Abstract
A balanced inflammatory response is crucial for the organism to defend against external infections, however, an exaggerated response may lead to detrimental effects, including tissue damage and even the onset of disease. Therefore, anti-inflammatory drugs are essential for the rational control of inflammation. In this study, we found that a previously screened peptide TaY (KEKKEVVEYGPSSYGYG) was able to inhibit the LPS-induced RAW264.7 inflammatory response by decreasing a series of proinflammatory cytokines, such as TNF-α, IL-6, and nitric oxide (NO). To elucidate the underlying mechanism, we conducted further investigations. Western blot analysis showed that TaY reduced the phosphorylation of key proteins (IKK-α/β, IκB-α,NF-κB (P65)) in the TLR4-NF-κB signaling pathway and inhibited the inflammatory response. Furthermore, molecular docking and molecular dynamic simulations suggested that TaY binds to the hydrophobic pocket of MD2 through hydrogen bonding and hydrophobic interactions, potentially competing with LPS for MD2 binding. Collectively, TaY is a promising candidate for the development of novel therapeutic strategies against inflammatory disorders.
1. Introduction
Inflammation is an immune response of the organism in response to irritation or infection, which is an important mechanism to maintain health and prevent infection [1,2,3,4]. However, excessive inflammation can damage the organism and further produce various diseases: asthma, arthritis, and atherosclerosis [3,5,6]. Macrophages play an important role in the pathogenesis of these diseases [7,8]. Among the external substances that stimulate inflammation, the most important is LPS, a lipopolysaccharide from the cell wall of Gram-negative bacteria that activates the toll-like receptor (TLR) 4 signaling pathway, which, in turn, activates the body’s inflammatory response and a series of downstream events [9].
LPS induces TLR4 dimerization by binding to the hydrophobic structural domain of the myeloid differentiation protein-2 (MD2) [10], thereby transducing the signal into the cell. This process is carried out through the MYD88-dependent and TRIF-dependent pathways, respectively. MYD88-dependent signaling mainly activates the inhibitory kappa B kinase (IKK) and mitogen-activated protein kinases (MAPK) pathways [11]. IKKα, IKKβ, and IKKγ form a complex that catalyzes the phosphorylation of IκB protein, leading to IκB protein degradation and promoting nuclear transcription factor-κB (NF-κB) nuclear translocation, and the transfer of NF-κB to the nucleus to regulate a variety of downstream inflammatory factors (TNF-α, IL-1β, IL-6, NO) and related genes (cyclooxygenase-2, COX-2; inducible nitric oxide synthase, iNOS) expression [11,12].
Therefore, developing anti-inflammatory drugs that target key proteins is very effective in the inflammatory pathway. Currently, the commonly used anti-inflammatory drugs are mainly non-steroidal anti-inflammatory drugs (NSAIDs) [13], which work by inhibiting COX which is the key enzyme responsible for the synthesis of prostaglandins and messenger molecules during inflammation [14,15]. However, the major issue with NSAIDS is the toxic side effects associated with long-term use [16,17,18]. Therefore, developing new anti-inflammatory drugs with high efficacy and low toxicity has become a topic of great interest.
Peptide drugs combine the advantages of small chemical molecules (<500 Da) and protein drugs (>5000 Da) because of their molecular weight [19]. Their small molecular weight allows them to be synthesized using chemical methods, which offer precise structure, easy quality control, and low production cost. Additionally, their therapeutic effects are similar to those of protein drugs, which have the advantages of low toxicity and high specificity [20,21]. These make peptide drug highly advantageous in the development of anti-inflammatory treatments.
In the previous study, we screened a peptide TaY (KEKKEVVEYGPSSYGYG), which demonstrated excellent anti-inflammatory activity by luciferase-based reporter gene cell screening. We utilized the RAW-NF-κB luciferase reporter gene to screen for compounds that can stimulate cellular immune modulation. In this process, we found that, although TaY showed a weak stimulatory effect on the signaling pathway, it significantly reduced the increase in luciferase activity induced by LPS stimulation (unpublished data). In order to investigate the anti-inflammatory mechanism of TaY, this study was conducted using the LPS-induced macrophage RAW264.7 inflammation model to reveal the molecular anti-inflammatory mechanisms of TaY.
2. Results and Discussion
Inflammation is a bioprotective response to microbial invasion that can cause cellular damage, in which pro-inflammatory mediators are released to promote disease symptoms [1,2]. LPS-mediated inflammation is the most common [22]. Macrophages, as an important immune cell type that responds to inflammation, produce a series of pro-inflammatory mediators including TNF-α, IL-6, IL-1β, NO, and PGE2 [7,23]. Excessive production of these pro-inflammatory mediators can damage local cells and further aggravate symptoms [24,25]. Therefore, inhibiting the production of pro-inflammatory mediators is an effective strategy to suppress inflammation.
2.1. TaY Reduced the Expression of Pro-Inflammatory Cytokines in LPS-Induced RAW264.7 Inflammation
In this study, we first analyzed the toxicity of TaY on RAW264.7, and as shown in the Figure 1a, TaY was not significantly toxic to RAW264.7 in the concentration range of 20–1000 μg/mL. Nitric oxide, as an important marker of the inflammatory response, plays a very important role in several inflammatory diseases [26], and its ease of detection makes it a frequently preferred assay in the screening of anti-inflammatory drugs [27,28]. So we first analyzed the level of TaY to reduce LPS-induced NO production. As predicted, 100 ng/mL of LPS-stimulated cells resulted in a significant increase in NO levels, and the results showed that TaY’s ability to reduce NO levels exhibited a dose-dependent enhancement, which was almost the same level as that of the control group at 100 ng/μL (Figure 1b). These results demonstrated that TaY does, indeed, possess a strong anti-inflammatory ability. Additionally, we simultaneously examined the expression of iNOS, a major NO-producing gene. The results showed that TaY reduced the LPS-induced elevation of iNOS protein at both the transcriptional and protein levels (Figure 1c–e).
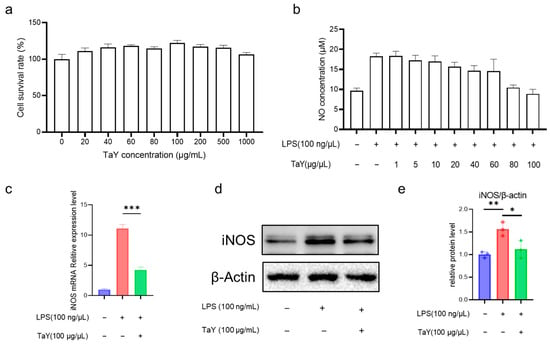
Figure 1.
TaY reduces the NO level of LPS-induced RAW264.7 inflammation. (a) Cytotoxicity assay of TaY on RAW264.7; (b) effect of different concentrations of TaY on NO production; (c) TaY reduces the mRNA expression level of iNOS; (d) Western blot results of TaY reducing the expression of iNOS proteins, β-actin as the reference protein; (e) grayscale analysis of Western blot results. The data are presented as the mean ± SD (n = 3). NS, p > 0.05; * p ≤ 0.05; ** p ≤ 0.01; and *** p ≤ 0.001.
The anti-inflammatory capacity of TaY was initially determined by the detection of NO, as well as the inducible enzyme, but in addition to NO, LPS stimulation caused an increase in the expression levels of several inflammatory factors (TNF-α, IL-6, and IL-1β) [11,12]. To further characterize the anti-inflammatory activity of TaY, we also evaluated the expression levels of the major pro-inflammatory cytokines TNF-α and IL-6 in RAW264.7 (Figure 2). As expected, the results were similar to those of NO and iNOS. TaY treatment was effective at decreasing the expression levels of pro-inflammatory factors, both at the transcriptional and protein levels (Figure 2).
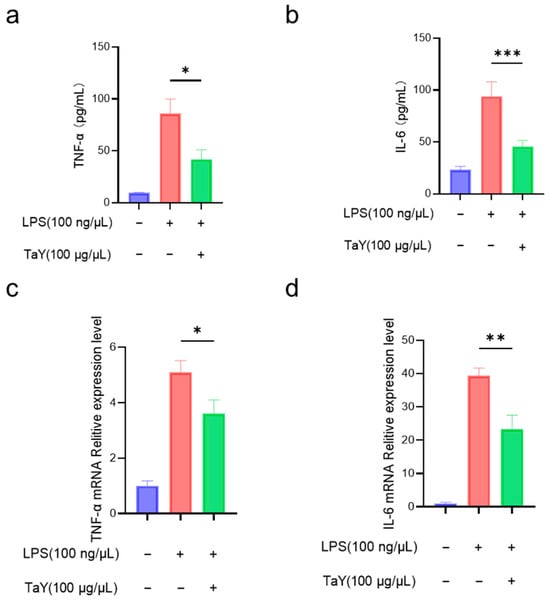
Figure 2.
TaY reduces the proinflammatory cytokine of LPS-induced RAW 264.7 inflammation; (a,b) ELISA results of TNF-α and IL-6; (c,d) RT-PCR results of TNF-α and IL-6. The data are presented as the mean ± SD (n = 3). * p ≤ 0.05; ** p ≤ 0.01; and *** p ≤ 0.001.
Based on the level of reduction of LPS-induced pro-inflammatory mediators by TaY, its anti-inflammatory ability is quite strong, and peptide anti-inflammatory agents have been reported in many cases, but most of them do not have that strong anti-inflammatory activity compared to TaY. For example, Histatin-1 roughly reduces the level of LPS-produced NO by half [29], whereas TaY in the present study at 100 ng/μL could almost completely bring the level of NO production to the same level as the control (Figure 1b). This remarkable efficacy not only positions TaY favorably among peptide anti-inflammatory drugs, but it is also relatively rare among the remaining types of anti-inflammatory drugs reported [28,30,31].
2.2. TaY Inhibited the LPS-Activated TLR4-NF-κB Signaling Pathway
NF-κB, as a transcription factor, plays a crucial role in the expression of genes related to inflammation and immunity [32]. The TLR4-NF-κB signaling pathway is activated following LPS-induced inflammation in RAW264.7 [33]. TLR4 first activates MYD88, which recruits a series of downstream protein kinases to activate IKK. IKK then promotes the phosphorylation, ubiquitination, and subsequent protease-mediated degradation of IκB, allowing NF-κB to be released and translocated to the nucleus, where it functions as a transcription factor that promotes the transcription of a range of inflammation-related genes (iNOS, COX-2, TNF-α, IL-1β, and IL-6) [34,35].
In the present study, we demonstrated that TaY reduced several inflammatory factors (TNF-α, IL-6, NO) in the LPS-induced inflammatory response. Next, we aimed to analyze the role of TaY in the TLR4-NF-κB signaling pathway. We first examined the protein expression levels in this pathway using Western blot. Previous studies have shown that LPS-induced NF-κB signaling is a dynamic process [36], so we first examined the dynamics of several key proteins in the NF-κB signaling pathway using our cells. The results indicate that iNOS expression increases over time, while p-IκBα expression peaks at 6 h and then begins to degrade. The expression of p-IKKα/β shows an increase from 3 h to 6 h, after which it is nearly indistinguishable from the control group. Similarly, p-p65 expression is also highest at 6 h. Based on the expression patterns of these proteins, we selected a 6 h LPS treatment duration for our formal experiments, and the results were shown in Figure 3. The results showed that TaY treatment resulted in a significant reduction in the phosphorylation levels of IKK-α/β (Figure 3b), followed by a decrease in the phosphorylation level of IκBα compared to the LPS-treated group (Figure 3c). This ultimately led to a reduction in the phosphorylation of P65 and decreased NF-κB entry into the nucleus (Figure 3d). These findings indicate a reduction in proinflammatory factors, including TNF-α, IL-6, and NO.
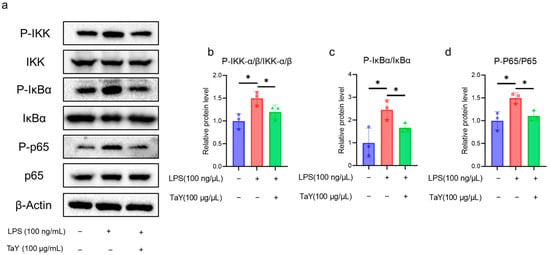
Figure 3.
TaY inhibits LPS activation of the TLR4/NF-κB signaling pathway. (a) Western blot results for IKK, IκBα, P65 proteins, and their phosphorylated forms, with β-Actin as the reference protein; (b–d) grayscale quantitative results for the proteins of P-IKK-α/β/IKK-α/β, P-IκBα/IκBα, and P-P65/P65, respectively. The data are presented as the mean ± SD (n = 3). * p ≤ 0.05.
Through our Western blot experiments, we determined that TaY exerts its anti-inflammatory activity by inhibiting the TLR4-NF-κB signaling pathway. NF-κB is a critical node in the development of anti-inflammatory drugs, as activated NF-κB translocates to the nucleus to activate the expression of multiple inflammatory genes [34,35,37]. In this study, TaY achieves its anti-inflammatory function by regulating the TLR4-NF-κB signaling pathway. This mechanism is similar to that of another peptide, STM28, which inhibits downstream signaling by binding to TLR4 to exert anti-inflammatory activity [38]. Additionally, it has also been shown that peptide can exert its anti-inflammatory effects through the AMPK signaling pathway [29,39].
2.3. TaY Exerts Its Anti-Inflammatory Function by Binding to the MD2 Hydrophobic Pocket
In the previous study, we demonstrated that TaY exerts its anti-inflammatory function by inhibiting the activation of the TLR4-NF-κB signaling pathway, however, the specific mechanism by which TaY inhibits this signaling remains unknown. Peptide anti-inflammatory pathways have been reported to neutralize LPS and block signaling by inhibiting TLR4 and its ligands [40,41,42,43]. For example, LL-37, an antimicrobial peptide identified in the human body, exerts its anti-inflammatory effects by neutralizing LPS [44,45], while LK2(6)A(L), a peptide derived from the skin secretions of Chinese brown frog Rana chensinensis, can alleviate LPS-induced acute lung injury in mice by binding to MD2 [46]. The MD2 protein is essential for LPS function, as LPS must bind to the hydrophobic pocket of MD2 to activate TLR4 [47]. MD2-deficient mice are unresponsive to LPS stimulation, and some synthetic LPS-like chemicals, such as Eritoran, have been shown to inhibit LPS-induced inflammation [48,49].
To determine whether TaY also exerts its anti-inflammatory function by competitively binding to MD2, we conducted molecular docking and molecular dynamics simulation to investigate the interaction between MD2 and TaY (Figure 4). The complex trajectory analysis showed a stable RMSD value at 5 Å after approximately 100 ns (Figure 4a), and the Rg value also stabilized at around 1.65 nm after the same duration (Figure 4b). We analyzed key interaction parameters between TaY and MD2, including electrostatic interactions, hydrogen bonds, and hydrophobic interactions (Figure 5b,c, Table 1). Current reports suggest that small molecules bind to residues Leu78, Asn86, Arg90, Glu92, Tyr102, Ser120, Phe121, Lys122, Ile124 of MD2 [47,49,50,51,52,53,54]. Our molecular docking results revealed hydrogen bonds between Lys1, Glu2, Lys4, Glu8, Ser13 of TaY and Pro88, Arg90, Lys91, Glu92, Lys128 of MD2. While Arg90, and Glu92 have been previously examined. Our findings indicate that Pro88, Lys91, and Lys128 also play significant roles in MD2 activation.
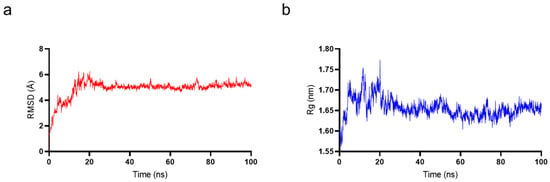
Figure 4.
Molecular dynamics simulation results of TaY and MD2. (a) The root mean square deviation (RMSD) value for MD2 and TaY. (b) The radius of gyration (Rg) value for MD2 and TaY.
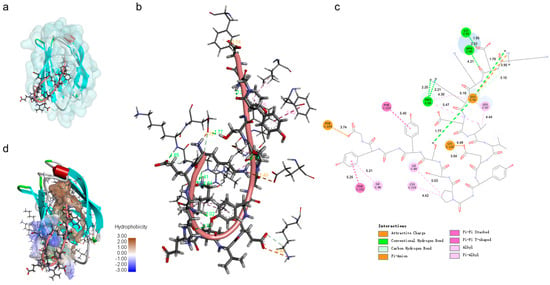
Figure 5.
TaY can bind to the hydrophobic pocket binding domains of MD2. The overall 3D conformation (a), local interaction 3D conformation (b), and 2D conformation (c) of TaY docked to MD2 (d). The hydrophobic interaction surface demonstrating the results of the docking of TaY to MD2.

Table 1.
Interactions between TaY and MD2.
The binding of TaY to MD2 predominantly involves residues 1–9. Previous studies have shown that small molecules are crucial for hydrogen bond formation with residues located on the exterior of the hydrophobic pocket when binding to MD2 [49,55]. Our results similarly indicate that the portion of TaY forming hydrogen bonds with MD2 is primarily concentrated at the MD2 pocket site (Figure 5a,d). Additionally, residues 10–17 of TaY formed strong hydrophobic interactions with the hydrophobic core of the MD2 hydrophobic pocket (TaY: Pro11, Try14, Try16) (Figure 5c, Table 1). These hydrophobic interactions enhanced the binding of TaY to MD2, giving TaY a competitive advantage over LPS in binding to the MD2 hydrophobic pocket. However, these results are based on simulations and we cannot provide a definitive conclusion that TaY exerts its anti-inflammatory activity by binding to MD2. In the future, we still need to validate the interactions between TaY and MD2 based on the results of molecular docking in the experiment.
3. Materials and Methods
3.1. Synthesis of Peptides
Peptides were synthesized using the standard Fmoc solid-phase method by GL Biochem Ltd. (Shanghai, China). The purity of the peptides was 95%, and their relative masses were determined by MALDI-TOF-MS.
3.2. Cell Culture
Mouse macrophages (RAW264.7) were purchased from the Shanghai Cell Bank, Institute of Cell Biology, Chinese Academy of Sciences, and cultured in Duchenne’s Modified Eagle’s Medium (DMEM; HyClone, Utah, USA). The DMEM was supplemented with 10% (v/v) fetal bovine serum (Procell, Wuhan, China) and 1% (v/v) penicillin/streptomycin (Solarbio, Beijing, China), and incubated at 37 °C in a humidified environment (5% CO2, 95% air).
3.3. Cytotoxicity Analysis
The toxicity of TaY on RAW264.7 cells was assessed using the CCK-8 kit. RAW264.7 cells were seeded into 96-well plates at a density of 3.0 × 105 cells per well and cultured for 12 h. After 12 h, the cells were treated with different concentrations of peptides (0, 20, 40, 60, 80, 100, 200, 500, and 1000 μg/mL) for 24 h. Subsequently, 10 μL of CCK-8 solution was added to each well according to the kit instructions and the cells were incubated for 2 h at 37 °C in the dark. The absorbance of the reaction solution was measured at 450 nm, and cell viability was calculated using the following formula:
AS is the absorbance of the well containing cells, CCK-8, and peptides; AB is the absorbance of the well containing medium and CCK-8, but without cells; and AC is the absorbance of the well containing cells and CCK-8, but without peptides.
3.4. Determination of Nitric Oxide Content
The LPS-induced inflammatory macrophage model was performed according to our previously described methodology [56]. LPS from E. coli 0111:B4 was purchased from Sigma-Aldrich (St. Louis, MO, USA). RAW264.7 cells were cultured in 96-well plates at a density of 3.0 × 105 cells per well for 12 h. After 12 h of incubation, different concentrations of peptides (0, 1, 5, 10, 20, 40, 60, 80, and 100 μg/mL) were added to each well and incubated for another 3 h. Then, LPS was added to each well at a final concentration of 100 ng/mL and incubated for 24 h. The cell culture supernatants were used for the NO assay. NO levels were quantified using Griess reagents according to the manufacturer’s instructions (Beyotime, Beijing, China). Specifically, 50 μL of Griess A and 50 μL of Griess B were sequentially added to 50 μL of culture medium supernatant, and the absorbance of the reaction mixture was measured at 540 nm.
3.5. Enzyme-Linked Immunosorbent Assay (ELISA)
RAW 264.7 cells were cultured in 6-well plates at a density of 2.0 × 106 cells per well for 12 h. After 12 h of incubation, 100 μg/mL of TaY was added to each well and incubated for another 3 h. Then, LPS was added to each well at a final concentration of 100 ng/mL and incubated for 24 h. After 24 h of incubation with LPS, cell supernatants were collected and then assayed using ELISA kits (Solarbio, Beijing, China) for IL-6, and TNF-α according to the manufacturer’s instructions.
3.6. RNA Isolation and Quantitative Real-Time PCR
RAW 264.7 cells were cultured in 6-well plates at a density of 2.0 × 106 cells per well for 12 h. After 12 h of incubation, 100 μg/mL of TaY was added to each well and incubated for another 3 h. Then, LPS was added to each well at a final concentration of 100 ng/mL and incubated for 24 h. The cell pellets were the collected for RNA isolation. RNA isolation: total RNA was isolated using TRIzol reagent (Solarbio, Beijing, China), and the RNA integrity of all samples was assessed by agarose gel electrophoresis and NanoDrop. All cDNAs were synthesized using the HiScript® III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the manufacturer’s instructions. Table 2 lists the primers used in this study. A two-step amplification method was used in this study: preincubation at 95 °C for 30 s, denaturation at 95 °C for 5 s, and extension at 60 °C for 30 s for a total of 40 cycles, followed by 95 °C for 5 s, 60 °C for 60 s, and melting at 95 °C for 1 s for a total of 1 cycle. The gene expression was normalized by comparing the expression of the target gene with that of the housekeeping gene β-actin. Results are reported as fold increase in gene expression relative to control samples.

Table 2.
Sequences of the primers used for RT-PCR assays.
3.7. Western Blot Analysis
RAW 264.7 cells were cultured in 6-well plates at a density of 2.0 × 106 cells per well for 12 h. After 12 h of incubation, 100 μg/mL of TaY were added to each well and incubated for another 3 h. Then, LPS was added to each well at a final concentration of 100 ng/mL and incubated for 6 h. The cell pellets were then collected for protein extraction. Protein extraction was performed using RIPA, with protease inhibitors added proportionally, and protein concentrations were determined using the BCA method. Protein electrophoresis was performed using Tris-Gly SDS-PAGE, followed by membrane transfer using PVDF membranes. The membranes were blocket with 5% skim milk. The primary antibody for the target protein was incubated overnight. Then HRP-coupled secondary antibody was used to bind to the primary antibody, and the target protein was visulalized using a chemiluminescence visualizer. The following antibody were used in this study: p65 (1:5000), p-p65 (1:2000), IκB-α (1:5000), p-IκB-α (1:2000), IKK-α/β (1:5000), β-actin (1:10000) were purchased from Abmart (Shanghai, China); iNOS (1:2000) was purchased from proteintech (Wuhan, Hubei, China), p-IKK-α/β (1:2000) was purchased from Cell Signaling Technology (Beverly, MA, USA). The secondary antibody HRP-Goat Anti-Rabbit IgG (1:5000) was purchased from huaixngbio (Beijing, China).
3.8. Molecular Docking
The three-dimensional (3D) structure of the hybrid peptide TaY was built using PEPFOLD3.5 (https://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD3/, accessed on 1 December 2016) [57]. The crystal structure of MD2 was obtained from Protein Data Bank (PDB ID: 2Z64). First, global rigid docking of TaY and MD2 was performed using HPEPDOCK (http://huanglab.phys.hust.edu.cn/hpepdock/, accessed on 2 July 2018) [58]. Local docking conformations were then optimized at the local server using ROSETTA’s FlexPepDock (http://flexpepdock.furmanlab.cs.huji.ac.il/, accessed on 27 May 2011), and the best docking conformations were selected based on scores [59]. Docking results were visualized using Discovery Studio 2021 and the interaction forces between the complex were analyzed.
3.9. Molecular Dynamics (MD) Simulations
GROMACS 2020.6 software was used to perform MD simulations of the YTP-TLR2 complex with the AMBER99SB-ILDN force field [60]. The complex was placed centrally in a dodecahedron box with a size of 1.2 nm and dissolved in water [46]. Na+/Cl− ions were added to maintain the system in a neutral environment [46]. Energy minimization was performed using the steepest descent algorithm and continued until the maximum force was less than 1000 kJ/mol/nm, with a step size of 0.01 [61]. NVT and NPT ensembles were simulated using the leap-frog algorithm for 1 ns, with the temperature and pressure set to 310 K and 1 bar, respectively [62]. Finally, a 200 ns MD simulation was performed. After the stimulation, the trajectory file was analyzed using GROMACS, and the root mean square deviation (RMSD) and radius of gyration (Rg) of the system were obtained.
3.10. Statistical Analysis
Statistical analysis was performed using GraphPad Prism v9.0. Student’s t-test was used for statistical comparisons. All data are expressed as mean ± SD of at least three independent experiments. Significance was claimed with p values ≤ 0.05. NS: p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001.
4. Conclusions
In conclusion, our results suggest that TaY has promising potential as a peptide anti-inflammatory agent, which can reduce the activation of the TLR4-NF-κB inflammatory signaling pathway by LPS through the competitive binding of MD2 protein to LPS. The binding of TaY to MD2 reduces the phosphorylation levels of key proteins in the TLR4-NF-κB signaling pathway (IKK-α/β, IκBα), leading to decrease NF-κB nuclear translocation and ultimately a reduction in the expression of inflammatory factors (TNF-α, IL-6, NO, iNOS). These results demonstrate the potential of this peptide as a novel anti-inflammatory drug. In the future, we need to study the targets of TaY and MD2 to gain a deeper understanding of the anti-inflammatory mechanism of TaY.
Author Contributions
Conceptualization, J.W., R.Z. and X.W.; data curation, Y.Z.; formal analysis, J.W.; funding acquisition, X.W., D.S. and R.Z.; investigation, Y.T. and Z.L.; methodology, J.W., Y.Z. and J.Z.; project administration, X.W., D.S. and R.Z.; resources, Y.T.; software, J.Z.; validation, S.C. and H.Z.; visualization, J.W. and X.Z.; writing—original draft, J.W.; writing—review and editing, J.W., Y.Z., J.Z., Z.A. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R & D projects of China (No. 2022YFD1300700 and 2022YFD1300903), the National Natural Science Foundation of China (No. 32402776), and the China Agricultural University-Jilin Xide Agricultural and Animal Husbandry Company Science and Technology Cooperation Program (No. 202405510410698).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The MD simulations in this work were supported by the High-Performance Computing Platform of the China Agricultural University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wolkowicz, P.; White, C.R.; Anantharamaiah, G.M. Apolipoprotein Mimetic Peptides: An Emerging Therapy against Diabetic Inflammation and Dyslipidemia. Biomolecules 2021, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Montgomery, K.E. Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancers 2012, 4, 618–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, M.; Xu, S.; Zhang, J.; Wang, Z.; Mao, X.; Yao, X.; Liu, C. Effect of luteolin on inflammatory responses in RAW264.7 macrophages activated with LPS and IFN-γ. J. Funct. Foods 2017, 32, 123–130. [Google Scholar] [CrossRef]
- Amir, M.; Somakala, K.; Ali, S. p38 MAP kinase inhibitors as anti inflammatory agents. Mini Rev. Med. Chem. 2013, 13, 2082–2096. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, S.; Lou, Z.; Gao, J. The macrophage polarization in inflammatory dermatosis and its potential drug candidates. Biomed. Pharmacother. 2023, 161, 114469. [Google Scholar] [CrossRef]
- Tang, D.; Cao, F.; Yan, C.; Fang, K.; Ma, J.; Gao, L.; Sun, B.; Wang, G. Extracellular Vesicle/Macrophage Axis: Potential Targets for Inflammatory Disease Intervention. Front. Immunol. 2022, 13, 705472. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, N.M.G.P.; Oliveira, L.S.; Gomes, M.T.R.; Carneiro, M.B.H.; Vieira, L.Q.; Oliveira, S.C.; Horta, M.F. Requirement of scavenger receptors for activation of the IRF-3/IFN-β/STAT-1 pathway in TLR4-mediated production of NO by LPS-activated macrophages. Nitric Oxide 2023, 134–135, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Miranda, G.M.; Santos, V.; Bessa, J.R.; Teles, Y.C.F.; Yahouédéhou, S.; Goncalves, M.S.; Ribeiro-Filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37s–42s. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef]
- Karthik, C.S.; Manukumar, H.M.; Ananda, A.P.; Nagashree, S.; Rakesh, K.P.; Mallesha, L.; Qin, H.L.; Umesha, S.; Mallu, P.; Krishnamurthy, N.B. Synthesis of novel benzodioxane midst piperazine moiety decorated chitosan silver nanoparticle against biohazard pathogens and as potential anti-inflammatory candidate: A molecular docking studies. Int. J. Biol. Macromol. 2018, 108, 489–502. [Google Scholar] [CrossRef]
- Wang, S.M.; Zha, G.F.; Rakesh, K.P.; Darshini, N.; Shubhavathi, T.; Vivek, H.K.; Mallesha, N.; Qin, H.L. Synthesis of benzo[d]thiazole-hydrazone analogues: Molecular docking and SAR studies of potential H(+)/K(+) ATPase inhibitors and anti-inflammatory agents. MedChemComm 2017, 8, 1173–1189. [Google Scholar] [CrossRef]
- Li, C.; Sridhara, M.B.; Rakesh, K.P.; Vivek, H.K.; Manukumar, H.M.; Shantharam, C.S.; Qin, H.L. Multi-targeted dihydrazones as potent biotherapeutics. Bioorg. Chem. 2018, 81, 389–395. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef]
- Otvos, L. The latest trends in peptide drug discovery and future challenges. Expert Opin. Drug Discov. 2024, 19, 869–872. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Ranasinghe, P.; Gunasekara, U.; Jeon, Y.J. Antioxidant and anti-inflammatory functionality of ten Sri Lankan seaweed extracts obtained by carbohydrase assisted extraction. Food Sci. Biotechnol. 2018, 27, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Nagatake, T.; Saika, A.; Kawai, S.; Node, E.; Hosomi, K.; Kunisawa, J. Induction of unique macrophage subset by simultaneous stimulation with LPS and IL-4. Front. Immunol. 2023, 14, 1111729. [Google Scholar] [CrossRef] [PubMed]
- Coveney, S.; Murphy, S.; Belton, O.; Cassidy, T.; Crowe, M.; Dolan, E.; de Gaetano, M.; Harbison, J.; Horgan, G.; Marnane, M.; et al. Inflammatory cytokines, high-sensitivity C-reactive protein, and risk of one-year vascular events, death, and poor functional outcome after stroke and transient ischemic attack. Int. J. Stroke 2022, 17, 163–171. [Google Scholar] [CrossRef]
- Chen, J.; Xuan, J.; Gu, Y.T.; Shi, K.S.; Xie, J.J.; Chen, J.X.; Zheng, Z.M.; Chen, Y.; Chen, X.B.; Wu, Y.S.; et al. Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomed. Pharmacother. 2017, 91, 208–219. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Carrillo-Perdomo, E.; Aller, A.; Giampieri, F.; Gasparrini, M.; González-Pérez, L.; Beltrán-Ayala, P.; Battino, M. Anti-inflammatory effect of Capuli cherry against LPS-induced cytotoxic damage in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 46–52. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Q.C.; Ren, Y.; Zhao, L.; Yang, F.; Zhang, Y.; Zhao, W.J.; Tan, Y.Z.; Shen, X.F. Ajudecumin A from Ajuga ovalifolia var. calantha exhibits anti-inflammatory activity in lipopolysaccharide-activated RAW264.7 murine macrophages and animal models of acute inflammation. Pharm. Biol. 2018, 56, 649–657. [Google Scholar] [CrossRef]
- Lee, S.M.; Son, K.N.; Shah, D.; Ali, M.; Balasubramaniam, A.; Shukla, D.; Aakalu, V.K. Histatin-1 Attenuates LPS-Induced Inflammatory Signaling in RAW264.7 Macrophages. Int. J. Mol. Sci. 2021, 22, 7856. [Google Scholar] [CrossRef]
- Marques, R.V.; Sestito, S.E.; Bourgaud, F.; Miguel, S.; Cailotto, F.; Reboul, P.; Jouzeau, J.Y.; Rahuel-Clermont, S.; Boschi-Muller, S.; Simonsen, H.T.; et al. Anti-Inflammatory Activity of Bryophytes Extracts in LPS-Stimulated RAW264.7 Murine Macrophages. Molecules 2022, 27, 1940. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg. Chem. 2017, 70, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Akashi, S.; Saitoh, S.; Wakabayashi, Y.; Kikuchi, T.; Takamura, N.; Nagai, Y.; Kusumoto, Y.; Fukase, K.; Kusumoto, S.; Adachi, Y.; et al. Lipopolysaccharide interaction with cell surface toll-like receptor 4-MD-2: Higher affinity than that with MD-2 or CD14. J. Exp. Med. 2003, 198, 1035–1042. [Google Scholar] [CrossRef]
- Zeng, K.W.; Zhang, T.; Fu, H.; Liu, G.X.; Wang, X.M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur. J. Pharmacol. 2012, 692, 29–37. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, J.; Zhu, S.; Zhao, J.; Ye, Q.; Xu, Y.; Dong, H.; Zheng, X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef]
- Covert, M.W.; Leung, T.H.; Gaston, J.E.; Baltimore, D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science 2005, 309, 1854–1857. [Google Scholar] [CrossRef]
- Durand, J.K.; Baldwin, A.S. Targeting IKK and NF-κB for Therapy. Adv. Protein Chem. Struct. Biol. 2017, 107, 77–115. [Google Scholar] [CrossRef]
- Sugiyama, K.; Muroi, M.; Tanamoto, K. A novel TLR4-binding peptide that inhibits LPS-induced activation of NF-κB and in vivo toxicity. Eur. J. Pharmacol. 2008, 594, 152–156. [Google Scholar] [CrossRef]
- Qin, X.; Liu, Z.; Nong, K.; Fang, X.; Chen, W.; Zhang, B.; Wu, Y.; Wang, Z.; Shi, H.; Wang, X.; et al. Porcine-derived antimicrobial peptide PR39 alleviates DSS-induced colitis via the NF-κB/MAPK pathway. Int. Immunopharmacol. 2024, 127, 111385. [Google Scholar] [CrossRef]
- Sharp, C.R.; DeClue, A.E.; Haak, C.E.; Honaker, A.R.; Reinero, C.R. Evaluation of the anti-endotoxin effects of polymyxin B in a feline model of endotoxemia. J. Feline Med. Surg. 2010, 12, 278–285. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.T.; Trujillo-Paez, J.V.; Umehara, Y.; Yue, H.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of Antimicrobial Peptides in Skin Barrier Repair in Individuals with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 7607. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Blencke, H.M.; Cheng, H.; Li, C. The antimicrobial effect of CEN1HC-Br against Propionibacterium acnes and its therapeutic and anti-inflammatory effects on acne vulgaris. Peptides 2018, 99, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Hosoda, H.; Nakamura, K.; Hu, Z.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Tabe, Y.; Nagaoaka, I. Antimicrobial cathelicidin peptide LL-37 induces NET formation and suppresses the inflammatory response in a mouse septic model. Mol Med. Rep. 2017, 16, 5618–5626. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Sun, C.; Shang, D. The antimicrobial peptide LK2(6)A(L) exhibits anti-inflammatory activity by binding to the myeloid differentiation 2 domain and protects against LPS-induced acute lung injury in mice. Bioorg. Chem. 2023, 132, 106376. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Schenk, M.; Lagovsky, I.; Illig, D.; Walz, C.; Rohlfs, M.; Conca, R.; Muise, A.M.; Snapper, S.B.; et al. Human MD2 deficiency-an inborn error of immunity with pleiotropic features. J. Allergy Clin. Immunol. 2023, 151, 791–796.e7. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, B.S.; Kim, J.I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef]
- Roh, E.; Lee, H.S.; Kwak, J.A.; Hong, J.T.; Nam, S.Y.; Jung, S.H.; Lee, J.Y.; Kim, N.D.; Han, S.B.; Kim, Y. MD-2 as the target of nonlipid chalcone in the inhibition of endotoxin LPS-induced TLR4 activity. J. Infect. Dis. 2011, 203, 1012–1020. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, X.; Chen, G.; Jiang, L.; Wang, Z.; Fang, Q.; Liu, X.; Wang, J.; Zhang, Y.; Wu, W.; et al. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br. J. Pharmacol. 2015, 172, 4391–4405. [Google Scholar] [CrossRef] [PubMed]
- Gradisar, H.; Keber, M.M.; Pristovsek, P.; Jerala, R. MD-2 as the target of curcumin in the inhibition of response to LPS. J. Leukoc. Biol. 2007, 82, 968–974. [Google Scholar] [CrossRef]
- Peluso, M.R.; Miranda, C.L.; Hobbs, D.J.; Proteau, R.R.; Stevens, J.F. Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: Structure-activity relationships and in silico binding to myeloid differentiation protein-2 (MD-2). Planta Med. 2010, 76, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Liu, Y.; Xiang, X.; Dong, Z. Paclitaxel ameliorates lipopolysaccharide-induced kidney injury by binding myeloid differentiation protein-2 to block Toll-like receptor 4-mediated nuclear factor-κB activation and cytokine production. J. Pharmacol. Exp. Ther. 2013, 345, 69–75. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Chen, L.; Liu, X.; Fu, W.; Zhang, Y.; Li, C.; Liang, G.; Cai, Y. Insights into the binding mode of curcumin to MD-2: Studies from molecular docking, molecular dynamics simulations and experimental assessments. Mol. Biosyst. 2015, 11, 1933–1938. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X.; et al. Optimization of Exopolysaccharide Produced by Lactobacillus plantarum R301 and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef]
- Lamiable, A.; Thevenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tuffery, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.Y. HPEPDOCK: A web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Raveh, B.; London, N.; Schueler-Furman, O. Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins 2010, 78, 2029–2040. [Google Scholar] [CrossRef]
- Georgoulia, P.S.; Glykos, N.M. Molecular simulation of peptides coming of age: Accurate prediction of folding, dynamics and structures. Arch. Biochem. Biophys. 2019, 664, 76–88. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Shuian, D.; Liu, J.; Zhao, W. Identification and Molecular Mechanism of Novel Immunomodulatory Peptides from Gelatin Hydrolysates: Molecular Docking, Dynamic Simulation, and Cell Experiments. J. Agric. Food Chem. 2023, 71, 2924–2934. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).