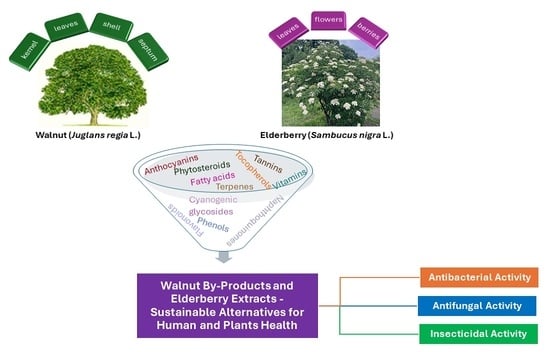
Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health
Abstract
1. Introduction
2. Walnut (Juglans regia L.) and Elderberry (Sambucus nigra L.) Characterization
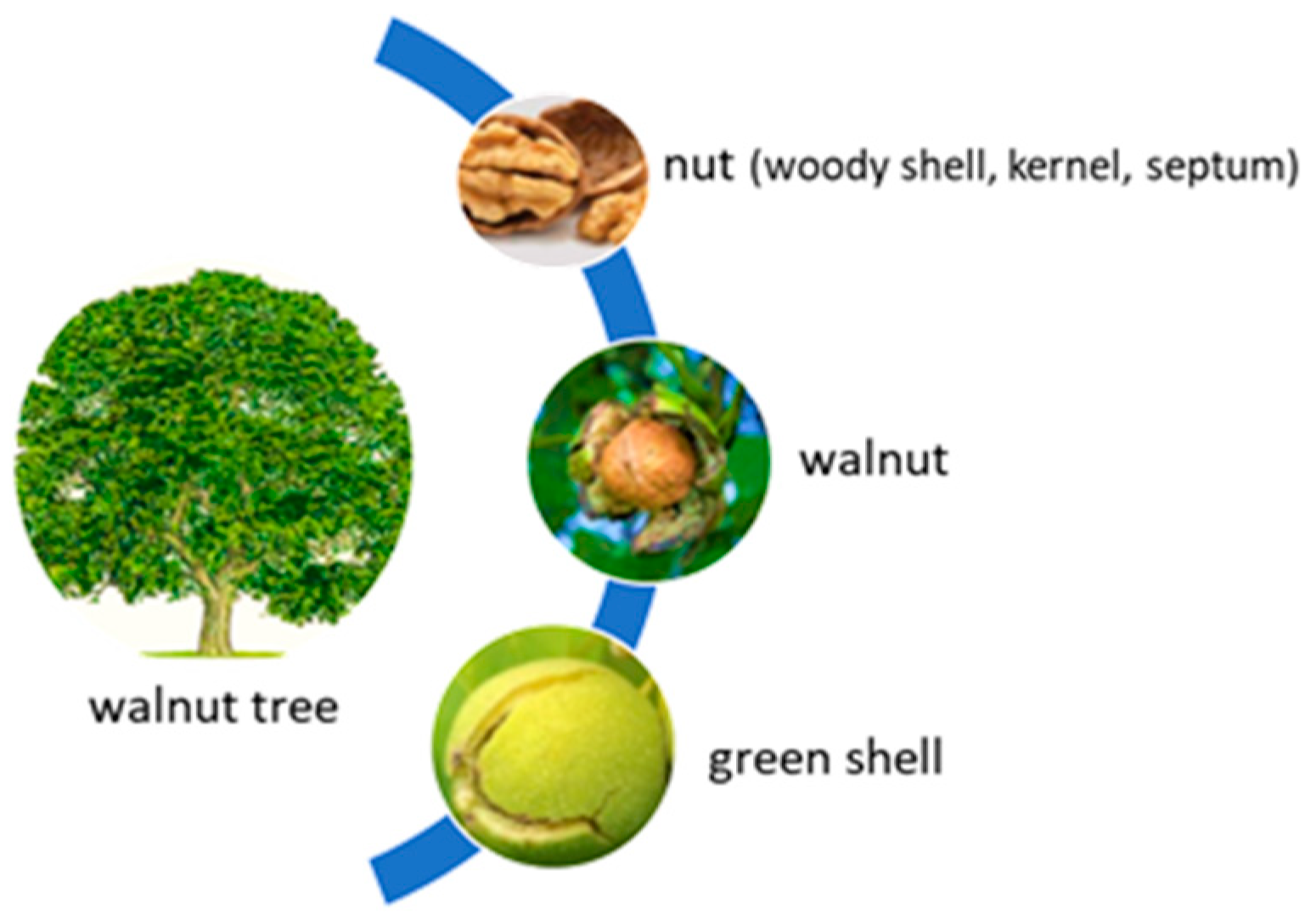
2.1. General Description
2.2. Chemical Composition
2.2.1. Chemical Composition of Walnut
- Chemical composition of the walnut kernel
- Chemical composition of walnut by-products
Chemical Composition of Walnut Leaves
Chemical Composition of Walnut Shell
Chemical Composition of the Walnut Septum
2.2.2. Chemical Composition of Elderberry
- Chemical composition of elderberry leaves
- Chemical composition of elderberry flowers
- Chemical composition of elderberries
3. Health Benefits and Applications of Walnut By-Products (Juglans regia L.) and Elderberry (Sambucus nigra L.) Extracts
3.1. Health Benefits and Applications of Walnut By-Products (Juglans regia L.) Extracts
3.1.1. Antibacterial Activity
3.1.2. Antifungal Activity
3.1.3. Insecticidal Activity
3.2. Health Benefits and Applications of Elderberry (Sambucus nigra L.) Extracts
3.2.1. Antibacterial Activity
3.2.2. Antifungal Activity
3.2.3. Insecticidal Activity
4. Common Applications—Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramawat, K.G.; Mérillon, J.-M. (Eds.) Bioactive Molecules and Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Tringali, C. (Ed.) Bioactive Compounds from Natural Sources. Isolation, Characterisation and Biological Properties; Taylor & Francis: London, UK, 2001. [Google Scholar]
- Colegate, S.M.; Molyneux, R.J. (Eds.) Bioactive Natural Products: Detection, Isolation, and Structural Determination; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of utilization vegetal extracts as natural plant protection products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Li, Y.; Chemat, F. Plant Based “Green Chemistry 2.0”; Springer: Singapore, 2019. [Google Scholar]
- Nicolescu, V.-N.; Rédei, K.; Vor, T.; Bastien, J.-C.; Brus, R.; Benčat, T.; Đodan, M.; Cvjetkovic, B.; Andrašev, S.; La Porta, N.; et al. A review of black walnut (Juglans nigra L.) ecology and management in Europe. Trees 2020, 34, 1087–1112. [Google Scholar] [CrossRef]
- Available online: https://agrobiznes.ro/2023-01-31-romania-ramane-cel-mai-mare-producator-de-nuci-din-ue-circa-20-000-de-ha-urmeaza-sa-intre-pe-rod/ (accessed on 19 September 2023).
- Available online: https://www.indexbox.io/search/production-walnut-romania/ (accessed on 5 October 2023).
- Institutul Național de Statistică. Producția Vegetală la Principalele Culturi în Anul 2021; Editura Institutului Național de Statistică: Bucharest, Romania, 2022. [Google Scholar]
- Popa, R.-G.; Bălăcescu, A.; Popescu, L.G. Organic walnut cultivation in intensive and super-intensive system—Sustainable investment. Case study: Gorj County, Romania. Sustainability 2023, 15, 1244. [Google Scholar] [CrossRef]
- Lozan, A.; Arndt, C. Report on the Status of Organic Agriculture and Industry in Romania; EkoConnect: Desden, Germany, 2022. [Google Scholar]
- Mocanu, M.L.; Amariei, S. Elderberries—A source of bioactive compounds with antiviral action. Plants 2022, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive compounds from elderberry: Extraction, health benefits, and food applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive potential of elderberry (Sambucus nigra L.): Antioxidant, antimicrobial activity, bioaccessibility and prebiotic potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ma, M.; Chen, Z.; Ma, A.; Li, S.; Xia, J.; Jia, Y. A review on extracts, chemical composition and product development of walnut Diaphragma juglandis fructus. Foods 2023, 12, 3379. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, M.; Sharma, M. A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. 2022, 88, 601–616. [Google Scholar] [CrossRef]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Mourabit, Y.; Bouyahya, A.; El Yadini, M.; Machich, O.; El Hajjaji, S.; El Boury, H.; Dakka, N.; et al. A review on medicinal uses, nutritional value, and antimicrobial, antioxidant, anti-inflammatory, antidiabetic, and anticancer potential related to bioactive compounds of J. regia. Food Rev. Int. 2023, 39, 6199–6249. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Food. 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Du, H.; Li, C.; Wen, Y.; Tu, Y.; Zhong, Y.; Yuan, Z.; Li, Y.; Liang, B. Secondary metabolites from pericarp of Juglans regia. Biochem. Syst. Ecol. 2014, 54, 88–91. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Davaran, S.; Moosavi-Movahedi, A.; Dastmalchi, S. Investigating the interaction of juglone (5-hydroxy-1, 4-naphthoquinone) with serum albumins using spectroscopic and in silico methods. J. Iran. Chem. Soc. 2017, 14, 1527–1540. [Google Scholar] [CrossRef]
- Tsasi, G.; Milošević-Ifantis, T.; Skaltsa, H. Phytochemical study of Juglans regia L. pericarps from Greece with a chemotaxonomic approach. Chem. Biodivers. 2016, 13, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Maleita, C.; Esteves, I.; Chim, R.; Fonseca, L.; Braga, M.E.M.; Abrantes, I.; de Sousa, H.C. Naphthoquinones from walnut husk residues show strong nematicidal activities against the root-knot nematode Meloidogyne hispanica. ACS Sustain. Chem. Eng. 2017, 5, 3390–3398. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut (Juglans regia L.) shell pyroligneous acid: Chemical constituents and functional applications. RSC Adv. 2018, 8, 22376. [Google Scholar] [CrossRef]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M.; Fabcic, J. Phenolics in walnut liqueur. Acta Hortic. 2007, 744, 451–454. [Google Scholar] [CrossRef]
- Upadhyay, V.; Kambhoja, S.; Harshaleena, A. Antifungal activity and preliminary phytochemical analysis of stem bark extracts of Juglans regia linn. Int. J. Pharm. Biol. Sci. Arch. 2010, 1, 442–447. [Google Scholar]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2019, 279, 279–287. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernández-Gutiérrez, A. Development of a rapid method to determine phenolic and other polar compounds in walnut by capillary electrophoresis–electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A 2008, 1209, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Hamedi, M.; Khodaiyan, F. Determination and characterization of kernel biochemical composition and functional compounds of Persian walnut oil. J. Food Sci. Technol. 2014, 51, 34–42. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Lipid class compositions, tocopherols and sterols of tree nut oils extracted with different solvents. J. Food Lipids 2008, 15, 81–96. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Pérez Rubio, A.G.; Pérez Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Rébufa, C.; Artaud, J.; Le Dréau, Y. Walnut (Juglans regia L.) oil chemical composition depending on variety, locality, extraction process and storage conditions: A comprehensive review. J. Food Compos. Anal. 2015, 110, 104534. [Google Scholar] [CrossRef]
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and quantification of major phenolic constituents in Juglans regia L. leaves: Healthy vs. infected leaves with Xanthomonas campestris pv. juglandis using HPLC-MS/MS. J. King Saud Univ. Sci. 2022, 34, 101890. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Pires, T.C.S.P.; Calhelha, R.C.; Alves, M.J.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crop. Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentao, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Nicu, A.I.; Pîrvu, L.; Stoian, G.; Vamanu, A. Antibacterial activity of ethanolic extracts from Fagus sylvatica L. and Juglans regia L. leaves. Farmacia 2018, 66, 483–486. [Google Scholar] [CrossRef]
- Bennacer, A.; Sahir-Halouane, F.; Aitslimane-Aitkaki, S.; Oukali, Z.; Oliveira, I.V.; Rahmouni, N.; Aissaoui, M. Structural characterization of phytochemical content, antibacterial, and antifungal activities of Juglans regia L. leaves cultivated in Algeria. Biocatal. Agric. Biotechnol. 2022, 40, 102304. [Google Scholar] [CrossRef]
- Nancy, P.; Manasi, M.; Varghese, A. Antiplaque activity of Juglans regia L. and characterization of juglone from Juglans regia L. Am. J. Biochem. Biotechnol. 2011, 7, 29–31. [Google Scholar] [CrossRef]
- Tociu, M.; Manolache, F.; Bălanucă, B.; Moroșan, A.; Stan, R. Superior valorisation of Juglans regia L. leaves of different maturity through the isolation of bioactive compounds. Molecules 2023, 28, 7328. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef]
- Wenzel, J.; Storer Samaniego, C.; Wang, L.; Burrows, L.; Tucker, E.; Dwarshuis, N.; Ammerman, M.; Zand, A. Antioxidant potential of Juglans nigra, black walnut, husks extracted using supercritical carbon dioxide with an ethanol modifier. Food Sci. Nutr. 2017, 5, 223–232. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Pandey, S.K.; Lal, M. A comparative study on antioxidant, anti-inflammatory, genotoxicity, antimicrobial activities and chemical composition of fruit and leaf essential oils of Litsea cubeba Pers from North-east India. Ind. Crops Prod. 2018, 125, 131–139. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Abdulwahid, M.Y.; Akinwande, A.A.; Kamarou, A.; Kamarou, M.; Romanovski, V.; Al-Qasem, I.A. The production of environmentally friendly building materials out of recycling walnut shell waste: A brief review. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Fordos, S.; Abid, N.; Gulzar, M.; Pasha, I.; Oz, F.; Shahid, A.; Khan, M.K.I.; Khaneghah, A.M.; Aadil, R.M. Recent development in the application of walnut processing by-products (walnut shell and walnut husk). Biomass Convers. Biorefinery 2023, 13, 14389–14411. [Google Scholar] [CrossRef]
- Kizatova, M.; Sultanova, M.; Baikenov, A.; Saduakas, A.; Akzhanov, N. Revealing the features of the composition of the walnut shell from the point of view of the possibility of its use in the food industry. East.-Eur. J. Enterp. Technol. 2022, 1, 49–55. [Google Scholar] [CrossRef]
- Genovese, C.; Cambria, M.T.; D’angeli, F.; Addamo, A.P.; Malfa, G.A.; Siracusa, L.; Pulvirenti, L.; Anfuso, C.D.; Lupo, G.; Salmeri, M. The double effect of walnut septum extract (Juglans regia L.) counteracts A172 glioblastoma cell survival and bacterial growth. Int. J. Oncol. 2020, 57, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Mateș, L.; Rusu, M.E.; Popa, D.-S. Phytochemicals and biological activities of walnut septum: A systematic review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Wang, D.; Mu, Y.; Dong, H.; Yan, H.; Hao, C.; Wang, X.; Zhang, L. Chemical constituents of the ethyl acetate extract from Diaphragma juglandis fructus and their inhibitory activity on nitric oxide production in vitro. Antioxidants 2018, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, Z.; Dai, S.; Che, X.; Liu, W. Identification and quantification of bioactive compounds in Diaphragma juglandis fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171727/nutrients (accessed on 5 October 2023).
- Mitroi, C.L.; Simescu, M.; Simescu, R.; Cugerean, M.I.; Cugerean, I.D.; Ştefan, I.D.; Popescu, S.G.; Velciov, A.B. Elderberry-functional product (review). J. Agroaliment. Process. Technol. 2022, 28, 268–272. [Google Scholar]
- Kołodziej, B.; Maksymiec, N.; Drożdżal, K.; Antonkiewicz, J. Effect of traffic pollution on chemical composition of raw elderberry (Sambucus nigra L.). J. Elem. 2012, 17, 67–78. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra extracts–natural antioxidants and antimicrobial compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Food. 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef] [PubMed]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W.; et al. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae). J. Etnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef] [PubMed]
- Kiprovski, B.; Malenčić, D.; Ljubojević, M.; Ognjanov, V.; Veberic, R.; Hudina, M.; Mikulic-Petkovsek, M. Quality parameters change during ripening in leaves and fruits of wild growing and cultivated elderberry (Sambucus nigra) genotypes. Sci. Hortic. 2021, 277, 109792. [Google Scholar] [CrossRef]
- Sandu Bălan (Tăbăcariu), A.; Patriciu, O.-I.; Ștefănescu, I.-A.; Ifrim, I.-L.; Fînaru, A.-L. Sambucus nigra L.: Characterization of Elderberry Extracts Obtained by Various Methods. In Proceedings of the Conference Proceedings Abstracts of the 18th International Conference of Constructive Design and Technological Optimization in Machine Building Field—OPROTEH 2023, Bacău, Romania, 11–13 May 2023. [Google Scholar]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Cvetanović, A.; Mahomoodally, F.M.; Radojković, M. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crop. Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Badim, H.; Salvador, Â.C.; Silvestre, A.J.D.; Santos, S.A.O.; Rocha, S.M.; Sousa, A.M.; Pereira, M.O.; Pereira Wilson, C.; Rocha, C.M.R.; et al. Chemical characterization of Sambucus nigra L. flowers aqueous extract and its biological implications. Biomolecules 2021, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Katragunta, K.; Wang, Y.H.; Ali, Z.; Srivedavyasasri, R.; Gafner, S.; Slimestad, R.; Khan, I.A. Chemical profiling and UHPLC-QToF analysis for the simultaneous determination of anthocyanins and flavonoids in Sambucus berries and authentication and detection of adulteration in elderberry dietary supplements using UHPLC-PDA-MS. J. Food Compos. Anal. 2022, 110, 104584. [Google Scholar] [CrossRef]
- Salvador, A.C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crop. Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia. J. Pharm. Anal. 2013, 3, 298–302. [Google Scholar] [CrossRef]
- Jaiswal, B.S.; Tailang, M. Juglans regia: A review of its traditional uses phytochemistry and pharmacology. Indo Am. J. Pharm. Res. 2017, 7, 390–398. [Google Scholar] [CrossRef]
- Mohan, S.; Panneerselvam, K. An investigation on antibacterial filler property of silver nanoparticles generated from Walnut shell powder by in situ process. Mater. Today Proc. 2021, 39, 368–372. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.G.; Swilaiman, S.; Ahmad, S.H.; Darogha, S.N. Antibacterial effect of Juglans regia against dental caries Streptococcus mutans. Pak. J. Med. Health Sci. 2020, 14, 1313–1317. [Google Scholar]
- Gomes, F.; Martins, N.; Ferreira, I.C.F.R.; Henriques, M. Anti-biofilm activity of hydromethanolic plant extracts against Staphylococcus aureus isolates from bovine mastitis. Heliyon 2019, 5, e01728. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]
- Rather, A.M.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Jabli, M.; Sebeia, N.; Boulares, M.; Faidi, K. Chemical analysis of the characteristics of Tunisian Juglans regia L. fractions: Antibacterial potential, gas chromatography–mass spectroscopy and a full investigation of their dyeing properties. Ind. Crop. Prod. 2017, 108, 690–699. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Moghadam, H.N.; Mahdavi-Ourtakand, M. Evaluating the anti-biofilm and antibacterial effects of Juglans regia L. extracts against clinical isolates of Pseudomonas aeruginosa. Microb. Pathog. 2018, 118, 285–289. [Google Scholar] [CrossRef]
- Huo, J.; Zhao, Z.; Hua, Z.; Fan, J.; Du, J.; Guo, B. Evaluation of Juglans regia L., root for wound healing via antioxidant, antimicrobial and anti-inflammatory activity. Indian J. Biochem. Biophys. 2020, 57, 304–311. [Google Scholar]
- D’Angeli, F.; Malfa, G.A.; Garozzo, A.; Li Volti, G.; Genovese, C.; Stivala, A.; Nicolosi, D.; Attanasio, F.; Bellia, F.; Ronsisvalle, S.; et al. Antimicrobial, antioxidant, and cytotoxic activities of Juglans regia L. pellicle extract. Antibiotics 2021, 10, 159. [Google Scholar] [CrossRef]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2019, 35, 2076–2081. [Google Scholar] [CrossRef]
- Kavuncuoglu, H.; Kavuncuoglu, E.; Karatas, S.M.; Benli, B.; Sagdic, O.; Yalcin, H. Prediction of the antimicrobial activity of walnut (Juglans regia L.) kernel aqueous extracts using artificial neural network and multiple linear regression. J. Microbiol. Methods 2018, 148, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Duda-Seiman, C.; Sinitean, A.; Negrea, P.; Negrea, A.-G.; Dugăeşescu, D.; Duda-Seiman, D.; Avram, S.; Putz, A.-M.; Bumbăcilă, B.; Irimia, G.-L.; et al. Extraction and antibacterial activity of natural compounds from walnuts (Juglans regia). New Front. Chem. 2017, 26, S4_P3. [Google Scholar]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef]
- Kheddouma, A.; Cherraben, Y. In vitro antimicrobial activity of Salvadora persica and Juglans regia extracts against microbial strains from oral cavity. Biocatal. Agric. Biotechnol. 2021, 33, 102003. [Google Scholar] [CrossRef]
- Jafer, F.N.; Naser, L. The biological activity of aqueous and methanolic extracts of Juglans regia on yeasts and pathologic bacteria. Arch. Clin. Microbiol. 2020, 11, 113. [Google Scholar] [CrossRef]
- Aldawood, T.; Alyousef, A.; Alyousef, S.; Aldosari, N.; Hussam, S.; Alhadad, A.; Bhaian, F.; Eldeen, D.S.; Abdul, N.S. Antibacterial effect of Juglans regia L bark extract at different concentrations against human salivary microflora. J. Oral Med. Oral Surg. Oral Pathol. Oral Radiol. 2017, 3, 214–217. [Google Scholar] [CrossRef]
- Mutha, M.; Deshpande, R.R.; Shep, S.; Deshpande, N.R.; Torne, R. Comparative evaluation of antibacterial properties of different extracts of Juglans regia (walnut) & Erethia laevis (Ajaan Vruksh) against salivary microflora. Int. J. Pharm. Clin. Res. 2015, 7, 151–153. [Google Scholar]
- Alkhawajah, A.M. Studies on the antimicrobial activity of Juglans regia. Am. J. Chin. Med. 1997, 25, 175–180. [Google Scholar] [CrossRef]
- Upadhyay, V.; Kambhoja, S.; Harsha, L.K. Antibacterial activity and preliminary phytochemical analysis of stem bark extract of Juglans regia linn. Pharmacologyonline 2010, 3, 274–279. [Google Scholar]
- Liu, S.; Cheng, S.; Jia, J.; Cui, J. Resource efficiency and environmental impact of juglone in Pericarpium Juglandis: A review. Front. Environ. Sci. 2022, 10, 999059. [Google Scholar] [CrossRef]
- Sandu Bălan (Tăbăcariu), A.; Patriciu, O.-I.; Ștefănescu, I.-A.; Ifrim, I.-L.; Fînaru, A.-L. Walnut (Juglans regia L.): By-Products Analysis and Capitalization. In Book of Abstracts of Conferința Națională de Chimie, ed., XXXVI, Călimănești-Căciulata, Romania, 4–7 October 2022. Available online: http://administrare.chimie.upb.ro/schr/doc/evenimente/2022/10/04/1/volum-de-rezumate/doc.pdf (accessed on 10 January 2024).
- Sandu Bălan (Tăbăcariu), A.; Patriciu, O.-I.; Ștefănescu, I.-A.; Ifrim, I.-L.; Fînaru, A.-L. Walnut (Juglans regia L.)—Present and Perspectives on Phytosanitary Activity. In Book of Abstracts of 5th International Conference of the Doctoral School—Excellence in Doctoral Studies through Innovation, Convergence and Interdisciplinarity, “Gheorghe Asachi” Technical University of Iasi, Iasi, Romania, 18–20 May 2022. Available online: https://conferinta-csd.tuiasi.ro/wp-content/uploads/2022/05/Book_of_Abstracts_CSD2022_WEB-1.pdf (accessed on 10 January 2024).
- Ameziane, N.; Boubaker, H.; Boudyach, H.; Msanda, F.; Jilal, A.; Ait Benaoumar, A. Antifungal activity of Moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 2007, 27, 273–277. [Google Scholar] [CrossRef]
- Wianowska, D.; Garbaczewska, S.; Cieniecka-Roslonkiewicz, A.; Dawidowicz, A.L.; Jankowska, A. Comparison of antifungal activity of extracts from different Juglans regia cultivars and juglone. Microb. Pathog. 2016, 100, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sehajpal, A.; Arora, S.; Kaur, P. Evaluation of plant extracts against Rhizoctonia solani causing sheath blight of rice. J. Plant Prot. Sci. 2009, 1, 25–30. [Google Scholar]
- Sadeghnezhad, R.; Enayati, A.; Ebrahimzadeh, M.A.; Azarnoosh, M.; Fazeli-Dinan, M. Toxicity and anti-feeding effects of walnut (Juglans regia L.) extract on Sitophilus oryzae L. (Coleoptera: Curculionidae). Fresenius Environ. Bull. 2020, 29, 325–331. [Google Scholar]
- Petrikovszki, R.; Tóthné Bogdányi, F.; Tóth, F.; Nagy, P. First report on the effect of aqueous extracts of Hungarian organic mulch materials on entomopathogenic and slug-parasitic nematodes. Acta Phytopathol. Entomol. Hung. 2019, 54, 279–288. [Google Scholar] [CrossRef]
- Erdogan, P. Oviposition deterrent activities of some plant extracts against tomato leaf miner, Tuta Absoluta meyrick (Lepidoptera: Gelehiidae). J. Bacteriol. Mycol. Open Access 2019, 7, 139–142. [Google Scholar] [CrossRef][Green Version]
- Civelek, H.S.; Çolak, A.M. Effects of some plant extracts and bensultap on Trichoferus griseus (Fabricius, 1792) (Coleoptera: Cerambycidae). World J. Agric. Sci. 2008, 4, 721–725. [Google Scholar]
- Jovanović, Z.; Kostić, M.; Popović, Z. Grain-protective properties of herbal extracts against the bean weevil Acanthoscelides obtectus Say. Ind. Crop. Prod. 2007, 26, 100–104. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Widhalm, J.R. Agricultural uses of juglone: Opportunities and challenges. Agronomy 2020, 10, 1500. [Google Scholar] [CrossRef]
- Khan, A.A.; Fazili, A.B.A.; Bhat, S.A.; Bhat, W.F.; Asghar, M.N.; Khan, M.S.; Bano, B. Purification, characterization and studies of a novel cysteine protease inhibitor from Juglans regia: Implications as a potential biopesticide. J. King Saud Univ. Sci. 2022, 34, 101829. [Google Scholar] [CrossRef]
- Terzić, M.; Majkić, T.; Beara, I.; Zengin, G.; Miljić, U.; Đurović, S.; Mollica, A.; Radojković, M. Elderberry (Sambucus nigra L.) wine as a novel potential functional food product. Food Biosci. 2022, 50, 102047. [Google Scholar] [CrossRef]
- Ramanauskiene, K.; Inkeniene, A.M.; Puidokaite, E.; Grigonis, A. Quality analysis of semisolid formulations with the liquid extract of elderflower (Sambucus nigra L.). Acta Pol. Pharm. 2019, 76, 1061–1071. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Barriga, A.; Albericio, F.; Romero, M.S.; Guzmán, F. Identification of peptides in flowers of Sambucus nigra with antimicrobial activity against aquaculture pathogens. Molecules 2018, 23, 1033. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Czyżowska, A.; Kręgiel, D. Antibacterial and antiadhesive activities of extracts from edible plants against soft drink spoilage by Asaia spp. J. Food Prot. 2017, 80, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Cioch, M.; Satora, P.; Skotniczny, M.; Semik-Szczurak, D.; Tarko, T. Characterisation of antimicrobial properties of extracts of selected medicinal plants. Pol. J. Microbiol. 2017, 66, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plants Res. 2010, 4, 1805–1809. [Google Scholar] [CrossRef]
- Salamon, I.; Şimşek Sezer, E.N.; Kryvtsova, M.; Labun, P. Antiproliferative and antimicrobial activity of anthocyanins from berry fruits after their isolation and freeze-drying. Appl. Sci. 2021, 11, 2096. [Google Scholar] [CrossRef]
- Pehlivan Karakaş, F.; Yildirim, A.; Türker, A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turk. J. Biol. 2021, 36, 641–652. [Google Scholar] [CrossRef]
- Tosun, M.N.; Demirel Zorba, N.N.; Yüceer, Y.K. Anti-quorum sensing and antitumor activity of Prunella vulgaris, Sambucus nigra, Calendula officinalis: Potential use in food industry. J. Microbiol. Biotechnol. Food Sci. 2021, 10, e2774. [Google Scholar] [CrossRef]
- Rodino, S.; Butu, A.; Butu, M.; Cornea, P.C. Comparative studies on antibacterial activity of licorice, elderberry and dandelion. Dig. J. Nanomater. Biostruct. 2015, 10, 947–955. [Google Scholar]
- Mohammadsadeghi, S.; Malekpour, A.; Zahedi, S.; Eskandari, F. The antimicrobial activity of elderberry (Sambucus nigra L.) extract against gram positive bacteria, gram negative bacteria and yeast. Res. J. Appl. Sci. 2013, 8, 240–243. [Google Scholar]
- Kačániová, M.; Miklášová, K.; Kunová, S.; Galovičová, L.; Borotová, P.; Válková, V.; Žiarovská, J.; Terentjeva, M. Antimicrobial and antioxidant activity of black elder, stinging nettle, marigold and ribwort plantain. Sci. Pap. Anim. Sci. Biotechnol. 2021, 54, 136–142. [Google Scholar]
- Allison, B.J.; Allenby, M.C.; Bryant, S.S.; Min, J.E.; Hieromnimon, M.; Joyner, P.M. Antibacterial activity of fractions from three Chumash medicinal plant extracts and in vitro inhibition of the enzyme enoyl reductase by the flavonoid jaceosidin. Nat. Prod. Res. 2016, 31, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Rodino, S.; Negoescu, C.; Butu, M.; Cornea, P.C. Preliminary investigation regarding the antifungal activity of Sambucus nigra extracts. Anim. Sci. Biotechnol. 2013, 70, 391–392. [Google Scholar]
- Puia, C.E.; Miclea, R.; Toader, I.; Cornoiu, I. Biological control of potential toxigenic fungi in straw used as bedding material for swine. Anim. Sci. Biotechnol. 2012, 69, 335–336. [Google Scholar]
- Jankowska, B.; Wojciechowicz-Żytko, E. Effect of aqueous extracts of black alder (Alnus glutinosa (LINNAEUS, 1753) GAERTNER, 1791) and elder (Sambucus nigra LINNAEUS, 1753) on the occurrence of Brevicoryne brassicae LINNAEUS, 1758 (Hemiptera, Aphidoidea), its parasitoid Diaeretiella rapae (M’INTOSH, 1855) (Hymenoptera, Ichneumonoidea) and predatory Syrphidae on white cabbage. Pol. J. Entomol. 2016, 85, 237–246. [Google Scholar] [CrossRef]
- Ahmad, M.; Saeed, F.; Mehjabeen; Jahan, N. Evaluation of insecticidal and anti-oxidant activity of selected medicinal plants. J. Pharmacogn. Phytochem. 2013, 2, 153–158. [Google Scholar]
- Ertürk, O. Antifeedant and toxicity effects of some plant extracts on Thaumetopoae solitaria Frey. (Lep.: Thaumetopoeidae). Turk. J. Biol. 2006, 30, 51–57. [Google Scholar]
- Moreira, C.D.; Santos, T.B.; Freitas, R.H.C.N.; Pacheco, P.A.F.; da Rocha, D.R. Juglone: A versatile natural platform for obtaining new bioactive compounds. Curr. Top. Med. Chem. 2021, 21, 2018–2045. [Google Scholar] [CrossRef]
- Sharma, N.; Ghosh, P.; Sharma, U.K.; Sood, S.; Sinha, A.K.; Gulati, A. Microwave-assisted efficient extraction and stability of juglone in different solvents from Juglans regia: Quantification of six phenolic constituents by validated RP-HPLC and evaluation of antimicrobial activity. Anal. Lett. 2009, 42, 2592–2609. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Zou, G.M.; Xi, M.H.; Hou, Y.J.; Shen, H.Y.; Ao, J.F.; Li, M.; Wang, J.; Luo, A.W. Juglone inhibits Listeria monocytogenes ATCC 19115 by targeting cell membrane and protein. Foods 2022, 11, 2558. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.F.C.; Stefani, V.; Brandeli, A. Use of 1,4-naphthoquinones for control of Erwinia carotovora. Can. J. Microbiol. 2004, 50, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Al-Shaikh, K.M.A.; Sobahi, T.R.; Khan, K.A.; Asiri, A.M. An Efficient approach to the synthesis of highly congested 9,10-dihydrophenanthrene-2,4-dicarbonitriles and their biological evaluation as antimicrobial agents. Molecules 2013, 18, 15704–15716. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, S.; Wang, M.; Peng, Y.; Dong, Y.; Li, X. Anticomplement and antimicrobial activities of flavonoids from Entada phaseoloides. Nat. Prod. Commun. 2012, 7, 867–871. [Google Scholar] [PubMed]
- de Almeida Roger, J.; Magro, M.; Spagnolo, S.; Bonaiuto, E.; Baratella, D.; Fasolato, L.; Vianello, F. Antimicrobial and magnetically removable tannic acid nanocarrier: A processing aid for Listeria monocytogenes treatment for food industry applications. Food Chem. 2018, 267, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, I.; Costa, R.; Madureira, S.; Borges, S.; Oliveira, A.L.; Pintado, M.; Baptista-Silva, S. Tannic acid tailored-made microsystems for wound infection. Int. J. Mol. Sci. 2023, 24, 4826. [Google Scholar] [CrossRef] [PubMed]
- Kernou, O.-N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of rosmarinic acid with its derivatives in the treatment of microbial pathogens. Molecules. 2023, 28, 4243. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.V.; Pereira, N.R.; Pereira, R.C.G.; Duarte, L.P.; Takahashi, J.A.; Silva, R.R. Synthesis and antimicrobial activity of ursolic acid ester derivatives. Chem. Biodivers. 2022, 19, e202100566. [Google Scholar] [CrossRef]
- Shukla, Y.N.; Srivastava, A.; Kumar, S.; Kumar, S. Phytotoxic and antimicrobial constituents of Argyreia speciosa and Oenothera biennis. J. Ethnopharmacol. 1999, 67, 241–245. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal activity of gallic acid in vitro and in vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.-L.; Gambari, R.; Kok, S.H.-L.; Lam, K.-H.; Tang, J.C.-O.; Bian, Z.-X.; Lee, K.K.-H.; Chui, C.-H. Non-toxic agarose/gelatin-based microencapsulation system containing gallic acid for antifungal application. Int. J. Mol. Med. 2015, 35, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-M.-C.; Seo, D.-J.; Lee, H.-B.; Kim, I.-S.; Kim, K.-Y.; Park, R.-D.; Jung, W.-J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar] [CrossRef]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of gallic acid derivatives. Arab. J. Chem. 2017, 10, S2870–S2880. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic acid and its derivatives: Antimicrobial drugs towards microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Gandhi, B.; Juliya, J.; Dileep, V.; Rajeswari Batchu, U.; Misra, S.; Kaki, S.S. Antioxidant and biological activities of novel structured monoacylglycerol derivatives with phenolic acids. Eur. J. Lipid Sci. Technol. 2021, 123, 2100055. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The role of hydroxycinnamic acid amide pathway in plant immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef]
- Wijayanti, E.D.; Safitri, A.; Siswanto, D.; Triprisila, L.F.; Fatchiyah, F. Antimicrobial activity of ferulic acid in Indonesian purple rice through toll-like receptor signaling. Makara J. Sci. 2021, 25, 247–257. [Google Scholar] [CrossRef]
- de Morais, M.C.; Perez-Castillo, Y.; Silva, V.R.; Santos, L.S.; Soares, M.B.P.; Bezerra, D.P.; de Castro, R.D.; de Sousa, D.P. Cytotoxic and antifungal amides derived from ferulic acid: Molecular docking and mechanism of action. Biomed Res. Int. 2021, 2021, 3598000. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Cibelli, F.; Baiano, A.; Carlucci, A.; Raimondo, M.L.; Campaniello, D.; Viggiani, I.; Bevilacqua, A.; Corbo, M.R. Removal ability and resistance to cinnamic and vanillic acids by fungi. Microorganisms 2020, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 13–14. [Google Scholar] [CrossRef]
- Sadeghi-Ghadi, Z.; Vaezi, A.; Ahangarkani, F.; Ilkit, M.; Ebrahimnejad, P.; Badali, H. Potent in vitro activity of curcumin and quercetin co-encapsulated in nanovesicles without hyaluronan against Aspergillus and Candida isolates. J. Mycol. Med. 2020, 30, 101014. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.-Y.; Xiong, S.-J.; Li, G.; Guo, C.-Q.; Zhou, J.; Ma, J.-W.; Yin, J.-L.; Liu, Y.-Q.; Zhu, Y.-X. Antifungal activity of ginger rhizome extract against Fusarium solani. Horticulturae 2022, 8, 983. [Google Scholar] [CrossRef]
- Yin, J.A.; Peng, X.D.; Lin, J.; Zhang, Y.; Zhang, J.; Gao, H.; Tian, X.; Zhang, R.; Zhao, G. Quercetin ameliorates Aspergillus fumigatus keratitis by inhibiting fungal growth, toll-like receptors and inflammatory cytokines. Int. Immunopharmacol. 2021, 93, 107435. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, F.; Darzi, G.N.; Mohammadi, M. Extraction and purification of ursolic acid from the apple peel and in vitro assessment of the biochemical antibacterial, antioxidant and wound healing characteristics. Appl. Food Biotechnol. 2022, 9, 17–30. [Google Scholar] [CrossRef]
- Hassan, S.; Hamed, S.; Almuhayawi, M.; Hozzein, W.; Selim, S.; AbdElgawad, H. Bioactivity of ellagic acid and velutin: Two phenolic compounds isolated from marine algae. Egypt. J. Bot. 2021, 61, 219–231. [Google Scholar] [CrossRef]
- Tavares, W.S.; Martin-Pastor, M.; Tavares, A.G.; Sousa, F.F.O. Biopharmaceutical activities related to ellagic acid, chitosan, and zein and their improvement by association. J. Food Sci. 2018, 83, 2970–2975. [Google Scholar] [CrossRef]
- Ielciu, I.; Niculae, M.; Pall, E.; Barbalata, C.; Tomuta, I.; Olah, N.-K.; Burtescu, R.F.; Benedec, D.; Oniga, I.; Hanganu, D. Antiproliferative and antimicrobial effects of Rosmarinus officinalis L. loaded liposomes. Molecules 2022, 27, 3988. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Chapter 9—β-Sitosterol as a functional bioactive. In A Centum of Valuable Plant Bioactives, 1st ed.; Mushtaq, M., Anwar, F., Eds.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 193–212. [Google Scholar]

| Compounds Class | Compound Name | References |
|---|---|---|
| Tannins | Glansreginin A | [27] |
| Ellagic acid derivatives | [27,29,30] | |
| Flavonoids | Procyanidin dimer 1 | [27,34] |
| Procyanidin trimer | [27,29,30,34] | |
| Catechin | [27,34] | |
| Hydroxybenzoic acids | Gallic acid | [27,29,30] |
| Hydroxycinnamic acids | 3-O-p-Coumaroylquinic acid | [27,29,30,34] |
| Ferulic acid glucoside | ||
| Chlorogenic acid (3-O-Caffeoylquinic acid) | ||
| Tocopherols | α-Tocopherol | [28,31,34] |
| γ-Tocopherol | ||
| δ-Tocopherol | ||
| Phytosteroids | Campesterol | [28,32,34] |
| β-Sitosterol | ||
| Stigmasterol | ||
| Avenasterol | ||
| Triterpenes derivatives | Cycloartenol | [33,34] |
| 2,4-Methyl cycloartenol | ||
| Aliphatic alcohols | Docosanol | |
| Tetracosanol | ||
| Hexacosanol | ||
| Fatty acids | Linoleic acid | [34] |
| Oleic acid | ||
| Linolenic acid | ||
| Palmitic acid |
| Compounds Class | Compound Name | References |
|---|---|---|
| Hydroxycinnamic acids | Neochlorogenic acid (5-O-Caffeoylquinic acid) | [35,36,37,38,39,40,42] |
| 3-p-Cumaroylquinic acid | ||
| Ferulic acid | ||
| Caffeic acid | ||
| p-Coumaric acid derivatives | ||
| Flavonoids | (+) Catechin | |
| (-) Epicatechin | ||
| Santin | ||
| Myricetin derivatives | ||
| Quercetin derivatives | ||
| Quercetin | ||
| Kaempferol derivatives | ||
| Naphthoquinones | Juglone (5-Hydroxy-1,4-naphthoquinone) | [34,35,41,42] |
| Hydrojuglone | ||
| Hydrojuglone derivatives | ||
| 1,4-Naphthoquinone |
| Compounds Class | Compound Name | References |
|---|---|---|
| Tannins | Ellagic acid | [20,43,44,45,46,49] |
| Tannic acid | ||
| Naphthoquinones | Juglone | [20,43,44,45,46,48] |
| 3-Methoxy-juglone | ||
| 3-Ethoxy-juglone | ||
| 8-Hydroxyquinoline | ||
| 1,4-Naphthoquinone | ||
| 5,8-Dihydroxy-1,4-naphthoquinone | ||
| 2-Hidroxi-1,4-naphthoquinone | ||
| Naphthoquinone glycosides | 1,4,5-Trihydroxynaphthalene-1,4-di-β-d-glucopyranoside | |
| 1,4,5-Trihydroxynaphthalene-1,5-di-O-β-d-glucopyranoside | ||
| 1,4,8-Trihydroxynaphthalene-1-O-β-d-glucopyranoside | ||
| Naphthalenone | (4R)-3,4-Dihydro-4-butoxy-5-hydroxy-naphthalen-1(2H)-one | |
| Tetralones | Regiolona | [20,43,44,45,46] |
| 5,8-Dihydroxy-4-methoxi-α-tetralone | ||
| 4,5-Dihydroxy-α-tetralone | ||
| Sclerone | ||
| Juglanone | ||
| Hydroxybenzoic acids | Gallic acid | [20,43,44,45,46,47,48,49] |
| Vanillic acid | ||
| Syringic acid | ||
| 2,3-Dihydroxybenzoic acid | ||
| Tyrosol | ||
| Hydroxycinnamic acids | Ferulic acid | |
| Sinapic acid | ||
| Rosmarinic acid | ||
| Flavonoids | (+) Catechin | [20,43,44,45,46,48] |
| Quercetin | ||
| (−) Epicatechin | [20,43,44,45,46,48] | |
| Myricetin | ||
| Apigenin | ||
| Rutin | ||
| Phytosteroids | β-Sitosterol | [19,20,43,44,45,46,47,48] |
| Stigmasterol | ||
| Daucosterol | ||
| Campesterol | ||
| Triterpenoids | Olenolic acid | [20,43,44,45,46,48] |
| Oleanolic acid | ||
| Corosolic acid | ||
| Ursolic acid | ||
| Sesquiterpenes | (+) Dehydrovomifoliol | |
| Blumenol A | ||
| Vitamins | Ascorbic acid | [20,49] |
| α-Tocopherol |
| Compounds Class | Compound Name | References |
|---|---|---|
| Hydroxybenzoic acids | Gallic acid | [50,51,52,53,54] |
| Protocatechuic acid | ||
| Vanillic acid | ||
| Tannins | 2-Galloyl hexose | [50,51,52,53] |
| Ellagic acid | ||
| Ellagic acid hexoside | ||
| Flavonoids | Epigallocatechin | [50,51,54] |
| Catechin | ||
| Epicatechin | ||
| Epigallocatechin gallate | ||
| Quercetin-3-O-glucoside | ||
| Quercitrin (Quercetin-3-O-rhamnoside) | ||
| Hydroxycinnamic acids | p-Coumaric acid | [50,51,52,53,54] |
| Compounds Class | Compound Name | References |
|---|---|---|
| Hydroxycinnamic acids | 5-p-Coumaroylquinic acid | [12,13,14,18,58,59,60,61,62] |
| Caffeic acid | ||
| Chlorogenic acid | ||
| Neochlorogenic acid | ||
| Flavonoids | Rutinoside-3-izorhamnetin | |
| Rutinoside-3-kaempferol | ||
| Quercetin acetyl glucoside | ||
| Quercetin-rutinoside | ||
| Anthocyanins | Cyanidin-3-p-coumaroyl sambubioside | |
| Cyanidin-glucoside | ||
| Pelargonidin-rutinoside | ||
| Cyanogenic glycosides | Sambunigrin | [58,59] |
| Compounds Class | Compound Name | References |
|---|---|---|
| Phenols and derivatives | p-Hydroxybenzoic acid | [18,58,64,65] |
| Protocatechuic acid | ||
| Gentisic acid | ||
| p-Coumaric acid | ||
| Quinic acid | ||
| Ferulic acid | ||
| Flavonoids | Naringenin | [58,60,64,65] |
| Catechin | ||
| Epicatechin | ||
| Quercetin | ||
| Isorhamnetin | ||
| Neochlorogenic acid | ||
| Kaempferol-3-O-glucoside | ||
| Quercetin-3-O-hexoside | ||
| Kaempferol | ||
| Rutin | ||
| Tannins | Ellagic acid | [18,60,64] |
| Terpenes | Ursolic acid | [64] |
| Compounds Class | Compound Name | References |
|---|---|---|
| Phenols | Chlorogenic acid | [12,13,14,18,60,62,66,67] |
| Neochlorogenic acid | ||
| 4-Caffeoylquinic acid | ||
| Flavonoids | Izorhamnetin-3-glucoside | |
| Izorhamnetin-3-rutinoside | ||
| Quercetin-acetyl-hexoside | ||
| Quercetin-glucoside | ||
| Quercetin-rutinoside | ||
| Anthocyanins | Cyanidin-sambubioside | |
| Cyanidin-diglucoside | ||
| Cyanidin-glucoside | ||
| Cyanidin-3-p-coumaroil sambubioside-5-glucoside | ||
| Fatty acids | Hexadecenoic acid | |
| Octadecanoic acid | ||
| Eicosanoic acid | ||
| Phytosterols | Campesterol | |
| Stigmasterol | ||
| β-Sitosterol | ||
| Triterpenoids | β-Amyrin | |
| Oleanolic acid | ||
| Ursolic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu-Bălan, A.; Ifrim, I.-L.; Patriciu, O.-I.; Ștefănescu, I.-A.; Fînaru, A.-L. Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health. Molecules 2024, 29, 498. https://doi.org/10.3390/molecules29020498
Sandu-Bălan A, Ifrim I-L, Patriciu O-I, Ștefănescu I-A, Fînaru A-L. Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health. Molecules. 2024; 29(2):498. https://doi.org/10.3390/molecules29020498
Chicago/Turabian StyleSandu-Bălan (Tăbăcariu), Anca, Irina-Loredana Ifrim, Oana-Irina Patriciu, Ioana-Adriana Ștefănescu, and Adriana-Luminița Fînaru. 2024. "Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health" Molecules 29, no. 2: 498. https://doi.org/10.3390/molecules29020498
APA StyleSandu-Bălan, A., Ifrim, I.-L., Patriciu, O.-I., Ștefănescu, I.-A., & Fînaru, A.-L. (2024). Walnut By-Products and Elderberry Extracts—Sustainable Alternatives for Human and Plant Health. Molecules, 29(2), 498. https://doi.org/10.3390/molecules29020498