Light-Controlled Interconvertible Self-Assembly of Non-Photoresponsive Suprastructures
Abstract
1. Introduction
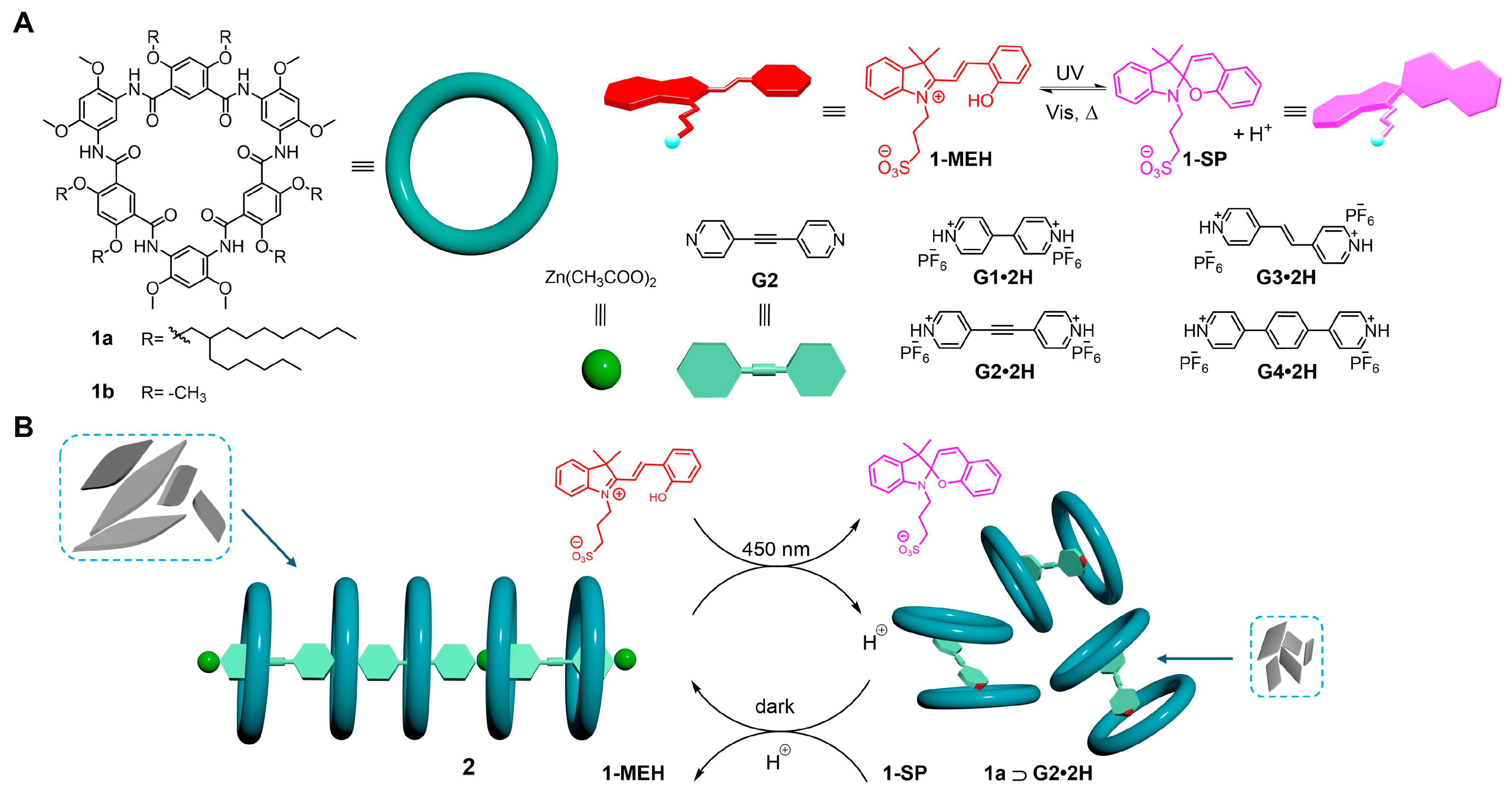
2. Results and Discussion
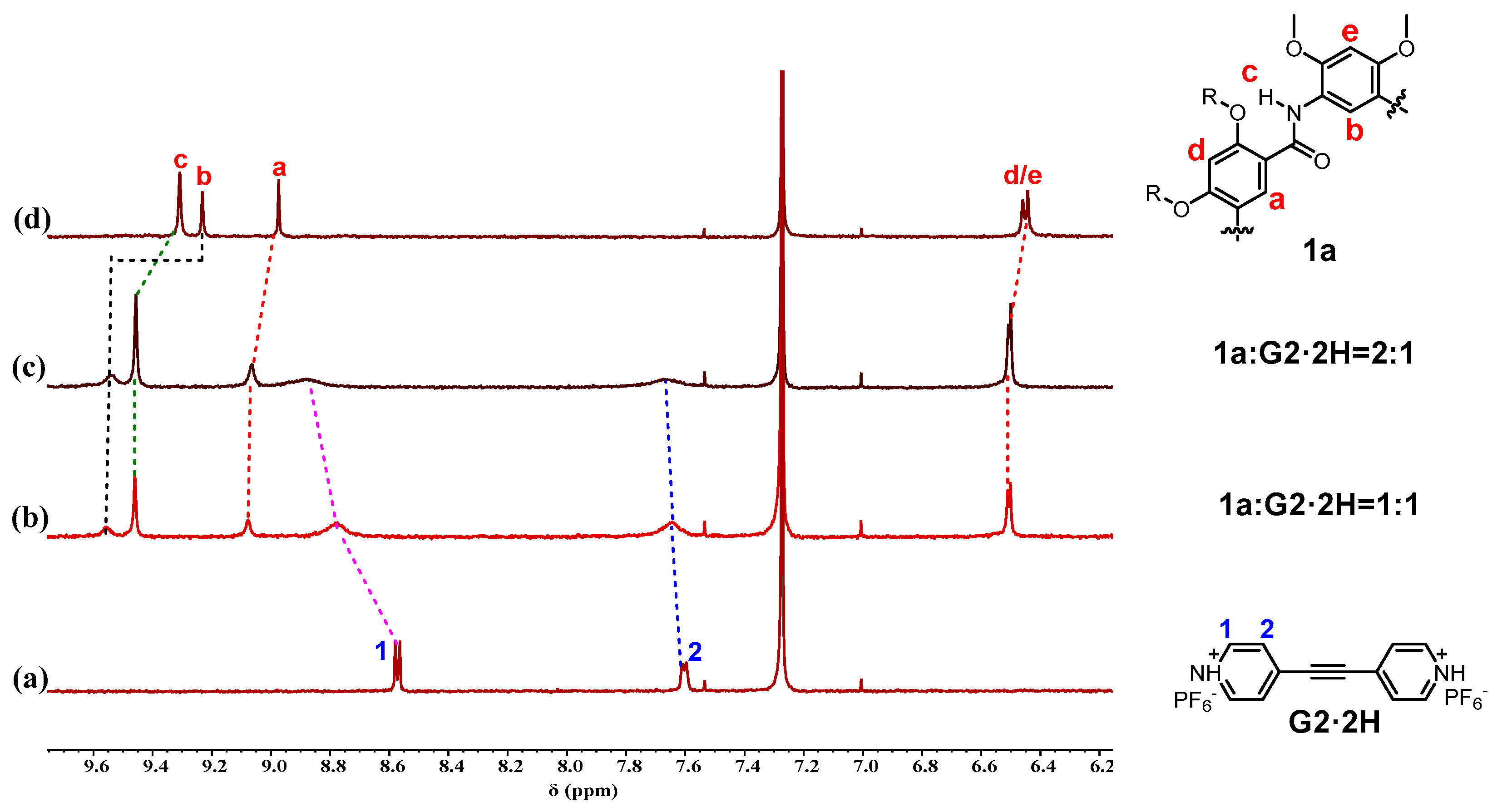
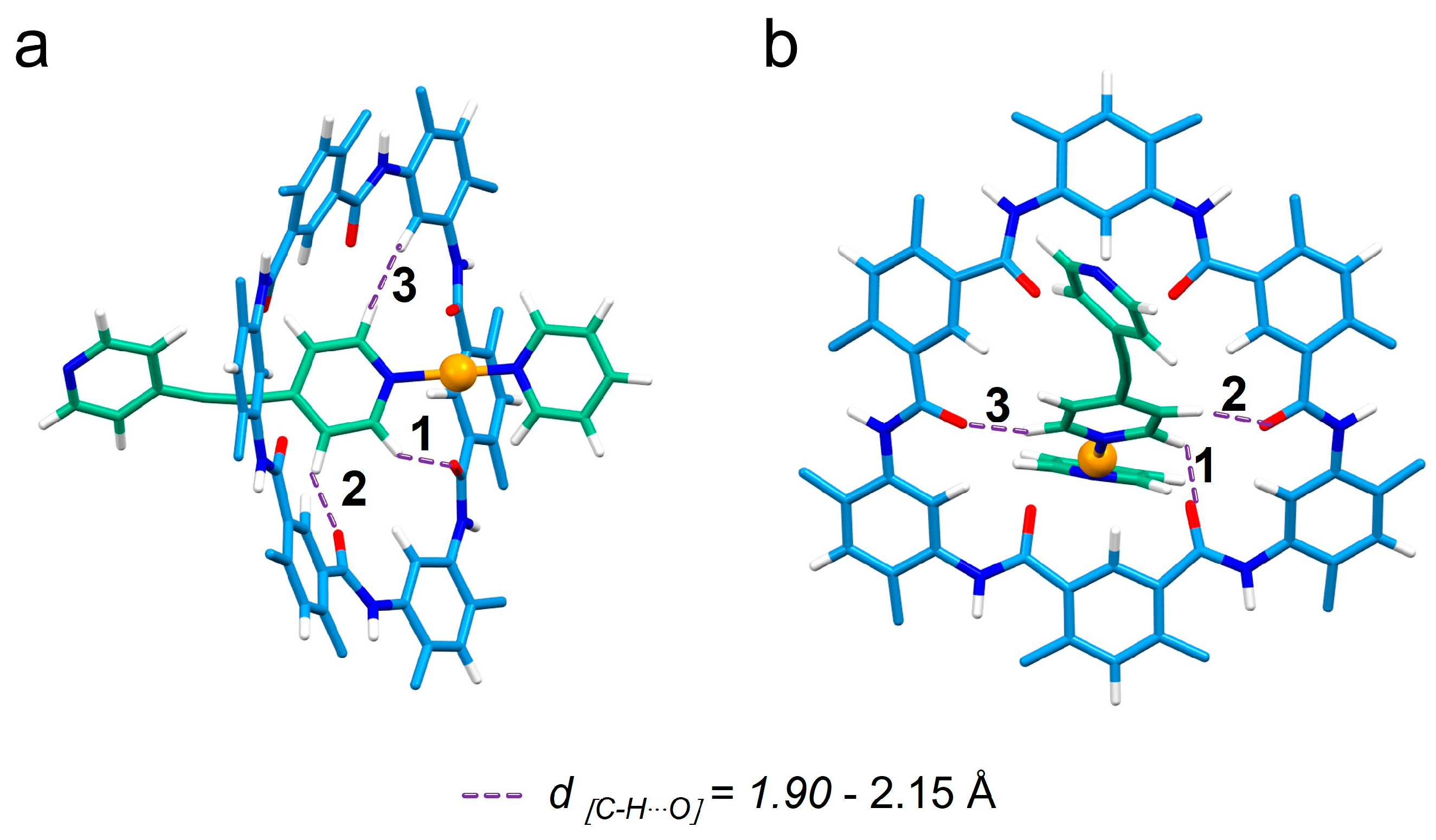
2.1. Host–Guest Interaction
2.2. Photoisomerization Behavior of 1-MEH
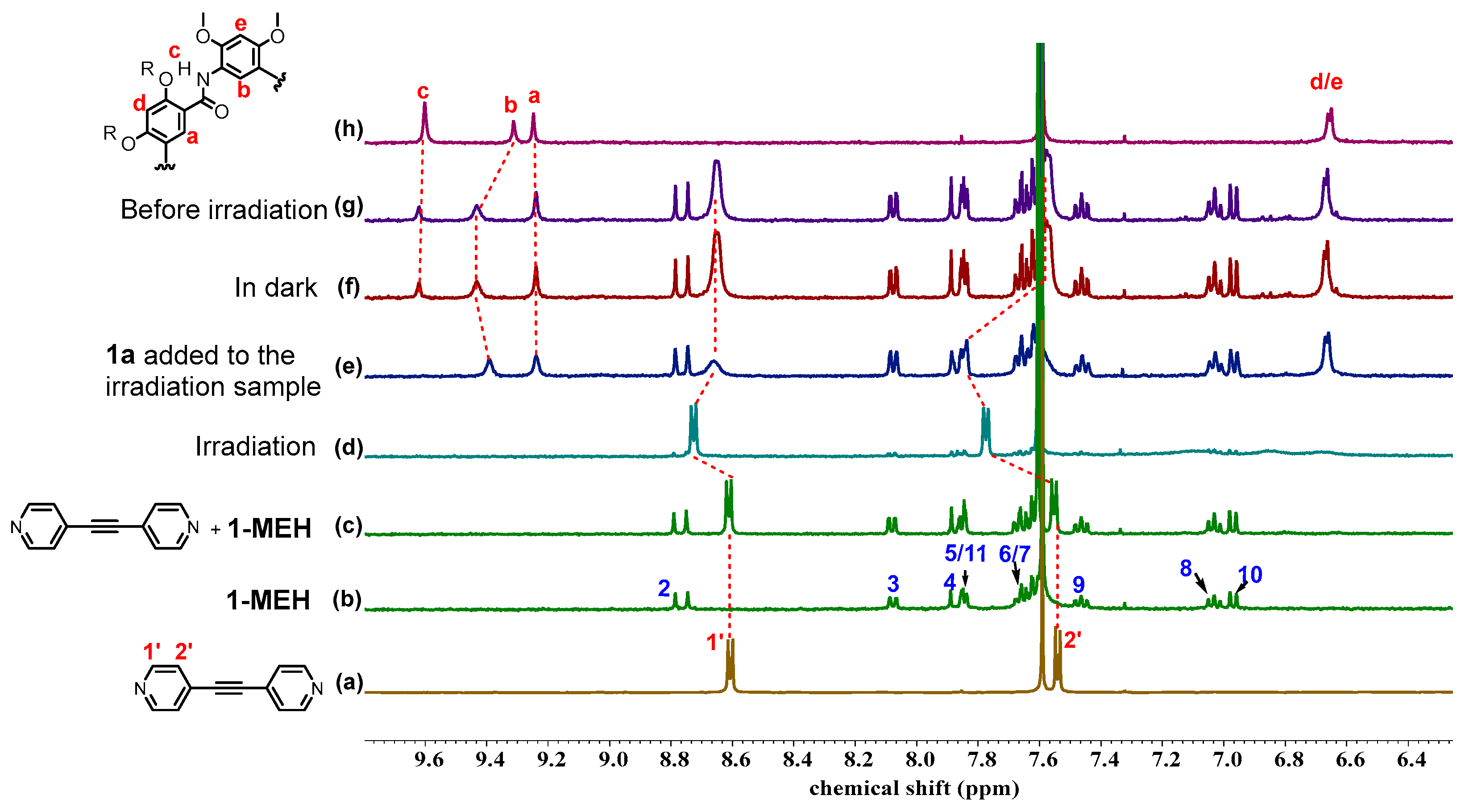
2.3. Photoswitchable Complexation with Non-Photoresponsive Macrocycle
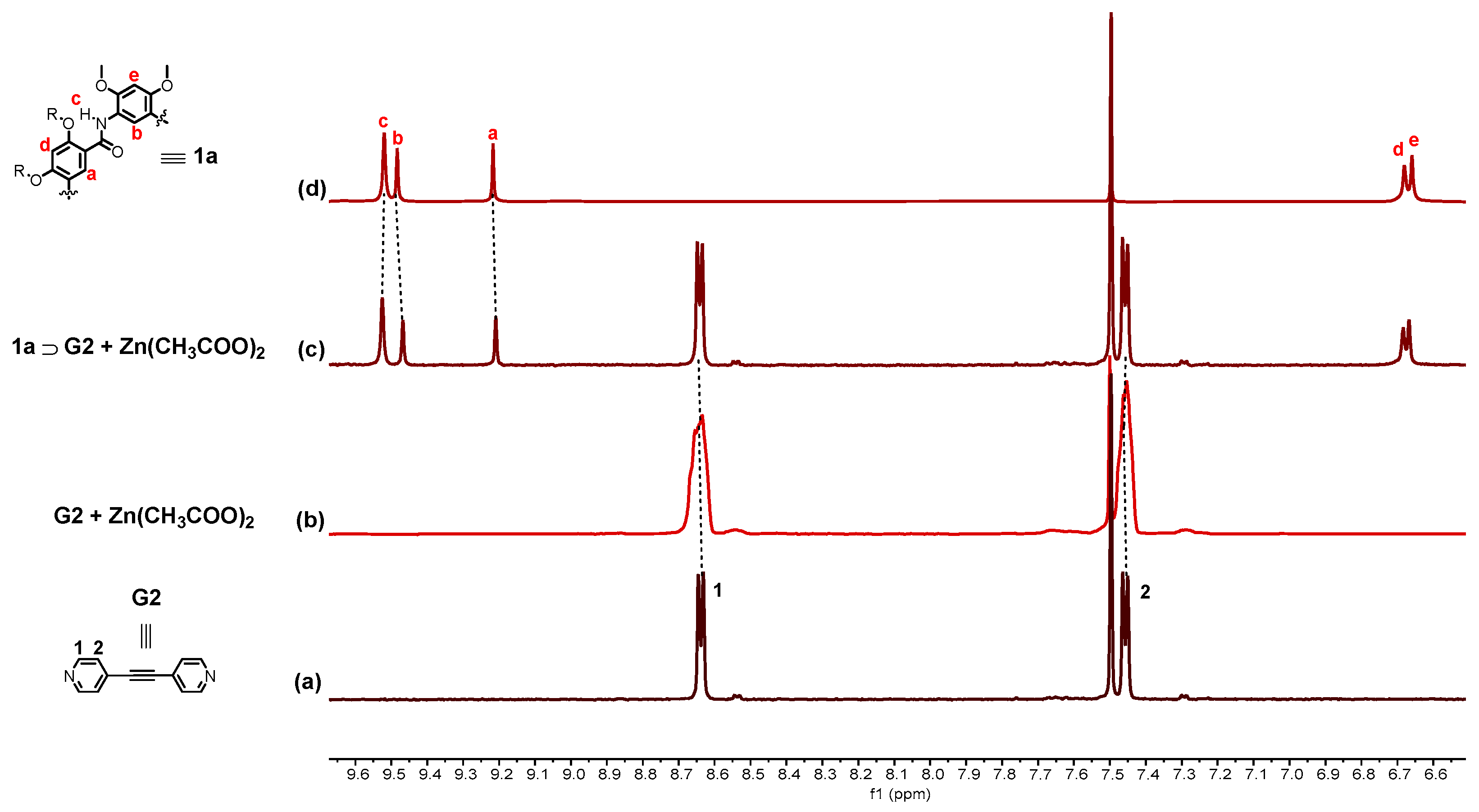
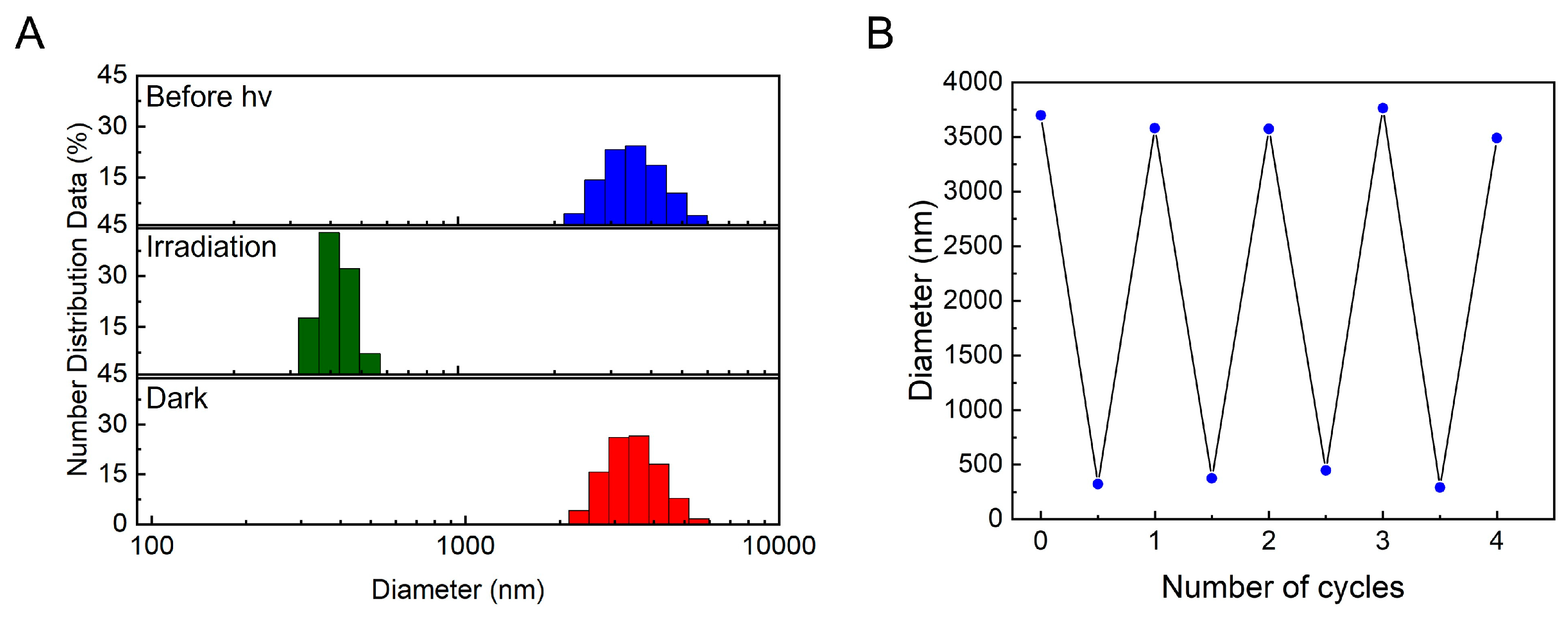
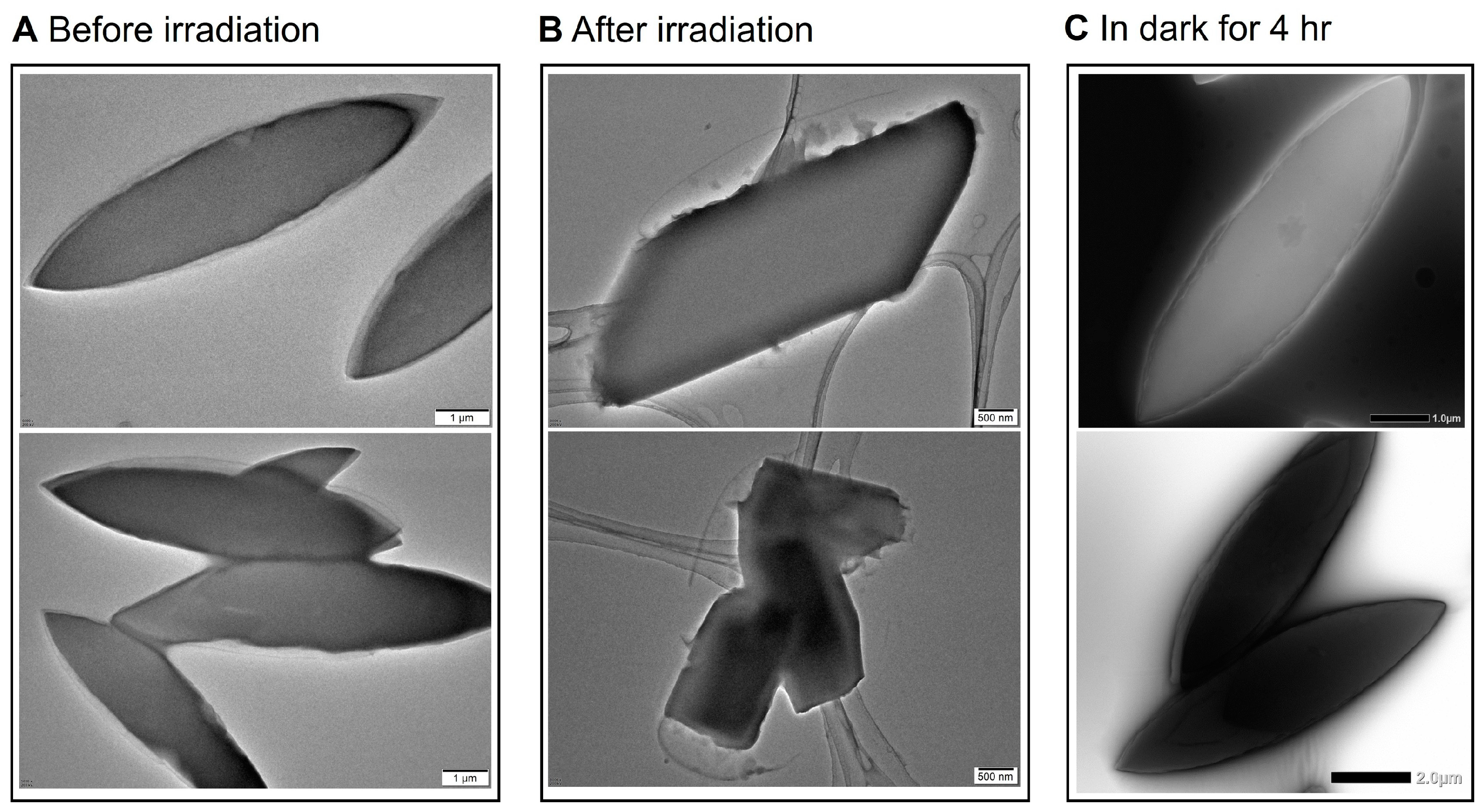
2.4. Controlled Reversible Polypseudorotaxane Formation Driven by Light
3. Materials and Methods
3.1. Materials and Reagents
3.2. Experimental Methods
3.3. Synthesis of Hydrogen-Bonded Macrocycle 1a and Guests G1·2H-G4·2H
3.4. DFT Calculations
3.5. Visualization of Noncovalent Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Moscoso, A.; Ballester, P. Light-Responsive Molecular Containers. Chem. Commun. 2017, 53, 4635–4652. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, S.; Kim, J.; Soh, S.; Grzybowski, B. Photoswitchable Catalysis Mediated by Dynamic Aggregation of Nanoparticles. J. Am. Chem. Soc. 2010, 132, 11018–11020. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Samanta, D.; Leizrowice, R.; Margulis, B.; Zhao, H.; Börner, M.; Udayabhaskararao, T.; Manna, D.; Klajn, R. Light-Controlled Self-Assembly of Non-Photoresponsive Nanoparticles. Nat. Chem. 2015, 7, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hu, X.; Li, Y.; Zou, X.; Xiong, S.; Lin, C.; Shen, Y.; Wang, L. Multistimuli-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[6]arene and SAINT Complexation for Controllable Drug Release. J. Am. Chem. Soc. 2014, 136, 10762–10769. [Google Scholar] [CrossRef]
- He, H.; Feng, M.; Chen, Q.; Zhang, X.; Zhan, H. Light-Induced Reversible Self-Assembly of Gold Nanoparticles Surface-Immobilized with Coumarin Ligands. Angew. Chem. Int. Ed. 2016, 55, 936–940. [Google Scholar] [CrossRef]
- Zhang, J.; Whitesell, J.; Fox, M. Photoreactivity of Self-Assembled Monolayers of Azobenzene or Stilbene Derivatives Capped on Colloidal Gold Clusters. Chem. Mater. 2001, 13, 2323–2331. [Google Scholar] [CrossRef]
- Klajn, R. Immobilized Azobenzenes for the Construction of Photoresponsive Materials. Pure Appl. Chem. 2010, 82, 2247–2279. [Google Scholar] [CrossRef]
- Silvi, S.; Arduini, A.; Pochini, A.; Secchi, A.; Tomasulo, M.; Raymo, F.; Baroncini, M.; Credi, A. A Simple Molecular Machine Operated by Photoinduced Proton Transfer. J. Am. Chem. Soc. 2007, 129, 13378–13379. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, D.; Li, M.; Yang, F.; Li, Z.; An, W.; Jiang, S.; Zheng, X.; Niu, C.; Qu, D. An Acid-Base Responsive Linear-Cyclic Polymer Rotaxane Molecular Shuttle with Fluorescence Signal Output. Chin. Chem. Lett. 2022, 33, 1533–1536. [Google Scholar] [CrossRef]
- Tatum, L.; Foy, J.; Aprahamian, I. Waste Management of Chemically Activated Switches: Using a Photoacid to Eliminate Accumulation of Side Products. J. Am. Chem. Soc. 2014, 136, 17438–17441. [Google Scholar] [CrossRef]
- Xu, F.; Feringa, B.L. Photoresponsive Supramolecular Polymers: From Light-Controlled Small Molecules to Smart Materials. Adv. Mater. 2023, 35, 2204413. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.; Lee, H.; Qu, Z.; Banks, R.; Phillips, R.; Thomson, M. Controlling Organization and Forces in Active Matter through Optically Defined Boundaries. Nature. 2019, 572, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Duan, H.; Zhan, T.; Hu, X.; Kong, L.; Zhang, K. Light-Fueled Dissipative Self-Assembly at Molecular and Macro-Scale Enabled by a Visible-Light-Responsive Transient Hetero-Complementary Quadruple Hydrogen Bond. Chin. Chem. Lett. 2023, 34, 107639. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, C. Switchable Complexation between (O-Methyl)6-2,6-helic[6]arene and Protonated Pyridinium Salts Controlled by Acid/Base and Photoacid. Org. Lett. 2017, 19, 3175. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jia, F.; Cui, J.; Lu, S.; Jiang, W. Light-Controlled Switching of a Non-Photoresponsive Molecular Shuttle. Org. Lett. 2017, 19, 2945–2948. [Google Scholar] [CrossRef]
- Gong, B.; Shao, Z. Self-Assembling Organic Nanotubes with Precisely Defined, Sub-Nanometer Pores: Formation and Mass Transport Characteristics. Acc. Chem. Res. 2013, 46, 2856–2866. [Google Scholar] [CrossRef]
- Ong, W.; Zeng, H. Rapid Construction of Shape-Persistent H-bonded Macrocycles via One-pot H-bonding-Assisted Macrocyclization. J. Incl. Phenom. Mol. Recognit. Chem. 2013, 76, 1–11. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Hou, J.; Li, Z. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef]
- Wu, X.; Liu, R.; Sathyamoorthy, B.; Yamato, K.; Liang, G.; Shen, L.; Ma, S.; Sukumaran, D.K.; Szyperski, T.; Fang, W.; et al. Discrete Stacking of Aromatic Oligoamide Macrocycles. J. Am. Chem. Soc. 2015, 137, 5879–5882. [Google Scholar] [CrossRef]
- Wang, X.; Ji, J.; Liu, Z.; Cai, Y.; Tang, J.; Shi, Y.; Yang, C.; Yuan, L. Chiroptical Sensing of Amino Acid Derivatives by Host–Guest Complexation with Cyclo[6]aramide. Molecules 2021, 26, 4064. [Google Scholar] [CrossRef]
- Ren, C.; Maurizot, V.; Zhao, H.; Shen, J.; Zhou, F.; Ong, W.Q.; Du, Z.; Zhang, K.; Su, H.; Zeng, H. Five-Fold-Symmetric Macrocyclic Aromatic Pentamers: High-Affinity Cation Recognition, Ion-Pair-Induced Columnar Stacking, and Nanofibrillation. J. Am. Chem. Soc. 2011, 133, 13930–13933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, J.; Sun, C.; Ren, C.; Zeng, H. Intramolecularly Hydrogen-Bonded Aromatic Pentamers as Modularly Tunable Macrocyclic Receptors for Selective Recognition of Metal Ions. J. Am. Chem. Soc. 2015, 137, 12055–12063. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Ren, C.; Ye, R.; Sun, C.; Chiad, K.; Chen, X.; Li, Z.; Xue, F.; Su, H.; Chass, G.A.; et al. Persistently Folded Circular Aromatic Amide Pentamers Containing Modularly Tunable Cation-Binding Cavities with High Ion Selectivity. J. Am. Chem. Soc. 2010, 132, 9564–9566. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Shen, J.; Zeng, H. One-Pot Synthesis of Strained Macrocyclic Pyridone Hexamers and Their High Selectivity toward Cu2+ Recognition. Org. Lett. 2015, 17, 5946–5949. [Google Scholar] [CrossRef]
- Helsel, A.; Brown, A.; Yamato, K.; Feng, W.; Yuan, L.; Clements, A.; Harding, S.; Szabo, G.; Shao, Z.; Gong, B. Highly Conducting Transmembrane Pores Formed by Aromatic Oligoamide Macrocycles. J. Am. Chem. Soc. 2008, 130, 15784–15785. [Google Scholar] [CrossRef]
- Kang, K.; Lohrman, J.; Nagarajan, S.; Chen, L.; Deng, P.; Shen, X.; Fu, K.; Feng, W.; Johnson, D.; Yuan, L. Convergent Ditopic Receptors Enhance Anion Binding upon Alkali Metal Complexation for Catalyzing the Ritter Reaction. Org. Lett. 2019, 21, 652–655. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Chen, L.; Hu, J.; Wen, C.; Zheng, Q.; Wu, L.; Zeng, H.; Gong, B.; Yuan, L. Liquid-Crystalline Mesogens Based on Cyclo[6]aramides: Distinctive Phase Transitions in Response to Macrocyclic Host-Guest Interactions. Angew. Chem. Int. Ed. 2015, 54, 11147–11152. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Wu, J.; Mai, X.; Qin, S.; Zhou, Y.; Yuan, D.; Li, X.; Feng, W.; Yuan, L. A Molecular Sheaf: Doubly Threaded [6]Rotaxane. Chem. Commun. 2024, 60, 5622–5625. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, T.A.; Zhong, Y.; Miller, D.P.; McGrath, J.K.; Scalzo, C.T.; Redington, M.C.; Zurek, E.; Gong, B. Ultra-Tight Host-Guest Binding with Exceptionally Strong Positive Cooperativity. Angew. Chem. Int. Ed. 2022, 61, e202213467. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Z.; Liu, S.; Li, X.; Chen, R.; Ren, Y.; Feng, W.; Yuan, L. Cyclo[6]aramide-Tropylium Charge Transfer Complex as a Colorimetric Chemosensor for Differentiation of Intimate and Loose Ion Pairs. Org. Lett. 2015, 17, 5950–5953. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, Z.; Wang, L.; Chen, L.; Cai, Y.; Deng, P.; Feng, W.; Li, X.; Yuan, L. A Dynamic Hydrogen-Bonded Azo-Macrocycle for Precisely Photo-Controlled Molecular Encapsulation and Release. Angew. Chem. Int. Ed. 2019, 58, 12519–12523. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, T.A.; Zhong, Y.; Sánchez, B.L.S.; Kauffmann, B.; McGrath, J.K.; Scalzo, C.; Miller, D.P.; Huc, I.; Zurek, E.; Ferrand, Y.; et al. Stable Pseudo[3]rotaxanes with Strong Positive Binding Cooperativity based on Shape-Persistent Aromatic Oligoamide Macrocycles. Chem. Commun. 2021, 57, 11645–11648. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.; Kannekanti, V.; Ye, Z.; Yang, Z.; Chen, L.; Cai, Y.; Zhu, B.; Feng, W.; Yuan, L. Light-Controlled Switchable Complexation by a Non-Photoresponsive Hydrogen-Bonded Amide Macrocycle. Org. Chem. Front. 2020, 7, 846–855. [Google Scholar] [CrossRef]
- Han, Y.; Meng, Z.; Chen, C. Acid/base Controllable Complexation of A Triptycene-Derived Macrotricyclic Host and Protonated 4,4’-Bipyridinium/Pyridinium Salts. Chem. Commun. 2016, 52, 590–593. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Zhang, Y.; Liu, Y. Fluorescent Supramolecular Polypseudorotaxane Architectures with Ru(ii)/tri(bipyridine) Centers as Multifunctional DNA Reagents. Chem. Commun. 2015, 51, 16127–16130. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host-Guest Interactions. Adv. Mater. 2024, 36, 2304249. [Google Scholar] [CrossRef]
- Han, X.-N.; Zong, Q.-S.; Han, Y.; Chen, C.-F. Pagoda[5]arene with Large and Rigid Cavity for the Formation of 1:2 Host-Guest Complexes and Acid/Base-Responsive Crystalline Vapochromic Properties. CCS Chem. 2022, 4, 318–330. [Google Scholar] [CrossRef]
- Hou, X.; Chen, X.; Bisoyi, H.; Qi, Q.; Xu, T.; Chen, D.; Li, Q. Light-Driven Aqueous Dissipative Pseudorotaxanes with Tunable Fluorescence Enabling Deformable Nano-Assemblies. ACS Appl. Mater. Interfaces 2023, 15, 11004–11015. [Google Scholar] [CrossRef]
- Chen, X.; Feng, W.; Bisoyi, H.; Zhang, S.; Chen, X.; Yang, H.; Li, Q. Light-Activated Photodeformable Supramolecular Dissipative Self-Assemblies. Nat. Commun. 2022, 13, 3216. [Google Scholar] [CrossRef]
- Rosario, R.; Gust, D.; Hayes, M.; Jahnke, F.; Springer, J.; Garcia, A.A. Photon-Modulated Wettability Changes on Spiropyran-Coated Surfaces. Langmuir 2002, 18, 8062–8069. [Google Scholar] [CrossRef]
- Pradeepruban, J.; Kshama, K.; Pintu, K. Merocyanines of Non-Activated Spiropyrans: Generation and Spectrokinetic Studies. ChemistrySelect 2018, 3, 11065–11070. [Google Scholar]
- Xu, Y.; Fei, J.; Li, G.; Yuan, T.; Li, Y.; Wang, C.; Li, X.; Li, J. Enhanced Photophosphorylation of a Chloroplast-Entrapping Long-Lived Photoacid. Angew. Chem. Int. Ed. 2017, 56, 12903–12907. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.S.A.; Sainoo, Y.; Takaoka, T.; Waizumi, H.; Wang, Z.; Alam, M.I.; Ando, A.; Arafune, R.; Komeda, T. Chemistry of the Photoisomerization and Thermal Reset of Nitro-Spiropyran and Merocyanine Molecules on the Channel of the MoS2 Field Effect Transistor. Phys. Chem. Chem. Phys. 2021, 23, 27273–27281. [Google Scholar] [CrossRef] [PubMed]
- Périllat, V.J.; Berton, C.; Pezzato, C. The Effect of Temperature on the Photoacidity of Merocyanine Photoacids in Water. Mater. Today Chem. 2022, 25, 100918. [Google Scholar] [CrossRef]
- Jia, L.-M.; Tong, J.; Yu, S.-Y. Neutral Coordination Polymers of Cobalt(II), Copper(II), Zinc(II) and Manganese(II) β-diketonate Complexes with Fluorescent Anthracene Dipyridine: Synthesis, Structure and Luminescence Properties. J. Photoch. Photobiol. A 2018, 355, 84–93. [Google Scholar] [CrossRef]
- Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, B.; Mennucci, G.A.; Petersson, H.; et al. Gaussian 09, Revision, B.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Brynn, H.; Thordarson, P. The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Li, X.; Yuan, X.; Deng, P.; Chen, L.; Ren, Y.; Wang, C.; Wu, L.; Feng, W.; Gong, B.; Yuan, L. Macrocyclic Shape-Persistency of Cyclo[6]aramide Results in Enhanced Multipoint Recognition for the Highly Efficient Template-Directed Synthesis of Rotaxanes. Chem. Sci. 2017, 8, 2091–2100. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]

| Guest | CHCl3/CH3CN | K1 (M−1) | K2 (M−1) | Ka (M−2) | α b |
|---|---|---|---|---|---|
| G1·2H | 1:1 | (2.05 ± 0.18) × 104 | (1.30 ± 0.06) × 104 | (2.70 ± 0.06) × 108 | 2.5 |
| G2·2H | 1:1 | (1.61 ± 0.03) × 104 | (4.41 ± 0.25) × 104 | (7.10 ± 0.53) × 109 | 10.9 |
| G3·2H | 1:1 | (8.06 ± 0.17) × 102 | (9.53 ± 0.03) × 102 | (7.68 ± 0.18) × 105 | 4.7 |
| G4·2H | 1:1 | (1.81 ± 0.08) × 104 | (1.38 ± 0.04) × 104 | (2.50 ± 0.03) × 108 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Kothapalli, S.S.K.; Yang, Z.; Guo, X.; Li, X.; Cai, Y.; Feng, W.; Yuan, L. Light-Controlled Interconvertible Self-Assembly of Non-Photoresponsive Suprastructures. Molecules 2024, 29, 4842. https://doi.org/10.3390/molecules29204842
Yu W, Kothapalli SSK, Yang Z, Guo X, Li X, Cai Y, Feng W, Yuan L. Light-Controlled Interconvertible Self-Assembly of Non-Photoresponsive Suprastructures. Molecules. 2024; 29(20):4842. https://doi.org/10.3390/molecules29204842
Chicago/Turabian StyleYu, Wentao, Sudarshana Santhosh Kumar Kothapalli, Zhiyao Yang, Xuwen Guo, Xiaowei Li, Yimin Cai, Wen Feng, and Lihua Yuan. 2024. "Light-Controlled Interconvertible Self-Assembly of Non-Photoresponsive Suprastructures" Molecules 29, no. 20: 4842. https://doi.org/10.3390/molecules29204842
APA StyleYu, W., Kothapalli, S. S. K., Yang, Z., Guo, X., Li, X., Cai, Y., Feng, W., & Yuan, L. (2024). Light-Controlled Interconvertible Self-Assembly of Non-Photoresponsive Suprastructures. Molecules, 29(20), 4842. https://doi.org/10.3390/molecules29204842







