Emerging Thermosensitive Probes Based on Triamino-Phenazinium Dyes
Abstract
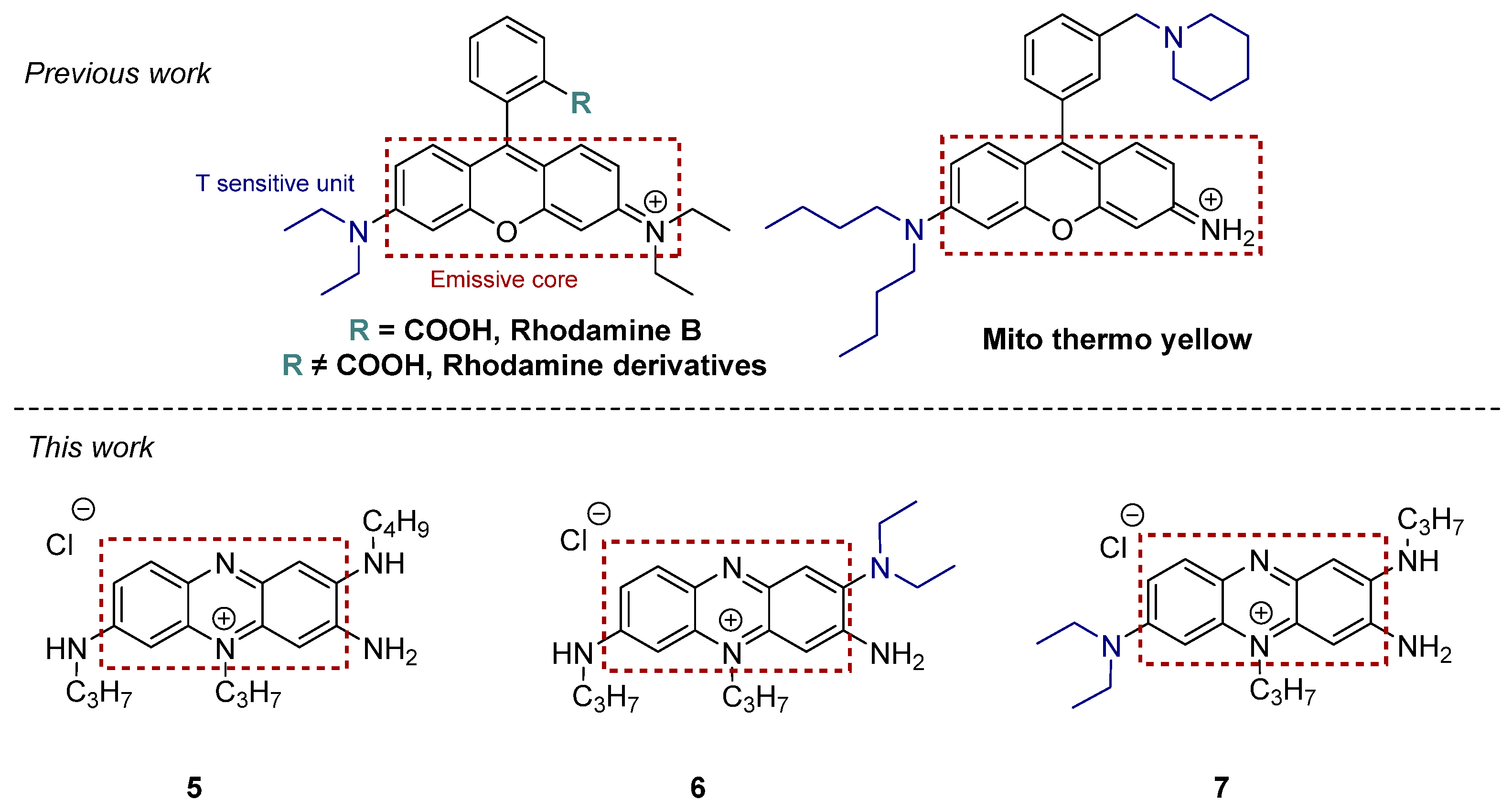
1. Introduction
2. Results
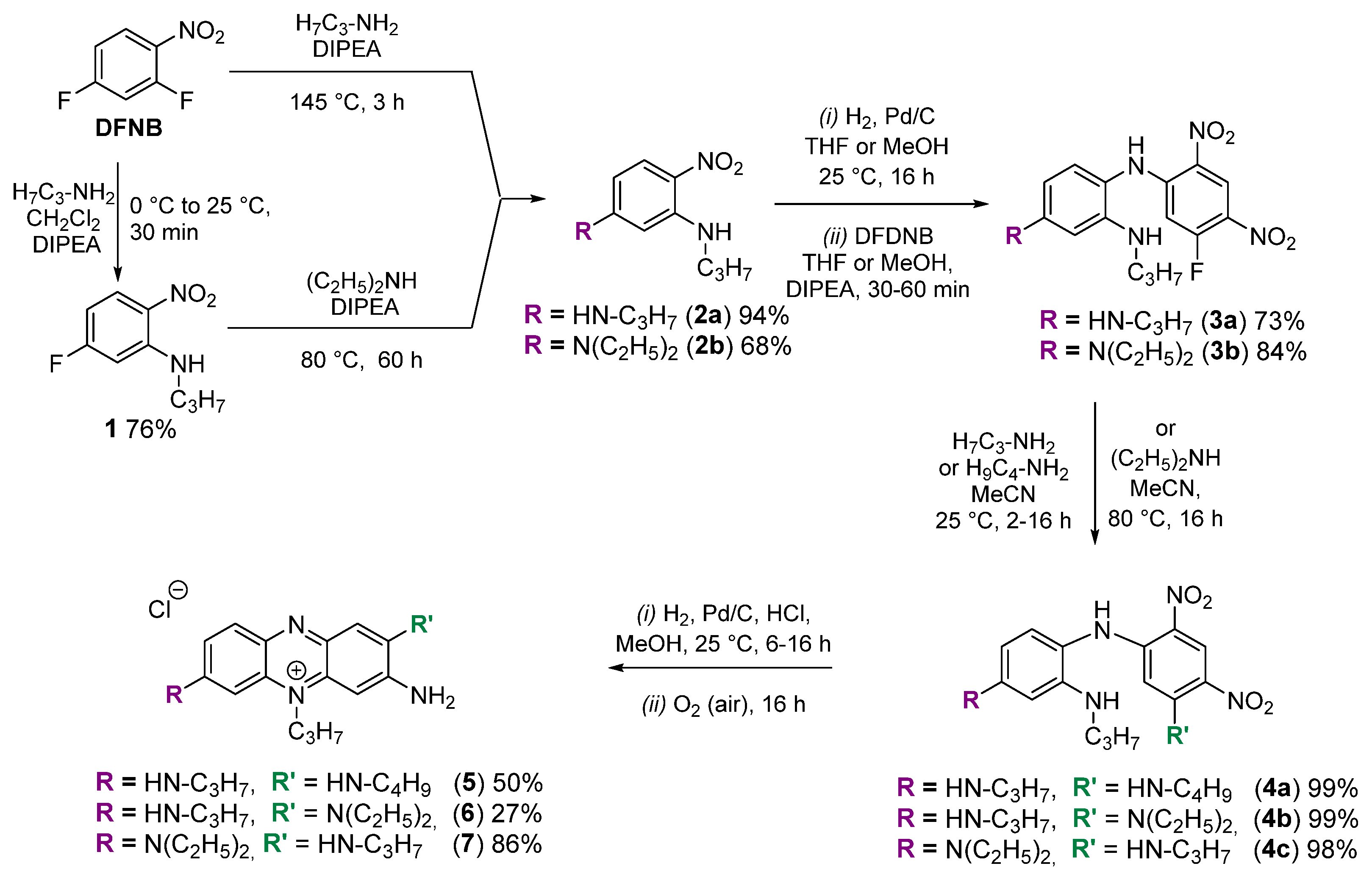
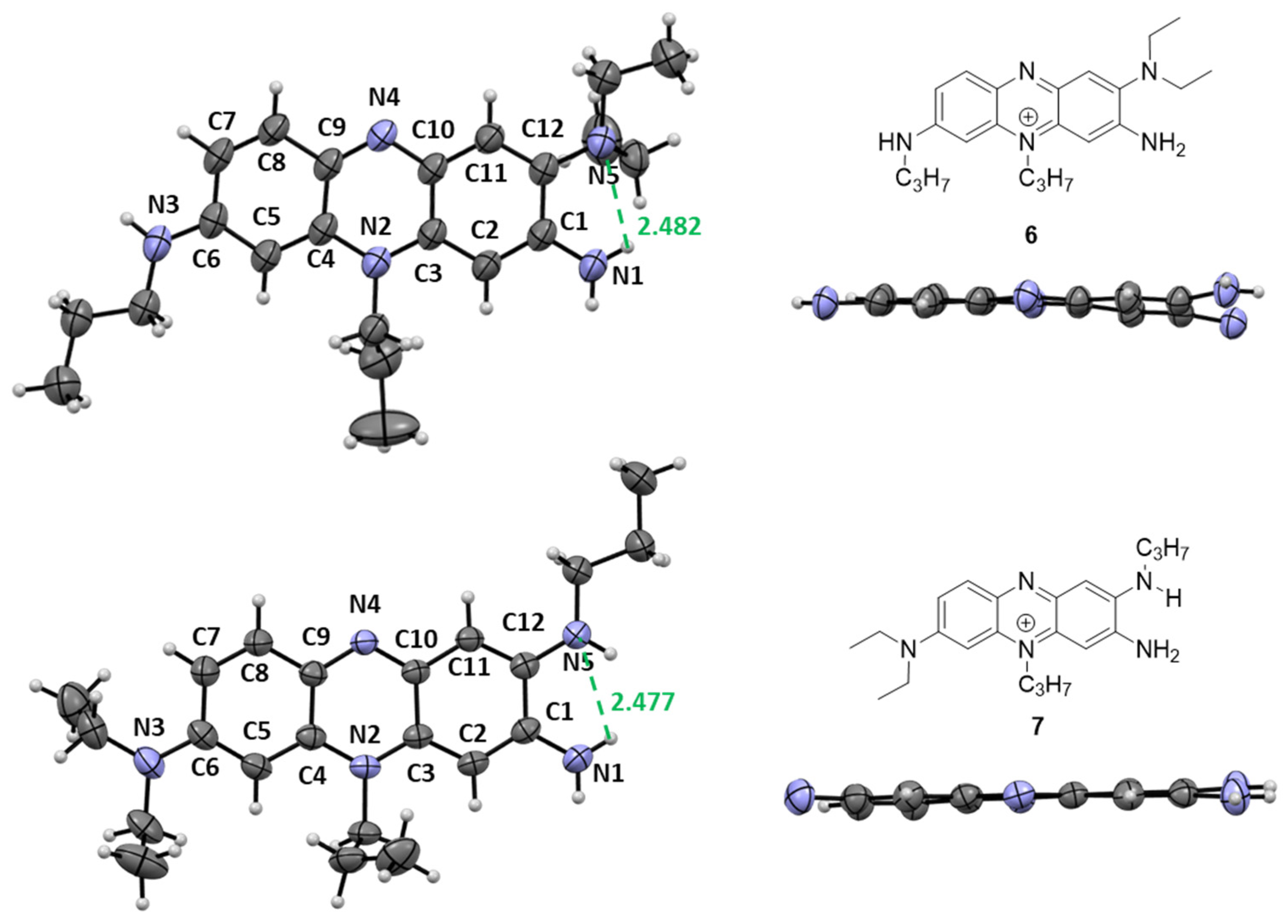
2.1. Synthesis and Characterization of Phenazinium Dyes
2.2. Optical Properties
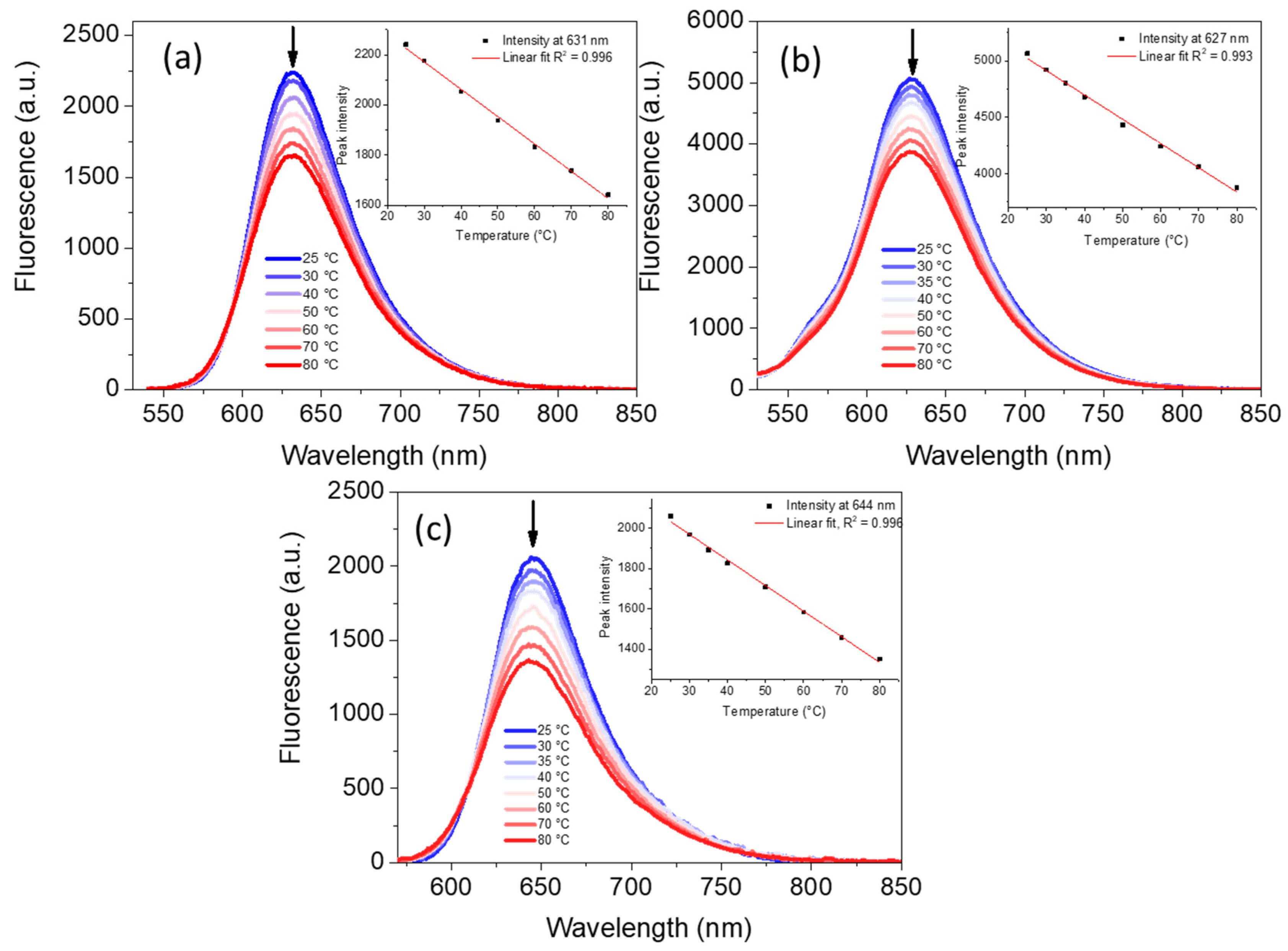
2.3. Temperature Sensitivity
3. Discussion
4. Materials and Methods
4.1. General Remarks and Analysis Conditions
4.2. Synthesis Protocols and Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Fu, M.; Bian, M.; Zhu, Q. Recent Progress on Small Molecular Temperature-Sensitive Fluorescent Probes. Biotechnol. Bioeng. 2023, 120, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Vetrone, F. Luminescence Nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the Nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Henriksen-Lacey, M.; Renero-Lecuna, C.; Liz-Marzán, L.M. Challenges for Optical Nanothermometry in Biological Environments. Chem. Soc. Rev. 2022, 51, 4223–4242. [Google Scholar] [CrossRef]
- Okabe, K.; Sakaguchi, R.; Shi, B.; Kiyonaka, S. Intracellular Thermometry with Fluorescent Sensors for Thermal Biology. Pflügers Arch.—Eur. J. Physiol. 2018, 470, 717–731. [Google Scholar] [CrossRef]
- Suzuki, M.; Plakhotnik, T. The Challenge of Intracellular Temperature. Biophys. Rev. 2020, 12, 593–600. [Google Scholar] [CrossRef]
- Uchiyama, S.; Gota, C. Luminescent Molecular Thermometers for the Ratiometric Sensing of Intracellular Temperature. Rev. Anal. Chem. 2017, 36, 20160021. [Google Scholar] [CrossRef]
- Homma, M.; Takei, Y.; Murata, A.; Inoue, T.; Takeoka, S. A Ratiometric Fluorescent Molecular Probe for Visualization of Mitochondrial Temperature in Living Cells. Chem. Commun. 2015, 51, 6194–6197. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, X.; Feng, J.; Li, S.; Li, Y. Organic Dye Thermometry. In Thermometry at the Nanoscale: Techniques and Selected Applications; Carlos, L.D., Palacio, F., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; ISBN 978-1-84973-904-7. [Google Scholar]
- Lakowicz, J.R.; Gryczynski, I.; Bogdanov, V.; Kusba, J. Light Quenching and Fluorescence Depolarization of Rhodamine B and Applications of This Phenomenon to Biophysics. J. Phys. Chem. 1994, 98, 334–342. [Google Scholar] [CrossRef]
- Ferguson, J.; Mau, A. Spontaneous and Stimulated Emission from Dyes. Spectroscopy of the Neutral Molecules of Acridine Orange, Proflavine, and Rhodamine B. Aust. J. Chem. 1973, 26, 1617–1624. [Google Scholar] [CrossRef]
- Snare, M.J.; Treloar, F.E.; Ghiggino, K.P.; Thistlethwaite, P.J. The Photophysics of Rhodamine B. J. Photochem. 1982, 18, 335–346. [Google Scholar] [CrossRef]
- Hinckley, D.A.; Seybold, P.G.; Borris, D.P. Solvatochromism and Thermochromism of Rhodamine Solutions. Spectrochim. Acta Part. A Mol. Spectrosc. 1986, 42, 747–754. [Google Scholar] [CrossRef]
- Paviolo, C.; Clayton, A.H.A.; McArthur, S.L.; Stoddart, P.R. Temperature Measurement in the Microscopic Regime: A Comparison between Fluorescence Lifetime- and Intensity-Based Methods. J. Microsc. 2013, 250, 179–188. [Google Scholar] [CrossRef]
- Arai, S.; Suzuki, M.; Park, S.-J.; Yoo, J.S.; Wang, L.; Kang, N.-Y.; Ha, H.-H.; Chang, Y.-T. Mitochondria-Targeted Fluorescent Thermometer Monitors Intracellular Temperature Gradient. Chem. Commun. 2015, 51, 8044–8047. [Google Scholar] [CrossRef]
- Chrétien, D.; Bénit, P.; Leroy, C.; El-Khoury, R.; Park, S.; Lee, J.Y.; Chang, Y.-T.; Lenaers, G.; Rustin, P.; Rak, M. Pitfalls in Monitoring Mitochondrial Temperature Using Charged Thermosensitive Fluorophores. Chemosensors 2020, 8, 124. [Google Scholar] [CrossRef]
- Huang, Z.; Li, N.; Zhang, X.; Wang, C.; Xiao, Y. Fixable Molecular Thermometer for Real-Time Visualization and Quantification of Mitochondrial Temperature. Anal. Chem. 2018, 90, 13953–13959. [Google Scholar] [CrossRef]
- Huang, Z.; Li, N.; Zhang, X.; Xiao, Y. Mitochondria-Anchored Molecular Thermometer Quantitatively Monitoring Cellular Inflammations. Anal. Chem. 2021, 93, 5081–5088. [Google Scholar] [CrossRef]
- Shen, F.; Yang, W.; Cui, J.; Hou, Y.; Bai, G. Small-Molecule Fluorogenic Probe for the Detection of Mitochondrial Temperature In Vivo. Anal. Chem. 2021, 93, 13417–13420. [Google Scholar] [CrossRef]
- Arai, S.; Lee, S.-C.; Zhai, D.; Suzuki, M.; Chang, Y.T. A Molecular Fluorescent Probe for Targeted Visualization of Temperature at the Endoplasmic Reticulum. Sci. Rep. 2014, 4, 6701. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; López-Duarte, I.; Duchemin, N.; Vu, T.-T.; Wu, Y.; Budynina, E.M.; Volkova, Y.A.; Peña Cabrera, E.; Ramírez-Ornelas, D.E.; Kuimova, M.K. Exploring Viscosity, Polarity and Temperature Sensitivity of BODIPY-Based Molecular Rotors. Phys. Chem. Chem. Phys. 2017, 19, 25252–25259. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Cornell, B.; Sherin, P.S.; Maleckaitė, K.; Kubánková, M.; Izquierdo, M.A.; Vu, T.T.; Volkova, Y.A.; Budynina, E.M.; Molteni, C.; et al. Cyclopropyl Substituents Transform the Viscosity-Sensitive BODIPY Molecular Rotor into a Temperature Sensor. ACS Sens. 2021, 6, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent Developments of BODIPY-Based Colorimetric and Fluorescent Probes for the Detection of Reactive Oxygen/Nitrogen Species and Cancer Diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Berneth, H. Azine Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-3-527-30385-4. [Google Scholar]
- Hughes, N. Chapter 45—Phenazine, Oxazine, Thiazine and Sulphur Dyes. In Rodd’s Chemistry of Carbon Compounds, 2nd ed.; Coffey, S., Ed.; Elsevier: Amsterdam, The Netherlands, 1964; pp. 403–436. ISBN 978-0-444-53345-6. [Google Scholar]
- Shimizu, T.; Miyamoto, E.; Shishido, M. Negative Charging Electrophotographic Photoreceptor. JP2011138030A, 14 January 2011. [Google Scholar]
- Raue, R.; Beecken, H. Toners for Electrophotography. DE3615571A1, 12 November 1987. [Google Scholar]
- Schurig, P.; Wichert, P.; Mueller, W.; Hentzschel, J.; Prezewowsky, E.; Klepzig, W. Color for Regenerating Textile Color Carrier. DD289897A7, 16 May 1991. [Google Scholar]
- Wada, H.; Kondo, F.; Takahashi, Y.; Hasegawa, H.; Ishii, K. Color for Regenerating Textile Color Carrier. JP48056252A, 7 August 1973. [Google Scholar]
- Dale, J.; Caro, H. Brit. Patent No. 3307, December 1863. [Google Scholar]
- Chen, Z.; Pascal, S.; Daurat, M.; Lichon, L.; Nguyen, C.; Godefroy, A.; Durand, D.; Ali, L.M.A.; Bettache, N.; Gary-Bobo, M.; et al. Modified Indulines: From Dyestuffs to In Vivo Theranostic Agents. ACS Appl. Mater. Interfaces 2021, 13, 30337–30349. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Qian, K.; Liu, H.; Wang, Z.; Gao, F.; Qu, C.; Dai, W.; Lin, D.; Chen, K.; et al. Bioinspired Large Stokes Shift Small Molecular Dyes for Biomedical Fluorescence Imaging. Sci. Adv. 2022, 8, eabo3289. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]

| Dye | Solvent | λmax (ε, M−1 cm−1) | λem | Φ [a] | Stokes Shift (cm−1) |
|---|---|---|---|---|---|
| 5 | MeCN DMF H2O | 552 (33,740) 550 (−) 566 (−) | 644 639 668 | 0.58 0.61 − | 2590 2530 2700 |
| 6 | MeCN DMF H2O | 531 (47,960) 545 (−) 538 (−) | 642 622 660 | 0.006 0.006 − | 3250 2270 3440 |
| 7 | MeCN DMF H2O | 579 (38,790) 572 (−) 583 (−) | 657 651 697 | 0.68 0.67 − | 2050 2120 2800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, T.; Brunel, F.; Camplo, M.; Siri, O. Emerging Thermosensitive Probes Based on Triamino-Phenazinium Dyes. Molecules 2024, 29, 4830. https://doi.org/10.3390/molecules29204830
Munteanu T, Brunel F, Camplo M, Siri O. Emerging Thermosensitive Probes Based on Triamino-Phenazinium Dyes. Molecules. 2024; 29(20):4830. https://doi.org/10.3390/molecules29204830
Chicago/Turabian StyleMunteanu, Tatiana, Frédéric Brunel, Michel Camplo, and Olivier Siri. 2024. "Emerging Thermosensitive Probes Based on Triamino-Phenazinium Dyes" Molecules 29, no. 20: 4830. https://doi.org/10.3390/molecules29204830
APA StyleMunteanu, T., Brunel, F., Camplo, M., & Siri, O. (2024). Emerging Thermosensitive Probes Based on Triamino-Phenazinium Dyes. Molecules, 29(20), 4830. https://doi.org/10.3390/molecules29204830







