Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters
Abstract
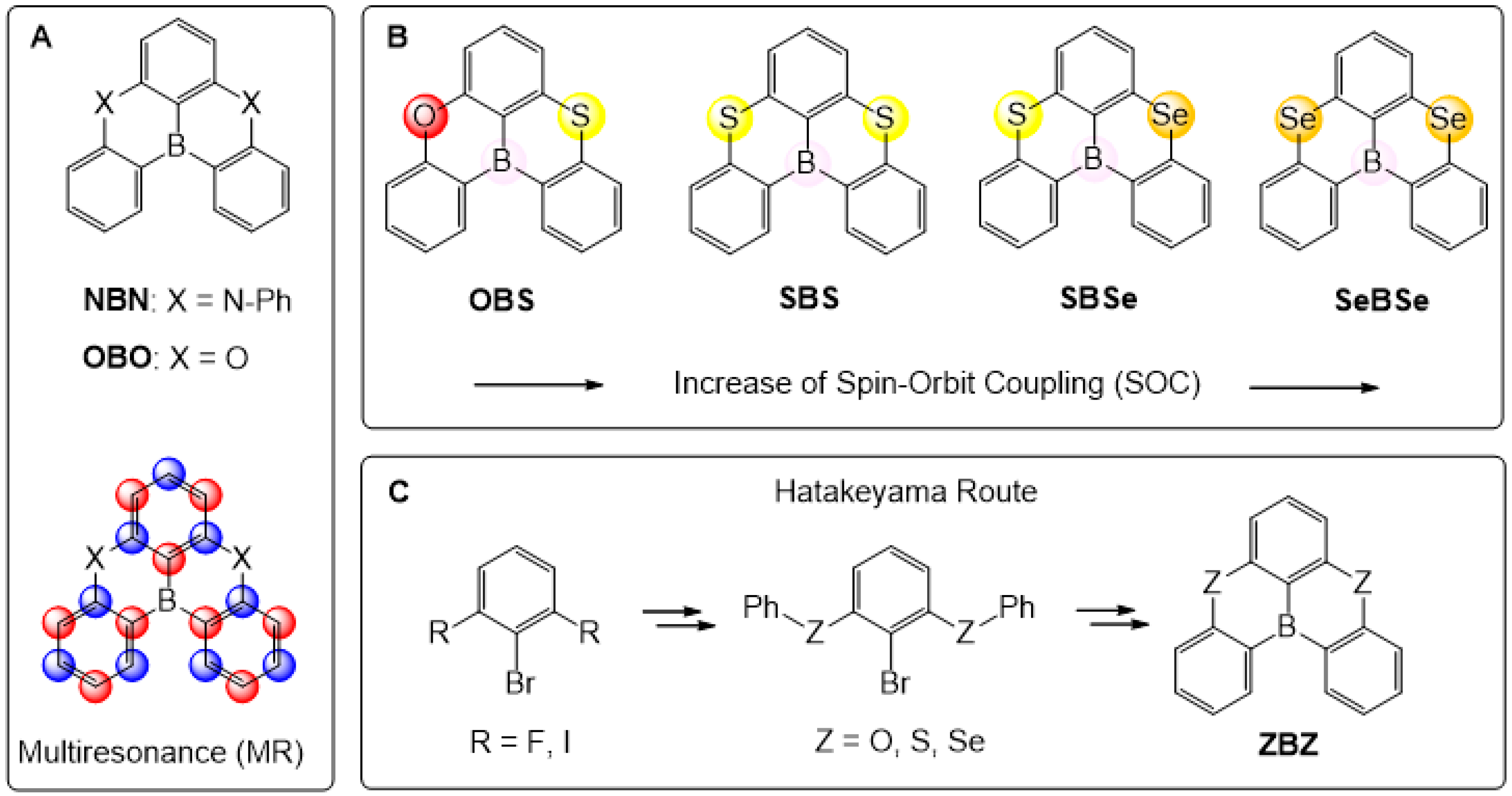
1. Introduction
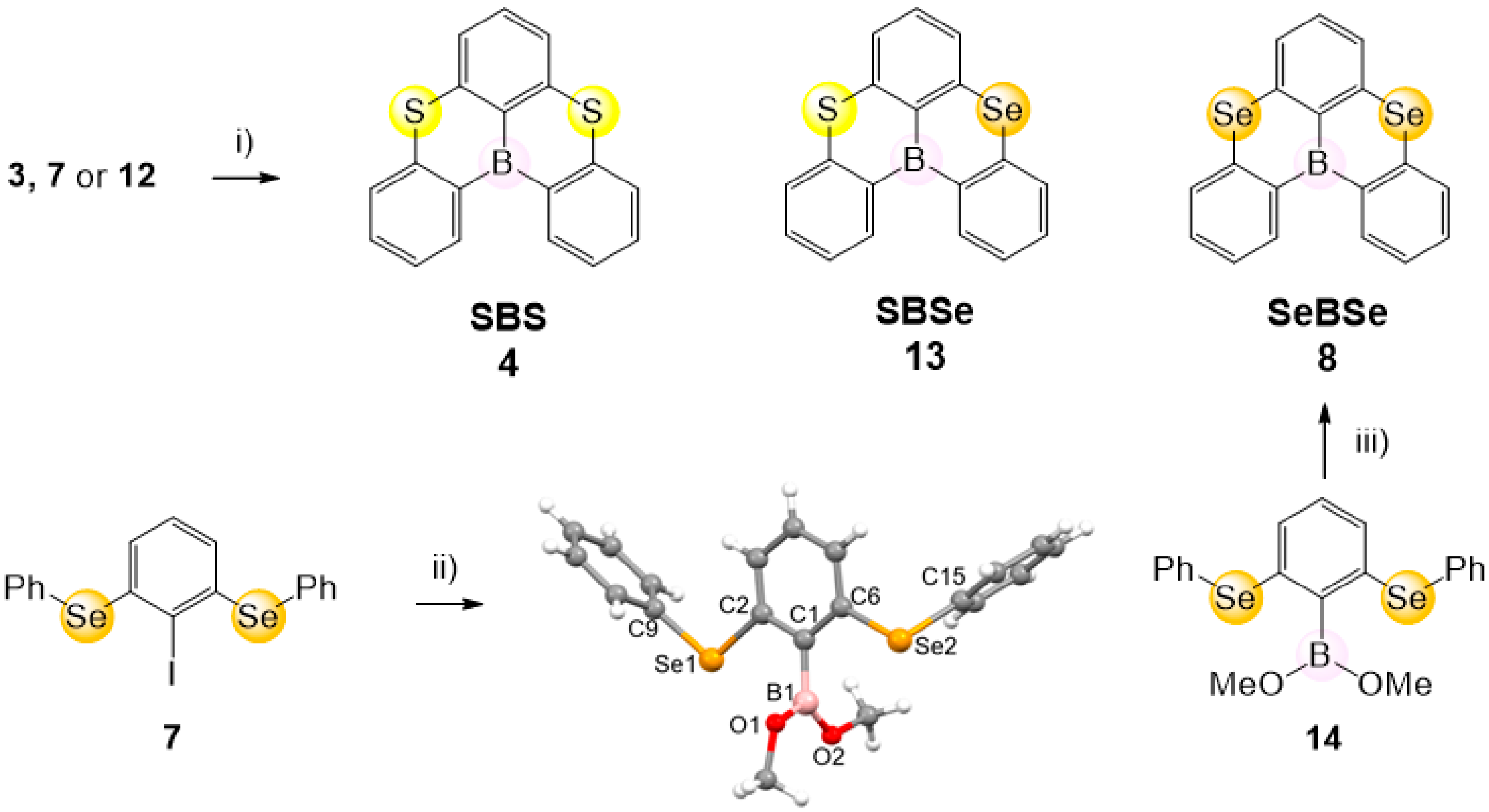
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Song, J.; Lee, H.; Jeong, E.G.; Choi, K.C.; Yoo, S. Organic light-emitting diodes: Pushing toward the limits and beyond. Adv. Mater. 2020, 32, 1907539. [Google Scholar] [CrossRef] [PubMed]
- Pode, R. Organic light emitting diode devices: An energy efficient solid state lighting for applications. Renew. Sustain. Energy Rev. 2020, 133, 110043. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lee, J.-H.; Lin, B.-Y.; Chen, S.; Wu, S.-T. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef]
- Geffroy, B.; Le Roy, P.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572–582. [Google Scholar] [CrossRef]
- Yersin, H.; Monkowius, U. Thermally activated delayed fluorescence and beyond. Photophysics and material design strategies. Adv. Photonics Res. 2024, 2400111. [Google Scholar] [CrossRef]
- Shi, Y.-Z.; Wu, H.; Wang, K.; Yu, J.; Oua, X.-M.; Zhang, X.-H. Recent progress in thermally activated delayed fluorescence emitters for nondoped organic light-emitting diodes. Chem. Sci. 2022, 13, 3625–3651. [Google Scholar] [CrossRef]
- Teng, J.-M.; Wang, Y.-W.; Chen, C.-F. Recent progress of narrowband TADF emitters and their applications in OLEDs. J. Mater. Chem. C 2020, 8, 11340–11353. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, X.; Yang, C. Thermally activated delayed fluorescence polymers and their application in organic light-emitting diodes. Prog. Polym. Sci. 2024, 158, 101892. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, Y.; Li, N.; Huang, Z.; Yang, C. Versatile boron-based thermally activated delayed fluorescence materials for organic light-emitting diodes. Aggregate 2022, 3, e182. [Google Scholar] [CrossRef]
- Kim, H.J.; Yasuda, T. Narrowband emissive thermally activated delayed fluorescence materials. Adv. Opt. Mater. 2022, 10, 2201714. [Google Scholar] [CrossRef]
- Du, M.; Zhou, J.; Luo, X.; Duan, L.; Zhang, D. A perspective on boron-based multiple resonance narrowband emitters and devices. Moore More 2024, 1, 5. [Google Scholar] [CrossRef]
- Naveen, K.R.; Konidena, R.K.; Keerthika, P. Neoteric advances in oxygen bridged triaryl boron-based delayed fluorescent materials for organic light emitting diodes. Chem. Rec. 2023, 23, e202300208. [Google Scholar] [CrossRef]
- Ahn, D.H.; Kim, S.W.; Lee, H.; Ko, I.J.; Karthik, D.; Lee, J.Y.; Kwon, J.H. Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors. Nat. Photonics 2019, 13, 540–546. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure blue thermally activated delayed fluorescence molecules: Efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef]
- Hirai, H.; Nakajima, K.; Nakatsuka, S.; Shiren, K.; Ni, J.; Nomura, S.; Ikuta, T.; Hatakeyama, T. One-step borylation of 1,3-diaryloxybenzenes towards efficient materials for organic light-emitting diodes. Angew. Chem. Int. Ed. 2015, 54, 13581–13585. [Google Scholar] [CrossRef]
- Mamada, M.; Hayakawa, M.; Ochi, J.; Hatakeyama, T. Organoboron-based multiple-resonance emitters: Synthesis, structure–property correlations, and prospects. Chem. Soc. Rev 2024, 53, 1624–1692. [Google Scholar] [CrossRef]
- Hu, Y.X.; Miao, J.; Hua, T.; Huang, Z.; Qi, Y.; Zou, Y.; Qiu, Y.; Xia, H.; Liu, H.; Cao, X.; et al. Efficient selenium-integrated TADF OLEDs with reduced roll-off. Nat. Photonics 2022, 16, 803–810. [Google Scholar] [CrossRef]
- Nagata, M.; Min, H.; Watanabe, E.; Fukumoto, H.; Mizuhata, Y.; Tokitoh, N.; Agou, T.; Yasuda, T. Fused-nonacyclic multi-resonance delayed fluorescence emitter based on ladder-thiaborin exhibiting narrowband sky-blue emission with accelerated reverse intersystem crossing. Angew. Chem. Int. Ed. 2021, 60, 20280–20285. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Min, H.; Yasuda, T. Ultrafast triplet–singlet exciton interconversion in narrowband blue organoboron emitters doped with heavy chalcogens. Angew. Chem. Int. Ed. 2022, 61, e202205684. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Yang, M.; Shibata, H.; Amanokura, N.; Yasuda, T. Achieving ultimate narrowband and ultrapure blue organic light-emitting diodes based on polycyclo-heteraborin multi-resonance delayed-fluorescence emitters. Adv. Mater. 2022, 34, 2107951. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, L.; Wang, X.; Yang, Q.; Li, W.; Tian, H.; Shao, S.; Wang, L.; Jing, X.; Wang, F. Novel boron- and sulfur-doped polycyclic aromatic hydrocarbon as multiple resonance emitter for ultrapure blue thermally activated delayed fluorescence polymers. Sci. China Chem. 2021, 64, 547–551. [Google Scholar] [CrossRef]
- Keshri, S.K.; Liu, G.; Yasuda, T. Ultrafast spin- flip exciton conversion and narrowband sky-blue luminescence in a fused polycyclic selenaborin emitter. Front. Chem. 2024, 12, 1375552. [Google Scholar] [CrossRef]
- Pratik, S.M.; Coropceanu, V.; Brédas, J.-L. Purely organic emitters for multiresonant thermally activated delay fluorescence: Design of highly efficient sulfur and selenium derivatives. ACS Mater. Lett. 2022, 4, 440–447. [Google Scholar] [CrossRef]
- Hagai, M.; Inai, N.; Yasuda, T.; Fujimoto, K.J.; Yanai, T. Extended theoretical modeling of reverse intersystem crossing for thermally activated delayed fluorescence materials. Sci. Adv. 2024, 10, eadk3219. [Google Scholar] [CrossRef]
- Chang, Y.; Wu, Y.; Wang, X.; Li, W.; Yang, Q.; Wang, S.; Shao, S.; Wang, L. Boron sulfur-doped polycyclic aromatic hydrocarbon emitters with multiple-resonance-dominated lowest excited states for efficient narrowband deep-blue emission. Chem. Eng. J. 2023, 451, 138545. [Google Scholar] [CrossRef]
- Gao, H.; Li, Z.; Pang, Z.; Qin, Y.; Liu, G.; Gao, T.; Dong, X.; Shen, S.; Xie, X.; Wang, P.; et al. Rational molecular design dtrategy for high-efficiency ultrapure blue TADF emitters: Symmetrical and rigid sulfur-bridged boron based acceptors. ACS Appl. Mater. Interfaces 2023, 15, 5529–5537. [Google Scholar] [CrossRef]
- Chang, Y.; Wu, Y.; Zhang, K.; Wang, S.; Wang, X.; Shao, S.; Wang, L. 1,8-diphenyl-carbazole-based boron, sulfur-containing multi-resonance emitters with suppressed aggregation emission for narrowband OLEDs. Dyes Pigments 2023, 220, 111678. [Google Scholar] [CrossRef]
- Ye, K.; Li, G.; Li, F.; Shi, C.; Jiang, Z.; Zhang, F.; Li, Q.; Su, J.; Song, D.; Yuan, A. B-embedded disulfide-bridged p-conjugated compounds: Structures and optical tuning. Phys. Chem. Chem. Phys. 2024, 26, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Krief, A. Synthesis of selenium and tellurium ylides and carbanions: Applications to organic synthesis. In The Chemistry of Organic Selenium and Tellurium Compound, 1st ed.; Patai, S., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 1987; Volume 2, pp. 677–764. [Google Scholar]
- Gilman, H.; Webb, F.J. The metalation of some sulfur-containing organic compounds. J. Am. Chem. Soc. 1949, 71, 4062–4066. [Google Scholar] [CrossRef]
- Dumont, W.; Bayet, P.; Krief, A. Cleavage of selenium compounds by butyllithium. A new, regiospecific, allyl alcohol synthon. Angew. Chem. Int. Ed. 1974, 13, 804–806. [Google Scholar] [CrossRef]
- Clarembeau, M.; Krief, A. Novel synthesis of benzyllithiums from benzylselenides. Tetrahedron Lett. 1985, 26, 1093–1096. [Google Scholar] [CrossRef]
- Clarembeau, M.; Krief, A. A novel method for the geminal dialkylation of the carbonyl group of aromatic aldehydes and ketones. Tetrahedron Lett. 1986, 27, 1719–1722. [Google Scholar] [CrossRef]
- Clarembeau, M.; Krief, A. Metallation of benzyl selenides and of α-aryl selenoacetals. Scope and limitations. Tetrahedron Lett. 1986, 27, 1723–1726. [Google Scholar] [CrossRef]
- Lide, D.R. Handbooks of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 9-65–9-71. [Google Scholar]
- Leroux, F.; Schlosser, M. The preparation of organolithium reagents and intermediates. In The Chemistry of Organolithium Compounds, 1st ed.; Rappoport, Z., Marek, I., Eds.; John Wiley & Sons, Ltd.: Hobooken, NJ, USA, 2004; pp. 435–493. [Google Scholar]
- Zender, E.; Karger, S.; Neubaur, R.; Virovets, A.; Lerner, H.-W.; Wagner, M. Green-Emitting extended B3,N2-doped polycyclic aromatic hydrocarbon with multiple resonance structure. Org. Lett. 2024, 26, 939–944. [Google Scholar] [CrossRef]
- Knöller, J.A.; Sönmez, B.; Matulaitis, T.; Gupta, A.K.; Zysman-Colman, E.; Laschat, S. A novel B,O,N-doped mesogen with narrowband MR-TADF emission. Chem. Commun. 2024, 60, 4459–4462. [Google Scholar] [CrossRef]
- Böser, R.; Haufe, L.C.; Freytag, M.; Jones, P.G.; Hörner, G.; Frank, R. Completing the series of boron-nucleophilic cyanoborates: Boryl anions of type NHC-B(CN)2−. Chem. Sci. 2017, 8, 6274–6280. [Google Scholar] [CrossRef]
- Böser, R.; Denker, L.; Frank, R. N-Heterocyclic carbene adducts of alkynyl functionalized 1,3,2-dithioborolanes. Molecules 2019, 24, 1690. [Google Scholar] [CrossRef]
- Böser, R.; Denker, L.; Frank, R. Benzyl borane NHC adducts: Beyond B−C bond scission. Chem. A Eur. J. 2019, 25, 10575–10579. [Google Scholar] [CrossRef] [PubMed]
- Dolati, H.; Haufe, L.C.; Denker, L.; Lorbach, A.; Grotjahn, R.; Hörner, G.; Frank, R. Two π-electrons make the difference—From BODIPY to BODIIM switchable fluorescent dyes. Chem. A Eur. J. 2020, 26, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Dolati, H.; Denker, L.; Trzaskowski, B.; Frank, R. Superseding β-diketiminato ligands: An amido imidazoline-2-imine ligand stabilizes the exhaustive series of B = X boranes (X = O, S, Se, Te). Angew. Chem. Int. Ed. 2021, 60, 4633–4639. [Google Scholar] [CrossRef] [PubMed]
- Güven, Z.; Denker, L.; Wullschläger, D.; Martínez, J.P.; Trzaskowski, B.; Frank, R. Reductive Al−B σ-bond formation in alumaboranes: Facile scission of polar multiple bonds. Angew. Chem. Int. Ed. 2022, 61, e202209502. [Google Scholar] [CrossRef] [PubMed]
- Denker, L.; Wullschläger, D.; Martínez, J.P.; Świerczewski, S.; Trzaskowski, B.; Tamm, M.; Frank, R. Cobalt(I)-catalyzed transformation of Si–H bonds: H/D exchange in hydrosilanes and hydrosilylation of olefins. ACS Catal. 2023, 13, 2586–2600. [Google Scholar] [CrossRef]
- Reshi, N.U.D.; Bockfeld, D.; Baabe, D.; Denker, L.; Martínez, J.P.; Trzaskowski, B.; Frank, R.; Tamm, M. Iron(I) and iron(II) amido-imidazolin-2-imine complexes as catalysts for H/D exchange in hydrosilanes. ACS Catal. 2024, 14, 1759–1772. [Google Scholar] [CrossRef]
- Denker, L.; Dolati, H.; Barthen, M.; Frank, R. Amino imidazolin-2-imine ligands in magnesium complexes: Approaches towards low-valent Mg(I) species. Z. Anorg. Allg. Chem. 2024, 650, e202300247. [Google Scholar] [CrossRef]
- Dolati, H.; Denker, L.; Martínez, J.P.; Trzaskowski, B.; Frank, R. Iminoboranes with parent B = NH entity: Imino group metathesis, nucleophilic reactivity and N−N coupling. Chem. Eur. J. 2023, 29, e202302494. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPRO Softw. Syst. version 1.171.39.46; Rigaku Corporation: Oxford, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güven, Z.; Dolati, H.; Wessel, L.; Frank, R. Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters. Molecules 2024, 29, 5819. https://doi.org/10.3390/molecules29245819
Güven Z, Dolati H, Wessel L, Frank R. Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters. Molecules. 2024; 29(24):5819. https://doi.org/10.3390/molecules29245819
Chicago/Turabian StyleGüven, Zeynep, Hadi Dolati, Leo Wessel, and René Frank. 2024. "Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters" Molecules 29, no. 24: 5819. https://doi.org/10.3390/molecules29245819
APA StyleGüven, Z., Dolati, H., Wessel, L., & Frank, R. (2024). Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters. Molecules, 29(24), 5819. https://doi.org/10.3390/molecules29245819





