Aluminium 8-Hydroxyquinolinate N-Oxide as a Precursor to Heterometallic Aluminium–Lanthanide Complexes
Abstract
1. Introduction
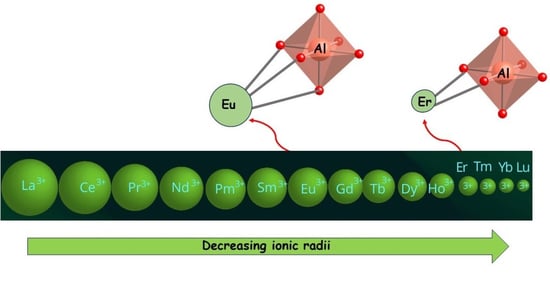
2. Results and Discussion
3. Materials and Methods
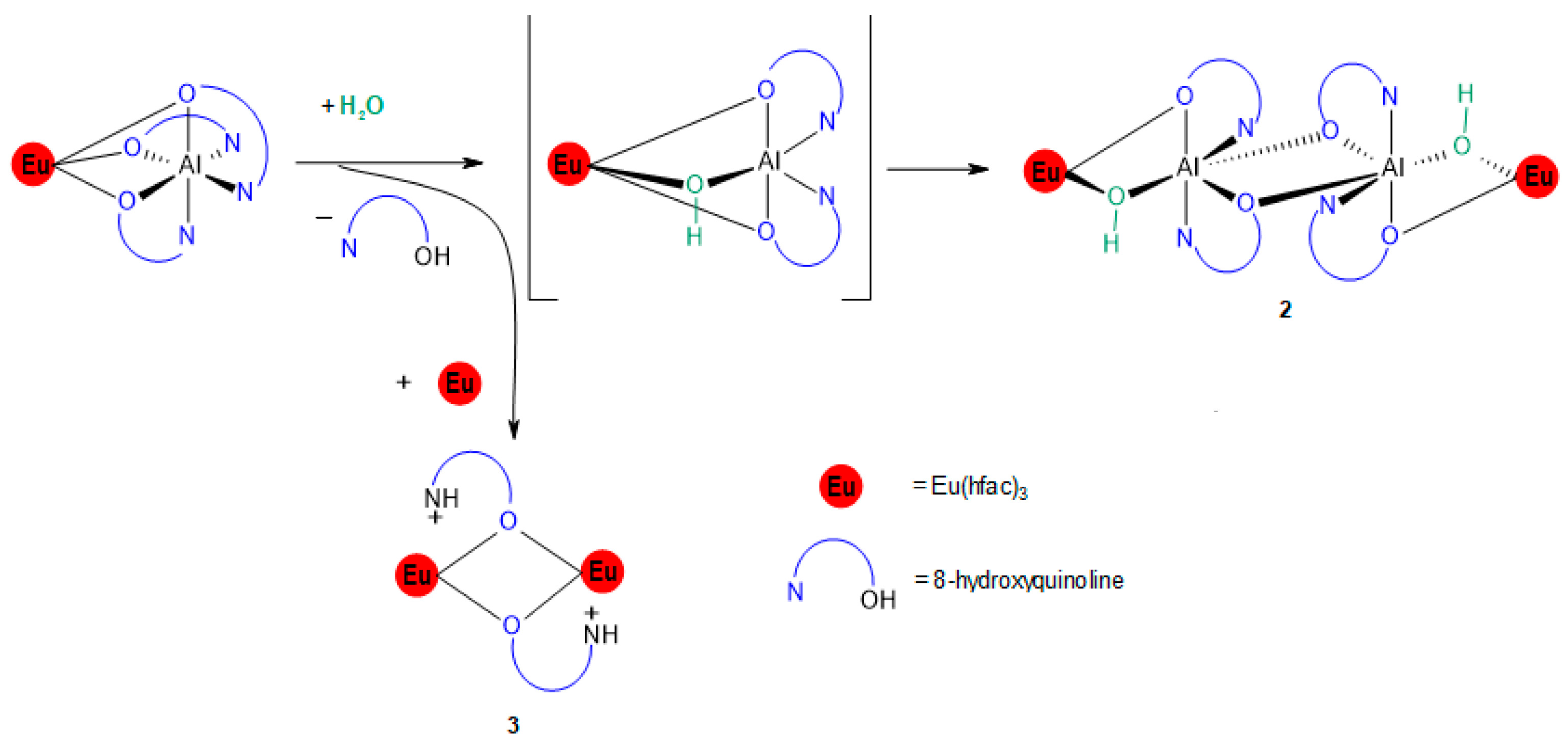
3.1. Reaction of [Eu(hfac)3] with [Alq3]
3.2. Synthesis of [Eu2(hfac)6(µ-Hq)2] (3)
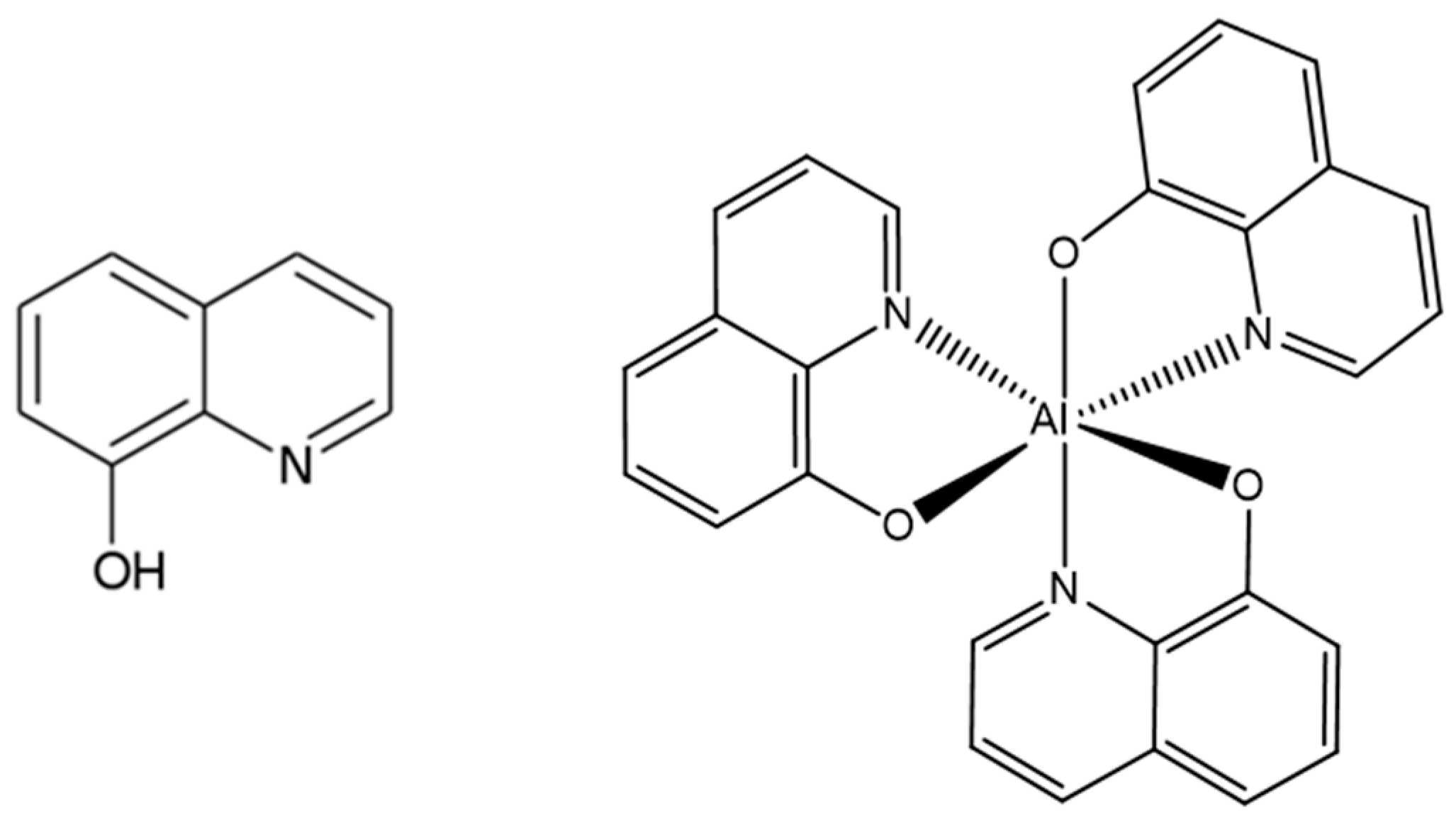
3.3. Synthesis of [Al(qNO)3] (4)
3.4. Synthesis of [Ln(hfac)3Al(qNO)3] (Ln = Eu, Gd and Er)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Xue, J.; Yang, G.-P.; Dang, L.-L.; Ma, L.-F.; Li, D.-S.; Wang, Y.-Y. Recent advances of functional heterometallic-organic framework (HMOF) materials: Design strategies and applications. Coord. Chem. Rev. 2022, 463, 214521. [Google Scholar] [CrossRef]
- Bao, S.-S.; Qin, M.-F.; Zheng, L.-M. Metal phosphonates incorporating metalloligands: Assembly, structures and properties. Chem. Commun. 2020, 56, 12090–12108. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-Metal MOFs: Unique Opportunities in Metal–Organic Framework (MOF) Functionality and Design. Angew. Chem. Int. Ed. 2019, 58, 15188–15205. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 289–290, 74–122. [Google Scholar] [CrossRef]
- Das, M.C.; Xiang, S.; Zhang, Z.; Chen, B. Functional Mixed Metal–Organic Frameworks with Metalloligands. Angew. Chem. Int. Ed. 2011, 50, 10510–10520. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, R. Metalloligands to material: Design strategies and network topologies. CrystEngComm 2016, 18, 9185–9208. [Google Scholar] [CrossRef]
- Kumar, G.; Gupta, R. Molecularly designed architectures—The metalloligand way. Chem. Soc. Rev. 2013, 42, 9403–9453. [Google Scholar] [CrossRef]
- Sørensen, M.A.; Weihe, H.; Vinum, M.G.; Mortensen, J.S.; Doerrer, L.H.; Bendix, J. Imposing high-symmetry and tuneable geometry on lanthanide centres with chelating Pt and Pd metalloligands. Chem. Sci. 2017, 8, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Spodine, E.; Audebrand, N.; Venegas-Yazigi, D.; Paredes-Garciá, V. Structural Versatility of 3d-CeIII Heterometallic Coordination Polymers Using CoII or CuII. Cryst. Growth Des. 2018, 18, 5155–5165. [Google Scholar] [CrossRef]
- Chandler, B.D.; Cramb, D.T.; Shimizu, G.K.H. Microporous Metal−Organic Frameworks Formed in a Stepwise Manner from Luminescent Building Blocks. J. Am. Chem. Soc. 2006, 128, 10403–10412. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, M.; Yang, Q.Y.; Fu, L.; Li, K.; Wei, S.C.; Su, C.Y. Dual-Emission from a Single-Phase Eu–Ag Metal–Organic Framework: An Alternative Way to Get White-Light Phosphor. Chem. Mater. 2012, 24, 1954–1960. [Google Scholar] [CrossRef]
- Huang, X.F.; Ma, J.X.; Liu, W.S. Lanthanide Metalloligand Strategy toward d–f Heterometallic Metal–Organic Frameworks: Magnetism and Symmetric-Dependent Luminescent Properties. Inorg. Chem. 2014, 53, 5922–5930. [Google Scholar] [CrossRef]
- Ma, J.X.; Guo, J.; Wang, H.; Li, B.; Yang, T.; Chen, B. Microporous Lanthanide Metal–Organic Framework Constructed from Lanthanide Metalloligand for Selective Separation of C2H2/CO2 and C2H2/CH4 at Room Temperature. Inorg. Chem. 2017, 56, 7145–7150. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Wang, L.F.; Chen, Y.C.; Zhang, Z.M.; Li, Q.W.; Tong, M.L. Magnetocaloric Properties of Heterometallic 3d–Gd Complexes Based on the [Gd(oda)3]3− Metalloligand. Chem.-A Eur. J. 2016, 22, 802–808. [Google Scholar] [CrossRef]
- Visconti, M.; Maggini, S.; Ciani, G.; Mercandelli, P.; Del Secco, B.; Prodi, L.; Sgarzi, M.; Zaccheroni, N.; Carlucci, L. New Lanthanide Metalloligands and Their Use for the Assembly of Ln–Ag Bimetallic Coordination Frameworks: Stepwise Modular Synthesis, Structural Characterization, and Optical Properties. Cryst. Growth Des. 2019, 19, 5376–5389. [Google Scholar] [CrossRef]
- Merkens, C.; Englert, U. Ordered bimetallic coordination networks featuring rare earth and silver cations. Dalton Trans. 2012, 41, 4664–4673. [Google Scholar] [CrossRef]
- Yin, J.C.; Qin, T.Z.; Hu, C.; He, G.M.; Zhao, B.W.; Zhang, C.; Wang, J. A copper(II)–gadolinium(III) heterometallic MOF: Synthesis, structure, and electrochemical property. Mater. Lett. 2017, 197, 221–223. [Google Scholar] [CrossRef]
- Armelao, L.; Belli Dell’Amico, D.; Bellucci, L.; Bottaro, G.; Ciattini, S.; Labella, L.; Manfroni, G.; Marchetti, F.; Mattei, C.A.; Samaritani, S. Homodinuclear Lanthanide Complexes with the Divergent Heterotopic 4,4′-Bipyridine N-Oxide (bipyMO) Ligand. Eur. J. Inorg. Chem. 2018, 2018, 4421–4428. [Google Scholar] [CrossRef]
- Bellucci, L.; Bottaro, G.; Labella, L.; Causin, V.; Marchetti, F.; Samaritani, S.; Belli Dell’Amico, D.; Armelao, L. Composition−Thermometric Properties Correlations in Homodinuclear Eu3+ Luminescent Complexes. Inorg. Chem. 2020, 59, 18156–18167. [Google Scholar] [CrossRef]
- Fioravanti, L.; Bellucci, L.; Armelao, L.; Bottaro, G.; Marchetti, F.; Pineider, F.; Poneti, G.; Samaritani, S.; Labella, L. Stoichiometry Controlled Assembly of Lanthanide Molecular Complexes of the heteroditopic divergent ligand 4′-(4-pyridil)-2,2′:6′,2”-terpyridine N-oxide in hypodentate or bridging coordination modes. Structural, Magnetic and Photoluminescence studies. Inorg. Chem. 2022, 61, 265–278. [Google Scholar] [CrossRef]
- Binnemans, K. Rare-Earth Beta-Diketonates. Handb. Phys. Chem. Rare Earths 2005, 35, 107–272. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Lip, H.C.; Louie, H.W.; Drew, M.G.B.; Hudson, M.J. Interaction of lanthanide shift reagents with co-ordination complexes; direct observation of nuclear magnetic resonance signals for free and complexed tris(pentane-2,4-dionato)cobalt(III) at ambient temperature, and X-ray crystal and molecular structure. J. Chem. Soc. Chem. Commun. 1977, 778. [Google Scholar] [CrossRef]
- Øwre, A.; Vinum, M.; Kern, M.; van Slageren, J.; Bendix, J.; Perfetti, M. Chiral, Heterometallic Lanthanide–Transition Metal Complexes by Design. Inorganics 2018, 6, 72. [Google Scholar] [CrossRef]
- Rogachev, A.Y.; Mironov, A.V.; Nemukhin, A.V. Experimental and theoretical studies of the products of reaction between Ln(hfa)3 and Cu(acac)2 (Ln = La, Y; acac = acetylacetonate, hfa = hexafluoroacetylacetonate). J. Mol. Struct. 2007, 831, 46–54. [Google Scholar] [CrossRef]
- Cosquer, G.; Pointillart, F.; Le Gal, Y.; Golhen, S.; Cador, O.; Ouahab, L. Ferromagnetic versus Antiferromagnetic Exchange Interactions in Tetrathiafulvalene-Based 3d/4f Heterobimetallic Complexes. Chem. Eur. J. 2011, 17, 12502–12511. [Google Scholar] [CrossRef]
- Kuz’mina, N.P.; Malkerova, I.P.; Alikhanyan, A.S.; Gleizes, A.N. The use of 3d-metal complexes as ligands to prepare volatile 4f–3d heterobimetallic complexes. J. Alloys Compd. 2004, 374, 315–319. [Google Scholar] [CrossRef]
- Jia, R.; Li, H.-F.; Chen, P.; Gao, T.; Sun, W.-B.; Li, G.-M.; Yan, P.-F. Synthesis, structure, and tunable white light emission of heteronuclear Zn2Ln2 arrays using a zinc complex as ligand. CrystEngComm 2016, 18, 917–923. [Google Scholar] [CrossRef]
- Chen, L.; Breeze, S.R.; Rousseau, R.J.; Wang, S.; Thompson, L.K. Polynuclear Copper-Lanthanide Complexes with Amino Alcohol Ligands. Syntheses, Structures, and Magnetic and Spectroscopic Studies of CuII(bdmmp)2(H2O), PrIIICuII(bdmmp)(bdmmpH)(µ-OH)(hfacac)3, [LaIIICuII(bdmmp)(bdmmpH)(µ-OH)(O2CCF3)3]2, and CuII4(bdmmp)2(µ4-O)(O2CCF3)4, (bdmmpH= 2,6-Bis[(dimethylamino)methyl]-4-methylphenol; hfacac = Hexafluoroacetylacetonato). Inorg. Chem. 1995, 34, 454–465. [Google Scholar] [CrossRef]
- Armelao, L.; Belli Dell’Amico, D.; Bottaro, G.; Bellucci, L.; Labella, L.; Marchetti, F.; Mattei, C.A.; Mian, F.; Pineider, F.; Poneti, G.; et al. 1D hetero-bimetallic regularly alternated 4f–3d coordination polymers based on N-oxide-4,4′-bipyridine (bipyMO) as a linker: Photoluminescence and magnetic properties. Dalton Trans. 2018, 47, 8337–8345. [Google Scholar] [CrossRef]
- Bellucci, L.; Bottaro, G.; Labella, L.; Marchetti, F.; Samaritani, S.; Belli Dell’Amico, D.; Armelao, L. 1D-Zigzag Eu3+/Tb3+ Coordination Chains as Luminescent Ratiometric Thermometers Endowed with Multicolor Emission. Materials 2021, 14, 6445. [Google Scholar] [CrossRef]
- Bellucci, L.; Babetto, L.; Bottaro, G.; Carlotto, S.; Labella, L.; Gallo, E.; Marchetti, F.; Samaritani, S.; Armelao, L. Competing excitation paths in Luminescent Heterobimetallic Ln-Al complexes: Unravelling interactions via experimental and theoretical investigations. iScience 2023, 26, 106614. [Google Scholar] [CrossRef]
- Fischbach, A.; Anwander, R. Rare-earth metals and aluminum getting close in Ziegler-type organometallics. Adv. Polym. Sci. 2006, 204, 155–281. [Google Scholar] [CrossRef]
- Xu, H.B.; Deng, J.G.; Kang, B. Designed synthesis and photophysical properties of multifunctional hybrid lanthanide complexes. RSC Adv. 2013, 3, 11367–11384. [Google Scholar] [CrossRef]
- Westin, G.; Ekstrand, Å.; Zangellini, E.; Börjesson, L. Preparation and optical studies of Er-doped Al-Si-Ti oxide glasses using the ErAl3(OPri)12 isolated Er-ion precursor. J. Phys. Chem. Solids 2000, 61, 67–74. [Google Scholar] [CrossRef]
- Khan, M.B.; Salah, ·N.; Khan, Z.H. Functional enhancement in Alq3 via metal doping and nanoscale synthesis: A review. Appl. Nanosci. 2022, 12, 1365–1385. [Google Scholar] [CrossRef]
- Brinkmann, M.; Gadret, G.; Muccini, M.; Taliani, C.; Masciocchi, N.; Sironi, A. Correlation between Molecular Packing and Optical Properties in Different Crystalline Polymorphs and Amorphous Thin Films of mer-Tris(8-hydroxyquinoline)aluminum(III). J. Am. Chem. Soc. 2000, 122, 5147–5157. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Miyamae, T.; Ito, E.; Noguchi, Y.; Ishii, H. Characterization of the Interactions between Alq3 Thin Films and Al Probed by Two-Color Sum-Frequency Generation Spectroscopy. J. Phys. Chem. C 2011, 115, 9551–9560. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; BreÂdas, J.L.; Lo Ègdlund, M.; et al. Electroluminescence in Conjugated Polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Hu, J.S.; Ji, H.X.; Cao, A.M.; Huang, Z.X.; Zhang, Y.; Wan, L.J.; Xia, A.D.; Yu, D.P.; Meng, X.M.; Lee, S.T. Facile solution synthesis of hexagonal Alq3 nanorods and their field emission properties. Chem. Commun. 2007, 3083–3085. [Google Scholar] [CrossRef]
- Aziz, H.; Popovic, Z.; Xie, S.; Hor, A.M.; Hu, N.X.; Tripp, C.; Xu, G. Humidity-induced crystallization of tris (8-hydroxyquinoline) aluminum layers in organic light-emitting devices. Appl. Phys. Lett. 1998, 72, 756–758. [Google Scholar] [CrossRef]
- Utz, M.; Chen, C.; Morton, M.; Papadimitrakopoulos, F. Ligand Exchange Dynamics in Aluminum Tris-(Quinoline-8-olate): A Solution State NMR Study. J. Am. Chem. Soc. 2003, 125, 1371–1375. [Google Scholar] [CrossRef]
- Xu, H.B.; Chen, X.M.; Zhang, Q.S.; Zhang, L.Y.; Chen, Z.N. Fluoride-enhanced lanthanide luminescence and white-light emitting in multifunctional Al3Ln2 (Ln = Nd, Eu, Yb) heteropentanuclear complexes. Chem. Commun. 2009, 2, 7318–7320. [Google Scholar] [CrossRef]
- Xu, H.B.; Zhang, L.Y.; Huang, X.; Li, B.; Chen, Z.N. Structures and Photophysical Properties of Homo- and Heteronuclear Lanthanide(III) Complexes with Bridging 2-Methyl-8-hydroxylquinoline (HMq) in the μ-Phenol Mode. Cryst. Growth Des. 2010, 10, 4101–4108. [Google Scholar] [CrossRef]
- Tsuboi, T.; Torii, Y. Selective Synthesis of Facial and Meridianal Isomers of Alq3. Mol. Cryst. Liq. Cryst. 2010, 529, 42–52. [Google Scholar] [CrossRef]
- Shrader, W.D.; Celebuski, J.; Kline, S.J.; Johnson, D. Synthesis of a novel hexadentate chelating agent based on 8-hydroxyquinoline. Tetrahedron Lett. 1988, 29, 1351–1354. [Google Scholar] [CrossRef]
- Zhang, S.G.; Xu, H.Y.; Shi, J.M. The 2:1 Adduct of 8-Hydroxyquinoline N-Oxide and Diaquabis(8-Hydroxy-Quinoline N-Oxide)Dithiocyanato-Cobalt(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m2083. [Google Scholar] [CrossRef]
- Shi, J.M.; Meng, X.Z.; Sun, Y.M.; Xu, H.Y.; Shi, W.; Cheng, P.; Liu, L.D. Magnetic Study of a One-Dimensional Mn(II) Coordination Polymer Dealing with π-π Stacking. J. Mol. Struct. 2009, 917, 164–169. [Google Scholar] [CrossRef]
- Gu, L.Q.; Li, J.M. Catena-Poly[[[Diaquabis(8-Hydroxy-Quinoline N-Oxide-ΚO 1)Cobalt(II)] μ-2,5-Dimethyl-Pyrazine 1,4-Dioxide- Κ2 O 1:O 4] Bis-(Perchlorate)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m401. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Z.; Wei, Z.-Z.; Qin, Q.-P.; Yang, L.; Liang, H. High anticancer activity and apoptosis- and autophagy-inducing properties of novel lanthanide(III) complexes bearing 8-hydroxyquinoline-N-oxide and 1,10-phenanthroline. Dalton Trans. 2021, 50, 5828–5834. [Google Scholar] [CrossRef] [PubMed]
- Montes, V.A.; Li, G.; Pohl, R.; Shinar, J.; Anzenbacher, P. Effective Color Tuning in Organic Light-Emitting Diodes Based on Aluminum Tris(5-Aryl-8-Hydroxyquinoline) Complexes. Adv. Mater. 2004, 16, 2001–2003. [Google Scholar] [CrossRef]
- Rajeswaran, M.; Blanton, T.N.; Tang, C.W.; Lenhart, W.C.; Switalski, S.C.; Giesen, D.J.; Antalek, B.J.; Pawlik, T.D.; Kondakov, D.Y.; Zumbulyadis, N.; et al. Structural, thermal, and spectral characterization of the different crystalline forms of Alq3, tris(quinolin-8-olato)aluminum(III), an electroluminescent material in OLED technology. Polyhedron 2009, 28, 835–843. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Taveira, R.J.S.; Costa, J.C.S.; Fernandes, A.M.; Melo, A.; Silva, A.M.S.; Santos, L.M.N.B.F. Understanding M–ligand bonding and mer-/facisomerism in tris(8-hydroxyquinolinate) metallic complexes. Phys. Chem. Chem. Phys. 2016, 18, 16555–16565. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Carlotto, A.; Babetto, L.; Carlotto, S.; Miozzi, M.; Seraglia, R.; Casarin, M.; Bottaro, G.; Rancan, M.; Armelao, L. Luminescent Thermometers: From a Library of Europium(III) β-Diketonates to a General Model for Predicting the Thermometric Behaviour of Europium-Based Coordination Systems. ChemPhotoChem 2020, 4, 674–684. [Google Scholar] [CrossRef]
- Rancan, M.; Tessarolo, J.; Carlotto, A.; Carlotto, S.; Rando, M.; Barchi, L.; Bolognesi, E.; Seraglia, R.; Bottaro, G.; Casarin, M.; et al. Adaptive helicity and chiral recognition in bright europium quadruple-stranded helicates induced by host-guest interaction. Cell Rep. Phys. Sci. 2022, 3, 100692. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Approach to the Frontier-Electron Theory of Chemical Reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Kameta, N.; Imura, H.; Ohashi, K.; Aoyama, T. Equilibrium and Spectroscopic Studies on the Complexation of Tris(1-(2-Thienyl)-4,4,4-Trifluoro-1,3-Butanedionato)Lanthanoids(III) with Tris(2,4-Pentanedionato)Cobalt(III) as Complex Ligand. Inorg. Chem. Commun. 1999, 2, 124–127. [Google Scholar] [CrossRef]
- Kameta, N.; Imura, H.; Ohashi, K.; Aoyama, T. Stability Constants of Inner- and Outer-Sphere Complexes of Hydrated Tris(1-(2-Thienyl)-4,4,4-Trifluoro-1,3-Butanedionato) Lanthanide(III) with Tris(2,4-Pentanedionato) Cobalt(III). Polyhedron 2002, 21, 805. [Google Scholar] [CrossRef]
- Bellucci, L.; Giordano, L.; Carlotto, S.; Poneti, G.; Rancan, M.; Samaritani, S.; Armelao, L.; Labella, L. Heterometallic [Ln(hfac)3Cu(acac)2] complexes with late 4f ions. Inorg. Chim. Acta 2023, 556, 121665. [Google Scholar] [CrossRef]
- Li, H.; Zhang, F.; Wang, Y.; Zheng, D. Synthesis and characterization of tris-(8-hydroxyquinoline)aluminum. Mater. Sci. Eng. B 2003, 100, 40–46. [Google Scholar] [CrossRef]
- Katakura, R.; Koide, Y. Configuration-Specific Synthesis of the Facial and Meridional Isomers of Tris(8-hydroxyquinolinate)aluminum (Alq3). Inorg. Chem. 2006, 45, 5730–5732. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Belli Dell’Amico, D.; Bellucci, L.; Bottaro, G.; Labella, L.; Marchetti, F.; Samaritani, S. A convenient synthesis of highly luminescent lanthanide 1D-zigzag coordination chains based only on 4,4′-bipyridine as connector. Polyhedron 2016, 119, 371–376. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Mortier, W.J. The Use of Global and Local Molecular Parameters for the Analysis of the Gas-Phase Basicity of Amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef] [PubMed]







| No. Bridging Ligands | Total Enthalpy/ΔH | Entropy/ −TΔS | Gibbs Free Energy/ΔG | ΔG Difference | |
|---|---|---|---|---|---|
| [La(hfac)3Al(qNO)3] | 2 | −46.30 | 23.77 | −22.53 | |
| [La(hfac)3Al(qNO)3] | 3 | −52.67 | 26.18 | −26.49 | −3.96 |
| [Eu(hfac)3Al(qNO)3] | 2 | −38.36 | 22.18 | −16.18 | |
| [Eu(hfac)3Al(qNO)3] | 3 | −42.46 | 22.43 | −20.03 | −3.85 |
| [Gd(hfac)3Al(qNO)3] | 2 | −39.82 | 15.91 | −23.91 | |
| [Gd(hfac)3Al(qNO)3] | 3 | −42.94 | 15.10 | −27.83 | −3.92 |
| [Er(hfac)3Al(qNO)3] | 2 | −53.02 | 22.08 | −30.94 | |
| [Er(hfac)3Al(qNO)3] | 3 | −50.04 | 21.01 | −29.02 | 1.92 |
| Identification Code | 2 | 3 | 5 · 0.5 Toluene | 7 |
| CCDC number | 2298051 | 2298049 | 2298048 | 2298050 |
| Empirical formula | C66H32Al2Eu2F36N4O18 | C48H20Eu2F36N2O14 | C45.5H25AlEuF18N3O12 | C42H21AlErF18N3O12 |
| Formula weight | 2210.83 | 1836.58 | 1326.62 | 1295.86 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P | P | P 2/c | P |
| a (Å) | 13.3724(5) | 11.9995(7) | 20.3737(6) | 13.433(3) |
| b (Å) | 17.3056(6) | 12.8030(7) | 12.9319(3) | 13.522(2) |
| c (Å) | 17.7157(7) | 13.1061(7) | 20.6857(6) | 14.240(3) |
| α (°) | 93.7570(10) | 107.517(2) | - | 91.094(6) |
| β (°) | 92.5010(10) | 112.709(2) | 106.1290(10) | 105.476(6) |
| γ (°) | 98.2140(10) | 105.019(2) | - | 101.144(6) |
| Volume (Å3) | 4043.1(3) | 1601.95(16) | 5235.6(2) | 2439.0(8) |
| Z | 2 | 1 | 4 | 2 |
| ρcalc (g cm−1) | 1.816 | 1.904 | 1.683 | 1.765 |
| μ (mm−1) | 1.709 | 2.104 | 1.340 | 1.870 |
| F(000) | 2152 | 884 | 2612 | 1266 |
| θ range (°) | 1.19 to 26.50 | 3.26 to 27.48 | 2.59 to 25.99 | 2.67 to 26.39 |
| Reflections collected | 151373 | 54307 | 48953 | 85152 |
| Data/restraints/parameters | 16,701/0/1153 | 7314/210/505 | 10,231/43/741 | 9875/204/805 |
| Goodness of fit on F2 | 1.019 | 1.132 | 1.054 | 1.125 |
| Final R1 [I ≥ 2σ(I)] | 0.0448 | 0.0380 | 0.0512 | 0.0510 |
| Final wR2 [I ≥ 2σ(I)] | 0.1191 | 0.0905 | 0.1290 | 0.1483 |
| Final R1 [all data] | 0.0684 | 0.0467 | 0.0791 | 0.0578 |
| Final wR2 [all data] | 0.1330 | 0.0995 | 0.1450 | 0.1559 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, E.; Bellucci, L.; Carlotto, S.; Bottaro, G.; Babetto, L.; Giordano, L.; Marchetti, F.; Samaritani, S.; Armelao, L.; Labella, L. Aluminium 8-Hydroxyquinolinate N-Oxide as a Precursor to Heterometallic Aluminium–Lanthanide Complexes. Molecules 2024, 29, 451. https://doi.org/10.3390/molecules29020451
Gallo E, Bellucci L, Carlotto S, Bottaro G, Babetto L, Giordano L, Marchetti F, Samaritani S, Armelao L, Labella L. Aluminium 8-Hydroxyquinolinate N-Oxide as a Precursor to Heterometallic Aluminium–Lanthanide Complexes. Molecules. 2024; 29(2):451. https://doi.org/10.3390/molecules29020451
Chicago/Turabian StyleGallo, Elisa, Luca Bellucci, Silvia Carlotto, Gregorio Bottaro, Luca Babetto, Luca Giordano, Fabio Marchetti, Simona Samaritani, Lidia Armelao, and Luca Labella. 2024. "Aluminium 8-Hydroxyquinolinate N-Oxide as a Precursor to Heterometallic Aluminium–Lanthanide Complexes" Molecules 29, no. 2: 451. https://doi.org/10.3390/molecules29020451
APA StyleGallo, E., Bellucci, L., Carlotto, S., Bottaro, G., Babetto, L., Giordano, L., Marchetti, F., Samaritani, S., Armelao, L., & Labella, L. (2024). Aluminium 8-Hydroxyquinolinate N-Oxide as a Precursor to Heterometallic Aluminium–Lanthanide Complexes. Molecules, 29(2), 451. https://doi.org/10.3390/molecules29020451