New Stable Gallium(III) and Indium(III) Complexes with Thiosemicarbazone Ligands: A Biological Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
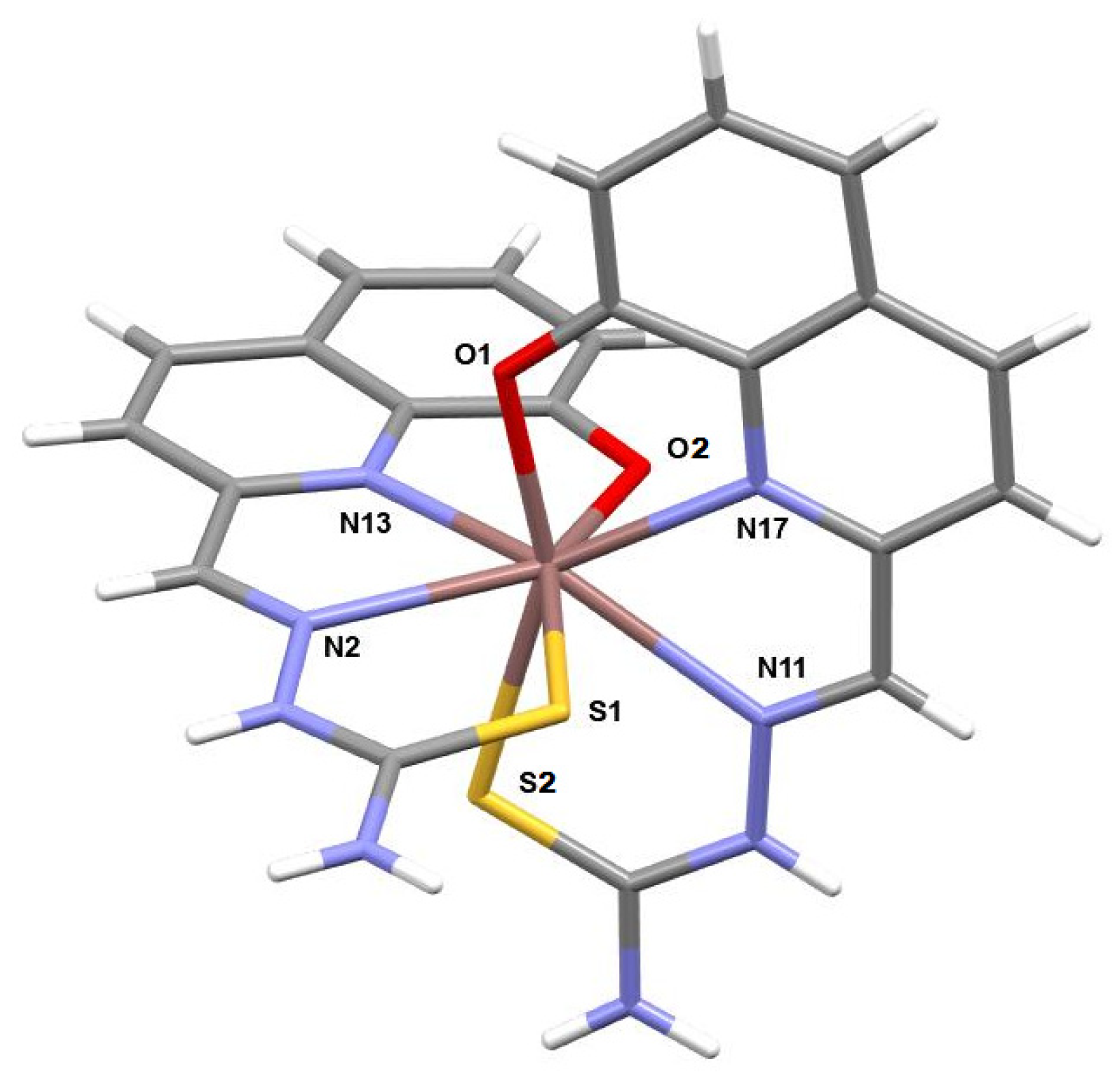
2.2. Structural Information
2.3. UV-Visible Titrations
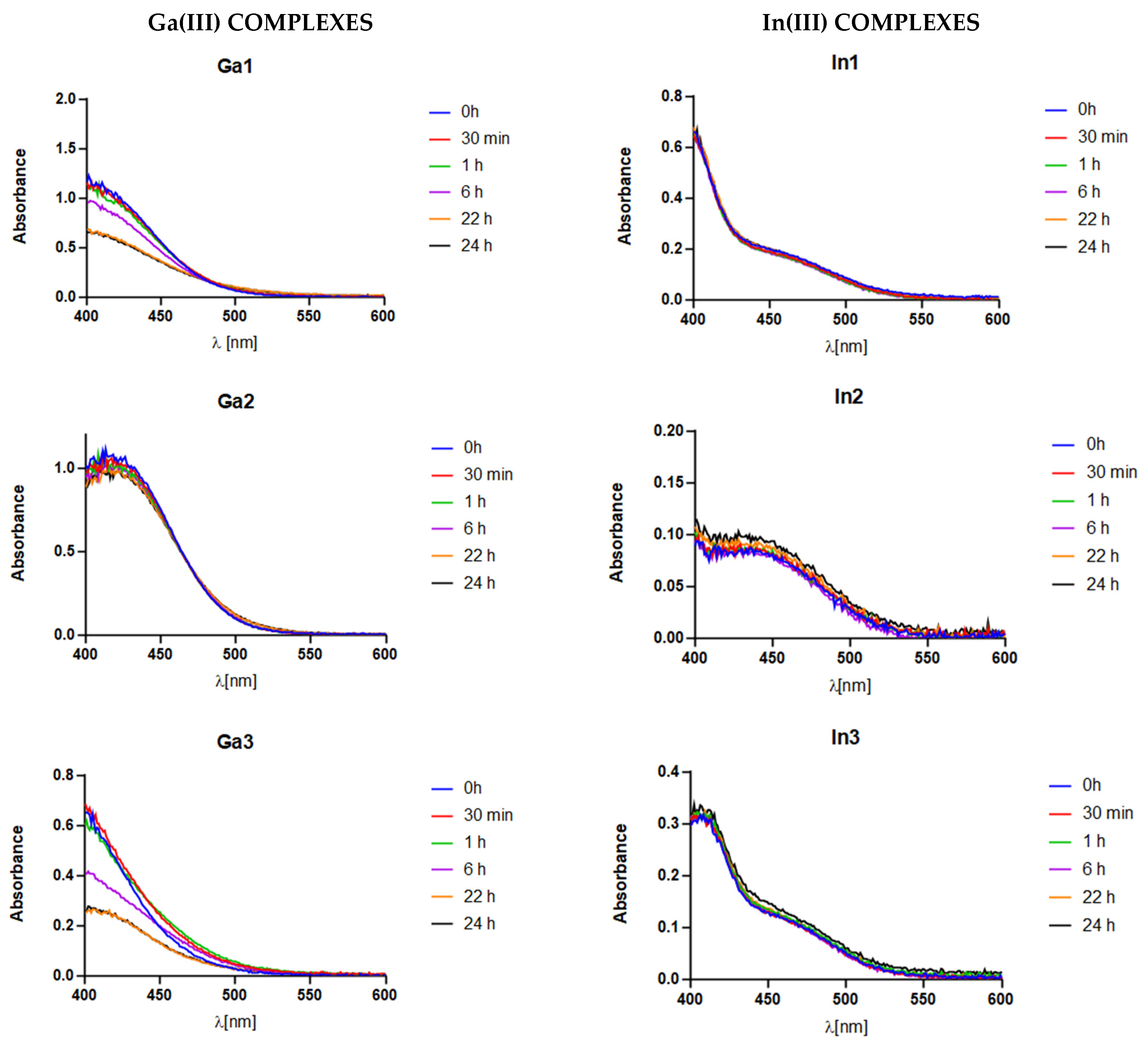
2.4. Stability Assay
2.5. Cytotoxicity Assay
2.6. Antibiotic Assay
3. Materials and Methods
3.1. Preparation of the Complexes
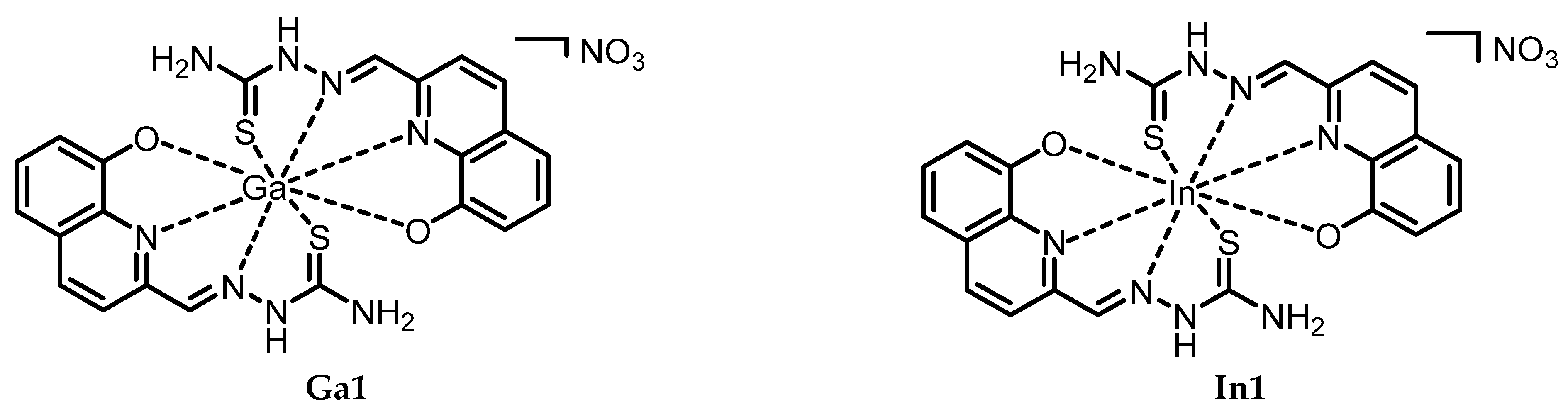
- Ga1. (((8-Hydroxyquinolin-2-yl)methylidene)amino)thiourea (L1; 51 mg, 0.21 mmol) was dissolved in MeOH (10 mL) at reflux. A solution of hydrated Ga(NO3)3 (52 mg, 0.20 mmol) in 3 mL in MeOH was then added dropwise to the ligand mixture and left stirring for 2 h at RT. A turbid dark yellow mixture was then obtained and cooled down to 0 °C in an ice bath. The yellow product was then separated via filtration (65%). Anal. Calcd. for C44H38Ga2N10O11S4 (2Ga1 + H2O): C, 41.86; N, 19.97; H, 3.03; S, 10.16. Exp.: C, 42.38; N, 19.21; H, 2.90; S, 9.42. IR (ATR, cm−1): 3418, 3228, 3139, 3076, and 2935 w (N-H, O-H, and C-H), 1526 s (C=N), 1385 m and 745 s (C=S). ESI-MS m/z (%): 313.5 ([Ga + L − 2H]+ 71.1), 558.6 ([Ga + 2L − 2H]+ 100.0). 1H-NMR (400 MHz, DMSO-d6): [ppm] 12.24 (s, 1H, NH), 8.86 (br, 1H, N=C-H), 8.48 (br, 1H, CH ar.), 8.44 (d, 1H, CH ar.), 8.32 (br, 1H, CH ar.), 8.29 (t, 1H, CH ar.), 7.52 (s, 1H, NH), 7.38 (d, 1H, CH ar.), 7.19 (s, 1H, NH). 13C-NMR (400 MHz, DMSO-d6): [ppm] 178.4 (C=N aliph.), 155.2 (C=S), 153.4 (C-OH), 151.9–150.5 (C=N ar.), 142.4 (C=C=N ar.), 139.3–138.8–138.1 (C=C=C ar.), 136.1 (C=CH-C ar., N-para), 130.0–128.8 (C=CH-C ar., OH-para), 128.4–128.1 (C=CH-C ar., N-meta), 120.0–118.4–117.8 (C=CH-C ar., OH-para), 114.1–113.5–112.1 (C=CH-C ar., OH-ortho).
- In1. (((8-Hydroxyquinolin-2-yl)methylidene)amino)thiourea (L1; 49 mg, 0.20 mmol) was dissolved in MeOH (10 mL) at reflux. A solution of hydrated In(NO3)3 (61 mg, 0.20 mmol) in 3 mL in MeOH was then added dropwise to the ligand mixture and left stirring for 2 h at RT. A clear dark red solution was then obtained and cooled down to 0 °C in an ice bath. The solvent was removed via rotary evaporation and the resulting dry solid was recovered with EtOH (10 mL). The solution was left at 0 °C overnight, and the product precipitated. After filtration, the product was washed with diethyl ether, to yield a red solid (21%). Anal. Calcd for C22H30InN9O11S2 (In1 + 6H2O): C, 34.07; N, 16.90; H, 3.90; S, 8.27. Exp.: C, 33.55; N, 17.16; H, 2.45; S, 7.76. IR (ATR, cm−1): 3451, 3286, 3186, and 3062 w (N-H, O-H, and C-H), 1575 m (C=N), 1385 s and 756 s (C=S). ESI-MS m/z (%): 359.2 ([In + L − 2H]+, 72.4), 605.2 ([In + 2L − 2H]+, 100.0). 1H-NMR (400 MHz, DMSO-d6): [ppm] 11.94 (s, 1H, HC=N-NH), 8.62 (d, 1H, CH ar.), 8.54 (s, 1H, NH), 8.52–8.33 (m, 5H, CH ar., +NH), 8.31 (s, 1H, N=C-H), 7.90 (d, 1H, CH ar.), 7.53–7.38 (m, 3H, CH ar.), 7.17–7.04 (m, 2H, CH ar.), 6.86 (d, 1H, CH ar.). 13C-NMR (400 MHz, DMSO-d6): [ppm] 178.5 (C=N aliph.), 158.9 (C=S), 152.8 (C-OH), 151.6 (C=N ar.), 141.3–140.8 (C=C=N ar.), 137.1 (C=C=C ar.), 136.3 (C=CH-C ar., N-para), 131.4 (C=CH-C ar., OH-para), 128.9–128.4 (C=CH-C ar., N-meta), 121.6–118.6–117.9 (C=CH-C ar., OH-para), 112.6–112.2–110.7 (C=CH-C ar., OH-ortho). XRD: CCDC No 2311577.
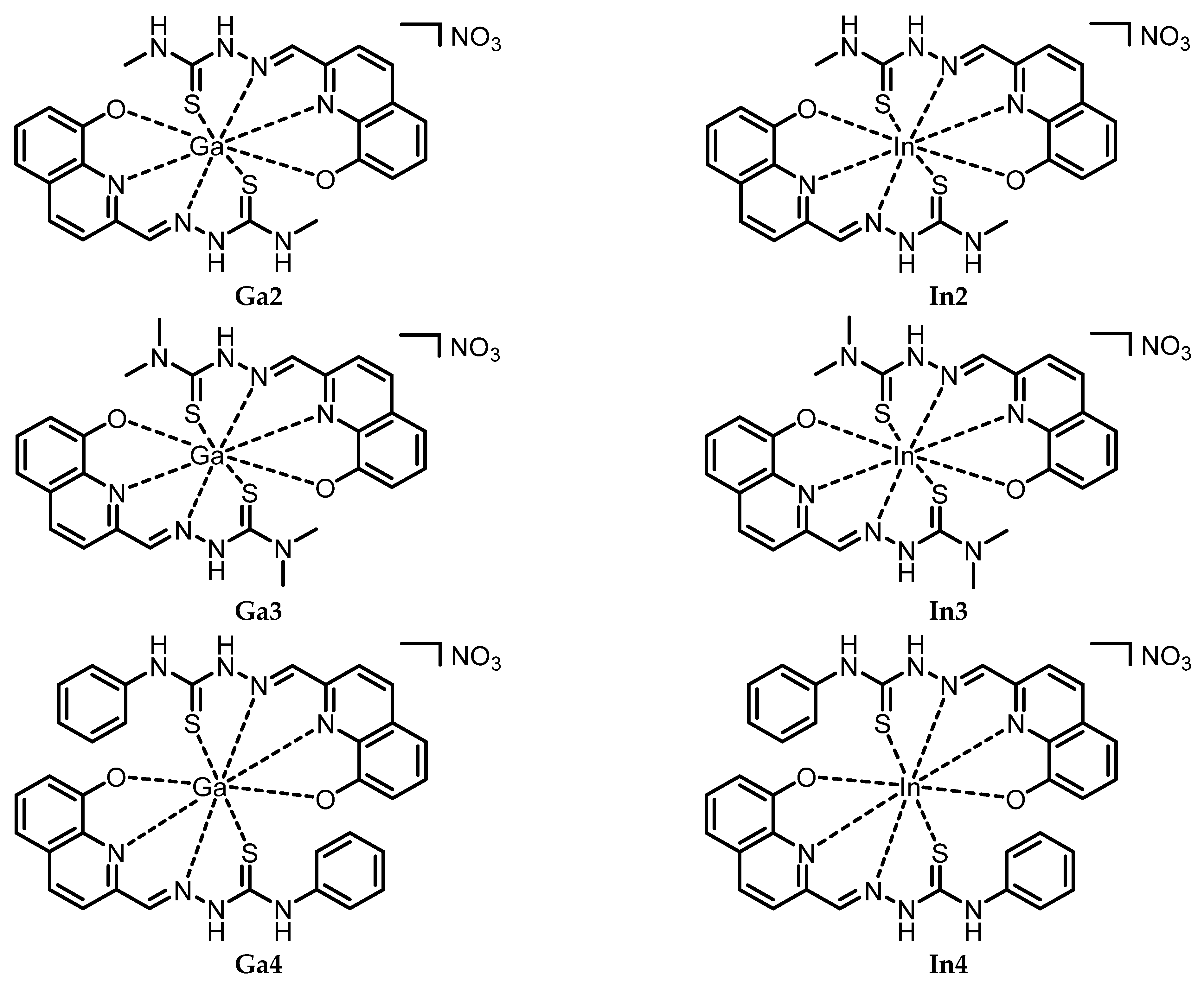
- Ga2. 1-(((8-Hydroxyquinolin-2-yl)methylidene)amino)-3-methylthiourea (L2; 49 mg, 0.19 mmol) was dissolved in MeOH (10 mL) at reflux. A solution of hydrated Ga(NO3)3 (48 mg, 0.19 mmol) in 3 mL in MeOH was then added dropwise to the ligand mixture and left stirring for 2 h at RT. A turbid dark yellow mixture was then obtained and cooled down to 0 °C in an ice bath. The yellow product is then separated via filtration (82%). Anal. Calcd for C48H50Ga2N18O13S4 (2Ga2 + 3H2O): C, 42.56; N, 18.61; H, 3.72; S, 9.47. Exp.: C, 42.58; N, 17.98; H, 3.53; S, 9.12. IR (ATR, cm−1): 3418, 3255, 3139, 3042 w (N-H, O-H, and C-H), 1584 (C=N), 1130 and 751 s (C=S). ESI-MS m/z (%): 587.3 ([Ga + 2L − 2H]+, 12.4). 1H-NMR (400 MHz, DMSO-d6): [ppm] 8.91 (s, 1H, N=C-H), 8.91 (br, 1H, C=CH=C N-para), 8.77 (s, 1H, NH), 8.66 (br, 1H, C=CH=C N-meta), 7.66 (t, 1H, C=CH=C OH-meta), 7.42 (dd, 1H, C=CH=C OH-ortho), 7.20 (dd, 1H, C=CH=C OH-para), 4.05 (s, 3H, CH3).13C NMR (400 MHz, DMSO-d6): [ppm] 181.7 (C=N aliph.), 155.8 (C=S), 151.2 (C-OH), 140.8 (C=N ar.), 136.6 (C=C=N ar.), 131.0 (C=C=C ar.), 129.4 (C=CH-C ar., N-para), 120.6 (C=CH-C ar., OH-para), 115.1 (C=CH-C ar., N-meta), 114.1 (C=CH-C ar., OH-para), 49.1 (C=CH-C ar., OH-ortho), 34.4 (CH3).
- In2. 1-(((8-Hydroxyquinolin-2-yl)methylidene)amino)-3-methylthiourea (L2; 49 mg, 0.19 mmol) was dissolved in EtOH (10 mL) at reflux. A solution of hydrated In(NO3)3 (58 mg, 0.19 mmol) in 3 mL in EtOH was then added dropwise to the ligand mixture and left stirring for 2 h. A turbid red mixture was then obtained and cooled down to 0 °C in an ice bath. After filtration, the product was washed with diethyl ether, to yield a red solid (54%). Anal. Calcd. for C24H30InN9O9S2 (In2 + 4H2O): C, 37.56; N, 16.43; H, 3.94 S, 8.35. Exp.: C, 38.07; N, 15.55; H, 3.11; S, 8.60. IR (ATR, cm−1): 3330 and 3167 w (N-H, O-H and C-H), 1589 m (C=N), 1138 and 742 s (C=S). ESI-MS m/z (%): 633.0 ([In + 2L − 2H]+, 100.0), 373.0 ([In + L − 2H]+, 24.7). 1H-NMR (400 MHz, DMSO-d6): [ppm] 9.84 (s, 1H, HC=N-NH), 8.91 (s, 1H, NH), 8.68 (s, 1H, NH), 8.63 (br, 1H, CH ar.), 8.62 (s, 1H, N-H), 8.56 (d, 1H, CH ar.), 8.32 (d, 1H, CH ar.), 8.02 (s, 1H, N=C-H), 7.98 (s, 1H, N=C-H), 7.87 (d, 1H, CH ar.), 7.48 (t, 1H, CH ar.), 7.45 (dd, 1H, CH ar.), 7.41 (dd, 1H, CH ar.), 7.11 (dd, 1H, CH ar.), 7.10 (t, 1H, CH ar.), 6.86 (dd, 1H, CH ar.), 6.77 (s, 1H, NH), 3.89 (s, 3H, CH3), 3.88 (s, 3H, CH3). 13C-NMR (400 MHz, DMSO-d6): [ppm] 182.2, 181.0 (C=N aliph.), 158.7 (C=S), 153.4 (C-OH), 151.7 (C=N ar.), 143.0, 141.1, 140.8 (C=C=N ar.), 138.1, 136.4 (C=C=C ar.), 131.0, 129.4 (C=CH-C ar., N-para), 128.8, 128.2, 127.5 (C=CH-C ar., OH-para), 121.4, 118.9 (C=CH-C ar., N-meta), 117.7 (C=CH-C ar., OH-para), 112.4, 111.9, 110.9 (C=CH-C ar., OH-ortho), 33.5, 32.9 (CH3).
- Ga3. 1-(((8-Hydroxyquinolin-2-yl)methylidene)amino)-3,3-dimethylthiourea (L3; 40 mg, 0.14 mmol) was dissolved in MeOH (10 mL) at reflux. A solution of hydrated Ga(NO3)3 (40 mg, 0.16 mmol) in 3 mL in MeOH was then added dropwise to the ligand mixture. A clear red solution was then obtained and left stirring at RT for 2 h. The resulting red solution was then cooled down to 0 °C in an ice bath. The solvent was removed via rotary evaporation and the resulting dry solid was recovered with EtOH (10 mL). The solution was left at 0 °C overnight, and the product precipitated. After filtration, the product was washed with diethyl ether, to yield a red solid (66%). Anal. Calcd. for C26H38GaN9O11S2 (Ga3 + 6H2O): C, 39.71; N, 16.03; H, 4.87; S, 8.15. Exp.: C, 39.74; N, 16.18; H, 3.96; S, 7.90. IR (ATR, cm−1): 3379, 3070 w (O-H and C-H), 1589 m (C=N), 1302 s and 729 m (C=S) ESI-MS m/z (%): 341.1 ([Ga + L − 2H]+, 100.0), 614.8 ([Ga + 2L − 2H]+, 3.8), 679.0 ([Ga + 2L + NO3− − H]+, 38.8). 1H-NMR (400 MHz, DMSO-d6): [ppm] 8.40 (s, 1H, N=C-H), 8.33 (d, 1H, CH ar.), 8.04 (d, 1H, CH ar.), 7.91 (s, 1H, N-H), 7.45 (dd, 1H, CH ar.), 7.39 (t, 1H, CH ar.), 7.14 (dd, 1H, CH ar.). 13C NMR (400 MHz, DMSO-d6): [ppm] 181.0, 180.7 (C=N aliph.), 153.0 (C=S), 152.0 (C-OH), 143.2 (C=N ar.), 136.8, 136.0 (C=C=N ar.), 129.9, 128.6, 128.1 (C=C=C ar.), (C=CH-C ar., N-para), 119.1 (C=CH-C ar., OH-para), 117.9, 117.6 (C=CH-C ar., N-meta), 114.3 (C=CH-C ar., OH-para), 112.3 (C=CH-C ar., OH-ortho), 42.4 (CH3).
- In3. 1-(((8-Hydroxyquinolin-2-yl)methylidene)amino)-3,3-dimethylthiourea (L3; 39 mg, 0.14 mmol) was dissolved in EtOH (10 mL) at reflux. A solution of hydrated In(NO3)3 (44 mg, 0.15 mmol) in 3 mL in EtOH was then added dropwise to the ligand mixture. A red solution was then obtained and left stirring at RT for 2 h. The red turbid mixture was then cooled down to 0 °C in an ice bath. After filtration, the product was washed with diethyl ether, to yield a red solid (47%). Anal. Calcd. for C26H32InN9O8S2 (In3 + 3H2O): C, 40.16; N, 16.21; H, 4.15; S, 8.25. Exp.: C, 40.69; N, 15.78; H, 3.76; S, 8.50. IR (ATR, cm−1): 3377, 2985 and 2902 w (C-H, O-H), 1573 m (C=N), 1316 and 740 s (C=S). ESI-MS m/z (%): 387.4 ([In + L − 2H]+, 20.5), 661.1 ([In + 2L − 2H]+, 55.1). 1H-NMR (400 MHz, DMSO-d6): [ppm] 8.75 (s, 1H, N=C-H), 8.60 (d, 1H, C=CH=C N-para), 7.95 (d, 1H, C=CH=C N-meta), 7.40 (br, 1H, C=CH=C OH-meta), 7.40 (br, 1H, C=CH=C OH-ortho), 7.27 (s, 1H, NH), 6.79 (br, 1H, C=CH=C OH-para).13C NMR (400 MHz, DMSO-d6): [ppm] 180.7, 178.2 (C=N aliph.), 159.1 (C=S), 153.2 (C-OH), 152.0 (C=N ar.), 143.5, 140.3, 139.9 (C=C=N ar.), 136.5 (C=C=C ar.), 131.0 (C=CH-C ar., N-para), 128.6, 128.1 (C=CH-C ar., OH-para), 120.7 (C=CH-C ar., N-meta), 117.8, 117.6 (C=CH-C ar., OH-para), 112.5, 112.2, 111.6, 110.1 (C=CH-C ar., OH-ortho), 42.4 (CH3).
- Ga4. 3-(((8-Hydroxyquinolin-2-yl)methylidene)amino)-1-phenylthiourea (L4; 51 mg, 0.16 mmol) was dissolved in MeOH (10 mL) at reflux. A solution of hydrated Ga(NO3)3 (38 mg, 0.15 mmol) in 3 mL in MeOH was then added dropwise to the ligand mixture. A clear orange solution was then obtained and left stirring at RT for 2 h. The resulting turbid orange mixture was then cooled down to 0 °C in an ice bath. After filtration, the product was washed with diethyl ether, to yield an orange solid (36%). Anal. Calcd. for C34H32GaN9O8S2 (Ga4 + 3H2O): C, 49.29; N, 15.22; H, 3.89; S, 7.74. Exp.: C, 50.02; N, 14.68; H, 3.37; S, 7.89. IR (ATR, cm−1): 3288, 3123 and 2988 w (N-H, O-H and C-H), 1529 m (C=N), 1316 and 698 m (C=S). ESI-MS m/z (%): 435.3 ([Ga + L + EtOH − 2H]+, 100.0), 757.3 ([Ga + 2L + EtOH − 2H]+, 7.7). 1H-NMR (400 MHz, DMSO-d6): [ppm] 12.65 (s, 2H, NH), 10.28 (s, 2H, NH), 8.52 (s, 1H, N=C-H), 8.36 (s, 1H, N=C-H), 7.58–7.24 (m, 20H, C-H ar.). 13C-NMR (400 MHz, DMSO): [ppm] 177.0, 168.2 (C=N aliph.), 163.0, 155.6 (C=S), 143.2, 141.7 (C-OH), 139.1, 136.0, 132.7, 132.2, 129.9, 127.9, 125.9, 125.5, 120.21, 119.9, 113.9, 113.0, 109.5, 108.0, 101.8, 100.8, 98.3, 87.0, 84.1, 68.2. (C ar.).
- In4. 3-(((8-Hydroxyquinolin-2-yl)methylidene)amino)-1-phenylthiourea (L4; 50 mg, 0.16 mmol) was dissolved in EtOH (10 mL) at reflux. A solution of hydrated In(NO3)3 (46 mg, 0.15 mmol) in 3 mL in EtOH was then added dropwise to the ligand mixture. A red solution was then obtained and left stirring at RT for 2 h. Afterwards, the solution was left for some hours at 0 °C. Diethyl ether (10 mL) was then added, and the solution was left at 0 °C for additional hours. The solvents were removed through rotary evaporation, and EtOH (3 mL) was used to recover the mixture, which was then left at 0 °C overnight, giving a red turbid mixture. After filtration, the product was washed with diethyl ether, to yield a red-orange solid (21%). Anal. Calcd. for C34H38InN9O11S2 (In4 + 6H2O): C, 44.02; N, 13.59; H, 4.13; S, 6.91. Exp.: C, 43.56; N, 14.09; H, 3.68; S, 6.78. IR (ATR, cm−1): 3029 and 2775 w (N-H, O-H and C-H), 1498 m (C=N), 1311 and 693 s (C=S). ESI-MS m/z (%): 435.3 ([In + L − 2H]+, 4.6), 481.3 ([In + L + EtOH − 2H]+, 1.8), 757.3 ([In + 2L − 2H]+, 15.6). 1H-NMR (400 MHz, DMSO-d6): [ppm] 9.83 (s, 1H, NH), 8.68 (s, 1H, N=CH), 8.56 (d, 1H, CH ar.), 7.81 (d, 1H CH ar.), 7.78 (dd, 2H, CH ar.), 7.41 (t, 1H, CH ar.), 7.31 (t, 2H, CH ar.), 7.04 (dd, 1H, CH ar.), 7.03 (t, 1H, CH ar.), 6.81 (dd, 1H, CH ar.). 13C NMR (400 MHz, DMSO-d6): [ppm] 177.1, 175.5 (C=N aliph.), 159.8, 153.9 (C=S), 152.2, 143.4 (C-OH), 143.2, 140.9, 140.6, 139.5, 138.7, 137.0, 136.6, 131.5, 129.9, 129.4, 129.0, 128.8, 128.7, 126.8, 126.1, 123.2, 121.8, 121.6, 119.2, 118.3, 112.6, 112.2, 110.6 (C ar.).
3.2. UV-Visible Titrations
3.3. Stability Assay
3.4. Cytotoxicity Assay
3.5. Antibiotic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef]
- Qi, J.; Deng, J.; Qian, K.; Tian, L.; Li, J.; He, K.; Huang, X.; Cheng, Z.; Zheng, Y.; Wang, Y. Novel 2-Pyridinecarboxaldehyde Thiosemicarbazones Ga(III) Complexes with a High Antiproliferative Activity by Promoting Apoptosis and Inhibiting Cell Cycle. Eur. J. Med. Chem. 2017, 134, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Y.; Gao, J.J.; Chen, X.; Ma, B.; Yang, Z.S.; Tang, J.; Wang, B.W.; Chen, T.; Wang, C.; Gao, S.; et al. A Gallium(III) Complex That Engages Protein Disulfide Isomerase A3 (PDIA3) as an Anticancer Target. Angew. Chem. Int. Ed. 2020, 59, 20147–20153. [Google Scholar] [CrossRef]
- Lessa, J.A.; Parrilha, G.L.; Beraldo, H. Gallium Complexes as New Promising Metallodrug Candidates. Inorganica Chim. Acta 2012, 393, 53–63. [Google Scholar] [CrossRef]
- Wang, Y.; Han, B.; Xie, Y.; Wang, H.; Wang, R.; Xia, W.; Li, H.; Sun, H. Combination of Gallium(III) with Acetate for Combating Antibiotic Resistant: Pseudomonas Aeruginosa. Chem. Sci. 2019, 10, 6099–6106. [Google Scholar] [CrossRef] [PubMed]
- Scaccaglia, M.; Rega, M.; Vescovi, M.; Pinelli, S.; Tegoni, M.; Bacci, C.; Pelosi, G.; Bisceglie, F. Gallium(III)-Pyridoxal Thiosemicarbazone Derivatives as Nontoxic Agents against Gram-Negative Bacteria. Metallomics 2022, 14, mfac070. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-Based Anti-Infectives: Targeting Microbial Iron-Uptake Mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Käkelä, M.; Luoto, P.; Viljanen, T.; Virtanen, H.; Liljenbäck, H.; Jalkanen, S.; Knuuti, J.; Roivainen, A.; Li, X.G. Adventures in Radiosynthesis of Clinical Grade [68Ga]Ga-DOTA-Siglec-9. RSC Adv. 2018, 8, 8051–8056. [Google Scholar] [CrossRef]
- Kaneta, K.; Takahama, H.; Tateishi, E.; Irie, Y.; Moriuchi, K.; Amano, M.; Okada, A.; Amaki, M.; Kiso, K.; Kanzaki, H.; et al. Clinical Outcomes of Radiologic Relapse in Patients with Cardiac Sarcoidosis Under Immunosuppressive Therapies. Am. J. Cardiol. 2023, 188, 24–29. [Google Scholar] [CrossRef]
- Matsumura, M.; Okada, A.; Yokoyama, H.; Sekiguchi, M.; Shimizu, A.; Tanaka, T.; Nangaku, M.; Takano, H. Usefulness of Gallium-67 Scintigraphy for Evaluating the Histopathological Activity in Interstitial Nephritis. Clin. Exp. Nephrol. 2023, 27, 251–261. [Google Scholar] [CrossRef]
- Conen, P.; Pennetta, F.; Dendl, K.; Hertel, F.; Vogg, A.; Haberkorn, U.; Giesel, F.L.; Mottaghy, F.M. [68 Ga]Ga-FAPI Uptake Correlates with the State of Chronic Kidney Disease. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, W.; Li, H.; Xia, W. Identification and Characterization of a Metalloprotein Involved in Gallium Internalization in Pseudomonas Aeruginosa. ACS Infect. Dis. 2019, 5, 1693–1697. [Google Scholar] [CrossRef]
- Oyen, W.J.G.; Claessens, R.A.M.J.; Van Horn, J.R.; Van der Meer, J.W.M.; Corstens, F.H.M. Scintigraphic Detection of Bone and Joint Infections with Indium-111-Labeled Nonspecific Polyclonal Human Immunoglobulin G. J. Nucl. Med. 1990, 31, 403–412. [Google Scholar]
- Sarko, D.; Eisenhut, M.; Haberkorn, U.; Mier, W. Bifunctional Chelators in the Design and Application of Radiopharmaceuticals for Oncological Diseases. Curr. Med. Chem. 2012, 19, 2667–2688. [Google Scholar] [CrossRef]
- Gray, H.W.; Cuthbert, I.; Richards, J.R. Clinical Imaging with Indium-111 Leukocytes: Uptake in Bowel Infarction. J. Nucl. Med. 1981, 22, 701–702. [Google Scholar]
- Makhlouf, A.; Hajdu, I.; Hartimath, S.V.; Alizadeh, E.; Wharton, K.; Wasan, K.M.; Badea, I.; Fonge, H. 111 In-Labeled Glycoprotein Nonmetastatic b (GPNMB) Targeted Gemini Surfactant-Based Nanoparticles against Melanoma: In Vitro Characterization and in Vivo Evaluation in Melanoma Mouse Xenograft Model. Mol. Pharm. 2019, 16, 542–551. [Google Scholar] [CrossRef]
- Shih, W.J. Indium 113m Perfusion Study and the Nonfunctioning Thyroid Nodule. J. Nucl. Med. 1975, 16, 1187–1188. [Google Scholar] [PubMed]
- Beraldo, H. Pharmacological Applications of Non-Radioactive Indium(III) Complexes: A Field yet to Be Explored. Coord. Chem. Rev. 2020, 419, 213375. [Google Scholar] [CrossRef]
- Pelosi, G. Thiosemicarbazone Metal Complexes: From Structure to Activity. Open Crystallogr. J. 2010, 3, 16–28. [Google Scholar] [CrossRef]
- Haribabu, J.; Balakrishnan, N.; Swaminathan, S.; Dorairaj, D.P.; Azam, M.; Subarkhan, M.K.M.; Chang, Y.L.; Hsu, S.C.N.; Štarha, P.; Karvembu, R. Michael Addition-Driven Synthesis of Cytotoxic Palladium(II) Complexes from Chromone Thiosemicarbazones: Investigation of Anticancer Activity through in Vitro and in Vivo Studies. New J. Chem. 2023, 47, 15748–15759. [Google Scholar] [CrossRef]
- Belicchi-Ferrari, M.; Bisceglie, F.; Pelosi, G.; Tarasconi, P. Heterocyclic Substituted Thiosemicarbazones and Their Cu(II) Complexes: Synthesis, Characterization and Studies of Substituent Effects on Coordination and DNA Binding. Polyhedron 2008, 27, 1361–1367. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Haribabu, J.; Dhanabalan, A.K.; Swaminathan, S.; Sun, S.; Dibwe, D.F.; Bhuvanesh, N.; Awale, S.; Karvembu, R. Thiosemicarbazone(s)-Anchored Water Soluble Mono- A Nd Bimetallic Cu(II) Complexes: Enzyme-like Activities, Biomolecular Interactions, Anticancer Property and Real-Time Live Cytotoxicity. Dalt. Trans. 2020, 49, 9411–9424. [Google Scholar] [CrossRef]
- Haribabu, J.; Srividya, S.; Mahendiran, D.; Gayathri, D.; Venkatramu, V.; Bhuvanesh, N.; Karvembu, R. Synthesis of Palladium(II) Complexes via Michael Addition: Antiproliferative Effects through ROS-Mediated Mitochondrial Apoptosis and Docking with SARS-CoV-2. Inorg. Chem. 2020, 59, 17109–17122. [Google Scholar] [CrossRef] [PubMed]
- Lessa, J.A.; Reis, D.C.; Mendes, I.C.; Speziali, N.L.; Rocha, L.F.; Pereira, V.R.A.; Melo, C.M.L.; Beraldo, H. Antimony(III) Complexes with Pyridine-Derived Thiosemicarbazones: Structural Studies and Investigation on the Antitrypanosomal Activity. Polyhedron 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Gou, Y.; Wang, J.; Chen, S.; Zhang, Z.; Zhang, Y.; Zhang, W.; Yang, F. A−N−heterocyclic Thiosemicarbazone Fe(III) Complex: Characterization of Its Antitumor Activity and Identification of Anticancer Mechanism. Eur. J. Med. Chem. 2016, 123, 354–364. [Google Scholar] [CrossRef]
- Reis, D.C.; Pinto, M.C.X.; Souza-Fagundes, E.M.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Antimony(III) Complexes with 2-Benzoylpyridine-Derived Thiosemicarbazones: Cytotoxicity against Human Leukemia Cell Lines. Eur. J. Med. Chem. 2010, 45, 3904–3910. [Google Scholar] [CrossRef]
- Scaccaglia, M.; Rega, M.; Bacci, C.; Giovanardi, D.; Pinelli, S.; Pelosi, G.; Bisceglie, F. Bismuth Complex of Quinoline Thiosemicarbazone Restores Carbapenem Sensitivity in NDM-1-Positive Klebsiella Pneumoniae. J. Inorg. Biochem. 2022, 234, 111887. [Google Scholar] [CrossRef]
- Hickey, J.L.; Crouch, P.J.; Mey, S.; Caragounis, A.; White, J.M.; White, A.R.; Donnelly, P.S. Copper(II) Complexes of Hybrid Hydroxyquinoline-Thiosemicarbazone Ligands: GSK3β Inhibition Due to Intracellular Delivery of Copper. Dalt. Trans. 2011, 40, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.X.; Ji, Y.M.; Lu, Y.L.; Li, M.X.; Wu, Y.Y.; Han, Q.X. Cadmium(II) and Indium(III) Complexes Derived from 2-Benzoylpyridine N(4)-Cyclohexylthiosemicarbazone: Synthesis, Crystal Structures, Spectroscopic Characterization and Cytotoxicity. Synth. Met. 2016, 219, 109–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Gou, Y.; Wang, J.; Yang, K.; Qi, J.; Zhou, Z.; Liang, S.; Liang, H.; Yang, F. Four Copper(II) Compounds Synthesized by Anion Regulation: Structure, Anticancer Function and Anticancer Mechanism. Eur. J. Med. Chem. 2016, 121, 399–409. [Google Scholar] [CrossRef]
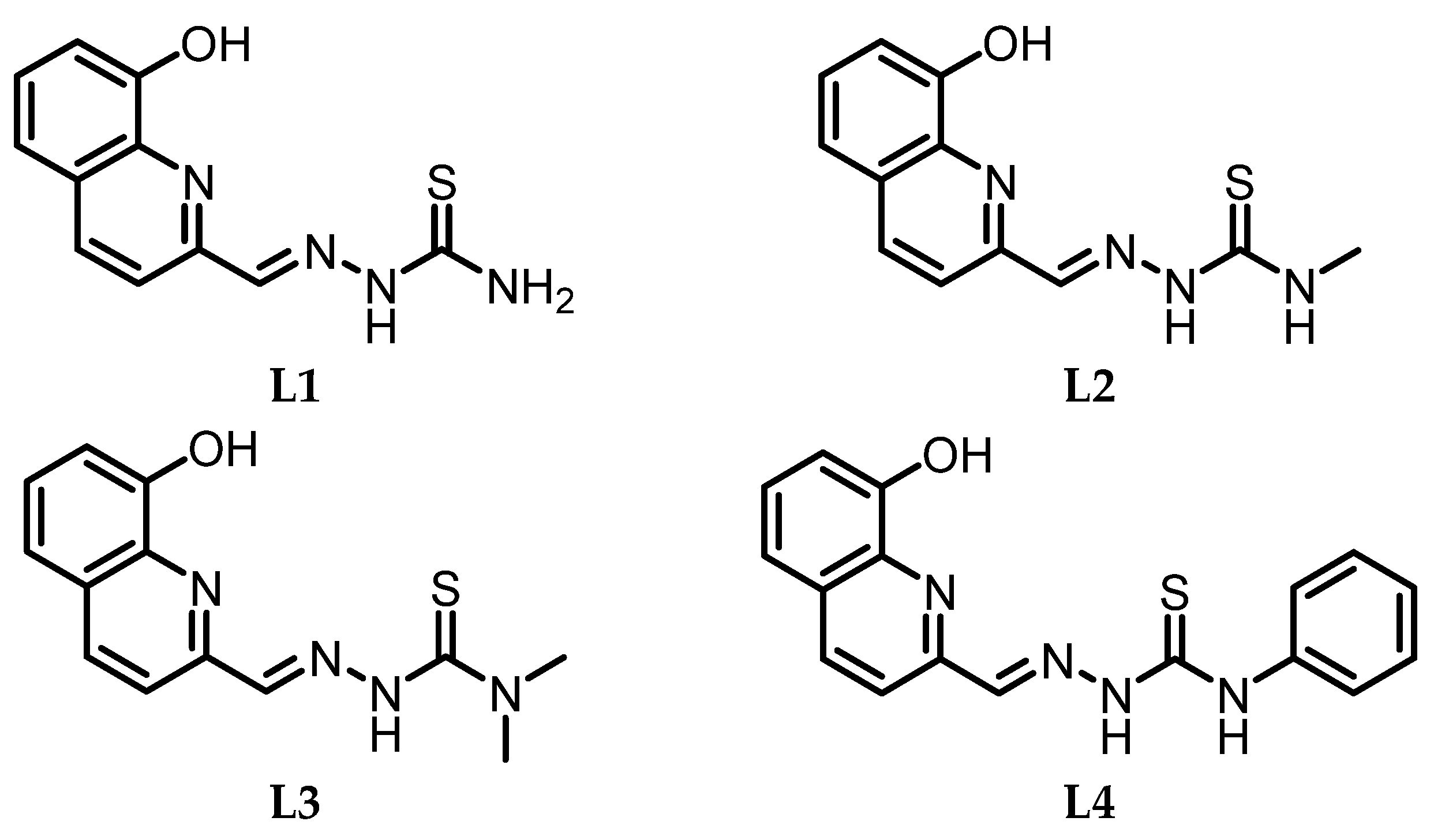


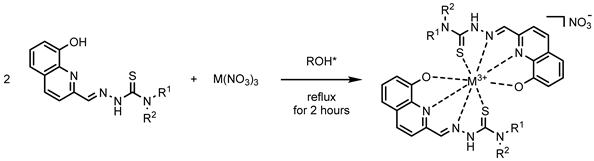 | ||
|---|---|---|
| M=Ga (Solvent: ROH *) | M=In (Solvent: ROH *) | |
| R1=H; R2=H | Ga1 (MeOH) | In1 (MeOH) |
| R1=Me; R2=H | Ga2 (MeOH) | In2 (EtOH) |
| R1=Me; R2=Me | Ga3 (MeOH) | In3 (EtOH) |
| R1=Ph; R2=H | Ga4 (MeOH) | In4 (EtOH) |
| Molecule 1 | Molecule 2 | ||
|---|---|---|---|
| In1-S3 | 2.6622(4) | In1-S1 | 2.6223(4) |
| In1-N11 | 2.563(1) | In1-N2 | 2.537(1) |
| In1-N17 | 2.329(1) | In1-N13 | 2.321(1) |
| In1-O1 | 2.223(1) | In1-O5 | 2.253(1) |
| Compound | MIC E. coli [μM] | MIC MRSA [μM] |
|---|---|---|
| L1 | >100 | >100 |
| L2 | >100 | >100 |
| L3 | >100 | 100 |
| L4 | >100 | 25 |
| Ga1 | >100 | >100 |
| In1 | >100 | >100 |
| Ga2 | >100 | >100 |
| In2 | >100 | 100–50 |
| Ga3 | >100 | 50 |
| In3 | >100 | >100 |
| Ga4 | >100 | 100 |
| In4 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verderi, L.; Scaccaglia, M.; Rega, M.; Bacci, C.; Pinelli, S.; Pelosi, G.; Bisceglie, F. New Stable Gallium(III) and Indium(III) Complexes with Thiosemicarbazone Ligands: A Biological Evaluation. Molecules 2024, 29, 497. https://doi.org/10.3390/molecules29020497
Verderi L, Scaccaglia M, Rega M, Bacci C, Pinelli S, Pelosi G, Bisceglie F. New Stable Gallium(III) and Indium(III) Complexes with Thiosemicarbazone Ligands: A Biological Evaluation. Molecules. 2024; 29(2):497. https://doi.org/10.3390/molecules29020497
Chicago/Turabian StyleVerderi, Lorenzo, Mirco Scaccaglia, Martina Rega, Cristina Bacci, Silvana Pinelli, Giorgio Pelosi, and Franco Bisceglie. 2024. "New Stable Gallium(III) and Indium(III) Complexes with Thiosemicarbazone Ligands: A Biological Evaluation" Molecules 29, no. 2: 497. https://doi.org/10.3390/molecules29020497
APA StyleVerderi, L., Scaccaglia, M., Rega, M., Bacci, C., Pinelli, S., Pelosi, G., & Bisceglie, F. (2024). New Stable Gallium(III) and Indium(III) Complexes with Thiosemicarbazone Ligands: A Biological Evaluation. Molecules, 29(2), 497. https://doi.org/10.3390/molecules29020497









