A Facile Ugi/Ullmann Cascade Reaction to Access Fused Indazolo-Quinoxaline Derivatives with Potent Anticancer Activity
Abstract
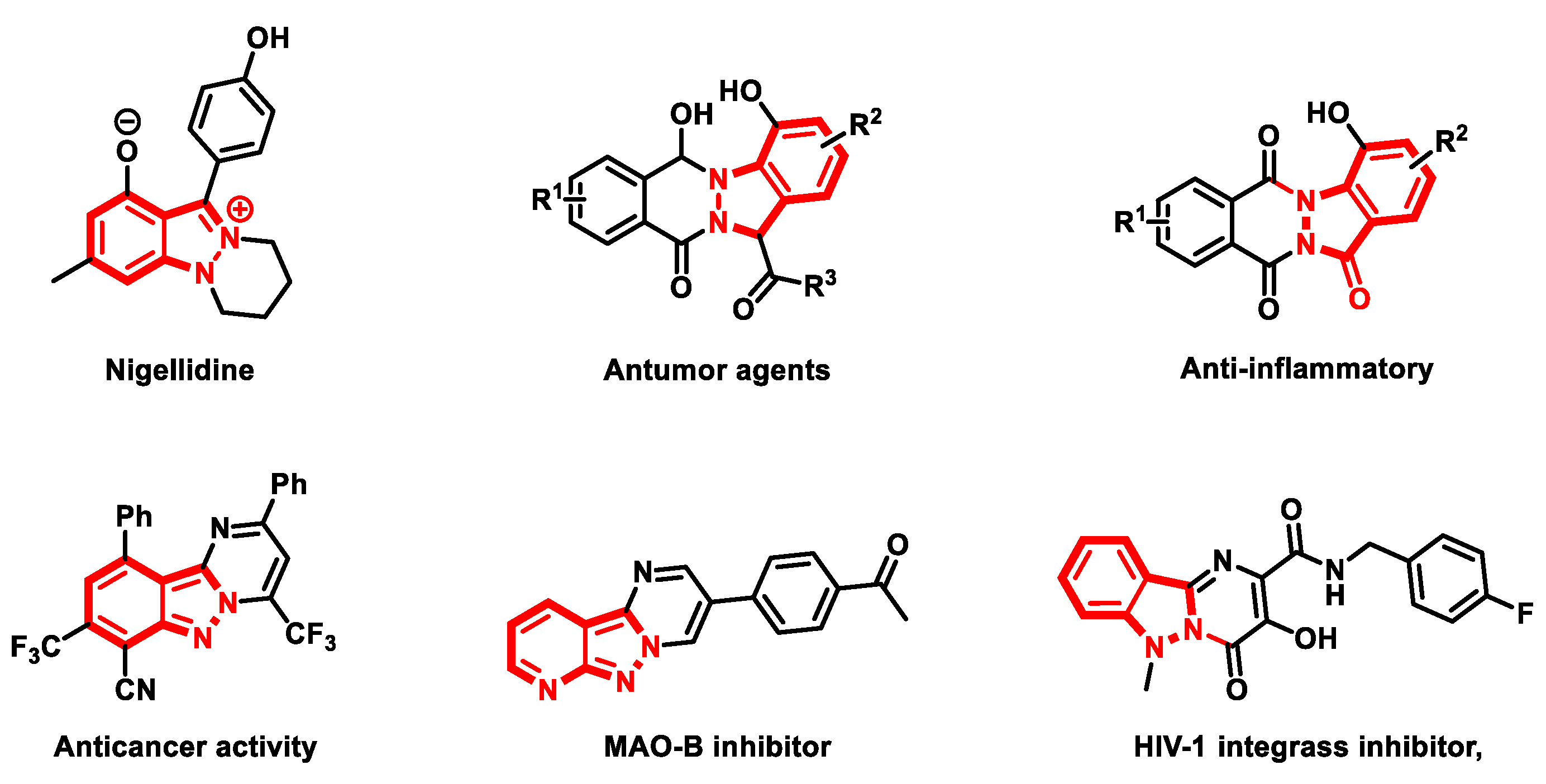
1. Introduction
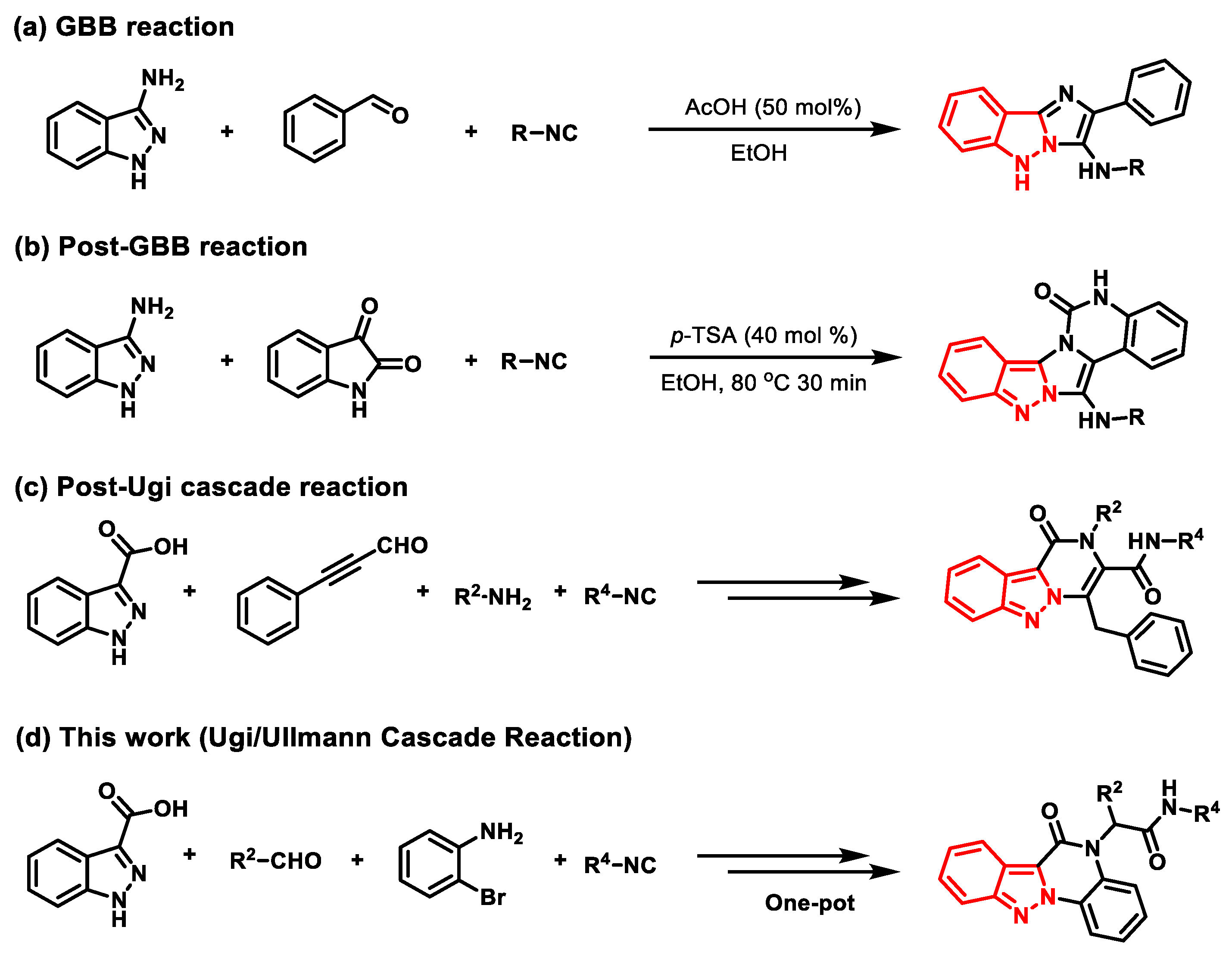
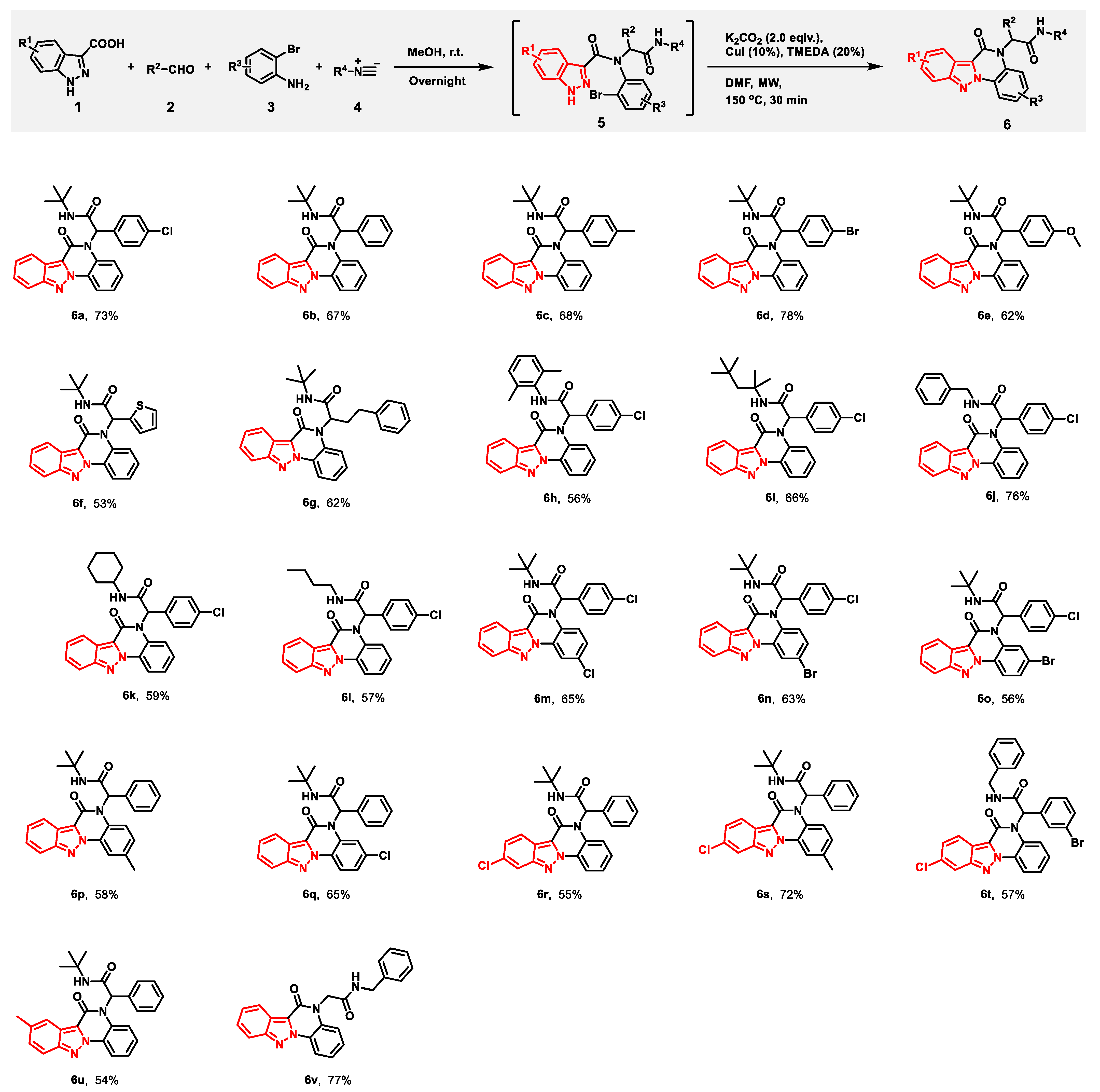
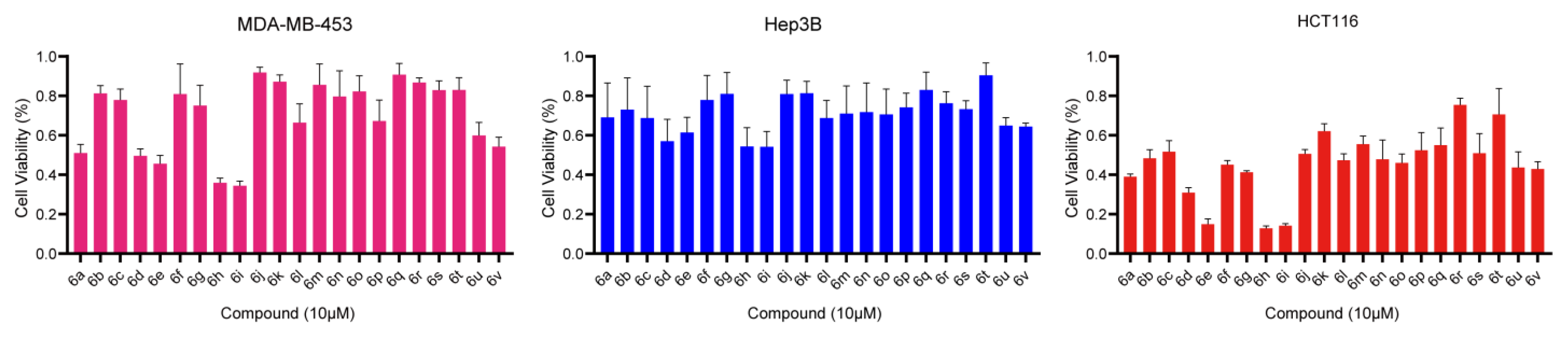
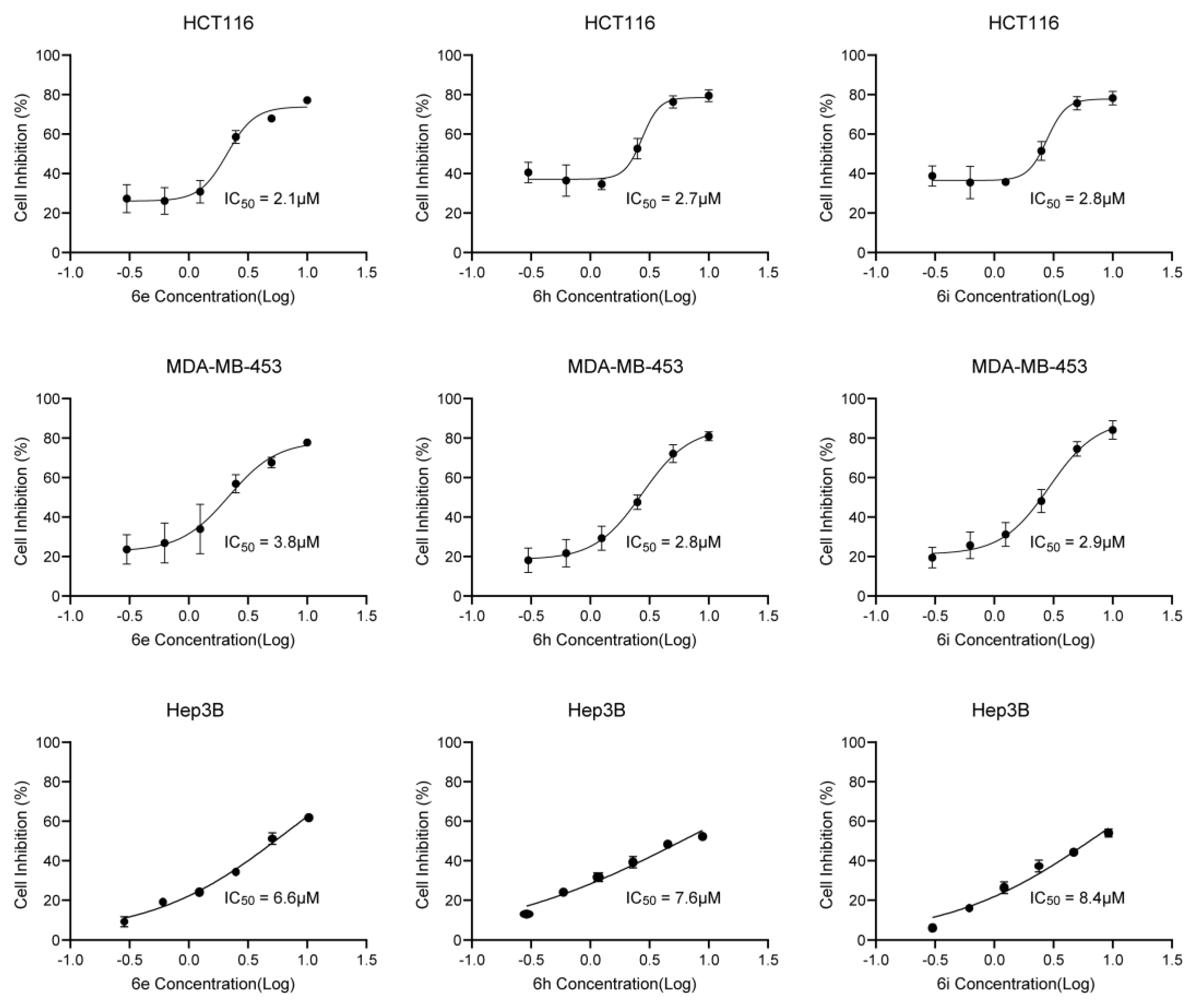
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Cell Lines and Culture
3.3. Cell Viability Assay
3.4. General Procedure for Synthesis of Compounds 6a–v
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flick, A.C.; Ding, H.X.; Leverett, C.A.; Kyne, R.E., Jr.; Liu, K.K.C.; Fink, S.J.; O’Donnell, C.J. Synthetic Approaches to the New Drugs Approved during 2015. J. Med. Chem. 2017, 60, 6480–6515. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.-M.; Damu, G.L.V.; Geng, R.-X.; Zhou, C.-H. Comprehensive Review in Current Developments of Imidazole-Based Medicinal Chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C.; Dinges, J. Bioactive Heterocyclic Compound Classes: Pharmaceuticals; Wiley-VCH: Singapore, 2010; pp. 3–16. [Google Scholar]
- Sharma, K.; Bhat, V.K. On Topological Descriptors of Polycyclic Aromatic Benzenoid Systems. Polycycl. Aromat. Comp. 2023, 43, 4111–4130. [Google Scholar] [CrossRef]
- Miao, Q. (Ed.) Polycyclic Arenes and Heteroarenes: Synthesis, Properties, and Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2016; ISBN 9783527338474. [Google Scholar]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Ghosh, S.; Hajra, A. Electrochemical Functionalization of Imidazopyridine and Indazole: An Overview. Adv. Synth. Catal. 2021, 363, 5047–5071. [Google Scholar] [CrossRef]
- Shafakat Ali, N.A.; Dar, B.; Pradhan, V.; Farooqui, M. Chemistry and Biology of Indoles and Indazoles: A Mini-Review. Mini-Rev. Med. Chem. 2013, 13, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, D.D.; Chapolikar, A.D.; Devkate, C.G.; Warad, K.D.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of Indazole Motifs and Their Medicinal Importance: An Overview. Eur. J. Med. Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef]
- Aman, W.; Lee, J.; Kim, M.; Yang, S.; Jung, H.; Hah, J.-M. Discovery of Highly Selective CRAF Inhibitors, 3-Carboxamido-2H-Indazole-6-Arylamide: In Silico FBLD Design, Synthesis and Evaluation. Bioorg. Med. Chem. Lett. 2016, 26, 1188–1192. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, G.; Liu, Y.; Shi, Y.; Gao, G.; Wu, D.; Lan, J.; You, J. Unparalleled Ease of Access to a Library of Biheteroaryl Fluorophores via Oxidative Cross-Coupling Reactions: Discovery of Photostable NIR Probe for Mitochondria. J. Am. Chem. Soc. 2016, 138, 4730–4738. [Google Scholar] [CrossRef]
- Liu, J.; Qian, C.; Zhu, Y.; Cai, J.; He, Y.; Li, J.; Wang, T.; Zhu, H.; Li, Z.; Li, W.; et al. Design, Synthesis and Evaluate of Novel Dual FGFR1 and HDAC Inhibitors Bearing an Indazole Scaffold. Bioorg. Med. Chem. 2018, 26, 747–757. [Google Scholar] [CrossRef]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Leger, J.M.; Deprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaitre, N.; Pehourcq, F.; et al. Synthesis, Antimalarial Activity, and Molecular Modeling of New Pyrrolo[1,2-a]quinoxalines, Bispyrrolo-[1,2-a]quinoxalines, Bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines, and Bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; et al. Synthesis and Evaluation of the Cytotoxic Activity of Novel Ethyl 4-[4-(4-substitutedpiperidin-1-yl)]-benzyl-phenylpyrrolo [1,2-a]quinoxaline-carboxylate Derivatives in Myeloid and Lymphoid Leukemia Cell Lines. Eur. J. Med. Chem. 2016, 113, 214–227. [Google Scholar] [CrossRef]
- Guillon, J.; LeBorgne, M.; Rimbault, C.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Baratin, S.; Marchivie, M.; Roche, S.; Bollacke, A.; et al. Synthesis and Biological Evaluation of Novel Substituted Pyrrolo[1,2-a]quinoxaline Derivatives as Inhibitors of the Human Protein Kinase CK2. Eur. J. Med. Chem. 2013, 65, 205–222. [Google Scholar] [CrossRef]
- Jones, E.D.; Vandegraaff, N.; Le, G.; Choi, N.; Issa, W.; Macfarlane, K.; Thienthong, N.; Winfield, L.J.; Coates, J.A.V.; Lu, L.; et al. Design of a series of bicyclic HIV-1 integrase inhibitors. Part 1: Selection of the scaffold. Bioorg. Med. Chem. Lett. 2010, 20, 5913–5917. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mason, K.A.; Ang, K.K.; Buchholz, T.; Valdecanas, D.; Mathur, A.; Buser-Doepner, C.; Toniatti, C.; Milas, L. MK-4827, a PARP-1/-2 inhibitor, strongly enhances response of human lung and breast cancer xenografts to radiation. Investig. New Drugs 2012, 30, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, F.; Berthelot, D.; De Cleyn, M.; Macdonald, G.; Minne, G.; Oehlrich, D.; Pieters, S.; Surkyn, M.; Trabanco, A.A.; Tresadern, G.; et al. Design and Synthesis of a Novel Series of Bicyclic Heterocycles as Potent γ-Secretase Modulators. J. Med. Chem. 2012, 55, 9089–9106. [Google Scholar] [CrossRef]
- Bridges, K.A.; Hirai, H.; Buser, C.A.; Brooks, C.; Liu, H.; Buchholz, T.A.; Molkentine, J.M.; Mason, K.A.; Meyn, R.E. MK-1775, a Novel Wee1 Kinase Inhibitor, Radiosensitizes p53-Defective Human Tumor Cells. Clin. Cancer Res. 2011, 17, 5638–5648. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Altamura, S.; Boueres, J.; Ferrigno, F.; Fonsi, M.; Giomini, C.; Lamartina, S.; Monteagudo, E.; Ontoria, J.M.; Orsale, M.V.; et al. Discovery of 2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): A Novel Oral Poly(ADPribose)polymerase (PARP) Inhibitor Efcacious in BRCA-1 and -2 Mutant Tumors. J. Med. Chem. 2009, 52, 7170–7185. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; De Oliveira, E.; Ottenhof, N.; Watters, J.; Brooks, D.; Demuth, T.; Shumway, S.D.; Mizuarai, S.; Hirai, H.; Maitra, A.; et al. MK-1775, a Potent Wee1 Inhibitor, Synergizes with Gemcitabine to Achieve Tumor Regressions, Selectively in p53-Defcient Pancreatic Cancer Xenografs. Clin. Cancer Res. 2011, 17, 2799–2806. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, C.; Yamane, M. Pd/Cu Cooperative Catalysis: An Efficient Synthesis of (3-isoindazolyl)allenes via Cross-Coupling of 2-Alkynyl Azobenzenes and Terminal Alkynes. Chem. Commun. 2017, 53, 2606–2609. [Google Scholar] [CrossRef]
- Guo, C.; Li, B.; Liu, H.; Zhang, X.; Zhang, X.; Fan, X. Synthesis of Fused or Spiro Polyheterocyclic Compounds via the Dehydrogenative Annulation Reactions of 2-Arylindazoles with Maleimides. Org. Lett. 2019, 21, 7189–7193. [Google Scholar]
- Lin, W.-C.; Yang, D.-Y. Visible Light Photoredox Catalysis: Synthesis of Indazolo[2,3-a]quinolines from 2-(2-Nitrophenyl)-1,2,3,4-tetrahydroquinolines. Org. Lett. 2013, 15, 4862–4865. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, S.-G.; Wang, L.-S.; Ma, J.-T.; Yu, Z.-C.; Wu, Y.-D.; Wu, A.-X. I2-Promoted gem-Diarylethene Involved Aza-Diels–Alder Reaction and Wagner–Meerwein Rearrangement: Construction of 2,3,4-Trisubstituted Pyrimido[1,2-b]indazole Skeletons. Org. Lett. 2023, 25, 3386–3390. [Google Scholar] [CrossRef]
- Balwe, S.G.; Jeong, Y.T. An Approach towards the Synthesis of Novel Fused Nitrogen Tricyclic Heterocyclic Scaffolds via GBB reaction. Org. Biomol. Chem. 2018, 16, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Balwe, S.G.; Jeong, Y.T. One-step Construction of Complex Polyheterocycles via a Sequential Post-GBB Cyclization/Spiro Ring Expansion Triggered by a [1,5]-Hydride Shift. Org. Chem. Front. 2018, 5, 1628–1632. [Google Scholar] [CrossRef]
- Xu, J.; Tan, H.-B.; Zhang, Y.-J.; Tang, D.-Y.; Zhan, F.; Li, H.; Chen, Z.-Z.; Xu, Z.-G. Catalyst-Free One-pot Synthesis of Densely Substituted Pyrazolepyrazines as Anti-colorectal Cancer Agents. Sci. Rep. 2020, 10, 9281. [Google Scholar] [CrossRef]
- Russo, C.; Brunelli, F.; Tron, G.C.; Giustiniano, M. Isocyanide-Based Multicomponent Reactions Promoted by Visible Light Photoredox Catalysis. Chem. Eur. J. 2023, 29, e202203150. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Hu, R.; Tang, B.Z. Multicomponent Reactions in Polymer Science. Macrmol. Rapid Comm. 2021, 42, 2100104. [Google Scholar] [CrossRef]
- Cimarelli, C. Multicomponent Reactions. Molecules 2019, 24, 2372. [Google Scholar] [CrossRef]
- Garbarino, S.; Ravelli, D.; Protti, S.; Basso, A. Photoinduced Multicomponent Reactions. Angew. Chem. Int. Ed. 2016, 55, 15476–15484. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multi-component Reactions: Advanced Tools for Sustainable Organic Synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Marcaccini, S.; Torroba, T. The Use of the Ugi Four-Component Condensation. Nat. Protoc. 2007, 2, 632–639. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, N.; Vachhani, D.D.; Van der Eycken, E.V. Metal-mediated Post-Ugi Transformations for the Construction of Diverse Heterocyclic Scaffolds. Chem. Soc. Rev. 2015, 44, 1836–1860. [Google Scholar] [CrossRef]
- Xu, Z.-G.; Li, S.-Q.; Meng, J.-P.; Tang, D.-Y.; He, L.-J.; Lei, J.; Lin, H.-K.; Li, H.-Y.; Chen, Z.-Z. Functionalized Spiroindolines with Anticancer Activity through a Metal-Free Post-Ugi Diastereoselective One-Pot Cascade Reaction. Chem. Eur. J. 2018, 24, 6732–6736. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Song, L.; Van der Eycken, E.V. Post-Ugi Cyclizations towards Polycyclic N-Heterocycles. Chem. Rec. 2023, 23, e202300095. [Google Scholar] [CrossRef]
- Fouad, M.A.; Abdel-Hamid, H.; Ayoup, M.S. Two Decades of Recent Advances of Ugi Reactions: Synthetic and Pharmaceutical applications. RSC Adv. 2020, 10, 42644–42681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Saxena, A.; Patra, R.; Ray, D.; Li, H.; Saha, B. Synthesis of Fused Polycyclic β-carboline Derivatives Using Ugi-4CR Followed by Cascade Cyclization. Mol. Divers. 2023, 27, 951–957. [Google Scholar] [CrossRef]
- Lei, J.; Meng, J.-P.; Tang, D.-Y.; Frett, B.; Chen, Z.-Z.; Xu, Z.-G. Recent Ad vances in the Development of Polycyclic Skeletons via Ugi Reaction Cascades. Mol. Divers. 2018, 22, 503–516. [Google Scholar] [CrossRef]
- Li, Z.; Song, L.; Meervelt, L.V.; Tian, G.; Van der Eycken, E.V. Cationic Gold (I)-Catalyzed Cascade Bicyclizations for Divergent Synthesis of (Spiro) Polyheterocycles. ACS Catal. 2018, 8, 6388–6393. [Google Scholar] [CrossRef]
- Liu, C.; Song, L.; Van Meervelt, L.; Peshkov, V.A.; Li, Z.; Van der Eycken, E.V. Palladium-Catalyzed Arylative Dearomatization and Subsequent Aromatization/Dearomatization/aza-Michael Addition: Access to Zephycarinatine and Zephygranditine Skeletons. Org. Lett. 2021, 23, 5065–5070. [Google Scholar] [CrossRef]
- Li, X.; Zarganes-Tzitzikas, T.; Kurpiewska, K.; Dömling, A. Amenamevir by Ugi-4CR. Green Chem. 2023, 25, 1322–1325. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; He, L.-J.; Luo, Y.-F.; Meng, J.-P.; Tang, D.-Y.; Li, H.; Chen, Z.-Z.; Xu, Z.-G. Dieckmann Condensation of Ugi N-Acylamino Amide Product: Facile Access to Functionalized 2,2-Disubstituted Indolin-3-ones. J. Org. Chem. 2022, 87, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.-H.; Wang, J.-L.; Song, G.-T.; Tang, D.-Y.; Yao, F.; Lin, H.; Yan, W.; Li, H.; Xu, Z.-G.; et al. Diversity-Oriented Synthesis of Imidazo-Dipyridines with Anticancer Activity via the Groebke-Blackburn-Bienaymé and TBAB-Mediated Cascade Reaction in One Pot. J. Org. Chem. 2019, 84, 12632–12638. [Google Scholar] [CrossRef]
- Yang, D.-L.; Qin, H.-X.; Zhang, N.-N.; Zhang, Y.-J.; Huang, J.-H.; Hu, C.-S.; Zhang, X.-X.; Li, Y.; He, L.-J. Pyrrolyldihydropyrazino [1,2- a]indoletrione Analogue Microtubule Inhibitor Induces Cell-Cycle Arrest and Apoptosis in Colorectal Cancer Cells. Molecules 2023, 28, 1948. [Google Scholar] [CrossRef] [PubMed]
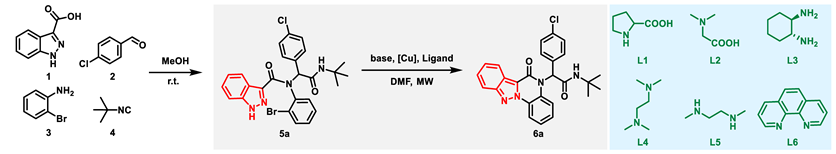 | ||||||
|---|---|---|---|---|---|---|
| Entry | Base (2.0 Eqiv.) | Cat. (10 mol%) | Ligand (20 mol%) | Temp. (°C) | Time (Min) | Yield of 6a (%) b |
| 1 | K2CO3 | / | / | MW 120 | 10 min | Trace |
| 2 | K2CO3 | / | / | MW 150 | 10 min | 25 |
| 3 | K2CO3 | / | / | MW 150 | 30 min | 55 |
| 4 | KOtBu | / | / | MW 150 | 30 min | 31 |
| 5 | Cs2CO3 | / | / | MW 150 | 30 min | 43 |
| 6 | TEA | / | / | MW 150 | 30 min | Trace |
| 7 | DBU | / | / | MW 150 | 30 min | 15 |
| 8 | DMAP | / | / | MW 150 | 30 min | 17 |
| 9 | K2CO3 | CuI | L1 | MW 150 | 30 min | 57 |
| 10 | K2CO3 | CuI | L2 | MW 150 | 30 min | 67 |
| 11 | K2CO3 | CuI | L3 | MW 150 | 30 min | 79 |
| 12 | K2CO3 | CuI | L4 | MW 150 | 30 min | 95 |
| 13 | K2CO3 | CuI | L5 | MW 150 | 30 min | 83 |
| 14 | K2CO3 | CuI | L6 | MW 150 | 30 min | 73 |
| 15 | K2CO3 | CuBr | L4 | MW 150 | 30 min | 65 |
| 16 | K2CO3 | Cu2O | L4 | MW 150 | 30 min | 53 |
| 17 | K2CO3 | Cu(OTf)2 | L4 | MW 150 | 30 min | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; He, L.; Qin, H.; Liu, Y.; Yang, B.; Xu, Z.; Yang, D. A Facile Ugi/Ullmann Cascade Reaction to Access Fused Indazolo-Quinoxaline Derivatives with Potent Anticancer Activity. Molecules 2024, 29, 464. https://doi.org/10.3390/molecules29020464
Li Y, He L, Qin H, Liu Y, Yang B, Xu Z, Yang D. A Facile Ugi/Ullmann Cascade Reaction to Access Fused Indazolo-Quinoxaline Derivatives with Potent Anticancer Activity. Molecules. 2024; 29(2):464. https://doi.org/10.3390/molecules29020464
Chicago/Turabian StyleLi, Yong, Liujun He, Hongxia Qin, Yao Liu, Binxin Yang, Zhigang Xu, and Donglin Yang. 2024. "A Facile Ugi/Ullmann Cascade Reaction to Access Fused Indazolo-Quinoxaline Derivatives with Potent Anticancer Activity" Molecules 29, no. 2: 464. https://doi.org/10.3390/molecules29020464
APA StyleLi, Y., He, L., Qin, H., Liu, Y., Yang, B., Xu, Z., & Yang, D. (2024). A Facile Ugi/Ullmann Cascade Reaction to Access Fused Indazolo-Quinoxaline Derivatives with Potent Anticancer Activity. Molecules, 29(2), 464. https://doi.org/10.3390/molecules29020464







