An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects
Abstract
1. Introduction
The Endocannabinoid System: Cannabinoid Receptors and Endocannabinoids
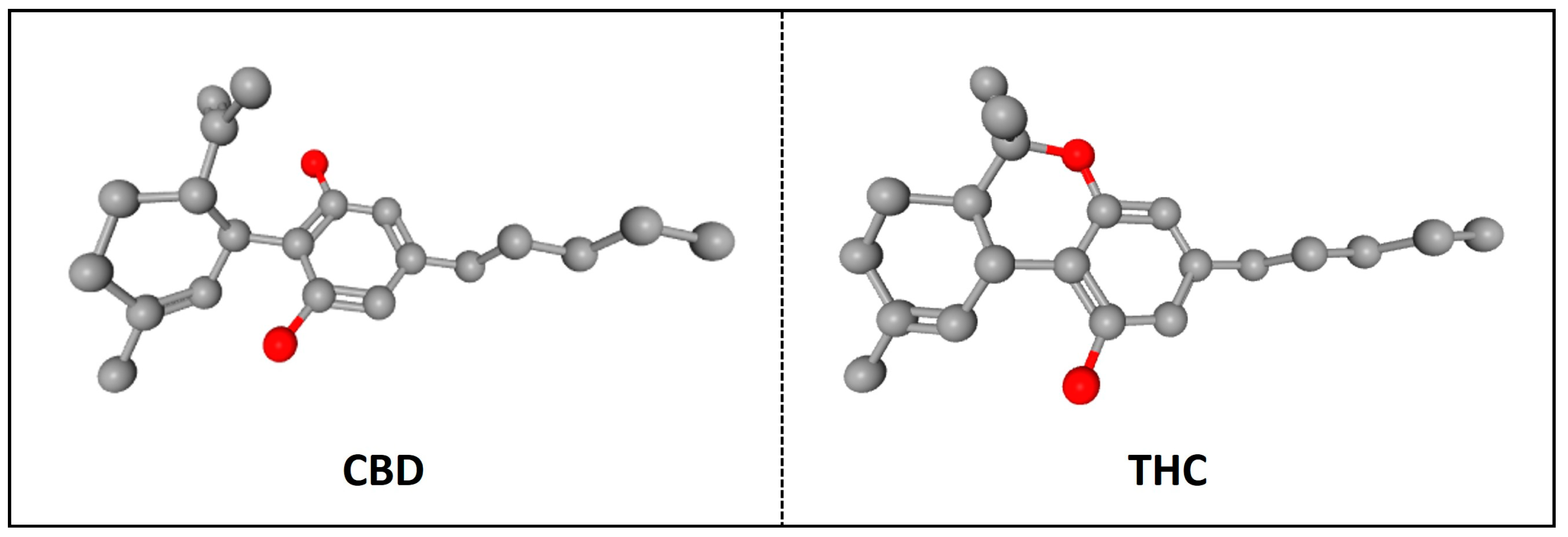
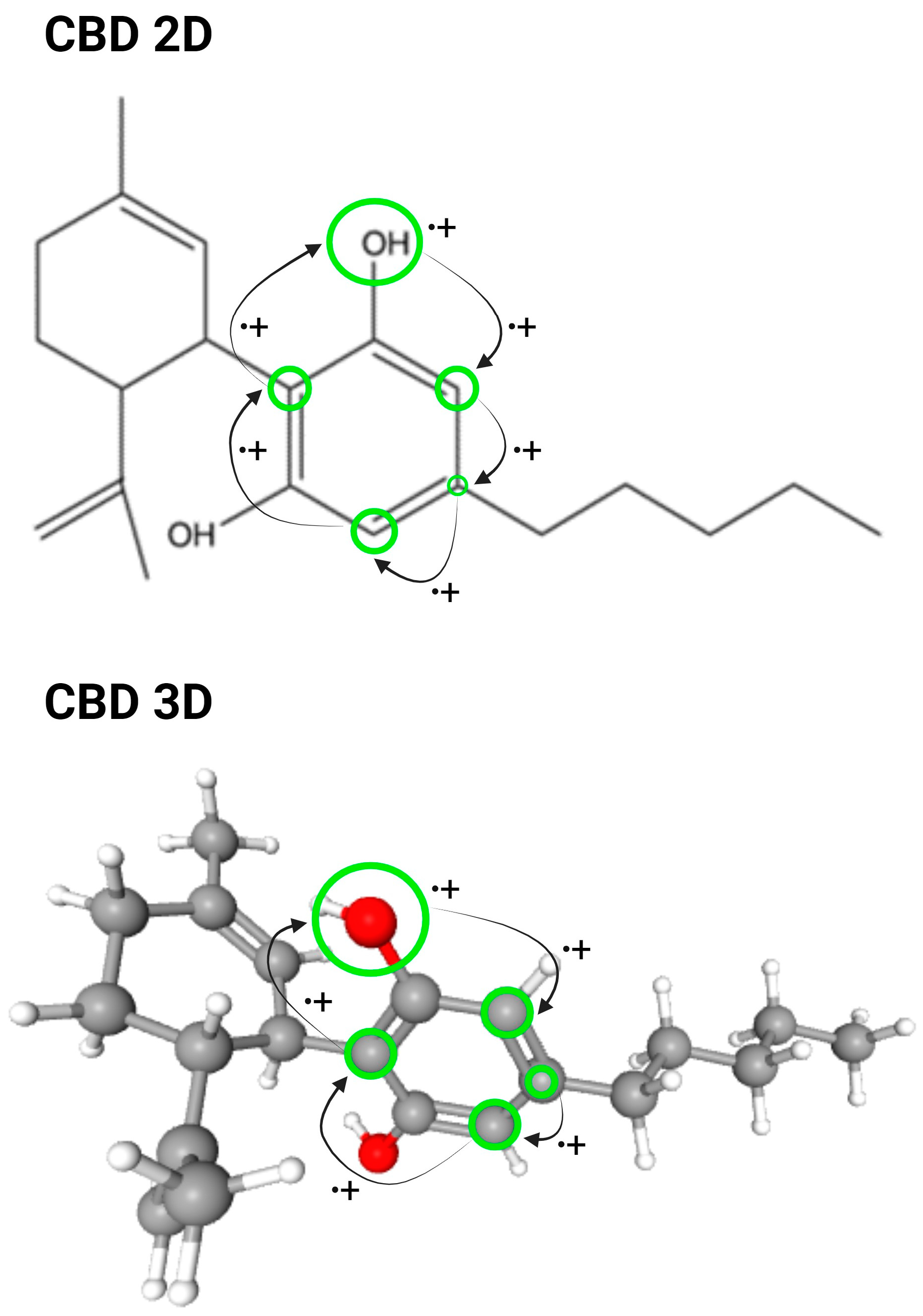
2. CBD: Structure, Sources, and Clinical Use
3. CBD as a Drug: Pharmacokinetics and Current Formulations
3.1. Absorption
- Oral administration
- Inhalation
- Intravenous administration
- Transdermal administration
3.2. Distribution
3.3. Metabolism
3.4. Excretion
3.5. Drug Interaction
3.6. Safety and Adverse Effects
3.7. Dosage
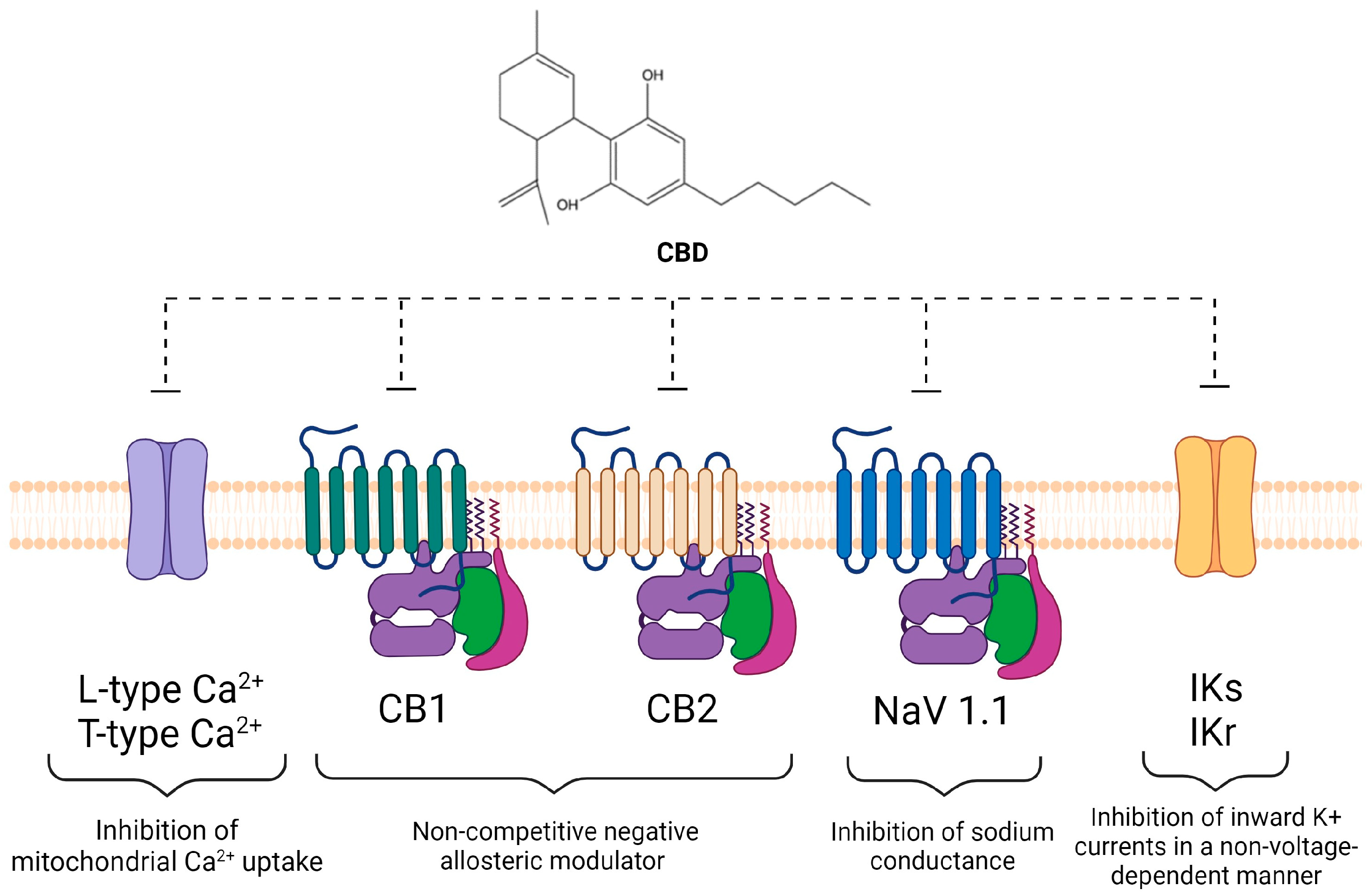
4. CBD as a Pharmacological Inhibitor
4.1. CBD Inhibition of Signaling in the Endocannabinoid System
4.2. CBD Modulates Intracellular Ion Concentrations and Inhibitor of Ion Channels
- Calcium and calcium channels
- Sodium and sodium channels
- Potassium and potassium channels
- Chloride and chloride channels
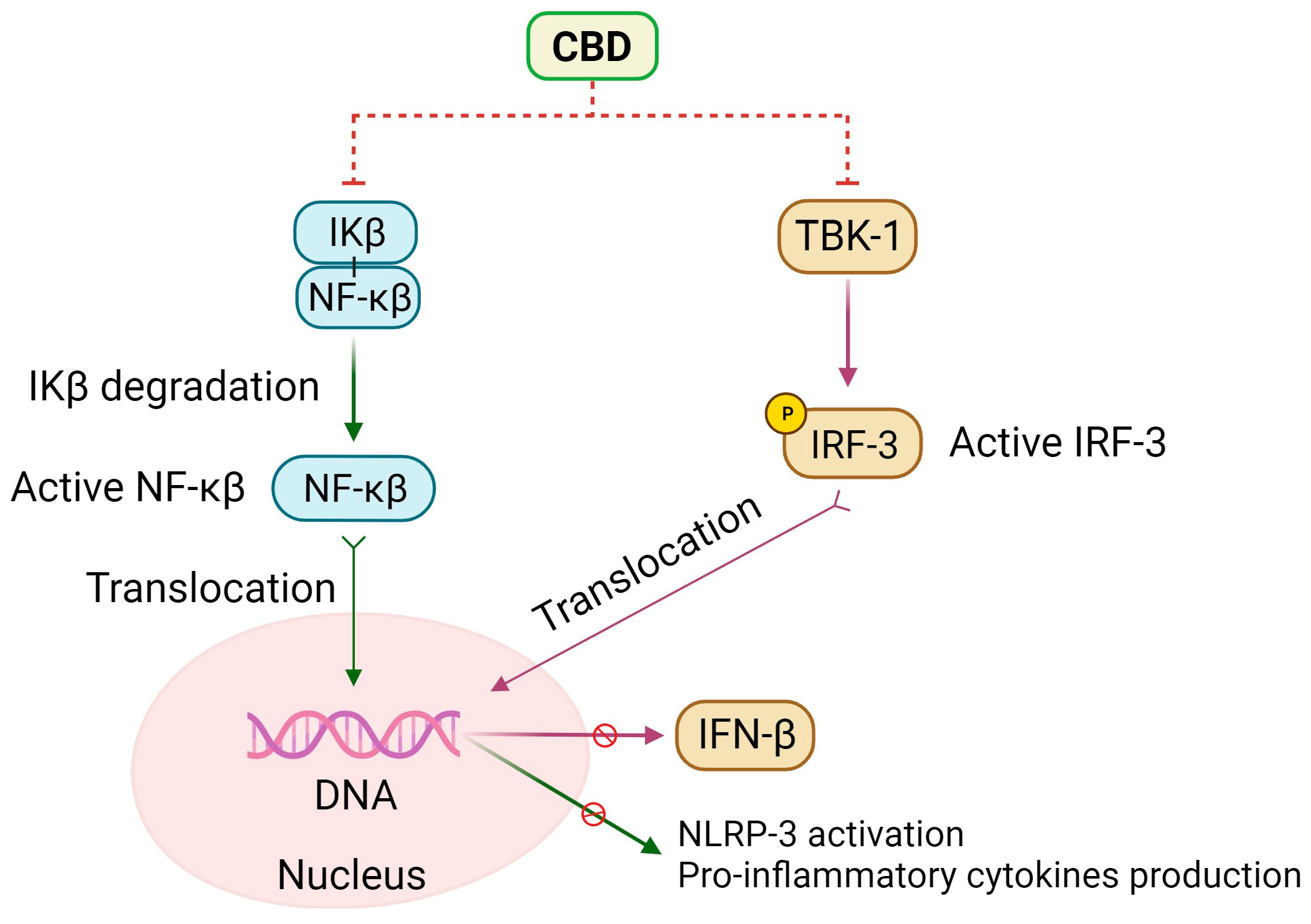
5. Anti-Inflammatory Effects of CBD
Additional Molecular and Cellular Effects of CBD
- Nuclear peroxisome proliferator-activated receptors (PPAR)
- Adenosine receptors
- GABAA receptors
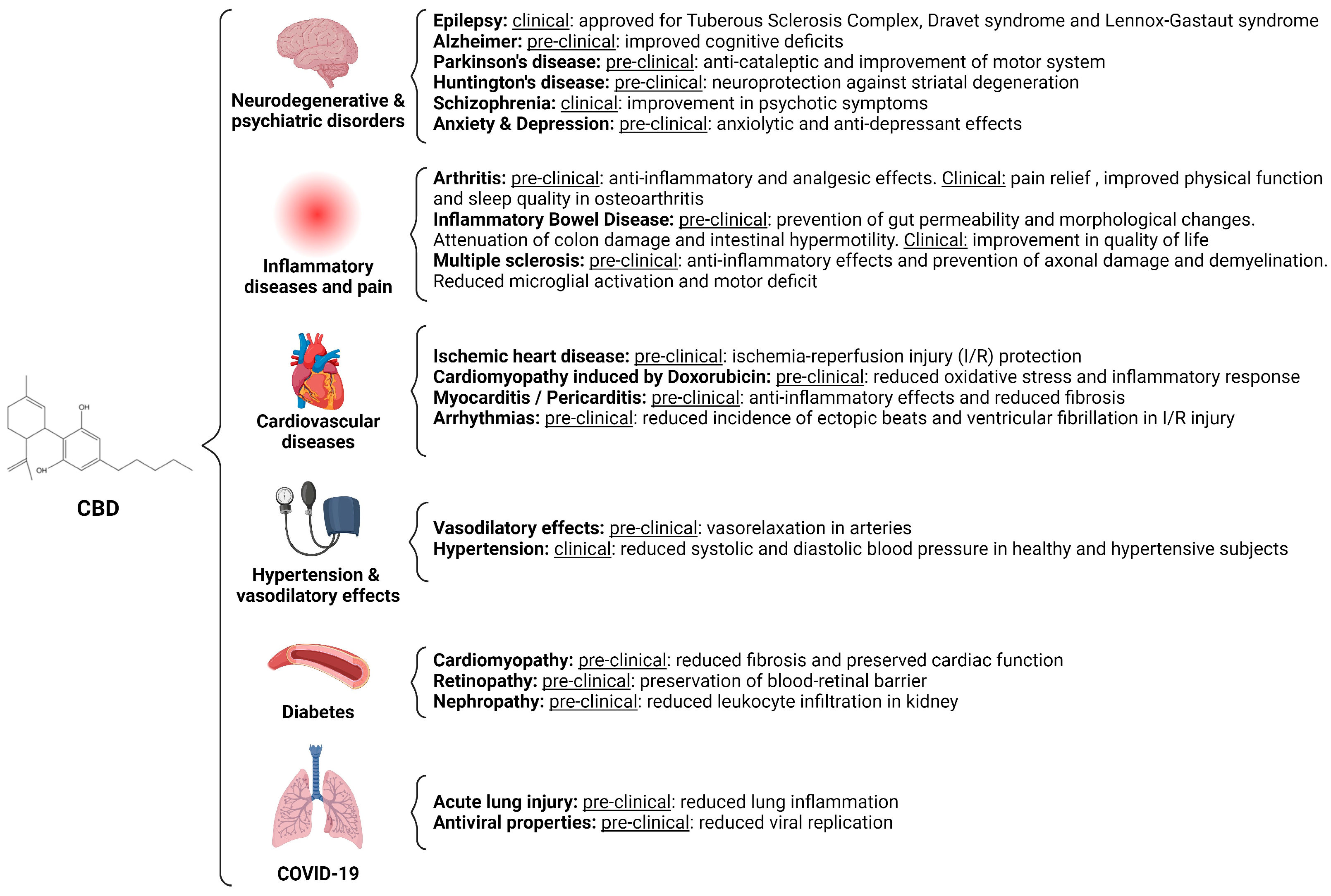
6. CBD as a Pharmacological Inhibitor, an Overview of Preclinical and Clinical Studies
6.1. CBD in Neurodegenerative and Psychiatric Disorders
- Epilepsy
- Alzheimer’s Disease
- Parkinson’s Disease
- Huntington’s Disease
- Schizophrenia
- Anxiety and depression
6.2. CBD in the Treatment of Pain and Autoinflammatory Diseases
- Arthritis
- Inflammatory Bowel Diseases
- Multiple sclerosis
6.3. CBD in Heart Disease
- Ischemic heart disease
- Toxic cardiomyopathy induced by Doxorubicin
- Myocarditis and pericarditis
- Arrhythmias
- Vasodilatory effects and Hypertension
6.4. Diabetes
- Diabetic cardiomyopathy
- Diabetic nephropathy
6.5. CBD in COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor Oxygenated Cannabinoids from High Potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Parker, L.A. Constituents of Cannabis Sativa. In Cannabinoids and Neuropsychiatric Disorders; Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–13. ISBN 978-3-030-57369-0. [Google Scholar]
- ElSohly, M.A.; Slade, D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Viana, M.D.B.; de Aquino, P.E.A.; Estadella, D.; Ribeiro, D.A.; Viana, G.S.D.B. Cannabis Sativa and Cannabidiol: A Therapeutic Strategy for the Treatment of Neurodegenerative Diseases? Med. Cannabis Cannabinoids 2022, 5, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Jarrahian, A. The Movement of N-Arachidonoylethanolamine (Anandamide) across Cellular Membranes. Chem. Phys. Lipids 2000, 108, 123–134. [Google Scholar] [CrossRef]
- Piomelli, D.; Beltramo, M.; Glasnapp, S.; Lin, S.Y.; Goutopoulos, A.; Xie, X.-Q.; Makriyannis, A. Structural Determinants for Recognition and Translocation by the Anandamide Transporter. Proc. Natl. Acad. Sci. USA 1999, 96, 5802–5807. [Google Scholar] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Karsak, M.; Cohen-Solal, M.; Freudenberg, J.; Ostertag, A.; Morieux, C.; Kornak, U.; Essig, J.; Erxlebe, E.; Bab, I.; Kubisch, C.; et al. Cannabinoid Receptor Type 2 Gene Is Associated with Human Osteoporosis. Hum. Mol. Genet. 2005, 14, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; van ’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of Bone Mass, Bone Loss and Osteoclast Activity by Cannabinoid Receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Juan-Picó, P.; Fuentes, E.; Javier Bermúdez-Silva, F.; Javier Díaz-Molina, F.; Ripoll, C.; Rodríguez de Fonseca, F.; Nadal, A. Cannabinoid Receptors Regulate Ca2+ Signals and Insulin Secretion in Pancreatic β-Cell. Cell Calcium 2006, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Ross, R.A. Cannabinoid Receptors and Their Ligands. Prostagland. Leukot. Essent. Fat. Acids PLEFA 2002, 66, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. Allosterism and Cannabinoid CB1 Receptors: The Shape of Things to Come. Trends Pharmacol. Sci. 2007, 28, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.L. Medical Uses of Marijuana? Nature 1993, 365, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; Gilliam, A.F.; Burch, D.F.; Roche, M.J.; Seltzman, H.H. Comparative Receptor Binding Analyses of Cannabinoid Agonists and Antagonists. J. Pharmacol. Exp. Ther. 1998, 285, 285–292. [Google Scholar]
- Petitet, F.; Jeantaud, B.; Reibaud, M.; Imperato, A.; Dubroeucq, M.-C. Complex Pharmacology of Natural Cannabivoids: Evidence for Partial Agonist Activity of Δ9-Tetrahydrocannabinol and Antagonist Activity of Cannabidiol on Rat Brain Cannabinoid Receptors. Life Sci. 1998, 63, PL1–PL6. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol Induces Rapid-Acting Antidepressant-like Effects and Enhances Cortical 5-HT/Glutamate Neurotransmission: Role of 5-HT1A Receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramirez-Rodriguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid Receptor CB1 Mediates Baseline and Activity-Induced Survival of New Neurons in Adult Hippocampal Neurogenesis. Cell Commun. Signal. 2010, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol Displays Unexpectedly High Potency as an Antagonist of CB1 and CB2 Receptor Agonists in Vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are Cannabidiol and Δ9-Tetrahydrocannabivarin Negative Modulators of the Endocannabinoid System? A Systematic Review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An Overview of Some Pharmacological Aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- PubChem Cannabidiol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/644019 (accessed on 6 December 2023).
- PubChem Dronabinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/16078 (accessed on 6 December 2023).
- Jones, P.G.; Falvello, L.; Kennard, O.; Sheldrick, G.M.; Mechoulam, R. Cannabidiol. Acta Crystallogr. Sect. B 1977, 33, 3211–3214. [Google Scholar] [CrossRef]
- Jung, B.; Lee, J.K.; Kim, J.; Kang, E.K.; Han, S.Y.; Lee, H.-Y.; Choi, I.S. Synthetic Strategies for (-)-Cannabidiol and Its Structural Analogs. Chem. Asian J. 2019, 14, 3749–3762. [Google Scholar] [CrossRef]
- Greenwich Biosciences. Epidiolex [Package Insert]; Greenwich Biosciences: Carlsbad, CA, USA, 2018. [Google Scholar]
- The Agriculture Improvement Act of 2018 (2018 Farm Bill). Available online: https://www.fs.usda.gov/managing-land/farm-bill (accessed on 30 November 2023).
- Maguire, R.F.; Wilkinson, D.J.; England, T.J.; O’Sullivan, S.E. The Pharmacological Effects of Plant-Derived versus Synthetic Cannabidiol in Human Cell Lines. Med. Cannabis Cannabinoids 2021, 4, 86–96. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Knaub, K.; Sartorius, T.; Dharsono, T.; Wacker, R.; Wilhelm, M.; Schön, C. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules 2019, 24, 2967. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary Fats and Pharmaceutical Lipid Excipients Increase Systemic Exposure to Orally Administered Cannabis and Cannabis-Based Medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlović, D.; Dujić, Ž.; Ainslie, P.N. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2020, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Abbotts, K.S.S.; Ewell, T.R.; Butterklee, H.M.; Bomar, M.C.; Akagi, N.; Dooley, G.P.; Bell, C. Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function. Nutrients 2022, 14, 2152. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A Systematic Review and Meta-Analysis of the Haemodynamic Effects of Cannabidiol. Front. Pharmacol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Nadulski, T.; Pragst, F.; Weinberg, G.; Roser, P.; Schnelle, M.; Fronk, E.-M.; Stadelmann, A.M. Randomized, Double-Blind, Placebo-Controlled Study About the Effects of Cannabidiol (CBD) on the Pharmacokinetics of Δ9-Tetrahydrocannabinol (THC) After Oral Application of THC Verses Standardized Cannabis Extract. Ther. Drug Monit. 2005, 27, 799. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-Dose Kinetics of Deuterium-Labelled Cannabidiol in Man after Smoking and Intravenous Administration. Biomed. Environ. Mass Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human Skin Permeation of Delta8-Tetrahydrocannabinol, Cannabidiol and Cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol Bioavailability after Nasal and Transdermal Application: Effect of Permeation Enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol—Transdermal Delivery and Anti-Inflammatory Effect in a Murine Model. J. Control. Release Off. J. Control. Release Soc. 2004, 93, 377–387. [Google Scholar] [CrossRef]
- Bardhi, K.; Coates, S.; Watson, C.J.W.; Lazarus, P. Cannabinoids and Drug Metabolizing Enzymes: Potential for Drug-Drug Interactions and Implications for Drug Safety and Efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Crippa, J.A.S. Safety and Side Effects of Cannabidiol, a Cannabis Sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.P.; McGuire, P. Adverse Effects of Cannabidiol: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Neuropsychopharmacology 2020, 45, 1799–1806. [Google Scholar] [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef]
- Schlag, A.K.; O’Sullivan, S.E.; Zafar, R.R.; Nutt, D.J. Current Controversies in Medical Cannabis: Recent Developments in Human Clinical Applications and Potential Therapeutics. Neuropharmacology 2021, 191, 108586. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016, 1, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in Patients with Seizures Associated with Lennox-Gastaut Syndrome (GWPCARE4): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V.; et al. Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Bebin, E.M.; Filloux, F.; Kwan, P.; Loftus, R.; Sahebkar, F.; Sparagana, S.; Wheless, J. Long-Term Cannabidiol Treatment for Seizures in Patients with Tuberous Sclerosis Complex: An Open-Label Extension Trial. Epilepsia 2022, 63, 426–439. [Google Scholar] [CrossRef]
- The INS011-14-029 Study Investigators; Wheless, J.W.; Dlugos, D.; Miller, I.; Oh, D.A.; Parikh, N.; Phillips, S.; Renfroe, J.B.; Roberts, C.M.; Saeed, I.; et al. Pharmacokinetics and Tolerability of Multiple Doses of Pharmaceutical-Grade Synthetic Cannabidiol in Pediatric Patients with Treatment-Resistant Epilepsy. CNS Drugs 2019, 33, 593–604. [Google Scholar] [CrossRef]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef]
- Morgan, C.J.A.; Das, R.K.; Joye, A.; Curran, H.V.; Kamboj, S.K. Cannabidiol Reduces Cigarette Consumption in Tobacco Smokers: Preliminary Findings. Addict. Behav. 2013, 38, 2433–2436. [Google Scholar] [CrossRef]
- Vuilleumier, C.; Scherbaum, N.; Bonnet, U.; Roser, P. Cannabinoids in the Treatment of Cannabis Use Disorder: Systematic Review of Randomized Controlled Trials. Front. Psychiatry 2022, 13, 867878. [Google Scholar]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complement. Med. 2019, 25, 392–397. [Google Scholar] [CrossRef]
- Shannon, S.; Opila-Lehman, J. Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. Perm. J. 2016, 20, 4. [Google Scholar] [CrossRef]
- Arnold, J.C.; McCartney, D.; Suraev, A.; McGregor, I.S. The Safety and Efficacy of Low Oral Doses of Cannabidiol: An Evaluation of the Evidence. Clin. Transl. Sci. 2023, 16, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, C.; Vadalà, M. A Therapeutic Effect of Cbd-Enriched Ointment in Inflammatory Skin Diseases and Cutaneous Scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, C.; Vadalà, M. Short-Term Efficacy of CBD-Enriched Hemp Oil in Girls with Dysautonomic Syndrome after Human Papillomavirus Vaccination. Isr. Med. Assoc. J. 2017, 19, 79–84. [Google Scholar] [PubMed]
- Lazarini-Lopes, W.; Campos-Rodriguez, C.; Garcia-Cairasco, N.; N’Gouemo, P.; Forcelli, P.A. Cannabidiol Attenuates Generalized Tonic-Clonic and Suppresses Limbic Seizures in the Genetically Epilepsy-Prone Rats (GEPR-3) Strain. Pharmacol. Rep. 2023, 75, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naya, N.; Nirar, A.H.; Kim, M.M.; Mauro, A.G.; Mezzaroma, E.; Narayan, P.; Hamer, A.; Bolton, J.; Abbate, A.; Toldo, S. Protective Effects of Cannabidiol in a Mouse Model of Acute Pericarditis. Circ. Res. 2022, 131, e169–e190. [Google Scholar] [CrossRef]
- Watanabe, K.; Kayano, Y.; Matsunaga, T.; Yamamoto, I.; Yoshimura, H. Inhibition of Anandamide Amidase Activity in Mouse Brain Microsomes by Cannabinoids. Biol. Pharm. Bull. 1996, 19, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Rakhshan, F.; Day, T.A.; Blakely, R.D.; Barker, E.L. Carrier-Mediated Uptake of the Endogenous Cannabinoid Anandamide in RBL-2H3 Cells. J. Pharmacol. Exp. Ther. 2000, 292, 960–967. [Google Scholar]
- Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide—Bisogno—2001—British Journal of Pharmacology—Wiley Online Library. Available online: https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1038/sj.bjp.0704327 (accessed on 16 February 2023).
- Bootman, M.D. Calcium Signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011171. [Google Scholar] [CrossRef]
- Ali, R.M.; Al Kury, L.T.; Yang, K.-H.S.; Qureshi, A.; Rajesh, M.; Galadari, S.; Shuba, Y.M.; Howarth, F.C.; Oz, M. Effects of Cannabidiol on Contractions and Calcium Signaling in Rat Ventricular Myocytes. Cell Calcium 2015, 57, 290–299. [Google Scholar] [CrossRef]
- Robertson-Gray, O.J.; Walsh, S.K.; Ryberg, E.; Lipina, C.; Wainwright, C.L. L-α-Lysophosphatidylinositol (LPI) Aggravates Myocardial Ischemia/Reperfusion Injury via a GPR55/ROCK-Dependent Pathway. Pharmacol. Res. Perspect. 2019, 7, e00487. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.J.; Anderson, L.L.; McGregor, I.S.; Arnold, J.C.; Petrou, S. Beyond CBD: Inhibitory Effects of Lesser Studied Phytocannabinoids on Human Voltage-Gated Sodium Channels. Front. Physiol. 2023, 14, 1081186. [Google Scholar] [CrossRef] [PubMed]
- Orvos, P.; Pászti, B.; Topal, L.; Gazdag, P.; Prorok, J.; Polyák, A.; Kiss, T.; Tóth-Molnár, E.; Csupor-Löffler, B.; Bajtel, Á.; et al. The Electrophysiological Effect of Cannabidiol on hERG Current and in Guinea-Pig and Rabbit Cardiac Preparations. Sci. Rep. 2020, 10, 16079. [Google Scholar] [CrossRef]
- Harding, E.K.; Souza, I.A.; Gandini, M.A.; Gadotti, V.M.; Ali, M.Y.; Huang, S.; Antunes, F.T.T.; Trang, T.; Zamponi, G.W. Differential Regulation of Cav3.2 and Cav2.2 Calcium Channels by CB1 Receptors and Cannabidiol. Br. J. Pharmacol. 2023, 180, 1616–1633. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, A.J.; Ryan, D.; Pertwee, R.G.; Platt, B. Cannabidiol-Induced Intracellular Ca2+ Elevations in Hippocampal Cells. Neuropharmacology 2006, 50, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of Recombinant Human T-Type Calcium Channels by Δ9-Tetrahydrocannabinol and Cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef]
- Drummond-Main, C.D.; Ahn, Y.; Kesler, M.; Gavrilovici, C.; Kim, D.Y.; Kiroski, I.; Baglot, S.L.; Chen, A.; Sharkey, K.A.; Hill, M.N.; et al. Cannabidiol Impairs Brain Mitochondrial Metabolism and Neuronal Integrity. Cannabis Cannabinoid Res. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, M.; Torres-López, L.; Valle-Reyes, J.S.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol Directly Targets Mitochondria and Disturbs Calcium Homeostasis in Acute Lymphoblastic Leukemia. Cell Death Dis. 2019, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef]
- Le Marois, M.; Ballet, V.; Sanson, C.; Maizières, M.-A.; Carriot, T.; Chantoiseau, C.; Partiseti, M.; Bohme, G.A. Cannabidiol Inhibits Multiple Cardiac Ion Channels and Shortens Ventricular Action Potential Duration in Vitro. Eur. J. Pharmacol. 2020, 886, 173542. [Google Scholar] [CrossRef]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules 2023, 28, 5980. [Google Scholar] [CrossRef]
- Isaev, D.; Shabbir, W.; Dinc, E.Y.; Lorke, D.E.; Petroianu, G.; Oz, M. Cannabidiol Inhibits Multiple Ion Channels in Rabbit Ventricular Cardiomyocytes. Front. Pharmacol. 2022, 13, 821758. [Google Scholar] [CrossRef]
- Gonca, E.; Darıcı, F. The Effect of Cannabidiol on Ischemia/Reperfusion-Induced Ventricular Arrhythmias: The Role of Adenosine A1 Receptors. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 76–83. [Google Scholar] [CrossRef]
- Topal, L.; Naveed, M.; Orvos, P.; Pászti, B.; Prorok, J.; Bajtel, Á.; Kiss, T.; Csupor-Löffler, B.; Csupor, D.; Baczkó, I.; et al. The Electrophysiological Effects of Cannabidiol on Action Potentials and Transmembrane Potassium Currents in Rabbit and Dog Cardiac Ventricular Preparations. Arch. Toxicol. 2021, 95, 2497–2505. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. Immunotargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Rothwarf, D.M.; Karin, M. The NF-κB Activation Pathway: A Paradigm in Information Transfer from Membrane to Nucleus. Sci.’s STKE 1999, 1999, re1. [Google Scholar] [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-Activated NF-κB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Széles, L.; Töröcsik, D.; Nagy, L. PPARgamma in Immunity and Inflammation: Cell Types and Diseases. Biochim. Biophys. Acta 2007, 1771, 1014–1030. [Google Scholar] [CrossRef]
- Sugiyama, H.; Nonaka, T.; Kishimoto, T.; Komoriya, K.; Tsuji, K.; Nakahata, T. Peroxisome Proliferator-Activated Receptors Are Expressed in Human Cultured Mast Cells: A Possible Role of These Receptors in Negative Regulation of Mast Cell Activation. Eur. J. Immunol. 2000, 30, 3363–3370. [Google Scholar] [CrossRef]
- Ricote, M.; Huang, J.; Fajas, L.; Li, A.; Welch, J.; Najib, J.; Witztum, J.L.; Auwerx, J.; Palinski, W.; Glass, C.K. Expression of the Peroxisome Proliferator-Activated Receptor γ (PPARγ) in Human Atherosclerosis and Regulation in Macrophages by Colony Stimulating Factors and Oxidized Low Density Lipoprotein. Proc. Natl. Acad. Sci. USA 1998, 95, 7614–7619. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.A.; Thomazy, V.A.; Evans, R.M. PPARγ Promotes Monocyte/Macrophage Differentiation and Uptake of Oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Welch, J.S.; Ricote, M.; Binder, C.J.; Willson, T.M.; Kelly, C.; Witztum, J.L.; Funk, C.D.; Conrad, D.; Glass, C.K. Interleukin-4-Dependent Production of PPAR-γ Ligands in Macrophages by 12/15-Lipoxygenase. Nature 1999, 400, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, A.; Grunebach, F.; Zobywlaski, A.; Denzlinger, C.; Brugger, W.; Brossart, P. Dendritic Cell Immunogenicity Is Regulated by Peroxisome Proliferator-Activated Receptor Γ1. J. Immunol. Am. Assoc. Immunol. 2022, 169, 1228–1235. [Google Scholar]
- Szatmari, I.; Gogolak, P.; Im, J.S.; Dezso, B.; Rajnavolgyi, E.; Nagy, L. Activation of PPARγ Specifies a Dendritic Cell Subtype Capable of Enhanced Induction of iNKT Cell Expansion. Immunity 2004, 21, 95–106. [Google Scholar] [CrossRef]
- Szatmari, I.; Vámosi, G.; Brazda, P.; Balint, B.L.; Benko, S.; Széles, L.; Jeney, V.; Özvegy-Laczka, C.; Szántó, A.; Barta, E.; et al. Peroxisome Proliferator-Activated Receptor γ-Regulated ABCG2 Expression Confers Cytoprotection to Human Dendritic Cells. J. Biol. Chem. 2006, 281, 23812–23823. [Google Scholar] [CrossRef]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the Cardiovascular System. Antioxid. Redox Signal. 2009, 11, 1415–1452. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of Adipogenesis in Fibroblasts by PPARγ2, a Lipid-Activated Transcription Factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-Dependent Vascular Actions of Cannabidiol in the Rat Aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol Protects an in Vitro Model of the Blood–Brain Barrier from Oxygen-Glucose Deprivation via PPARγ and 5-HT1A Receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The Plant Cannabinoid Δ9-Tetrahydrocannabivarin Can Decrease Signs of Inflammation and Inflammatory Pain in Mice. Br. J. Pharmacol. 2010, 160, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Critical Role of Mast Cells and Peroxisome Proliferator–Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J. Immunol. 2015, 194, 5211–5222. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.; Thangavelu, C.S.; Joloya, E.M.; Kuo, A.; Li, Z.; Blumberg, B. Cannabidiol Promotes Adipogenesis of Human and Mouse Mesenchymal Stem Cells via PPARγ by Inducing Lipogenesis but Not Lipolysis. Biochem. Pharmacol. 2022, 197, 114910. [Google Scholar] [CrossRef]
- Haskó, G.; Cronstein, B.N. Adenosine: An Endogenous Regulator of Innate Immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an Equilibrative Nucleoside Transporter by Cannabidiol: A Mechanism of Cannabinoid Immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef]
- Pandolfo, P.; Silveirinha, V.; Santos-Rodrigues, A.D.; Venance, L.; Ledent, C.; Takahashi, R.N.; Cunha, R.A.; Köfalvi, A. Cannabinoids Inhibit the Synaptic Uptake of Adenosine and Dopamine in the Rat and Mouse Striatum. Eur. J. Pharmacol. 2011, 655, 38–45. [Google Scholar] [CrossRef]
- Viczjan, G.; Szilagyi, A.; Takacs, B.; Ovari, I.; Szekeres, R.; Tarjanyi, V.; Erdei, T.; Teleki, V.; Zsuga, J.; Szilvassy, Z.; et al. The Effect of a Long-Term Treatment with Cannabidiol-Rich Hemp Extract Oil on the Adenosinergic System of the Zucker Diabetic Fatty (ZDF) Rat Atrium. Front. Pharmacol. 2022, 13, 1043275. [Google Scholar] [CrossRef]
- The Neuroprotective Effect of Cannabidiol in an In Vitro Model of Newborn Hypoxic–Ischemic Brain Damage in Mice Is Mediated by CB 2 and Adenosine Receptors. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S096999610900309X?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS096999610900309X%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 20 February 2023).
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a Non-Psychotropic Plant-Derived Cannabinoid, Decreases Inflammation in a Murine Model of Acute Lung Injury: Role for the Adenosine A2A Receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol Provides Long-Lasting Protection against the Deleterious Effects of Inflammation in a Viral Model of Multiple Sclerosis: A Role for A2A Receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABAA Receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Rasile, M.; Corradini, I.; Matteoli, M. Environmental Regulation of the Chloride Transporter KCC2: Switching Inflammation off to Switch the GABA on? Transl. Psychiatry 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Gaeta, A.; Cannata, B.; Pinzaglia, C.; Aronica, E.; Morano, A.; Cifelli, P.; Palma, E. GABAergic Neurotransmission in Human Tissues Is Modulated by Cannabidiol. Life 2022, 12, 2042. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Carlini, E.A. Toward Drugs Derived from Cannabis. Naturwissenschaften 1978, 65, 174–179. [Google Scholar] [CrossRef]
- Carlini, E.A.; Cunha, J.M. Hypnotic and Antiepileptic Effects of Cannabidiol. J. Clin. Pharmacol. 1981, 21, 417S–427S. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Cannabidiol in Dravet Syndrome Study Group Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Devinsky, O.; Verducci, C.; Thiele, E.A.; Laux, L.C.; Patel, A.D.; Filloux, F.; Szaflarski, J.P.; Wilfong, A.; Clark, G.D.; Park, Y.D.; et al. Open-Label Use of Highly Purified CBD (Epidiolex®) in Patients with CDKL5 Deficiency Disorder and Aicardi, Dup15q, and Doose Syndromes. Epilepsy Behav. 2018, 86, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Mazurkiewicz-Bełdzińska, M.; Chin, R.F.; Gil-Nagel, A.; Gunning, B.; Halford, J.J.; Mitchell, W.; Scott Perry, M.; Thiele, E.A.; Weinstock, A.; et al. Long-Term Safety and Efficacy of Add-on Cannabidiol in Patients with Lennox-Gastaut Syndrome: Results of a Long-Term Open-Label Extension Trial. Epilepsia 2021, 62, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol Attenuates Seizures and Social Deficits in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- Vilela, L.R.; Lima, I.V.; Kunsch, É.B.; Pinto, H.P.P.; de Miranda, A.S.; Vieira, É.L.M.; de Oliveira, A.C.P.; Moraes, M.F.D.; Teixeira, A.L.; Moreira, F.A. Anticonvulsant Effect of Cannabidiol in the Pentylenetetrazole Model: Pharmacological Mechanisms, Electroencephalographic Profile, and Brain Cytokine Levels. Epilepsy Behav. 2017, 75, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kwan Cheung, K.A.; Peiris, H.; Wallace, G.; Holland, O.J.; Mitchell, M.D. The Interplay between the Endocannabinoid System, Epilepsy and Cannabinoids. Int. J. Mol. Sci. 2019, 20, 6079. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, M.; Onetti, Y.; Cortés-Montero, E.; Garzón, J.; Sánchez-Blázquez, P. Cannabidiol Enhances Morphine Antinociception, Diminishes NMDA-Mediated Seizures and Reduces Stroke Damage via the Sigma 1 Receptor. Mol. Brain 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered Cannabidiol and Clobazam: Preclinical Evidence for Both Pharmacodynamic and Pharmacokinetic Interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Karl, T. In Vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer’s Disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo, L.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in Vivo Blunts Beta-Amyloid Induced Neuroinflammation by Suppressing IL-1beta and iNOS Expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and Other Cannabinoids Reduce Microglial Activation in Vitro and in Vivo: Relevance to Alzheimer’s Disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic Cannabidiol Treatment Improves Social and Object Recognition in Double Transgenic APPswe/PS1Δ E9 Mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-Term Cannabidiol Treatment Prevents the Development of Social Recognition Memory Deficits in Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S. Open-Label Trial of a Cannabidiol Solution for the Treatment of Behavioral Symptoms in Older Adults with Alzheimer’s Dementia; NIH: Bethesda, MD, USA, 2023.
- Okhravi, H. Effects of THC-Free CBD Oil on Agitation in Patients with Alzheimer’s Disease; NIH: Bethesda, MD, USA, 2023.
- García-Arencibia, M.; González, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Evaluation of the Neuroprotective Effect of Cannabinoids in a Rat Model of Parkinson’s Disease: Importance of Antioxidant and Cannabinoid Receptor-Independent Properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.F.; Levin, R.; Suiama, M.A.; Diana, M.C.; Gouvêa, D.A.; Almeida, V.; Santos, C.M.; Lungato, L.; Zuardi, A.W.; Hallak, J.E.; et al. Cannabidiol Prevents Motor and Cognitive Impairments Induced by Reserpine in Rats. Front. Pharmacol. 2016, 28, 343. [Google Scholar]
- Gomes, F.V.; Del Bel, E.A.; Guimarães, F.S. Cannabidiol Attenuates Catalepsy Induced by Distinct Pharmacological Mechanisms via 5-HT1A Receptor Activation in Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 43–47. [Google Scholar] [CrossRef]
- Giuliano, C.; Francavilla, M.; Ongari, G.; Petese, A.; Ghezzi, C.; Rossini, N.; Blandini, F.; Cerri, S. Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 8920. [Google Scholar] [CrossRef]
- University of Colorado. A Randomized, Double Blind, Placebo-Controlled Crossover Study of Tolerability and Efficacy of Cannabidiol (CBD) on Tremor in Parkinson’s Disease; NIH: Bethesda, MD, USA, 2019.
- Fumoleau, P.; Chevallier, B.; Kerbrat, P.; Krakowski, Y.; Misset, J.L.; Maugard-Louboutin, C.; Dieras, V.; Azli, N.; Bougon, N.; Riva, A.; et al. A Multicentre Phase II Study of the Efficacy and Safety of Docetaxel as First-Line Treatment of Advanced Breast Cancer: Report of the Clinical Screening Group of the EORTC. Ann. Oncol. 1996, 7, 165–171. [Google Scholar] [CrossRef]
- Sagredo, O.; Ramos, J.A.; Decio, A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabidiol Reduced the Striatal Atrophy Caused 3-Nitropropionic Acid in Vivo by Mechanisms Independent of the Activation of Cannabinoid, Vanilloid TRPV1 and Adenosine A2A Receptors. Eur. J. Neurosci. 2007, 26, 843–851. [Google Scholar] [CrossRef]
- Sagredo, O.; González, S.; Aroyo, I.; Pazos, M.R.; Benito, C.; Lastres-Becker, I.; Romero, J.P.; Tolón, R.M.; Mechoulam, R.; Brouillet, E.; et al. Cannabinoid CB2 Receptor Agonists Protect the Striatum against Malonate Toxicity: Relevance for Huntington’s Disease. Glia 2009, 57, 1154–1167. [Google Scholar] [CrossRef]
- López-Sendón Moreno, J.L.; García Caldentey, J.; Trigo Cubillo, P.; Ruiz Romero, C.; García Ribas, G.; Alonso Arias, M.A.A.; García de Yébenes, M.J.; Tolón, R.M.; Galve-Roperh, I.; Sagredo, O.; et al. A Double-Blind, Randomized, Cross-over, Placebo-Controlled, Pilot Trial with Sativex in Huntington’s Disease. J. Neurol. 2016, 263, 1390–1400. [Google Scholar] [CrossRef]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled Clinical Trial of Cannabidiol in Huntington’s Disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, S.; Kloiber, S.; Le Foll, B. Effects of Cannabidiol (CBD) in Neuropsychiatric Disorders: A Review of Pre-Clinical and Clinical Findings. Prog. Mol. Biol. Transl. Sci. 2019, 167, 25–75. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.A.; Guimarães, F.S. Cannabidiol Inhibits the Hyperlocomotion Induced by Psycotomimetic Drugs in Mice. Eur. J. Paharmacol. 2005, 512, 199–205. [Google Scholar] [CrossRef]
- Long, L.E.; Chesworth, R.; Huang, X.-F.; McGregor, I.S.; Arnold, J.C.; Karl, T. A Behavioural Comparison of Acute and Chronic Delta9-Tetrahydrocannabinol and Cannabidiol in C57BL/6JArc Mice. Int. J. Neuropsychopharmacol. 2010, 13, 861–876. [Google Scholar] [CrossRef] [PubMed]
- PRIME PubMed|Cannabidiol Attenuates Haloperidol-Induced Catalepsy and c-Fos Protein Expression in the Dorsolateral Striatum via 5-HT1A Receptors in Mice. Available online: https://www.unboundmedicine.com/medline/citation/27131780/Cannabidiol_attenuates_haloperidol_induced_catalepsy_and_c_Fos_protein_expression_in_the_dorsolateral_striatum_via_5_HT1A_receptors_in_mice_ (accessed on 11 December 2023).
- Renard, J.; Loureiro, M.; Rosen, L.G.; Zunder, J.; de Oliveira, C.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol Counteracts Amphetamine-Induced Neuronal and Behavioral Sensitization of the Mesolimbic Dopamine Pathway through a Novel mTOR/p70S6 Kinase Signaling Pathway. J. Neurosci. 2016, 36, 5160–5169. [Google Scholar] [CrossRef]
- Pedrazzi, J.F.C.; Issy, A.C.; Gomes, F.V.; Guimarães, F.S.; Del-Bel, E.A. Cannabidiol Effects in the Prepulse Inhibition Disruption Induced by Amphetamine. Psychopharmacology 2015, 232, 3057–3065. [Google Scholar] [CrossRef]
- Marco, E.M.; García-Gutiérrez, M.S.; Bermúdez-Silva, F.-J.; Moreira, F.A.; Guimarães, F.; Manzanares, J.; Viveros, M.-P. Endocannabinoid System and Psychiatry: In Search of a Neurobiological Basis for Detrimental and Potential Therapeutic Effects. Front. Behav. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef]
- Gururajan, A.; Taylor, D.A.; Malone, D.T. Effect of Cannabidiol in a MK-801-Rodent Model of Aspects of Schizophrenia. Behav. Brain Res. 2011, 222, 299–308. [Google Scholar] [CrossRef]
- Uribe-Mariño, A.; Francisco, A.; Castiblanco-Urbina, M.A.; Twardowschy, A.; Salgado-Rohner, C.J.; Crippa, J.A.S.; Hallak, J.E.C.; Zuardi, A.W.; Coimbra, N.C. Anti-Aversive Effects of Cannabidiol on Innate Fear-Induced Behaviors Evoked by an Ethological Model of Panic Attacks Based on a Prey vs the Wild Snake Epicrates Cenchria Crassus Confrontation Paradigm. Neuropsychopharmacology 2012, 37, 412–421. [Google Scholar] [CrossRef]
- Marinho, A.L.Z.; Vila-Verde, C.; Fogaça, M.V.; Guimarães, F.S. Effects of Intra-Infralimbic Prefrontal Cortex Injections of Cannabidiol in the Modulation of Emotional Behaviors in Rats: Contribution of 5HT₁A Receptors and Stressful Experiences. Behav Brain Res. 2015, 286, 49–56. [Google Scholar] [CrossRef]
- Campos, A.C.; Ferreira, F.R.; Guimarães, F.S. Cannabidiol Blocks Long-Lasting Behavioral Consequences of Predator Threat Stress: Possible Involvement of 5HT1A Receptors. J. Psychiatr. Res. 2012, 46, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Resstel, L.B.M.; Joca, S.R.L.; Moreira, F.A.; Corrêa, F.M.A.; Guimarães, F.S. Effects of Cannabidiol and Diazepam on Behavioral and Cardiovascular Responses Induced by Contextual Conditioned Fear in Rats. Behav. Brain Res. 2006, 172, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.D.P.; Campos, A.C.; Bortoli, V.C.D.; Zangrossi, H.; Guimarães, F.S.; Zuardi, A.W. Intra-Dorsal Periaqueductal Gray Administration of Cannabidiol Blocks Panic-like Response by Activating 5-HT1A Receptors. Behav. Brain Res. 2010, 213, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT1A Receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef]
- Sartim, A.G.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effect of Cannabidiol Injection into the Ventral Medial Prefrontal Cortex-Possible Involvement of 5-HT1A and CB1 Receptors. Behav. Brain Res. 2016, 303, 218–227. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A Randomized, Double-Blind, Placebo-Controlled Study of Daily Cannabidiol for the Treatment of Canine Osteoarthritis Pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef]
- Gamble, L.-J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Philpott, H.T.; O’Brien, M.; McDougall, J.J. Attenuation of Early Phase Inflammation by Cannabidiol Prevents Pain and Nerve Damage in Rat Osteoarthritis. Pain 2017, 158, 2442–2451. [Google Scholar] [CrossRef]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal Cannabidiol Reduces Inflammation and Pain-Related Behaviours in a Rat Model of Arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The Nonpsychoactive Cannabis Constituent Cannabidiol Is an Oral Anti-Arthritic Therapeutic in Murine Collagen-Induced Arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Tingting, R.; Zurmahr, J.; Classen, T.; Schneider, M.; Pongratz, G. Cannabidiol (CBD): A Killer for Inflammatory Rheumatoid Arthritis Synovial Fibroblasts. Cell Death Dis. 2020, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- NYU Langone Health. The Use of Cannabidiol (CBD) in Pain Reduction for Knee Osteoarthritis. A Double-Blind, Randomized Control Study; NIH: Bethesda, MD, USA, 2023.
- Ranganath, V. Randomized, Double Blind, Placebo-Controlled Trial to Evaluate the Safety and Tolerability of Cannabidiol (CBD) in Moderate to Severe Rheumatoid Arthritis; NIH: Bethesda, MD, USA, 2023.
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a Treatment for Arthritis and Joint Pain: An Exploratory Cross-Sectional Study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Arendt-Nielsen, L.; Duch, K.S.; Kristensen, S. Cannabidiol Treatment in Hand Osteoarthritis and Psoriatic Arthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Pain 2022, 163, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and Palmitoylethanolamide Are Anti-Inflammatory in the Acutely Inflamed Human Colon. Clin. Sci. 2017, 131, 2611–2626. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Janjua, T.I.; Martin, J.H.; Begun, J.; Popat, A. Cannabidiol—Help and Hype in Targeting Mucosal Diseases. J. Control. Release 2023, 365, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Aviello, G.; Romano, B.; Orlando, P.; Capasso, R.; Maiello, F.; Guadagno, F.; Petrosino, S.; Capasso, F.; Di Marzo, V.; et al. Cannabidiol, a Safe and Non-Psychotropic Ingredient of the Marijuana Plant Cannabis Sativa, Is Protective in a Murine Model of Colitis. J. Mol. Med. 2009, 87, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef]
- Schicho, R.; Storr, M. Topical and Systemic Cannabidiol Improves Trinitrobenzene Sulfonic Acid Colitis in Mice. Pharmacology 2012, 89, 149–155. [Google Scholar] [CrossRef]
- Jamontt, J.; Molleman, A.; Pertwee, R.; Parsons, M. The Effects of Δ9-Tetrahydrocannabinol and Cannabidiol Alone and in Combination on Damage, Inflammation and in Vitro Motility Disturbances in Rat Colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-Induced Hyperpermeability of the Human Gut In Vitro and In Vivo-A Randomized, Placebo-Controlled, Double-Blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.S.; Sia, T.C.; Wattchow, D.A.; Smid, S.D. Interleukin 17A Evoked Mucosal Damage Is Attenuated by Cannabidiol and Anandamide in a Human Colonic Explant Model. Cytokine 2014, 65, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol Attenuates Chemically-Induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharmacol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Naftali, T.; Bar-Lev Schleider, L.; Almog, S.; Meiri, D.; Konikoff, F.M. Oral CBD-Rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn’s Disease, a Randomised Controlled Trial. J. Crohns Colitis 2021, 15, 1799–1806. [Google Scholar] [CrossRef]
- Naftali, T.; Mechulam, R.; Marii, A.; Gabay, G.; Stein, A.; Bronshtain, M.; Laish, I.; Benjaminov, F.; Konikoff, F.M. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig. Dis. Sci. 2017, 62, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Pilot Study of Cannabidiol-Rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol Inhibits Pathogenic T Cells, Decreases Spinal Microglial Activation and Ameliorates Multiple Sclerosis-like Disease in C57BL/6 Mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef]
- Giacoppo, S.; Soundara Rajan, T.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Purified Cannabidiol, the Main Non-Psychotropic Component of Cannabis Sativa, Alone, Counteracts Neuronal Apoptosis in Experimental Multiple Sclerosis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4906–4919. [Google Scholar]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target Regulation of PI3K/Akt/mTOR Pathway by Cannabidiol in Treatment of Experimental Multiple Sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.-J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L.F. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. 2021, 16, 346–362. [Google Scholar] [CrossRef] [PubMed]
- González-García, C.; Torres, I.M.; García-Hernández, R.; Campos-Ruíz, L.; Esparragoza, L.R.; Coronado, M.J.; Grande, A.G.; García-Merino, A.; Sánchez López, A.J. Mechanisms of Action of Cannabidiol in Adoptively Transferred Experimental Autoimmune Encephalomyelitis. Exp. Neurol. 2017, 298, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Longoria, V.; Parcel, H.; Toma, B.; Minhas, A.; Zeine, R. Neurological Benefits, Clinical Challenges, and Neuropathologic Promise of Medical Marijuana: A Systematic Review of Cannabinoid Effects in Multiple Sclerosis and Experimental Models of Demyelination. Biomedicines 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J. Mechanisms of Cannabidiol (CBD) in Persons with Multiple Sclerosis (MS): The Role of Sleep and Pain Phenotype; NIH: Bethesda, MD, USA, 2023.
- Krylatov, A.V.; Ugdyzhekova, D.S.; Bernatskaya, N.A.; Maslov, L.N.; Mekhoulam, R.; Pertwee, R.G.; Stephano, G.B. Activation of Type II Cannabinoid Receptors Improves Myocardial Tolerance to Arrhythmogenic Effects of Coronary Occlusion and Reperfusion. Bull. Exp. Biol. Med. 2001, 131, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Ugdyzhekova, D.S.; Bernatskaya, N.A.; Stefano, J.B.; Graier, V.F.; Tam, S.W.; Mekhoulam, R. Endogenous Cannabinoid Anandamide Increases Heart Resistance to Arrhythmogenic Effects of Epinephrine: Role of CB1 and CB2 Receptors. Bull. Exp. Biol. Med. 2001, 131, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Ugdyzhekova, D.S.; Krylatov, A.V.; Bernatskaya, N.A.; Maslov, L.N.; Mechoulam, R.; Pertwee, R.G. Activation of Cannabinoid Receptors Decreases the Area of Ischemic Myocardial Necrosis. Bull. Exp. Biol. Med. 2002, 133, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Underdown, N.J.; Hiley, C.R.; Ford, W.R. Anandamide Reduces Infarct Size in Rat Isolated Hearts Subjected to Ischaemia–Reperfusion by a Novel Cannabinoid Mechanism. Br. J. Pharmacol. 2005, 146, 809–816. [Google Scholar] [CrossRef]
- Durst, R.; Danenberg, H.; Gallily, R.; Mechoulam, R.; Meir, K.; Grad, E.; Beeri, R.; Pugatsch, T.; Tarsish, E.; Lotan, C. Cannabidiol, a Nonpsychoactive Cannabis Constituent, Protects against Myocardial Ischemic Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H3602–H3607. [Google Scholar] [CrossRef]
- Franco-Vadillo, A.; Toledo-Blass, M.; Rivera-Herrera, Z.; Guevara-Balcazar, G.; Orihuela-Rodriguez, O.; Morales-Carmona, J.A.; Kormanovski-Kovzova, A.; Lopez-Sanchez, P.; Rubio-Gayosso, I.; Castillo-Hernandez, M.D.C. Cannabidiol-mediated RISK PI3K/AKT and MAPK/ERK Pathways Decreasing Reperfusion Myocardial Damage. Pharmacol. Res. Perspect. 2021, 9, e00784. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute Administration of Cannabidiol in Vivo Suppresses Ischaemia-Induced Cardiac Arrhythmias and Reduces Infarct Size When given at Reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.-S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective Effect of Cannabidiol in Rats Exposed to Doxorubicin Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.M.; Kim, S.R.; Hong, S.H.; Choi, H.-S.; Seo, H.S.; Shin, Y.C.; Ko, S.-G. Cucurbitacin D Induces Cell Cycle Arrest and Apoptosis by Inhibiting STAT3 and NF-κB Signaling in Doxorubicin-Resistant Human Breast Carcinoma (MCF7/ADR) Cells. Mol. Cell. Biochem. 2015, 409, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-κB Pathway. Med. Sci. Monit. 2019, 25, 6085–6096. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Oh, J.G. SERCA2a: A Prime Target for Modulation of Cardiac Contractility during Heart Failure. BMB Rep. 2013, 46, 237–243. [Google Scholar] [CrossRef]
- Lee, W.-S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Hask’, G.; Čiháková, D.; Mechoulam, R.; Pacher, P. Cannabidiol Limits T Cell–Mediated Chronic Autoimmune Myocarditis: Implications to Autoimmune Disorders and Organ Transplantation. Mol. Med. 2016, 22, 136–146. [Google Scholar] [CrossRef]
- Cardiol Therapeutics Inc. Impact of CardiolRx on Myocardial Recovery in Acute Myocarditis. A Double-Blind, Placebo-Controlled Trial; NIH: Bethesda, MD, USA, 2023.
- Cardiol Therapeutics Inc. Impact of CardiolRxTM on Recurrent Pericarditis an Open Label Pilot Study; NIH: Bethesda, MD, USA, 2023.
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A Single Dose of Cannabidiol Reduces Blood Pressure in Healthy Volunteers in a Randomized Crossover Study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol Improves Vasorelaxation in Zucker Diabetic Fatty Rats through Cyclooxygenase Activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol Causes Endothelium-Dependent Vasorelaxation of Human Mesenteric Arteries via CB1 Activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- McQueen, D.S.; Bond, S.M.; Smith, P.J.W.; Balali-Mood, K.; Smart, D. Cannabidiol Lacks the Vanilloid VR1-Mediated Vasorespiratory Effects of Capsaicin and Anandamide in Anaesthetised Rats. Eur. J. Pharmacol. 2004, 491, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.H.F.; Crestani, C.C.; Gomes, F.V.; Guimarães, F.S.; Correa, F.M.A.; Resstel, L.B.M. Cannabidiol Injected into the Bed Nucleus of the Stria Terminalis Modulates Baroreflex Activity through 5-HT1A Receptors. Pharmacol. Res. 2010, 62, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Granjeiro, É.M.; Gomes, F.V.; Guimarães, F.S.; Corrêa, F.M.A.; Resstel, L.B.M. Effects of Intracisternal Administration of Cannabidiol on the Cardiovascular and Behavioral Responses to Acute Restraint Stress. Pharmacol. Biochem. Behav. 2011, 99, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Zuardi: Transcultural Evaluation of a Self-Evaluation…. Available online: https://scholar.google.com/scholar_lookup?title=Transcultural%20evaluation%20of%20a%20self-evaluation%20scale%20of%20subjective%20states&journal=J%20Brasileiro%20Psiquiatr&volume=131&pages=403-406&publication_year=1981&author=Zuardi%2CAW&author=Karniol%2CIG (accessed on 3 February 2023).
- Fusar-Poli, P.; Allen, P.; Bhattacharyya, S.; Crippa, J.A.; Mechelli, A.; Borgwardt, S.; Martin-Santos, R.; Seal, M.L.; O’Carrol, C.; Atakan, Z.; et al. Modulation of Effective Connectivity during Emotional Processing by Δ9-Tetrahydrocannabinol and Cannabidiol. Int. J. Neuropsychopharmacol. 2010, 13, 421–432. [Google Scholar] [CrossRef]
- Crippa, J.A.; Zuardi, A.W.; Martín-Santos, R.; Bhattacharyya, S.; Atakan, Z.; McGuire, P.; Fusar-Poli, P. Cannabis and Anxiety: A Critical Review of the Evidence. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 515–523. [Google Scholar] [CrossRef]
- Kumric, M.; Bozic, J.; Dujic, G.; Vrdoljak, J.; Dujic, Z. Chronic Effects of Effective Oral Cannabidiol Delivery on 24-h Ambulatory Blood Pressure and Vascular Outcomes in Treated and Untreated Hypertension (HYPER-H21-4): Study Protocol for a Randomized, Placebo-Controlled, and Crossover Study. J. Pers. Med. 2022, 12, 1037. [Google Scholar] [CrossRef]
- CDC What Is Diabetes? Available online: https://www.cdc.gov/diabetes/basics/diabetes.html (accessed on 8 January 2024).
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol Attenuates High Glucose-Induced Endothelial Cell Inflammatory Response and Barrier Disruption. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.-T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and Blood-Retinal Barrier-Preserving Effects of Cannabidiol in Experimental Diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, and Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Ramos-González, M.; Lozano, O.; Jerjes-Sánchez, C.; García-Rivas, G. Therapeutic Applications of Cannabinoids in Cardiomyopathy and Heart Failure. Oxidative Med. Cell. Longev. 2020, 2020, 4587024. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Zeira, M.; Reich, S.; Slavin, S.; Raz, I.; Mechoulam, R.; Gallily, R. Cannabidiol Arrests Onset of Autoimmune Diabetes in NOD Mice. Neuropharmacology 2008, 54, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Hidalgo, B.; García-Martín, A.; Muñoz, E.; González-Mariscal, I. Detrimental Effect of Cannabidiol on the Early Onset of Diabetic Nephropathy in Male Mice. Pharmaceuticals 2021, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol Attenuates Cisplatin-Induced Nephrotoxicity by Decreasing Oxidative/Nitrosative Stress, Inflammation, and Cell Death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol Improves Lung Function and Inflammation in Mice Submitted to LPS-Induced Acute Lung Injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Park, J.G.; Cho, K.-H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of Antiviral Potencies of Cannabinoids against SARS-CoV-2 Using Computational and in Vitro Approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Ohtsuki, T.; Chen, S.-N.; Friesen, J.B.; Drayman, N.; Mohamed, A.; Dann, C.; et al. Cannabidiol Inhibits SARS-CoV-2 Replication and Promotes the Host Innate Immune Response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In Search of Preventive Strategies: Novel High-CBD Cannabis Sativa Extracts Modulate ACE2 Expression in COVID-19 Gateway Tissues. Aging 2020, 12, 22425–22444. [Google Scholar] [CrossRef]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Chibane, F.; Costigliola, V.; Yu, J.C.; Vaibhav, K.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020, 5, 197–201. [Google Scholar] [CrossRef]
- Salles, É.L.; Khodadadi, H.; Jarrahi, A.; Ahluwalia, M.; Paffaro, V.A.; Costigliola, V.; Yu, J.C.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol (CBD) Modulation of Apelin in Acute Respiratory Distress Syndrome. J. Cell. Mol. Med. 2020, 24, 12869–12872. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Pacheco, J.C.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; Osório, F.D.L.; Loureiro, S.R.; Dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; et al. Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. 2022, 7, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Bod Australia. Safety and Tolerability of Full Spectrum Cannabidiol Dominant Medicinal Cannabis in Treating Symptoms Associated with Long COVID: A Feasibility Study; NIH: Bethesda, MD, USA, 2023.
- Feng, Y.; Chen, F.; Yin, T.; Xia, Q.; Liu, Y.; Huang, G.; Zhang, J.; Oyen, R.; Ni, Y. Pharmacologic Effects of Cannabidiol on Acute Reperfused Myocardial Infarction in Rabbits: Evaluated with 3.0T Cardiac Magnetic Resonance Imaging and Histopathology. J. Cardiovasc. Pharmacol. 2015, 66, 354–363. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Polizio, A.H.; Abbate, A.; Toldo, S.; Mezzaroma, E. An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules 2024, 29, 473. https://doi.org/10.3390/molecules29020473
Martinez Naya N, Kelly J, Corna G, Golino M, Polizio AH, Abbate A, Toldo S, Mezzaroma E. An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules. 2024; 29(2):473. https://doi.org/10.3390/molecules29020473
Chicago/Turabian StyleMartinez Naya, Nadia, Jazmin Kelly, Giuliana Corna, Michele Golino, Ariel H. Polizio, Antonio Abbate, Stefano Toldo, and Eleonora Mezzaroma. 2024. "An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects" Molecules 29, no. 2: 473. https://doi.org/10.3390/molecules29020473
APA StyleMartinez Naya, N., Kelly, J., Corna, G., Golino, M., Polizio, A. H., Abbate, A., Toldo, S., & Mezzaroma, E. (2024). An Overview of Cannabidiol as a Multifunctional Drug: Pharmacokinetics and Cellular Effects. Molecules, 29(2), 473. https://doi.org/10.3390/molecules29020473









