Formulating Electrolytes for 4.6 V Anode-Free Lithium Metal Batteries
Abstract
1. Introduction
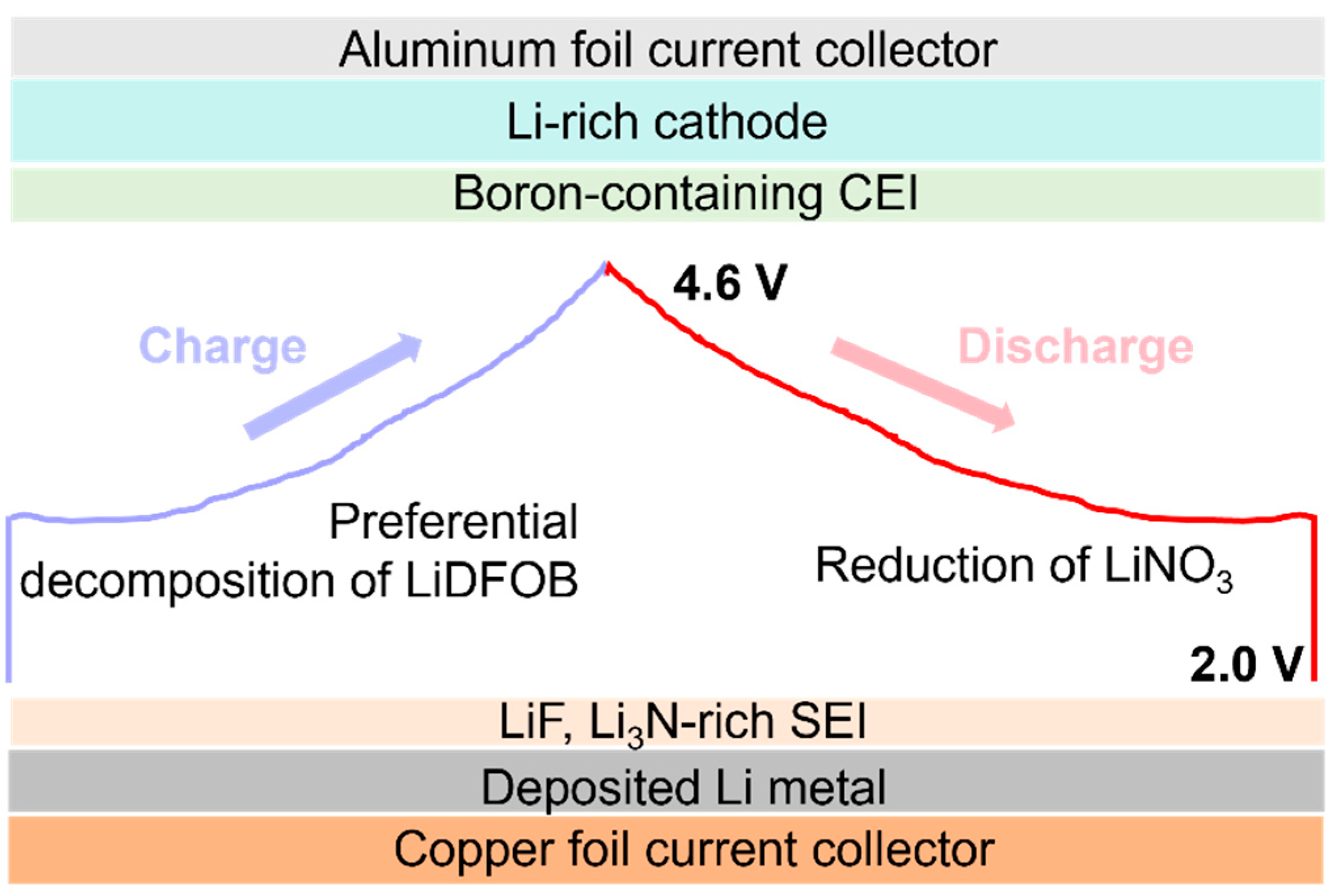
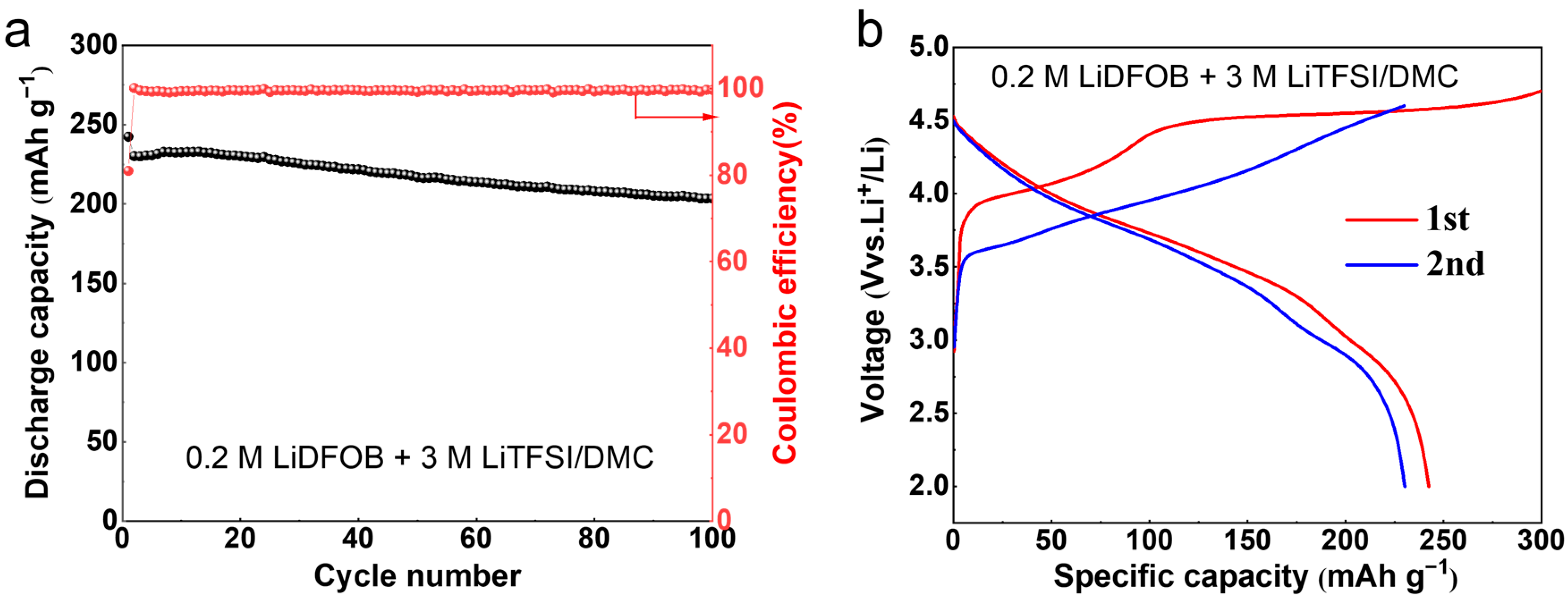
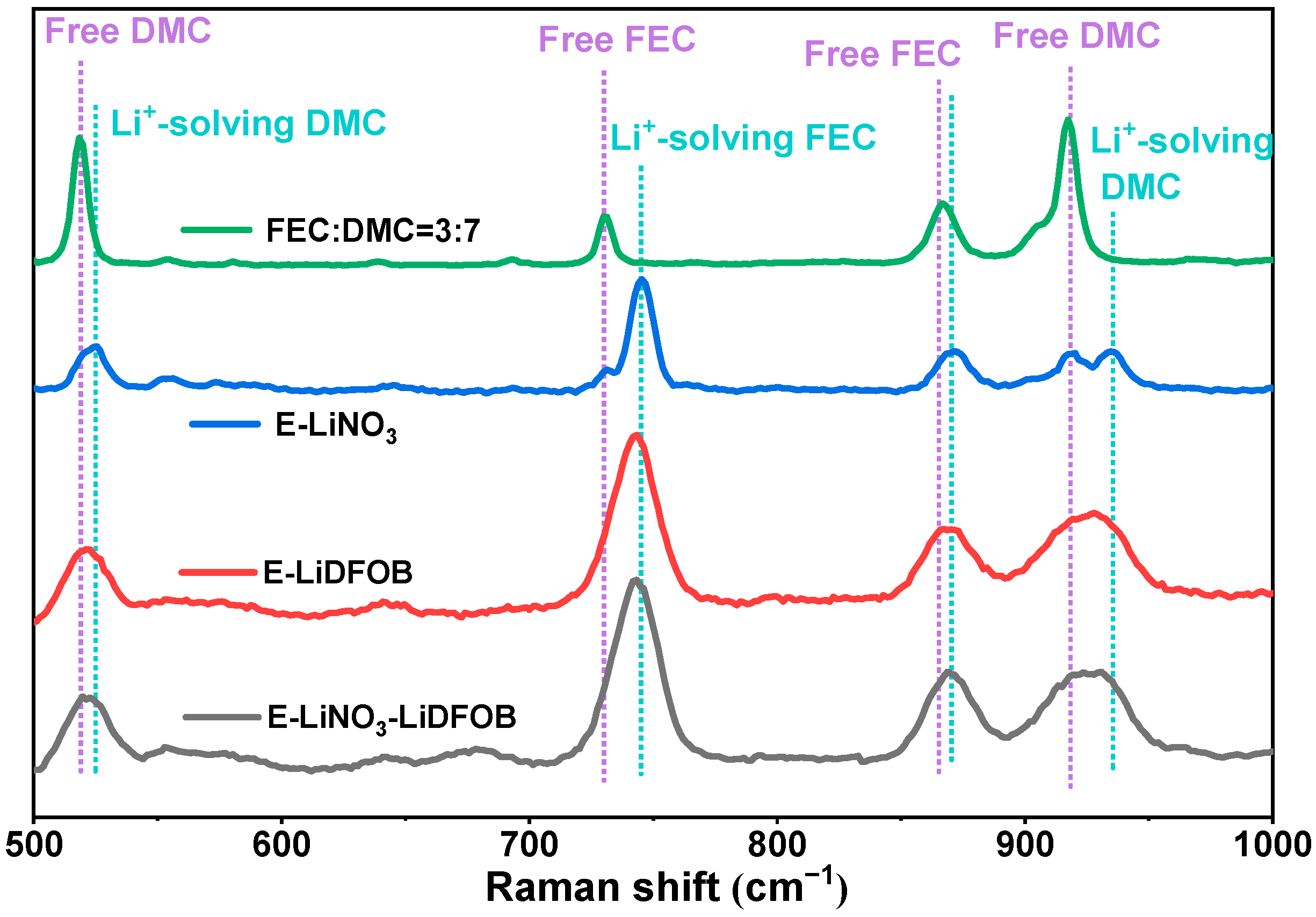
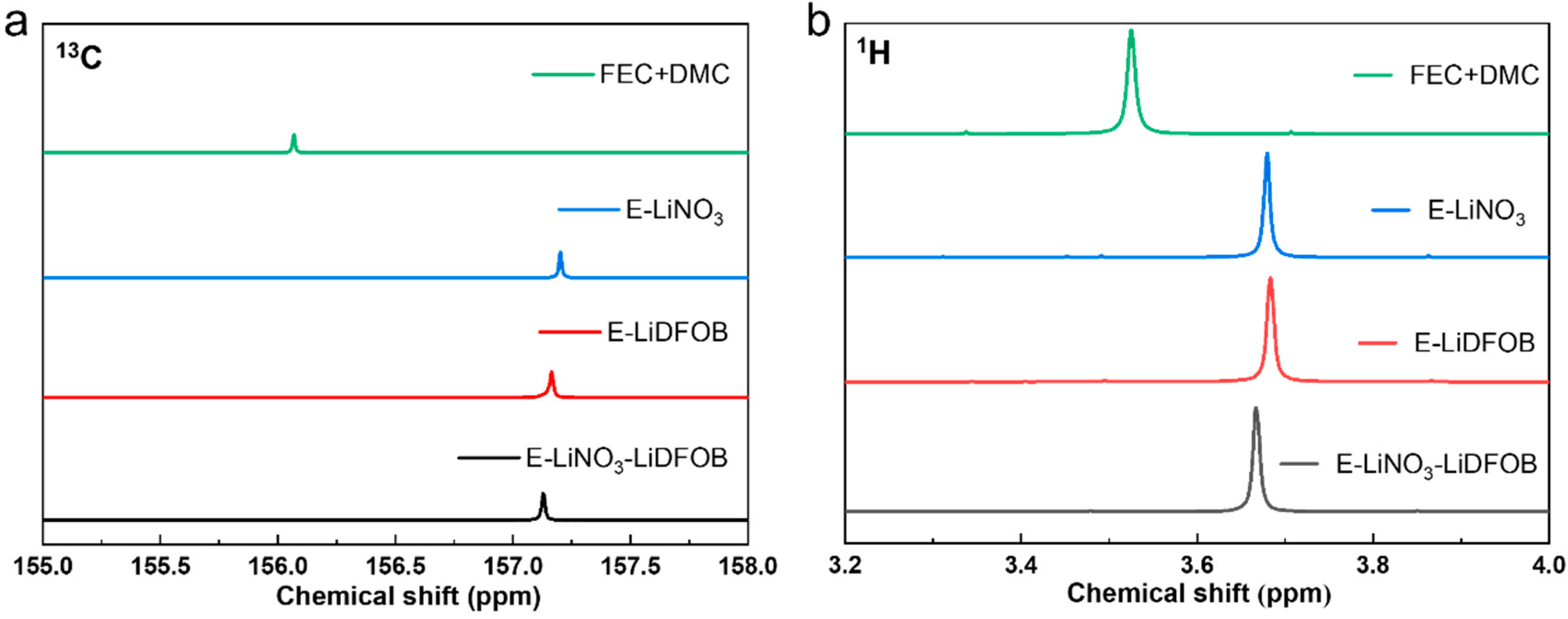
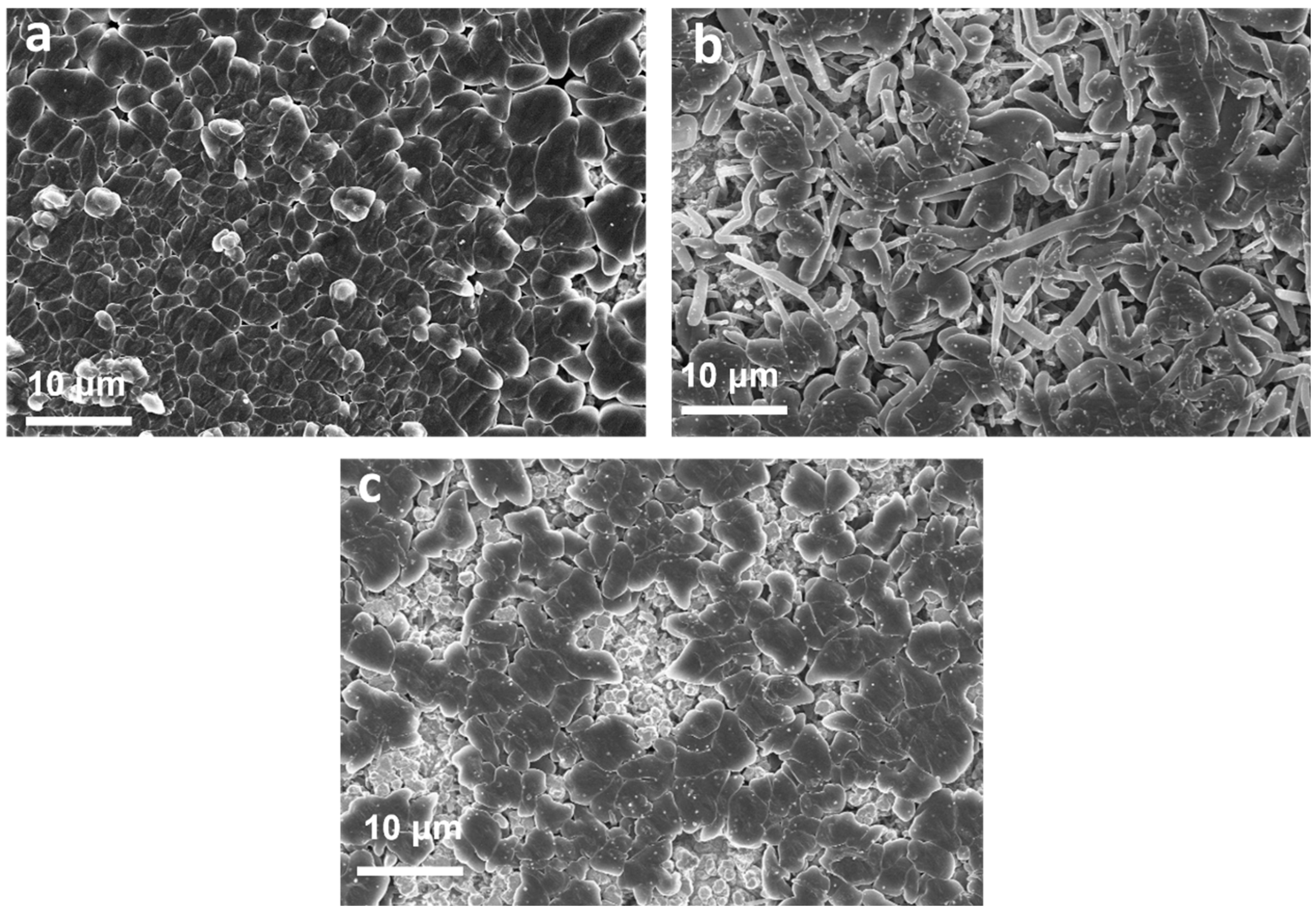
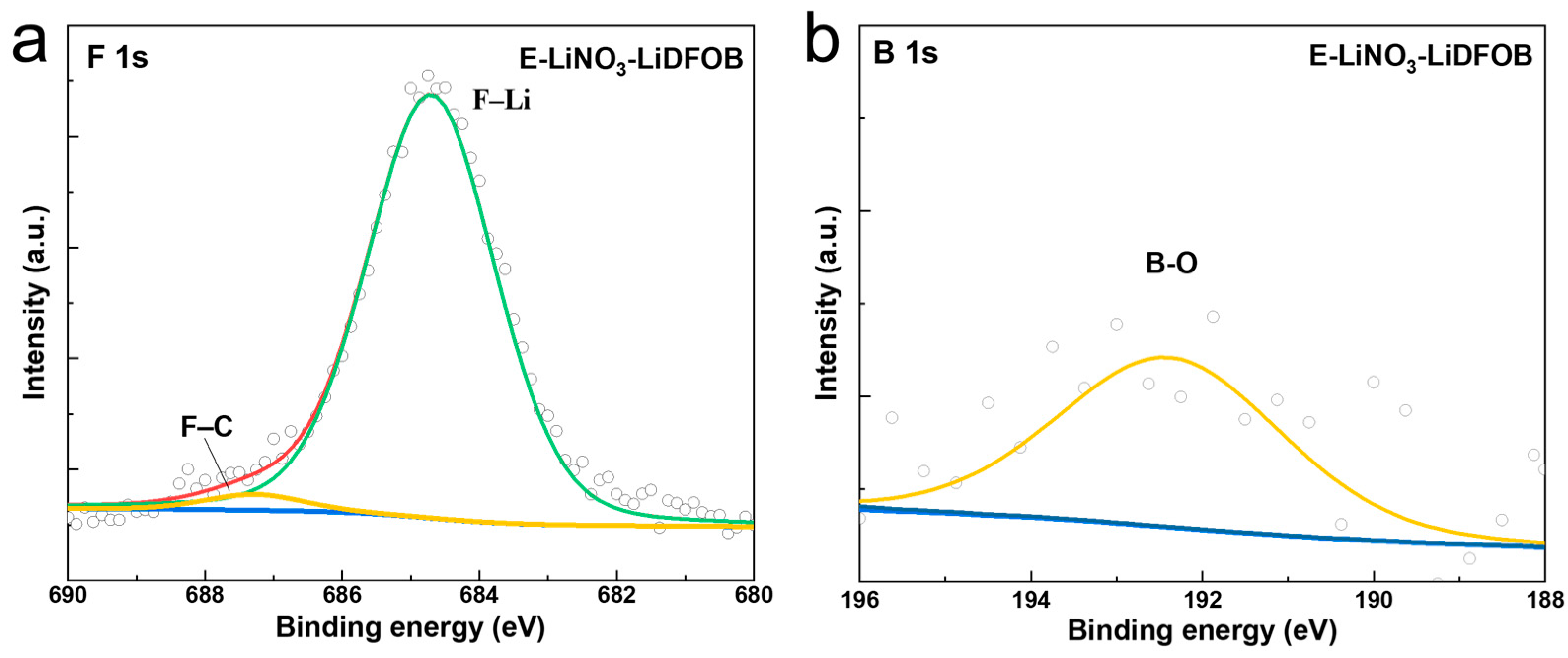
2. Results and Discussion
3. Method
3.1. Materials
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Yu, X.; Qin, X.; Li, B.; Kang, F. Carbon sphere-templated synthesis of porous yolk-shell ZnCo2O4 spheres for high-performance lithium storage. J. Alloy. Compd. 2019, 780, 65–71. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond-a 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ye, L.; Zhao, X.; Zhang, P.; Yang, J. Electronic modulation and structural engineering of carbon-based anodes for low-temperature lithium-ion batteries: A review. Molecules 2023, 28, 2108. [Google Scholar] [CrossRef] [PubMed]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Ge, B.; Hu, L.; Yu, X.; Wang, L.; Fernandez, C.; Yang, N.; Liang, Q.; Yang, Q.H. Engineering triple-phase interfaces around the anode towards practical alkali metal-air batteries. Adv. Mater. 2024, 36, 2400937. [Google Scholar] [CrossRef]
- Hu, L.; Deng, J.; Lin, Y.; Liang, Q.; Ge, B.; Weng, Q.; Bai, Y.; Li, Y.; Deng, Y.; Chen, G.; et al. Restructuring electrolyte solvation by a versatile diluent toward beyond 99.9% Coulombic efficiency of sodium plating/stripping at ultralow temperatures. Adv. Mater. 2024, 36, 2312161. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.; Zhu, Y.; Lin, M.C.; Chen, H.; Li, Y.Y.; Hung, W.H.; Zhou, B.; Wang, X.; Bai, Y.; et al. High-safety and high-energy-density lithium metal batteries in a novel ionic-liquid electrolyte. Adv. Mater. 2020, 32, e2001741. [Google Scholar] [CrossRef]
- Qian, J.F.; Adams, B.D.; Zheng, J.M.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.C.; Hu, J.Z.; Zhang, J.G. Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Shao, A.; Tang, X.; Zhang, M.; Bai, M.; Ma, Y. Challenges, strategies, and prospects of the anode-free lithium metal batteries. Adv. Energ. Sust. Res. 2022, 3, 2100197. [Google Scholar] [CrossRef]
- Su, L.; Charalambous, H.; Cui, Z.; Manthiram, A. High-efficiency, anode-free lithium–metal batteries with a close-packed homogeneous lithium morphology. Energy Environ. Sci. 2022, 15, 843–854. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv. Energy Mater. 2020, 11, 2003092. [Google Scholar] [CrossRef]
- Jin, C.B.; Liu, T.F.; Sheng, O.W.; Li, M.; Liu, T.C.; Yuan, Y.F.; Nai, J.W.; Ju, Z.J.; Zhang, W.K.; Liu, Y.J.; et al. Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nat. Energy 2021, 6, 378–387. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Z.; Wang, Z.; Wang, X.; Chen, W.; Wang, J.; Zhong, W.; Ma, R. Suppressing local dendrite hotspots via current density redistribution using a superlithiophilic membrane for stable lithium metal anode. Adv. Sci. 2023, 10, e2206995. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-free full cells: A pathway to high-energy density lithium-metal batteries. Adv. Energy Mater. 2021, 11, 2000804. [Google Scholar] [CrossRef]
- Liang, P.; Sun, H.; Huang, C.L.; Zhu, G.; Tai, H.C.; Li, J.; Wang, F.; Wang, Y.; Huang, C.J.; Jiang, S.K.; et al. A nonflammable high-voltage 4.7 V anode-free lithium battery. Adv. Mater. 2022, 34, e2207361. [Google Scholar] [CrossRef]
- Mao, M.; Ji, X.; Wang, Q.; Lin, Z.; Li, M.; Liu, T.; Wang, C.; Hu, Y.S.; Li, H.; Huang, X.; et al. Anion-enrichment interface enables high-voltage anode-free lithium metal batteries. Nat. Commun. 2023, 14, 1082. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yan, K.; Wan, S.; He, F.; Sun, B.; Wang, G. Towards high-energy-density lithium-ion batteries: Strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mater. 2021, 34, 716–734. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, X.; Cao, R.; Engelhard, M.H.; Liu, Y.; Hu, D.; Mei, D.; Zheng, J.; Zhao, W.; Li, Q.; et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Ren, X.D.; Zou, L.F.; Jiao, S.H.; Mei, D.H.; Engelhard, M.H.; Li, Q.Y.; Lee, H.Y.; Niu, C.J.; Adams, B.D.; Wang, C.M.; et al. High-concentration ether electrolytes for stable high-voltage lithium metal batteries. ACS Energy Lett. 2019, 4, 896–906. [Google Scholar] [CrossRef]
- Teng, W.; Wu, J.; Liang, Q.; Deng, J.; Xu, Y.; Liu, Q.; Wang, B.; Ma, T.; Nan, D.; Liu, J.; et al. Designing advanced liquid electrolytes for alkali metal batteries: Principles, progress, and perspectives. Energy Environ. Mater. 2022, 6, e12355. [Google Scholar] [CrossRef]
- Ma, T.; Deng, J.; Lin, Y.; Liang, Q.; Hu, L.; Wang, X.; Liu, J.; Zhao, X.; Li, Y.; Nan, D.; et al. Li-rich organosulfur cathode with boosted kinetics for high-energy lithium-sulfur batteries. Energy Environ. Mater. 2024, 7, e12704. [Google Scholar] [CrossRef]
- Zheng, H.; Hu, Z.; Liu, P.; Xu, W.; Xie, Q.; He, W.; Luo, Q.; Wang, L.; Gu, D.; Qu, B.; et al. Surface Ni-rich engineering towards highly stable Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials. Energy Storage Mater. 2020, 25, 76–85. [Google Scholar] [CrossRef]
- Mao, M.; Huang, B.; Li, Q.; Wang, C.; He, Y.-B.; Kang, F. In-situ construction of hierarchical cathode electrolyte interphase for high performance LiNi0.8Co0.1Mn0.1O2/Li metal metal battery. Nano Energy 2020, 78, 105282. [Google Scholar] [CrossRef]
- Li, X.; Zheng, J.; Engelhard, M.H.; Mei, D.; Li, Q.; Jiao, S.; Liu, N.; Zhao, W.; Zhang, J.G.; Xu, W. Effects of imide-orthoborate dual-salt mixtures in organic carbonate electrolytes on the stability of lithium metal batteries. ACS Appl. Mater. Interfaces 2018, 10, 2469–2479. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Rong, S.; Zhao, K.; He, K.; Cheng, S.; Sun, Y.; Xiang, H. Multifunctional acetamide additive combined with LiNO3 co-assists low-concentration electrolyte interfacial stability for lithium metal batteries. ACS Appl. Mater. Interfaces 2023, 15, 53405–53416. [Google Scholar] [CrossRef]
- Schedlbauer, T.; Krüger, S.; Schmitz, R.; Schmitz, R.W.; Schreiner, C.; Gores, H.J.; Passerini, S.; Winter, M. Lithium difluoro(oxalato)borate: A promising salt for lithium metal based secondary batteries? Electrochim. Acta 2013, 92, 102–107. [Google Scholar] [CrossRef]
- Hobold, G.M.; Lopez, J.; Guo, R.; Minafra, N.; Banerjee, A.; Shirley Meng, Y.; Shao-Horn, Y.; Gallant, B.M. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 2021, 6, 951–960. [Google Scholar] [CrossRef]
- Akkinepally, B.; Reddy, I.N.; Manjunath, V.; Reddy, M.V.; Mishra, Y.K.; Ko, T.J.; Zaghib, K.; Shim, J. Temperature effect and kinetics, LiZr2(PO4)3 and Li1.2Al0.2Zr1.8(PO4)3 and electrochemical properties for rechargeable ion batteries. Int. J. Energy Res. 2022, 46, 14116–14132. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.Q.; Liu, M.C.; Zhang, H.; Cheng, S.J.; Xie, J. Balanced solvation/de-solvation of electrolyte facilitates Li-ion intercalation for fast charging and low-temperature Li-ion batteries. Nano Energy 2022, 98, 107265. [Google Scholar] [CrossRef]
- Bai, Z.; Ying, Z.; Zhang, F.; Wang, W.; Huang, Z.; Yang, T.; Li, W.; Dong, W.; Yan, J.; Lin, C.; et al. Enabling high stability of Co-free LiNiO2 cathode via a sulfide-enriched cathode electrolyte interface. ACS Energy Lett. 2024, 9, 2717–2726. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Lin, H.; Hu, L.; Zhan, C.; Weng, Q.; Yu, X.; Sun, X.; Zhang, Q.; Mo, J.; Li, B. Formulating Electrolytes for 4.6 V Anode-Free Lithium Metal Batteries. Molecules 2024, 29, 4831. https://doi.org/10.3390/molecules29204831
Deng J, Lin H, Hu L, Zhan C, Weng Q, Yu X, Sun X, Zhang Q, Mo J, Li B. Formulating Electrolytes for 4.6 V Anode-Free Lithium Metal Batteries. Molecules. 2024; 29(20):4831. https://doi.org/10.3390/molecules29204831
Chicago/Turabian StyleDeng, Jiaojiao, Hai Lin, Liang Hu, Changzhen Zhan, Qingsong Weng, Xiaoliang Yu, Xiaoqi Sun, Qianlin Zhang, Jinhan Mo, and Baohua Li. 2024. "Formulating Electrolytes for 4.6 V Anode-Free Lithium Metal Batteries" Molecules 29, no. 20: 4831. https://doi.org/10.3390/molecules29204831
APA StyleDeng, J., Lin, H., Hu, L., Zhan, C., Weng, Q., Yu, X., Sun, X., Zhang, Q., Mo, J., & Li, B. (2024). Formulating Electrolytes for 4.6 V Anode-Free Lithium Metal Batteries. Molecules, 29(20), 4831. https://doi.org/10.3390/molecules29204831






