The Role of –OEt Substituents in Molybdenum-Assisted Pentathiepine Formation—Access to Diversely Functionalized Azines
Abstract
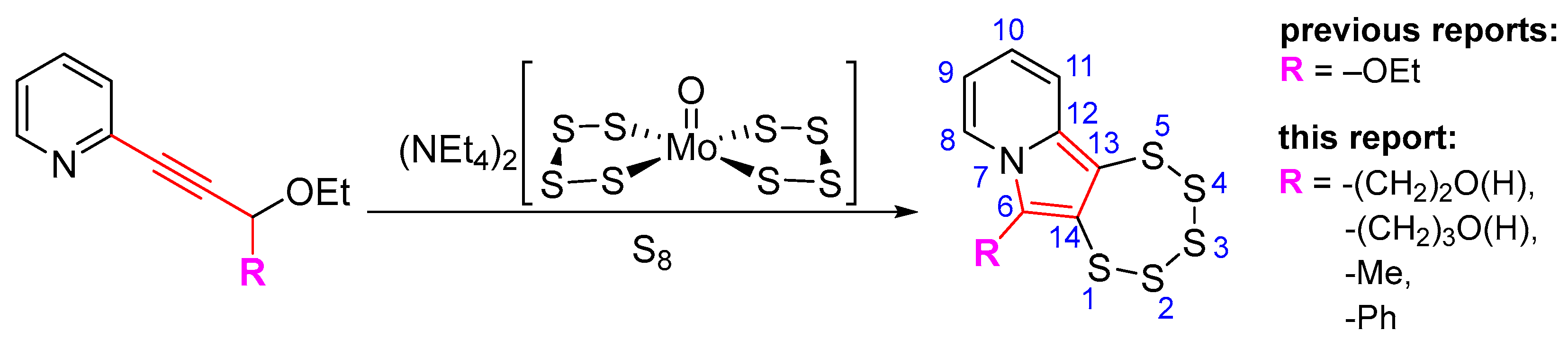
1. Introduction
2. Results and Discussion
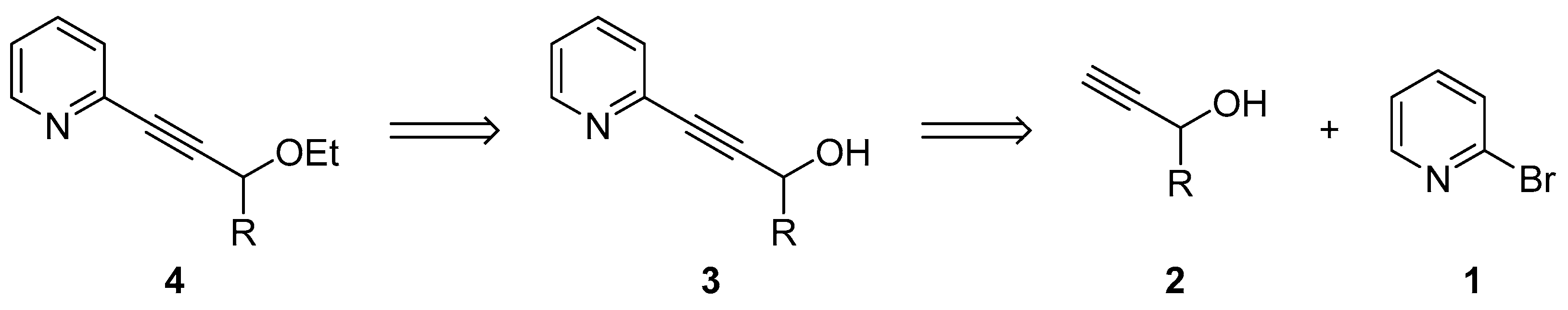
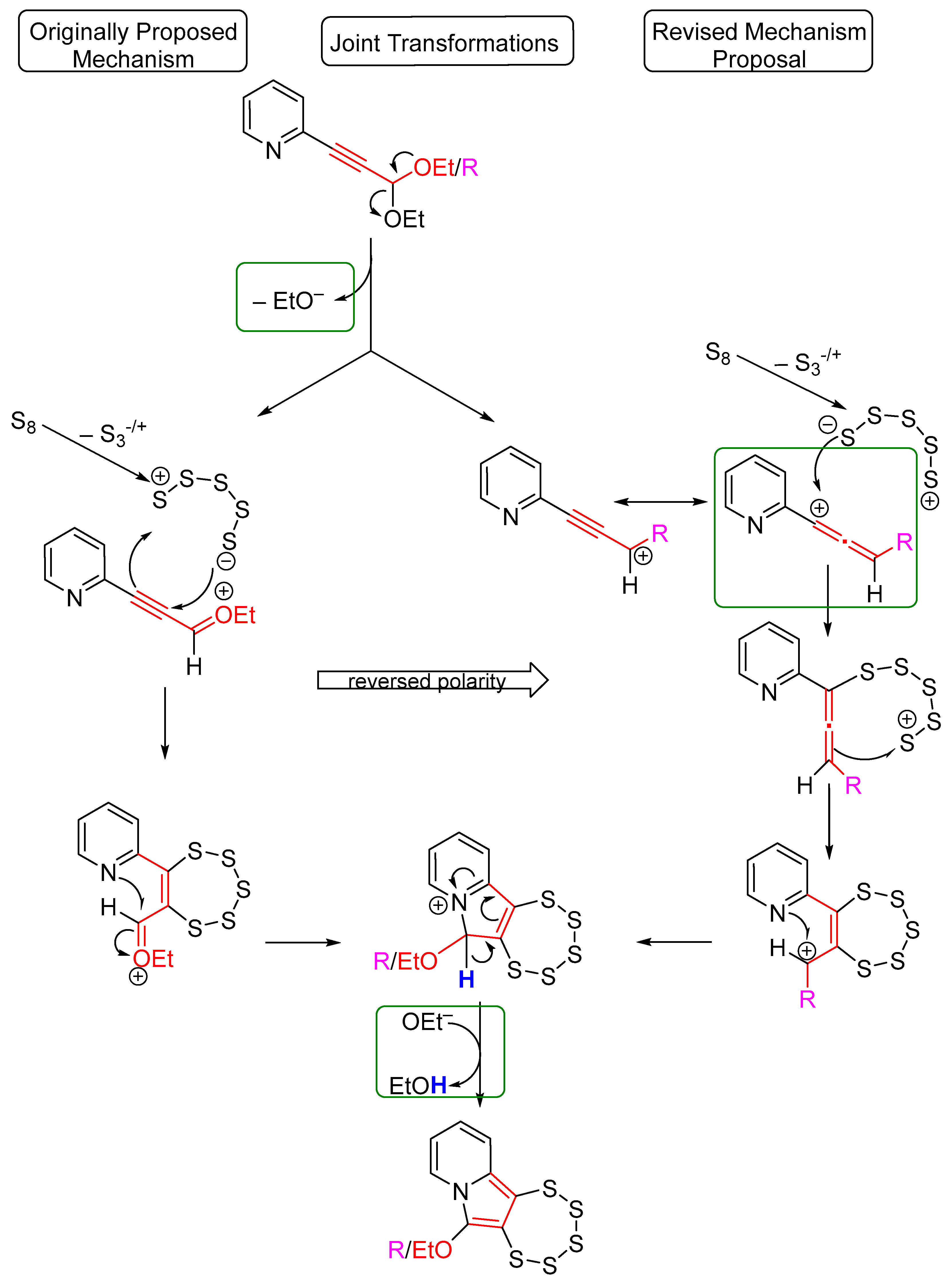
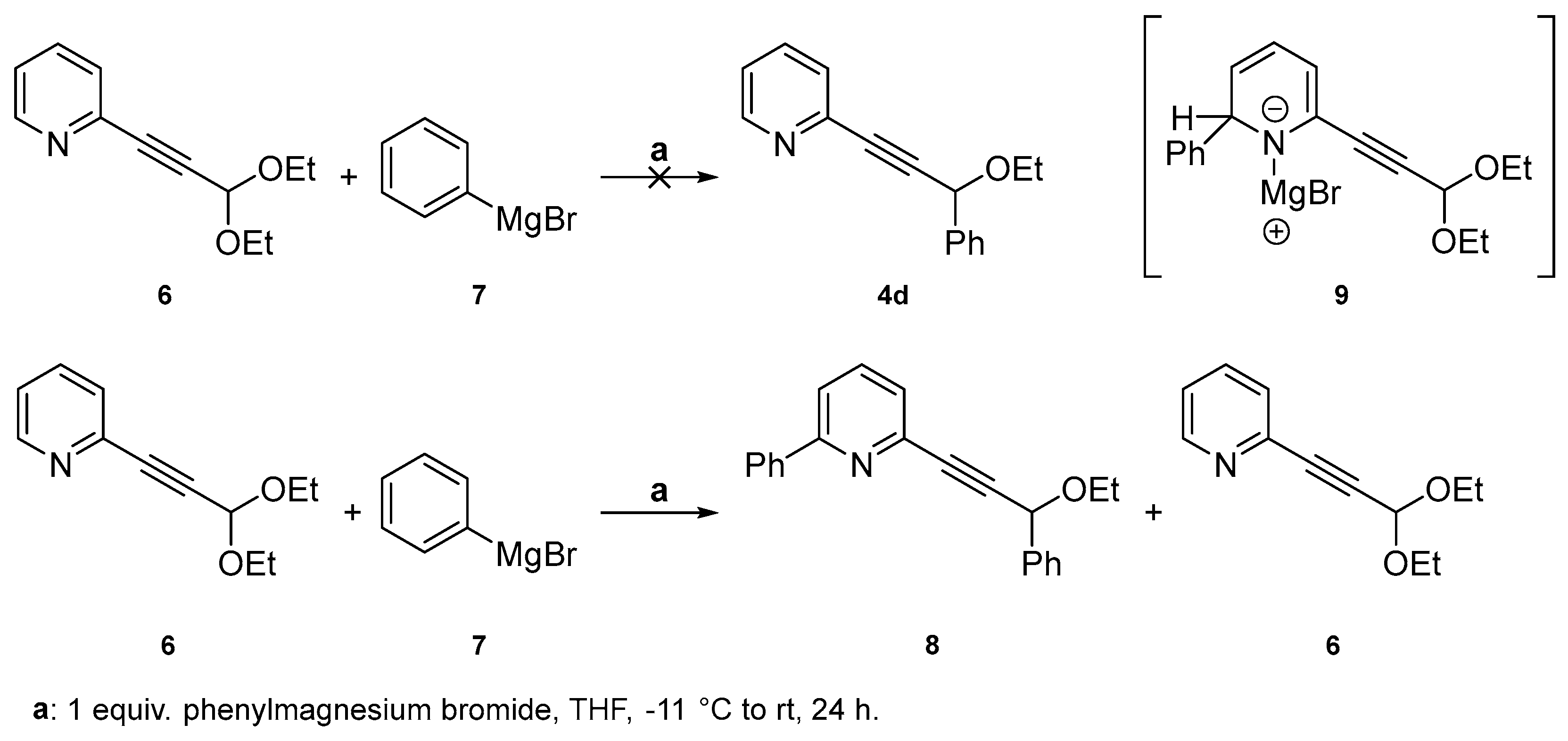
2.1. Testing the Postulated Mechanism—An Overview
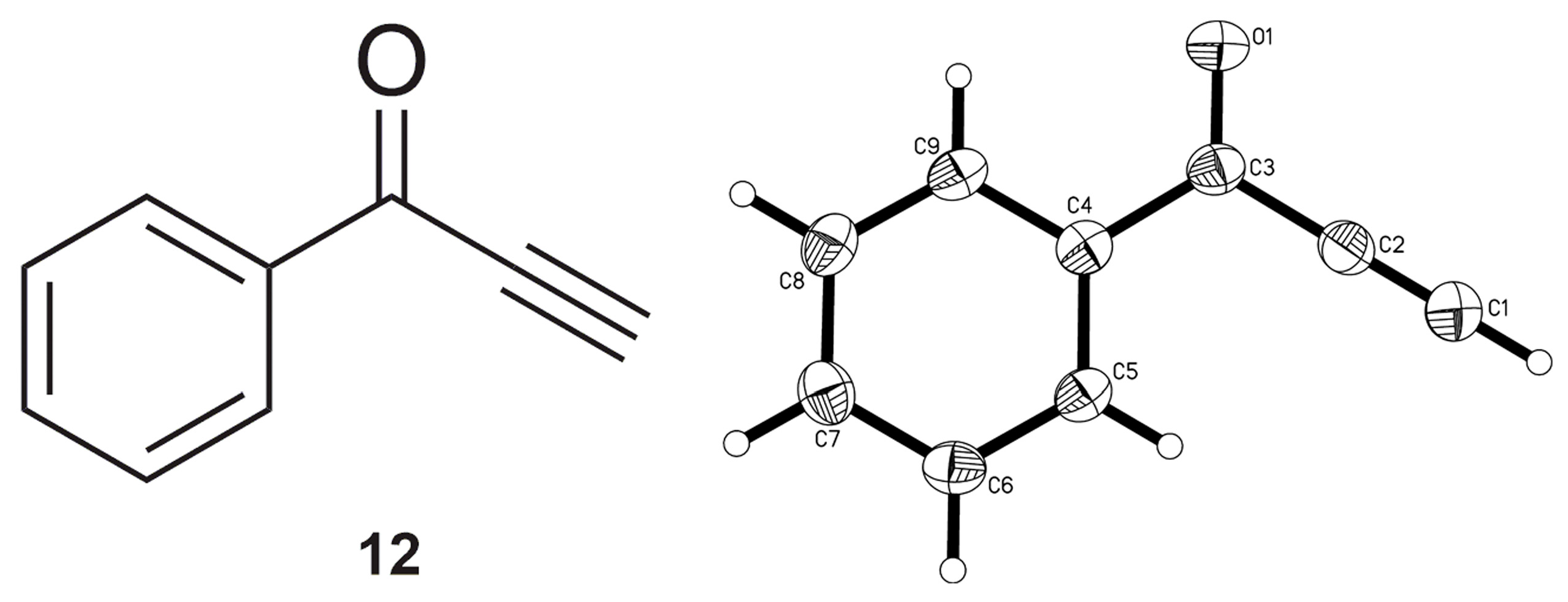
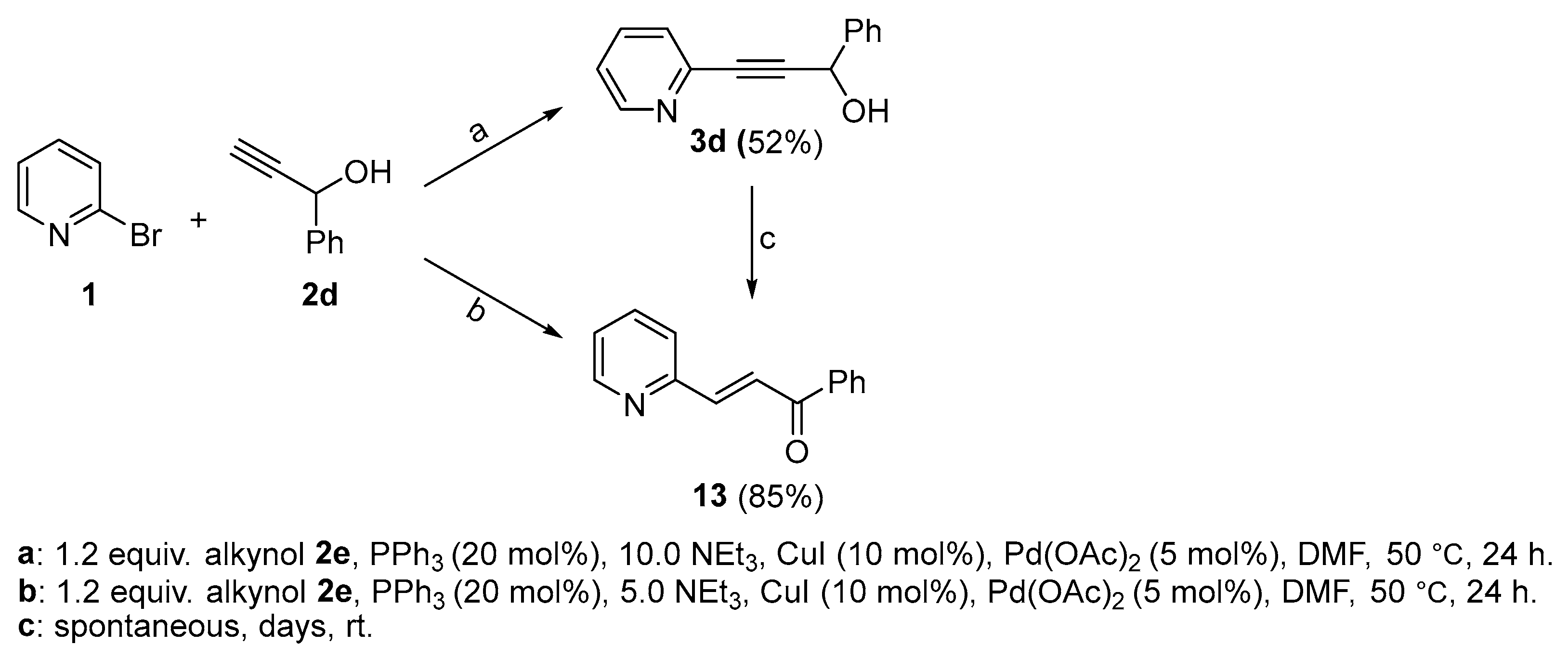
2.2. Complete Absence of –OEt
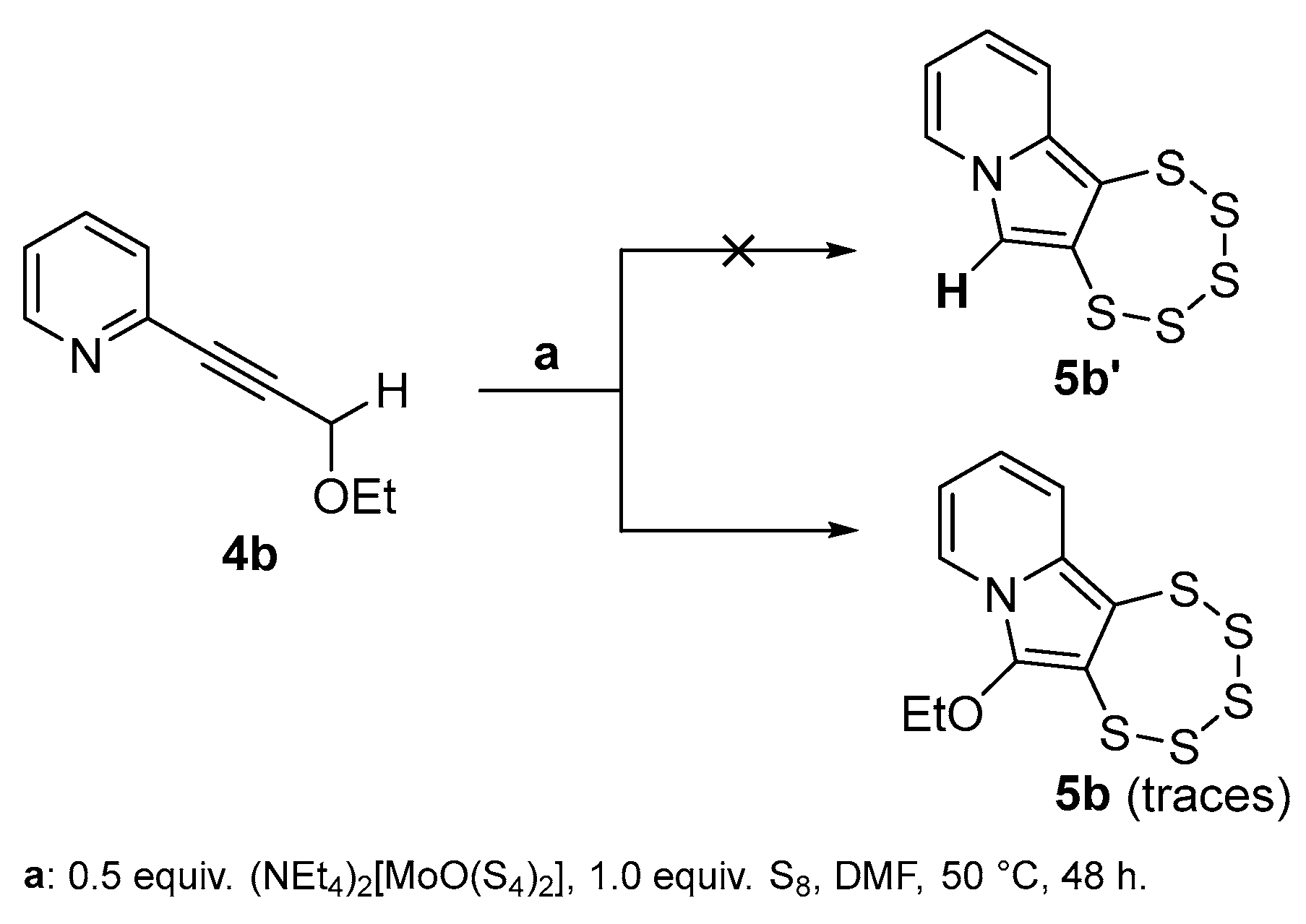
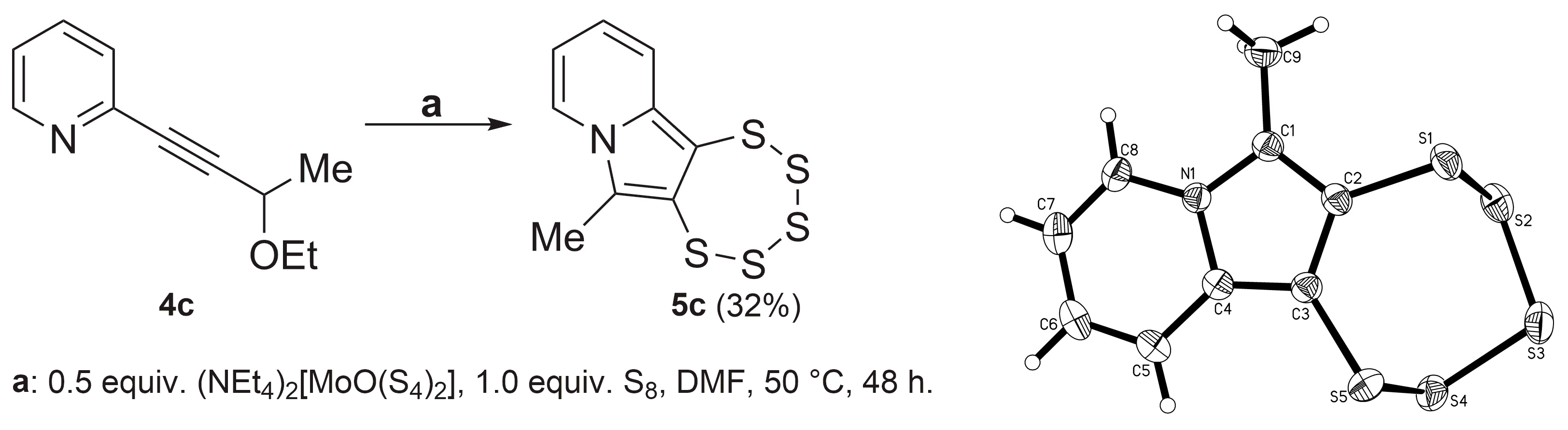
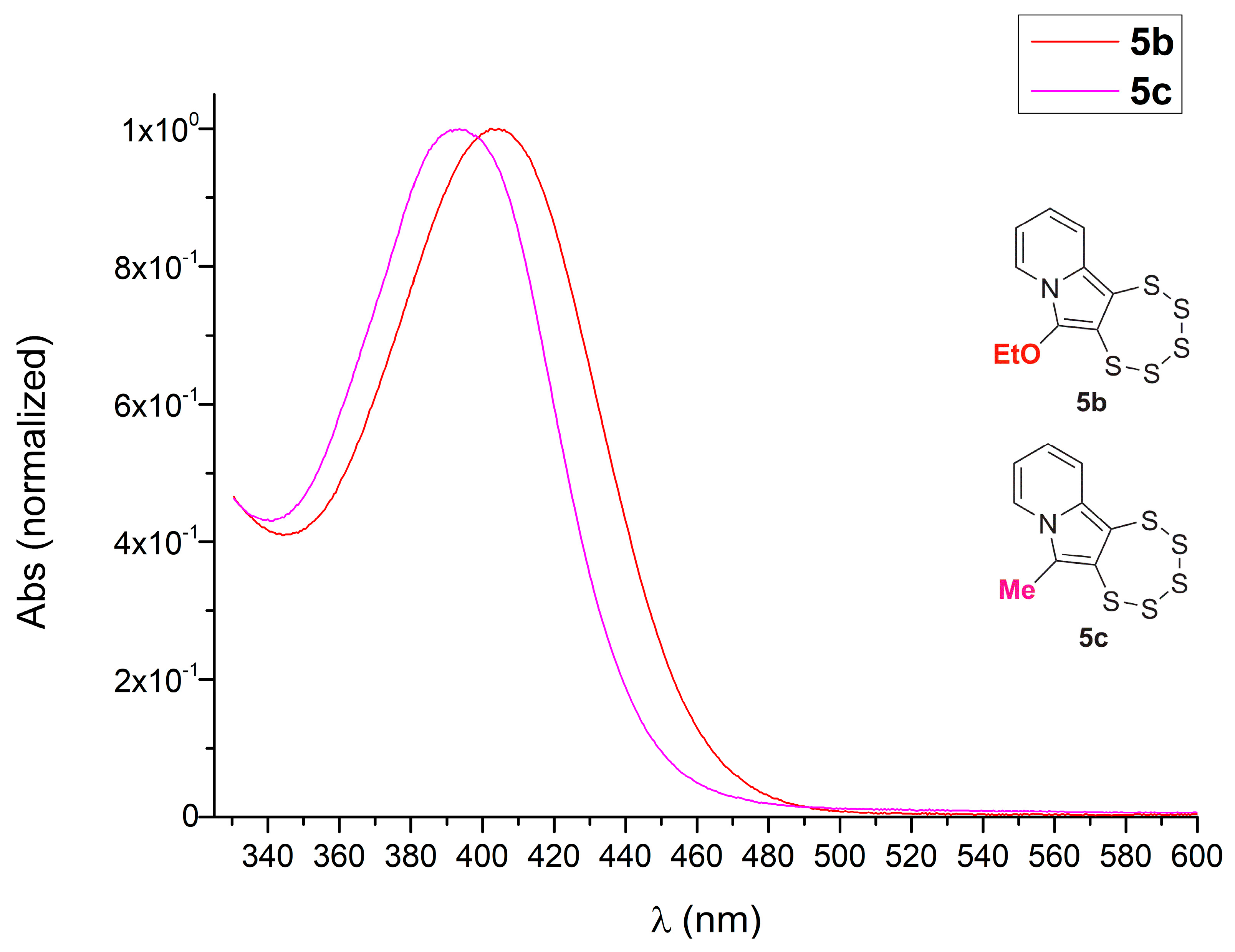
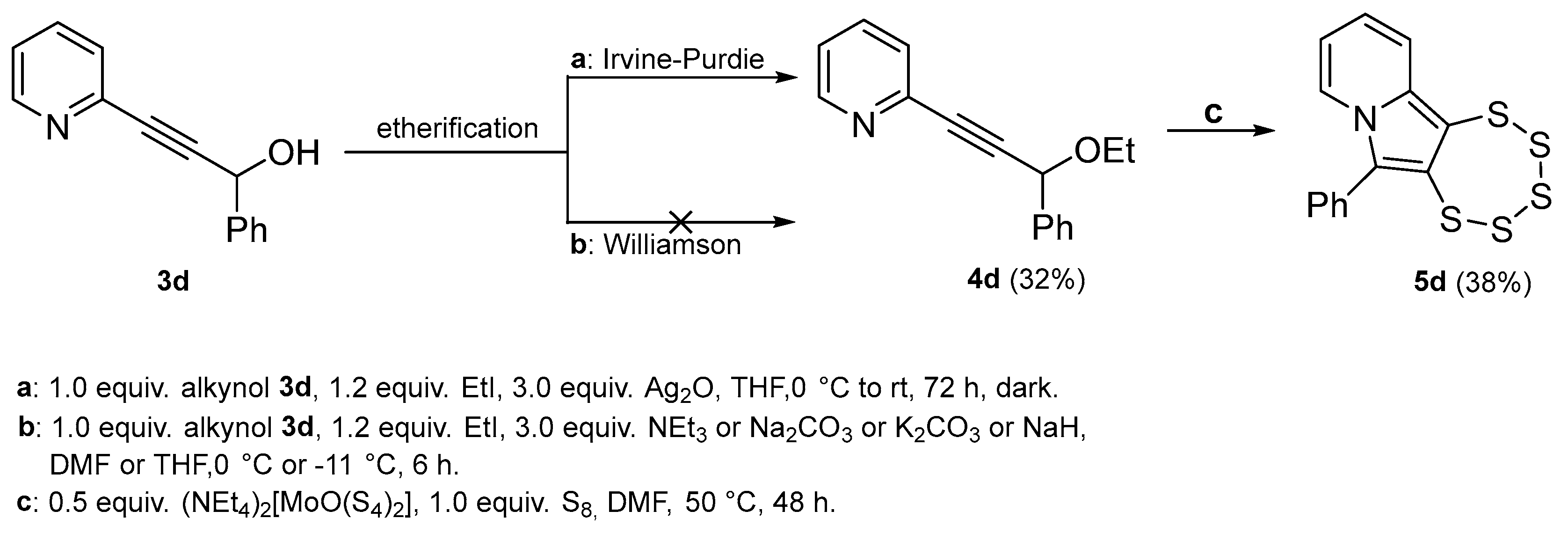
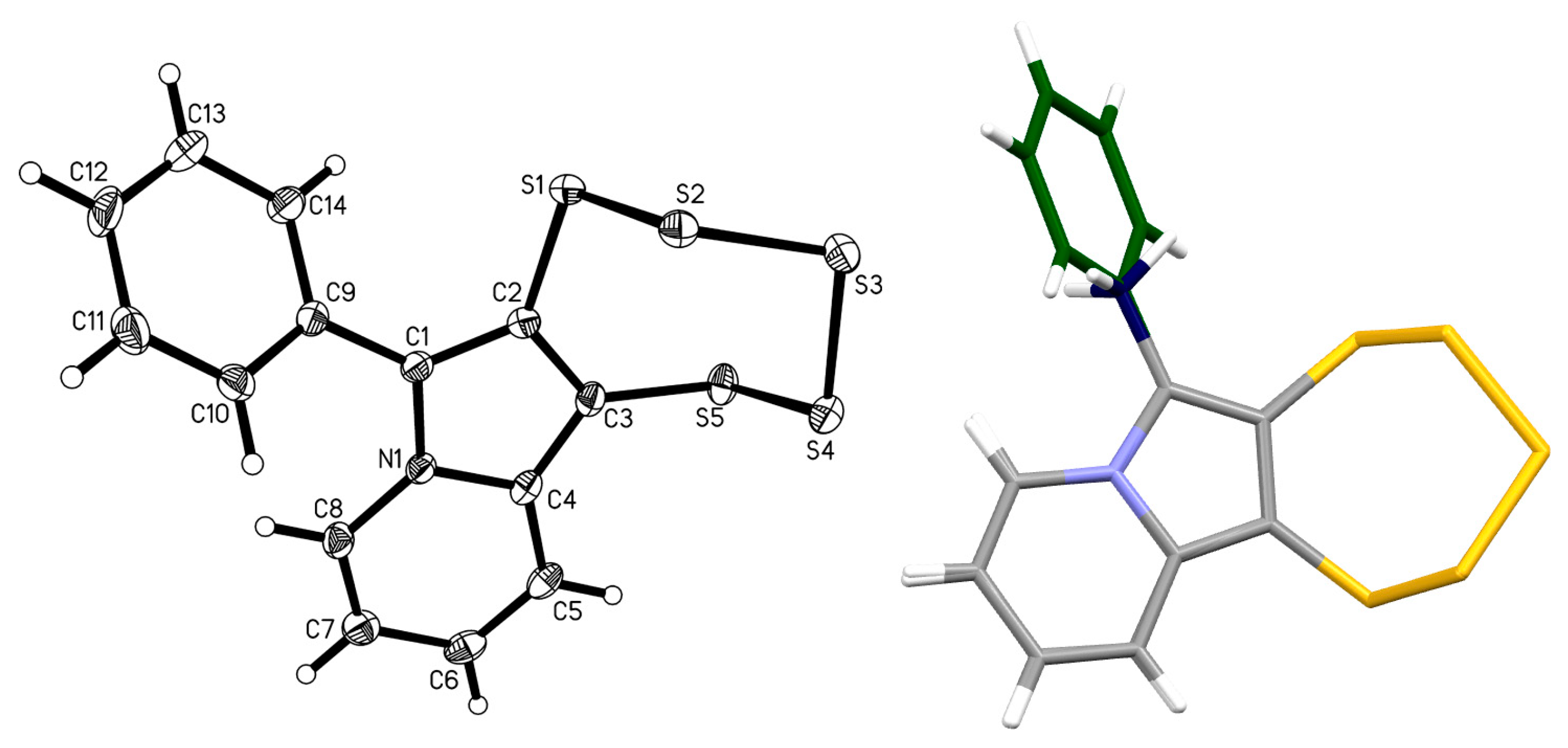
2.3. Presence of a Single –OEt
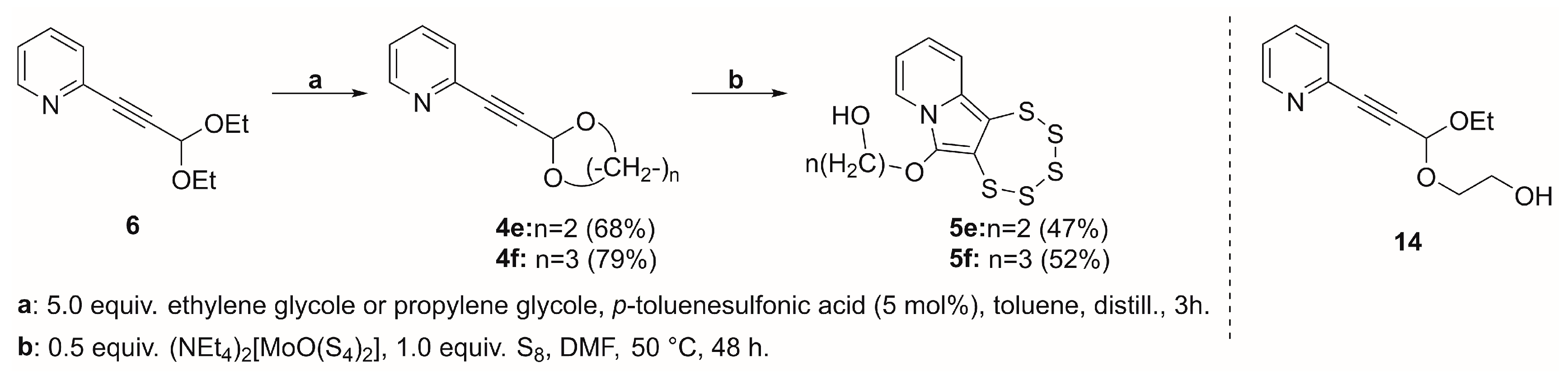
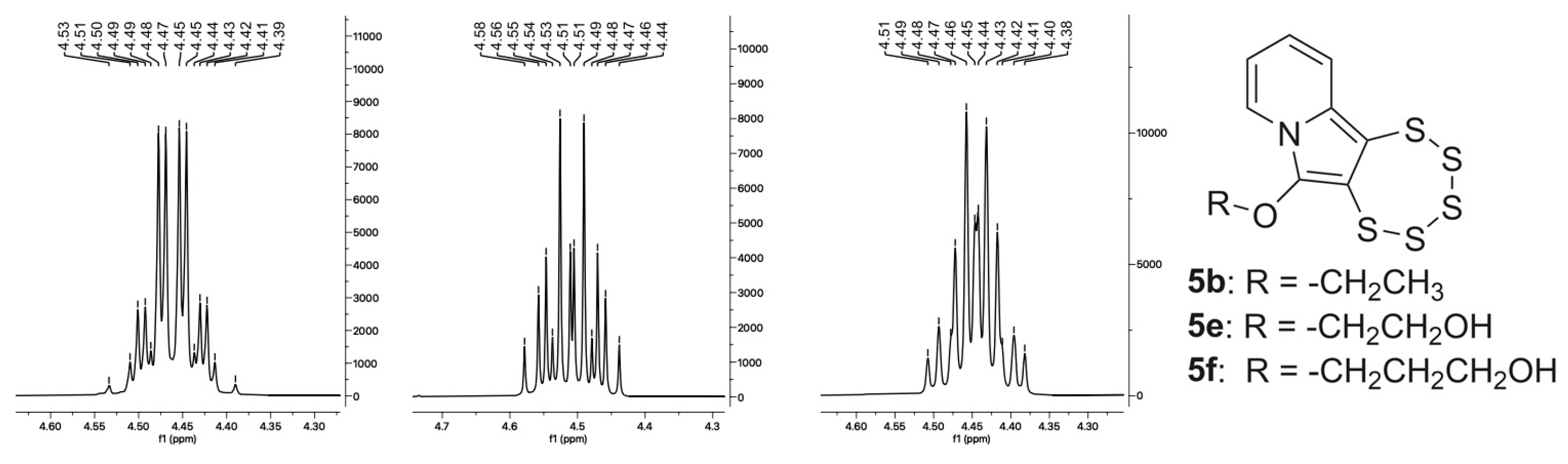
2.4. Cyclic Acetal Precursors
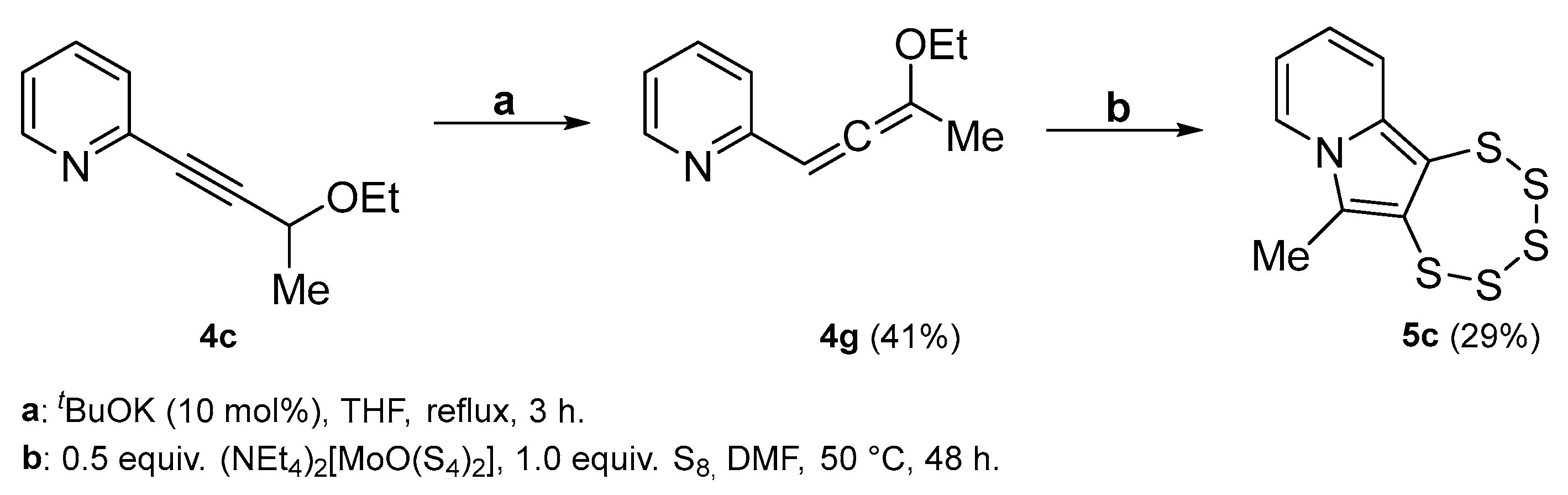
2.5. Allene Precursor Variation
3. Materials and Methods
3.1. Materials, Methods, and Instrumentation
3.1.1. General Experimental Procedures
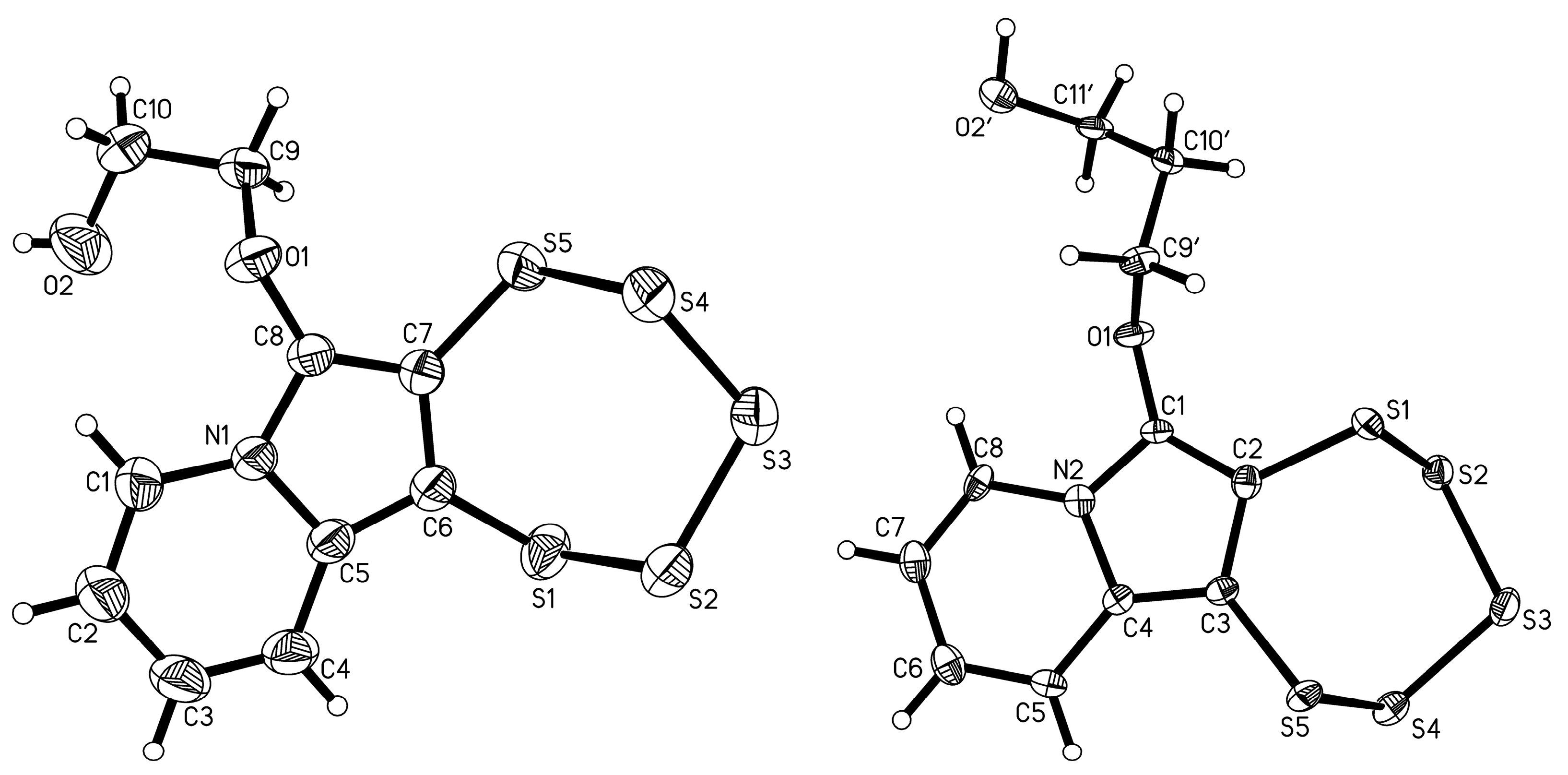
3.1.2. Singe-Crystal X-ray Diffraction
3.2. Syntheses
3.2.1. Sonogashira Cross-Coupling Reactions
3.2.2. Ethylation via Williamson Reaction
3.2.3. Ethylation via Irvine–Purdie Reaction
3.2.4. Aldehyde Reduction
3.2.5. Nucleophilic Attack on Diethyl Acetal
3.2.6. Diethyl Acetal Exchange Reaction
3.2.7. Tautomerization from Alkyne to Allene
3.2.8. Synthetic Procedures for Pentathiepine Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arumugam, V.; Venkatesan, M.; Ramachandran, K.; Sundaresan, U.; Ramachandran, S. Ascidians. In Encyclopedia of Marine Biotechnology; Kim, S.-K., Ed.; Wiley VCH: Weinheim, Germany, 2020; pp. 2487–2508. [Google Scholar] [CrossRef]
- Jacobsen, L.M.; Tallarita, R.; Bandaru, S.S.M.; Schulzke, C. Chapter 14 Synthesis of pharmacologically significant pentathiepins: A journey from harsh to mild conditions. In Non-Conventional Synthesis; György, K., Bubun, B., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2024; pp. 403–466. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Korchagina, D.V.; Baev, D.S.; Vassiliev, P.M.; Volcho, K.P.; Salakhutdinov, N.F. Antimicrobial Activity of Substituted Benzopentathiepin-6-amines. J. Antibiot. 2019, 72, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Rybalova, T.V.; Rogachev, A.D.; Khomenko, T.M.; Volcho, K.P.; Salakhutdinov, N.F. Molecular and Supramolecular Structure of 8-(Trifluoromethyl)Benzo[f][1,2,3,4,5] Pentathiepin-6-Amine—A Representative of Condensed Arene Pentathiepines. J. Struct. Chem. 2018, 59, 1753–1758. [Google Scholar] [CrossRef]
- Mahendran, A.; Ghogare, A.A.; Bittman, R.; Arthur, G.; Greer, A. Synthesis and antiproliferative properties of a new ceramide analog of varacin. Chem. Phys. Lipids 2016, 194, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Biorg. Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Fehér, F.; Langer, M. Contribution to the chemistry of sulfur, no. 104 Synthesis of pentathiepin and benzopentathiepin. Tetrahedron Lett. 1971, 12, 2125–2126. [Google Scholar] [CrossRef]
- Davidson, B.S.; Molinski, T.F.; Barrows, L.R.; Ireland, C.M. Varacin: A novel benzopentathiepin from Lissoclinum vareau that is cytotoxic toward a human colon tumor. J. Am. Chem. Soc. 1991, 113, 4709–4710. [Google Scholar] [CrossRef]
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development. Nat. Product. Bioprospecting 2017, 7, 1–111. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; St»lhandske, C. Transformation of isatin with P4S10 to pentathiepino[6,7-b]indole in one step. Tetrahedron Lett. 1994, 35, 5279–5282. [Google Scholar] [CrossRef]
- Chenard, B.L.; Harlow, R.L.; Johnson, A.L.; Vladuchick, S.A. Synthesis, structure, and properties of pentathiepins. J. Am. Chem. Soc. 1985, 107, 3871–3879. [Google Scholar] [CrossRef]
- Rewcastle, G.W.; Janosik, T.; Bergman, J. Reactions of 2-lithiated indoles with elemental sulfur. Formation of pentathiepino[6,7-b]indoles and indoline-2-thiones. Tetrahedron 2001, 57, 7185–7189. [Google Scholar] [CrossRef]
- Sato, R.; Ohyama, T.; Kawagoe, T.; Baba, M.; Nakajo, S.; Kimura, T.; Ogawa, S. Synthesis and characterization of functionalized benzopentathiepins. Heterocycles 2001, 55, 145–154. [Google Scholar] [CrossRef]
- Zubair, M.; Ghosh, A.C.; Schulzke, C. The unexpected and facile molybdenum mediated formation of tri- and tetracyclic pentathiepins from pyrazine-alkynes and sulfur. Chem. Commun. 2013, 49, 4343–4345. [Google Scholar] [CrossRef] [PubMed]
- Tallarita, R.; Jacobsen, L.M.; Elvers, B.J.; Richter, S.; Bandaru, S.S.M.; Correia, J.V.; Schulzke, C. Synthesis of Seven Indolizine-Derived Pentathiepines: Strong Electronic Structure Response to Nitro Substitution in Position C-9. Molecules 2024, 29, 216. [Google Scholar] [CrossRef] [PubMed]
- Behnisch-Cornwell, S.; Bandaru, S.S.M.; Napierkowski, M.; Wolff, L.; Zubair, M.; Urbainsky, C.; Lillig, C.; Schulzke, C.; Bednarski, P.J. Pentathiepins: A Novel Class of Glutathione Peroxidase 1 Inhibitors that Induce Oxidative Stress, Loss of Mitochondrial Membrane Potential and Apoptosis in Human Cancer Cells. ChemMedChem 2020, 15, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Biala, G.; Kedzierska, E.; Kruk-Slomka, M.; Orzelska-Gorka, J.; Hmaidan, S.; Skrok, A.; Kaminski, J.; Havrankova, E.; Nadaska, D.; Malik, I. Research in the Field of Drug Design and Development. Pharmaceuticals 2023, 16, 1283. [Google Scholar] [CrossRef] [PubMed]
- Naredla, R.R.; Klumpp, D.A. Contemporary Carbocation Chemistry: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 6905–6948. [Google Scholar] [CrossRef]
- Li, J.J. Carbocation Chemistry: Applications in Organic Synthesis; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Williamson, A. XLV. Theory of ætherification. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1850, 37, 350–356. [Google Scholar] [CrossRef]
- Wolff, L.; Bandaru, S.S.M.; Eger, E.; Lam, H.N.; Napierkowski, M.; Baecker, D.; Schulzke, C.; Bednarski, P.J. Comprehensive Evaluation of Biological Effects of Pentathiepins on Various Human Cancer Cell Lines and Insights into Their Mode of Action. Int. J. Mol. Sci. 2021, 22, 7631. [Google Scholar] [CrossRef]
- Grard, J. Several reactions of propargylic acetal. Compt. Rend. 1929, 189, 541–543. [Google Scholar]
- González-Bello, C.; Castedo, L. Six-Membered Heterocycles: Pyridines. In Modern Heterocyclic Chemistry; Alvarez-Builla, J., Vaquero, J.J., Barluenga, J., Eds.; Wiley VCH: Weinheim, Germany, 2011; pp. 1431–1525. [Google Scholar] [CrossRef]
- Barnes, R.A. Properties and Reactions of Pyridine and its Hydrogenated Derivatives. In The Chemistry of Heterocyclic Compounds; Klingsberg, E., Ed.; Interscience Publishers, Inc.: New York, NY, USA, 1960; pp. 1–97. [Google Scholar] [CrossRef]
- Tenenbaum, L.E. Alkylpyridines and Arylpyridines. In The Chemistry of Heterocyclic Compounds; Klingsberg, E., Ed.; Interscience Publishers, Inc.: New York, NY, USA, 1961; pp. 155–298. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W. Pentathiepins. Chem. Rev. 2004, 104, 2617–2630. [Google Scholar] [CrossRef] [PubMed]
- Napierkowski, M.; Janke, U.; Rong, A.; Delcea, M.; Bandaru, S.S.M.; Schulzke, C.; Bednarski, P.J. Liposomal formulation of model pentathiepin improves solubility and stability toward glutathione while preserving anticancer activity. Arch. Pharm. 2023, 356, e2300087. [Google Scholar] [CrossRef] [PubMed]
- Ieronimo, G.; Palmisano, G.; Maspero, A.; Marzorati, A.; Scapinello, L.; Masciocchi, N.; Cravotto, G.; Barge, A.; Simonetti, M.; Ameta, K.L.; et al. A novel synthesis of N-hydroxy-3-aroylindoles and 3-aroylindoles. Org. Biomol. Chem. 2018, 16, 6853–6859. [Google Scholar] [CrossRef] [PubMed]
- Kueny-Stotz, M.; Isorez, G.; Chassaing, S.; Brouillard, R. Straightforward Synthesis of Highly Hydroxylated Phloroglucinol-Type 3-Deoxyanthocyanidins. Synlett 2007, 2007, 1067–1070. [Google Scholar] [CrossRef]
- Fu, R.; Liu, Y.; Wu, T.; Zhang, X.; Zhu, Y.; Luo, J.; Zhang, Z.; Jiang, Y. Metal-free synthesis of β-aminoketones by the reductive hydroamination of ynones. Chem. Commun. 2022, 58, 3525–3528. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.M. Side Reactions in a Grignard Synthesis. J. Chem. Educ. 1999, 76, 76. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Kiel, A.; Herten, D.-P.; Rominger, F.; Müller, T.J.J. Synthesis, Structure and Emission Properties of Spirocyclic Benzofuranones and Dihydroindolones: A Domino Insertion–Coupling–Isomerization– Diels–Alder Approach to Rigid Fluorophores. Chem. Eur. J. 2008, 14, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Williamson Ether Synthesis. In Comprehensive Organic Name Reactions and Reagents; Wang, Z., Ed.; Wiley VCH: Weinheim, Germany, 2010; pp. 3026–3030. [Google Scholar] [CrossRef]
- Purdie, T.; Irvine, J.C. The alkylation of sugars. J. Chem. Soc., Trans. 1903, 83, 1021–1037. [Google Scholar] [CrossRef]
- Wang, Z. Irvine-Purdie Methylation. In Comprehensive Organic Name Reactions and Reagents; Wang, Z., Ed.; Wiley VCH: Weinheim, Germany, 2010; pp. 1526–1529. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Greer, A. On the Origin of Cytotoxicity of the Natural Product Varacin. A Novel Example of a Pentathiepin Reaction That Provides Evidence for a Triatomic Sulfur Intermediate. J. Am. Chem. Soc. 2001, 123, 10379–10386. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Brzostowska, E.M.; Greer, A. The Role of Amine in the Mechanism of Pentathiepin (Polysulfur) Antitumor Agents. J. Am. Chem. Soc. 2003, 125, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Brzostowska, E.M.; Paulynice, M.; Bentley, R.; Greer, A. Planar Chirality due to a Polysulfur Ring in Natural Pentathiepin Cytotoxins. Implications of Planar Chirality for Enantiospecific Biosynthesis and Toxicity. Chem. Res. Toxicol. 2007, 20, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Wuts, P.G.M.; Green, T.W. Chapter 4: Protection for the Carbonyl Group. In Greene’s Protective Groups in Organic Synthesis; Wuts, P.G.M., Green, T.W., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 431–532. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Green, T.W. Chapter 5: Protection for the Carboxyl Group. In Greene’s Protective Groups in Organic Synthesis; Wuts, P.G.M., Green, T.W., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 533–646. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, Y.; Zhang, H.; Chen, J.; Liu, Y. Highly Efficient Synthesis of Functionalized Indolizines and Indolizinones by Copper-Catalyzed Cycloisomerizations of Propargylic Pyridines. J. Org. Chem. 2007, 72, 7783–7786. [Google Scholar] [CrossRef] [PubMed]
- Deagostino, A.; Prandi, C.; Toppino, A.; Venturello, P. Palladium-catalysed Heck reaction on 1,2-dien-1-ols: A stereoselective synthesis of α-arylated α,β-unsaturated aldehydes. Tetrahedron 2008, 64, 10344–10349. [Google Scholar] [CrossRef]
- Hoff, S.; Brandsma, L.; Arens, J.F. Preparation, metallation and alkylation of allenyl ethers. Recl. Trav. Chim. Pays-Bas-J. Roy. Neth. Chem. Soc. 1968, 87, 916–924. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
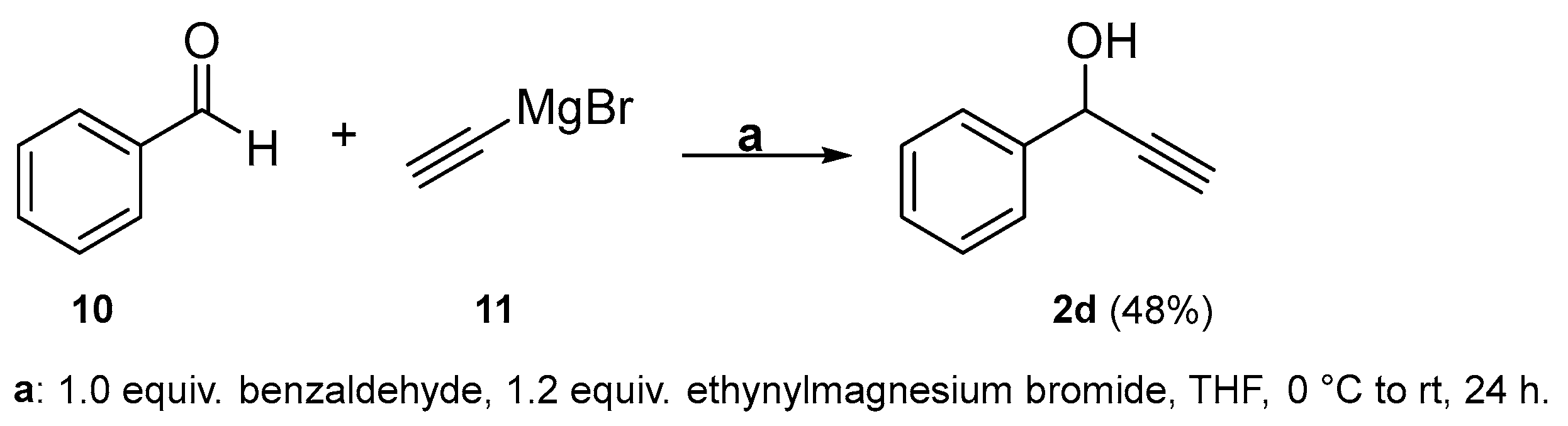
| Starting Material | Product | Yield (%) | ||
|---|---|---|---|---|
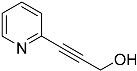 | 3a | None | / | |
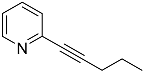 | 4′a | None | / | |
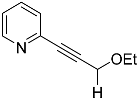 | 4b | 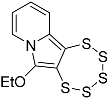 | 5b | Traces 1 |
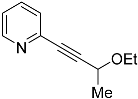 | 4c | 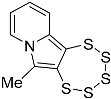 | 5c | 32 2 |
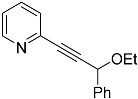 | 4d | 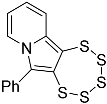 | 5d | 38 |
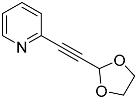 | 4e | 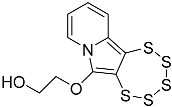 | 5e | 47 |
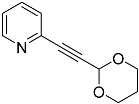 | 4f | 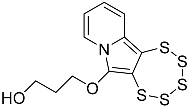 | 5f | 52 |
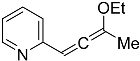 | 4g | 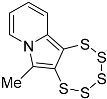 | 5c | 29 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallarita, R.; Jacobsen, L.M.; Bandaru, S.S.M.; Elvers, B.J.; Schulzke, C. The Role of –OEt Substituents in Molybdenum-Assisted Pentathiepine Formation—Access to Diversely Functionalized Azines. Molecules 2024, 29, 3806. https://doi.org/10.3390/molecules29163806
Tallarita R, Jacobsen LM, Bandaru SSM, Elvers BJ, Schulzke C. The Role of –OEt Substituents in Molybdenum-Assisted Pentathiepine Formation—Access to Diversely Functionalized Azines. Molecules. 2024; 29(16):3806. https://doi.org/10.3390/molecules29163806
Chicago/Turabian StyleTallarita, Roberto, Lukas M. Jacobsen, Siva S. M. Bandaru, Benedict J. Elvers, and Carola Schulzke. 2024. "The Role of –OEt Substituents in Molybdenum-Assisted Pentathiepine Formation—Access to Diversely Functionalized Azines" Molecules 29, no. 16: 3806. https://doi.org/10.3390/molecules29163806
APA StyleTallarita, R., Jacobsen, L. M., Bandaru, S. S. M., Elvers, B. J., & Schulzke, C. (2024). The Role of –OEt Substituents in Molybdenum-Assisted Pentathiepine Formation—Access to Diversely Functionalized Azines. Molecules, 29(16), 3806. https://doi.org/10.3390/molecules29163806






