Application and Challenge of Metalloporphyrin Sensitizers in Noninvasive Dynamic Tumor Therapy
Abstract
1. Introduction
2. The Possible Therapeutic Mechanism of PDT and SDT
3. Application of Metalloporphyrins in PDT
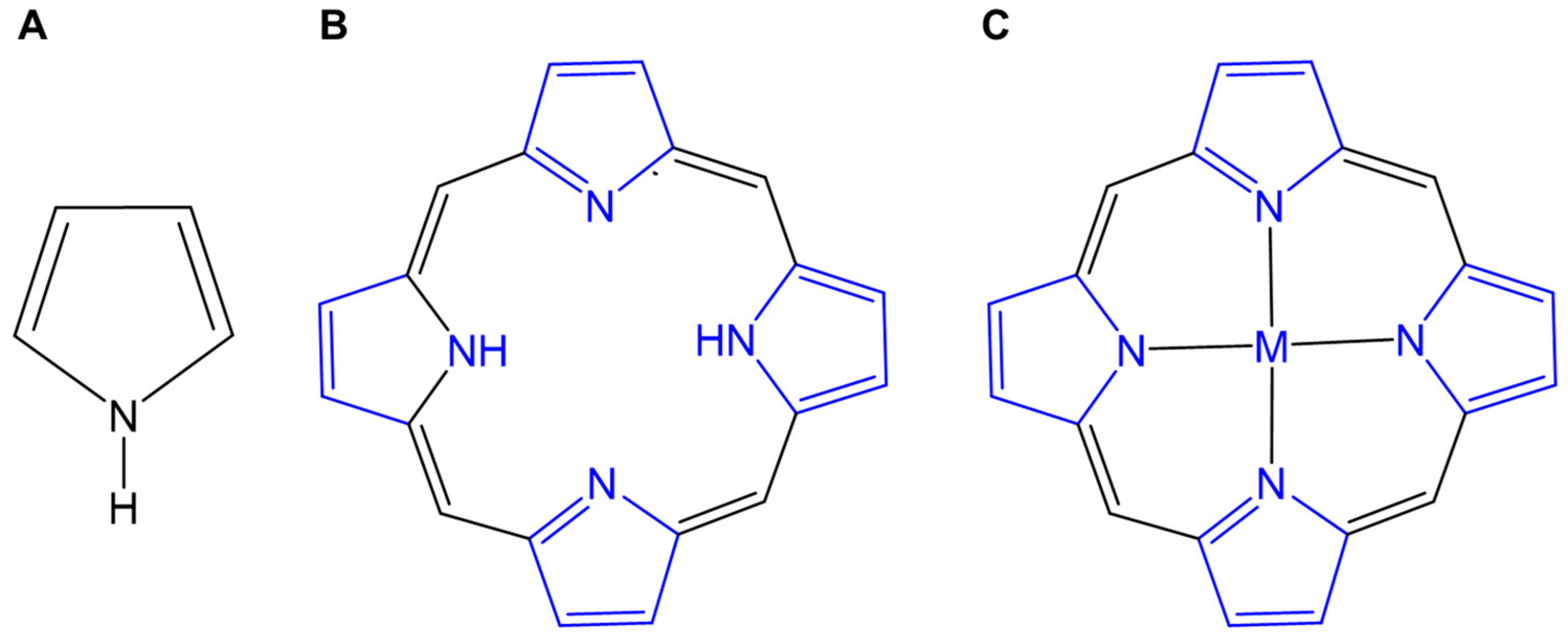
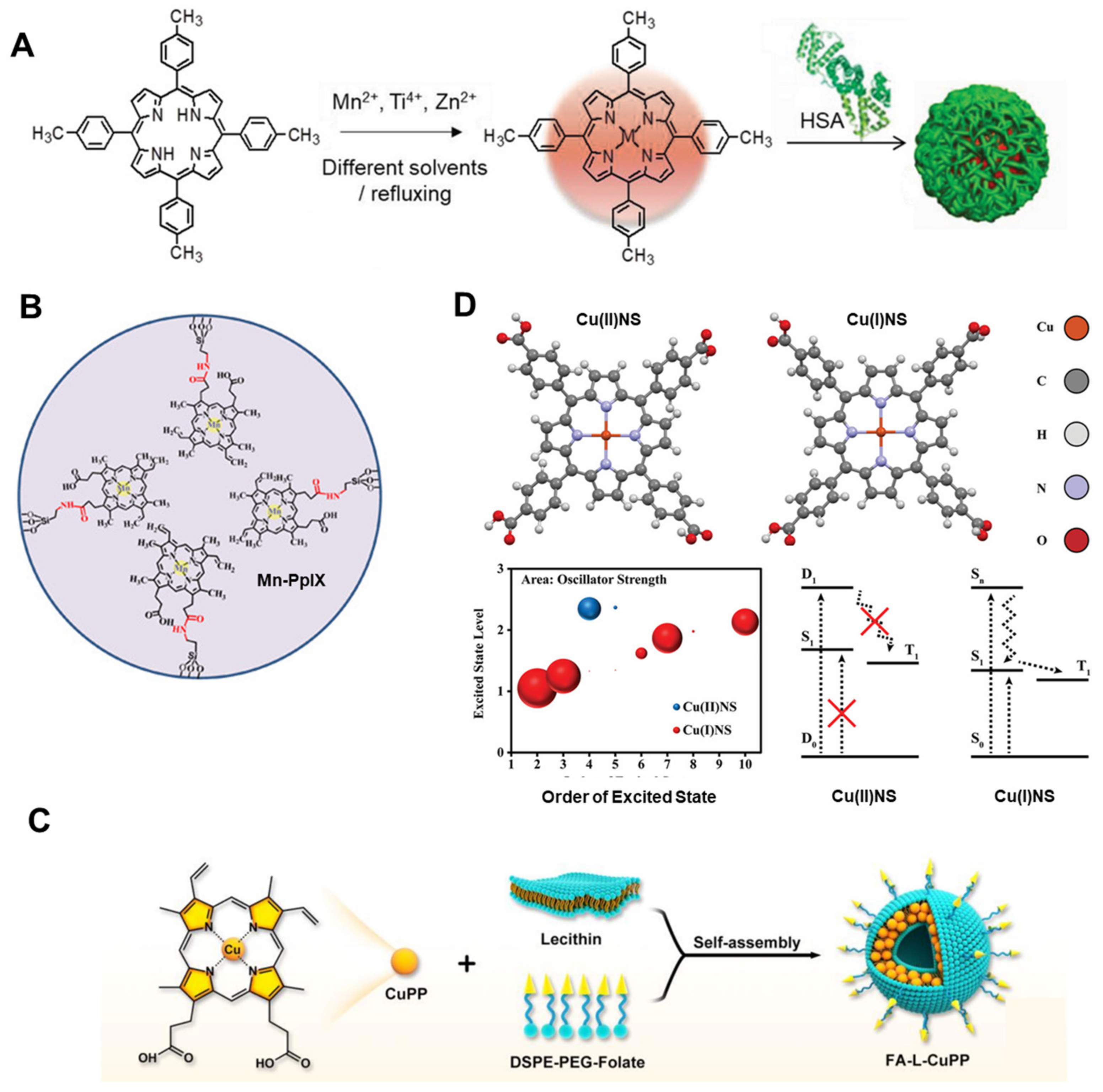
3.1. The Characteristic of Metalloporphyrins as Photosensitizers
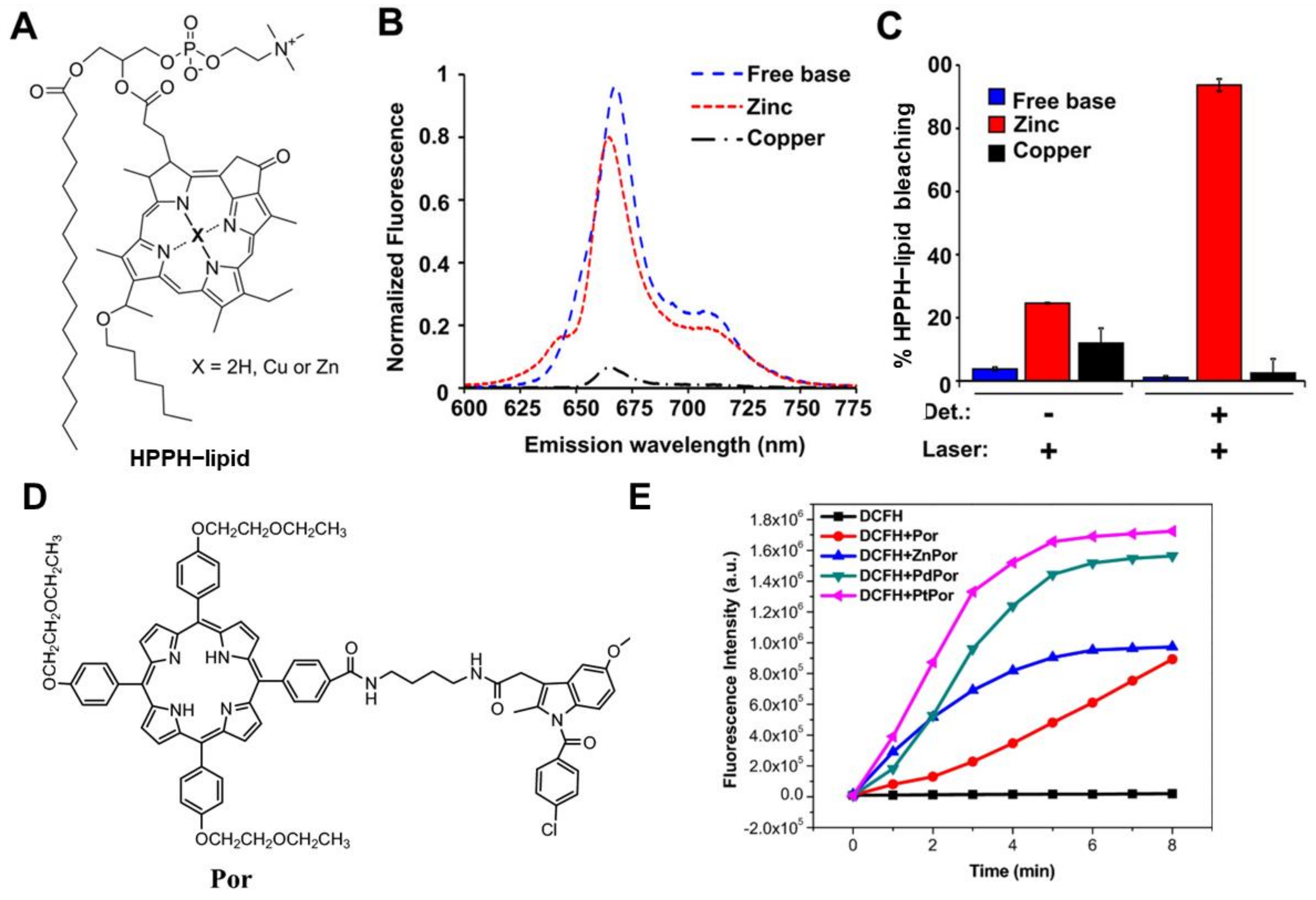
3.2. Assisted Enhanced PDT
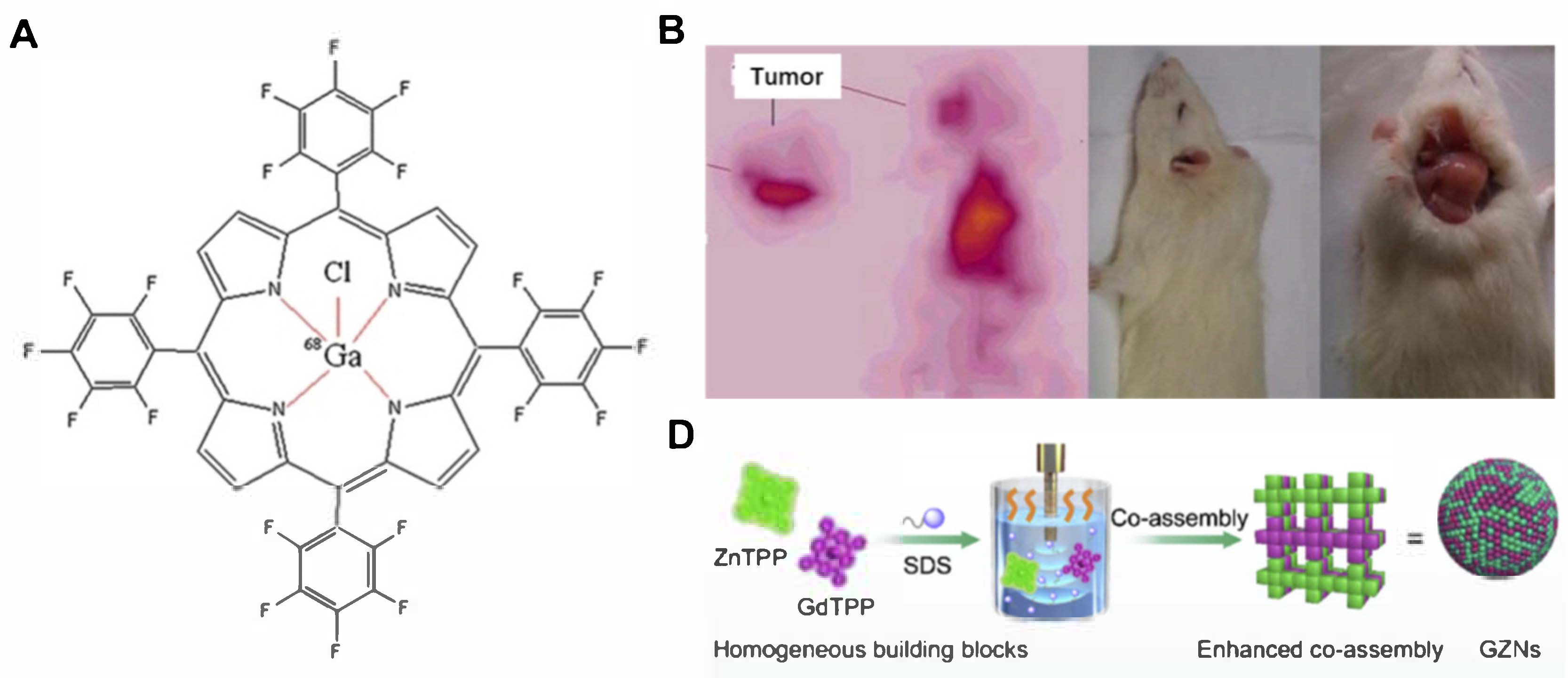
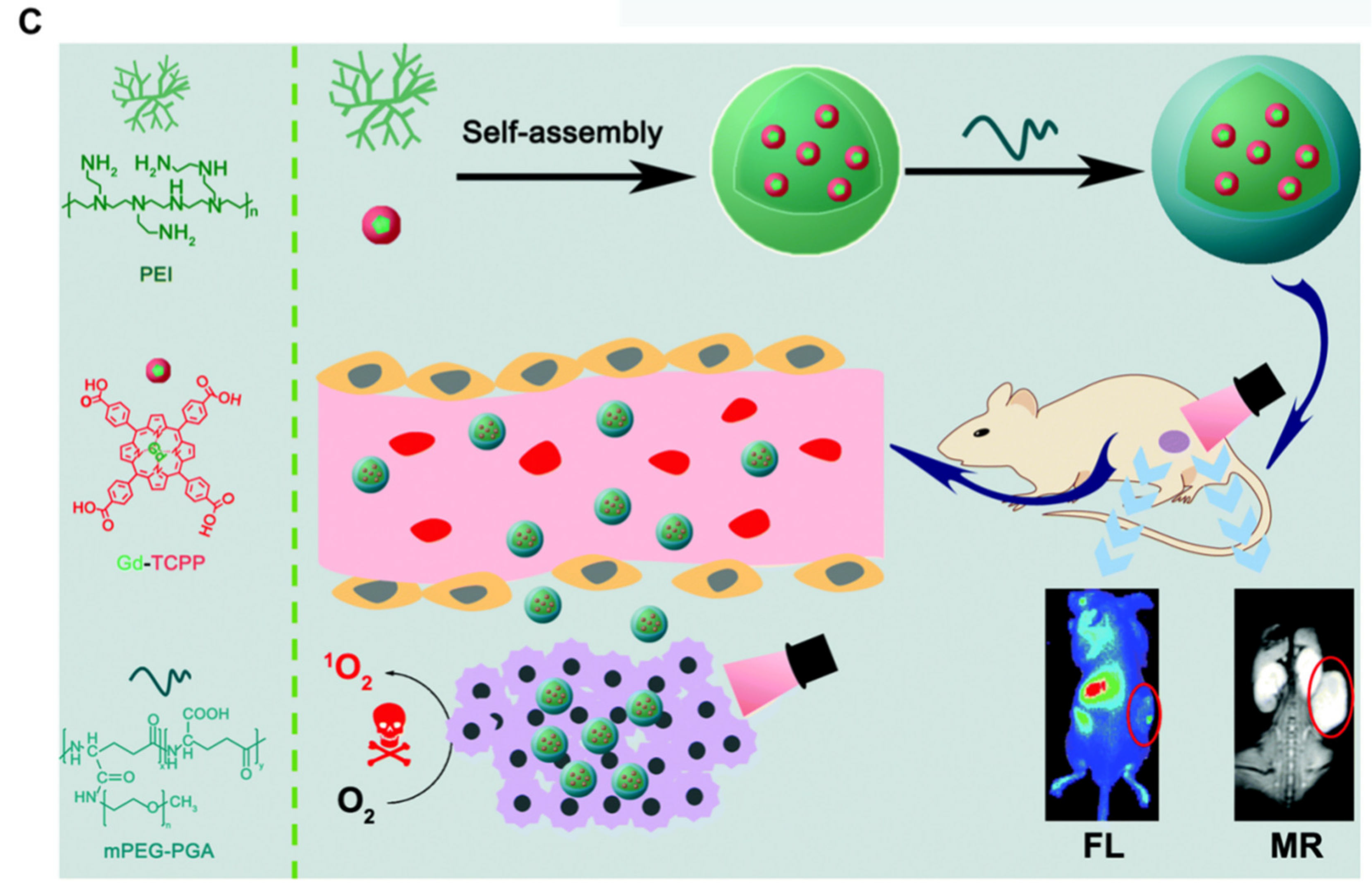
3.3. Image-Guided PDT
3.4. Metalloporphyrin Frameworks for PDT
4. Application of Metalloporphyrins in SDT
4.1. The Characteristics of Metalloporphyrins Chelated by Different Metal Centers for SDT
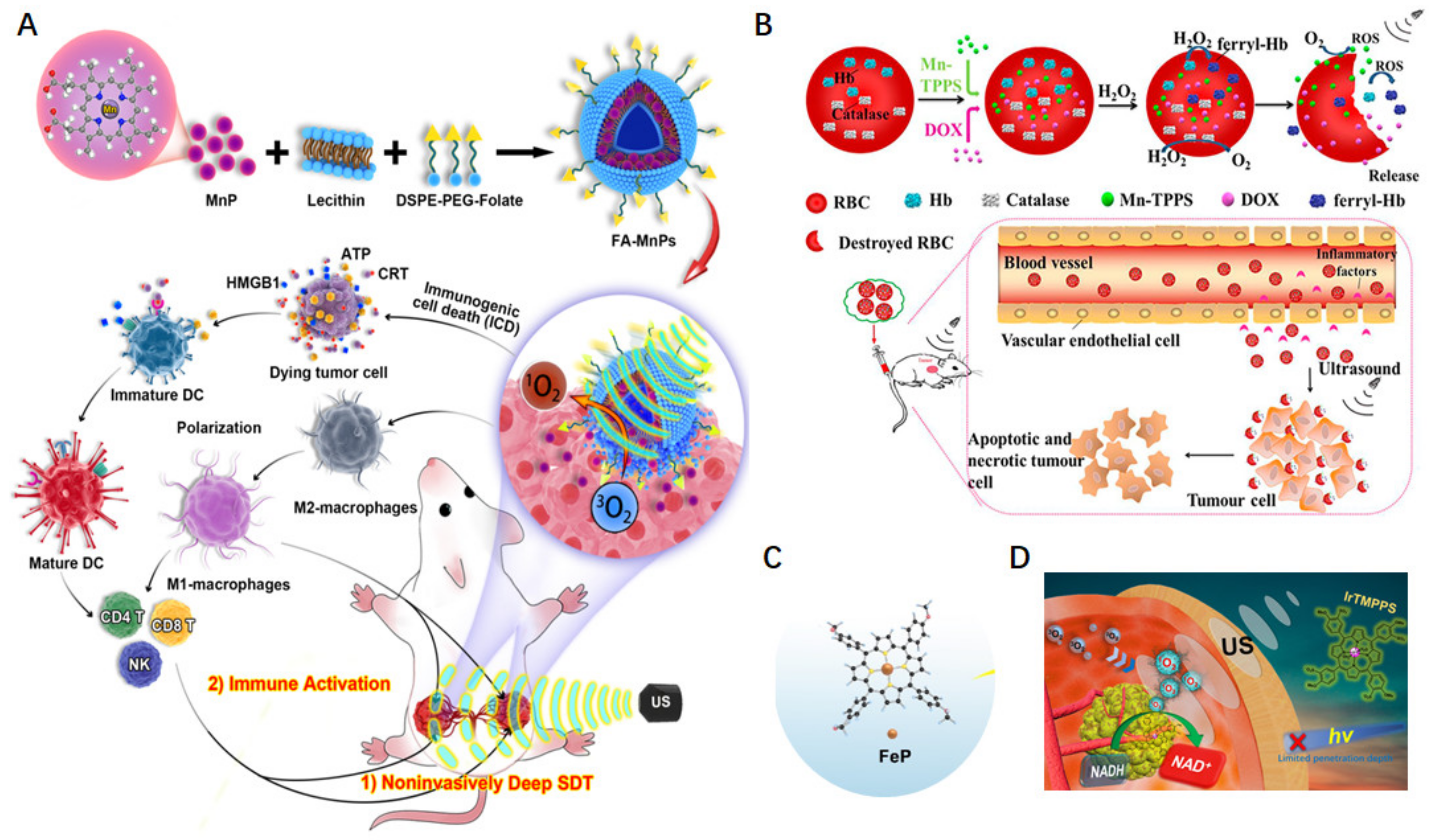
4.2. Synergistically Enhanced SDT Effect by Modulating Tumor Microenvironment
4.3. Metalloporphyrin Frameworks as Sonosensitizers for Enhanced Antitumor Effect
5. Combined PDT and SDT Based on Metalloporphyrins
6. Pharmacokinetics of Metalloporphyrins in Cancer Therapy
7. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Ilic, M.; Ilic, I. Cancer mortality in Serbia, 1991–2015: An age-period-cohort and joinpoint regression analysis. Cancer Commun. 2018, 38, 10. [Google Scholar] [CrossRef] [PubMed]
- Rollin, G.; Lages, J.; Shepelyansky, D.L. Wikipedia network analysis of cancer interactions and world influence. PLoS ONE 2019, 14, e0222508. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, M.; Meng, H.; Liu, Y.; Niu, W.; Zhou, Y.; Zhao, R.; Duan, Y.; Zeng, Z.; Li, X.; et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci. China Life Sci. 2019, 62, 640–647. [Google Scholar] [CrossRef]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Diesendruck, Y.; Benhar, I. Novel immune check point inhibiting antibodies in cancer therapy—Opportunities and challenges. Drug Resist. Updates 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Phour, A.; Gaur, V.; Banerjee, A.; Bhattacharyya, J. Recombinant protein polymers as carriers of chemotherapeutic agents. Adv. Drug Deliv. Rev. 2022, 190, 114544. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, P.; Wang, J.; Fang, Y.; Hwang, K.C. Advances of medical nanorobots for future cancer treatments. J. Hematol. Oncol. 2023, 16, 74. [Google Scholar] [CrossRef]
- Varadé, J.; Magadán, S.; González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell. Mol. Immunol. 2020, 18, 805–828. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Chang, M.; Wang, Z.; Dong, C.; Zhou, R.; Chen, L.; Huang, H.; Feng, W.; Wang, Z.; Wang, Y.; Chen, Y. Ultrasound-Amplified Enzyodynamic Tumor Therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis. Adv. Mater. 2022, 35, 2208817. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Yue, Y.; Li, Z.; Qiao, L.; Qi, G.; Ping, Y.; Liu, B. Smart Nanoassembly Enabling Activatable NIR Fluorescence and ROS Generation with Enhanced Tumor Penetration for Imaging-Guided Photodynamic Therapy. Adv. Mater. 2024, 36, 2404296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Yang, H.; Yu, L.; Xu, Y.; Sharma, A.; Yin, P.; Li, X.; Kim, J.S.; Sun, Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021, 50, 11227–11248. [Google Scholar] [CrossRef]
- Gong, F.; Cheng, L.; Yang, N.; Gong, Y.; Ni, Y.; Bai, S.; Wang, X.; Chen, M.; Chen, Q.; Liu, Z. Preparation of TiH(1.924) nanodots by liquid-phase exfoliation for enhanced sonodynamic cancer therapy. Nat. Commun. 2020, 11, 3712. [Google Scholar] [CrossRef]
- Di, Y.; Deng, R.; Liu, Z.; Mao, Y.; Gao, Y.; Zhao, Q.; Wang, S. Optimized strategies of ROS-based nanodynamic therapies for tumor theranostics. Biomaterials 2023, 303, 122391. [Google Scholar]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar]
- Liang, G.; Sadhukhan, T.; Banerjee, S.; Tang, D.; Zhang, H.; Cui, M.; Montesdeoca, N.; Karges, J.; Xiao, H. Reduction of Platinum(IV) Prodrug Hemoglobin Nanoparticles with Deeply Penetrating Ultrasound Radiation for Tumor-Targeted Therapeutically Enhanced Anticancer Therapy. Angew. Chem. Int. Ed. 2023, 62, e202301074. [Google Scholar] [CrossRef]
- Wu, A.; Jiang, L.; Xia, C.; Xu, Q.; Zhou, B.; Jin, Z.; He, Q.; Guo, J. Ultrasound-Driven Piezoelectrocatalytic Immunoactivation of Deep Tumor. Adv. Sci. 2023, 6, 2303016. [Google Scholar] [CrossRef]
- Guo, S.; Sun, X.; Cheng, J.; Xu, H.; Dan, J.; Shen, J.; Zhou, Q.; Zhang, Y.; Meng, L.; Cao, W.; et al. Apoptosis of THP-1 macrophages induced by protoporphyrin IX-mediated sonodynamic therapy. Int. J. Nanomed. 2013, 8, 2239–2246. [Google Scholar]
- Yumita, N.; Iwase, Y.; Nishi, K.; Komatsu, H.; Takeda, K.; Onodera, K.; Fukai, T.; Ikeda, T.; Umemura, S.; Okudaira, K.; et al. Involvement of reactive oxygen species in sonodynamically induced apoptosis using a novel porphyrin derivative. Theranostics 2012, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yu, W.; Chen, S.; Wang, Z.; Tian, Z.; He, J.; Liu, Y. Application of metalloporphyrin sensitizers for the treatment or diagnosis of tumors. J. Chem. Res. 2022, 46. [Google Scholar] [CrossRef]
- El-Mageed, A.I.A.A.; Ogawa, T. Metal Ion Effect on the Supramolecular Structures of Metalloporphyrins on Single-Walled Carbon Nanotube Surface. Appl. Surf. Sci. 2018, 462, 904–912. [Google Scholar]
- Liao, M.; Cui, J.; Yang, M.; Wei, Z.; Xie, Y.; Lu, C. Photoinduced electron transfer in metalloporphyrins. J. Mol. Struct. 2022, 1267, 133591. [Google Scholar] [CrossRef]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2010, 40, 340–362. [Google Scholar] [CrossRef]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Fomicheva, M.; Petrov, R.; Yabbarov, N. Metalloporphyrins in Medicine: From History to Recent Trends. ACS Appl. Bio Mater. 2020, 3, 8146–8171. [Google Scholar] [CrossRef]
- Jiang, L.; Chee, P.L.; Gao, J.; Gan, C.R.R.; Owh, C.; Lakshminarayanan, R.; Jiang, S.; Hor, T.S.A.; Loh, X.J. A New Potent Antimicrobial Metalloporphyrin. Chem.-Asian J. 2021, 16, 1007–1015. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin Nanoparticles: Coordinating Diverse Theranostic Functions. Coord. Chem. Rev. 2017, 379, 99–120. [Google Scholar]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. [Google Scholar] [CrossRef] [PubMed]
- Campanholi, K.d.S.S.; Braga, G.; da Silva, J.B.; da Rocha, N.L.; de Francisco, L.M.B.; de Oliveira, É.L.; Bruschi, M.L.; de Castro-Hoshino, L.V.; Sato, F.; Hioka, N.; et al. Biomedical Platform Development of a Chlorophyll-Based Extract for Topic Photodynamic Therapy: Mechanical and Spectroscopic Properties. Langmuir 2018, 34, 8230–8244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, H.; Ran, G.; Yao, Y.; Yang, Z.-S.; Ning, Y.; Yu, Y.; Zhang, R.; Peng, X.-X.; Wu, J.; et al. Bioinspired Design of seco-Chlorin Photosensitizers to Overcome Phototoxic Effects in Photodynamic Therapy. Angew. Chem. Int. Ed. 2022, 61, e202204330. [Google Scholar] [CrossRef] [PubMed]
- Klaper, M.; Fudickar, W.; Linker, T. Role of Distance in Singlet Oxygen Applications: A Model System. J. Am. Chem. Soc. 2016, 138, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Dai, Z. The molecular design of and challenges relating to sensitizers for cancer sonodynamic therapy. Mater. Chem. Front. 2020, 4, 2223–2234. [Google Scholar] [CrossRef]
- Lafond, M.; Yoshizawa, S.; Umemura, S.-I. Sonodynamic Therapy: Advances and Challenges in Clinical Translation. J. Ultrasound Med. 2019, 38, 567–580. [Google Scholar] [CrossRef]
- Wu, J.; Nyborg, W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008, 60, 1103–1116. [Google Scholar] [CrossRef]
- Misík, V.; Riesz, P. Free Radical Intermediates in Sonodynamic Therapy. Ann. N. Y. Acad. Sci. 2006, 899, 335–348. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Wang, W.; Cao, T.; Zhang, L.; Wang, Z.; Chi, X.; Shi, T.; Wang, H.; He, X.; et al. High-yield porphyrin production through metabolic engineering and biocatalysis. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Praneeth, V.K.K.; Näther, C.; Peters, G.; Lehnert, N. Spectroscopic properties and electronic structure of five- and six-coordinate iron(II) porphyrin NO complexes: Effect of the axial N-donor ligand. Inorg. Chem. 2006, 45, 2797–2811. [Google Scholar] [CrossRef]
- Carter, K.A.; Wang, S.; Geng, J.; Luo, D.; Shao, S.; Lovell, J.F. Metal Chelation Modulates Phototherapeutic Properties of Mitoxantrone-Loaded Porphyrin-Phospholipid Liposomes. Mol. Pharm. 2016, 13, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, M.; Zhang, J.; Zhu, S.; Shi, M.; Wang, K. Metalloporphyrin-indomethacin conjugates as new photosensitizers for photodynamic therapy. JBIC J. Biol. Inorg. Chem. 2018, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Kalt, M.; Larocca, M.; Babu, V.; Spingler, B. Potent PBS/Polysorbate-Soluble Transplatin-Derived Porphyrin-Based Photosensitizers for Photodynamic Therapy. Inorg. Chem. 2021, 60, 9416–9426. [Google Scholar] [CrossRef] [PubMed]
- El-Mahalawy, A.M.; Nawar, A.M.; Wassel, A.R. Efficacy assessment of metalloporphyrins as functional materials for photodetection applications: Role of central tetrapyrrole metal ions. J. Mater. Sci. 2022, 57, 15413–15439. [Google Scholar]
- Batinic-Haberle, I.; Tovmasyan, A.; Huang, Z.; Duan, W.; Du, L.; Siamakpour-Reihani, S.; Cao, Z.; Sheng, H.; Spasojevic, I.; Alvarez Secord, A. H(2)O(2)-Driven Anticancer Activity of Mn Porphyrins and the Underlying Molecular Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 6653790. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, C.; Chen, G.; Liu, B.; Deng, X.; Wei, Y.; Xia, J.; Xing, B.; Ma, P.A.; Lin, J. Synthesis and Optimization of MoS2@Fe3O4-ICG/Pt(IV) Nanoflowers for MR/IR/PA Bioimaging and Combined PTT/PDT/Chemotherapy Triggered by 808 nm Laser. Adv. Sci. 2017, 4, 1600540. [Google Scholar] [CrossRef]
- Baglia, R.A.; Zaragoza, J.P.T.; Goldberg, D.P. Biomimetic Reactivity of Oxygen-Derived Manganese and Iron Porphyrinoid Complexes. Chem. Rev. 2017, 117, 13320–13352. [Google Scholar] [CrossRef]
- Chen, H.; Ma, A.; Yin, T.; Chen, Z.; Liang, R.; Pan, H.; Shen, X.; Zheng, M.; Cai, L. In Situ Photocatalysis of TiO–Porphyrin-Encapsulated Nanosystem for Highly Efficient Oxidative Damage against Hypoxic Tumors. ACS Appl. Mater. Interfaces 2020, 12, 12573–12583. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.; Si, W.; Zhong, X.; Cai, Y.; Zou, J.; Shao, J.; Yang, Z.; Dong, X. Zinc(II) Metalated Porphyrins as Photothermogenic Photosensitizers for Cancer Photodynamic/Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 10, 238–247. [Google Scholar] [CrossRef]
- Tao, C.; Ren, Q.; Yu, N.; Wen, M.; Qiu, P.; Niu, S.; Chen, Z.; Li, K.; Xie, D. Design of multifunctional theranostic nanoplatforms with glutathione-triggered H2S generation and H2S-activitated multi-modal therapy. Chem. Eng. J. 2024, 495, 153602–153613. [Google Scholar] [CrossRef]
- Fu, X.; Cai, Z.; Fu, S.; Cai, H.; Li, M.; Gu, H.; Jin, R.; Xia, C.; Lui, S.; Song, B.; et al. Porphyrin-Based Self-Assembled Nanoparticles for PET/MR Imaging of Sentinel Lymph Node Metastasis. ACS Appl. Mater. Interfaces 2024, 16, 27139–27150. [Google Scholar] [CrossRef] [PubMed]
- Mouraviev, V.; Venkatraman, T.N.; Tovmasyan, A.; Kimura, M.; Tsivian, M.; Mouravieva, V.; Polascik, T.J.; Wang, H.; Amrhein, T.J.; Batinic-Haberle, I.; et al. Mn porphyrins as novel molecular magnetic resonance imaging contrast agents. J. Endourol. 2012, 26, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, Y.; Jalilian, A.R.; Amini, M.M.; Ardaneh, K.; Rahiminejad, A.; Bolourinovin, F.; Moradkhani, S.; Majdabadi, A. Development of a (68)Ga-Fluorinated Porphyrin Complex as a Possible PET Imaging Agent. Nucl. Med. Mol. Imaging 2012, 46, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ogawa, K.; Kiwada, T.; Odani, A. Water-soluble metalloporphyrinates with excellent photo-induced anticancer activity resulting from high tumor accumulation. J. Inorg. Biochem. 2017, 170, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, J.; Hou, M.; Yang, M.; Yi, C. Gadolinium–porphyrin based polymer nanotheranostics for fluorescence/magnetic resonance imaging guided photodynamic therapy. Nanoscale 2021, 13, 16197–16206. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhong, Y.; Zou, Y.; Wang, C.; Wu, H.; Lee, A.; Yang, W.; Wang, X.; Liu, Y.; et al. Central metal-derived co-assembly of biomimetic GdTPP/ZnTPP porphyrin nanocomposites for enhanced dual-modal imaging-guided photodynamic therapy. Biomaterials 2019, 229, 119576–119588. [Google Scholar] [CrossRef]
- Gao, P.; Hussain, M.Z.; Zhou, Z.; Warnan, J.; Elsner, M.; Fischer, R.A. Zr-based metalloporphyrin MOF probe for electrochemical detection of parathion-methyl. Biosens. Bioelectron. 2024, 261, 116515. [Google Scholar] [CrossRef]
- Leng, F.; Liu, H.; Ding, M.; Lin, Q.-P.; Jiang, H.-L. Boosting Photocatalytic Hydrogen Production of Porphyrinic MOFs: The Metal Location in Metalloporphyrin Matters. ACS Catal. 2018, 8, 4583–4590. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, Y.; Liu, M.; Wang, J.; Pei, R. Synthesis of Metal–Organic Framework Nanosheets with High Relaxation Rate and Singlet Oxygen Yield. Chem. Mater. 2018, 30, 7511–7520. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Lu, X.; Wu, P.; Sun, Z.; Chu, H.; Peng, H. Controllable growth of drug-encapsulated metal-organic framework (MOF) on porphyrinic MOF for PDT/chemo-combined therapy. Mater. Des. 2023, 228, 111861. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [PubMed]
- Horváth, O.; Valicsek, Z.; Fodor, M.A.; Major, M.M.; Imran, M.; Grampp, G.; Wankmüller, A. Visible light-driven photophysics and photochemistry of water-soluble metalloporphyrins. Coord. Chem. Rev. 2016, 325, 59–66. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, P.; Xie, J.; Song, C.; Tang, C.; Wang, Y.; Yin, X.; Cai, Y.; Han, W.; Dong, X. Carrier-free nano-integrated strategy for synergetic cancer anti-angiogenic therapy and phototherapy. Chem. Sci. 2019, 10, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Yin, H.-H.; Xu, X.-H.; Xu, G.; Zhang, Y.; Zhou, B.-G.; Yue, W.-W.; Liu, C.; Sun, L.-P.; Xu, H.-X.; et al. Tumor Metabolism-Engineered Composite Nanoplatforms Potentiate Sonodynamic Therapy via Reshaping Tumor Microenvironment and Facilitating Electron–Hole Pairs’ Separation. Adv. Funct. Mater. 2020, 30, 2000326. [Google Scholar] [CrossRef]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound-Activated Sensitizers and Applications. Angew. Chem. 2020, 59, 14212–14233. [Google Scholar] [CrossRef]
- Liang, S.; Yao, J.; Liu, D.; Rao, L.; Chen, X.; Wang, Z. Harnessing Nanomaterials for Cancer Sonodynamic Immunotherapy. Adv. Mater. 2023, 35, 2211130. [Google Scholar] [CrossRef]
- Chen, J.; Luo, H.; Liu, Y.; Zhang, W.; Li, H.; Luo, T.; Zhang, K.; Zhao, Y.; Liu, J. Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano 2017, 11, 12849–12862. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, X.; Cheng, L.; Liu, Y.; Chen, Y.; Jiang, Z.; Liu, J. Multiple stimuli-responsive nanosystem for potent, ROS-amplifying, chemo-sonodynamic antitumor therapy. Bioact. Mater. 2021, 15, 355–371. [Google Scholar] [CrossRef]
- Yuan, M.; Liang, S.; Zhou, Y.; Xiao, X.; Liu, B.; Yang, C.; Ma, P.A.; Cheng, Z.; Lin, J. A Robust Oxygen-Carrying Hemoglobin-Based Natural Sonosensitizer for Sonodynamic Cancer Therapy. Nano Lett. 2021, 21, 6042–6050. [Google Scholar] [CrossRef]
- Yumita, N.; Okudaira, K.; Momose, Y.; Umemura, S.-I. Sonodynamically induced apoptosis and active oxygen generation by gallium-porphyrin complex, ATX-70. Cancer Chemother. Pharmacol. 2010, 66, 1071–1078. [Google Scholar] [CrossRef]
- Kuroki, M.; Hachimine, K.; Abe, H.; Shibaguchi, H.; Kuroki, M.; Maekawa, S.-I.; Yanagisawa, J.; Kinugasa, T.; Tanaka, T.; Yamashita, Y. Sonodynamic therapy of cancer using novel sonosensitizers. Anticancer Res. 2007, 27, 3673–3678. [Google Scholar] [PubMed]
- Giuntini, F.; Foglietta, F.; Marucco, A.M.; Troia, A.; Dezhkunov, N.V.; Pozzoli, A.; Durando, G.; Fenoglio, I.; Serpe, L.; Canaparo, R. Insight into ultrasound-mediated reactive oxygen species generation by various metal-porphyrin complexes. Free Radic. Biol. Med. 2018, 121, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Chen, H.; Cui, Y.; Luo, Z.; Liang, R.; Wu, Z.; Chen, Z.; Yin, T.; Ni, J.; Zheng, M.; et al. Metalloporphyrin Complex-Based Nanosonosensitizers for Deep-Tissue Tumor Theranostics by Noninvasive Sonodynamic Therapy. Small 2019, 15, e1804028. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Qian, X.; Chen, Y.; Yu, L.; Lin, H.; Wang, L.; Zhu, Y.; Shi, J. Metalloporphyrin-Encapsulated Biodegradable Nanosystems for Highly Efficient Magnetic Resonance Imaging-Guided Sonodynamic Cancer Therapy. J. Am. Chem. Soc. 2017, 139, 1275–1284. [Google Scholar] [CrossRef]
- Ma, A.; Ran, H.; Wang, J.; Ding, R.; Lu, C.; Liu, L.; Luo, Y.; Chen, H.; Yin, T. An Urchin-Shaped Copper-Based Metalloporphyrin Nanosystem as a Sonosensitizer for Sonodynamic Therapy. Nanomaterials 2022, 12, 209. [Google Scholar] [CrossRef]
- Wang, H.; Guo, J.; Lin, W.; Fu, Z.; Ji, X.; Yu, B.; Lu, M.; Cui, W.; Deng, L.; Engle, J.W.; et al. Open-Shell Nanosensitizers for Glutathione Responsive Cancer Sonodynamic Therapy. Adv. Mater. 2022, 34, 2110283–2110290. [Google Scholar] [CrossRef]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Ma, A.; Yin, T.; Chen, Z.; Liang, R.; Qiu, Y.; Zheng, M.; Cai, L. Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer. Biomaterials 2021, 269, 120639. [Google Scholar] [CrossRef]
- Du, B.; Yan, X.; Ding, X.; Wang, Q.; Du, Q.; Xu, T.; Shen, G.; Yao, H.; Zhou, J. Oxygen Self-Production Red Blood Cell Carrier System for MRI Mediated Cancer Therapy: Ferryl-Hb, Sonodynamic, and Chemical Therapy. ACS Biomater. Sci. Eng. 2018, 4, 4132–4143. [Google Scholar] [CrossRef]
- Yin, T.; Chen, H.; Ma, A.; Pan, H.; Chen, Z.; Tang, X.; Huang, G.; Liao, J.; Zhang, B.; Zheng, M.; et al. Cleavable collagenase-assistant nanosonosensitizer for tumor penetration and sonodynamic therapy. Biomaterials 2023, 293, 121992. [Google Scholar] [CrossRef]
- Xie, J.; Liang, C.; Luo, S.; Pan, Z.; Lai, Y.; He, J.; Chen, H.; Ren, Q.; Huang, H.; Zhang, Q.; et al. Water-Soluble Iridic–Porphyrin Complex for Non-invasive Sonodynamic and Sono-oxidation Therapy of Deep Tumors. ACS Appl. Mater. Interfaces 2021, 13, 27934–27944. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, J.; Qiu, H.; Zhang, C.; Huang, P.; Mao, Z.; Tong, W. Erythrocyte Membrane-Camouflaged PCN-224 Nanocarriers Integrated with Platinum Nanoparticles and Glucose Oxidase for Enhanced Tumor Sonodynamic Therapy and Synergistic Starvation Therapy. ACS Appl. Mater. Interfaces 2021, 13, 24532–24542. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Du, J.; Yang, J.; Li, Y.; Ohulchanskyy, T.Y.; Fang, X.; Chen, G. Metalloporphyrin MOFs-Based Nanoagent Enabling Tumor Microenvironment Responsive Sonodynamic Therapy of Intracranial Glioma Signaled by NIR-IIb Luminescence Imaging. Adv. Funct. Mater. 2023, 34, 2307816–2307828. [Google Scholar] [CrossRef]
- Niu, H.; Chen, J.; Jin, J.; Qi, X.; Bai, K.; Shu, C.; Wu, A.; Xiao, Y.; Wu, C.; Bu, H.; et al. Engineering metalloporphyrin-integrated nanosystems for targeted sono-/chemo- dynamic therapy of leptomeningeal carcinomatosis through intrathecal administration. Chem. Eng. J. 2022, 437, 135373. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Liu, Q.; Wang, X. Sinoporphyrin sodium triggered sono-photodynamic effects on breast cancer both in vitro and in vivo. Ultrason. Sonochem. 2016, 31, 437–448. [Google Scholar] [CrossRef]
- Geng, P.; Li, Y.; Macharia, D.K.; Ren, X.; Meng, R.; Wang, W.; Lan, H.; Xiao, S. One Stone, Three Birds: Design and Synthesis of “All-in-One” Nanoscale Mn-Porphyrin Coordination Polymers for Magnetic Resonance Imaging-Guided Synergistic Photodynamic-Sonodynamic Therapy. J. Colloid Interface Sci. 2024, 660, 1021–1029. [Google Scholar] [CrossRef]
- Xu, P.; Wen, C.; Gao, C.; Liu, H.; Li, Y.; Guo, X.; Shen, X.-C.; Liang, H. Near-Infrared-II-Activatable Self-Assembled Manganese Porphyrin-Gold Heterostructures for Photoacoustic Imaging-Guided Sonodynamic-Augmented Photothermal/Photodynamic Therapy. ACS Nano 2023, 18, 713–727. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Li, D.; Zhu, L.; Cai, X.; Liu, L.; Pan, H.; Ma, A. Application and Challenge of Metalloporphyrin Sensitizers in Noninvasive Dynamic Tumor Therapy. Molecules 2024, 29, 4828. https://doi.org/10.3390/molecules29204828
Ouyang J, Li D, Zhu L, Cai X, Liu L, Pan H, Ma A. Application and Challenge of Metalloporphyrin Sensitizers in Noninvasive Dynamic Tumor Therapy. Molecules. 2024; 29(20):4828. https://doi.org/10.3390/molecules29204828
Chicago/Turabian StyleOuyang, Jiacheng, Dan Li, Lizhen Zhu, Xiaoyuan Cai, Lanlan Liu, Hong Pan, and Aiqing Ma. 2024. "Application and Challenge of Metalloporphyrin Sensitizers in Noninvasive Dynamic Tumor Therapy" Molecules 29, no. 20: 4828. https://doi.org/10.3390/molecules29204828
APA StyleOuyang, J., Li, D., Zhu, L., Cai, X., Liu, L., Pan, H., & Ma, A. (2024). Application and Challenge of Metalloporphyrin Sensitizers in Noninvasive Dynamic Tumor Therapy. Molecules, 29(20), 4828. https://doi.org/10.3390/molecules29204828






