All-Nitrogen Energetic Material Cubic Gauche Polynitrogen: Plasma Synthesis and Thermal Performance
Abstract
1. Introduction
2. Results and Discussion
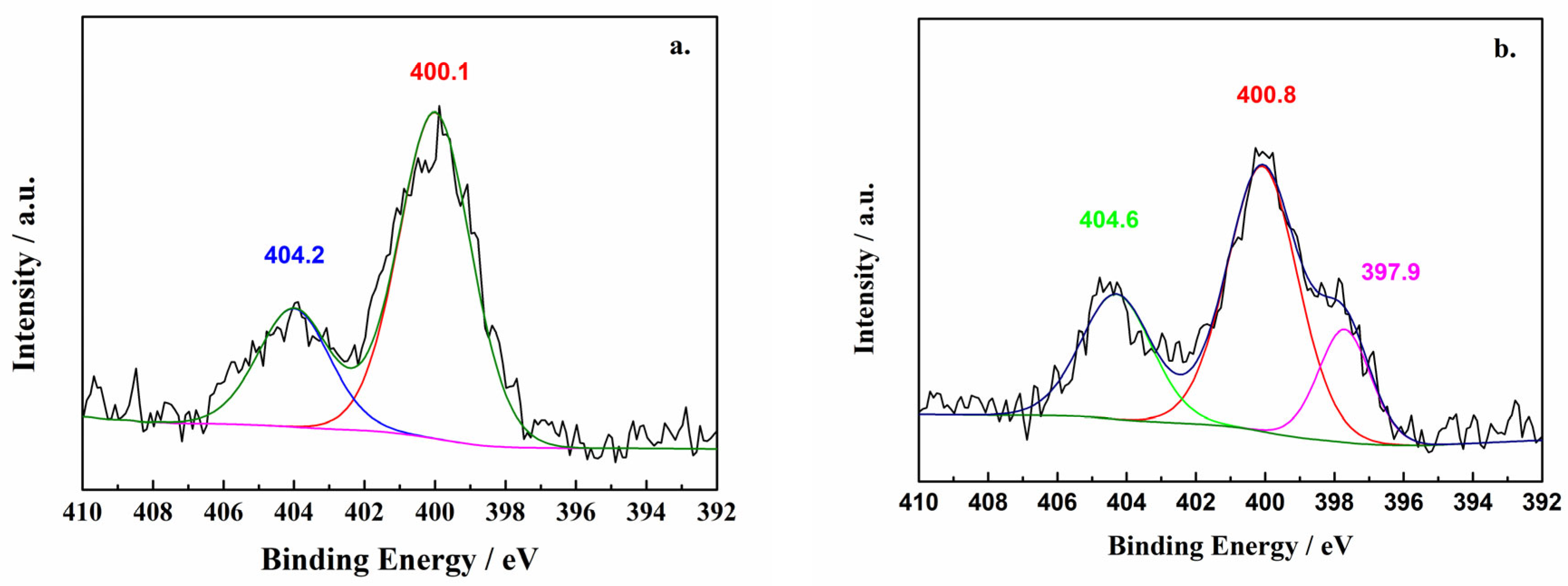
2.1. X-ray Photoelectron Spectroscopy
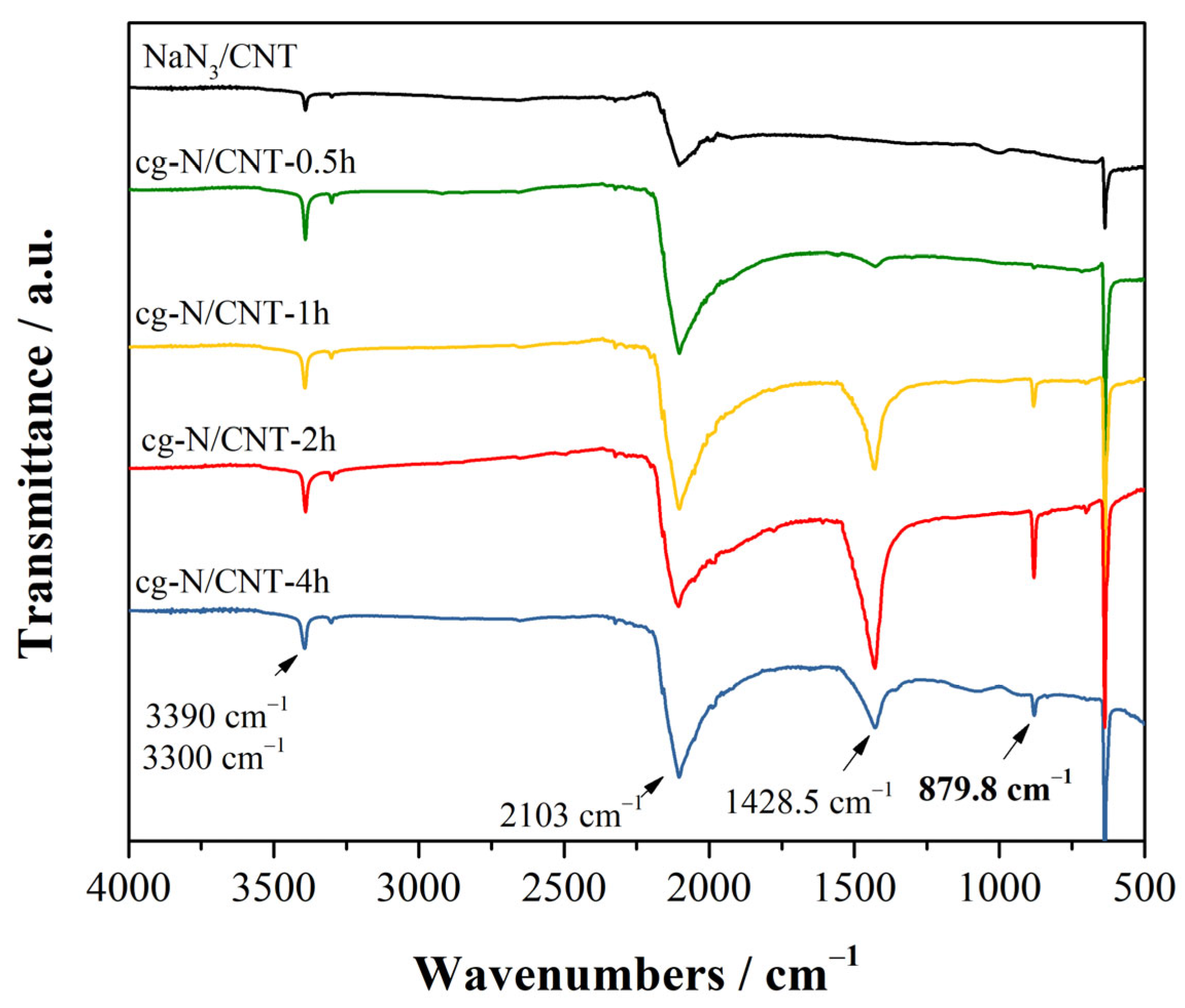
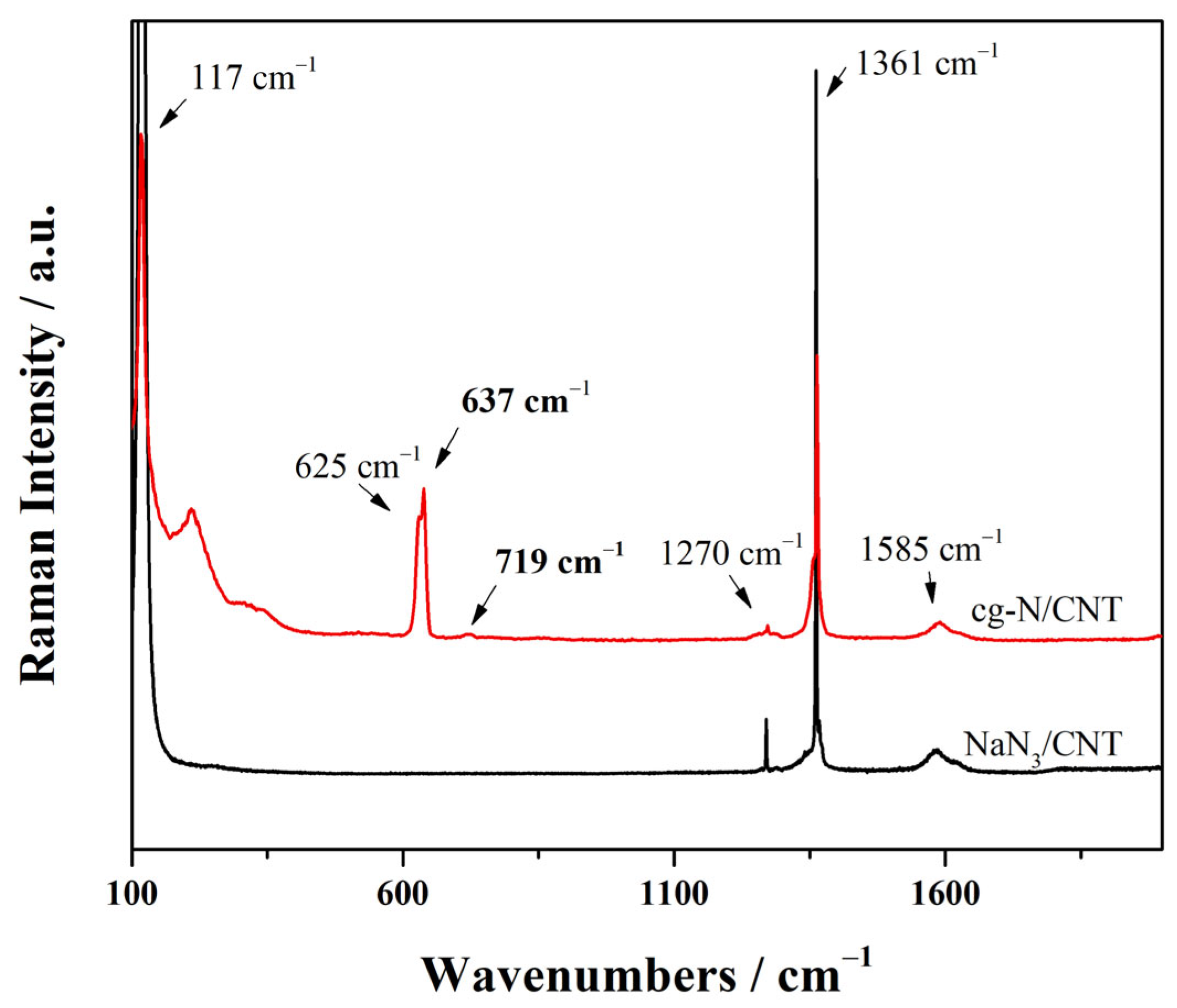
2.2. ATR-FTIR and Raman Spectroscopy
2.3. X-ray Powder Diffraction
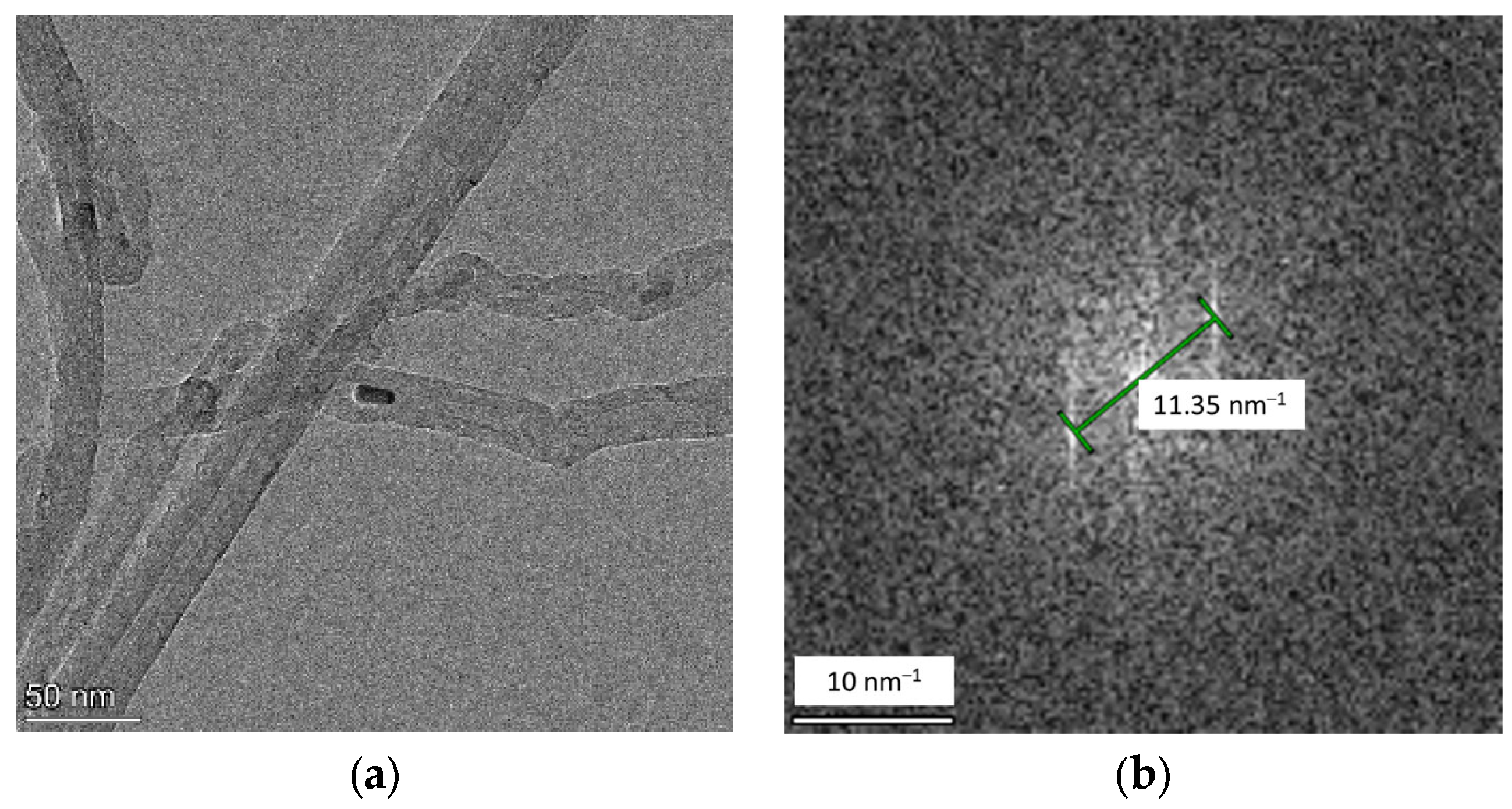
2.4. Transmission Electron Microscopy
2.5. Thermal Decomposition Performance of cg-N/CNT
2.6. Non-Isothermal Decomposition Kinetics
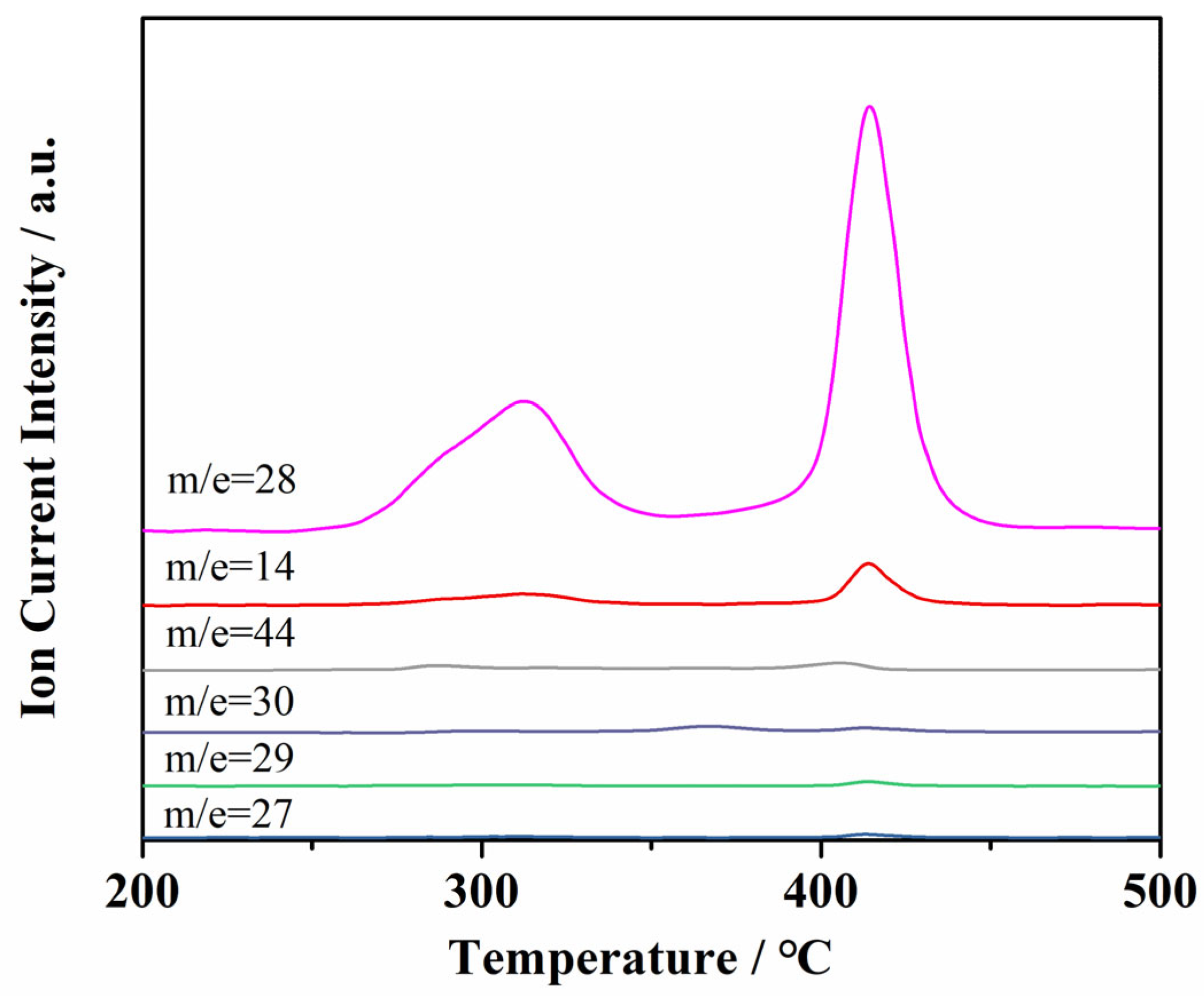
2.7. TG-DSC-FTIR-MS Analysis
3. Materials and Methods
3.1. Materials
3.2. Experimental Methods
3.3. Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Adeniyi, A.O. Solid Nitrogen and Nitrogen-rich Compounds as High Energy Density Materials. Phys. Status Solidi B 2021, 258, 2000588. [Google Scholar] [CrossRef]
- Li, Y.; Pang, S.P. Progress of All-nitrogen Ultrahigh-energetic Materials. Chin. J. Energ. Mater. 2012, 1, 9–16. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Lee, G.S.; Mitchell, A.R.; Schmidt, R.D. A review of energetic materials synthesis. Thermochim. Acta 2002, 384, 187–204. [Google Scholar] [CrossRef]
- Samartzis, P.C.; Wodtke, A.M. All-nitrogen Chemistry: How Far are we from N60? Int. Rev. Phys. Chem. 2006, 4, 527–552. [Google Scholar] [CrossRef]
- Christe, K. Recent Advances in the Chemistry of N5+, N5 and High-Oxygen Compounds. Propellants Explos. Pyrotech. 2007, 32, 194–204. [Google Scholar] [CrossRef]
- Östmark, H.; Wallin, S.; Goede, P. High Energy Density Materials (HEDM): Overview, Theory and Synthetic Efforts at FOI. Cent. Eur. J. Energetic Mater. 2007, 4, 83–108. [Google Scholar]
- Xu, Y.; Ding, L.; Yang, F.; Li, D.; Lu, M. LiN5: A novel pentazolate salt with high nitrogen content. Chem. Eng. J. 2021, 429, 132399. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W.; Sheehy, J.A.; Boatz, A. N5+: A Novel Homoleptic Polynitrogen Ion as a High Energy Density Material. Angew. Chem. Int. Ed. 1999, 38, 2004–2009. [Google Scholar] [CrossRef]
- Haiges, R.; Schneider, S.; Schroer, T.; Christe, K.O. High-Energy-Density Materials: Synthesis and Characterization of N5+[P(N3)6]−, N5+ [B(N3)4]−, N5+ [HF2]−·n HF, N5+ [BF4]−, N5+ [PF6]−, and N5+ [SO3F]−. Angew. Chem. Int. Ed. 2004, 37, 4919–4924. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Shen, C.; Lin, Q.; Wang, P.; Lu, M. A series of energetic metal pentazolate hydrates. Nature 2017, 549, 78–81. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, C.; Hu, B.C.; Yu, C.; Lu, M. Synthesis and characterization of the pentazolate anion cyclo-N5 in (N5)6(H3O)3(NH4)4Cl. Science 2017, 355, 374–376. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, C.; Jiang, C.; Yang, C.; Du, Y.; Zhao, Y.; Hu, B.C.; Zhang, Z.; Christe, K.O. Synthesis of AgN5 and its extended 3D energetic framework. Nat. Commun. 2018, 9, 1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, C.; Hu, B.; Yu, C.; Sun, C. A Symmetric Co(N5)2(H2O)4 4H2O High-Nitrogen Compound Formed by Cobalt(II) Cation Trapping of a Cyclo-N5 Anion. Angew. Chem. Int. Ed. 2017, 56, 4512–4514. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, H.; Shen, J.; Wu, B.; Ju, X.; Lu, C.; Cheng, G. 4-(1-Amino-5-aminotetrazolyl)methyleneimino-3-methylfuroxan and Its Derivatives: Synthesis, Characterization, and Energetic Properties. Eur. J. Inorg. Chem. 2014, 7, 1231–1238. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.; Wu, B.; Ju, X.; Lu, C.; Cheng, G. Synthesis and Characterization of a Stable, Catenated N 11 Energetic Salt. Angew. Chem. Int. Ed. 2013, 52, 4875–4877. [Google Scholar] [CrossRef] [PubMed]
- McMahan, A.K.; LeSar, R. Pressure dissociation of solid nitrogen under 1 mbar. Phys. Rev. Lett. 1985, 17, 1929–1932. [Google Scholar] [CrossRef]
- Mailhiot, C.; Yang, L.; McMahan, A.K. Polymeric nitrogen. Phys. Rev. B 1992, 22, 14419–14435. [Google Scholar] [CrossRef] [PubMed]
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A.; Dzivenko, D.A.; Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 2004, 3, 558–563. [Google Scholar] [CrossRef]
- Li, L.; Pu, M.; Feng, L.; Qi, L.; Zhang, L. Synthesis of Cubic Gauche Nitrogen (cg-N) under High Pressure and High Temperature. Chin. J. High Press. Phys. 2018, 32, 020102. [Google Scholar] [CrossRef]
- Lipp, M.J.; Klepeis, J.P.; Baer, B.J.; Cynn, H.; Yoo, C.S. Transformation of molecular nitrogen to nonmolecular phases at megabar pressures by direct laser heating. Phys. Rev. B 2007, 76, 014113. [Google Scholar] [CrossRef]
- Laniel, D.; Geneste, G.; Weck, G.; Mezouar, M.; Loubeyre, P. Hexagonal Layered Polymeric Nitrogen Phase Synthesized near 250 GPa. Phys. Rev. Lett. 2019, 122, 066001. [Google Scholar] [CrossRef] [PubMed]
- Laniel, D.; Winkler, B.; Fedotenko, T.; Pakhomova, A.S.; Dubrovinskaia, N. High-pressure polymeric nitrogen allotrope with the black phosphorus structure. Phys. Rev. Lett. 2020, 124, 216001. [Google Scholar] [CrossRef]
- Tomasino, D.; Kim, M.; Smith, J.; Yoo, C.S. Pressure-induced symmetry-lowering transition in dense nitrogen to layered polymeric nitrogen (lp-n) with colossal raman intensity. Phys. Rev. Lett. 2014, 113, 205502. [Google Scholar] [CrossRef]
- Li, L.; Tang, Q.Q.; Zhang, F.; Liu, S.; Wu, B.-B.; Zhou, C.-Y. Evidence for a New Extended Solid of Nitrogen. Chin. Phys. Lett. 2020, 37, 068101. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, S.; Du, M.; Shi, X.; Zhao, Z.; Guo, L.; Liu, B.; Liu, R.; Wang, P.; Liu, B. High-pressure-induced structural and chemical transformations in NaN3. J. Phys. Chem. C 2020, 124, 19904–19910. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Wei, Q.; Wang, H.; Wu, Z. Novel high-pressure phase with pseudo-benzene “N6” molecule of LiN3. EPL-Europhys. Lett. 2013, 101, 26004. [Google Scholar] [CrossRef]
- Eremets, M.I.; Popov, M.Y.; Trojan, I.A.; Denisov, V.N.; Boehler, R.; Hemley, R.J. Polymerization of nitrogen in sodium azide. J. Chem. Phys. 2004, 22, 10618–10623. [Google Scholar] [CrossRef]
- Li, D.; Zhu, P.; Jiang, J.; Li, M.; Liu, B.; Wang, X.; Cui, Q.; Zhu, H. High-Pressure Raman and Infrared Spectroscopic Studies of Cesium Azide. J. Phys. Chem. C 2016, 120, 27013–27018. [Google Scholar] [CrossRef]
- Ji, C.; Zheng, R.; Hou, D.; Zhu, H.; Wu, J.; Chyu, M.; Ma, Y. Pressure-induced phase transition in potassium azide up to 55 GPa. J. Appl. Phys. 2012, 111, 112613. [Google Scholar] [CrossRef]
- Ding, K.W. Experimental observation of TiN12+ cluster and theoretical investigation of its stable and metastable isomers. Chem. Sci. 2015, 6, 4723–4729. [Google Scholar] [CrossRef]
- Ding, K.W.; Chen, H.J.; Xu, H.; Yang, B.; Ge, Z.X.; Lu, C.; Zheng, W.J. Identification of octahedral coordinated ZrN12+ cationic clusters by mass spectrometry and structure searches. Dalton Trans. 2021, 50, 10187–10192. [Google Scholar] [CrossRef]
- Ding, K.W.; Xu, H.G.; Yang, Y.; Li, T.Q.; Chen, Z.; Ge, Z.X.; Zhu, W.; Zheng, W.J. Mass Spectrometry and Theoretical Investigation of VN n+(n = 8, 9, and 10) Clusters. J. Phys. Chem. A 2018, 122, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.W.; Li, T.Q.; Xu, H.G.; Su, H.P.; Liu, Y.; Zheng, W.J.; Ge, Z.X. Formation and Detection of Lithium-nitrogen Clusters with High Nitrogen Content. Chin. J. Energet. Mater. 2018, 41, 447–450. [Google Scholar] [CrossRef]
- Wu, Z.; Benchafia, E.M.; Iqbal, Z.; Wang, X.Q. N8 Polynitrogen Stabilized on Multi-Wall Carbon Nanotubes for Oxygen-Reduction Reactions at Ambient Conditions. Angew. Chem. Int. Ed. 2014, 53, 12555–12559. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wu, Z.; Hu, M.; Alzaim, S.; Young, J.; Dong, J.; Chang, J.; Zhuang, H.; Benchafia, E.M.; Yang, Y. Rational synthesis of polymeric nitrogen N8− with ultraviolet irradiation and its oxygen reduction reaction mechanism study with in situ shell-isolated nanoparticle-enhanced raman spectroscopy. ACS Catal. 2021, 21, 13034–13040. [Google Scholar] [CrossRef]
- Benchafia, E.M.; Yao, Z.; Yuan, G.; Chou, T.; Piao, H.; Wang, X.; Iqbal, Z. Cubic gauche polymeric nitrogen under ambient conditions. Nat. Commun. 2017, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Alzaim, S.; Li, S.; Benchafia, E.L.; Young, J.; Ravindra, N.M.; Iqbal, Z.; Wang, X. Synthesis and Stabilization of Cubic Gauche Polynitrogen under Radio-Frequency Plasma. Chem. Mater. 2022, 34, 4712–4720. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Liang, Q.; Liu, X.; Weng, Q.; Liu, J.; Yang, Y.; Dai, Z.; Ding, K.; Bando, Y. Amorphous Phosphorus/Nitrogen-Doped Graphene Paper for Ultrastable Sodium-Ion Batteries. Nano Lett. 2016, 16, 2054–2060. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D.; King, R.C. Handbook of X-ray Photoelectron Spectroscop; Perkin-Elmer: Waltham, MA, USA, 1992; p. 227. [Google Scholar]
- Razvan, C. Raman spectra and lattice dynamics of cubic gauche nitrogen. J. Chem. Phys. 2007, 127, 144510. [Google Scholar] [CrossRef]
- Onoe, T.; Iwamoto, S.; Inoue, M. Synthesis and activity of the Pt catalyst supported on CNT. Catal. Commu. 2007, 8, 701–706. [Google Scholar] [CrossRef]
- Trojan, I.A.; Eremets, M.I.; Medvedev, S.A.; Gavriliuk, A.G.; Prakapenka, V.B. Transformation from molecular to polymeric nitrogen at high pressures and temperatures: In situ X-ray diffraction study. Appl. Phys. Lett. 2008, 93, 091907. [Google Scholar] [CrossRef]
- Aravind, S.L.; Paramashivan, S.S.; Mahadevan, S. Thermo-kinetic studies of NaN3/KNO3 air bag gas generant mixture. J. Therm. Anal. Calorim. 2019, 136, 2183–2193. [Google Scholar] [CrossRef]
- Eslami, A.; Hosseini, S.G.; Asadi, V. The effect of microencapsulation with nitrocellulose on thermal properties of sodium azide particles. Prog. Org. Coat. 2009, 65, 269–274. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Yi, Q.H.; Liang, D.; Ma, Q.; Huang, M.; Tan, B.S.; Liu, Y.; Chi, Y. Synthesis, Crystal Structure, and Thermal Behavior of 3-(4-Aminofurazan-3-yl)-4-(4-nitrofurazan-3-yl)furazan(ANTF). Propellants Explos. Pyrotech. 2016, 41, 906–911. [Google Scholar] [CrossRef]
- Gao, Y.C.; Li, M.; Yang, W.T.; Hu, R.; Zhang, Y.C. Thermal Decomposition Performance of CL-20-Based Ultraviolet Curing Propellants. Propellants Explos. Pyrotech. 2022, 47, e202100335. [Google Scholar] [CrossRef]




| Sample Component | Heating Rate β (°C/min) | Exothermic Peak Tp (°C) | Apparent Activation Energy Ea (kJ/mol) | Pre-Exponential Constant lnAk | |
|---|---|---|---|---|---|
| Kissinger | Ozawa | ||||
| cg-N | 5 | 408.1 | 84.7 | 91.9 | 12.8 |
| 10 | 429.0 | ||||
| 20 | 464.8 | ||||
| 25 | 476.9 | ||||
| NaN3 | 5 | 397.6 | 184.9 | 191.2 | 31.8 |
| 10 | 412.3 | ||||
| 20 | 422.9 | ||||
| 25 | 426.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, C.; Li, J.; Ding, K.; Guo, S.; Jia, Y. All-Nitrogen Energetic Material Cubic Gauche Polynitrogen: Plasma Synthesis and Thermal Performance. Molecules 2024, 29, 504. https://doi.org/10.3390/molecules29020504
Qu C, Li J, Ding K, Guo S, Jia Y. All-Nitrogen Energetic Material Cubic Gauche Polynitrogen: Plasma Synthesis and Thermal Performance. Molecules. 2024; 29(2):504. https://doi.org/10.3390/molecules29020504
Chicago/Turabian StyleQu, Chenxi, Jiale Li, Kewei Ding, Songsong Guo, and Yating Jia. 2024. "All-Nitrogen Energetic Material Cubic Gauche Polynitrogen: Plasma Synthesis and Thermal Performance" Molecules 29, no. 2: 504. https://doi.org/10.3390/molecules29020504
APA StyleQu, C., Li, J., Ding, K., Guo, S., & Jia, Y. (2024). All-Nitrogen Energetic Material Cubic Gauche Polynitrogen: Plasma Synthesis and Thermal Performance. Molecules, 29(2), 504. https://doi.org/10.3390/molecules29020504