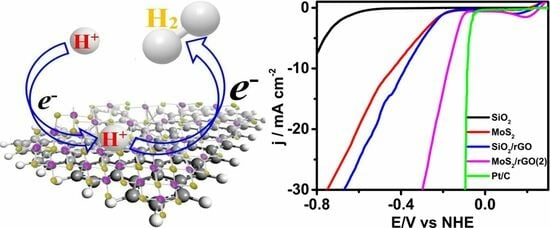
Nanocatalysis MoS2/rGO: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
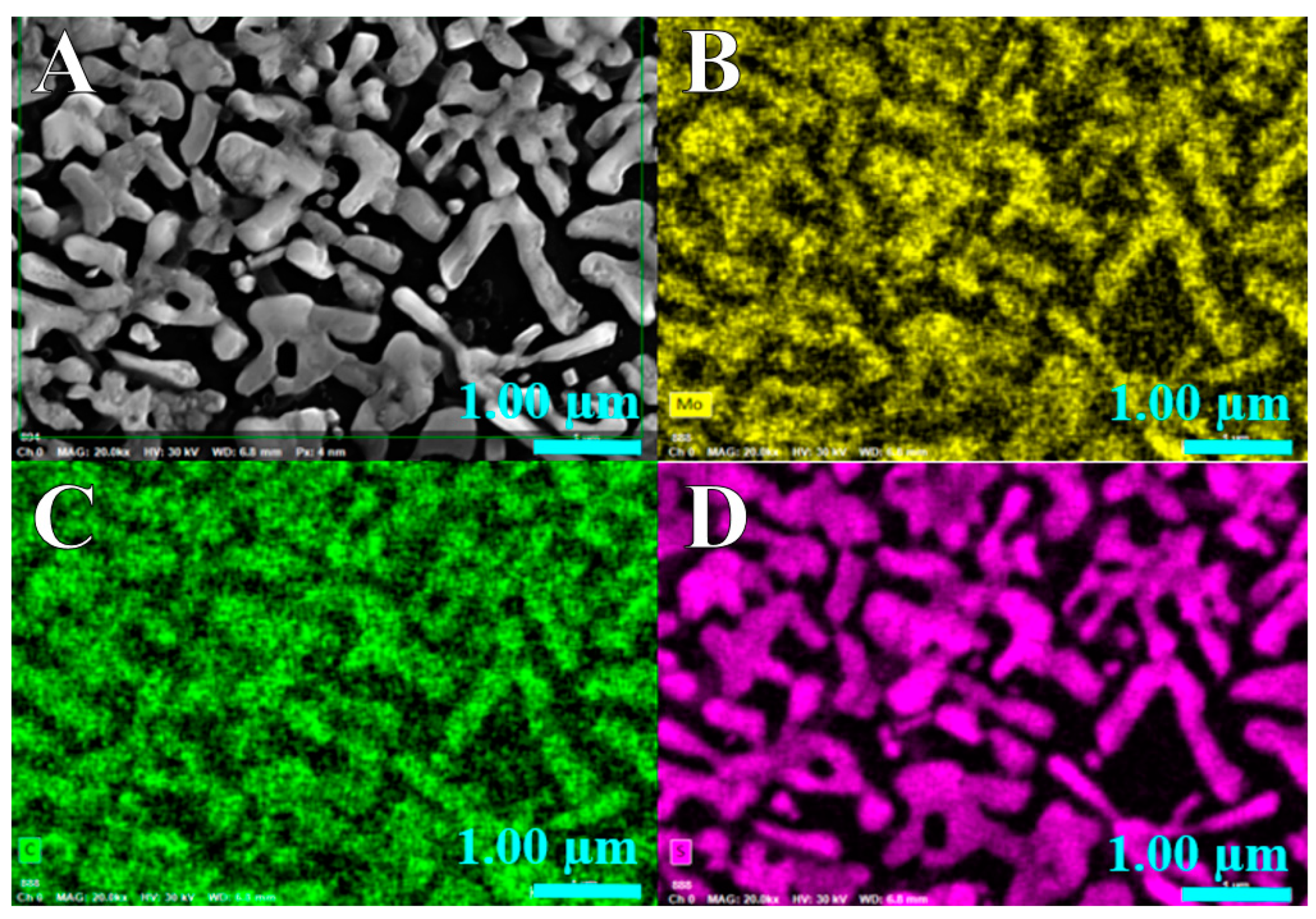
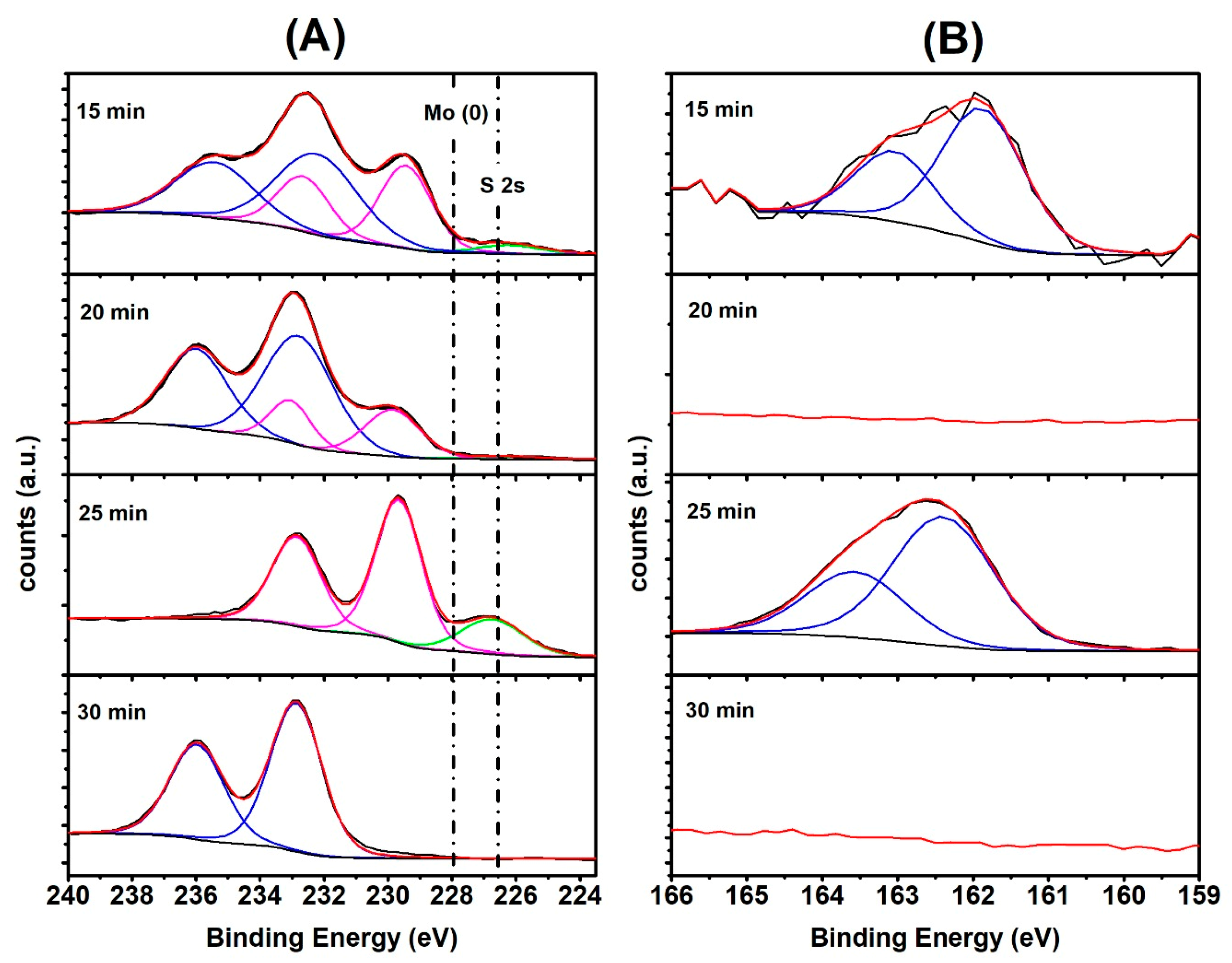
2.1. Materials’ Characterization by SEM, EDX, XPS and Raman
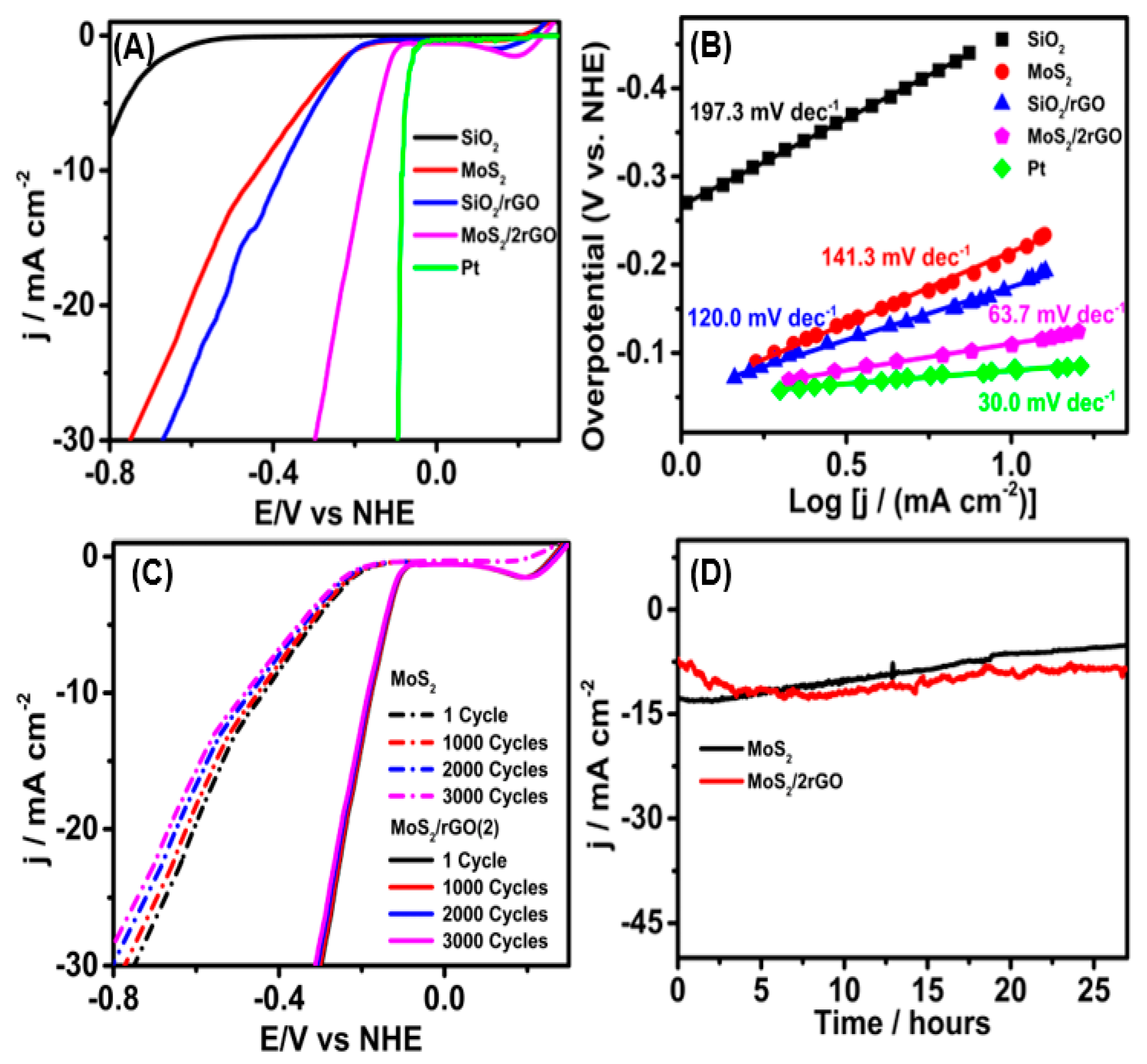
2.2. Electrochemical Characterization
3. Experimental Section
3.1. Chemicals and Apparatus
3.2. Materials Synthesis
3.3. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegelman, R.L.; Milner, P.J.; Kim, E.J.; Weston, S.C.; Long, J.R. Challenges and Opportunities for Adsorption-Based CO2 Capture from Natural Gas Combined Cycle Emissions. Energy Environ. Sci. 2019, 12, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.B.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; et al. Enhanced Catalytic Activity in Strained Chemically Exfoliated WS2 Nanosheets for Hydrogen Evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A Comprehensive Review on Cultivation and Harvesting of Microalgae for Biodiesel Production: Environmental Pollution Control and Future Directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jia, J.; Wang, A.; Zhao, L.; Xiong, T.; Liu, H.; Zhou, W. Confined Distribution of Platinum Clusters on MoO2 Hexagonal Nanosheets with Oxygen Vacancies as a High-Efficiency Electrocatalyst for Hydrogen Evolution Reaction. Nano Energy 2019, 62, 127–135. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, C.; Liu, K.; Hung, S.-F.; Ou, D.; Chen, H.M.; Fu, G.; Zheng, N. Identifying the Electrocatalytic Sites of Nickel Disulfide in Alkaline Hydrogen Evolution Reaction. Nano Energy 2017, 41, 148–153. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Fernandes, C.G.; Hood, Z.D.; Peng, R.; Wu, Z.; Dourado, A.H.B.; Parreira, L.S.; de Oliveira, D.C.; Camargo, P.H.C.; de Torresi, S.I.C. PdPt-TiO2 Nanowires: Correlating Composition, Electronic Effects and O-Vacancies with Activities towards Water Splitting and Oxygen Reduction. Appl. Catal. B Environ. 2020, 277, 119177. [Google Scholar] [CrossRef]
- Meng, X.; Yu, L.; Ma, C.; Nan, B.; Si, R.; Tu, Y.; Deng, J.; Deng, D.; Bao, X. Three-Dimensionally Hierarchical MoS2/Graphene Architecture for High-Performance Hydrogen Evolution Reaction. Nano Energy 2019, 61, 611–616. [Google Scholar] [CrossRef]
- Lai, G.-J.; Lyu, L.-M.; Huang, Y.-S.; Lee, G.-C.; Lu, M.-P.; Perng, T.-P.; Lu, M.-Y.; Chen, L.-J. Few-Layer WS2–MoS2 in-Plane Heterostructures for Efficient Photocatalytic Hydrogen Evolution. Nano Energy 2021, 81, 105608. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Xie, J.-Q.; Ye, H.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Co-Fe-P Nanotubes Electrocatalysts Derived from Metal-Organic Frameworks for Efficient Hydrogen Evolution Reaction under Wide PH Range. Nano Energy 2019, 56, 225–233. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Sukeri, A.; Bertotti, M. Fabrication of Nanoporous Gold-Islands via Hydrogen Bubble Template: An Efficient Electrocatalyst for Oxygen Reduction and Hydrogen Evolution Reactions. Int. J. Hydrogen Energy 2019, 44, 15001–15008. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhao, Y.; Wu, Y.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. Research Status and Progress in Degradation of Organic Pollutants via Hydrogen Evolution Reaction and Oxygen Evolution Reaction in Wastewater Electrochemical Treatment. Int. J. Hydrogen Energy 2023, 48, 33746–33762. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Gao, W.; Mao, Z.; Hu, E.; Gao, X.; Zhang, J.; Chen, Z. Strategies to Enhance the Electrocatalytic Behavior of Metal Selenides for Hydrogen Evolution Reaction: A Review. Int. J. Hydrogen Energy 2023, 48, 36722–36749. [Google Scholar] [CrossRef]
- Hai, X.; Zhou, W.; Wang, S.; Pang, H.; Chang, K.; Ichihara, F.; Ye, J. Rational Design of Freestanding MoS2 Monolayers for Hydrogen Evolution Reaction. Nano Energy 2017, 39, 409–417. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; Lu, Q.; Zhang, X.; Tan, C.; Zhang, H. Three-Dimensional Architectures Constructed from Transition-Metal Dichalcogenide Nanomaterials for Electrochemical Energy Storage and Conversion. Angew. Chemie Int. Ed. 2018, 57, 626–646. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum Single-Atom and Cluster Catalysis of the Hydrogen Evolution Reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef]
- Rivera, J.G.; Garcia-Garcia, R.; Coutino-Gonzalez, E.; Orozco, G. Hydrogen Evolution Reaction on Metallic Rhenium in Acid Media with or without Methanol. Int. J. Hydrogen Energy 2019, 44, 27472–27482. [Google Scholar] [CrossRef]
- Erdogan, F.O.; Celik, C.; Turkmen, A.C.; Sadak, A.E.; Cucu, E. Hydrogen Sorption Studies of Palladium Decorated Graphene Nanoplatelets and Carbon Samples. Int. J. Hydrogen Energy 2023, 48, 21476–21486. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, C.-Q.; Liu, W.; Hung, S.-F.; Bin Yang, H.; Gao, J.; Cai, W.; Chen, H.M.; Li, J.; Liu, B. Coordination Engineering of Iridium Nanocluster Bifunctional Electrocatalyst for Highly Efficient and PH-Universal Overall Water Splitting. Nat. Commun. 2020, 11, 4246. [Google Scholar] [CrossRef]
- Volpato, G.A.; Muneton Arboleda, D.; Brandiele, R.; Carraro, F.; Sartori, G.B.; Cardelli, A.; Badocco, D.; Pastore, P.; Agnoli, S.; Durante, C.; et al. Clean Rhodium Nanoparticles Prepared by Laser Ablation in Liquid for High Performance Electrocatalysis of the Hydrogen Evolution Reaction. Nanoscale Adv. 2019, 1, 4296–4300. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Liu, Q.; Asiri, A.M.; Sun, X. Closely Interconnected Network of Molybdenum Phosphide Nanoparticles: A Highly Efficient Electrocatalyst for Generating Hydrogen from Water. Adv. Mater. 2014, 26, 5702–5707. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, K.; Sinha, S.; Kumar, S.; Kumar, S. Simulation of Steam Reforming of Biogas in an Industrial Reformer for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 26809–26824. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Zhu, B.; Wan, X.; Hua, S.; Tang, H. A Bottom-up Method to Construct Ru-Doped FeP Nanosheets on Foam Iron with Ultra-High Activity for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2023, 48, 4686–4693. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, D.; Zhang, X.; Lv, K.; Wang, F.; Dong, B.; Wang, S.; Yang, C.; Li, J.; Yang, F.; et al. Self-Supported Ceramic Electrode of 1T-2H MoS2 Grown on the TiC Membrane for Hydrogen Production. Chem. Mater. 2021, 33, 6217–6226. [Google Scholar] [CrossRef]
- Som, N.N.; Jha, P.K. Hydrogen Evolution Reaction of Metal Di-Chalcogenides: ZrS2, ZrSe2 and Janus ZrSSe. Int. J. Hydrogen Energy 2020, 45, 23920–23927. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Yang, T.-Y.; Le, P.A.; Yen, P.-J.; Chueh, Y.-L.; Wei, K.-H. New Simultaneous Exfoliation and Doping Process for Generating MX 2 Nanosheets for Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 14786–14795. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Zhang, B.; Ji, X.; Xu, K.; Chen, C.; Miao, L.; Jiang, J. The Mechanism of Hydrogen Adsorption on Transition Metal Dichalcogenides as Hydrogen Evolution Reaction Catalyst. Phys. Chem. Chem. Phys. 2017, 19, 10125–10132. [Google Scholar] [CrossRef]
- Wu, L.; Hofmann, J.P. Comparing the Intrinsic HER Activity of Transition Metal Dichalcogenides: Pitfalls and Suggestions. ACS Energy Lett. 2021, 6, 2619–2625. [Google Scholar] [CrossRef]
- Cardoso, G.L.; Piquini, P.C.; Ahuja, R. From Monolayers to Nanotubes: Toward Catalytic Transition-Metal Dichalcogenides for Hydrogen Evolution Reaction. Energy Fuels 2021, 35, 6282–6288. [Google Scholar] [CrossRef]
- Cao, Y. Roadmap and Direction toward High-Performance MoS2 Hydrogen Evolution Catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, H.; Wang, B.; He, W.; Dong, H.; Sui, L.; Gan, Z.; Ma, S.; Pang, B.; Dong, L.; et al. Synthesis and Photocatalytic Performance of MoS2/Polycrystalline Black Phosphorus Heterojunction Composite. Int. J. Hydrogen Energy 2021, 46, 3530–3538. [Google Scholar] [CrossRef]
- Jatav, S.; Furlan, K.P.; Liu, J.; Hill, E.H. Heterostructured Monolayer MoS2 Nanoparticles toward Water-Dispersible Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 19813–19822. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, J.; Guan, J.; Ji, J.; Zhou, M.; Meng, L.; Chen, M.; Zhou, W.; Liu, Y.; Zhang, X. Enhanced Hydrogen Evolution Reaction Performance of MoS2 by Dual Metal Atoms Doping. Int. J. Hydrogen Energy 2022, 47, 23191–23200. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Abdollahi, S.H.; Safavi, A. Sugar-Based Natural Deep Eutectic Mixtures as Green Intercalating Solvents for High-Yield Preparation of Stable MoS 2 Nanosheets: Application to Electrocatalysis of Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 5896–5906. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Cheng, D. Identification of the Anti-Triangular Etched MoS2 with Comparative Activity with Commercial Pt for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2020, 45, 33457–33465. [Google Scholar] [CrossRef]
- Noori, Y.J.; Thomas, S.; Ramadan, S.; Smith, D.E.; Greenacre, V.K.; Abdelazim, N.; Han, Y.; Beanland, R.; Hector, A.L.; Klein, N.; et al. Large-Area Electrodeposition of Few-Layer MoS2 on Graphene for 2D Material Heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 49786–49794. [Google Scholar] [CrossRef]
- Teng, W.; Wang, Y.; Huang, H.; Li, X.; Tang, Y. Enhanced Photoelectrochemical Performance of MoS2 Nanobelts-Loaded TiO2 Nanotube Arrays by Photo-Assisted Electrodeposition. Appl. Surf. Sci. 2017, 425, 507–517. [Google Scholar] [CrossRef]
- Hyeon, Y.; Jung, S.-H.; Jang, W.; Kim, M.; Kim, B.-S.; Lee, J.-H.; Nandanapalli, K.R.; Jung, N.; Whang, D. Unraveling the Factors Affecting the Electrochemical Performance of MoS2–Carbon Composite Catalysts for Hydrogen Evolution Reaction: Surface Defect and Electrical Resistance of Carbon Supports. ACS Appl. Mater. Interfaces 2019, 11, 5037–5045. [Google Scholar] [CrossRef]
- Lin, J.; Wang, P.; Wang, H.; Li, C.; Si, X.; Qi, J.; Cao, J.; Zhong, Z.; Fei, W.; Feng, J. Defect-Rich Heterogeneous MoS2/NiS2 Nanosheets Electrocatalysts for Efficient Overall Water Splitting. Adv. Sci. 2019, 6, 1900246. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Andashti, P.; Ramezani, Z.; Zare-Shahabadi, V.; Torabi, P. Rapid and Green Synthesis of Highly Luminescent MoS2 Quantum Dots via Microwave Exfoliation of MoS2 Powder and Its Application as a Fluorescence Probe for Cortisol Detection in Human Saliva. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129048. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Carmona, E.R.; Villacorta, A.; Carevic, F.S.; Marcos, R. Sustainable Use of Mining and Electronic Waste for Nanomaterial Synthesis with Technological Applications: State of the Art and Future Directions. Green Chem. Lett. Rev. 2023, 16, 2260401. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Yu, Y.; Huang, S.; Yang, W.; Cao, L. Activating MoS2 for PH-Universal Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2017, 139, 16194–16200. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, Z.; Shen, S.; Chen, Y.; Du, Z.; Tao, W.; Xu, A.; Ye, X.; Zhong, W.; Feng, S. One-Step Method to Achieve Multiple Decorations on Lamellar MoS2 to Synergistically Enhance the Electrocatalytic HER Performance. J. Alloys Compd. 2020, 834, 155217. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Pan, F.; Zhu, Z.; Sun, H.; Tang, Y.; Fu, G. Plasma-induced Mo-doped Co3O4 with Enriched Oxygen Vacancies for Electrocatalytic Oxygen Evolution in Water Splitting. Carbon Energy 2023, 5, e279. [Google Scholar] [CrossRef]
- Saravia, L.P.H.; Anandhakumar, S.; Parussulo, A.L.A.; Matias, T.A.; Caldeira da Silva, C.C.; Kowaltowski, A.J.; Araki, K.; Bertotti, M. Development of a Tetraphenylporphyrin Cobalt (II) Modified Glassy Carbon Electrode to Monitor Oxygen Consumption in Biological Samples. J. Electroanal. Chem. 2016, 775, 72–76. [Google Scholar] [CrossRef]
- Chang, S.; Xuan, Y.; Duan, J.; Zhang, K. High-Performance Electroreduction CO2 to Formate at Bi/Nafion Interface. Appl. Catal. B Environ. 2022, 306, 121135. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, T.; Duan, J.; Jiang, L.; Hu, X.; Zhao, H.; Zhu, J.; Chen, S.; Wang, X. Two-Dimensional Nanomesh Arrays as Bifunctional Catalysts for N2 Electrolysis. ACS Catal. 2020, 10, 11371–11379. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Matias, T.A.; Saravia, L.P.H.; Nakamura, M.; Bernardes, J.S.; Bertotti, M.; Araki, K. Synergic Effects Enhance the Catalytic Properties of Alpha-Ni(OH)2-FeOCPc@rGO Composite for Oxygen Evolution Reaction. Electrochim. Acta 2018, 267, 161–169. [Google Scholar] [CrossRef]
- Rudra, S.; Seo, H.W.; Sarker, S.; Kim, D.M. Supercapatteries as Hybrid Electrochemical Energy Storage Devices: Current Status and Future Prospects. Molecules 2024, 29, 243. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.; Weinberg, G.; Mestl, G. Raman Spectroscopy of Molybdenum Oxides. Phys. Chem. Chem. Phys. 2002, 4, 812–821. [Google Scholar] [CrossRef]
- Wang, X.; Cormier, C.R.; Khosravi, A.; Smyth, C.M.; Shallenberger, J.R.; Addou, R.; Wallace, R.M. In Situ Exfoliated 2D Molybdenum Disulfide Analyzed by XPS. Surf. Sci. Spectra 2020, 27, 014019. [Google Scholar] [CrossRef]
- Iranmahboob, J.; Gardner, S.D.; Toghiani, H.; Hill, D.O. XPS Study of Molybdenum Sulfide Catalyst Exposed to CO and H2. J. Colloid Interface Sci. 2004, 270, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Li, W.; Huo, J.; Liao, T.; Cheng, N.; Du, Y.; Zhu, M.; Li, Y.; Zhang, J. Metal-Ion Bridged High Conductive RGO-M-MoS2 (M = Fe3+, Co2+, Ni2+, Cu2+ and Zn2+) Composite Electrocatalysts for Photo-Assisted Hydrogen Evolution. Appl. Catal. B Environ. 2019, 246, 129–139. [Google Scholar] [CrossRef]
- Idrees, M.; Amin, B.; Chen, Y.; Yan, X. Computation Insights of MoS2-CrXY (X≠Y S, Se, Te) van Der Waals Heterostructure for Spintronic and Photocatalytic Water Splitting Applications. Int. J. Hydrogen Energy 2023, 51, 1217–1228. [Google Scholar] [CrossRef]
- Saravia, L.P.H.; Sukeri, A.; Gonçalves, J.M.; Aguirre-Araque, J.S.; Brandão, B.B.N.S.; Matias, T.A.; Nakamura, M.; Araki, K.; Toma, E.H.; Bertotti, M. CoTRP/Graphene Oxide Composite as Efficient Electrode Material for Dissolved Oxygen Sensors. Electrochim. Acta 2016, 222, 1682–1690. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W. Symbiotic Oxides in Catalysts. Nat. Catal. 2023, 6, 985–986. [Google Scholar] [CrossRef]
- Wei, S.; Xing, P.; Tang, Z.; Wang, Y.; Dai, L. Spindle-Shaped Cobalt-Doped Iron Phosphide Anchored on Three-Dimensional Graphene Electrocatalysis for Hydrogen Evolution Reactions in Both Acidic and Alkaline Media. J. Power Sources 2023, 555, 232414. [Google Scholar] [CrossRef]
- Fang, W.; Wang, J.; Hu, Y.; Cui, X.; Zhu, R.; Zhang, Y.; Yue, C.; Dang, J.; Cui, W.; Zhao, H.; et al. Metal-Organic Framework Derived Fe-Co-CN/Reduced Graphene Oxide for Efficient HER and OER. Electrochim. Acta 2021, 365, 137384. [Google Scholar] [CrossRef]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous Cobalt Boride (Co2B) as a Highly Efficient Nonprecious Catalyst for Electrochemical Water Splitting: Oxygen and Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1502313. [Google Scholar] [CrossRef]
- Kuang, P.; Tong, T.; Fan, K.; Yu, J. In Situ Fabrication of Ni–Mo Bimetal Sulfide Hybrid as an Efficient Electrocatalyst for Hydrogen Evolution over a Wide PH Range. ACS Catal. 2017, 7, 6179–6187. [Google Scholar] [CrossRef]
- Huang, J.; Li, F.; Liu, B.; Zhang, P. Ni2P/RGO/NF Nanosheets As a Bifunctional High-Performance Electrocatalyst for Water Splitting. Materials 2020, 13, 744. [Google Scholar] [CrossRef]
- Lin, Z.; Li, K.; Tong, Y.; Wu, W.; Cheng, X.; Wang, H.; Chen, P.; Diao, P. Engineering Coupled NiSx-WO2.9 Heterostructure as PH-Universal Electrocatalyst for Hydrogen Evolution Reaction. ChemSusChem 2023, 16, e202201985. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, T.; Liu, T.; Lv, K.; Wu, Z.; Wang, D. Tungsten Phosphide (WP) Nanoparticles with Tunable Crystallinity, W Vacancies, and Electronic Structures for Hydrogen Production. Electrochim. Acta 2019, 323, 134798. [Google Scholar] [CrossRef]
- Srividhya, G.; Viswanathan, C.; Ponpandian, N. Interfacing NiV Layered Double Hydroxide with Sulphur-Doped g-C3N4 as a Novel Electrocatalyst for Enhanced Hydrogen Evolution Reaction through Volmer–Heyrovský Mechanism. Energy Adv. 2023, 2, 1464–1475. [Google Scholar] [CrossRef]

| Material | Overpotential η10 (mV) | Tafel Slope (mV/dec) | Ref. |
|---|---|---|---|
| MoS2/rGO(2) | 176 | 63.7 | This work |
| MoS2 | 447 | 141.3 | This work |
| Fe–Co–CN/rGO-700 | 213 | 97 | [61] |
| Co2B NPs | 328 | 92.4 | [62] |
| NiS2/MoS2 HNW | 204 | 65 | [63] |
| NiO/rGO/NF | 268 | 100 | [64] |
| NiSx-WO2.9/NF | 220 | 66 | [65] |
| WP nanoparticles | 254 | 65 | [66] |
| S-gCN/NiV | 560 | 79 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Olivos, F.; Hernández-Saravia, L.P.; Nelson, R.; Perez, M.d.l.A.; Villalobos, F. Nanocatalysis MoS2/rGO: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Molecules 2024, 29, 523. https://doi.org/10.3390/molecules29020523
Guzmán-Olivos F, Hernández-Saravia LP, Nelson R, Perez MdlA, Villalobos F. Nanocatalysis MoS2/rGO: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Molecules. 2024; 29(2):523. https://doi.org/10.3390/molecules29020523
Chicago/Turabian StyleGuzmán-Olivos, Fernando, Lucas Patricio Hernández-Saravia, Ronald Nelson, Maria de los Angeles Perez, and Francisco Villalobos. 2024. "Nanocatalysis MoS2/rGO: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction" Molecules 29, no. 2: 523. https://doi.org/10.3390/molecules29020523
APA StyleGuzmán-Olivos, F., Hernández-Saravia, L. P., Nelson, R., Perez, M. d. l. A., & Villalobos, F. (2024). Nanocatalysis MoS2/rGO: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Molecules, 29(2), 523. https://doi.org/10.3390/molecules29020523