The Experimental and Modeling Study on the Thermodynamic Equilibrium Hydrate Formation Pressure of Helium-Rich Natural Gas in the Presence of Tetrahydrofuran
Abstract
1. Introduction
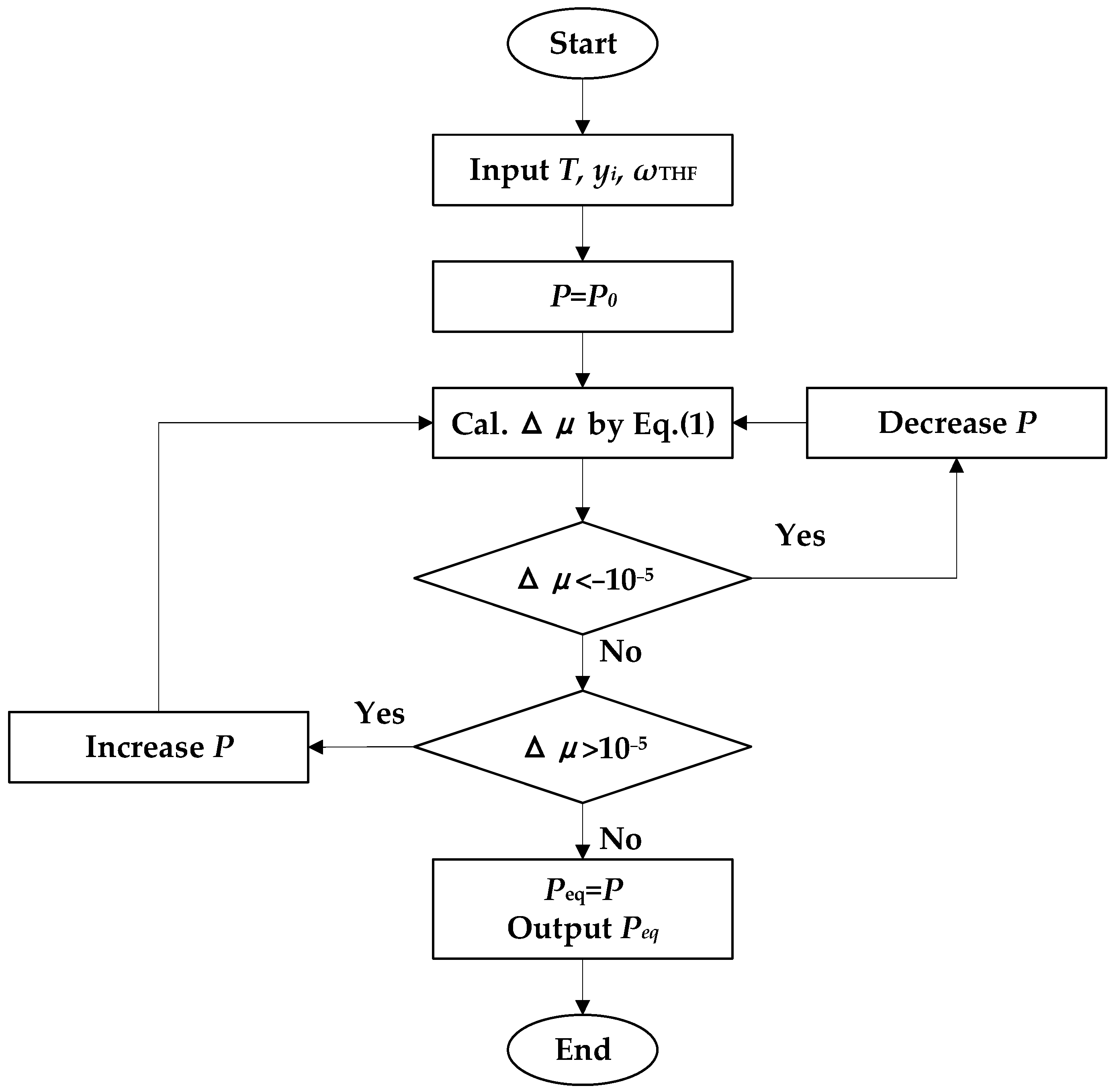
2. Model
3. Results
3.1. Single Gas System
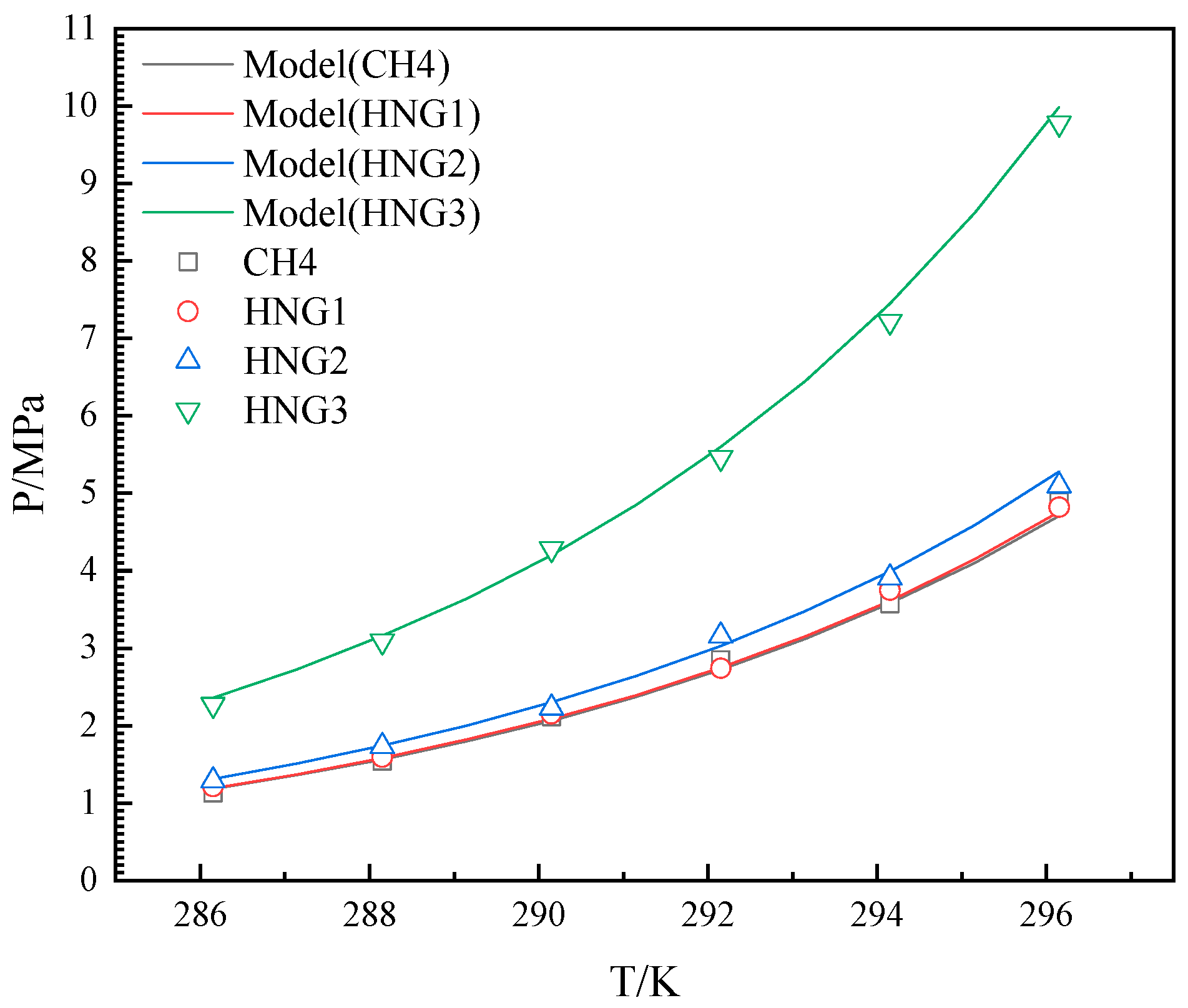
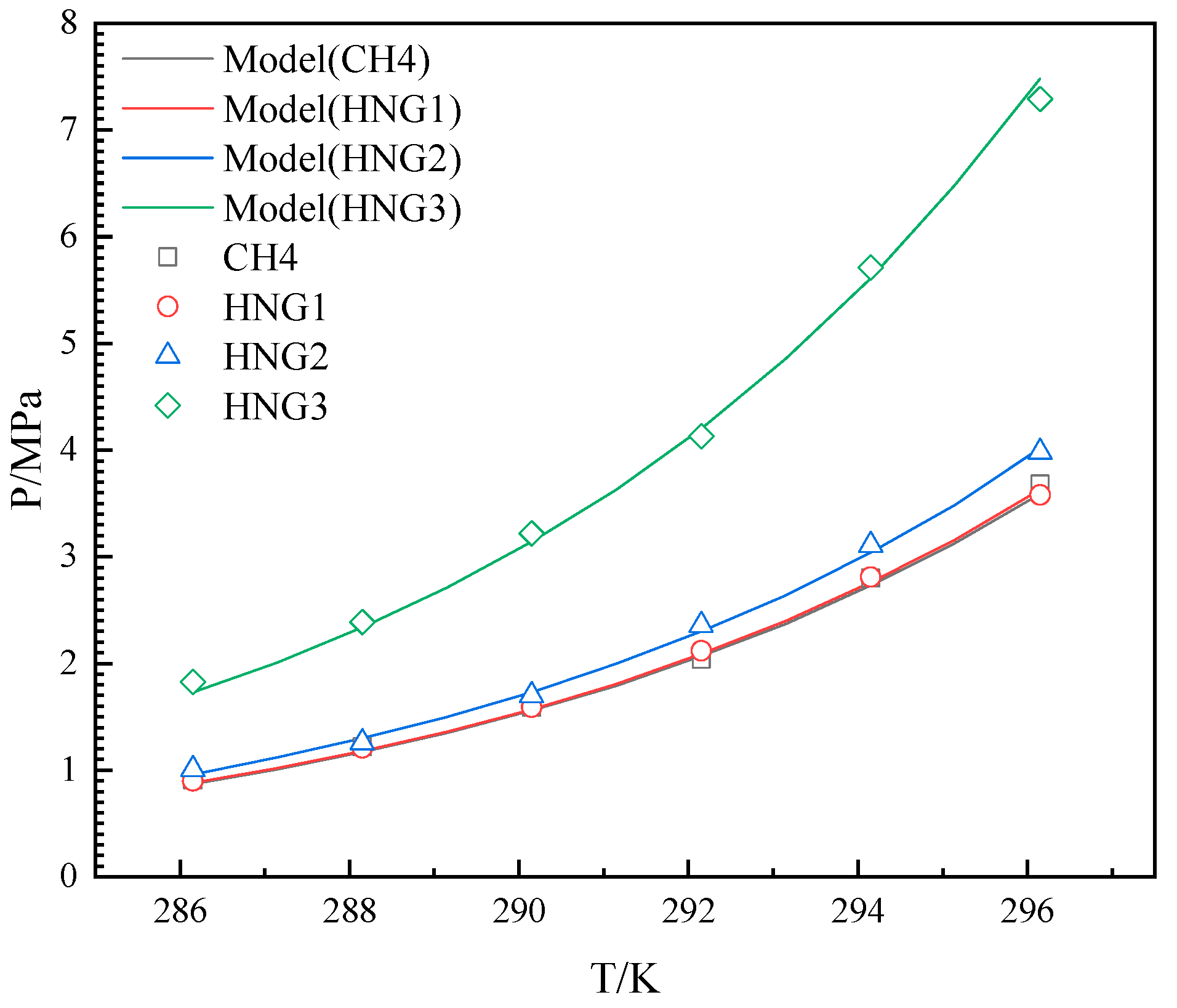
3.2. Binary Gas System
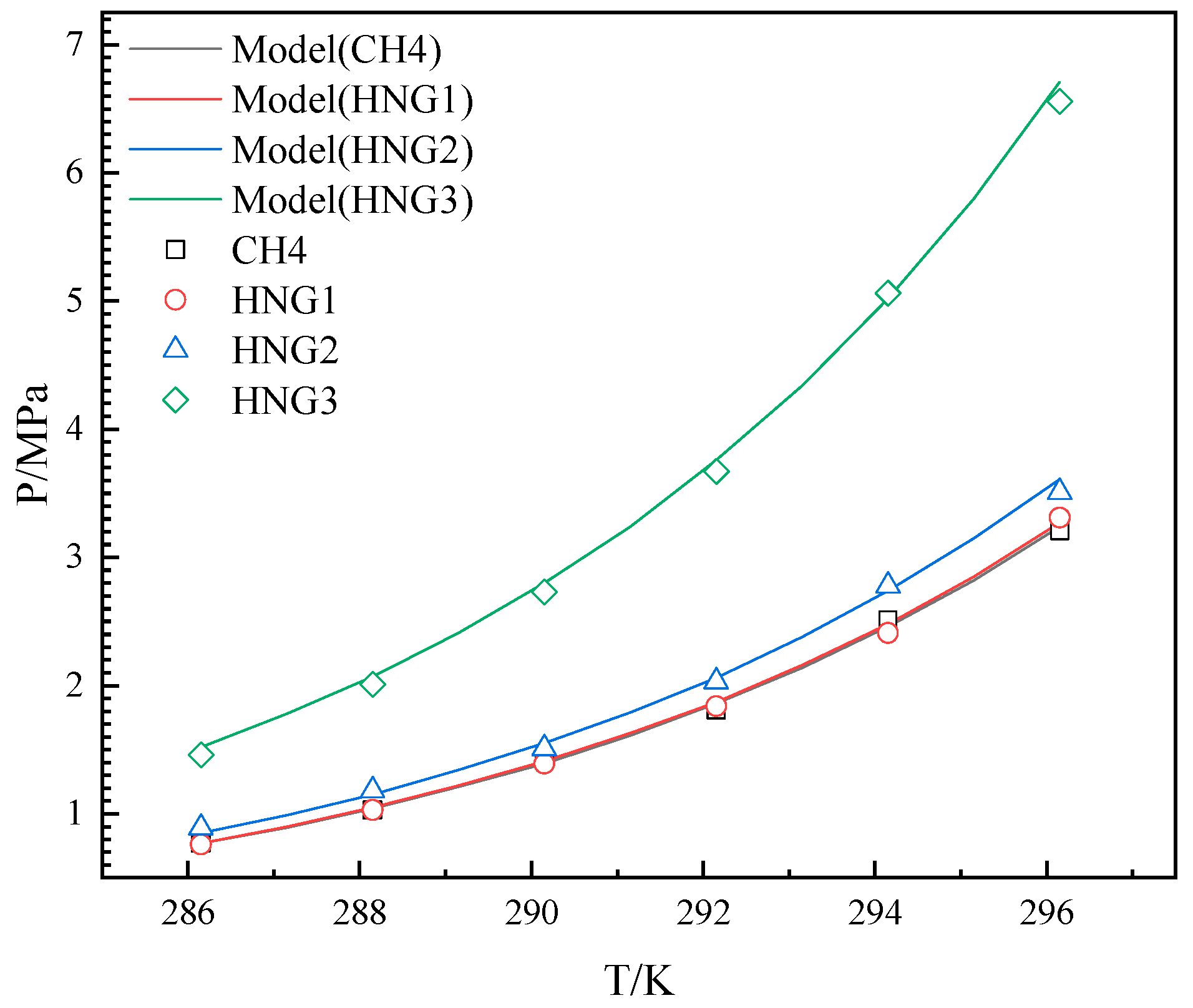
3.3. Ternary Gas System
4. Discussion
4.1. The Effects of Helium
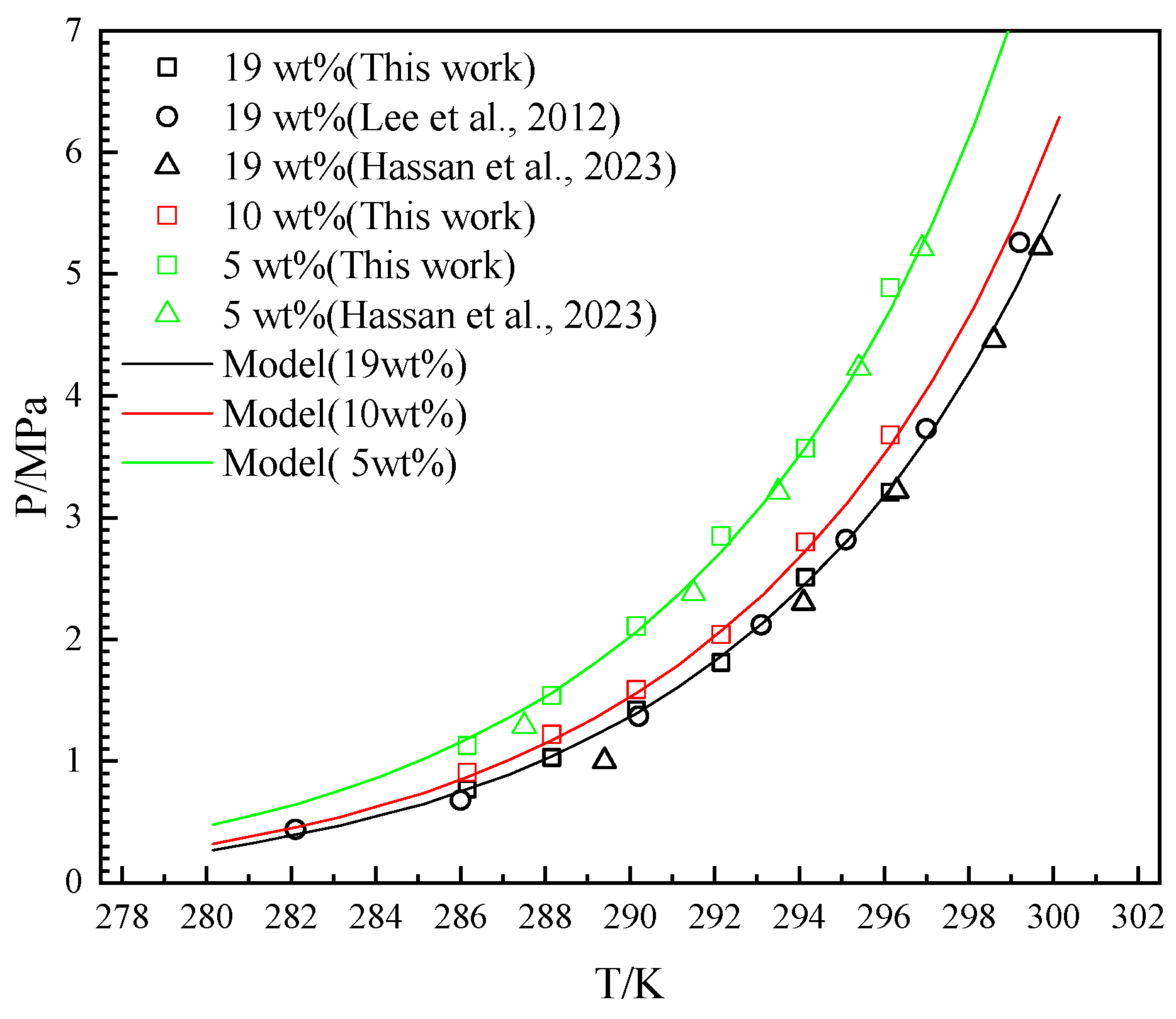
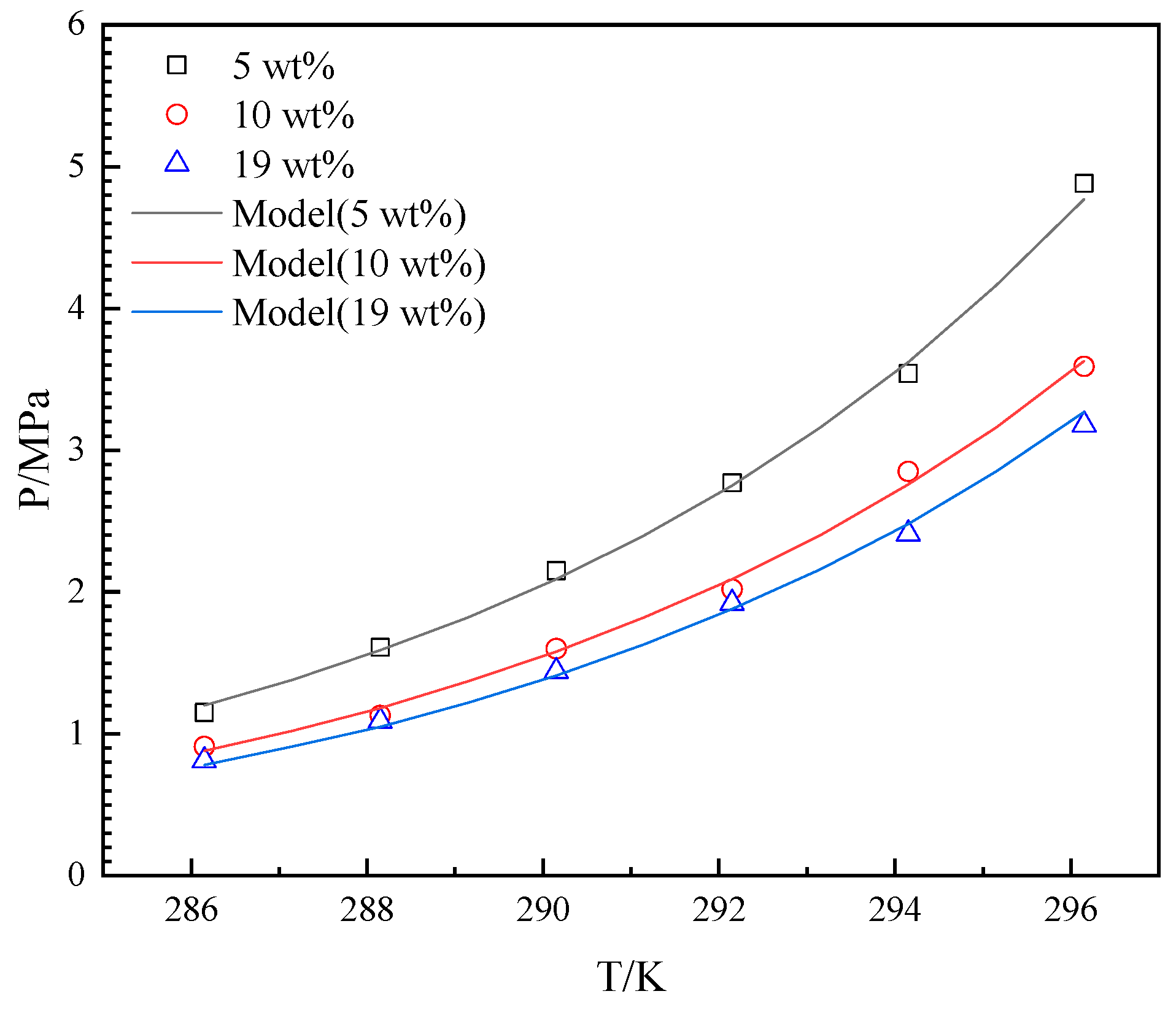
4.2. The Effects of THF
4.3. The Effects on Peq in the Model
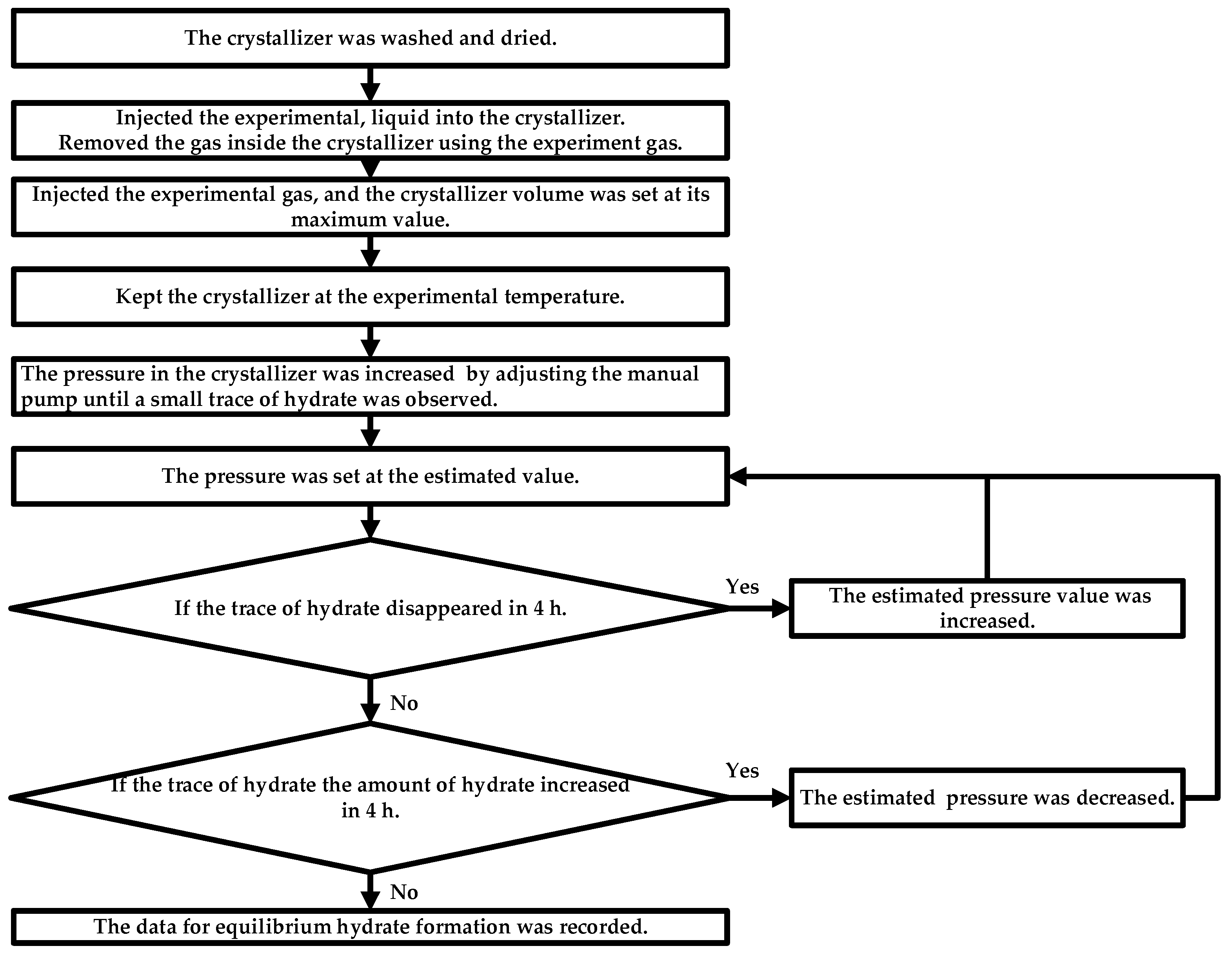
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviation | |
| ARD | average relative deviation |
| EoS | equation of state |
| GF | goodness of fit |
| HBGS | hydrate-based gas separation |
| HNG | helium-rich natural gas |
| NG | natural gas |
| PT | Patel–Teja |
| sI | structure I |
| sII | structure II |
| THF | tetrahydrofuran |
| Symbols | |
| the Antoine parameters of | |
| the binary interaction parameter between gas i and THF in the hydrate | |
| a | the parameters of PT EoS |
| the | |
| b | the parameters of PT EoS |
| the | |
| the Langmuir constant of gas i | |
| c | the parameters of PT EoS |
| F | the constant associated with the gas molecule in the PT EoS |
| correction factor for | |
| the fugacity of CH4, CO2, and helium | |
| the fugacity of THF in the liquid phase | |
| the fugacity of THF in the basic hydrate | |
| the Peq for HNG | |
| the Peq for pure CH4 | |
| Peq | thermodynamic equilibrium hydrate formation pressure |
| the difference in Peq | |
| saturated vapor pressure of THF | |
| R | the gas constant (8.314 J K−1 mol−1) |
| the mole volumes of water | |
| the mole volumes of THF | |
| the mole fractions of water | |
| the mole fractions of THF | |
| the | |
| the mole fractions of gases in the gas phase | |
| the | |
| the | |
| the activity of THF | |
| the activity of water | |
| β | the parameter of hydrate structure |
| the activity coefficients of water | |
| the activity coefficients of THF | |
| ζ | the constant associated with the gas molecule in the PT EoS |
| the occupation fraction of the linked cages in hydrates filled by gas i | |
| the parameter of Wilson model | |
| the parameter of Wilson model | |
| the parameter of Wilson model | |
| the parameter of Wilson model | |
| the ratio of the linked-cage number to the water-molecule number | |
| Δµ | the difference in chemical potential |
| the mass fraction of THF |
Appendix A. Calculation for the Fugacity of Gases
| Gases | ζ | F |
|---|---|---|
| CH4 | 0.324 | 0.455336 |
| CO2 | 0.309 | 0.707727 |
| helium | 0.329 | 0.452413 |
| THF | 0.313 | 0.733317 |

References
- Zhang, P.; Gong, C.; Zhou, T.; Du, P.; Song, J.; Shi, M.; Wang, X.; Gu, X. Helium extraction from natural gas using DD3R zeolite membranes. Chin. J. Chem. Eng. 2022, 49, 122–129. [Google Scholar] [CrossRef]
- Wu, X.; Jia, P.; Jia, W.; Li, C. A new process for high-efficiency crude helium extraction and purification from natural gas. Gas Sci. Eng. 2024, 124, 205278. [Google Scholar] [CrossRef]
- Grenev, I.V.; Gavrilov, V.Y. High-throughput screening of Metal−Organic frameworks for helium recovery from natural gas. Microporous Mesoporous Mater. 2024, 368, 113021. [Google Scholar] [CrossRef]
- Al-Sobhi, S.A.; Alnouss, A.; Alsaba, W.; Elkamel, A. Sustainable design and analysis for helium extraction from sale gas in liquefied natural gas production. J. Nat. Gas Sci. Eng. 2022, 102, 104599. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Liu, W.; Zhang, D.; Li, X. Main characteristics and effectiveness analysis of potential helium source rocks in crust-source helium-rich natural gas reservoirs. J. Nat. Gas Geosci. 2024, 9, 209–218. [Google Scholar] [CrossRef]
- Choi, S.; Sultan, M.M.B.; Alsuwailem, A.A.; Zuabi, S.M. Preparation and characterization of multilayer thin-film composite hollow fiber membranes for helium extraction from its mixtures. Sep. Purif. Technol. 2019, 222, 152–161. [Google Scholar] [CrossRef]
- Yu, L.; Mayne, B.; Nobandegani, M.S.; Grekou, T.; Hedlund, J. Recovery of helium from natural gas using MFI membranes. J. Membr. Sci. 2022, 644, 120113. [Google Scholar] [CrossRef]
- Weh, R.; Xiao, G.; Pouya, E.S.; May, E.F. Direct helium recovery from natural gas by dual reflux pressure swing adsorption cascade. Chem. Eng. J. 2022, 450, 137894. [Google Scholar] [CrossRef]
- Krishnan, K.; Potter, A.L.; Koh, C.A.; Carreon, M.A. Helium recovery from natural gas over CC3 membranes. J. Membr. Sci. Lett. 2023, 3, 100042. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, Y.; Liu, Z.; Xu, T.; Sun, Q.; Liu, A.; Yang, L.; Gong, J.; Guo, X. The hydrate-based separation of hydrogen and ethylene from fluid catalytic cracking dry gas in presence of n-octyl-β-d-glucopyranoside. Int. J. Hydrogen Energy 2022, 47, 31350–31369. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, P.; Li, H. Optimization of co-production process of cryogenic helium concentration and liquefied natural gas. Appl. Therm. Eng. 2023, 225, 120153. [Google Scholar] [CrossRef]
- Sun, Q.; Yuan, G.; Liu, Z.; Gao, J.; Wang, Y.; Guo, X.; Yang, L. Directional separation of hydrogen-containing gas mixture by hydrate-membrane coupling method. Int. J. Hydrogen Energy 2022, 47, 14580–14588. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Z.; Wu, Y.; Luo, J.; Liu, Z.; Wang, Y.; Sun, Q.; Guo, X. Simulation and optimization of hydrogen separation from hydrogenation tail gas by hydrate-membrane coupled method. Int. J. Hydrogen Energy 2024, 64, 58–64. [Google Scholar] [CrossRef]
- Han, G.; Lee, W.; Kim, M.; Lee, J.W.; Ahn, Y. Hydrogen separation from hydrogen-compressed natural gas blends through successive hydrate formations. Chem. Eng. J. 2024, 483, 149409. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Liu, Y.; Sun, Q.; Xu, Z.; Liu, A.; Wang, Y.; Guo, X. Thermodynamic effects of the interaction of multiple solutes and dodecahedral-cage deformation on the semi-clathrate hydrate formation with CH4-CO2. Chem. Eng. Sci. 2023, 269, 118468. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, M.; Pang, W.; Liu, Z.; Xu, Z.; Lei, X. The Phase Equilibria of Natural Gas Hydrate in the Presence of 1,3-Dimethylcyclohexane and Octyl-β-D-glucopyranoside. Molecules 2024, 29, 3604. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Nouri, S.; Aghajanloo, M.; Mohammadi, A.H.; Mohammadi, S. Experimental measurements and thermodynamic modeling of hydrate dissociation conditions for CO2 + THF + MgCl2 + water systems. Fluid Phase Equilib. 2023, 564, 113626. [Google Scholar] [CrossRef]
- Smith, C.; Barifcani, A.; Pack, D. Helium substitution of natural gas hydrocarbons in the analysis of their hydrate. J. Nat. Gas Sci. Eng. 2016, 35, 1293–1300. [Google Scholar] [CrossRef]
- Li, Z.; Han, T.; Lai, W.; Ma, J.; Zhang, Y.; Wu, Q.; Wang, C.; Liao, C.; Luo, S. Enhanced plasticization resistance of hollow fiber membranes for helium recovery from natural gas based on a novel thermally crosslinkable polyimide. J. Membr. Sci. 2023, 688, 122126. [Google Scholar] [CrossRef]
- Maekawa, T. Gas Hydrate Formation for Mixtures of Methane + Helium and Ethane + Helium. J. Chem. Eng. Data 2003, 48, 1283–1285. [Google Scholar] [CrossRef]
- Lee, Y.; Kawamura, T.; Yamamoto, Y.; Yoon, J. Phase Equilibrium Studies of Tetrahydrofuran (THF) + CH4, THF + CO2, CH4 + CO2, and THF + CO2 + CH4 Hydrates. J. Chem. Eng. Data 2012, 57, 3543–3548. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Z.; Qiu, X.; Zhu, M.; Li, C.; Zhang, A.; Li, J.; Li, C.; Huang, H. Hydrate Dissociation Equilibrium Conditions for Carbon Dioxide + Tetrahydrofuran. J. Chem. Eng. Data 2017, 62, 812–815. [Google Scholar] [CrossRef]
- Kumar, A.; Yeo, C.S.H.; Kumar, S.; Linga, P. Calorimetric Assessment of Ternary Methane–Carbon Dioxide–Tetrahydrofuran (CH4–CO2–THF) Hydrates: Application in Storage and Transport of CO2 Lean Natural Gas. Energy Fuels 2021, 35, 13249–13255. [Google Scholar] [CrossRef]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Stubos, A.K.; Martin, A.; Rovetto, L.J.; Florusse, L.J.; Peters, C.J. Experimental and computational investigation of the sII binary He-THF hydrate. J. Phys. Chem. B 2011, 115, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Hu, Z.; Li, Y.; Sun, Q.; Liu, A.; Yang, L.; Gong, J.; Guo, X. The Thermodynamic and Kinetic Effects of Sodium Lignin Sulfonate on Ethylene Hydrate Formation. Energies 2021, 14, 3291. [Google Scholar] [CrossRef]
- Khurana, M.; Veluswamy, H.P.; Daraboina, N.; Linga, P. Thermodynamic and kinetic modelling of mixed CH4-THF hydrate for methane storage application. Chem. Eng. J. 2019, 370, 760–771. [Google Scholar] [CrossRef]
- Zheng, R.; Li, X.; Negahban, S. Phase boundary of gas hydrates in single and mixed electrolyte solutions: Using a novel unified equation of state. J. Mol. Liq. 2022, 345, 117825. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, Z.; Li, X.; Fan, Z.; Negahban, S. Structural and dynamic analyses of CH4-C2H6-CO2 hydrates using thermodynamic modeling and molecular dynamic simulation. J. Chem. Thermodyn. 2022, 169, 106749. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, X.; Chapman, W.G.; Liu, A.; Yang, L.; Zhang, J. Vapor–hydrate two-phase and vapor–liquid–hydrate three-phase equilibrium calculation of THF/CH4/N2 hydrates. Fluid Phase Equilib. 2015, 401, 70–76. [Google Scholar] [CrossRef]
- Chen, G.; Guo, T. Thermodynamic modeling of hydrate formation based on new concepts. Fluid Phase Equilib. 1996, 122, 43–65. [Google Scholar] [CrossRef]
- Chen, G.; Guo, T. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Patel, N.C.; Teja, A.S. A new cubic equation of state for fluids and fluid mixtures. Chem. Eng. Sci. 1982, 37, 463–473. [Google Scholar] [CrossRef]
- Ivanov, E.V. To the issue of temperature-dependent behavior of standard molar volumes of components in the binary system (water + tetrahydrofuran) at ambient pressure. J. Chem. Thermodyn. 2014, 72, 37–43. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, J.; Gao, J.; Xu, Z.; Wang, Y.; Guo, X. Experimental and Modeling Study on Phase Equilibria of the Methane + Ethane Gas Mixture. J. Chem. Eng. Data 2023, 68, 2345–2352. [Google Scholar] [CrossRef]
- Hassan, H.; Romanos, J. Effects of Sea Salts on the Phase Behavior and Synthesis of Methane Hydrates + THF: An Experimental and Theoretical Study. Ind. Eng. Chem. Res. 2023, 62, 12305–12314. [Google Scholar] [CrossRef]
- Zheng, R.; Fan, Z.; Li, X.; Negahban, S. Phase behavior of high-pressure CH4-CO2 hydrates in NaCl solutions. Fuel 2020, 280, 118549. [Google Scholar] [CrossRef]




| (Pa) | (K) | (K) | |
|---|---|---|---|
| () | 6.2728 × 10−15 | 4879.29 | 23.01 |
| 1.6464 × 10−11 | 2799.66 | 15.90 | |
| 6.0000 × 10−12 | 2034.89 | 6.31 |
| Gases | Liquids | ARD | GF | SD |
|---|---|---|---|---|
| HNG1 | 5 wt% THF | 1.7% | 0.999 | 0.068 |
| HNG2 | 2.5% | 0.998 | 0.101 | |
| HNG3 | 2.7% | 0.999 | 0.151 | |
| HNG1 | 10 wt%THF | 1.8% | 0.999 | 0.036 |
| HNG2 | 2.6% | 0.998 | 0.050 | |
| HNG3 | 2.7% | 0.999 | 0.108 | |
| HNG1 | 19 wt% THF | 1.7% | 0.999 | 0.037 |
| HNG2 | 2.6% | 0.999 | 0.053 | |
| HNG3 | 2.5% | 0.999 | 0.086 | |
| HNG4 | 5 wt% THF | 2.3% | 0.999 | 0.065 |
| 10 wt% THF | 2.8% | 0.998 | 0.055 | |
| 19 wt% THF | 2.9% | 0.999 | 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, G.; Lu, F.; Ren, Q.; Xu, Z.; Fan, S.; Sun, Q.; Wang, Y.; Guo, X. The Experimental and Modeling Study on the Thermodynamic Equilibrium Hydrate Formation Pressure of Helium-Rich Natural Gas in the Presence of Tetrahydrofuran. Molecules 2024, 29, 4827. https://doi.org/10.3390/molecules29204827
Liu Z, Zhang G, Lu F, Ren Q, Xu Z, Fan S, Sun Q, Wang Y, Guo X. The Experimental and Modeling Study on the Thermodynamic Equilibrium Hydrate Formation Pressure of Helium-Rich Natural Gas in the Presence of Tetrahydrofuran. Molecules. 2024; 29(20):4827. https://doi.org/10.3390/molecules29204827
Chicago/Turabian StyleLiu, Zengqi, Guangqi Zhang, Fangfang Lu, Qiyuan Ren, Zhen Xu, Shiguang Fan, Qiang Sun, Yiwei Wang, and Xuqiang Guo. 2024. "The Experimental and Modeling Study on the Thermodynamic Equilibrium Hydrate Formation Pressure of Helium-Rich Natural Gas in the Presence of Tetrahydrofuran" Molecules 29, no. 20: 4827. https://doi.org/10.3390/molecules29204827
APA StyleLiu, Z., Zhang, G., Lu, F., Ren, Q., Xu, Z., Fan, S., Sun, Q., Wang, Y., & Guo, X. (2024). The Experimental and Modeling Study on the Thermodynamic Equilibrium Hydrate Formation Pressure of Helium-Rich Natural Gas in the Presence of Tetrahydrofuran. Molecules, 29(20), 4827. https://doi.org/10.3390/molecules29204827








