Abstract
The high electrons and holes recombination rate of ZnIn2S4 significantly limits its photocatalytic performance. Herein, a simple in situ photodeposition strategy is adopted to introduce the cocatalyst cobalt phosphate (Co-Pi) on ZnIn2S4, aiming at facilitating the separation of electron–hole by promoting the transfer of photogenerated holes of ZnIn2S4. The study reveals that the composite catalyst has superior photocatalytic performance than blank ZnIn2S4. In particular, ZnIn2S4 loaded with 5% Co-Pi (ZnIn2S4/5%Co-Pi) has the best photocatalytic activity, and the H2 production rate reaches 3593 μmol·g−1·h−1, approximately double that of ZnIn2S4 alone. Subsequent characterization data demonstrate that the introduction of the cocatalyst Co-Pi facilitates the transfer of ZnIn2S4 holes, thus improving the efficiency of photogenerated carrier separation. This investigation focuses on the rational utilization of high-content and rich cocatalysts on earth to design low-cost and efficient composite catalysts to achieve sustainable photocatalytic hydrogen evolution.
1. Introduction
Rapid economic and social development depends on fossil fuels. However, due to the non-renewable nature of fossil fuels and the detrimental impact on the environment, it is imperative that we urgently seek sustainable energy sources capable of replacing them [1,2,3,4,5]. Hydrogen (H2) energy, as a clean and renewable energy source, is one of the most promising alternative energy sources for fossil fuels [6,7,8]. Among various H2 production methods, solar-driven water splitting for H2 production is considered as a green and sustainable solar energy conversion technology, which can relieve the pressure of energy dilemma and environmental pollution [9,10,11,12]. Consequently, there is an urgent need to develop photocatalysts with high performance to promote the application of photocatalytic H2 evolution technology [13]. Nowadays, due to their remarkable light absorption properties and special electronic structures, metal sulfides have become a hot topic in the field of solar energy conversion technology.
As a ternary sulfide, ZnIn2S4 has attracted global attention from researchers on account of its favorable layered structure, simple synthesis, good photostability and suitable electronic band structure [14,15]. In particular, the flower-like structure has a high surface area and improves the light absorption through multiple reflections, which plays an important role in enhancing the photocatalytic performance [16,17,18]. However, due to the high recombination rate of photogenerated electron–hole pairs, pure ZnIn2S4 exhibits low photocatalytic activity [19,20,21,22]. To address this problem, the rational introduction of cocatalyst is a viable approach to optimize the activity and stability of ZnIn2S4 [23]. Among the many cocatalysts, cobalt phosphate (Co-Pi) has demonstrated remarkable ability to transfer photogenerated holes from different light-collecting semiconductors in previous studies and has been reported to improve their overall performance [24]. Therefore, the rational introduction of the holes cocatalyst Co-Pi into ZnIn2S4 is expected to obtain a cost-effective and efficient composite photocatalyst to promote photocatalytic H2 evolution. Moreover, in situ photodeposition is considered to be a promising method to enhance the photocatalytic activity of semiconductors, due to its advantages such as close contact, simple preparation and directional loading [25,26,27]. Consequently, rationally introducing Co-Pi into ZnIn2S4 by in situ photodeposition is expected to promote the migration of photogenerated holes of ZnIn2S4, thereby improving the photocatalytic performance of the composite photocatalyst.
Herein, we prepare the ZnIn2S4 nanoflower substrate material by the hydrothermal method, and the hybrid catalyst is constructed by in situ photodeposition of cobalt phosphate (Co-Pi) on ZnIn2S4 nanoflower. The ZnIn2S4/Co-Pi composite exhibits a significantly enhanced performance in the photocatalytic H2 evolution compared to pure ZnIn2S4. Notably, the optimal ZnIn2S4/5%Co-Pi photocatalytic H2 production rate is 3593 μmol·g−1·h−1, which surpasses most similar hybrid cocatalyst systems reported in the literature (Table 1). The photo/electrochemical tests and photoluminescence (PL) confirm that the photogenerated carrier separation efficiency of the composite catalyst is significantly improved. This work aims to provide insights for designing cost-effective and efficient mixed catalysts to enhance overall photocatalytic performance through rationally exploiting earth-abundant cocatalysts.

Table 1.
Comparison of the hydrogen production properties of the ZnIn2S4-based catalysts.
2. Results and Discussion
The preparation process diagram of the ZnIn2S4/Co-Pi (ZIS/Co-Pi) composite is shown in Figure 1a. Initially, ZnIn2S4 (ZIS) nanoflower is prepared by a one-step hydrothermal process. Subsequently, Co-Pi is introduced to ZIS nanoflower by in situ photodeposition to obtain ZIS/Co-Pi composites. Due to the best photocatalytic H2 production performance of ZnIn2S4/5%Co-Pi (Z5CP), we mainly discuss this proportion of the composites in the subsequent characterization. According to Figure S1a,b, the color of ZIS nanoflower changes significantly before and after in situ photodeposition, with pure ZIS appearing as bright yellow, and Z5CP appearing as yellowish green. The morphology and microstructure of different samples are obtained by field emission scanning electron microscopy (FESEM). As depicted in Figure 1b, pure ZIS presents a spherical flower-like structure with a diameter of about 1 μm. The SEM image of Z5CP (Figure 1c) shows that Z5CP inherits the flower-like structure of ZIS. Notably, the flower-like structure can provide a number of active sites, and multiple layers of petals enable light to be reflected multiple times, which leads to enhanced light absorption [36,37]. In addition, the SEM image of Z5CP shows that the Co-Pi nanoparticles are highly dispersed, and no large Co-Pi particles were observed. As presented in Figure 1d, transmission electron microscopy (TEM) characterization further confirms the spherical flower-like structure of ZIS. Moreover, Figure 1e shows that the Co-Pi nanoparticles are attached to the ZIS nanoflower, proving the successful synthesis of Z5CP composites. As depicted in Figure 1f, the lattice distance of Z5CP is about 0.297 nm corresponding to the (104) crystal face of ZIS, and the Co-Pi synthesized by in situ photodeposition is amorphous. Furthermore, the EDS spectra (Figure S2) and the element mapping results (Figure 1g) confirm the existence of Zn, In, S, P, O, and Co elements in Z5CP. The spatial distribution of Zn, In, S, O, P, and Co elements in the elemental mapping images of Z5CP composite shows that Co-Pi grows uniformly on the surface of ZIS nanoflower.
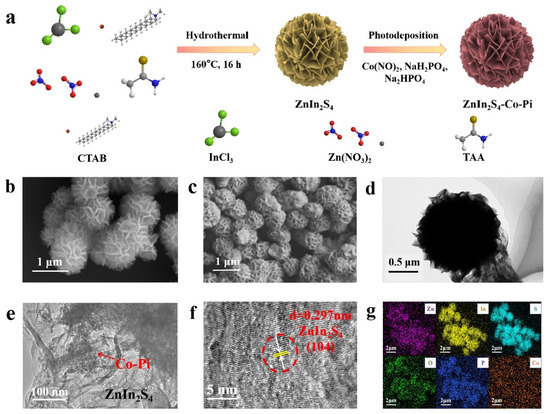
Figure 1.
(a) Diagram illustrating the synthesis of ZIS/Co-Pi. (b,c) FESEM images of ZIS and Z5CP. (d–f) TEM images of Z5CP. (g) Mapping analysis results of Z5CP.
The phase structure and crystallinity are analyzed by the X-ray diffraction (XRD) map. Figure 2a displays the XRD spectra of both ZIS and Z5CP. For ZIS, the strong diffraction peaks at 27.5° and 47.2° belong to the (102) and (110) faces of hexagonal ZIS (JCPDS No.65-2023) [38]. For Z5CP composites, the XRD diffraction curve closely resembles that of ZIS except that there is a faint peak at 55.6° belonging to the (202) face of hexagonal ZIS, indicating that ZIS remains a stable crystal structure after coupling with Co-Pi [39]. However, in the Z5CP composite, the characteristic diffraction peak of Co-Pi is not observed due to the amorphous nature of in situ photodeposition of Co-Pi [40,41]. The optical characteristics of the photocatalysts are analyzed by UV-visible diffuse reflection spectroscopy (DRS). As depicted in Figure 2b, the pure ZIS displays a clear absorption edge around 520 nm, indicating a band gap of about 2.44 eV [42]. Compared with pure ZIS, the absorption intensity of Z5CP hybrid in the visible range (520~750 nm) increases with the strong absorption of Co-Pi, indicating that the introduction of Co-Pi can improve the visible light response of ZIS. Moreover, Figure 2b shows that there is no significant shift in absorption edge for the Z5CP composite, indicating that the Co-Pi cocatalyst only deposits on the ZIS surface and does not bind with the crystal lattice.
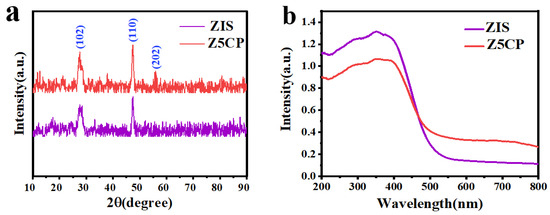
Figure 2.
(a) X-ray diffraction (XRD) patterns and (b) UV–vis diffuse reflectance spectra (DRS) of ZIS and Z5CP.
The chemical composition and elemental states of Z5CP composite are further determined by X-ray photoelectron spectroscopy (XPS). As presented in Figure 3a, Zn, In, S, Co, and P elements exist in the hybrid products, which further demonstrates the successful photodeposition of Co-Pi on the surface of ZIS nanoflower. As shown in Figure 3b, the XPS spectrum of Zn 2p exhibits two distinct peaks at 1045 and 1022 eV, which correspond to the binding energies of Zn 2p1/2 and Zn 2p3/2 of Zn2+, respectively. From the XPS spectrum of In 3d (Figure 3c), two peaks that center on binding energies 452.4 and 444.8 eV are respectively associated with In 3d3/2 and In 3d5/2, which indicate the +3 state of In. Moreover, as presented in Figure 3d, the peaks of 162.9 and 161.7 eV belong to S 2p1/2 and S 2p3/2, confirming the presence of S2−. In the XPS spectrum of Co 2p (Figure 3e), the peak of Co 2p3/2 is at 781.3 eV (satellite peak at 784.3 eV), indicating the presence of Co2+ in the Z5CP composite [43,44,45]. In addition, the peak of P 2p (Figure 3f) at 133.5 eV indicates that P presents in the form of phosphate groups, which further proves the successful synthesis of Z5CP [46].
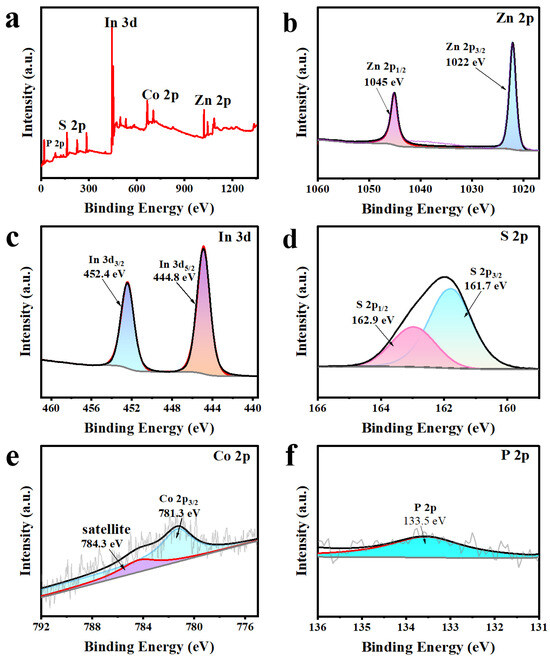
Figure 3.
(a) XPS spectra of Z5CP, high-resolution spectra of (b) Zn 2p, (c) In 3d, (d) S 2p, (e) Co 2p, (f) P 2p.
Photocatalytic H2 production is performed with triethanolamine (TEOA) as the hole scavenger, and the photocatalytic properties of pure ZIS and different proportions of ZIS/Co-Pi composites under visible light are investigated. Figure 4a is a diagram of the photocatalytic activity of ZIS and composite with 1%, 5%, and 10% Co-Pi (hereinafter shown as Z1CP, Z5CP, and Z10CP, respectively). As shown in Figure 4a, due to the fast photogenerated electron–hole recombination rate, the pure ZIS is less active and the H2 evolution rate is only 1832 μmol∙g−1∙h−1. After the introduction of Co-Pi cocatalyst, Z1CP, Z5CP, and Z10CP all show better H2 evolution performance compared with blank ZIS. With the increase in Co-Pi content, the hydrogen yield increases gradually. However, when the Co-Pi content increases further, the H2 evolution activity decreases, which may be due to the remarkable shielding effect of Co-Pi, thereby decreasing the photocatalytic active sites [47]. In particular, the Z5CP composite shows the highest H2 evolution rate (3593 μmol∙g−1∙h−1), approximately two times higher than that of ZIS alone. This can be attributed to the fact that in situ photodeposition of Co-Pi promotes the transfer of photogenerated holes and reduces the recombination rate of photogenerated carriers. As shown in Table 1, the Z5CP composite prepared in this work has optimal photocatalytic H2 production properties compared with the photocatalytic H2 production activities of some representative ZIS-based composites reported in recent years. In addition, the stability of Z5CP is tested by the cyclic test. As depicted in Figure 4b, after five cycles, no apparent deactivation has been observed for Z5CP composite, indicating the excellent stability of Z5CP composite.
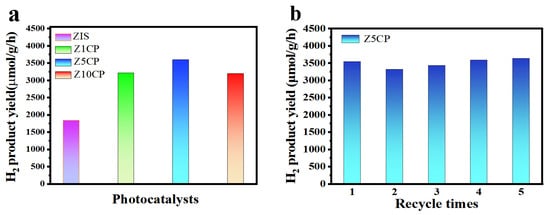
Figure 4.
(a) Photocatalytic H2 production over pure ZIS and Z5CP composites. (b) Stability plots of the photocatalytic H2 production by Z5CP.
Photo/electrochemical tests are used to further characterize material reducing capacity and photogenerated carrier transfer efficiency. Linear sweep voltammetry (LSV) is first used to determine the H2 evolution performance of ZIS and Z5CP samples. Figure 5a shows the polarization curve of ZIS and Z5CP composites. It can be seen that the overpotential of Z5CP is less than ZIS at the same current density, indicating that the H2 evolution performance of Z5CP is better than that of ZIS [48]. The kinetics of photocatalysis in different samples can be compared by the Tafel slope. As shown in Figure S3, the Tafel slope of the Z5CP composite (0.21 V/decade) is smaller than that of ZIS (0.24 V/decade), indicating the better reduction effect and interfacial charge transfer efficiency of Z5CP, which is consistent with the photocatalytic H2 production activity as well as other characterization results [49]. These results further demonstrate that Z5CP has faster reaction kinetics and excellent interface carrier separation efficiency. To study the charge separation and transfer of these ZIS/Co-Pi composites, instantaneous photocurrent (IT), electrochemical impedance spectroscopy (EIS) and steady-state photoluminescence (PL) spectra are measured on the ZIS and Z5CP samples [50]. As illustrated in Figure 5b, the optical current density of ZIS is small, indicating that the photogenerated carrier separation efficiency of ZIS is poor. However, it is found that after the introduction of Co-Pi, the optical current density of Z5CP is significantly improved compared with that of pure ZIS, indicating that Z5CP has better separation efficiency of electron (e−) and hole (h+) [51,52,53,54,55]. As shown in Figure 5c, the radius of curvature of Z5CP composite is smaller than ZIS, indicating that the charge transfer resistance of Z5CP is lower, which improves the separation and transfer rate of photogenerated carriers, thus enhancing the photocatalytic activity [56,57,58,59,60]. Furthermore, Figure 5d describes the steady−state photoluminescence (PL) spectra test of the sample. As shown in Figure 5d, the PL intensity of Z5CP is significantly lower than that of blank ZIS, indicating that the addition of cocatalyst Co-Pi effectively inhibits the recombination of photogenerated carriers [61,62,63,64,65]. Taken together, the results of these photo/electrochemical tests validate the improved separation and transfer of photogenerated charges in Z5CP, leading to the enhanced performance of photocatalytic H2 evolution.
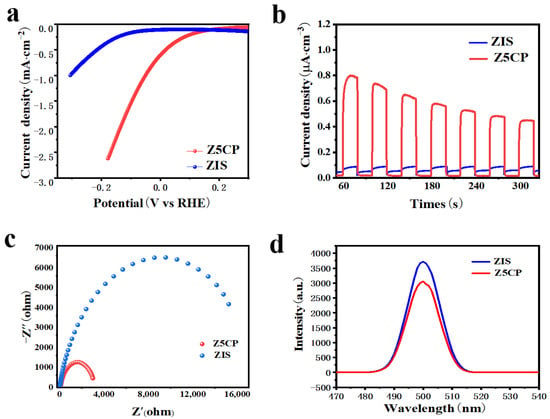
Figure 5.
(a) Polarization curves. (b) Transient photocurrent spectra. (c) EIS Nyquist plots. (d) Steady−state photoluminescence (PL) emission spectra with an excitation wavelength of 500 nm.
The information of chemical reaction area of the blank ZIS and the composite material Z5CP is obtained by the cyclic voltammetry test (CV). Figure 6a,b show the cyclic voltammetry (CV) curves of the blank ZIS and Z5CP composites, respectively. As illustrated in Figure 6c, the double-layer capacitance of Z5CP composite (3.99 μF·cm−2) is significantly larger than ZIS (1.83 μF·cm−2), which strongly proves that Z5CP has more active sites area than ZIS [45]. In addition, the flat charged position (Efb) of the original ZIS is measured with Mott–Schottky (MS). Generally, the slope of the positive one indicates that the semiconductor is an intrinsic n-type semiconductor [51]. As can be seen from Figure 6d, ZIS belongs to the n-type semiconductor. Moreover, Figure S4 shows the detailed fitting parameters of MS. According to the x-intercept of the block, its Efb is determined to be −0.52 V (vs. Ag/AgCl). In general, the conduction band position of n-type semiconductors is about 0.2 V more negative than that of Efb [66,67,68]. Therefore, the conduction charge position (ECB) of the ZIS is −0.72 V (vs. Ag/AgCl). From the formula ENHE = EAg/AgCl + 0.20 V, the ECB of ZIS is −0.52 V (vs. NHE). According to the band gap of ZIS (2.44 eV), the valence band potential (EVB) of ZIS is 1.92 V (vs. NHE).
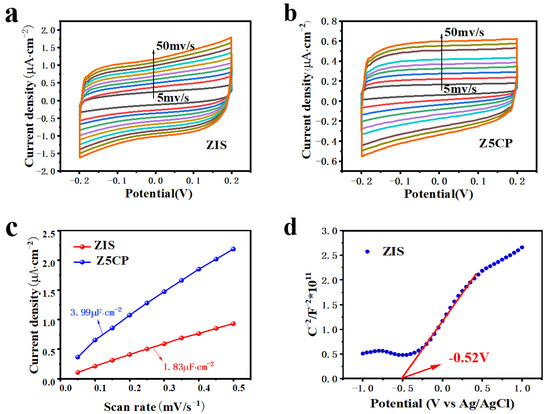
Figure 6.
(a,b) Cyclic voltammetry curves of the ZIS and Z5CP. (c) Current density scan rate plot. (d) Mott−Schottky plots for ZIS.
Combined with the above experiments and characterization, we propose a viable mechanism for photocatalytic H2 production of Z5CP under visible light. As shown in Figure 7, under visible light irradiation, Z5CP effectively absorbs the photon energy, and then the electrons on the valence band (VB) are excited and transition to the conduction band (CB), and the corresponding positive electric holes are generated on the valence band (VB). The electron (e−) migrated to the semiconductor surface binds to the H+ adsorbed in water to form H2. However, ZIS has a high electrons and holes recombination rate; therefore, its photocatalytic activity is limited. Notably, Co-Pi has the excellent property of transferring photogenerated holes, and the holes of ZIS are transferred to Co-Pi and drive cycles to catalyze the Co2+/3+ → Co4+→ Co2+/3+ reaction [24]. At the same time, ZIS rapidly exports holes to oxidize the sacrificial reagent of triethanolamine (TEOA); therefore, the resulting photogenerated hole (h+) is effectively separated and consumed by it. Therefore, the photogenerated carrier separation efficiency of the composite photocatalyst Z5CP is improved, which allows more electrons to transfer to the catalyst surface to react with H+ to produce more H2. This is also the main factor for the significant improvement of the photocatalytic H2 evolution performance of Z5CP composite.
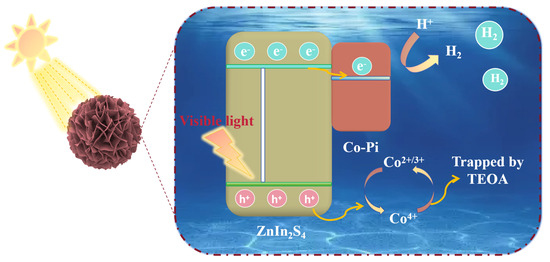
Figure 7.
Mechanism diagram of Z5CP in the visible light-driven photocatalytic H2 production reaction.
3. Experimental Section
3.1. Materials
Concentrated sulfuric acid (H2SO4), triethanolamine (C6H15NO3, TEOA), anhydrous ethanol (C2H5OH), N,N-dimethylformamide (C3H7NO), disodium hydrogen phosphate dihydrate (Na2HPO4·2H2O), and sodium dihydrogen phosphate tetrahydrate (NaH2PO4·4H2O) are supplied by Xilong Scientific Co., Ltd. (Shantou, China). Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), cetyltrimethylammonium bromide (C19H42BrN, CTAB), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), indium chloride tetrahydrate (InCl3·4H2O), and Nafion solution (5 wt%) (C9HF17O5S) are supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
3.2. Synthesis of ZnIn2S4 (ZIS)
Typically, Zn(NO3)2·6H2O (304.2 mg), InCl3·4H2O (624.4 mg), and cetyltrimethyl-ammonium bromide (CTAB) (230.6 mg) were added to a beaker containing 20 mL of deionized water and magnetically stirred for 30 min. Then, the thioacetamide (604.8 mg) was added to a beaker containing 10 mL deionized water and mixed to the above solution. Afterwards, the mixture was added to a Teflon liner and stirred for 30 min, and the liner was transferred to stainless steel autoclave heating in an oven at 433 K for 16 h. After cooling, the products were separated by filtration and washed several times with deionized water and ethanol. The resulting samples were dried under vacuum at 333 K for 12 h. Ultimately, a bright yellow solid was obtained.
3.3. Synthesis of ZnIn2S4/Co-Pi (ZIS/Co-Pi)
In a typical experiment, the prepared 200 mL (0.1 mol/L) NaH2PO4 and 200 mL (0.1 mol/L) Na2HPO4 solution were mixed and adjusted with pH to around 7. Subsequently, 80 mL of neutral buffer was measured, and the calculated amount of Co(NO3)2·6H2O was added to make it evenly dispersed by ultrasound. Furthermore, 40 mg of ZnIn2S4 was weighed and introduced into the aforementioned system which was then sealed using a sealing ring with several ventilation holes. Then, the system was subjected to Ar gas flow under magnetic stirring for 30 min followed by irradiation from a xenon lamp while maintaining stirring for an additional duration of 60 min after sealing. After the photodeposition, the samples were filtered with deionized water, and the samples were obtained after vacuum drying at 333 K for 12 h. The loading amount of Co-Pi in ZIS/xCo-Pi was altered by changing the amount of Co(NO3)2·6H2O. In the experimental design, the loading ratios of deposited Co-Pi in ZnIn2S4 are 1%, 5%, and 10%, respectively.
3.4. Activity Evaluation of Photocatalytic H2 Evolution
Photocatalytic H2 production was performed in a 50 mL airtight quartz reactor. In the entire quartz reactor, 5 mg of the catalyst was dispersed into a solution containing 5 mL of deionized water and 1 mL of triethanolamine (TEOA). Before the reaction, high purity Ar was injected into the quartz reactor for 30 min to exhaust the residual air in the reactor. A 300 W xenon lamp (λ > 420 nm) was selected as the light source, and after 2 h of illumination, 1 mL of gas was extracted into the gas chromatograph (thermal conductivity detector TCD, Agilent Technologies GC 7820A, Santa Clara, CA, USA) to detect the hydrogen yield obtained after the reaction. In order to evaluate the stability of ZIS/Co-Pi composite, the photocatalyst was separated and centrifuged. The recovered photocatalyst is then subjected to a subsequent cycle under the same conditions.
3.5. Characterization Methods
The morphological characteristics were tested through scanning electron microscopy (SEM, FESEM ZEISS sigma 500, Oberkochen, Batenwerburg, Germany) and transmission electron microscopy (TEM, Jeol JEM-2100F instrument, Jeol, Akishima, Tokyo). The determination of crystal structures was determined by X-ray diffraction (XRD) with Cu Kα (λ = 0.15406 nm, Bruker D8 Advance, Billerica, MA, USA). The surface composition of the samples was determined by X-ray photoelectron spectrometer (XPS, Thermo Fisher, K-Alpha, Waltham, MA, USA). The UV-visible diffuse reflectance spectrometer (DRS, Shimadzu UV-2600, Kyoto, Japan) was used to test the optical response of the catalyst. Photoluminescence (PL) spectra were obtained using a spectrofluorometer (FLS 980, Edinburgh Instruments Ltd., Edinburgh, UK) with an excitation wavelength of 500 nm. Furthermore, all the electrochemical measurements of the photocurrent, the electrochemical impedance spectra (EIS), the Mott–Schottky (MS), cyclic voltammetry (CV), and linear sweep voltammetry (LSV) curves were carried out in the three-electrode cell, in which Ag/AgCl was used as a reference electrode, a Pt wire was used as a counter electrode, and an indium in oxide (ITO) conductive glass was used with the samples as a working electrode in 0.1 M Na2SO4 electrolyte (pH = 7.56), all measurements were carried out on CH instruments CHI-660E electrochemical workstation (Shanghai Chenhua CHI-660E, Shanghai, China).
4. Conclusions
In summary, we synthesize spherical ZnIn2S4 nanoflower substrate material by the hydrothermal method, and reasonably construct a novel photocatalyst of indium zinc sulfide/cobalt phosphate (ZnIn2S4/Co-Pi) hybrid photocatalyst by the in situ photodeposition method. In the presence of cocatalyst cobalt phosphate (Co-Pi), the hybrid photocatalyst shows outstanding photocatalytic hydrogen evolution performance. Through changing the photodeposition amount of Co-Pi, it is observed that the highest H2 production rate of indium zinc sulfide (ZnIn2S4/5% Co-Pi) loaded with 5% cobalt phosphate (Co-Pi) is 3593 μmol·g−1·h−1, which is significantly higher than that of pure ZnIn2S4. The steady-state photoluminescence (PL) and electrochemical impedance spectroscopy (EIS) of the photocatalyst show that ZnIn2S4/Co-Pi composite has weaker PL intensity and lower charge transport resistance than blank ZnIn2S4, demonstrating that the hybrid photocatalyst has faster electron transfer and charge separation. Simultaneously, the larger double-layer capacitance and smaller overpotential of catalyst indicate that ZnIn2S4/Co-Pi composite has larger active area and better hydrogen evolution performance. This work makes reasonable use of the earth-abundant cocatalysts to design low-cost and efficient composite catalysts to promote the prospect of photocatalytic hydrogen evolution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29020465/s1, Figure S1: Schematic representation of the samples for ZnIn2S4 (a) and ZnIn2S4-5%Co-Pi (b); Figure S2: EDS spectrum of ZnIn2S4-5%Co-Pi; Figure S3: Tafel slope plots for ZnIn2S4 and ZnIn2S4-5%Co-Pi; Figure S4: Mott-Schottky plots for ZnIn2S4.
Author Contributions
Conceptualization, Y.W. (Yonghui Wu) and K.L.; methodology, Z.W.; software, Y.W. (Yonghui Wu) and Z.W.; validation, Y.W. (Yu Wei), Y.Y. and J.W.; formal analysis, Y.S.; investigation, Y.Y.; resources, K.L. and B.W.; data curation, K.Y. (Kai Yang); writing—original draft preparation, Y.W. (Yonghui Wu); writing—review and editing, K.L.; visualization, Y.W. (Yonghui Wu); supervision, K.L.; project administration, K.Y. and B.W.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from Jiangxi Provincial Natural Science Foundation (20224BAB203018), Postdoctoral Research Projects of Jiangxi Province (2021RC11, 204302600031), Jiangxi Province “Double Thousand Plan” (jxsq2023102143), High Level Talent Research Launch Project of JXUST (205200100518), Jiangxi University of Science and Technology students’ innovation and entrepreneurship training program (Preparation of graphene aerogel/semiconductor composite photocatalytic materials and their performance research, 202210407022), National Natural Science Foundation of China (21962006, 21902132), Jiangxi Provincial Academic and Technical Leaders Training Program-Young Talents (20204BCJL23037), Program of Qingjiang Excellent Young Talents, JXUST (JXUSTQJBJ2020005). The authors would like to thank Chen Weiwei from Shiyanjia Lab (www.shiyanjia.com) for the XPS analysis and Jiangxi Qianvi New Materials Co., Ltd. for SEM analysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gong, Y.-N.; Zhong, W.; Li, Y.; Qiu, Y.; Zheng, L.; Jiang, J.; Jiang, H.-L. Regulating Photocatalysis by Spin-State Manipulation of Cobalt in Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 16723–16731. [Google Scholar] [CrossRef]
- Su, B.; Zheng, M.; Lin, W.; Lu, X.F.; Luan, D.; Wang, S.; Lou, X.W. S-Scheme Co9S8@Cd0.8Zn0.2S-DETA Hierarchical Nanocages Bearing Organic CO2 Activators for Photocatalytic Syngas Production. Adv. Energy Mater. 2023, 13, 2203290. [Google Scholar] [CrossRef]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Zhai, Q.; Roy, A.K.; Dai, L. Charge transfer of carbon nanomaterials for efficient metal-free electrocatalysis. Interdiscip. Mater. 2022, 1, 28–50. [Google Scholar] [CrossRef]
- Yang, F.; Hu, P.; Yang, F.; Hua, X.-J.; Chen, B.; Gao, L.; Wang, K.-S. Photocatalytic applications and modification methods of two-dimensional nanomaterials: A review. Tungsten 2023. [Google Scholar] [CrossRef]
- Camara, F.; Gavaggio, T.; Dautreppe, B.; Chauvin, J.; Pécaut, J.; Aldakov, D.; Collomb, M.-N.; Fortage, J. Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water. Molecules 2022, 27, 6614. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Xu, J.; Cao, S.; Yu, J. Promoting intramolecular charge transfer of graphitic carbon nitride by donor–acceptor modulation for visible-light photocatalytic H2 evolution. Interdiscip. Mater. 2022, 1, 294–308. [Google Scholar] [CrossRef]
- Ma, M.-Y.; Yu, H.-Z.; Deng, L.-M.; Wang, L.-Q.; Liu, S.-Y.; Pan, H.; Ren, J.-W.; Maximov, M.Y.; Hu, F.; Peng, S.-J. Interfacial engineering of heterostructured carbon-supported molybdenum cobalt sulfides for efficient overall water splitting. Tungsten 2023, 5, 589–597. [Google Scholar] [CrossRef]
- Wu, K.; Shang, Y.; Li, H.; Wu, P.; Li, S.; Ye, H.; Jian, F.; Zhu, J.; Yang, D.; Li, B.; et al. Synthesis and Hydrogen Production Performance of MoP/a-TiO2/Co-ZnIn2S4 Flower-like Composite Photocatalysts. Molecules 2023, 28, 4350. [Google Scholar] [CrossRef]
- Yoshimura, N.; Yoshida, M.; Kobayashi, A. Efficient Hydrogen Production by a Photoredox Cascade Catalyst Comprising Dual Photosensitizers and a Transparent Electron Mediator. J. Am. Chem. Soc. 2023, 145, 6035–6038. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Lin, X.; Tang, Z.-R.; Xu, Y.-J. Silicon nanowires@Co3O4 arrays film with Z-scheme band alignment for hydrogen evolution. Catal. Today 2019, 335, 294–299. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-Functionalized Titanium Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Lin, Y.-D.; Hao, Y.; Chen, H.-N.; Guo, Z.-W.; Li, X.-X.; Zheng, S.-T. Recent advances in polyoxoniobate-catalyzed reactions. Tungsten 2022, 4, 81–98. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent advances in designing ZnIn2S4-based heterostructured photocatalysts for hydrogen evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Wan, L.; Ding, H.; Li, H.; Wang, X.; Zhang, W. Hollow core–shell Co9S8@ZnIn2S4/CdS nanoreactor for efficient photothermal effect and CO2 photoreduction. Appl. Catal. B 2022, 311, 121255. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, D.; Yao, X.; Dong, Z.; Li, X.; Ma, S.; Liu, J.; Zhang, D.; Li, H.; Pu, X.; et al. Highly efficient flower-like ZnIn2S4/CoFe2O4 photocatalyst with p-n type heterojunction for enhanced hydrogen evolution under visible light irradiation. J. Colloid Interface Sci. 2023, 641, 26–35. [Google Scholar] [CrossRef]
- Jiang, X.; Kong, D.; Luo, B.; Wang, M.; Zhang, D.; Pu, X. Preparation of magnetically retrievable flower-like AgBr/BiOBr/NiFe2O4 direct Z-scheme heterojunction photocatalyst with enhanced visible-light photoactivity. Colloids Surf. A 2022, 633, 127880. [Google Scholar] [CrossRef]
- Shi, R.; Yang, P.; Song, X.; Wang, J.; Che, Q.; Zhang, A. ZnO flower: Self-assembly growth from nanosheets with exposed {1 1¯ 0 0} facet, white emission, and enhanced photocatalysis. Appl. Surf. Sci. 2016, 366, 506–513. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, M.-Q.; Xu, Y.-J. A low-temperature and one-step method for fabricating ZnIn2S4–GR nanocomposites with enhanced visible light photoactivity. J. Mater. Chem. A 2014, 2, 14401. [Google Scholar] [CrossRef]
- Jin, P.; Wang, L.; Ma, X.; Lian, R.; Huang, J.; She, H.; Zhang, M.; Wang, Q. Construction of hierarchical ZnIn2S4@PCN-224 heterojunction for boosting photocatalytic performance in hydrogen production and degradation of tetracycline hydrochloride. Appl. Catal. B 2021, 284, 119762. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.-J.; Cui, W.-J.; Guo, Y.-H.; Wang, T.-W.; Yao, Z.-W.; Shi, Y.; Zhao, H.; Liu, J.; Hu, Z.-Y.; et al. Nickel clusters accelerating hierarchical zinc indium sulfide nanoflowers for unprecedented visible-light hydrogen production. J. Colloid Interface Sci. 2022, 608, 504–512. [Google Scholar] [CrossRef]
- Ding, Y.; Maitra, S.; Wang, C.; Halder, S.; Zheng, R.; Barakat, T.; Roy, S.; Chen, L.H.; Su, B.L. Vacancy defect engineering in semiconductors for solar light-driven environmental remediation and sustainable energy production. Interdiscip. Mater. 2022, 1, 213–255. [Google Scholar] [CrossRef]
- Busser, G.W.; Mei, B.; Pougin, A.; Strunk, J.; Gutkowski, R.; Schuhmann, W.; Willinger, M.-G.; Schlögl, R.; Muhler, M. Photodeposition of Copper and Chromia on Gallium Oxide: The Role of Co-Catalysts in Photocatalytic Water Splitting. ChemSusChem 2014, 7, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-Q.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Earth-Abundant MoS2 and Cobalt Phosphate Dual Cocatalysts on 1D CdS Nanowires for Boosting Photocatalytic Hydrogen Production. Langmuir 2019, 35, 11056–11065. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, W. Dynamically Monitoring the Photodeposition of Single Cocatalyst Nanoparticles on Semiconductors via Fluorescence Imaging. Anal. Chem. 2021, 93, 11915–11919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Mao, Q.; Jian, L.; Dong, Y.; Zhu, Y. Photodeposition of earth-abundant cocatalysts in photocatalytic water splitting: Methods, functions, and mechanisms. Chin. J. Catal. 2022, 43, 1774–1804. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Li, D.; Tang, J.; Huang, W. Isoelectric point-controlled preferential photodeposition of platinum on Cu2O-TiO2 composite surfaces. Chin. Chem. Lett. 2019, 30, 985–988. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, P.; Yang, L.; Zhai, H.; Liu, H.; Chen, J.; Ren, R.; Tan, X.; Pan, J. Sulfur vacancy and p-n junction synergistically boosting interfacial charge transfer and separation in ZnIn2S4/NiWO4 heterostructure for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 634, 817–826. [Google Scholar] [CrossRef]
- Qu, Y.; Ren, J.; Sun, D.; Yu, Y. Synergetic control of specific orientation and self-distribution of photoelectrons in micro-nano ZnIn2S4/black phosphorus quantum dots (BPQDs) heterojunction to enhance photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 642, 204–215. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, L.; Peng, S. Synthesis of oriented J type ZnIn2S4@CdIn2S4 heterojunction by controllable cation exchange for enhancing photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 650, 266–274. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Kong, X.Y.; Tan, L.-L.; Putri, L.K.; Chai, S.-P. Non-metal doping induced dual p-n charge properties in a single ZnIn2S4 crystal structure provoking charge transfer behaviors and boosting photocatalytic hydrogen generation. Appl. Catal. B 2023, 325, 122372. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, S.-A.; Ma, Y.; Chi, D.-J.; Chen, R.; Long, H.-M.; Chun, T.-J.; Liu, S.-J.; Qian, F.-P.; Zhang, K. N-doped C-coated MoO2/ZnIn2S4 heterojunction for efficient photocatalytic hydrogen production. Rare Met. 2023, 42, 1195–1204. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, W.; Chen, F.; Wang, X.; Yu, H. In situ cascade growth-induced strong coupling effect toward efficient photocatalytic hydrogen evolution of ReS2/ZnIn2S4. Appl. Catal. B 2023, 328, 122493. [Google Scholar] [CrossRef]
- Wu, K.; Jiang, R.; Zhao, Y.; Mao, L.; Gu, X.; Cai, X.; Zhu, M. Hierarchical NiCo2S4/ZnIn2S4 heterostructured prisms: High-efficient photocatalysts for hydrogen production under visible-light. J. Colloid Interface Sci. 2022, 619, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shang, H.; Zhou, J.; Ma, X.; Gao, X.; Wang, D.; Zhang, B.; Zhao, Y. Heterostructured core–shell CoS1.097@ZnIn2S4 nanosheets for enhanced photocatalytic hydrogen evolution under visible light. Chem. Eng. J. 2023, 457, 141192. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Q.; Guo, E.; Wei, M.; Pang, Y. Hierarchical Co9S8/ZnIn2S4 Nanoflower Enables Enhanced Hydrogen Evolution Photocatalysis. Energy Fuels 2022, 36, 4541–4548. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, M.; Yan, D.; Hu, X.; Fan, J.; Sun, T.; Liu, E. S-Scheme Co9S8 Nanoflower/Red Phosphorus Nanosheet Heterojunctions for Enhanced Photocatalytic H2 Evolution. ACS Appl. Nano Mater. 2023, 6, 14478–14487. [Google Scholar] [CrossRef]
- Liang, Q.; Gao, W.; Liu, C.; Xu, S.; Li, Z. A novel 2D/1D core-shell heterostructures coupling MOF-derived iron oxides with ZnIn2S4 for enhanced photocatalytic activity. J. Hazard. Mater. 2020, 392, 122500. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Z.; Zhang, M.; Wang, M.; Wu, R.; Shi, X.; Luo, B.; Zhang, D.; Pu, X.; Li, H. A novel direct Z-scheme heterojunction BiFeO3/ZnFe2O4 photocatalyst for enhanced photocatalyst degradation activity under visible light irradiation. J. Alloys Compd. 2022, 912, 165185. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L. In situ synthesis of cobalt–phosphate (Co–Pi) modified g-C3N4 photocatalysts with enhanced photocatalytic activities. Appl. Catal. B 2013, 142–143, 414–422. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Sui, D.; Jiang, W.; Liu, F.; Gu, X.; Zhao, Y.; Ying, P.; Mao, L.; Cai, X.; et al. In Situ Photodeposition of Cobalt Phosphate (CoHxPOy) on CdIn2S4 Photocatalyst for Accelerated Hole Extraction and Improved Hydrogen Evolution. Nanomaterials 2023, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Construction of Hierarchical Hollow Co9S8/ZnIn2S4 Tubular Heterostructures for Highly Efficient Solar Energy Conversion and Environmental Remediation. Angew. Chem. Int. Ed. 2020, 59, 8255–8261. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, S.K.; Vijayarajan, V.S.; Rishi Krishna, B.S.; Veluswamy, P.; Neppolian, B. Cobalt phosphate hydroxide loaded g-C3N4 photocatalysts and its hydrogen production activity. Int. J. Hydrogen Energy 2020, 45, 7562–7573. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Wei, Y.; Xie, L.; Chen, H.-Q.; Wang, J.; Yang, K.; Zou, L.-X.; Deng, T.; Lu, K.-Q. Decorating CdS with cobaltous hydroxide and graphene dual cocatalyst for photocatalytic hydrogen production coupled selective benzyl alcohol oxidation. Mol. Catal. 2024, 553, 113738. [Google Scholar] [CrossRef]
- Wei, Y.; Hao, J.-G.; Zhang, J.-L.; Huang, W.-Y.; Ouyang, S.-B.; Yang, K.; Lu, K.-Q. Integrating Co(OH)2 nanosheet arrays on graphene for efficient noble-metal-free EY-sensitized photocatalytic H2 evolution. Dalton Trans. 2023, 52, 13923–13929. [Google Scholar] [CrossRef]
- Ai, G.; Mo, R.; Li, H.; Zhong, J. Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 2015, 7, 6722–6728. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, L.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. MoS2 Quantum Dots-Modified Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution. ChemSusChem 2018, 11, 1108–1113. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, X.-C.; Liu, C.; Zeng, J.-M.; Qi, X.-P. Co3O4/stainless steel catalyst with synergistic effect of oxygen vacancies and phosphorus doping for overall water splitting. Tungsten 2022, 5, 100–108. [Google Scholar] [CrossRef]
- Mu, P.; Zhou, M.; Yang, K.; Chen, X.; Yu, Z.; Lu, K.; Huang, W.; Yu, C.; Dai, W. Cd0.5Zn0.5S/CoWO4 Nanohybrids with a Twinning Homojunction and an Interfacial S-Scheme Heterojunction for Efficient Visible-Light-Induced Photocatalytic CO2 Reduction. Inorg. Chem. 2021, 60, 14854–14865. [Google Scholar] [CrossRef]
- Jiang, X.; Gong, H.; Liu, Q.; Song, M.; Huang, C. In situ construction of NiSe/Mn0.5Cd0.5S composites for enhanced photocatalytic hydrogen production under visible light. Appl. Catal. B 2020, 268, 118439. [Google Scholar] [CrossRef]
- Li, M.; Zhang, D.; Zhou, h.; Sun, K.; Ma, X.; Dong, M. Construction of hollow tubular Co9S8/ZnSe S-scheme heterojunctions for enhanced photocatalytic H2 evolution. Int. J. Hydrogen Energy 2023, 48, 5126–5137. [Google Scholar] [CrossRef]
- Li, J.-Y.; Qi, M.-Y.; Xu, Y.-J. Efficient splitting of alcohols into hydrogen and C–C coupled products over ultrathin Ni-doped ZnIn2S4 nanosheet photocatalyst. Chin. J. Catal. 2022, 43, 1084–1091. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Lee, Y.J.; Tan, L.-L.; Putri, L.K.; Low, J.; Mohamed, A.R.; Chai, S.-P. Self-activated superhydrophilic green ZnIn2S4 realizing solar-driven overall water splitting: Close-to-unity stability for a full daytime. Nat. Commun. 2023, 14, 7676. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Bi, Y. Subnanometric Bismuth Clusters Confined in Pyrochlore-Bi2Sn2O7 Enable Remarkable CO2 Photoreduction. Angew. Chem. Int. Ed. 2023, 63, e202316459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-K.; Liu, J.; Zhang, L.; Dong, L.-Z.; Li, S.-L.; Kan, Y.-H.; Li, D.-S.; Lan, Y.-Q. Monometallic Catalytic Models Hosted in Stable Metal–Organic Frameworks for Tunable CO2 Photoreduction. ACS Catal. 2019, 9, 1726–1732. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Li, Y.-H.; Zhang, F.; Qi, M.-Y.; Chen, X.; Tang, Z.-R.; Yamada, Y.M.A.; Anpo, M.; Conte, M.; Xu, Y.-J. Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat. Commun. 2020, 11, 5181. [Google Scholar] [CrossRef]
- Guan, X.; Qian, Y.; Zhang, X.; Jiang, H.L. Enaminone-Linked Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2023, 62, e202306135. [Google Scholar] [CrossRef]
- Gao, J.-X.; Tian, W.-J.; Zhang, H.-Y. Progress of Nb-containing catalysts for carbon dioxide reduction: A minireview. Tungsten 2022, 4, 284–295. [Google Scholar] [CrossRef]
- Zou, J.; Wu, S.; Liu, Y.; Sun, Y.; Cao, Y.; Hsu, J.-P.; Shen Wee, A.T.; Jiang, J. An ultra-sensitive electrochemical sensor based on 2D g-C3N4/CuO nanocomposites for dopamine detection. Carbon 2018, 130, 652–663. [Google Scholar] [CrossRef]
- Hu, M.; Wu, C.; Feng, S.; Hua, J. A High Crystalline Perylene-Based Hydrogen-Bonded Organic Framework for Enhanced Photocatalytic H2O2 Evolution. Molecules 2023, 28, 6850. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-Q.; Chen, Y.; Xin, X.; Xu, Y.-J. Rational utilization of highly conductive, commercial Elicarb graphene to advance the graphene-semiconductor composite photocatalysis. Appl. Catal. B 2018, 224, 424–432. [Google Scholar] [CrossRef]
- Su, B.; Kong, Y.; Wang, S.; Zuo, S.; Lin, W.; Fang, Y.; Hou, Y.; Zhang, G.; Zhang, H.; Wang, X. Hydroxyl-Bonded Ru on Metallic TiN Surface Catalyzing CO2 Reduction with H2O by Infrared Light. J. Am. Chem. Soc. 2023, 145, 27415–27423. [Google Scholar] [CrossRef]
- Luo, D.; Peng, L.; Wang, Y.; Lu, X.; Yang, C.; Xu, X.; Huang, Y.; Ni, Y. Highly efficient photocatalytic water splitting utilizing a WO3−x/ZnIn2S4 ultrathin nanosheet Z-scheme catalyst. J. Mater. Chem. A 2021, 9, 908–914. [Google Scholar] [CrossRef]
- Tang, C.; Bao, T.; Li, S.; Wang, X.; Rao, H.; She, P.; Qin, J.-S. Bioinspired 3D penetrating structured micro-mesoporous NiCoFe-LDH@ZnIn2S4 Z-scheme heterojunction for simultaneously photocatalytic H2 evolution coupled with benzylamine oxidation. Appl. Catal. B 2024, 342, 123384. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Jin, Z. Rational design of a cobalt sulfide/bismuth sulfide S-scheme heterojunction for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 592, 237–248. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Jin, Z. Synergistic effect of MoS2 over WP photocatalyst for promoting hydrogen production. J. Solid State Chem. 2020, 288, 121419. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zeng, G.; Jiang, L.; Zhong, H.; Xie, Y.; Wang, H.; Chen, X.; Wang, H. Highly efficient photocatalytic activity and mechanism of Yb3+/Tm3+ codoped In2S3 from ultraviolet to near infrared light towards chromium (VI) reduction and rhodamine B oxydative degradation. Appl. Catal. B 2018, 225, 8–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).