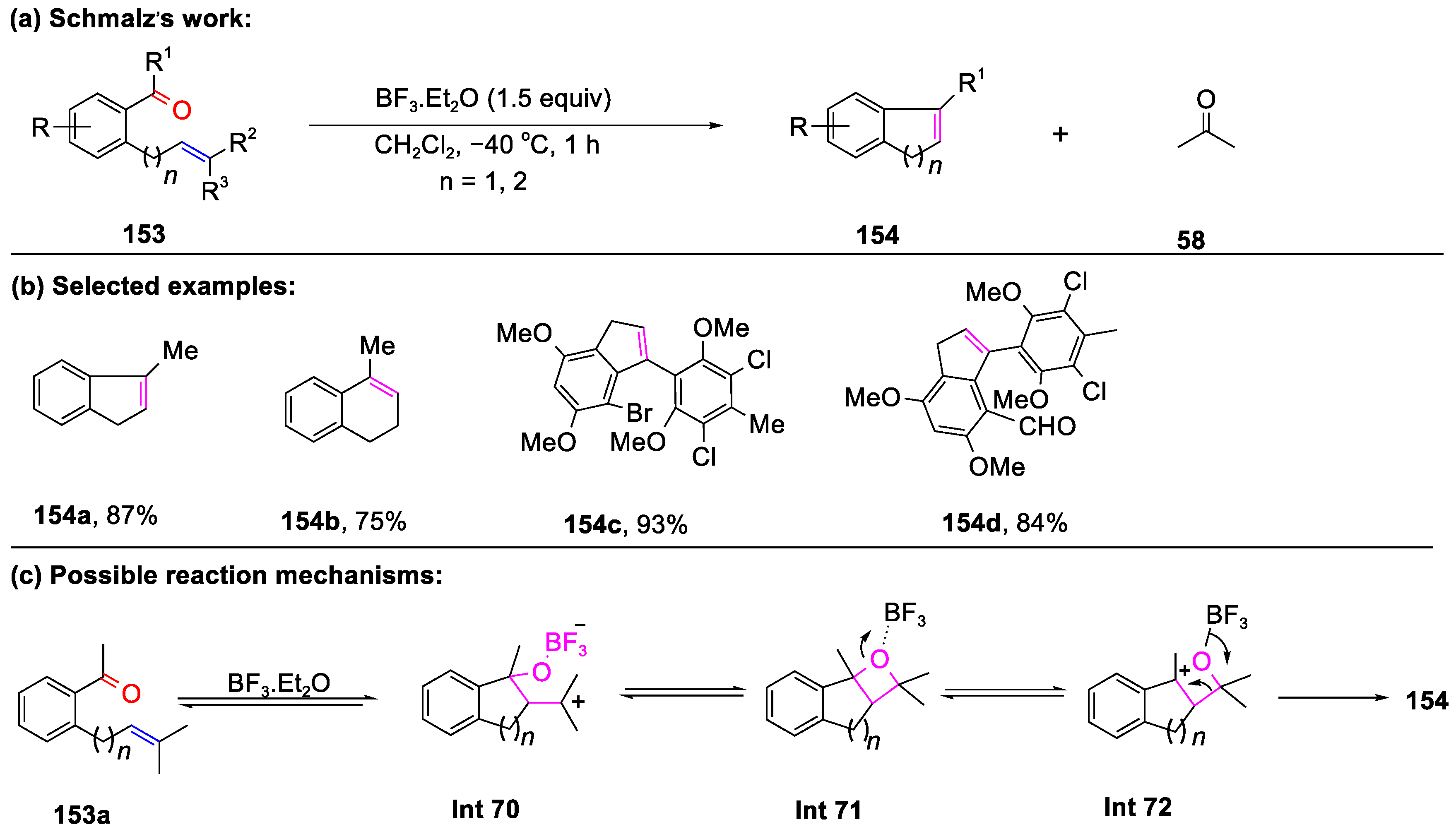
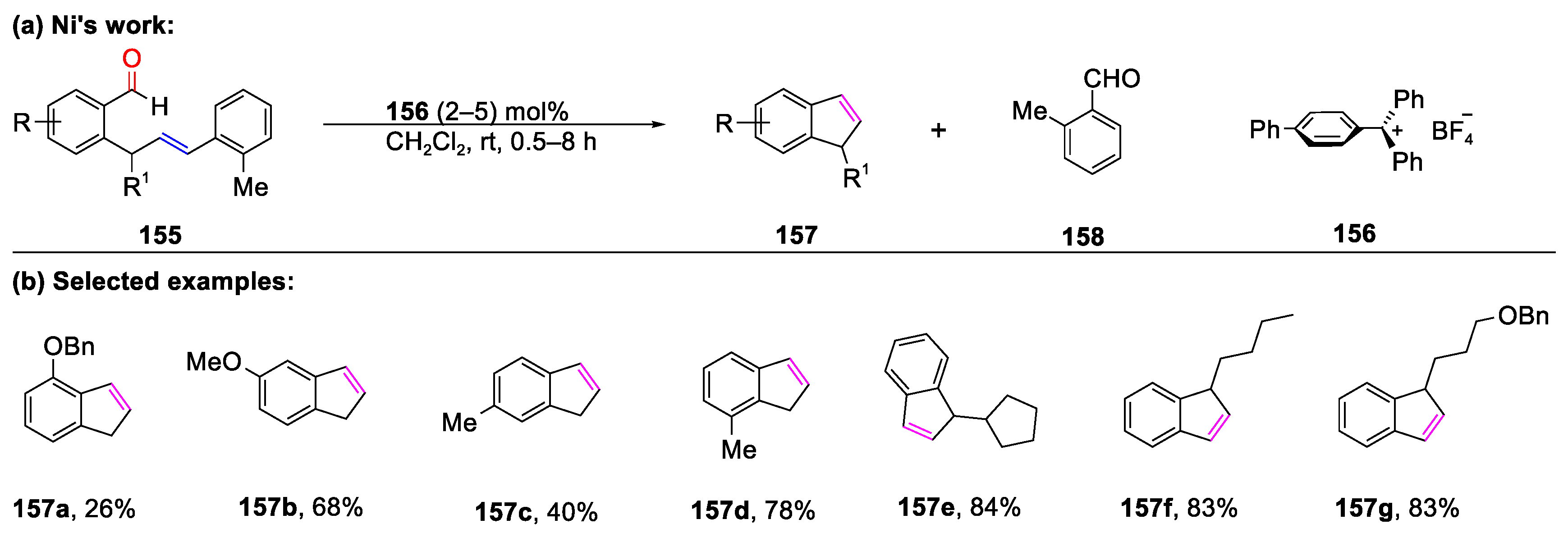
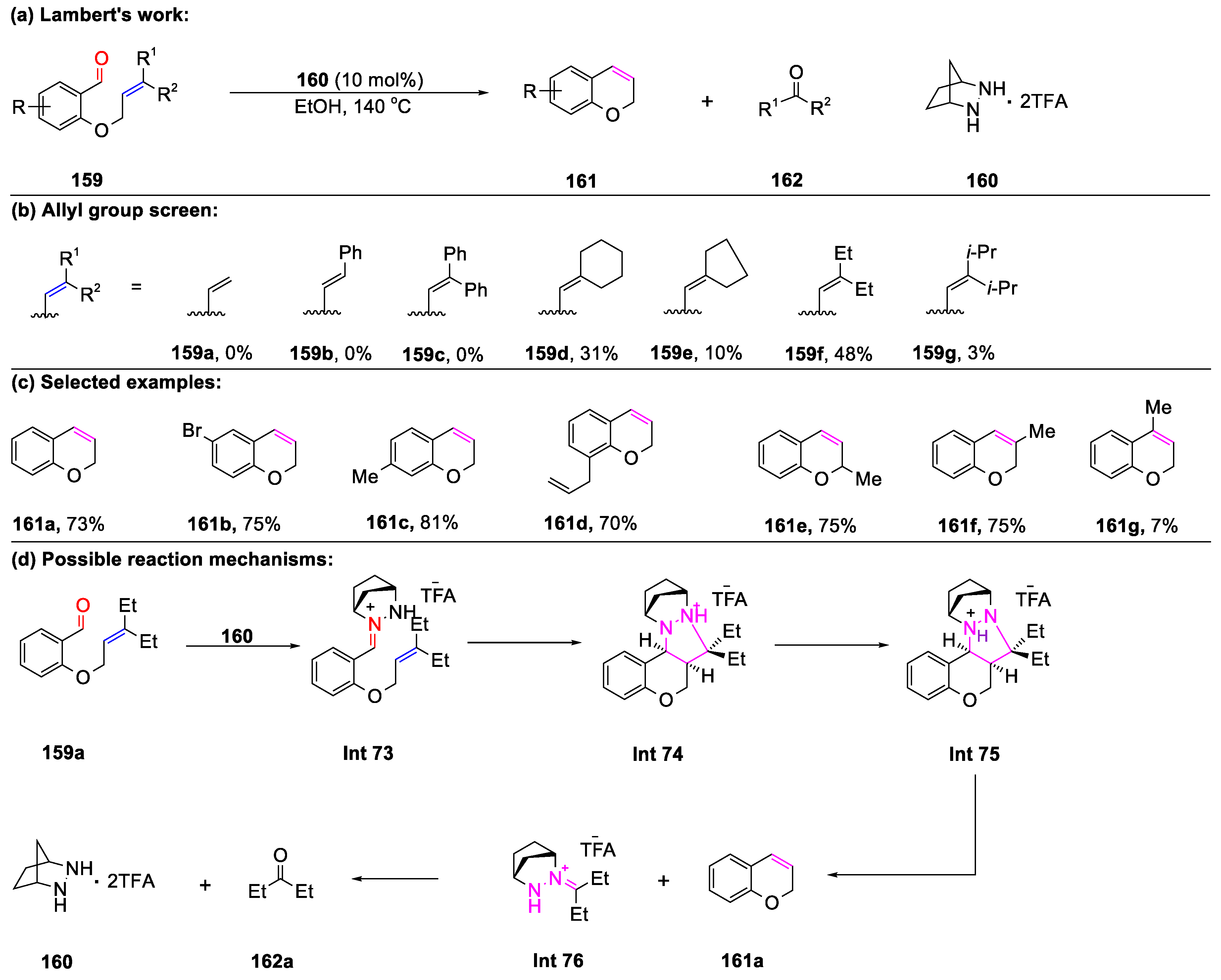

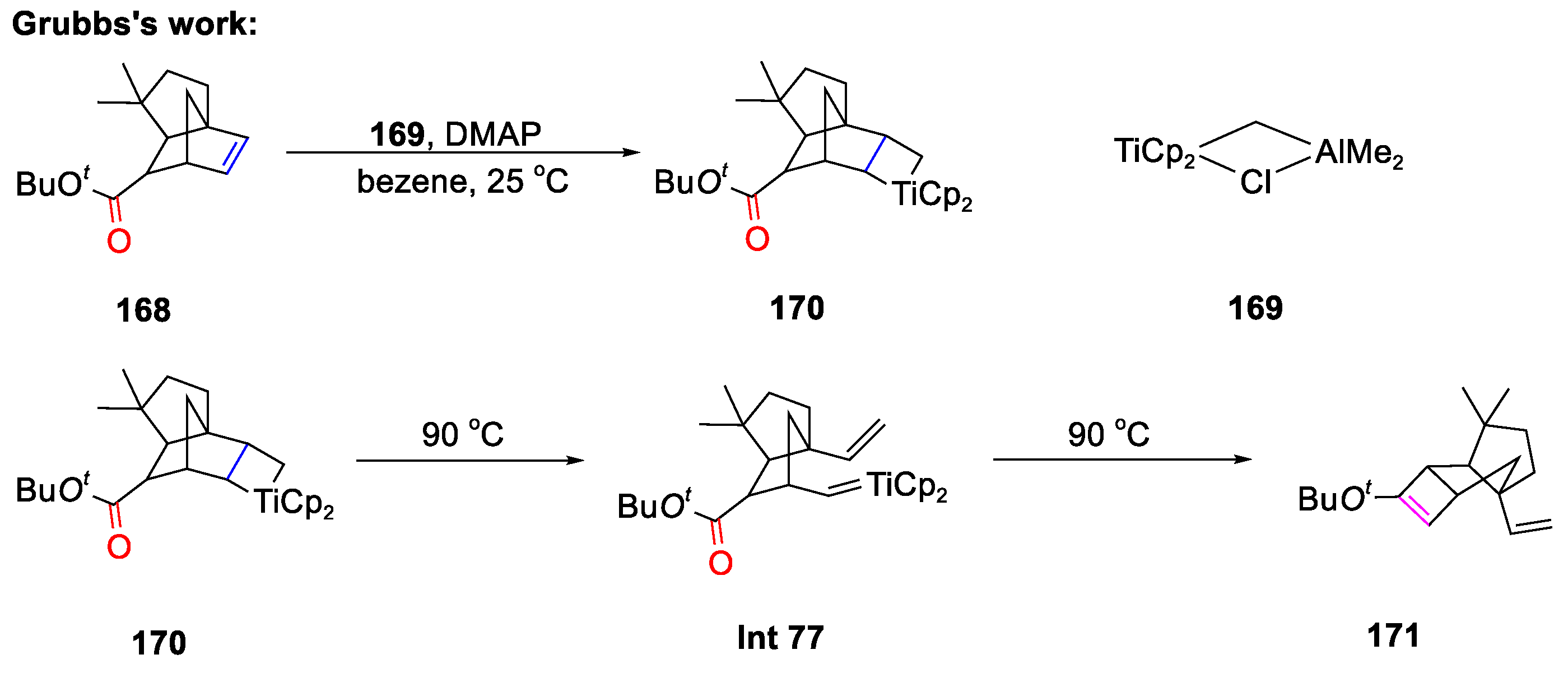

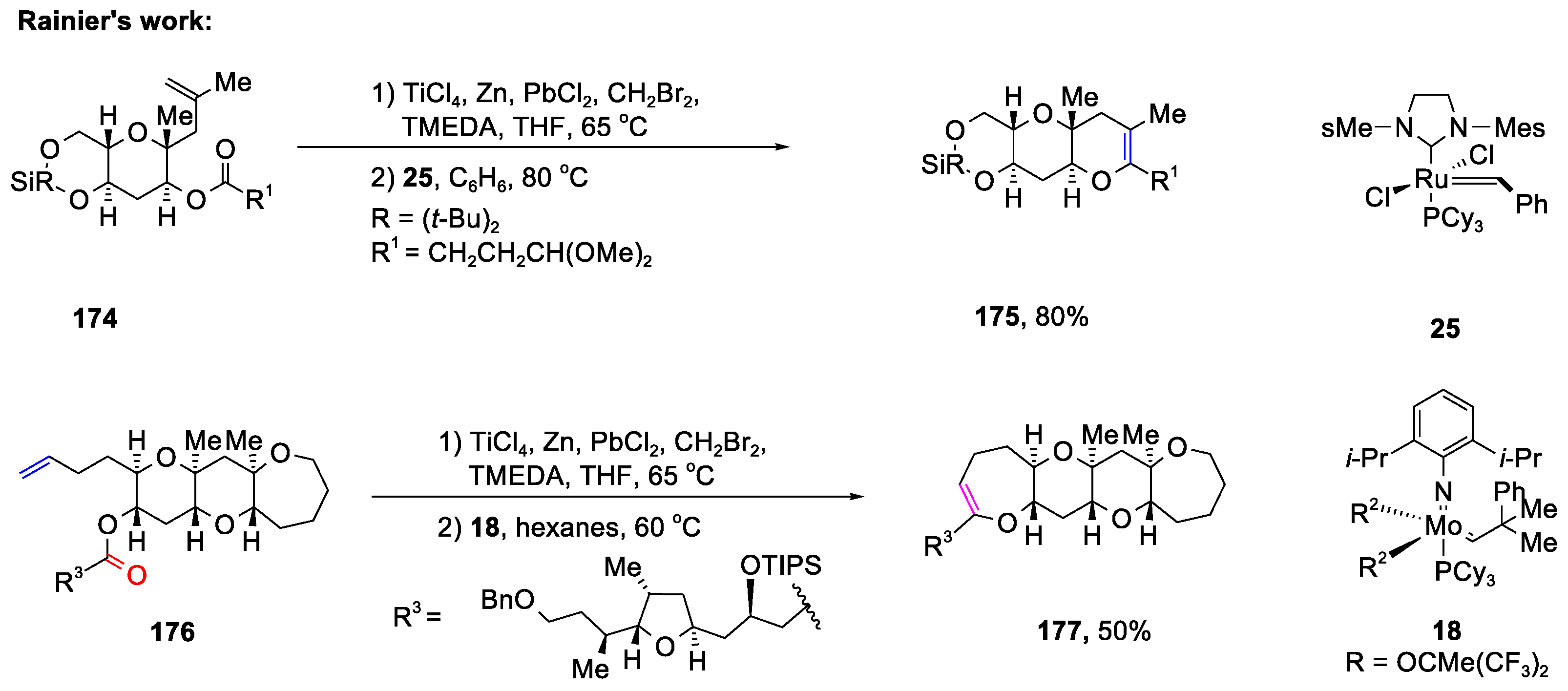


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X. Cyclization Strategies in Carbonyl–Olefin Metathesis: An Up-to-Date Review. Molecules 2024, 29, 4861. https://doi.org/10.3390/molecules29204861
Zhang X. Cyclization Strategies in Carbonyl–Olefin Metathesis: An Up-to-Date Review. Molecules. 2024; 29(20):4861. https://doi.org/10.3390/molecules29204861
Chicago/Turabian StyleZhang, Xiaoke. 2024. "Cyclization Strategies in Carbonyl–Olefin Metathesis: An Up-to-Date Review" Molecules 29, no. 20: 4861. https://doi.org/10.3390/molecules29204861
APA StyleZhang, X. (2024). Cyclization Strategies in Carbonyl–Olefin Metathesis: An Up-to-Date Review. Molecules, 29(20), 4861. https://doi.org/10.3390/molecules29204861




