A Review of Macrocycles Applied in Electrochemical Energy Storge and Conversion
Abstract
1. Introduction
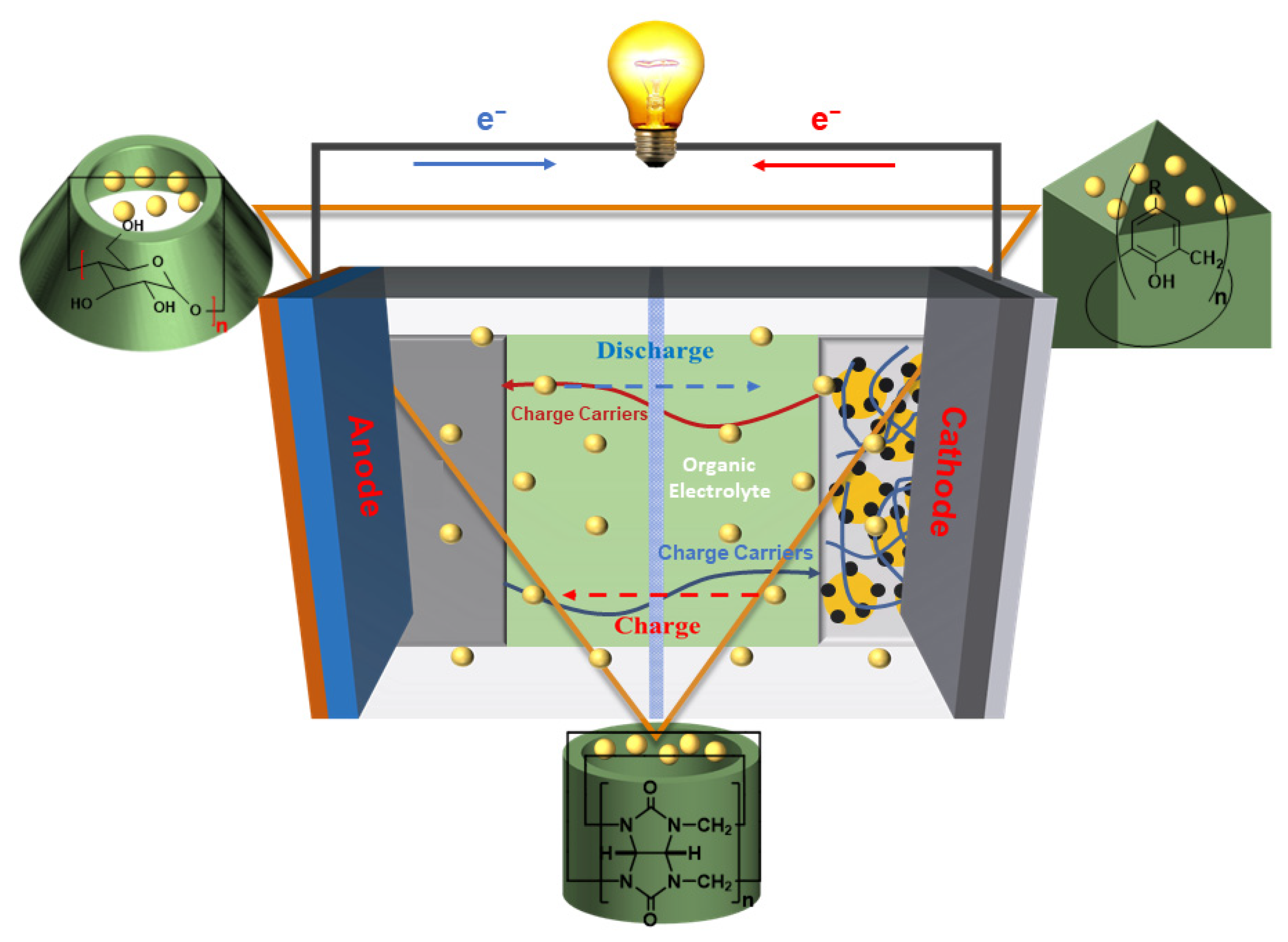
2. CDs in Electrochemical Energy Storage and Conversion
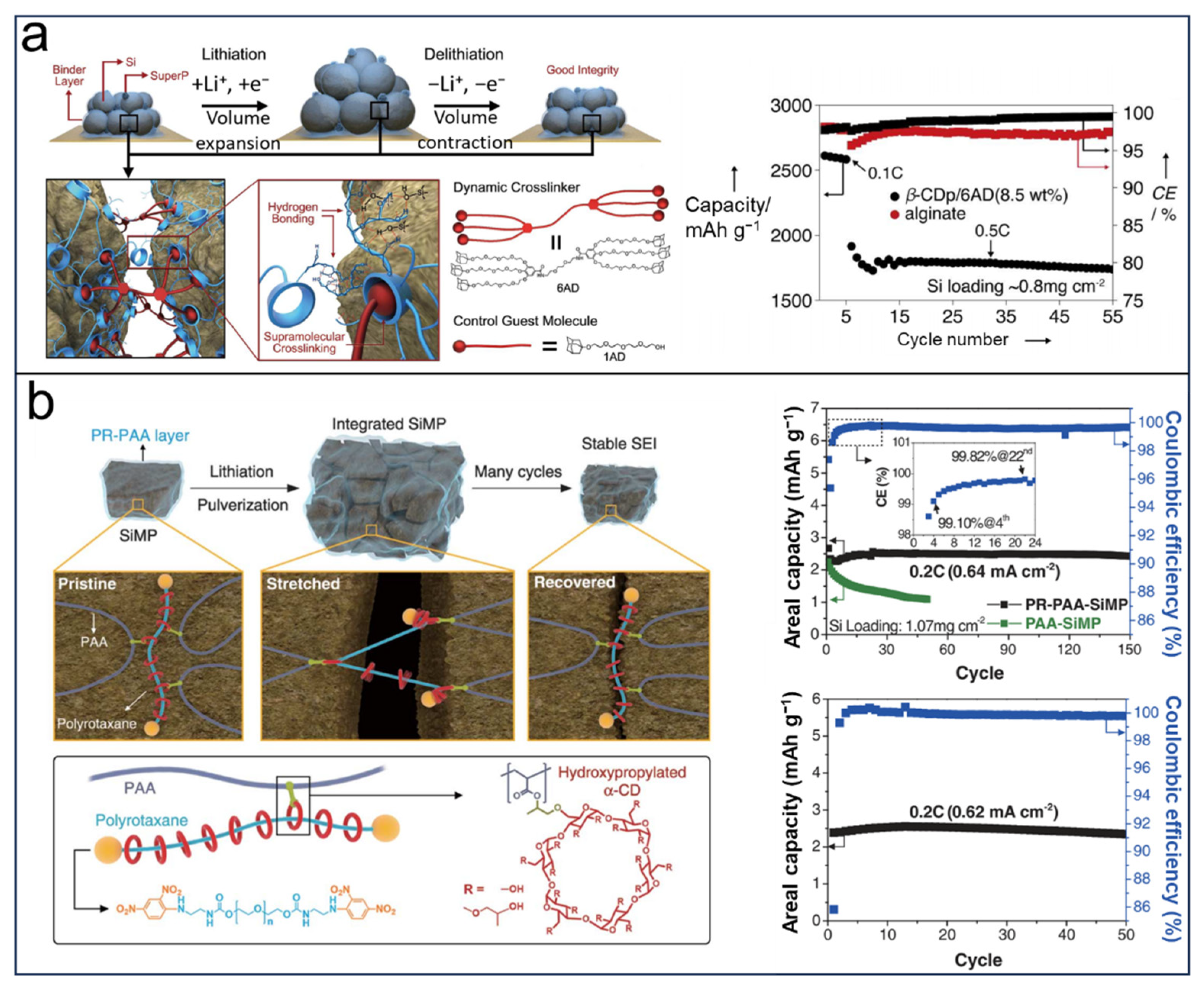
2.1. CDs in LIBs

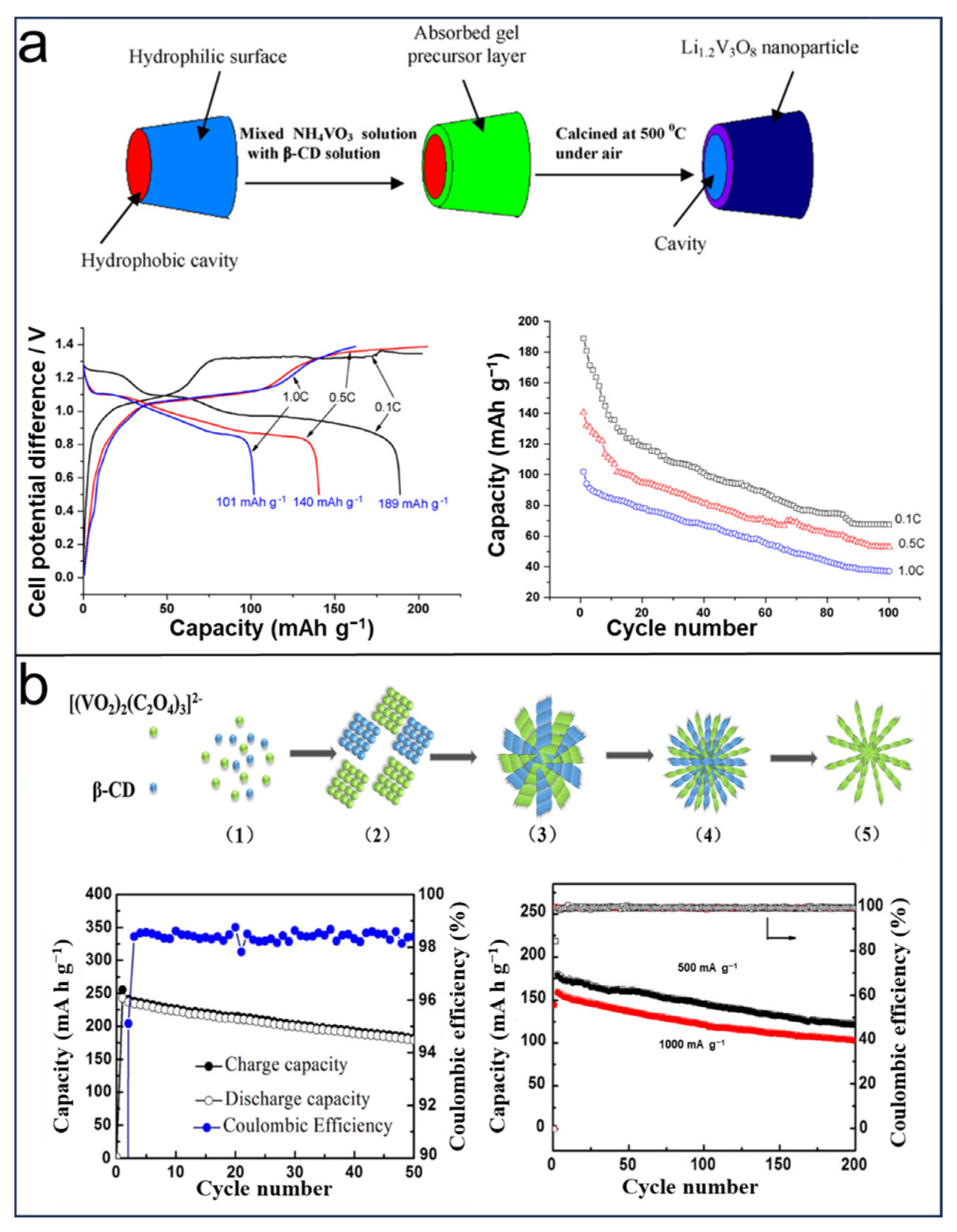
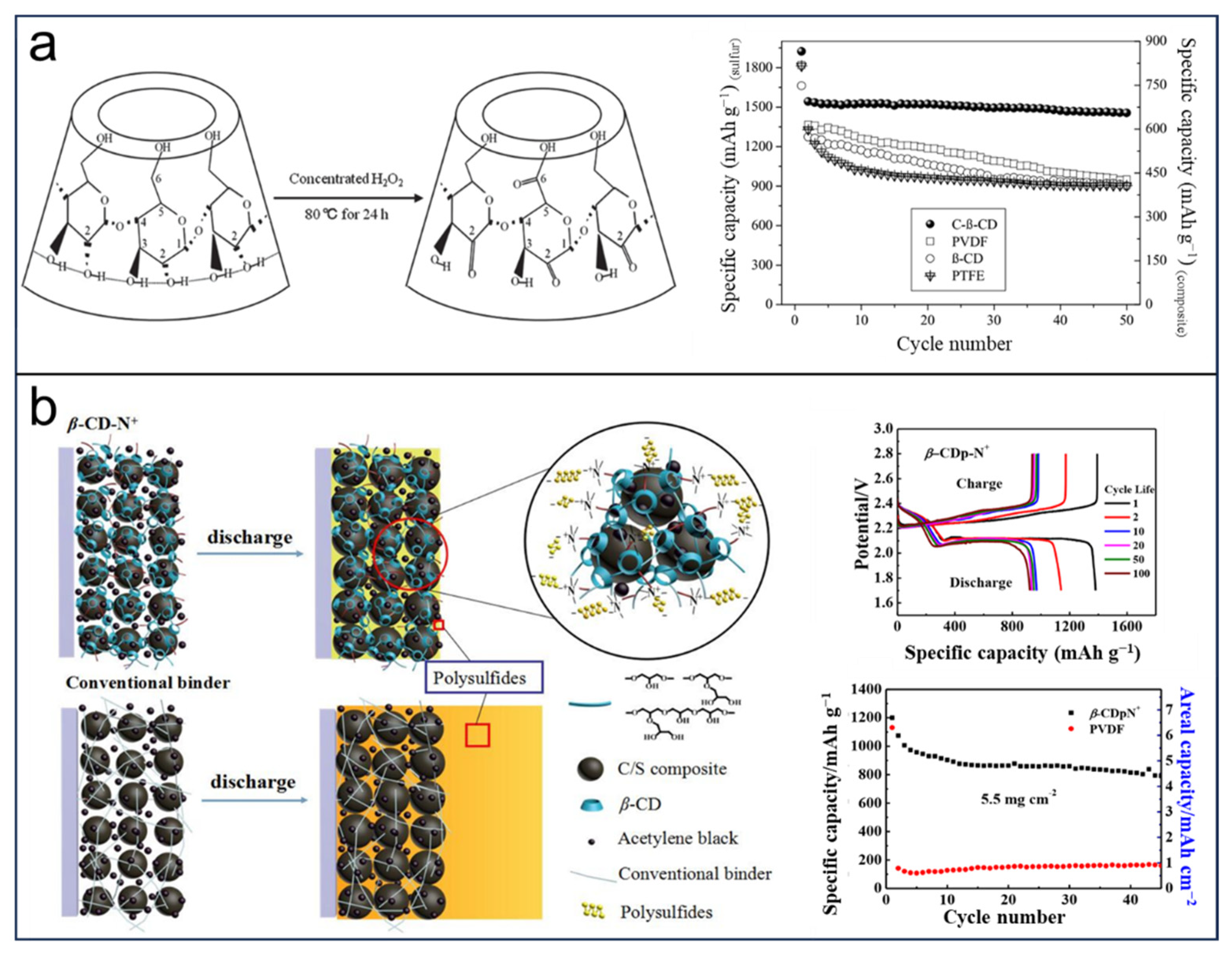
2.2. CDs in LSBs
2.3. CDs in Other Batteries
2.4. CDs in Supercapacitors
3. CAs in Electrochemical Energy Storage and Conversion
3.1. CAs in LIBs
3.2. CAs in SIBs
3.3. CAs in Other Batteries

3.4. CAs in Supercapacitors
4. CBs in Electrochemical Energy Storage and Conversion
CBs in LSBs
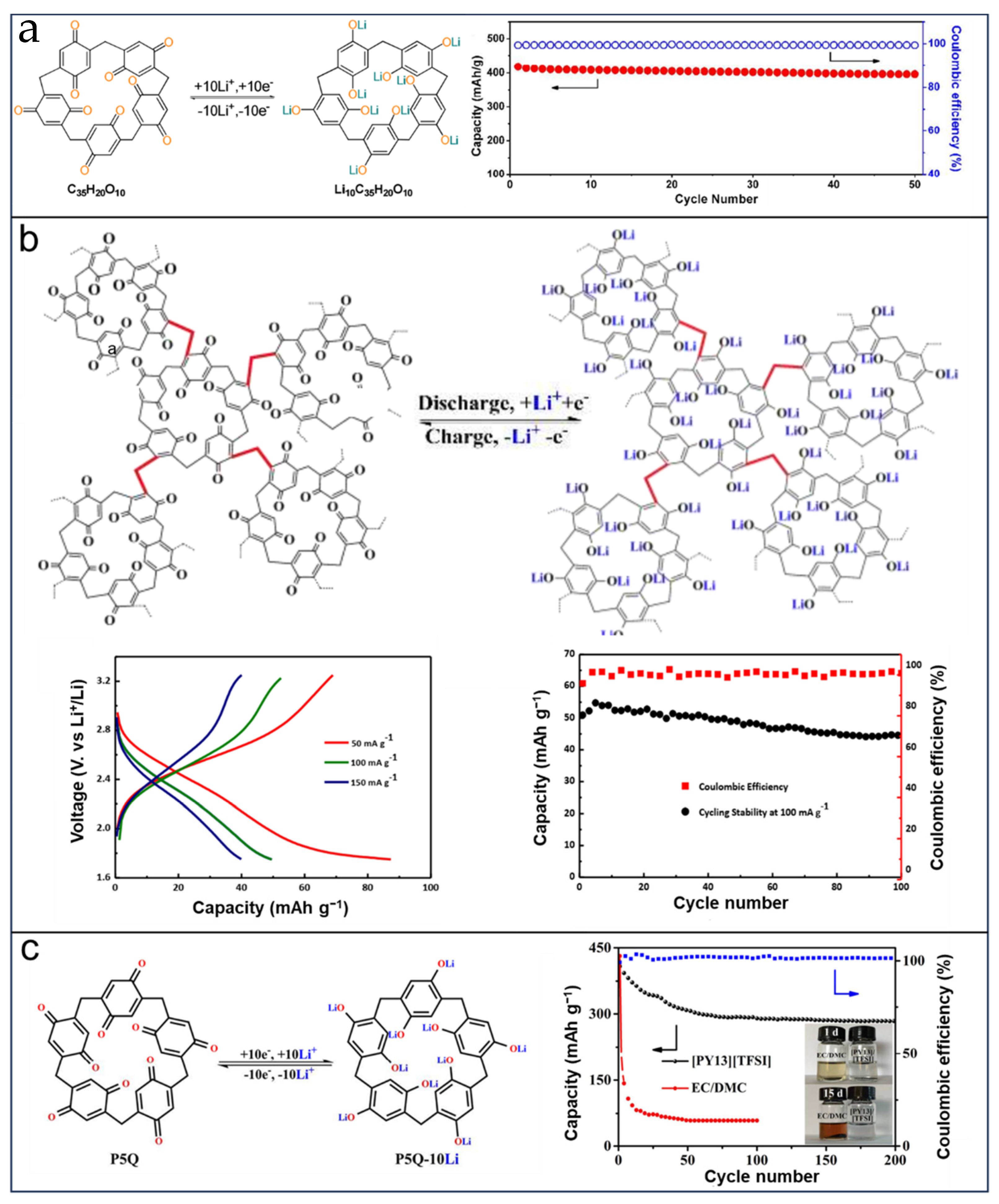
5. PAs in Electrochemical Energy Storage and Conversion
5.1. PAs in LIBs
5.2. PAs in SIBs
5.3. PAs in Supercapacitors
5.4. Computation Studies of PAs in Electrochemical Energy Storage and Conversion
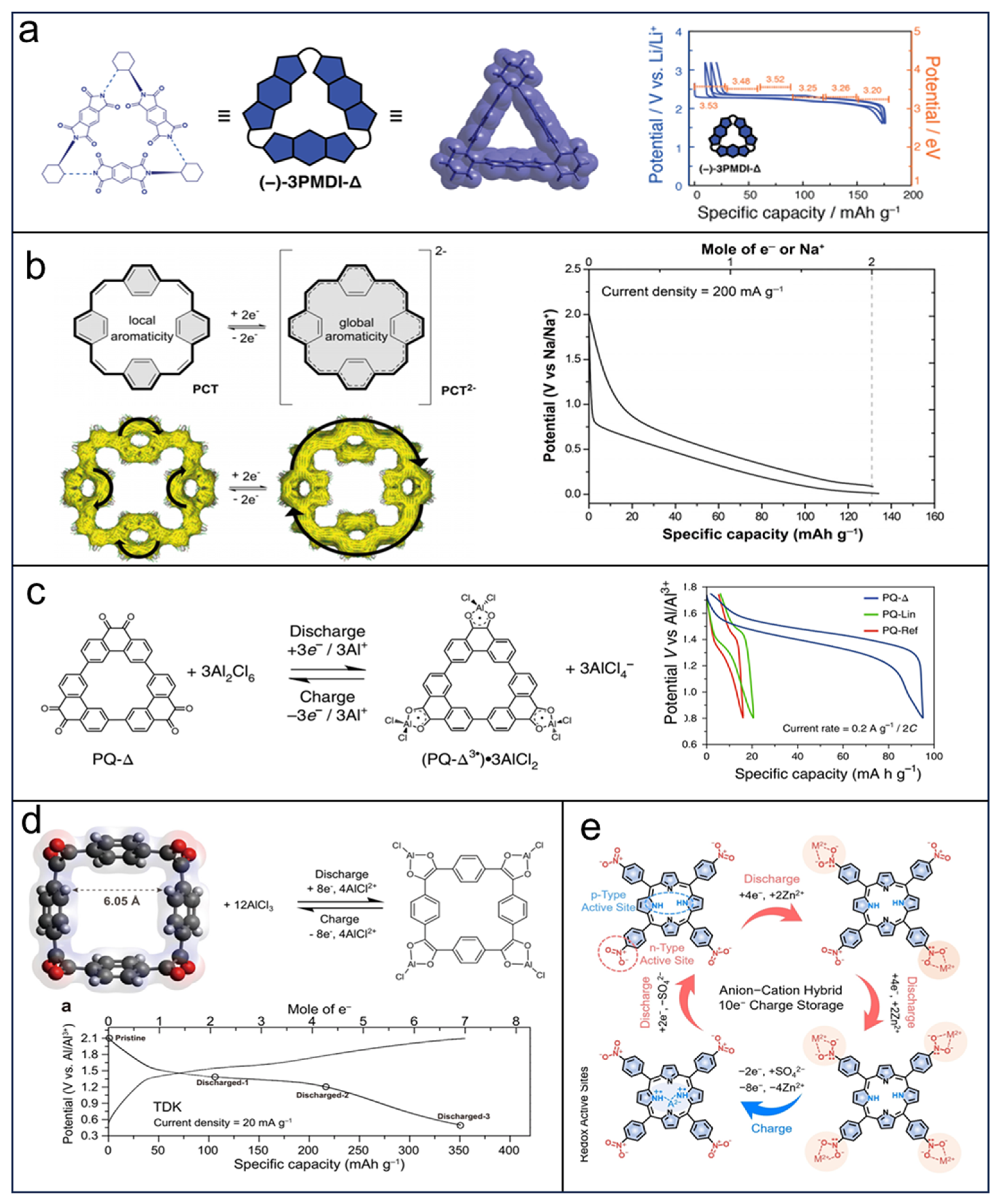
6. Other Macrocycles in Electrochemical Energy Storage and Conversion
7. Limitation and Prospects for Macrocycles in Electrochemical Energy Storage and Conversion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EES/EEC | Electrochemical energy storge and conversion |
| LIBs | Lithium-ion batteries |
| LSBs | Lithium-S batteries |
| SIBs | Sodium-ion batteries |
| MIBs | Magnesium-ion batteries |
| ZIBs | Zinc-ion batteries |
| PIBs | Potassium-ion battery |
| ARLBs | Aqueous rechargeable lithium batteries |
| AOFBs | Aqueous organic flow batteries |
| MOFs | Metal organic frameworks |
| COFs | Covalent organic frameworks |
| CDs | Cyclodextrins |
| CAs | Calixarenes |
| CBs | Cucurbiturils |
| PAs | Pillararenes |
| CNTs | Carbon nanotubes |
| GO | Graphene oxide |
| RGO | Reduced graphene oxide |
| β-CDp | β-cyclodextrin polymer |
| PAA | Poly-(acrylic acid) |
| EES/EEC | Electrochemical energy storge and conversion |
| LIBs | Lithium-ion batteries |
| PR | Pseudo-rotaxanes |
| C-β-CD | Carbonyl-β-cyclodextrin |
| poly-CDQA | Poly-β-cyclodextrin quaternary ammonium |
| rGO@β-CDP@S | Reduced graphene oxide and cyclodextrin polymers and S |
| VFB | Vanadium redox flow battery |
| AEM | Anion exchange membrane |
| HPSf-Im | Hydroxyl polysulfone with imidazolium function alization |
| HPNSC | Hierarchically porous N and S doped carbon |
| ECSA | Electrochemically active surface area |
| PEG-AD | adamantine end-capped poly(ethylene oxide) polymer linker |
| PMnCD | Porous carbon nanofiber/MnO2 composites derived from polyacrylonitrile CD |
| CQs | Calix[n]quinones |
| C4Q | Calix[4]quinone |
| PMA/PEG | Poly(methacrylate)/poly(ethylene glycol) |
| GPE | Gel polymer electrolyte |
| C6Q | Calix[6]quinone |
| PCE | Plastic crystal electrolyte |
| CMK-3 | Order mesoporous carbon |
| IL | Ionic liquid |
| PEO | Poly(ethylene oxide) |
| SWCNTs | Single wall carbon nanotubes |
| [PY13][TFSI] | N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)amide |
| AABs | Aqueous aluminum batteries |
| Al(OTF)3 | Aluminum trifluoromethanesulfonate |
| ZBs | Zinc batteries |
| PANI | Polyaniline |
| SC6 | Sulphatocalix[6]arene |
| PANI-ES | Sulphatocalix[6]arene doped PANI |
| CBC | Subnanoporous carbon |
| MMIMBF4 | 1-methyl-3-methylimidazole tetrafluoroborate |
| CPE | Composite polymer electrolyte |
| P5Q | Pillar[5]quinone |
| PMA | Poly(methacrylate) |
| PEG | Poly(ethylene glycol) |
| POP | Porous organic polymers |
| SPCC | Supramolecular coordination compounds |
| DFT | Density functional theory |
| PMDI-Δ | Triangular pyromellitic diimide |
| PCT | [2.2.2.2]paracyclophane-1,9,17,25-tetraene |
| PQ | Phenanthrenequinone |
| TDK | Tetradiketone |
| TNP | Tetranitroporphyrin |
References
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research Development on K-Ion Batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-Ion Batteries: A Look into the Future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-Based Materials as Supercapacitor Electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon Materials for Supercapacitor Application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-J.; Yue, J.; Chen, Z.; Feng, X.-X.; Zhang, J.; Yin, Y.-X.; Zhang, L.; Zheng, J.-C.; Luo, Y.; Xin, S.; et al. Asymmetric Fire-Retardant Quasi-Solid Electrolytes for Safe and Stable High-Voltage Lithium Metal Battery. Energy Mater. Adv. 2024, 5, 0076. [Google Scholar] [CrossRef]
- Zhang, J.; Chou, J.; Luo, X.-X.; Yang, Y.-M.; Yan, M.-Y.; Jia, D.; Zhang, C.-H.; Wang, Y.-H.; Wang, W.-P.; Tan, S.-J.; et al. A Fully Amorphous, Dynamic Cross-Linked Polymer Electrolyte for Lithium-Sulfur Batteries Operating at Subzero-Temperatures. Angew. Chem. Int. Ed. 2024, 63, e202316087. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.-H.; Park, N.-Y.; Kim, J.-M.; Hwang, J.-Y.; Sun, Y.-K. Forming Robust and Highly Li-Ion Conductive Interfaces in High-Performance Lithium Metal Batteries Using Chloroethylene Carbonate Additive. Adv. Energy Sustain. Res. 2024, 5, 2300151. [Google Scholar] [CrossRef]
- Yan, M.; Wang, C.-Y.; Fan, M.; Zhang, Y.; Xin, S.; Yue, J.; Zeng, X.-X.; Liang, J.-Y.; Song, Y.-X.; Yin, Y.-X.; et al. In Situ Derived Mixed Ion/Electron Conducting Layer on Top of a Functional Separator for High-Performance, Dendrite-Free Rechargeable Lithium-Metal Batteries. Adv. Funct. Mater. 2024, 34, 2301638. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Z.; Zhang, Q.; Xie, W.; Chen, J. Regulating Electrostatic Interaction between Hydrofluoroethers and Carbonyl Cathodes toward Highly Stable Lithium–Organic Batteries. J. Am. Chem. Soc. 2024, 146, 1100–1108. [Google Scholar] [CrossRef]
- Hou, X.; Lu, Y.; Ni, Y.; Zhang, D.; Zhao, Q.; Chen, J. Synthesis of a Class of Oxocarbons (C4O4, C5O5) and the Application as High-Capacity Cathode Materials for Lithium-Ion Batteries. Sci. China Chem. 2023, 66, 2780–2784. [Google Scholar] [CrossRef]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF Nanosheets for Electrochemical Energy Storage and Conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving High Energy Density and High-Power Density with Pseudocapacitive Materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Long, C.; Dong, B.; Fang, D.; Liu, Z.; Zhao, Y.; Li, X.; Fan, J.; Chen, S.; et al. Achieving Both High Voltage and High Capacity in Aqueous Zinc-Ion Battery for Record High Energy Density. Adv. Funct. Mater. 2019, 29, 1906142. [Google Scholar] [CrossRef]
- Evarts, E.C. Lithium Batteries: To the Limits of Lithium. Nature 2015, 526, S93–S95. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, R. The Rechargeable Revolution: A Better Battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Rangom, Y.; Kwok, C.Y.; Pang, Q.; Nazar, L.F. Interwoven MXene Nanosheet/Carbon-Nanotube Composites as Li-S Cathode Hosts. Adv. Mater. 2017, 29, 1603040. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 33, 2004689. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium Intercalation into Graphite to Realize High-Voltage/High-Power Potassium-Ion Batteries and Potassium-Ion Capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Kaveevivitchai, W.; Jacobson, A.J. High-Capacity Rechargeable Magnesium-Ion Batteries Based on a Microporous Molybdenum–Vanadium Oxide Cathode. Chem. Mater. 2016, 28, 4593–4601. [Google Scholar] [CrossRef]
- Li, B.; Masse, R.; Liu, C.; Hu, Y.; Li, W.; Zhang, G.; Cao, G. Kinetic Surface Control for Improved Magnesium-Electrolyte Interfaces for Magnesium Ion Batteries. Energy Storage Mater. 2019, 22, 96–104. [Google Scholar] [CrossRef]
- Rashad, M.; Asif, M.; Wang, Y.; He, Z.; Ahmed, I. Recent Advances in Electrolytes and Cathode Materials for Magnesium and Hybrid-Ion Batteries. Energy Storage Mater. 2020, 25, 342–375. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Zong, Q.; Zhuang, Y.; Liu, C.; Kang, Q.; Wu, Y.; Zhang, J.; Wang, J.; Tao, D.; Zhang, Q.; Cao, G. Dual Effects of Metal and Organic Ions Co-Intercalation Boosting the Kinetics and Stability of Hydrated Vanadate Cathodes for Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2301480. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Yoo, J.; Kwon, G.; Ko, Y.; Kang, K. Organic Batteries for a Greener Rechargeable World. Nat. Rev. Mater. 2023, 8, 54–70. [Google Scholar] [CrossRef]
- Li, M.; Hicks, R.P.; Chen, Z.; Luo, C.; Guo, J.; Wang, C.; Xu, Y. Electrolytes in Organic Batteries. Chem. Rev. 2023, 123, 1712–1773. [Google Scholar] [CrossRef] [PubMed]
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful Organic Materials for Secondary Batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. Organic Quinones towards Advanced Electrochemical Energy Storage: Recent Advances and Challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Xin, S.; Zhang, X.; Wang, L.; Yu, H.; Chang, X.; Zhao, Y.-M.; Meng, Q.; Xu, P.; Zhao, C.-Z.; Chen, J.; et al. Roadmap for Rechargeable Batteries: Present and Beyond. Sci. China Chem. 2024, 67, 13–42. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-Type Solid-State Fast Li Ion Conductors for Li Batteries: Critical Review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All Solid-State Polymer Electrolytes for High-Performance Lithium Ion Batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Z.; Wu, J.; Yan, T.; Shen, L.; Shi, Z.; Wu, Y.; Pan, X.; Zhang, L.; Zhang, Q.; et al. Tuning Dual-Atom Mediator toward High-Rate Bidirectional Polysulfide Conversion in Li-S Batteries. J. Energy Chem. 2023, 87, 462–472. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Tan, S.-J.; Lu, Z.-Y.; Xu, D.-X.; Chen, H.-X.; Zhang, C.-H.; Zhang, X.-S.; Li, G.; Zhao, Y.-M.; Chen, W.-P.; et al. Insights into Anion-Solvent Interactions to Boost Stable Operation of Ether-Based Electrolytes in Pure-SiOx||LiNi0.8Mn0.1Co0.1O2 Full Cells. Angew. Chem. Int. Ed. 2023, 62, e202305988. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, J.-Q.; Peng, H.-J.; Titirici, M.-M.; Xiang, R.; Chen, R.; Liu, Q.; Zhang, Q. A Review of Functional Binders in Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802107. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Sun, W.; Yang, J.; Gong, X.; Liu, R. Multifunctional MOF-Based Separator Materials for Advanced Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2021, 8, 2001941. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Wang, D.; Gao, T.; Song, J.; Zhou, P.; Xu, Z.; Yang, Z.; Xiao, N.; Guo, S. Single Atom Array Mimic on Ultrathin MOF Nanosheets Boosts the Safety and Life of Lithium–Sulfur Batteries. Adv. Mater. 2020, 32, 1906722. [Google Scholar] [CrossRef]
- Gebert, F.; Knott, J.; Gorkin, R.; Chou, S.-L.; Dou, S.-X. Polymer Electrolytes for Sodium-Ion Batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- John, B.; Cheruvally, G. Polymeric Materials for Lithium-Ion Cells. Polym. Adv. Technol. 2017, 28, 1528–1538. [Google Scholar] [CrossRef]
- Mallinson, J.; Collins, I. Macrocycles in New Drug Discovery. Future Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Chen, F.; Fan, Z.; Zhu, Y.; Sun, H.; Yu, J.; Jiang, N.; Zhao, S.; Lai, G.; Yu, A.; Lin, C.-T.; et al. β-Cyclodextrin-Immobilized Ni/Graphene Electrode for Electrochemical Enantiorecognition of Phenylalanine. Materials 2020, 13, 777. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Wang, Q.; Zhe, T.; Bai, Y.; Bu, T.; Zhang, M.; Wang, L. Electrochemical Behavior of Reduced Graphene Oxide/Cyclodextrins Sensors for Ultrasensitive Detection of Imidacloprid in Brown Rice. Food Chem. 2020, 333, 127495. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes. Acc. Chem. Res. 1983, 16, 161–170. [Google Scholar] [CrossRef]
- Gutsche, C.D. The Calixarenes. In Structural Chemistry; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–47. [Google Scholar]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-Ray Crystal Structures of Cucurbit[n]Uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.-J.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y. Pillararene-Based Assemblies: Design Principle, Preparation and Applications. Chem. Eur. J. 2013, 19, 16862–16879. [Google Scholar] [CrossRef]
- Desoky, M.M.H.; Caldera, F.; Brunella, V.; Ferrero, R.; Hoti, G.; Trotta, F. Cyclodextrins for Lithium Batteries Applications. Materials 2023, 16, 5540. [Google Scholar] [CrossRef]
- Xie, J.; Peng, H.-J.; Huang, J.-Q.; Xu, W.-T.; Chen, X.; Zhang, Q. A Supramolecular Capsule for Reversible Polysulfide Storage/Delivery in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2017, 56, 16223–16227. [Google Scholar] [CrossRef]
- Cheng, C.; Li, Z.H.; Zhan, X.Y.; Xiao, Q.Z.; Lei, G.T.; Zhou, X.D. A Macaroni-like Li1.2V3O8 Nanomaterial with High Capacity for Aqueous Rechargeable Lithium Batteries. Electrochim. Acta 2010, 55, 4627–4631. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Z.; Wang, L.; Wang, S.; Li, H.; Tao, Z.; Shi, J.; Guan, L.; Chen, J. Quasi-Solid-State Rechargeable Lithium-Ion Batteries with a Calix[4]Quinone Cathode and Gel Polymer Electrolyte. Angew. Chem. Int. Ed. 2013, 52, 9162–9166. [Google Scholar] [CrossRef]
- Ahmad, A.; Meng, Q.; Melhi, S.; Mao, L.; Zhang, M.; Han, B.-H.; Lu, K.; Wei, Z. A Hierarchically Porous Hypercrosslinked and Novel Quinone Based Stable Organic Polymer Electrode for Lithium-Ion Batteries. Electrochim. Acta 2017, 255, 145–152. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Huang, W.; Zhang, Q. Recent Progress in Calix[n]Quinone (N = 4, 6) and Pillar[5]Quinone Electrodes for Secondary Rechargeable Batteries. Batter. Supercaps 2020, 3, 476–487. [Google Scholar] [CrossRef]
- Esmaeilpour, D.; Broscheit, J.A.; Shityakov, S. Cyclodextrin-Based Polymeric Materials Bound to Corona Protein for Theranostic Applications. Int. J. Mol. Sci. 2022, 23, 3505. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-Based Nanosponges: A Critical Review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin–Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Frag. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Lesh, G.C.; Huggins, R.A. All-Solid Lithium Electrodes with Mixed-Conductor Matrix. J. Electrochem. Soc. 1981, 128, 725. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotech. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured Materials for Advanced Energy Conversion and Storage Devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Kwon, T.; Jeong, Y.K.; Deniz, E.; AlQaradawi, S.Y.; Choi, J.W.; Coskun, A. Dynamic Cross-Linking of Polymeric Binders Based on Host–Guest Interactions for Silicon Anodes in Lithium Ion Batteries. ACS Nano 2015, 9, 11317–11324. [Google Scholar] [CrossRef]
- Komaba, S.; Shimomura, K.; Yabuuchi, N.; Ozeki, T.; Yui, H.; Konno, K. Study on Polymer Binders for High-Capacity SiO Negative Electrode of Li-Ion Batteries. J. Phys. Chem. C 2011, 115, 13487–13495. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.; Coskun, A.; Choi, J.W. Highly Elastic Binders Integrating Polyrotaxanes for Silicon Microparticle Anodes in Lithium Ion Batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.-M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q.; et al. Role of Polyacrylic Acid (PAA) Binder on the Solid Electrolyte Interphase in Silicon Anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Fan, L.; Gao, L.; Kong, X.; Li, S.; Li, J.; Hong, X.; Lu, Y. Tuning the LUMO Energy of an Organic Interphase to Stabilize Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 644–650. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chen, X.; Hou, L.-P.; Li, B.-Q.; Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Regulating Anions in the Solvation Sheath of Lithium Ions for Stable Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 411–416. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Yin, L.; Shi, Y.; Sun, C.; An, B.; Cheng, H.-M.; Li, F. An Anion-Tuned Solid Electrolyte Interphase with Fast Ion Transfer Kinetics for Stable Lithium Anodes. Adv. Energy Mater. 2020, 10, 1903843. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Li, Q.; Yang, H.; Khoshi, M.R.; Xu, Y.; Hwang, S.; Chen, L.; Ji, X.; Yang, C.; et al. Electrolyte Design for LiF-Rich Solid–Electrolyte Interfaces to Enable High-Performance Microsized Alloy Anodes for Batteries. Nat. Energy 2020, 5, 386–397. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Chen, D.; Lv, C.; Jiao, Y.; Chen, G. One-Dimensional Co3O4 Nanonet with Enhanced Rate Performance for Lithium Ion Batteries: Carbonyl-β-Cyclodextrin Inducing and Kinetic Analysis. Chem. Eng. J. 2017, 321, 31–39. [Google Scholar] [CrossRef]
- Li, P.; Chen, G.; Zhang, N.; Ma, R.; Liu, X. β-Cyclodextrin as Lithium-Ion Diffusion Channel with Enhanced Kinetics for Stable Silicon Anode. Energy Environ. Mater. 2021, 4, 72–80. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Wei, Z.-Y.; Jin, B.; Zhong, X.-B.; Wang, H.; Zhang, W.-X.; Liang, J.-C.; Jiang, Q. Hydrothermal Synthesis of Copper Sulfide with Novel Hierarchical Structures and Its Application in Lithium-Ion Batteries. Appl. Surf. Sci. 2013, 277, 268–271. [Google Scholar] [CrossRef]
- Wang, S.-X.; Chen, S.; Wei, Q.; Zhang, X.; Wong, S.Y.; Sun, S.; Li, X. Bioinspired Synthesis of Hierarchical Porous Graphitic Carbon Spheres with Outstanding High-Rate Performance in Lithium-Ion Batteries. Chem. Mater. 2015, 27, 336–342. [Google Scholar] [CrossRef]
- Wang, Y.; Takahashi, K.; Lee, K.H.; Cao, G.Z. Nanostructured Vanadium Oxide Electrodes for Enhanced Lithium-Ion Intercalation. Adv. Funct. Mater. 2006, 16, 1133–1144. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, M.; Huang, Q.; Wang, D.; Yu, R.; Wang, J.; Zheng, Z.; Wang, D. V2O5 Textile Cathodes with High Capacity and Stability for Flexible Lithium-Ion Batteries. Adv. Mater. 2020, 32, 1906205. [Google Scholar] [CrossRef]
- Yue, Y.; Liang, H. Micro- and Nano-Structured Vanadium Pentoxide (V2O5) for Electrodes of Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602545. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Shen, B.; Xia, Z.; Li, Y.; Wu, Y.; Li, Q. Facile Synthesis of Mesoporous NH4V4O10 Nanoflowers with High Performance as Cathode Material for Lithium Battery. J. Mater. Sci. 2018, 53, 2045–2053. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Shu, Y.; Ma, L.; Zhang, X.; Ding, Y. Anchoring Polysulfides in Hierarchical Porous Carbon Aerogel via Electric-Field-Responsive Switch for Lithium Sulfur Battery. Electrochim. Acta 2019, 293, 458–465. [Google Scholar] [CrossRef]
- Ni, L.; Yang, G.; Wang, Q.; Duan, S.; Shen, C.; Xie, J.; Niu, G.; Li, H.; Chen, M.; Diao, G. Supramolecular Complexation of Polysulfides by β-Cyclodextrin Polymer Functionalized Graphene Hybrid Cathode for High-Performance Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 21, 378–389. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Monroe, C.W.; Yang, J.; Nuli, Y. Carbonyl-β-Cyclodextrin as a Novel Binder for Sulfur Composite Cathodes in Rechargeable Lithium Batteries. Adv. Funct. Mater. 2013, 23, 1194–1201. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, W.; Wang, A.; Yuan, K.; Jin, Z.; Yang, Y. Multidimensional Polycation β-Cyclodextrin Polymer as an Effective Aqueous Binder for High Sulfur Loading Cathode in Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2015, 7, 26257–26265. [Google Scholar] [CrossRef]
- Jin, K.; Zhou, X.; Liu, Z. Graphene/Sulfur/Carbon Nanocomposite for High Performance Lithium-Sulfur Batteries. Nanomaterials 2015, 5, 1481–1492. [Google Scholar] [CrossRef]
- Ren, X.; Sun, Q.; Zhu, Y.; Sun, W.; Li, Y.; Lu, L. Unveiling the Role of Hydroxyl Architecture on Polysulfide Trapping for High-Performance Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 4023–4032. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium Electrolyte Studies for the Vanadium Redox Battery-A Review. Chem. Sus. Chem. 2016, 9, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of Material Research and Development for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the All-Vanadium Redox Flow Battery for Energy Storage: A Review of Technological, Financial and Policy Aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy 2017, 15, 010801. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Ma, L.; Qaisrani, N.A.; Gong, S.; Li, P.; Zhang, F.; He, G. Cyclodextrin Templated Nanoporous Anion Exchange Membrane for Vanadium Flow Battery Application. J. Membr. Sci. 2019, 586, 98–105. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Liu, Y.; Jin, S.; Fell, E.M.; Wang, B.; Gordon, R.G.; Aziz, M.J.; Yang, Z.; Xu, T. Functioning Water-Insoluble Ferrocenes for Aqueous Organic Flow Battery via Host–Guest Inclusion. ChemSusChem 2021, 14, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Y.; Wang, Q.; Liao, Z.; Zhang, N.; Guo, Y.; Xiang, Z. Hierarchically Porous Metal-Free Carbon with Record High Mass Activity for Oxygen Reduction and Zn-Air Batteries. J. Mater. Chem. A 2019, 7, 9831–9836. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, W.; Wu, Z.; Sun, C.; Cai, Y.; Yang, G.; Chen, M.; Piao, Y.; Diao, G. Supramolecular Assembled Three-Dimensional Graphene Hybrids: Synthesis and Applications in Supercapacitors. Appl. Surf. Sci. 2017, 396, 412–420. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, A.; Song, X.; Shu, D.; Yi, F.; Zhong, J.; Zeng, R.; Zhao, S.; Meng, T. Supramolecule-Inspired Fabrication of Carbon Nanoparticles In Situ Anchored Graphene Nanosheets Material for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 26775–26782. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, Y.; Jin, X.; Yan, B.; Diao, G.; Piao, Y. Supramolecule-Assisted Synthesis of Cyclodextrin Polymer Functionalized Polyaniline/Carbon Nanotube with Core-Shell Nanostructure as High-Performance Supercapacitor Material. Electrochim. Acta 2020, 331, 135345. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, Y.A.; Kim, B.-H. Electrospun Polyacrylonitrile/Cyclodextrin-Derived Hierarchical Porous Carbon Nanofiber/MnO2 Composites for Supercapacitor Applications. Carbon 2020, 164, 296–304. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Cesium Separation from Radioactive Waste by Extraction and Adsorption Based on Crown Ethers and Calixarenes. Nucl. Eng. Technol. 2020, 52, 328–336. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Homden, D.M.; Redshaw, C. The Use of Calixarenes in Metal-Based Catalysis. Chem. Rev. 2008, 108, 5086–5130. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Hu, X.-Y.; Guo, D.-S. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 2768–2794. [Google Scholar] [CrossRef]
- Huang, W.; Zheng, S.; Zhang, X.; Zhou, W.; Xiong, W.; Chen, J. Synthesis and Application of Calix[6]Quinone as a High-Capacity Organic Cathode for Plastic Crystal Electrolyte-Based Lithium-Ion Batteries. Energy Storage Mater. 2020, 26, 465–471. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Zheng, S.; Zhou, W.; Xie, J.; Yang, Z.; Zhang, Q. Calix[6]Quinone as High-Performance Cathode for Lithium-Ion Battery. Sci. China Mater. 2020, 63, 339–346. [Google Scholar] [CrossRef]
- Genorio, B.; Pirnat, K.; Cerc-Korosec, R.; Dominko, R.; Gaberscek, M. Electroactive Organic Molecules Immobilized onto Solid Nanoparticles as a Cathode Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2010, 122, 7380–7382. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, H.; Yan, B.; Hu, J.; Huang, W. High-Capacity Organic Electrode Material Calix[4] Quinone/CMK-3 Nanocomposite for Lithium Batteries. Sci. China Mater. 2018, 61, 1285–1290. [Google Scholar] [CrossRef]
- Blazejczyk, A.; Wieczorek, W.; Kovarsky, R.; Golodnitsky, D.; Peled, E.; Scanlon, L.G.; Appetecchi, G.B.; Scrosati, B. Novel Solid Polymer Electrolytes with Single Lithium-Ion Transport. J. Electrochem. Soc. 2004, 151, A1762. [Google Scholar] [CrossRef]
- Kalita, M.; Bukat, M.; Ciosek, M.; Siekierski, M.; Chung, S.H.; Rodríguez, T.; Greenbaum, S.G.; Kovarsky, R.; Golodnitsky, D.; Peled, E.; et al. Effect of Calixpyrrole in PEO–LiBF4 Polymer Electrolytes. Electrochim. Acta 2005, 50, 3942–3948. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, J.; Huang, W. An Inorganic–Organic Nanocomposite Calix[4]Quinone (C4Q)/CMK-3 as a Cathode Material for High-Capacity Sodium Batteries. Inorg. Chem. Front. 2017, 4, 1806–1812. [Google Scholar] [CrossRef]
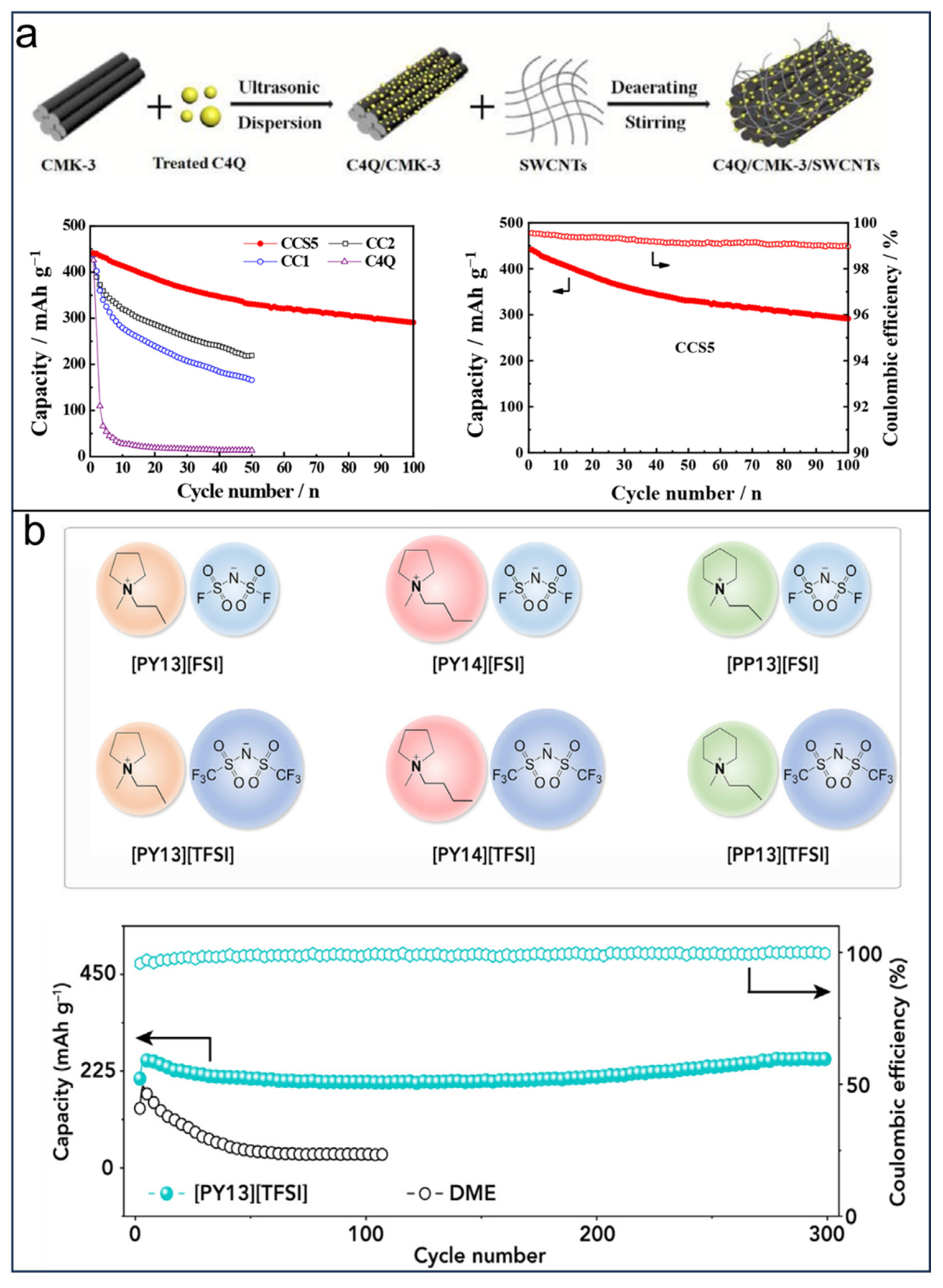
- Yan, B.; Wang, L.; Huang, W.; Zheng, S.; Hu, P.; Du, Y. High-Capacity Organic Sodium Ion Batteries Using a Sustainable C4Q/CMK-3/SWCNT Electrode. Inorg. Chem. Front. 2019, 6, 1977–1985. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Zhang, W.; Huang, W.; Yan, B.; Li, H.; Yu, S. Combination of High Performance Organic Cathode Calix[4]Quinone and Practical Biocarbon in Sodium-Ion Batteries. Org. Electron. 2020, 82, 105702. [Google Scholar] [CrossRef]
- Wang, X.; Shang, Z.; Yang, A.; Zhang, Q.; Cheng, F.; Jia, D.; Chen, J. Combining Quinone Cathode and Ionic Liquid Electrolyte for Organic Sodium-Ion Batteries. Chem 2019, 5, 364–375. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.-F.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.-L.; Lei, H.; Yu, Z.; Chen, L.-L.; Wang, M.; Jiao, S. Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Lu, Y.; Shi, R.; Ma, Y.; Yan, Z.; Zhang, K.; Chen, J. High-Energy-Density Quinone-Based Electrodes with [Al(OTF)]2+ Storage Mechanism for Rechargeable Aqueous Aluminum Batteries. Adv. Funct. Mater. 2021, 31, 2102063. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the Material and Structural Design of Zinc Anode towards High-Performance Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, W.; Luo, Z.; Liu, L.; Lu, Y.; Li, Y.; Li, L.; Hu, J.; Ma, H.; Chen, J. High-Capacity Aqueous Zinc Batteries Using Sustainable Quinone Electrodes. Sci. Adv. 2018, 4, eaao1761. [Google Scholar] [CrossRef]
- Waghmode, B.J.; Soni, R.; Patil, K.R.; Malkhede, D.D. Calixarene Based Nanocomposite Materials for High-Performance Supercapacitor Electrode. New J. Chem. 2017, 41, 9752–9761. [Google Scholar] [CrossRef]
- Correia, H.D.; Chowdhury, S.; Ramos, A.P.; Guy, L.; Demets, G.J.-F.; Bucher, C. Dynamic Supramolecular Polymers Built from Cucurbit[n]Urils and Viologens. Poly. Int. 2019, 68, 572–588. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Lin, J.-X.; Cao, M.-N.; Cao, R. Cucurbituril: A Promising Organic Building Block for the Design of Coordination Compounds and Beyond. Coordin. Chem. Rev. 2013, 257, 1334–1356. [Google Scholar] [CrossRef]
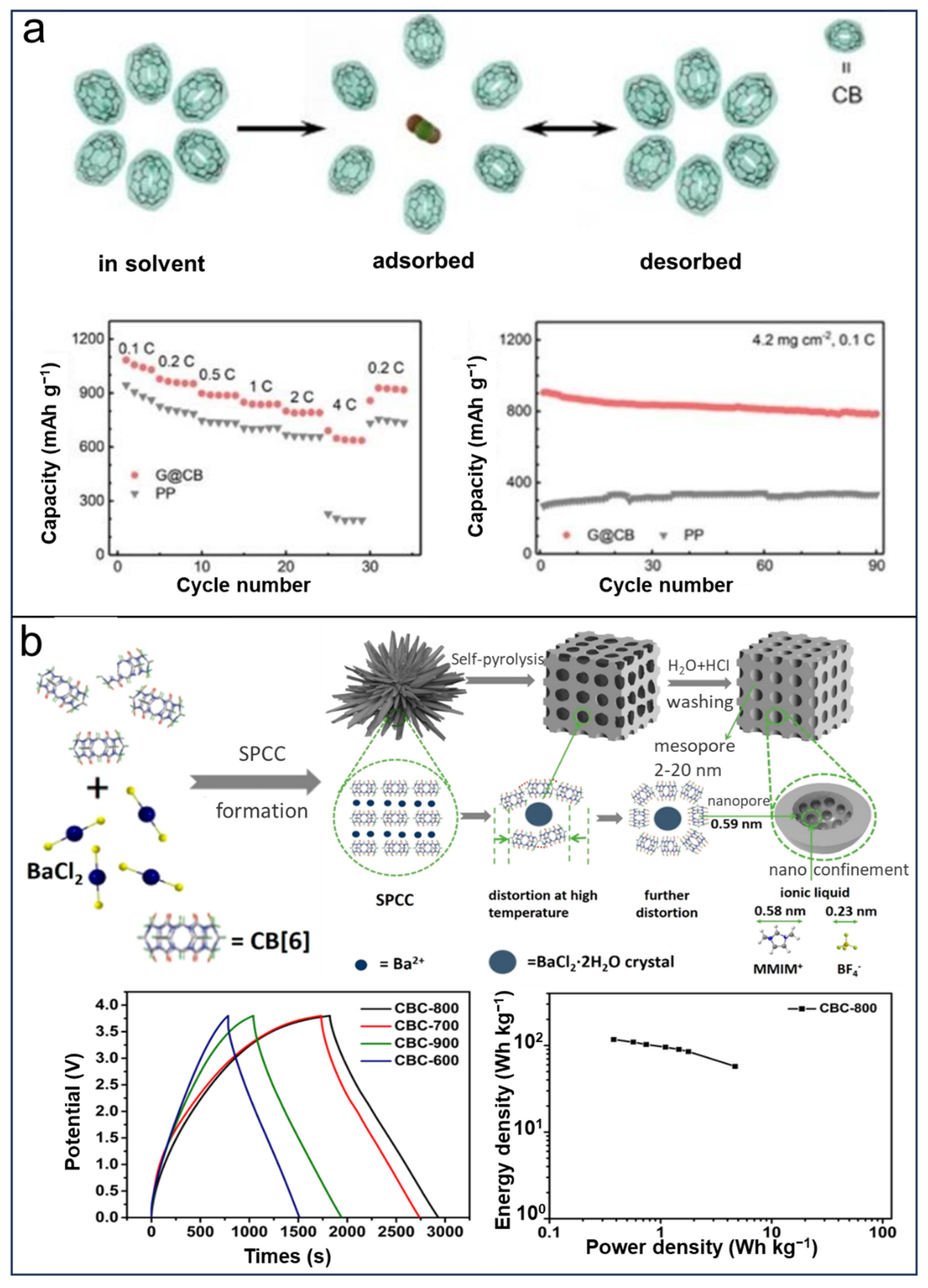
- Cui, J.; Yin, J.; Meng, J.; Liu, Y.; Liao, M.; Wu, T.; Dresselhaus, M.; Xie, Y.; Wu, J.; Lu, C.; et al. Supermolecule Cucurbituril Subnanoporous Carbon Supercapacitor (SCSCS). Nano Lett. 2021, 21, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Xu, F.; Liang, T.; Wen, H.; Tian, W. Pillararene-Based Supramolecular Polymers. Chem. Commun. 2019, 55, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hong, M.; Guo, D.; Shi, J.; Tao, Z.; Chen, J. All-Solid-State Lithium Organic Battery with Composite Polymer Electrolyte and Pillar[5]Quinone Cathode. J. Am. Chem. Soc. 2014, 136, 16461–16464. [Google Scholar] [CrossRef]
- Sun, H.; Xiong, W.; Zhou, W.; Zhang, W.; Wang, L.; Huang, W. High Performance Lithium-Ion Batteries with Pillar[5]Quinone/Ion-Liquid System. Org. Electron. 2020, 83, 105743. [Google Scholar] [CrossRef]
- Xiong, W.; Huang, W.; Zhang, M.; Hu, P.; Cui, H.; Zhang, Q. Pillar[5]Quinone–Carbon Nanocomposites as High-Capacity Cathodes for Sodium-Ion Batteries. Chem. Mater. 2019, 31, 8069–8075. [Google Scholar] [CrossRef]
- Guo, F.; Xiao, P.; Yan, B.; Hahn, M.; Kong, Y.; Zhang, W.; Piao, Y.; Diao, G. One-Pot Synthesis of Hydrazide-Pillar[5]Arene Functionalized Reduced Graphene Oxide for Supercapacitor Electrode. Chem. Eng. J. 2020, 391, 123511. [Google Scholar] [CrossRef]
- Bhatt, M.D.; O’Dwyer, C. Recent Progress in Theoretical and Computational Investigations of Li-Ion Battery Materials and Electrolytes. Phys. Chem. Chem. Phys. 2015, 17, 4799–4844. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Fisher, C.A.J. Lithium and Sodium Battery Cathode Materials: Computational Insights into Voltage, Diffusion and Nanostructural Properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Sendek, A.D.; Cubuk, E.D.; Antoniuk, E.R.; Cheon, G.; Cui, Y.; Reed, E.J. Machine Learning-Assisted Discovery of Solid Li-Ion Conducting Materials. Chem. Mater. 2019, 31, 342–352. [Google Scholar] [CrossRef]
- Huan, L.; Xie, J.; Chen, M.; Diao, G.; Zhao, R.; Zuo, T. Theoretical Investigation of Pillar[4]Quinone as a Cathode Active Material for Lithium-Ion Batteries. J. Mol. Model. 2017, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shi, H.; Shen, C.; Huan, L.; He, M.; Chen, M. Heteroatom-Bridged Pillar[4]Quinone: Evolutionary Active Cathode Material for Lithium-Ion Battery Using Density Functional Theory. J. Chem. Sci. 2021, 133, 2. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Jia, P.-P.; Xu, L.; Yang, H.-B. BODIPY-Based Macrocycles. Chem. Soc. Rev. 2020, 49, 5678–5703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Stoddart, J.F. Molecular Triangles: A New Class of Macrocycles. Acc. Chem. Res. 2021, 54, 2027–2039. [Google Scholar] [CrossRef]
- Kim, D.J.; Hermann, K.R.; Prokofjevs, A.; Otley, M.T.; Pezzato, C.; Owczarek, M.; Stoddart, J.F. Redox-Active Macrocycles for Organic Rechargeable Batteries. J. Am. Chem. Soc. 2017, 139, 6635–6643. [Google Scholar] [CrossRef]
- Kim, D.J.; Yoo, D.-J.; Otley, M.T.; Prokofjevs, A.; Pezzato, C.; Owczarek, M.; Lee, S.J.; Choi, J.W.; Stoddart, J.F. Rechargeable Aluminium Organic Batteries. Nat. Energy 2019, 4, 51–59. [Google Scholar] [CrossRef]
- Eder, S.; Yoo, D.-J.; Nogala, W.; Pletzer, M.; Santana Bonilla, A.; White, A.J.P.; Jelfs, K.E.; Heeney, M.; Choi, J.W.; Glöcklhofer, F. Switching between Local and Global Aromaticity in a Conjugated Macrocycle for High-Performance Organic Sodium-Ion Battery Anodes. Angew. Chem. Int. Ed. 2020, 59, 12958–12964. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.-J.; Heeney, M.; Glöcklhofer, F.; Choi, J.W. Tetradiketone Macrocycle for Divalent Aluminium Ion Batteries. Nat. Commun. 2021, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Miao, L.; Duan, H.; Lv, Y.; Gan, L.; Liu, M. Multielectron Redox-Bipolar Tetranitroporphyrin Macrocycle Cathode for High-Performance Zinc-Organic Batteries. Angew. Chem. Int. Ed. 2024, 63, e202401049. [Google Scholar] [CrossRef]


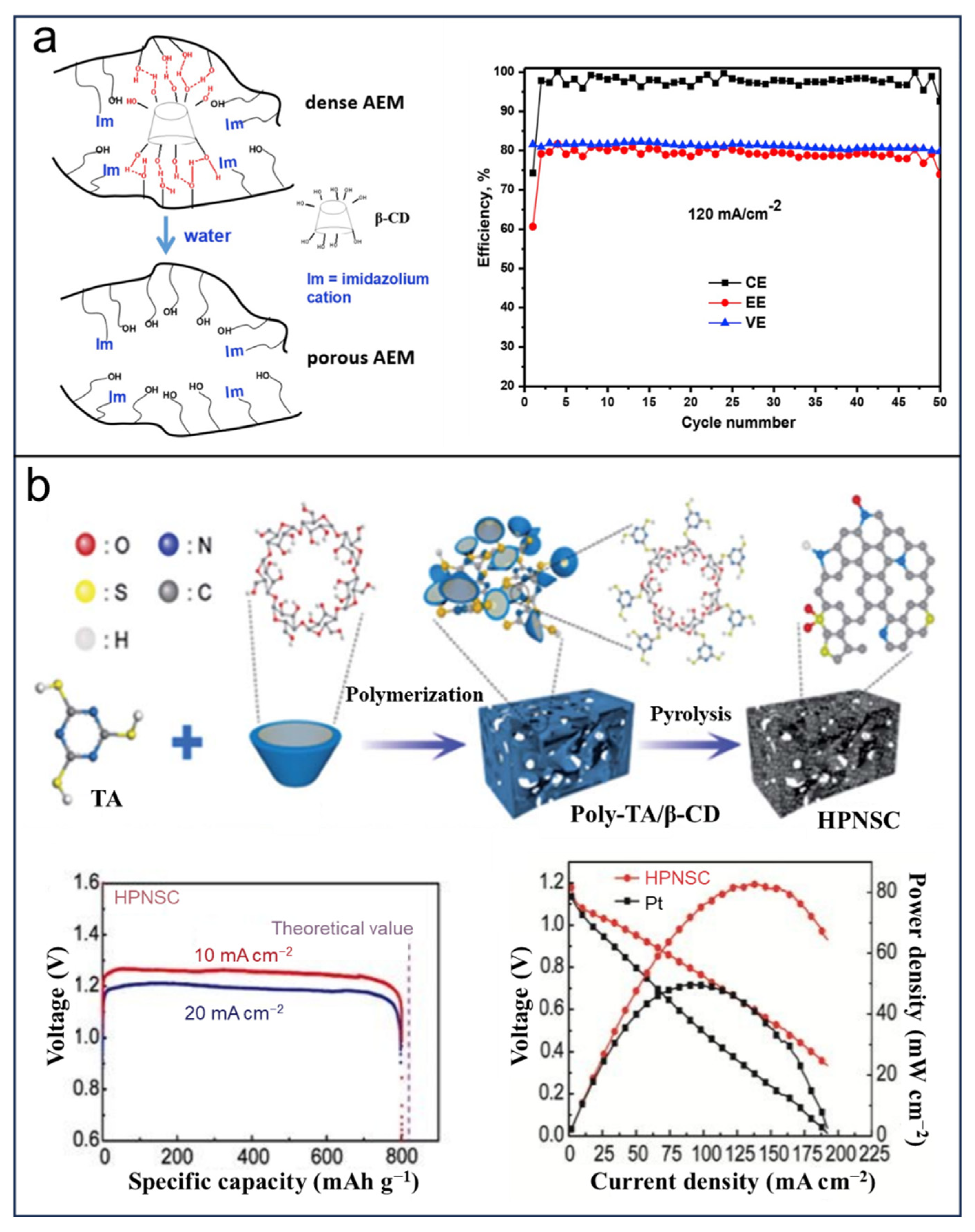

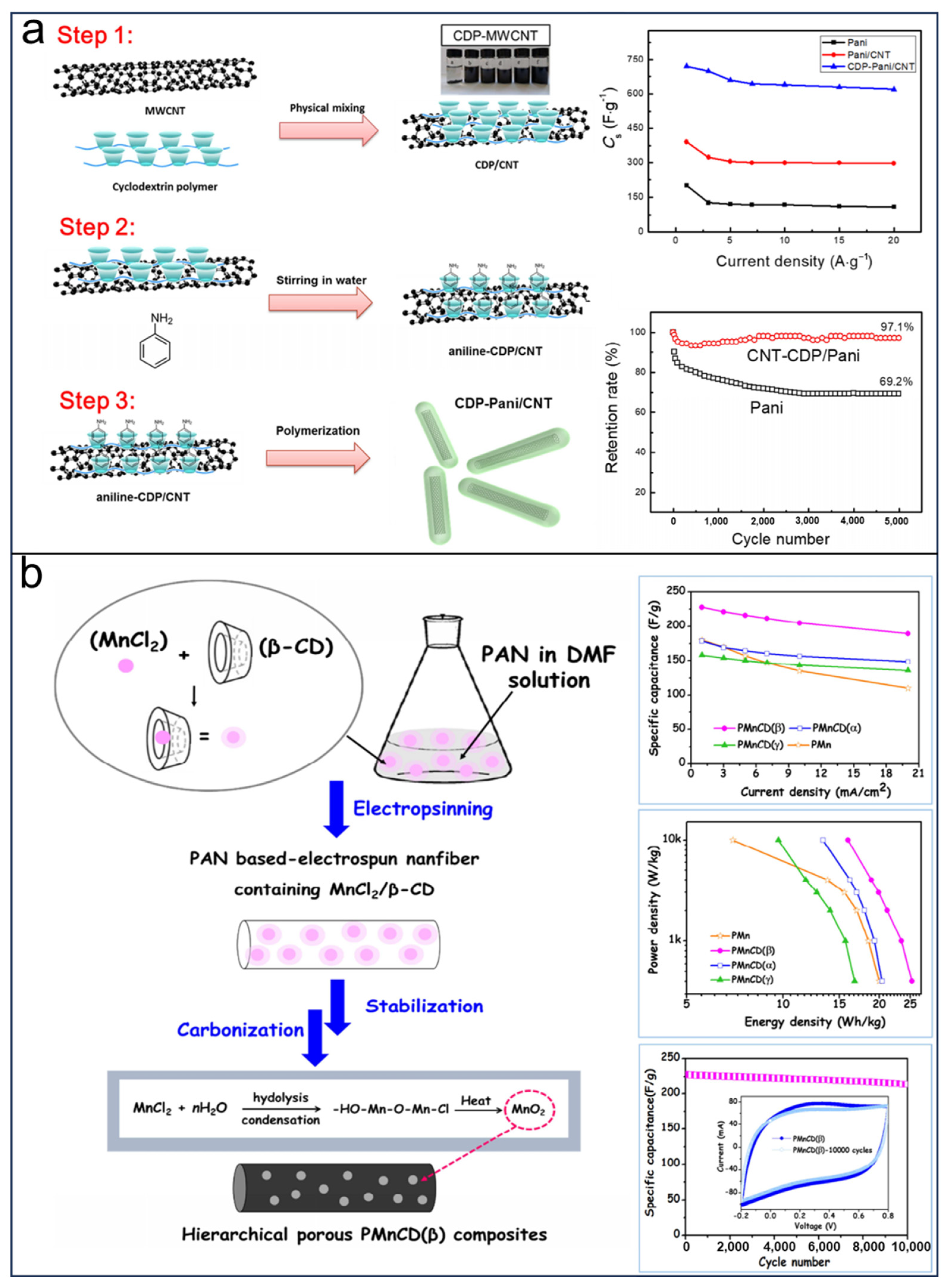



| Application | Devices | Synthesis | Specific Capacity or Capacitance | Working Voltage | Cycle Life (Cycle) | Advantage/ Disadvantage | Ref. |
|---|---|---|---|---|---|---|---|
| β-CDp/binder | LIBs | Polymerization | ~1600 mAh g−1 | 0–1.0 V | 150 (retention 90%) | Strong crosslinking/low rate | [80] |
| PR-PAA/binder | LIBs | Polymerization | 2971 mAh g−1 | 50 (retention 98%) | High reversibility/complicated synthesis | [82] | |
| C-β-CD/binder | LIBs | Oxidation | 982 mAh g−1 | 0.02–3 V | 200 (retention 626 mAh g−1) | Improving rate/poor cycle life | [88] |
| β-CD/channel | LIBs | Adsorption | 2562 mAh g−1 | 0.01–1.5 V | 200 (retention 1944 mAh g−1) | Promoting Li+ transport/poor cycle life | [89] |
| β-CD/template | ARLBs | Sol–gel route | 189 mAh g−1 | 0.5–1.4 V | 100 (retention 36%) | High porous structure/poor cycle life | [68] |
| β-CD/template | LIBs | Hydrothermal synthesis | 94 mAh g−1 | 1.8–2.6 V | 30 (retention 99%) | High stability/low capacity | [90] |
| α-CD/template | LISs | Hydrothermal synthesis | 700 mAh g−1 | 0.005–3 V | 50 (retention < 80%) | High rate/poor cycle life | [91] |
| β-CD-NH4V4O10/template | LIBs | Hydrothermal synthesis | 200 mAh g−1 | 2–4 V | 200 (retention 64.9%) | High Li+ transition, 3D porous/poor cycle life | [95] |
| poly-CDQA/adsorption | LSBs | Melt diffusion | 1307 mAh g−1 | 1.5–3.0 V | 100 (retention 84%) | High adsorption of polysulfides/poor cycle life | [96] |
| rGO@β-CDP@S/receptor | LSBs | Crosslinking | 1329 mAh g−1 | 1.7–2.8 V | 300 (retention 85.8%) | Host–guest interaction/complicated synthesis | [97] |
| C-β-CD/binder | LSBs | Oxidation | 1542.7 mA h g(sulfur)−1 | 1–3 V | 50 (retention 1456 mA h g(sulfur)−1) | Strong bonding strength/high decay rate | [98] |
| β-CDp-N+/binder | LSBs | Polycondensation | 1380 mAh g−1 | 1.7–2.8 V | 100 (retention 928 mAh g−1) | High initial capacity/high decay rate | [99] |
| Carbonβ-CD/interlayer | LSBs | Hydrothermal synthesis | ~1400 mAh g−1 | 1–3 V | 100 (retention 63.8%) | Increased conductivity/high decay rate | [100] |
| β-CD/template | VFBs | Cast | - | 0.8–1.65 V | 50 (retention 80% of energy efficiency) | Easily fabrication/high decay rate | [106] |
| hydroxypropyl-β-CD | AOFBs | Mixing | - | 0.3–1.1 V | 0.041% per cycle | High solubility/instability | [107] |
| β-CD polymer/template | ZIBs | Pyrolysis | ~800 mAh g−1 | 0–1 V | - | High ECSA/low lifespan | [108] |
| rGO@β-CDP@PEG-AD/host | Supercapacitors | Mixing | 163 F g−1 | −1 to 0 V | 10,000 (retention 80%) | Host–guest interaction/complicated fabrication | [109] |
| Carbon β-CD/Carbon | Supercapacitors | Hydrothermal reduction | 310.8 F g−1/at 0.5 A g−1 | −1 to −0.2 V | 10,000 (retention 100%) | Enlarging interlayer spacing/- | [110] |
| CDP/host | Supercapacitors | Mixing | 107.4 F·g−1 at 1 A·g−1 | −0.2 to 0.8 V | 5000 (retention 97%) | Guest-recognition capability/complicated fabrication | [111] |
| PMnCD/template | Supercapacitors | Electrospun, Hydrolysis | 228 F g−1 at 1 mAcm−2 | −0.2 to 0.8 V | 10,000 (retention 94%) | Hierarchical porous structure/complicated fabrication | [112] |
| Application | Devices | Synthesis | Specific Capacity or Capacitance | Working Voltage | Cycle Life (Cycle) | Advantage/Disadvantage | Ref. |
|---|---|---|---|---|---|---|---|
| C4Q/cathode | LIBs | Diazocoupling reaction, reduction, oxidation | 422 mAh g−1 | 1.5–3.5 V | 100 (retention 379 mAh g−1) | Coupling 8 Li/high solubility | [69] |
| C6Q/cathode | LIBs | Diazocoupling reaction, reduction, oxidation | 425 mAh g−1 at 0.05 C | 1.3–3.7 V | 500 (retention 405 mAh g−1) | High insolubility/poor cycle life at high rate | [117] |
| C6Q/cathode | LIBs | Diazocoupling reaction, reduction, oxidation | 423 mAh g−1 | 1.3–3.7 V | 300 (retention 195 mAh g−1) | Coupling 12 Li/high solubility in LiPF6 | [118] |
| C4Q/cathode | LIBs | Stirred | 39 mAh g−1 | 2–4 V | - | Increasing insolubility/losing capacity | [119] |
| C4Q-CMK-3/cathode | LIBs | Ultrasonicated | 427 mAh g−1 | 1.5–3.5 V | 100 (retention 58.7%) | High conductivity/poor cycle life | [120] |
| C4Q-CMK-3/cathode | SIBs | Ultrasonicated | 438 mAh g−1 | 1.2–4.2 V | 50 (retention 219 mAh g−1) | High conductivity/poor cycle life | [123] |
| C4Q/CMK-3/SWCNTs/cathode | SIBs | Stirring | 440 mAh g−1 | 1.2–4.2 V | 100 (retention 290 mAh g−1) | High conductivity/poor cycle life | [124] |
| C4Q-[PY13][TFSI]/cathode | SIBs | Diazocoupling reaction, reduction, oxidation | 863 Wh kg−1 (energy density) | 1.2–3.7 V | 300 (retention 99.7%) | High cycle life/low density | [126] |
| C4Q/cathode | AABs | Diazocoupling reaction, reduction, oxidation | 400 mAh g−1 | 0.5–1.5 V | 50 (retention 81%) | Great capacity/low cycle performance | [129] |
| C4Q/cathode | ZBs | Diazocoupling reaction, reduction, oxidation | 335 mAh g−1 | 0.2–1.75 V | 500 (retention 87%) | High capacity/high solubility | [131] |
| PANI-ES/SC6-MoS2/stabilizer | Supercapacitor | Stirring | 691 F g−1 | 5000 (retention 91%) | High capacitance/- | [132] |
| Application | Devices | Synthesis | Specific Capacity or Capacitance | Working Voltage | Cycle Life (Cycle) | Advantage/Disadvantage | Ref. |
|---|---|---|---|---|---|---|---|
| PQ5/cathode | LIBs | Oxidation | 418 mAh g−1 | 1.8–3.3 V | 50 (retention 94.7%) | High capacity/high solubility | [140] |
| Poly-P5Q/cathode | LIBs | Polymerization | 105 mAh g−1 | 1.75–3.25 V | 100 retention 82.3%) | High porous/high solubility | [70] |
| P5Q/cathode | LIBs | Oxidation | 408 mAh g−1 | 1.2–3.9 V | 200 (retention 70%) | High capacity/high fade rate | [141] |
| P5Q-CMK-3/cathode | SIBs | Ultrasonic mixing | 418 mAh g−1 | 1.5–4.2 V | 300 (retention 69.4%) | High capacity/high fade rate | [142] |
| RGO-HP5A/working electrode | Supercapacitor | Stirring | 331 F·g−1 (calculation) | −0.2 to 0.8 V | 10,000 (retention 93% at 5 A·g−1) | High cycle life/low capacitance | [143] |
| Application | Devices | Synthesis | Specific Capacity or Capacitance | Working Voltage | Cycle Life (Cycle) | Advantage/Disadvantage | Ref. |
|---|---|---|---|---|---|---|---|
| PMDI-Δ/anode | LIBs | Refluxing in acetic acid | 163 mAh g−1 | 1.6–3.2 V | 50 (retention 86 mAh g−1) | Single output voltage/low cycle life | [151] |
| PCT/anode | SIBs | Wittig reaction | 133 mAh g−1 | 0.01–2 V | 500 (retention 100%) | High stability/low capacity | [153] |
| PQ-Δ/cathode | AIBs | Ni(COD)2/2,2′-bipyridine/COD | 94 mAh g−1 | 0.7–1.75 V | 200 (retention 82 mAh g−1) | High stability/low capacity | [152] |
| TDK/cathode | AIBs | Benzoin condensation | 350 mAh g−1 | 0.4–2.1 V | 8000 (retention 78%) | High stability/complicate synthesis | [154] |
| TNP/cathode | ZIBs | BF3-Et2O,DDQ,Triethylamine | 338 mAh g−1 | 0.4–1.7 V | 5000 (retention 98.3) | High stability/- | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Fu, D.; Ji, Q.; Yang, Z. A Review of Macrocycles Applied in Electrochemical Energy Storge and Conversion. Molecules 2024, 29, 2522. https://doi.org/10.3390/molecules29112522
Zhu Q, Fu D, Ji Q, Yang Z. A Review of Macrocycles Applied in Electrochemical Energy Storge and Conversion. Molecules. 2024; 29(11):2522. https://doi.org/10.3390/molecules29112522
Chicago/Turabian StyleZhu, Qijian, Danfei Fu, Qing Ji, and Zhongjie Yang. 2024. "A Review of Macrocycles Applied in Electrochemical Energy Storge and Conversion" Molecules 29, no. 11: 2522. https://doi.org/10.3390/molecules29112522
APA StyleZhu, Q., Fu, D., Ji, Q., & Yang, Z. (2024). A Review of Macrocycles Applied in Electrochemical Energy Storge and Conversion. Molecules, 29(11), 2522. https://doi.org/10.3390/molecules29112522




