Abstract
This review highlights the advantages of high-precision liquid chromatography with an electrochemical detector (HPLC-ECD) in detecting and quantifying biological samples obtained through intracerebral microdialysis, specifically the serotonergic and dopaminergic systems: Serotonin (5-HT), 5-hydroxyindolacetic acid (5-HIAA), 3,4-dihydroxyphenylacetic acid (DOPAC), dopamine (DA), 3-metoxytryptamin (3-MT) and homovanillic acid (HVA). Recognized for its speed and selectivity, HPLC enables direct analysis of intracerebral microdialysis samples without complex derivatization. Various chromatographic methods, including reverse phase (RP), are explored for neurotransmitters (NTs) and metabolites separation. Electrochemical detector (ECD), particularly with glassy carbon (GC) electrodes, is emphasized for its simplicity and sensitivity, aimed at enhancing reproducibility through optimization strategies such as modified electrode materials. This paper underscores the determination of limits of detection (LOD) and quantification (LOQ) and the linear range (L.R.) showcasing the potential for real-time monitoring of compounds concentrations. A non-exhaustive compilation of literature values for LOD, LOQ, and L.R. from recent publications is included.
1. Introduction
Monoamine neurotransmitters (NTs) are a class of NTs that include brain chemicals involved in the transmission of signals between nerve cells, or neurons. The two main types of monoamine NTs are catecholamines and indolamines: catecholamines include dopamine (DA), norepinephrine (NE), and epinephrine (E) and indolamines include serotonin (5-hydroxytryptamine or 5-HT).
These monoamine NTs play a crucial role in the regulation of various physiological and behavioral processes, such as the regulation of mood, sleep, appetite, reward, attention, and stress. Imbalances in the functioning of monoamine NTs are associated with various neurological and psychiatric disorders.
1.1. The Serotonergic System
The serotonergic system utilizes 5-HT as a neurotransmitter and plays a pivotal role in regulating diverse physiological and psychological processes, including mood, sleep, appetite, thermoregulation, and heart rate [1].
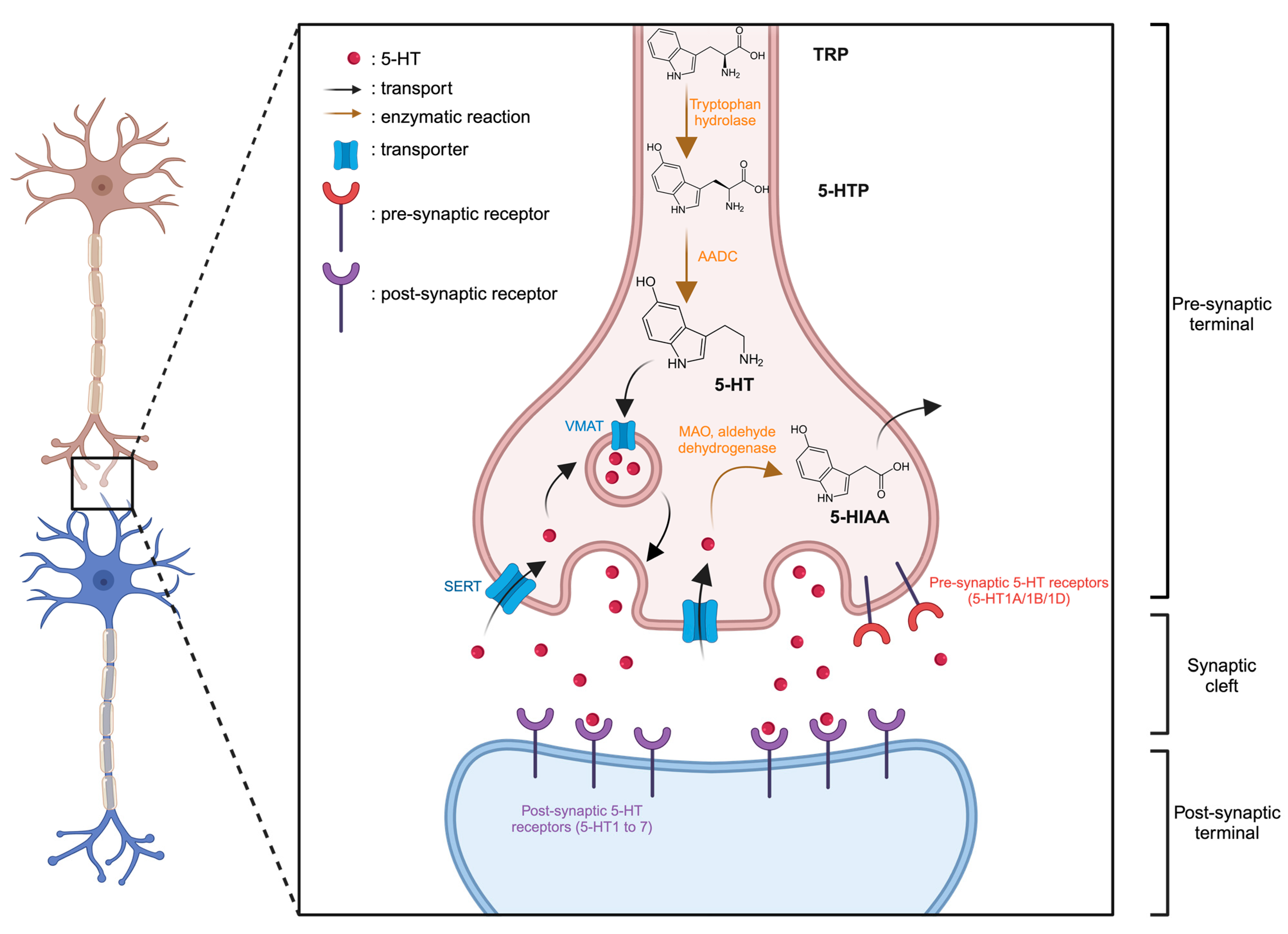
5-HT is synthesized from the essential amino acid tryptophan (TRP). Within neurons, TRP undergoes conversion to 5-hydroxytryptophan (5-HTP), catalyzed by the tryptophan hydroxylase enzyme. Subsequently, an enzymatic reaction involving aromatic amino acid decarboxylase (AADC) transforms 5-HTP into 5-HT (Figure 1). Following synthesis, the 5-HT is stored in vesicles via the vesicular monoamine transporter (VMAT) within the nerve endings of serotonergic neurons. Upon the arrival of an action potential at the nerve ending and the membrane depolarization, it triggers the release of 5-HT from vesicles into the synaptic cleft. Here, it can interact with receptors located on the postsynaptic membrane, known as serotonin receptors or 5-HT receptors. Extracellular 5-HT may also bind and activate inhibitory receptors expressed on 5-HT neurons (5-HT1A/1B/1D) thereby exerting negative feedback on its own release. Intracellular excess of 5-HT can be sequentially broken down by monoamine oxidase (MAO) and aldehyde dehydrogenase, resulting in the accumulation of 5-hydroxyindoleacetic acid (5-HIAA) in the intracellular space. All these receptors are present in the central nervous system and various tissues throughout the body. The effects of 5-HT vary based on the specific receptors it binds to.
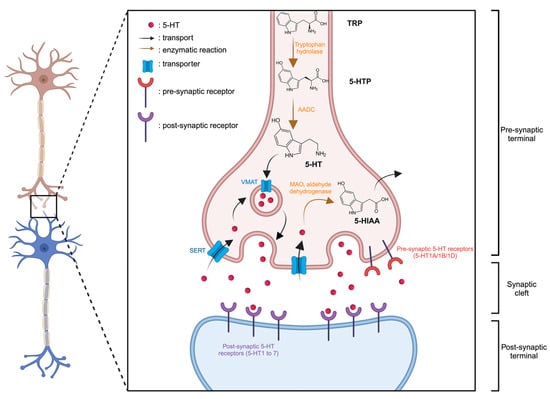
Figure 1.
Representation of serotonergic synapse. Created with BioRender.com accessed on 15 January 2024.
Dysfunctions in the serotonergic system may contribute to several disorders, including neurodegenerative disease like Parkinson’s [2] or Alzheimer’s [3,4], and also psychiatric disorders such as anxiety [5] and depression [6].
1.2. The Dopaminergic System
The dopaminergic system is a neurotransmission system in the brain that uses DA as a neurotransmitter. DA is a chemical compound that plays a crucial role in various brain functions, including the regulation of motivation [7], pleasure, reward, and movement [8].
The dopaminergic system is composed of several neural pathways, with the most well-known being the mesolimbic, mesocortical, and nigrostriatal pathways. It plays a key role in various physiological and behavioral processes, and its dysfunction is associated with several neurological and psychiatric disorders. For example, overactivity of the dopaminergic system is linked to schizophrenia [9,10], while deficiency is associated with Parkinson’s disease [11]. Medications that modulate dopaminergic activity are often used to treat these disorders.
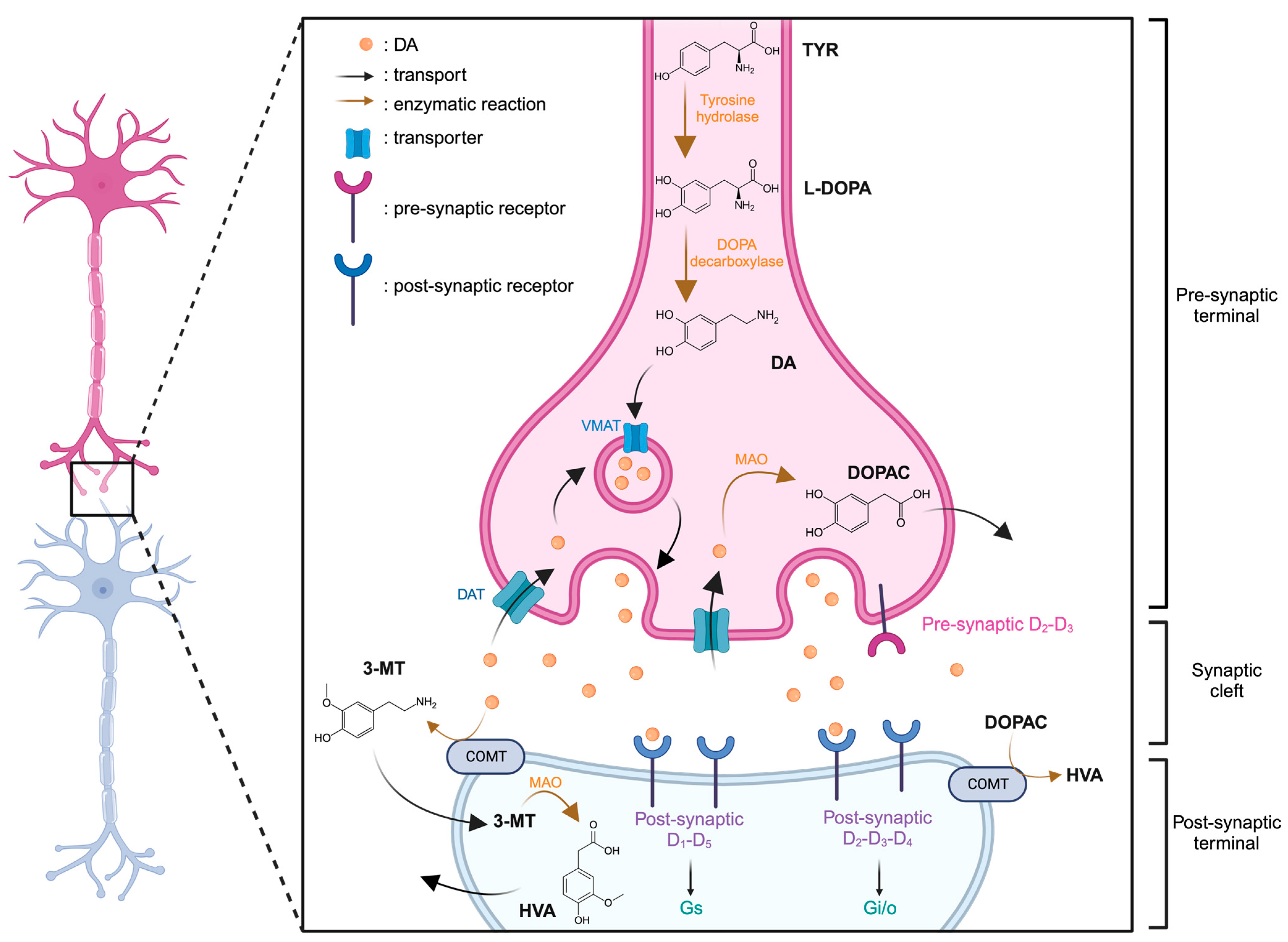
DA is synthesized in the neuron from tyrosine (TYR) through a two-step enzymatic reaction involving first, the tyrosine hydroxylase, synthesizing the levodopa (L-DOPA) and then, the DOPA decarboxylase. The DA can then be introduced into vesicles by the VMAT and released into the synaptic cleft. Degradation of DA can be catalyzed by MAO into DOPAC within the neuron. Two pathways are possible for its transformation into homovanillic acid (HVA), either through catecholamine-O-methyl-transferase (COMT) and MAO sequentially, with 3-metoxytryptamine (3-MT) as an intermediate, or by directly degrading DOPAC via COMT (Figure 2).
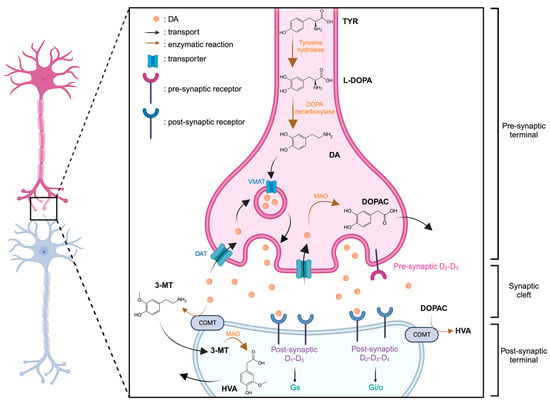
Figure 2.
Representation of dopaminergic synapse. Created with BioRender.com accessed on 15 January 2024.
Two additional catecholamines, derived from dopamine and integral to the neurotransmitter family, are norepinephrine (NE) and epinephrine (E). E plays a dual role, functioning as a neurotransmitter within the central nervous system and as a hormone within the adrenal medulla. NE, conversely, acts as a metabolic precursor to epinephrine and operates as a neurotransmitter in the central nervous system. Both NE and E are crucial in regulating fundamental processes such as the sleep-wake cycle, vigilance, stress response [12], emotions [13], learning [14], and memory [15]. Their multifaceted roles underscore their significance in orchestrating key physiological and cognitive functions within the intricate framework of the human body.
2. Microdialysis Experiment
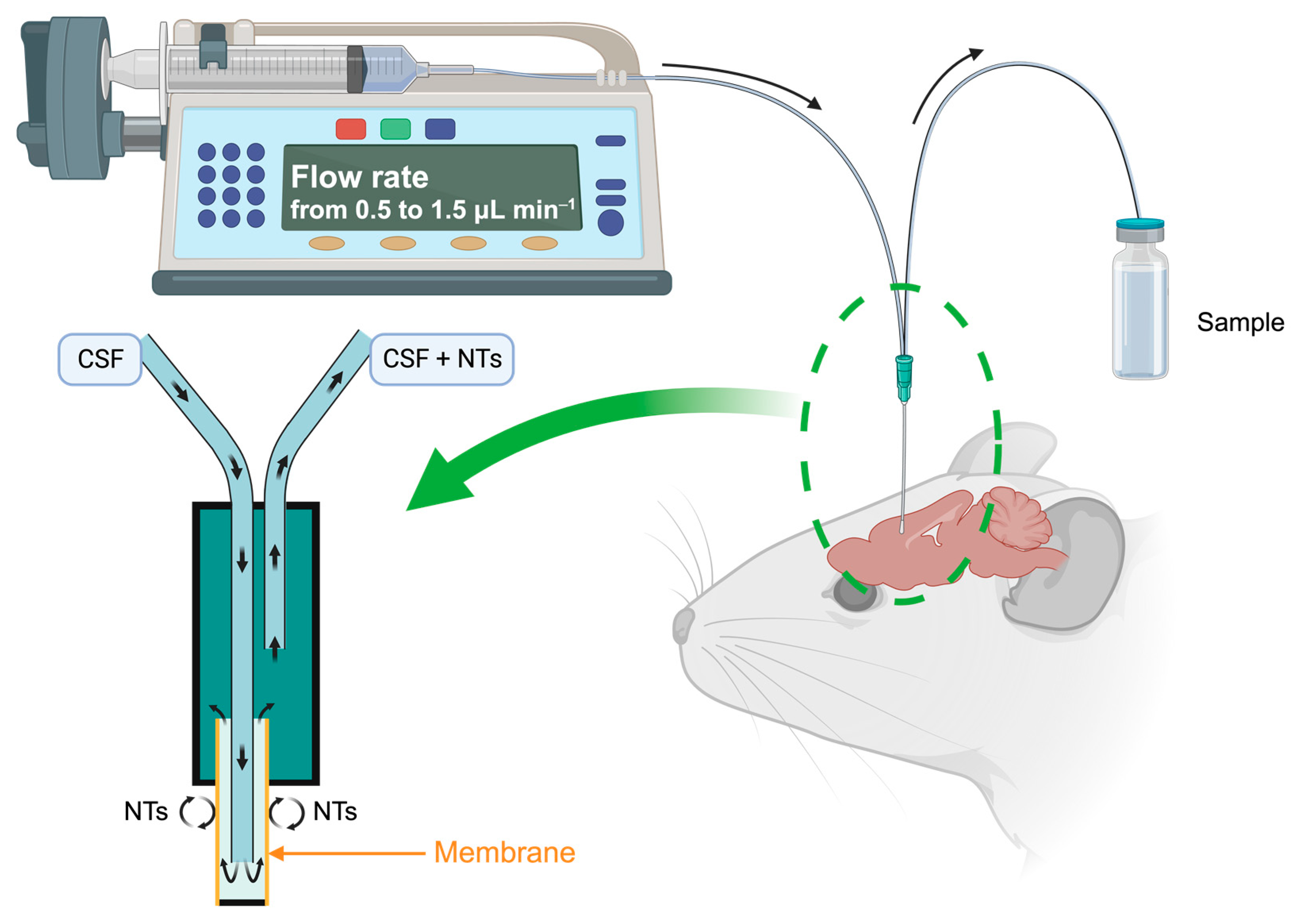
Microdialysis, employed for the past forty years [16,17], is a real-time sampling technique facilitating the analysis of extracellular NTs [18,19]. It functions on the principle of passive diffusion of small molecular-weight compounds through a porous membrane, transitioning from the region with the highest NTs concentration, namely the synaptic extracellular space, to the less concentrated area, the dialysis probe perfused with a neurotransmitter-free buffer solution at physiological pH. This technique allows the collection of dialysates at variable intervals, with flow rates ranging from 0.5 to 1.5 μL min−1, dependent on the experimental setup and brain area studied (Figure 3). These samples, which include monoamines and their metabolites, play a crucial role in comprehending the functioning of the serotonin and dopamine systems and associated disorders.
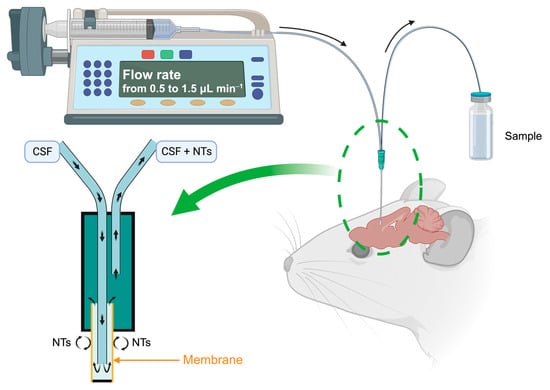
Figure 3.
Schematic principle of the microdialysis on a brain mouse. Created with BioRender.com accessed on 15 January 2024.
The reported sensitivity limit for 5-HT in various scientific publications is approximately 0.5 fmol per sample with a signal-to-noise ratio = 2 [20]. To comprehensively grasp the serotonergic and dopaminergic systems functionalities and its associated disorders and diseases, a combination of microdialysis sampling methods and high-performance analytical techniques is crucial for quantifying variations in extracellular NTs and metabolite concentrations.
3. Analytical Methods for Detection and Quantification of NTs and Metabolites
The analysis methods have been developed for the detection and quantification of NTs in vivo to understand specific neurotransmission dysfunctions in neurodegenerative diseases or in psychiatric disorders.
3.1. Gas Chromatography and Capillary Electrophoresis
Gas chromatography (GC) [21,22] and capillary electrophoresis (CE) [23,24] are analytical techniques used in these research areas. However, they have several drawbacks: GC requires the use of compound derivatization to make them more volatile [25], and CE is a limited technique for the analysis of complex mixtures. HPLC, on the other hand, allows for the analysis of biological samples from microdialysis without the need for chemical derivatization or extraction steps.
3.2. HPLC
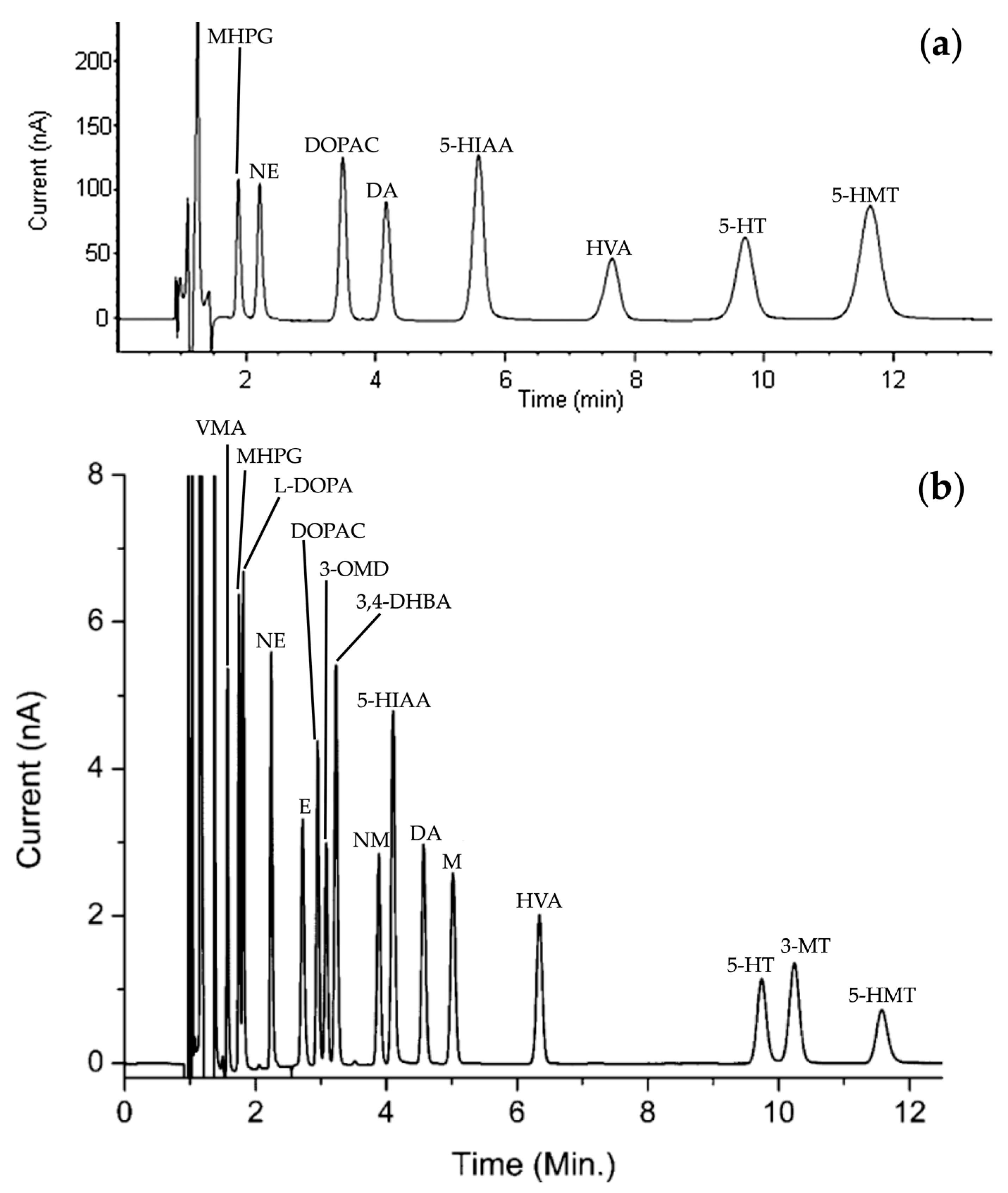
Since the late 1980s, research teams led by Donzanti et al. [26], Peinado et al. [27], and Rogers et al. [28] have focused their studies on the detection of amino acid neurotransmitters, particularly γ-aminobutyric acid (GABA), achieving a sensitivity of about 0.1 nM. HPLC analysis of NTs is characterized by its speed and selectivity. Typically, a reverse-phase (RP) system is used to separate NTs due to their hydrophobic nature. For instance, Bidel et al. developed an HPLC method for the separation of monoamines and their metabolites (Figure 4a). They achieved a total elution time of about 12 min, a minimum resolution between each peak of 2.2, and a number of theoretical plates (N) ranging from 2900 to 3800 [29]. These values are largely sufficient to validate the use of HPLC.
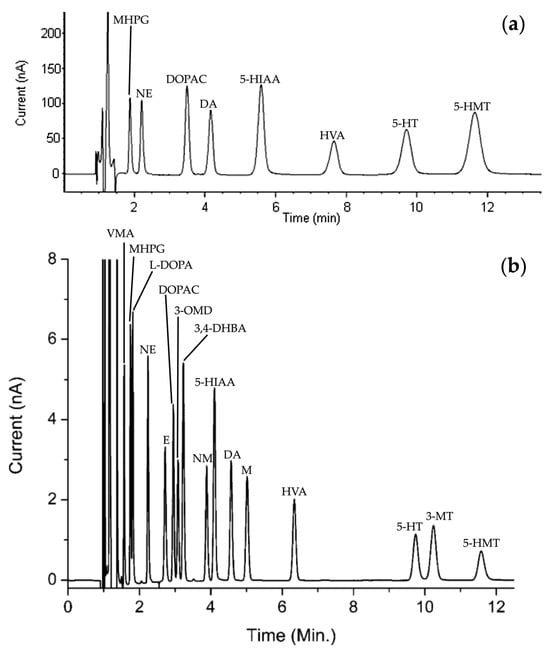
Figure 4.
HPLC chromatogram reprinted and adapted with permission from [29] Copyright 2016 John Wiley and Sons (a) and UHPLC chromatogram reprinted and adapted with permission from [30] Copyright 2013 American Chemical Society (b) of a standards solution containing of each NTs and metabolites: DOPAC, DA, 5-HIAA, HVA, 3-MT and 5-HT. For information, other NTs and metabolites have been presented: 3-methoxy-4-hydroxyphenylglycol (MHPG), norepinephrine (NE), 5-hydroxymethyl tolterodine (5-HMT), D,L-4-hydroxy-3-methoxy-mandelic acid (VMA), L-3,4-dihydroxyphenylalanine (L-DOPA), epinephrine (E), 3,4-dihydroxybenzylamine hydrobromide (3,4-DHBA), 3-methoxy-L-tyrosine (3-OMD) and D,L-metanephirine (M).
By way of comparison, Reinhoud et al. developed a UHPLC method similar conditions (see Section 3.3 for more details) under reverse phase conditions, enabling the separation of NTs in a 12 min run time elution (Figure 4b). The columns of UHPLC have smaller particle sizes. Consequently, chromatographic peaks will be finer and better defined, allowing for improved separation of compounds. Moreover, the number of theoretical plates in this study has been estimated to be close to 200,000 [30].
More recently, hydrophilic interaction liquid chromatography (HILIC) columns have been employed [31,32]. These columns offer reduced system pressure, allowing for enhanced flow rates and shorter analysis times. For example, in the study by Zhou et al., the use of a low-granularity column (1.7 µm) combined with a flow rate of 0.4 mL min−1 is employed. These experimental conditions result in very short analysis times, on the order of 4 min. It is noteworthy that the Multiple Reaction Monitoring (MRM) mode of the mass spectrometer enhances the selectivity of the measurements [33].
The utilization of HILIC column necessitates coupling with mass spectrometry because of its incompatibility with the salts used with the electrochemical detection.
3.3. Detection of NTs and Their Metabolites
Various types of detectors can be employed, including fluorescence detectors (FLD) [34,35,36], mass spectrometry (MS) [37,38,39], sensors and biosensors [40,41,42] or electrochemical detector (ECD). ECD holds an advantage over MS detectors due to its lower operating cost and easy maintenance. For instance, weekly polishing of a glassy carbon (GC) working electrode with alumina powder is adequate to maintaining a reflective surface, thereby enhancing result repeatability and reproducibility.
ECD has also been extensively utilized for the detection and quantification of NTs and their metabolites [43,44,45,46]. It proves to be a straightforward and sensitive method for quantifying small amounts of NTs. One of its notable benefits is its simplicity and minimal maintenance.
Several types of electrochemical detectors exist, including coulometric and amperometric detectors. The principle of the coulometric detector lies in the measurement of the current charge produced during electrolysis at a constant potential. Compounds are then oxidized or reduced almost entirely. Coulometric detectors are less sensitive to variations in flow rate and temperature than amperometric detectors. However, electrodes are easily contaminated due to their large electrolysis surface area. The measurement of the charge during the electrochemical reaction is based on Faraday’s law (Equation (1)).
with Q the charge (C or A s), n the number of electrons exchanges during the electrochemical detection, F the Faraday’s constant (96,485 C mol−1) and NO the number of moles of the O compound.
Amperometric detection generally provides high sensitivity and the response remains linear over a wide range of concentrations. For these reasons, they are more widespread than coulometric detectors. Compared to coulometric detection, electrolysis consumes only about 10% of the analyte. The value of the measured current in an amperometric detector depends on the geometry of the electrode used (Table 1).

Table 1.
Limiting current equation as a function of geometry electrode used during the amperometric detection [47].
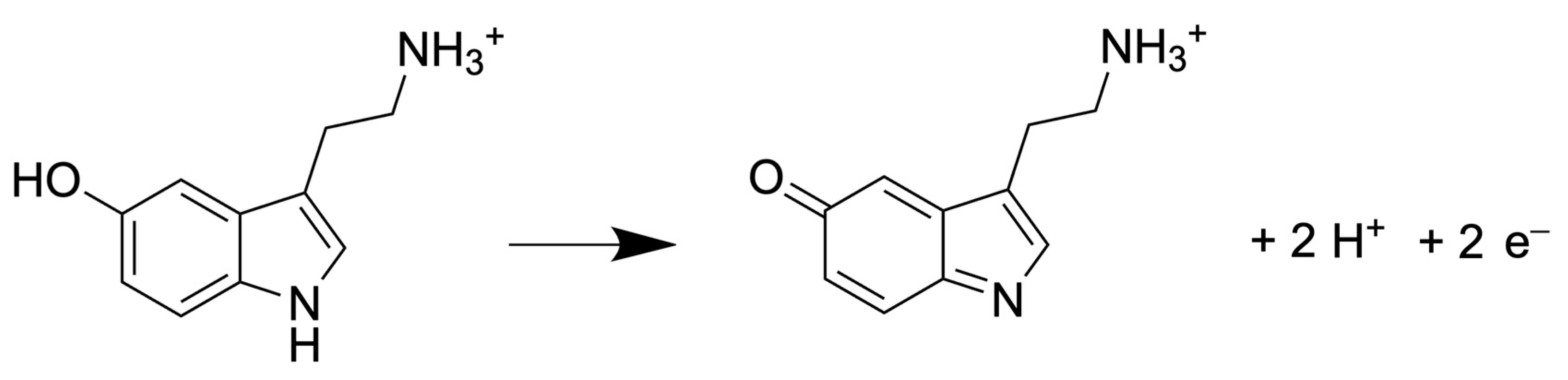
Regardless of the detector used, the number of electrons exchanged determines the measured current during the analysis. In case of detecting 5-HT, the number of electrons exchanges is 2 during the oxidation, as shown in Figure 5.
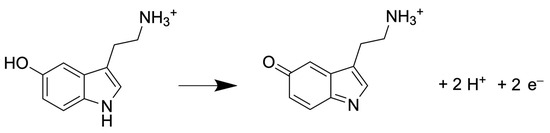
Figure 5.
Half reaction of 5-HT oxidation [48].
For electroactive compounds, charge can be correlated with the moles of the considered compound, extending to concentration. It is crucial to emphasize that the number of electrons exchanged in the electrochemical reaction is contingent on the applied potential. If the overvoltage is insufficient, then the electrochemical reaction remains incomplete.
ECD has some limitations: this detector is not universal and compounds must be electroactive to be detectable. In the same domain, GABA, a neurotransmitter involved in neurodegenerative diseases [49,50], can be analyzed by HPLC-ECD. However, GABA is not electroactive and requires chemical derivatization. In the Panrod et al. study, two derivatization pathway involving aldehydes moieties have been employed to be able to detect GABA [51]. Therefore, the development of a single analytical method for all NTs with an electrochemical detector appears to be challenging in light of the mentioned limitations.
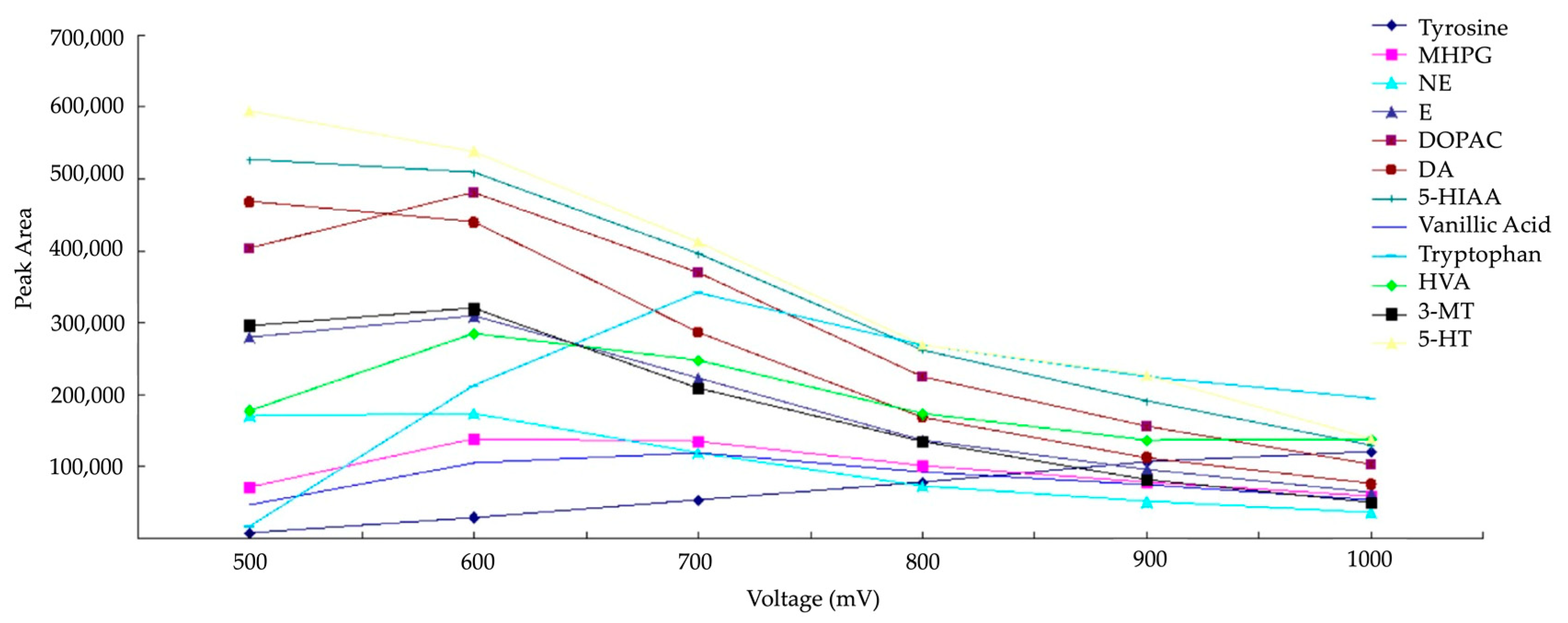
The two most frequently employed working electrode materials are GC and boron-doped diamond (BDD). These materials showcase diverse physicochemical characteristics: the BDD electrode boasts the largest electrochemical window, facilitating the detection of numerous electroactive compounds. However, as indicated in the study by Zhang et al. in 2016, a high overpotential seems to reduce the peak area of the analytes (Figure 6). The sensitivity of electrode passivation is higher for a BDD electrode compared to a GC electrode [52]. Consequently, it seems that reproducibility is superior with a GC electrode.
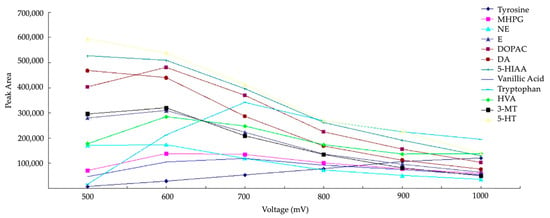
Figure 6.
Hydrodynamic voltammograms of monoamines NTs on BDD working electrode reprinted and modified with permission from [53] Copyright 2016 Elsevier.
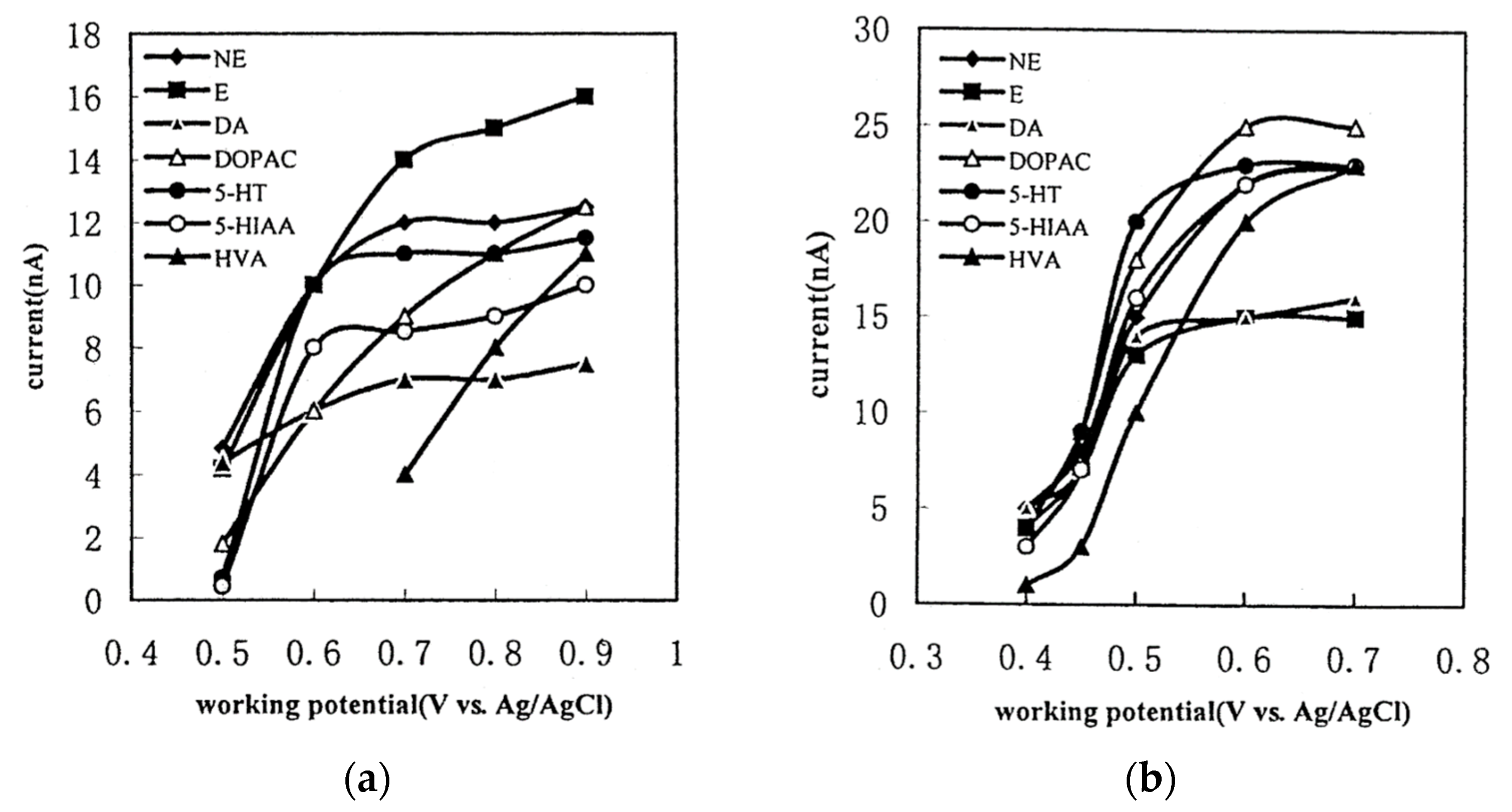
The GC electrode exhibits a narrower electrochemical window compared to the BDD electrode. Consequently, the analysis range is restricted, and the oxidation of water impedes the detection of compounds with high oxidation potentials [54]. Analyzing compounds with potentials involving reduction is, to our knowledge, impossible due to the reduction of dissolved oxygen [55]. In Figure 7a, the detection current is depicted as a function of the potential applied for monoamines NTs with a GC working electrode. Ultimately, the choice between the two electrodes depends on the application when the compounds have relatively low oxidation potentials. Then, it is preferable to use a GC working electrode.
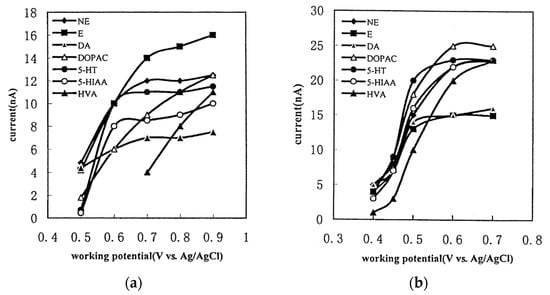
Figure 7.
Hydrodynamic voltammograms of monoamines NTs and their main metabolites on GC working electrode (a) and P-pABA modified working electrode (b) reprinted with permission from [56] Copyright 2001 Elsevier.
To enhance the sensitivity of NTs detection, two optimizations are considered: firstly, increasing the surface-to-volume ratio of the electrochemical cell, and secondly, using a modified electrode material to enhance its physicochemical characteristics. In the study by Xu et al., the working electrode on GC is modified through an electrochemical reaction with poly(para-aminobenzoic acid) (P-pABA) (Figure 7b) [56]. With a 0.7 V vs. Ag/AgCl potential detection, the sensitivity of measurement improved from 8.5 nA to 23 nA, 11 nA to 23 nA, 7 nA to 16 nA, 9 nA to 25 nA and 4 nA to 23 nA for 5-HIAA, 5-HT, DA, DOPAC and HVA, respectively. Augmenting sensitivity enhances the quantification of NTs.
In conclusion, the ECD is widely employed for the detection of monoamines due to its user-friendly nature and low maintenance cost. Its sensitivity enables the quantification of very low concentrations of NTs. To expand the capabilities of this detector, it would be possible to develop chemical derivatizations either directly on the compounds or on the electrode surface. These chemical modifications could induce a broader spectrum of applications.
3.4. Limit of Detection and Quantification
By convention, the limit of detection (LOD) and limit of quantification (LOQ) are established at signal-to-noise ratios of 3 and 10, respectively. The linear range (L.R.) denotes the measurement zone where the device-recorded signal is directly proportional to the quantity or concentration of the substance being measured.
Table 2 compiles the various values of LOD, LOQ, and the linear range for the NTs and their metabolites. Additionally, the different experimental conditions have been reported for informational purposes. The studies are ranked based on the particle size of the chromatographic C18 column used, from smallest to largest. The list of presented publications is not exhaustive. Other articles have been published on the use of HPLC-ECD but they did not detail the analytical development and LOD/LOQ of NTs. Those articles were not included in this summary table. For instance, we can cite several recent works by Guo et al. (2023) [57], Lei et al. (2023) [58], and Wang et al. (2022) [59].
According to these studies, the sensitivity of the targeted molecules can approach values close to 10−9 mol L−1 for HPLC system and less, close to 10−11 mol L−1, in UHPLC cases. As mentioned earlier, the use of columns with small particle sizes refines elution peaks and consequently lowers quantification limits. However, using such columns induces high back pressure. These low values facilitate the collection of microdialysis samples in the range of microliter, enabling real-time monitoring of neurotransmitter concentrations.
In electrochemical detection processes, the use of salts becomes crucial to increase conductivity and consequently achieve optimal performance. Typically, the aqueous phase of the mobile phase incorporates essential components, such as chelating compounds (e.g., sodium citrate, EDTA), which play a role in preventing coagulation [60], an anionic surfactant (sodium dodecyl sulfate, octane sulfonic acid) to prevent interference with lipids and enhance the retention of anionic compounds [61], and finally, a buffer (phosphate, citrate).
Most of the time, the pH of the mobile phase is buffered in an acidic environment. The more acidic the eluent, the faster the compounds will elute. Additionally, some compounds are sensitive and degrade more rapidly at basic pH. For instance, this is the case with DA, which oxidizes at a significant rate under basic pH conditions: the half-life at pH 5.6, 7.1, and 7.4 is 13.5 days, 19.7 min, and 4.95 min, respectively [62].
Moreover, the mobile phase, predominantly consisting of the aqueous phase with salts, is often augmented with methanol and/or acetonitrile. This strategic addition enhances the eluent’s apolar characteristics, ensuring a comprehensive and effective electrochemical detection setup.

Table 2.
Recent values of separation, detection and quantification of compounds involved in serotonergic system and their application (non-exhaustive list). All values provided have been standardized to the same unit (mol L−1) for the purpose of comparability.
Table 2.
Recent values of separation, detection and quantification of compounds involved in serotonergic system and their application (non-exhaustive list). All values provided have been standardized to the same unit (mol L−1) for the purpose of comparability.
| Reference | HPLC-ECD Conditions | Application | ||||||
|---|---|---|---|---|---|---|---|---|
| Reinhoud et al. (2013) [30] | Methods and Application | C18 column (1.0 × 100 mm, 1.7 µm particles size) Flow rate = 50 µL min−1 Column temperature = 37 °C Injection volume = 5 µL Mobile phase: 100 mM phosphoric acid, 100 mM citric acid, 8 mM KCl, 0.1 mM EDTA and 2.8 mM OSA. ACN/H2O (8/92; v/v) pH = 3 Detection: GC working electrode (+0.65 V vs. Ag/AgCl) | Development of analytical method for the determination of monoamines concentrations and application to a microdialysis in a prefrontal cortex. | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DA | DOPAC | HVA | |||
| LOD | 8.3 × 10−11 | 3.5 × 10−11 | 4.2 × 10−11 | 5.0 × 10−11 | 4.7 × 10−11 | |||
| Ferry et al. (2014) [63] | Methods and Application | C18 column (0.32 × 100 mm, 1.9 µm particles size) Flow rate = 8.5 µL min−1 Column temperature = 40 °C Injection volume = 1 µL Mobile phase: 140 mM potassium phosphate, 8 mM KCl, 0.1 mM EDTA, 6 mM OSA. MeOH/H2O (6/94; v/v) pH = 5 (adjusted with 10 mM NaOH) Detection: GC working electrode (+0.45 V vs. Ag/AgCl) | Development of analytical method for the determination of monoamines concentrations and application to microdialysis in the dorsal hippocampus | |||||
| Detection (mol L−1) | 5-HT | DA | 3-MT | |||||
| LOD | 1.5 × 10−9 | 7.5 × 10−10 | 1.5 × 10−9 | |||||
| LOQ | 5.0 × 10−9 | 2.5 × 10−9 | 5.0 × 10−9 | |||||
| Schou-Pedersen et al. (2016) [64] | Methods and Application | C18 column (4.6 × 100 mm, 2.6 µm particles size) Flow rate = 0.8 mL min−1 Column temperature = 30 °C Injection volume = 20 µLMobile phase: 70 mM potassium dihydrogen phosphate, 2 mM OSA, 0.1 mM EDTA. MeOH/H2O (10/90; v/v) pH = 3.12 (adjusted with 1 M citric acid) Detection: Porous graphite working electrode (+0.40 V vs. Pd) | Development of chromatographic method for the quantification of monoamine NTs from sub-regions of guinea pig brain (intracellular and extracellular). | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DOPAC | DA | HVA | |||
| LOQ | 8.8 × 10−9 | 3.8 × 10−9 | 3.6 × 10−9 | 1.0 × 10−8 | 1.2 × 10−8 | |||
| L.R. | 8.8 × 10−9–1.0 × 10−6 | 3.8 × 10−9–1.0 × 10−6 | 3.6 × 10−9–1.0 × 10−6 | 1.0 × 10−8–7.5 × 10−7 | 1.2 × 10−8–5.0 × 10−7 | |||
| Van Dam et al. (2014) [65] | Methods and Application | C18 column (1.0 × 250 mm, 3.0 µm particles size) Flow rate = 40 µL min−1 Column temperature = from 30 °C to 36 °C Injection volume = 50 µL Mobile phase: 8 mM KCl, 50 mM phosphoric acid, 50 mM citric acid, 0.1 mM EDTA, from 1.8 to 2.2 mM OSA. MeOH/H2O (from 13/87 to 17/83; v/v) pH = from 3.0 to 3.6 (adjusted with NaOH) Detection: GC working electrode (from +0.63 V to +0.67 V vs. Ag/AgCl) | Development of analytical method for the quantification of biogenic amines and metabolites in human brain tissue. | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DOPAC | DA | HVA | |||
| L.R. | 5.3 × 10−10–1.8 × 10−7 | 2.2 × 10−10–7.7 × 10−8 | 2.8 × 10−10–1.6 × 10−7 | 2.7 × 10−10–1.8 × 10−7 | 5.8 × 10−10–3.2 × 10−7 | |||
| Pantiya et al. (2024) [66] | Methods and Application | C18 column (3.0 × 500 mm, 2.6 µm particles size) Flow rate = 0.5 mL min−1 Column temperature = 25 °C Injection volume = 25 µLMobile phase: 130 mM sodium phosphate monobasic, 20 mM orthophosphoric acid, 2 mM sodium dodecyl sulfate, 50 µM EDTA. ACN, MeOH, H2O (5/10/95; v/v/v) pH = 3.2 (adjusted with phosphate buffer) Detection: GC working electrode (+0.50 V vs. Ag/AgCl) | Development of analytical method for the quantification of NTs and metabolites in brain mice microdialysates. | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DA | HVA | ||||
| LOD | 3.1 × 10−10 | 5.4 × 10−10 | 3.7 × 10−10 | 4.1 × 10−10 | ||||
| LOQ | 3.9 × 10−10 | 5.8 × 10−10 | 4.2 × 10−10 | 4.3 × 10−10 | ||||
| Allen et al. (2017) [67] | Methods and Application | C18 column (3.2 × 150 mm, 3.0 µm particles size) Flow rate = 0.6 mL min−1 Column temperature = N/A Injection volume = 40 µLMobile phase: 100 mM sodium acetate, 20 mM citric acid, 0.38 mM sodium octyl sulfate, 0.15 mM EDTA. ACN/H2O (5/95; v/v) pH = 3.3 (adjusted with glacial acetic acid) Detection: Dual working electrode (−0.22 V and +0.375 V) | Development of analytical method for the quantification of monoamines and metabolites in brain tissue of mice. | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DOPAC | DA | HVA | |||
| LOD | 1.7 × 10−9 | 6.5 × 10−10 | 7.4 × 10−10 | 8.2 × 10−10 | 4.1 × 10−10 | |||
| LOQ | 3.6 × 10−9 | 1.3 × 10−9 | 1.5 × 10−9 | 1.6 × 10−9 | 1.4 × 10−9 | |||
| L.R. | 3.6 × 10−9–1.7 × 10−4 | 1.3 × 10−9–7.9 × 10−5 | 1.5 × 10−9–8.9 × 10−5 | 1.6 × 10−9–2.0 × 10−4 | 1.4 × 10−9–8.2 × 10−5 | |||
| Yardimci et al. (2023) [68] | Methods and Application | C18 column (4.6 × 250 mm, 5.0 µm particles size) Flow rate = 1 mL min−1 Column temperature = 36 °C Injection volume = 20 µL Mobile phase: 35 mM citric acid, 19 mM sodium citrate, 0.16 mM EDTA, 1.1 mM heptasulfonic acid. Glacial acetic acid/tetrahydrofuran/MeOH/H2O (0.11/0.3/2.5/97.085; v/v/v/v) pH = 4.9 (adjusted with 10 M NaOH) Detection: GC working electrode (+0.50 V vs. Ag/AgCl) | Analysis in hypothalamic and subcortical nuclei | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DA | DOPAC | ||||
| LOQ | 5.7 × 10−7 | 5.2 × 10−7 | 6.5 × 10−7 | 6.0 × 10−7 | ||||
| Du et al. (2018) [69] | Methods and Application | C18 column (4.6 × 250 mm, 5.0 µm particles size) Flow rate = 1 mL min−1 Column temperature = 25 °C Injection volume = 20 µL Mobile phase: 25 mM sodium citrate, 0.01 mM EDTA. ACN/H2O (5/95; v/v) pH = 4.5 (adjusted with 1 M acetic acid) Detection: BDD working electrode (+0.70 V vs. Ag/AgCl) | Analytical method development | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | ||||||
| LOD | 2.1 × 10−8 | 1.6 × 10−8 | ||||||
| LOQ | 2.8 × 10−8 | 4.2 × 10−8 | ||||||
| L.R. | 2.8 × 10−8–1.1 × 10−6 | 2.6 × 10−8–2.6 × 10−6 | ||||||
| Zhang et al. (2016) [53] | Methods and Application | C18 column (4.6 × 250 mm, 5.0 µm particles size) Flow rate = 1 mL min−1 Column temperature = 25 °C Injection volume = 20 µL Mobile phase: 25 mM sodium citrate, 0.01 mM EDTA. ACN/H2O (5/95; v/v) pH = 4.5 (adjusted with 1 M acetic acid) Detection: BDD working electrode (+0.70 V vs. Ag/AgCl) | Determination of the concentrations of monoamines NTs in rat cortex and hippocampus tissues. | |||||
| Detection (mol L−1) | 5-HT | 5-HIAA | DA | DOPAC | 3-MT | HVA | ||
| LOD | 2.3 × 10−8 | 1.1 × 10−8 | 2.6 × 10−8 | 1.2 × 10−8 | 3.6 × 10−8 | 2.7 × 10−8 | ||
| LOQ | 8.5 × 10−8 | 3.1 × 10−8 | 9.8 × 10−8 | 4.8 × 10−8 | 1.2 × 10−7 | 8.2 × 10−8 | ||
| L.R. | 8.5 × 10−8–1.4 × 10−6 | 3.1 × 10−8–7.9 × 10−7 | 9.8 × 10−8–2.3 × 10−6 | 6.0 × 10−8–3.0 × 10−6 | 1.2 × 10−7–1.8 × 10−6 | 8.2 × 10−8–1.4 × 10−6 | ||
| Jiang et al. (2015) [70] | Methods and Application | C18 column (4.6 × 250 mm, 5.0 µm particles size) Flow rate = 1 mL min−1 Column temperature = 30 °C Injection volume = 10 µL Mobile phase: 50 mM potassium dihydrogen phosphate, 0.1 mM octane sulfonic acid. MeOH/H2O (5/95; v/v) pH = N/A Detection: GC working electrode (+0.70 V vs. Ag/AgCl) | Development of the analytical method for monoamines NTs in human urine. | |||||
| Detection (mol L−1) | 5-HT | DA | ||||||
| LOD | 6.1 × 10−8 | 3.9 × 10−8 | ||||||
| Lokhande et al. (2022) [71] | Methods and Application | C18 column (4.6 × 250 mm, 5.0 µm particles size) Flow rate = 1.3 mL min−1 Column temperature = 35 °C Injection volume = 20 µL Mobile phase: 50 mM potassium dihydrogen phosphate, 0.99 mM SOS and 53 µM EDTA. MeOH/H2O (12/88; v/v) pH = 2.5 (adjusted with 85% phosphoric acid) Detection: BDD working electrode (+0.70 V vs. Ag/AgCl) | Development of the analytical method for metabolites quantification in human CSF. | |||||
| Detection (mol L−1) | 5-HIAA | HVA | ||||||
| LOQ | 6.5 × 10−8 | 6.9 × 10−8 | ||||||
| L.R. | 6.5 × 10−8–2.6 × 10−6 | 6.9 × 10−8–2.7 × 10−6 | ||||||
Abreviations: N/A: not available, LOD: limit of detection, LOQ: limit of quantification, L.R.: linear range, EDTA: ethylendiaminetetraacetic acid, OSA: octane sulfonic acid, SOS: sodium octane sulfonate, ACN: acetonitrile, MeOH: methanol.
4. Conclusions
To study the brain serotonergic and dopaminergic systems, researchers employ intracerebral microdialysis coupled with high-performance liquid chromatography (HPLC) and electrochemical detector (ECD). HPLC, especially in reverse phase conditions, is a widely used separation technique for NT analysis, offering rapid and selective results. Electrochemical detection, particularly with glassy carbon electrodes, proves to be a sensitive and cost-effective method for quantifying NTs and their metabolites with values close to 10−9 mol L−1 for HPLC and 10−11 mol L−1 with UHPLC.
Optimizations, such as increasing the surface-to-volume ratio of the electrochemical cell and modifying electrode materials can enhance sensitivity. The determination of limits of detection and quantification, as well as the linear range, is crucial for evaluating the analytical performance of the HPLC-ECD system. Overall, the combination of microdialysis, HPLC, and ECD provides a powerful approach for understanding the dynamics of NTs and their metabolites, contributing to advancements in neuroscientific research and potential clinical applications.
It should be noted, however, that the use of ECD for neurotransmitters is becoming less common. Indeed, mass spectrometry is becoming more affordable, and the preventive maintenance of the analyzer is becoming simpler. Consequently, due to its high sensitivity and selectivity, mass spectrometry is currently the most common detector in analysis laboratories.
Research perspectives in the field of high-performance liquid chromatography with an electrochemical detector offer a fertile ground for improving the sensitivity and specificity of neurotransmitter detection. One promising avenue involves exploring the modification of the electrochemical detector’s surface. By altering surface properties, such as roughness or chemical composition, one can optimize the interaction with neurotransmitters, thereby enhancing detection efficiency.
Another research direction focuses on modifying the characteristics of the electrochemical cell itself, including its surface and dimensions. By adjusting these parameters, the signal-to-noise ratio can be increased, which is crucial for precise measurements. Electrochemical cells with an increased surface area can enhance the likelihood of interaction with analytes, thereby improving detection. Moreover, optimized dimensions can facilitate a clearer electrochemical response and reduce unwanted interferences.
By combining these approaches, it becomes conceivable to achieve quantification limits comparable to those obtained with mass spectrometry detectors, paving the way for significant advancements in understanding neurochemical mechanisms. These research endeavors hold the promise of bringing substantial improvements to neurotransmitter detection, opening new perspectives for understanding neurological disorders and mental illnesses.
Author Contributions
B.P.G.: review design and writing, G.G.: referencing and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agence Nationale pour la recherche, grant number AIS-in-DEP (ANR-22-CE37-0018 Neuroscience Intégrative et Cognitive).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Factor, S.A.; McDonald, W.M.; Goldstein, F.C. The Role of Neurotransmitters in the Development of Parkinson’s Disease-related Psychosis. Eur. J. Neurol. 2017, 24, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Tajeddinn, W.; Fereshtehnejad, S.; Ahmed, M.; Yoshitake, T.; Kehr, J.; Shahnaz, T.; Milovanovic, M.; Behbahani, H.; Hoglund, K.; Winblad, B.; et al. Association of Platelet Serotonin Levels in Alzheimer’s Disease with Clinical and Cerebrospinal Fluid Markers. J. Alzheimer Dis. 2016, 53, 621–630. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, J.; Zhou, P.; Li, J.; Gao, H.; Xia, Y.; Wang, Q. Neurotransmitter Receptors and Cognitive Dysfunction in Alzheimer’s Disease and Parkinson’s Disease. Prog. Neurobiol. 2012, 97, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Dreyer, J.-L. Anxiety and Ethanol Consumption in Socially Defeated Mice; Effect of Hippocampal Serotonin Transporter Knockdown. Behav. Brain Res. 2023, 451, 114508. [Google Scholar] [CrossRef]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and Neuroplasticity—Links between Molecular, Functional and Structural Pathophysiology in Depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef]
- Yagishita, S. Transient and Sustained Effects of Dopamine and Serotonin Signaling in Motivation-related Behavior. Psychiatry Clin. Neurosci. 2020, 74, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Di Porzio, U.; Viggiano, D.; De Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Weinstein, J.J.; Chohan, M.O.; Slifstein, M.; Kegeles, L.S.; Moore, H.; Abi-Dargham, A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry 2017, 81, 31–42. [Google Scholar] [CrossRef]
- Di Forti, M.; Lappin, J.M.; Murray, R.M. Risk Factors for Schizophrenia—All Roads Lead to Dopamine. Eur. Neuropsychopharmacol. 2007, 17, S101–S107. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s Disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.L. Epinephrine Biosynthesis: Hormonal and Neural Control During Stress. Cell. Mol. Neurobiol. 2006, 26, 889–898. [Google Scholar] [CrossRef]
- Harrison, N.A.; Morgan, R.; Critchley, H.D. From Facial Mimicry to Emotional Empathy: A Role for Norepinephrine? Soc. Neurosci. 2010, 5, 393–400. [Google Scholar] [CrossRef]
- Alves, E.; Lukoyanov, N.; Serrão, P.; Moura, D.; Moreira-Rodrigues, M. Epinephrine Increases Contextual Learning through Activation of Peripheral Β2-Adrenoceptors. Psychopharmacology 2016, 233, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B.; Roozendaal, B.; McGaugh, J.L. Role of Norepinephrine in Mediating Stress Hormone Regulation of Long-Term Memory Storage: A Critical Involvement of the Amygdala. Biol. Psychiatry 1999, 46, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Tossman, U.; Ungerstedt, U. Microdialysis in the Study of Extracellular Levels of Amino Acids in the Rat Brain. Acta Physiol. Scand. 1986, 128, 9–14. [Google Scholar] [CrossRef]
- Ungerstedt, U.; Hallström, Å. In Vivo Microdialysis-a New Approach to the Analysis of Neurotransmitters in the Brain. Life Sci. 1987, 41, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Zuo, H.; Stobaugh, J.F.; Lunte, C.E.; Lunte, S.M. Continuous in Vivo Monitoring of Amino Acid Neurotransmitters by Microdialysis Sampling with Online Derivatization and Capillary Electrophoresis Separation. Anal. Chem. 1995, 67, 594–599. [Google Scholar] [CrossRef]
- Van Schoors, J.; Lens, C.; Maes, K.; Michotte, Y.; Smolders, I.; Van Eeckhaut, A. Reassessment of the Antioxidative Mixture for the Challenging Electrochemical Determination of Dopamine, Noradrenaline and Serotonin in Microdialysis Samples. J. Chromatogr. B 2015, 998–999, 63–71. [Google Scholar] [CrossRef]
- Guiard, B.; Lanfumey, L.; Gardier, A. Microdialysis Approach to Study Serotonin Outflow in Mice Following Selective Serotonin Reuptake Inhibitors and Substance P (Neurokinin 1) Receptor Antagonist Administration: A Review. Curr. Drug Targets 2006, 7, 187–201. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, B.; Sun, Y.; Gao, J.; Lian, K. Analysis of 5-Hydroxytryptamine and Its Related Indoles in Cerebrospinal Fluid of Leukemic Children by Gas Chromatography-Mass Spectrometry. J. Lab. Med. 2020, 44, 41–45. [Google Scholar] [CrossRef]
- Shi, H.; Wang, B.; Niu, L.; Cao, M.; Kang, W.; Lian, K.; Zhang, P. Trace Level Determination of 5-Hydroxytryptamine and Its Related Indoles in Amniotic Fluid by Gas Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 143, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Whang, C. Capillary Electrophoresis of Monoamines and Catechol with Indirect Chemiluminescence Detection. Electrophoresis 1999, 20, 2533–2538. [Google Scholar] [CrossRef]
- Benturquia, N.; Couderc, F.; Sauvinet, V.; Orset, C.; Parrot, S.; Bayle, C.; Renaud, B.; Denoroy, L. Analysis of Serotonin in Brain Microdialysates Using Capillary Electrophoresis and Native Laser-Induced Fluorescence Detection. Electrophoresis 2005, 26, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Hattox, S.E.; Murphy, R.C. Mass Spectrometry and Gas Chromatography of Trimethylsilyl Derivatives of Catecholamine Related Molecules. Biol. Mass Spectrom. 1978, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Donzanti, B.A.; Yamamoto, B.K. An Improved and Rapid HPLC-EC Method for the Isocratic Separation of Amino Acid Neurotransmitters from Brain Tissue and Microdialysis Perfusates. Life Sci. 1988, 43, 913–922. [Google Scholar] [CrossRef]
- Peinado, J.M.; McManus, K.T.; Myers, R.D. Rapid Method for Micro-Analysis of Endogenous Amino Acid Neurotransmitters in Brain Perfusates in the Rat by Isocratic HPLC-EC. J. Neurosci. Methods 1986, 18, 269–276. [Google Scholar] [CrossRef]
- Rogers, K.L.; Philibert, R.A.; Allen, A.J.; Molitor, J.; Wilson, E.J.; Dutton, G.R. HPLC Analysis of Putative Amino Acid Neurotransmitters Released from Primary Cerebellar Cultures. J. Neurosci. Methods 1987, 22, 173–179. [Google Scholar] [CrossRef]
- Bidel, F.; Corvaisier, S.; Jozet-Alves, C.; Pottier, I.; Dauphin, F.; Naud, N.; Bellanger, C. An HPLC-ECD Method for Monoamines and Metabolites Quantification in Cuttlefish (Cephalopod) Brain Tissue: Biogenic Monoamines and Metabolites in Cuttlefish (Cephalopod) Brain. Biomed. Chromatogr. 2016, 30, 1175–1183. [Google Scholar] [CrossRef]
- Reinhoud, N.J.; Brouwer, H.-J.; Van Heerwaarden, L.M.; Korte-Bouws, G.A.H. Analysis of Glutamate, GABA, Noradrenaline, Dopamine, Serotonin, and Metabolites Using Microbore UHPLC with Electrochemical Detection. ACS Chem. Neurosci. 2013, 4, 888–894. [Google Scholar] [CrossRef]
- Tufi, S.; Lamoree, M.; De Boer, J.; Leonards, P. Simultaneous Analysis of Multiple Neurotransmitters by Hydrophilic Interaction Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1395, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chirita, R.-I.; West, C.; Finaru, A.-L.; Elfakir, C. Approach to Hydrophilic Interaction Chromatography Column Selection: Application to Neurotransmitters Analysis. J. Chromatogr. A 2010, 1217, 3091–3104. [Google Scholar] [CrossRef]
- Zhou, G.-S.; Yuan, Y.-C.; Yin, Y.; Tang, Y.-P.; Xu, R.-J.; Liu, Y.; Chen, P.-D.; Yin, L.; Duan, J.-A. Hydrophilic Interaction Chromatography Combined with Ultrasound-Assisted Ionic Liquid Dispersive Liquid–Liquid Microextraction for Determination of Underivatized Neurotransmitters in Dementia Patients’ Urine Samples. Anal. Chim. Acta 2020, 1107, 74–84. [Google Scholar] [CrossRef]
- Boulghobra, A.; Bonose, M.; Billault, I.; Pallandre, A. A Rapid and Sensitive Method for the Quantification of Dopamine and Serotonin Metabolites in Cerebrospinal Fluid Based on UHPLC with Fluorescence Detection. J. Chromatogr. B 2022, 1200, 123264. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.E.; Fico, D.; Pennetta, A.; Malitesta, C.; Nicolardi, G.; Lofrumento, D.D.; De Nuccio, F.; La Pesa, V. A Rapid and Simple Method for the Determination of 3,4-Dihydroxyphenylacetic Acid, Norepinephrine, Dopamine, and Serotonin in Mouse Brain Homogenate by HPLC with Fluorimetric Detection. J. Pharm. Biomed. Anal. 2014, 98, 266–270. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Mu, H.; Bai, Y.-H.; Yu, H.; Hu, Y.-M. A Rapid Method for the Determination of Dopamine in Porcine Muscle by Pre-Column Derivatization and HPLC with Fluorescence Detection. J. Pharm. Anal. 2011, 1, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Carrera, V.; Sabater, E.; Vilanova, E.; Sogorb, M.A. A Simple and Rapid HPLC–MS Method for the Simultaneous Determination of Epinephrine, Norepinephrine, Dopamine and 5-Hydroxytryptamine: Application to the Secretion of Bovine Chromaffin Cell Cultures. J. Chromatogr. B 2007, 847, 88–94. [Google Scholar] [CrossRef]
- Xu, N.; Qiu, C.; Wang, W.; Wang, Y.; Chai, C.; Yan, Y.; Zhu, D. HPLC/MS/MS for Quantification of Two Types of Neurotransmitters in Rat Brain and Application: Myocardial Ischemia and Protection of Sheng-Mai-San. J. Pharm. Biomed. Anal. 2011, 55, 101–108. [Google Scholar] [CrossRef]
- Kovac, A.; Somikova, Z.; Zilka, N.; Novak, M. Liquid Chromatography–Tandem Mass Spectrometry Method for Determination of Panel of Neurotransmitters in Cerebrospinal Fluid from the Rat Model for Tauopathy. Talanta 2014, 119, 284–290. [Google Scholar] [CrossRef]
- Shao, Z.; Chang, Y.; Venton, B.J. Carbon Microelectrodes with Customized Shapes for Neurotransmitter Detection: A Review. Anal. Chim. Acta 2022, 1223, 340165. [Google Scholar] [CrossRef]
- Polo, E.; Kruss, S. Nanosensors for Neurotransmitters. Anal. Bioanal. Chem. 2016, 408, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; McCracken, S.; Hossain, M.F.; Slaughter, G. Electrochemical Detection of Neurotransmitters. Biosensors 2020, 10, 101. [Google Scholar] [CrossRef]
- Dicgory, G.L.; Buckett, W.R. An Automated Method to Measure Monoamines and Metabolites Using Elevated Temperature Reversed Phase HPLC with Electrochemical Detection Application to Striatal Dopamine and Hippocampal Serotonin Turnover. J. Pharmacol. Methods 1984, 11, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Cannazza, G.; Di Stefano, A.; Mosciatti, B.; Braghiroli, D.; Baraldi, M.; Pinnen, F.; Sozio, P.; Benatti, C.; Parenti, C. Detection of Levodopa, Dopamine and Its Metabolites in Rat Striatum Dialysates Following Peripheral Administration of l-DOPA Prodrugs by Mean of HPLC–EC. J. Pharm. Biomed. Anal. 2005, 36, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.J.; Fillenz, M. Measurement of Noradrenaline Synthesis in Rat Hippocampal Synaptosomes Using HPLC with ECD. J. Neurosci. Methods 1985, 13, 231–238. [Google Scholar] [CrossRef]
- Saito, H.; Murai, S.; Abe, E.; Masuda, Y.; Itoh, T. Rapid and Simultaneous Assay of Monoamine Neurotransmitters and Their Metabolites in Discrete Brain Areas of Mice by HPLC with Coulometric Detection. Pharmacol. Biochem. Behav. 1992, 42, 351–356. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications; John Wiley & Sons Inc.: New York, NY, USA, 2001; ISBN 0-471-04372-9. [Google Scholar]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A Review on Electrochemical Detection of Serotonin Based on Surface Modified Electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef]
- Błaszczyk, J.W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front. Neurosci. 2016, 10, 239. [Google Scholar] [CrossRef]
- Nägga, K.; Bogdanovic, N.; Marcusson, J. GABA Transporters (GAT-1) in Alzheimer’s Disease. J. Neural Transm. 1999, 106, 1141–1149. [Google Scholar] [CrossRef]
- Panrod, K.; Tansirikongkol, A.; Panapisal, V. Comparison of Validated High-Performance Liquid Chromatography Methods Using Two Derivatizing Agents for Gamma-Aminobutyric Acid Quantification. Thai J. Pharm. Sci. 2016, 40, 203–208. [Google Scholar]
- Narmadha, M.; Noel, M.; Suryanarayanan, V. Relative Deactivation of Boron-Doped Diamond (BDD) and Glassy Carbon (GC) Electrodes in Different Electrolyte Media Containing Substituted Phenols—Voltammetric and Surface Morphologic Studies. J. Electroanal. Chem. 2011, 655, 103–110. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Luo, Y.; Shang, J.; Jiang, X. Simultaneous Determination of Eleven Compounds Related to Metabolism of Bioamines in Rat Cortex and Hippocampus by HPLC-ECD with Boron-Doped Diamond Working Electrode. J. Pharm. Biomed. Anal. 2016, 118, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gotti, G.; Evrard, D.; Gros, P. Simultaneous Electrochemical Detection of Oxygen (O2) and Hydrogen Peroxide (H2O2) in Neutral Media. Int. J. Electrochem. Sci. 2023, 18, 100262. [Google Scholar] [CrossRef]
- Gotti, G.; Fajerwerg, K.; Evrard, D.; Gros, P. Kinetics of Dioxygen Reduction on Gold and Glassy Carbon Electrodes in Neutral Media. Int. J. Electrochem. Sci. 2013, 8, 12643–12657. [Google Scholar] [CrossRef]
- Xu, F.; Gao, M.; Shi, G.; Wang, L.; Zhang, W.; Xue, J.; Jin, L.; Jin, J. Simultaneous Detection of Monoamines in Rat Striatal Microdialysate at Poly(Para-Aminobenzoic Acid) Modified Electrode by High-Performance Liquid Chromatography. Anal. Chim. Acta 2001, 439, 239–246. [Google Scholar] [CrossRef]
- Guo, Y.-X.; Xia, C.-Y.; Yan, Y.; Han, Y.; Shi, R.; He, J.; Wang, Y.-M.; Wang, Z.-X.; Zhang, W.-K.; Xu, J.-K. Loganin Improves Chronic Unpredictable Mild Stress-Induced Depressive-like Behaviors and Neurochemical Dysfunction. J. Ethnopharmacol. 2023, 308, 116288. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chen, H.; Chen, K. Effects of Bone Marrow Mesenchymal Stem Cells on Myelin Repair and Emotional Changes of a Cuprizone-Induced Demyelination Model. J. Integr. Neurosci. 2023, 22, 40. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Lian, W.-W.; He, J.; He, X.-L.; Wang, Y.-M.; Pan, C.-H.; Li, M.; Zhang, W.-K.; Liu, L.-Q.; Xu, J.-K. Cornuside Ameliorates Cognitive Impairments in Scopolamine Induced AD Mice: Involvement of Neurotransmitter and Oxidative Stress. J. Ethnopharmacol. 2022, 293, 115252. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Moradi, M.; Pirsaheb, M.; Esrafili, A.; Jafari, A.J.; Gholami, M.; Vasseghian, Y.; Antolini, E.; Dragoi, E.-N. Enhanced Photocatalytic Inactivation of E. Coli by Natural Pyrite in Presence of Citrate and EDTA as Effective Chelating Agents: Experimental Evaluation and Kinetic and ANN Models. J. Environ. Chem. Eng. 2019, 7, 102906. [Google Scholar] [CrossRef]
- Broch, S.C.; Celia García Alvarez-Coque, M.; Broch, S.C.; Esteve-Romero, J.S. Liquid Chromatographic Determination of Some Thiazide Diuretics in Pharmaceuticals with a Sodium Dodecyl Sulfate Mobile Phase. Analyst 1998, 123, 301–306. [Google Scholar] [CrossRef]
- Umek, N.; Geršak, B.; Vintar, N.; Šoštarič, M.; Mavri, J. Dopamine Autoxidation Is Controlled by Acidic pH. Front. Mol. Neurosci. 2018, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B.; Gifu, E.-P.; Sandu, I.; Denoroy, L.; Parrot, S. Analysis of Microdialysate Monoamines, Including Noradrenaline, Dopamine and Serotonin, Using Capillary Ultra-High Performance Liquid Chromatography and Electrochemical Detection. J. Chromatogr. B 2014, 951–952, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Schou-Pedersen, A.M.V.; Hansen, S.N.; Tveden-Nyborg, P.; Lykkesfeldt, J. Simultaneous Quantification of Monoamine Neurotransmitters and Their Biogenic Metabolites Intracellularly and Extracellularly in Primary Neuronal Cell Cultures and in Sub-Regions of Guinea Pig Brain. J. Chromatogr. B 2016, 1028, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, D.; Vermeiren, Y.; Aerts, T.; De Deyn, P.P. Novel and Sensitive Reversed-Phase High-Pressure Liquid Chromatography Method with Electrochemical Detection for the Simultaneous and Fast Determination of Eight Biogenic Amines and Metabolites in Human Brain Tissue. J. Chromatogr. A 2014, 1353, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Pantiya, P.; Guiard, B.P.; Gotti, G. Sensitive and Fast Detection of Monoamines and Their Metabolites by High-Performance Liquid Chromatography Coupled with an Electrochemical Detector (HPLC-ECD) under Isocratic Conditions. Application to Intracerebral Microdialysis in Mice Treated by Fluoxetine and Atomoxetine. Chromatographia 2024, in press. [Google Scholar]
- Allen, S.A.; Rednour, S.; Shepard, S.; Pond, B.B. A Simple and Sensitive High-performance Liquid Chromatography–Electrochemical Detection Assay for the Quantitative Determination of Monoamines and Respective Metabolites in Six Discrete Brain Regions of Mice. Biomed. Chromatogr. 2017, 31, e3998. [Google Scholar] [CrossRef]
- Yardimci, A.; Ertugrul, N.U.; Ozgen, A.; Ozbeg, G.; Ozdede, M.R.; Ercan, E.C.; Canpolat, S. Effects of Chronic Irisin Treatment on Brain Monoamine Levels in the Hypothalamic and Subcortical Nuclei of Adult Male and Female Rats: An HPLC-ECD Study. Neurosci. Lett. 2023, 806, 137245. [Google Scholar] [CrossRef]
- Du, T.; Cui, T.; Qiu, H.; Wang, N.; Huang, D.; Jiang, X. Simultaneous Determination of Tryptophan, Kynurenine, Kynurenic Acid and Two Monoamines in Rat Plasma by HPLC-ECD/DAD. J. Pharm. Biomed. Anal. 2018, 158, 8–14. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Y.; Chen, Y.; Ma, M.; Tan, Y.; Tang, H.; Chen, B. Determination of Monoamine Neurotransmitters in Human Urine by Carrier-Mediated Liquid-Phase Microextraction Based on Solidification of Stripping Phase. Talanta 2015, 144, 356–362. [Google Scholar] [CrossRef]
- Lokhande, R.V.; Bhagure, G.R.; Dherai, A.J.; Naik, P.R.; Udani, V.P.; Desai, N.A.; Ashavaid, T.F. Analytical Method Validation for Estimation of Neurotransmitters (Biogenic Monoamines) from Cerebrospinal Fluid Using High Performance Liquid Chromatography. Ind. J. Clin. Biochem. 2022, 37, 85–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).