Enhanced Immobilization of Enzymes on Plasma Micro-Nanotextured Surfaces and Microfluidics: Application to HRP
Abstract
1. Introduction
2. Results
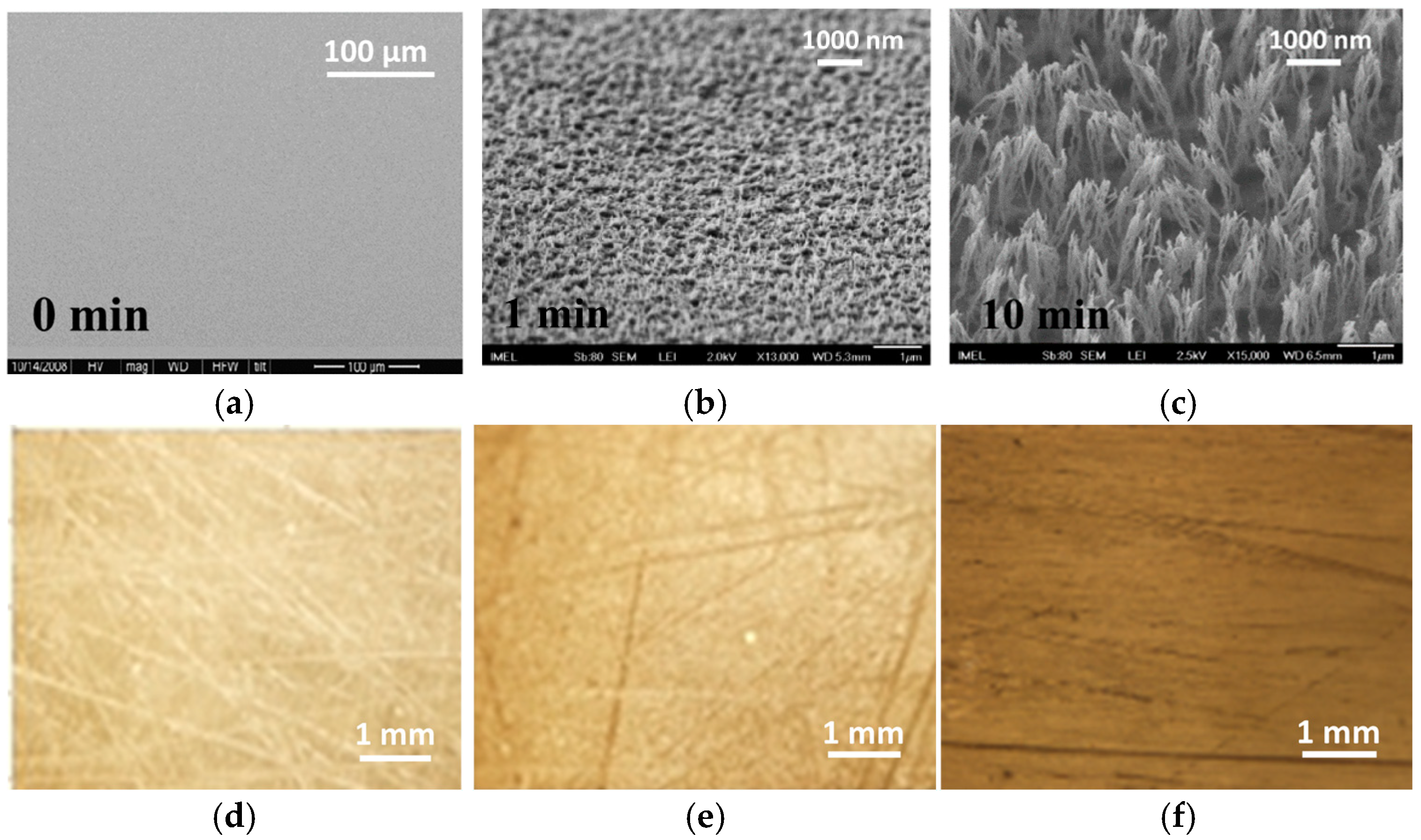
2.1. Plasma Micro-Nanotexturing and Enzyme Immobilization on PMMA Open Surfaces
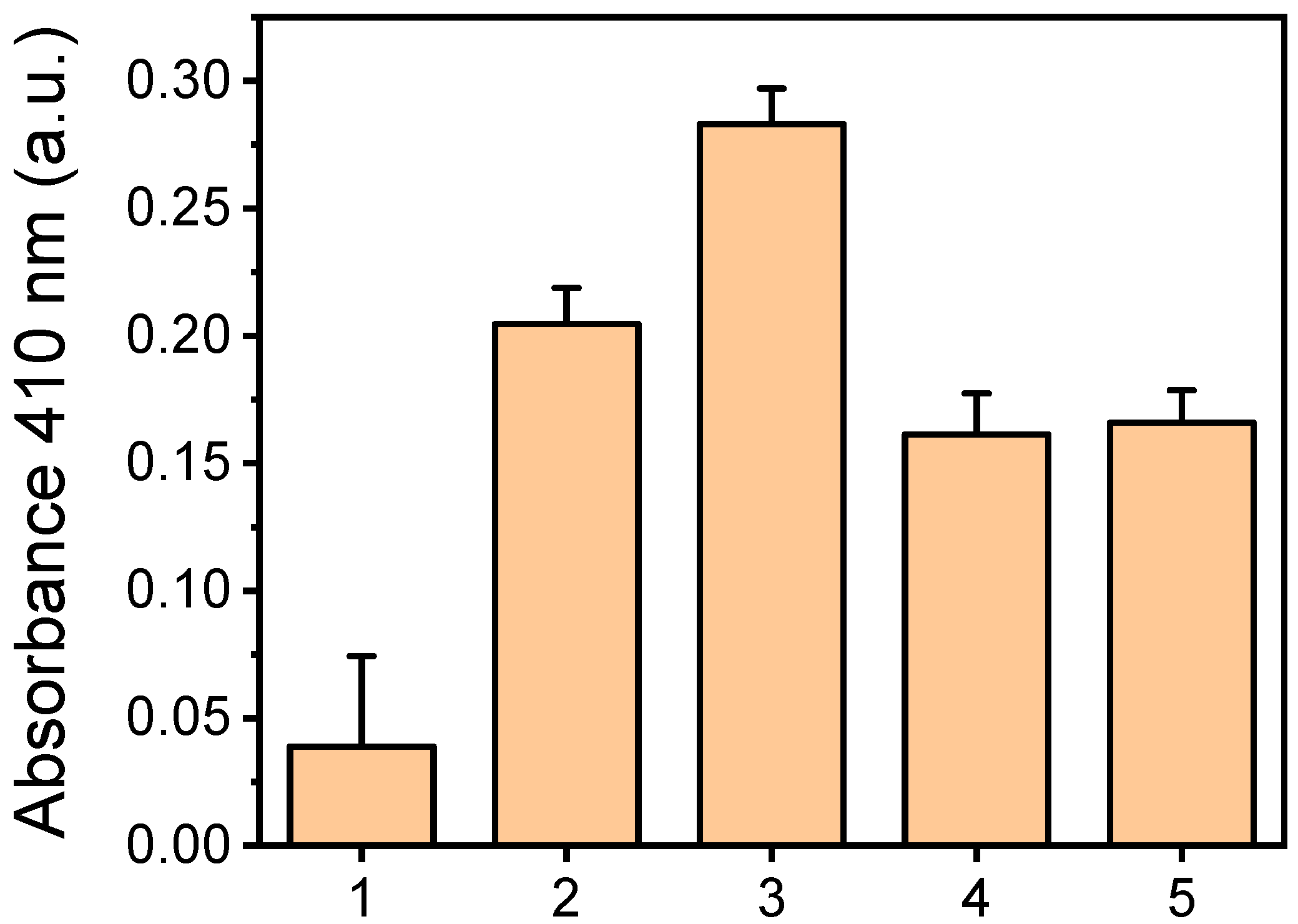
2.2. Selection of Method for HRP Immobilization to Oxygen-Plasma-Treated PMMA Surfaces
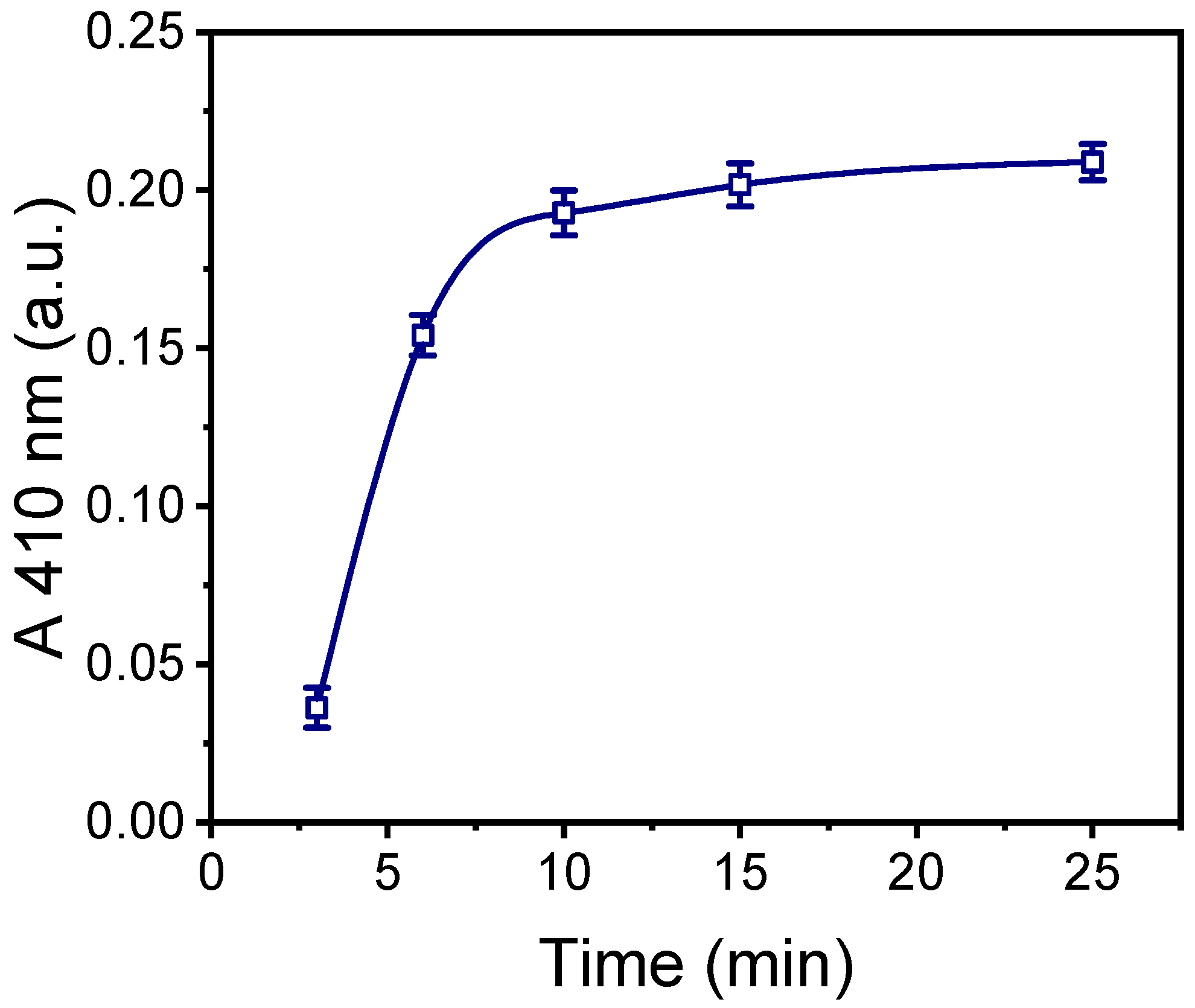
2.3. Optimization of HRP Immobilization by Physical Adsorption
2.4. Comparison with the Untreated PMMA Surfaces
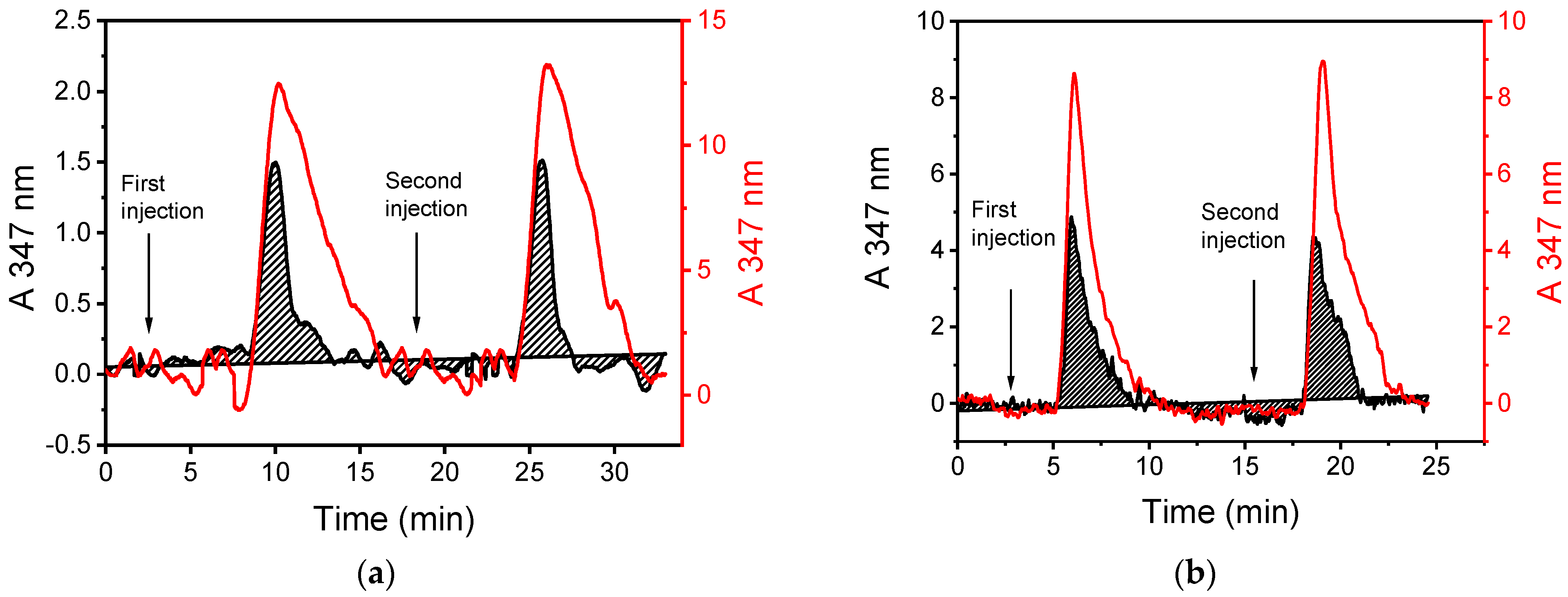
2.5. Evaluation of Plasma-Nanotextured Microreactor Performance
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Fabrication of Open PMMA Surfaces
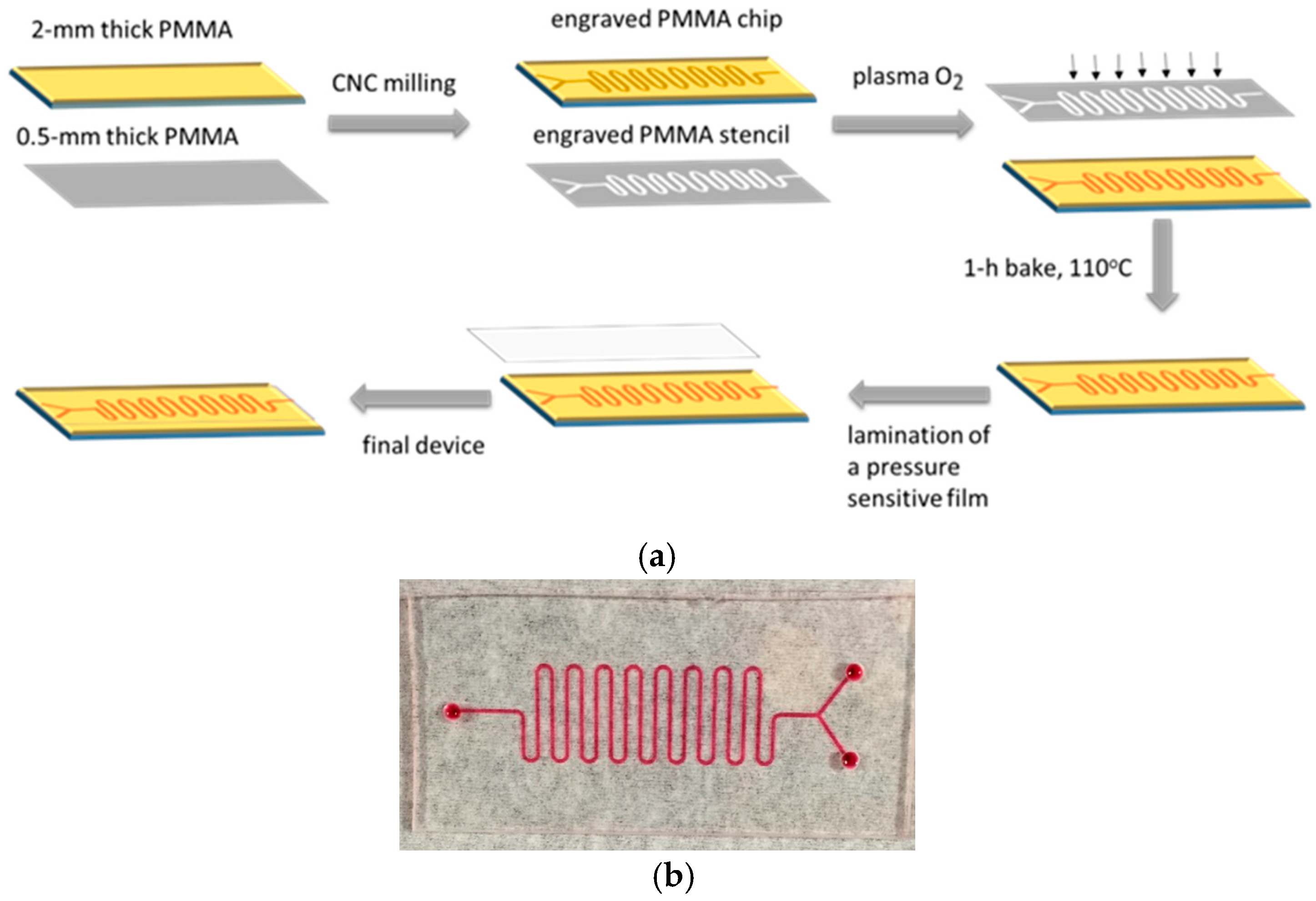
4.3. Microreactor Fabrication Process
4.4. Immobilization of HRP on Open PMMA Surfaces
4.4.1. Physical Adsorption
4.4.2. Covalent Bonding through EDC/NHS
4.4.3. Affinity Immobilization via Streptavidin
4.5. Evaluation of Immobilized Enzyme Activity of Open PMMA Surfaces
4.6. Evaluation of Immobilized Enzyme Quantity
4.7. Enzyme Immobilization on Oxygen-Plasma-Treated Microchannels and HRP Activity Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.R. Immobilized enzymes: A comprehensive review. Bull. Natl. Res. Cent. 2021, 45, 207. [Google Scholar] [CrossRef]
- Grubhofer, N.; Schleith, L. Modified ion exchangers as specific adsorbents. Naturwissenschaften 1953, 40, 508. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M.; Ghodousian, A. Techniques for immobilizing enzymes to create durable and effective biocatalysts. Res. Chem. 2024, 7, 101486. [Google Scholar] [CrossRef]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme immobilization: An overview on methods, support material, and applications of immobilized enzymes. Adv. Food. Nutr. Res. 2016, 79, 179–211. [Google Scholar] [CrossRef] [PubMed]
- Borges Reis, C.L.; Almeida de Sousa, E.Y.; de França Serpa, J.; Casemiro Oliveira, R.; Sousa dos Santos, J.C. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Naldi, M.; Tramarin, A.; Bartolini, M. Immobilized enzyme-based analytical tools in the -omics era: Recent advances. J. Pharm. Biomed. Anal. 2018, 160, 222–237. [Google Scholar] [CrossRef]
- Wouters, B.; Currivan, S.A.; Abdulhussain, N.; Hankemeier, T.; Schoenmakers, P.J. Immobilized-enzyme reactors integrated into analytical platforms: Recent advances and challenges. TrAC Trend Anal. Chem. 2021, 144, 116419. [Google Scholar] [CrossRef]
- Meller, K.; Szumski, M.; Buszewski, B. Microfluidic reactors with immobilized enzymes—Characterization, dividing, perspectives. Sens. Actuator B Chem. 2017, 244, 84–106. [Google Scholar] [CrossRef]
- Lukyanenko, K.A.; Denisov, I.; Yakimov, A.S.; Esimbekova, E.; Belousov, K.; Bukatin, A.; Kukhtevich, I.V.; Sorokin, V.V.; Evstrapov, A.A.; Belobrov, P.I. Analytical enzymatic reactions in microfluidic systems. Appl. Biochem. Microbiol. 2017, 53, 775–780. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Q.; Shao, L.; Jiacef, Y.; Zhang, X. Microfluidic immobilized enzyme reactors for continuous biocatalysis. React. Chem. Eng. 2020, 5, 9–32. [Google Scholar] [CrossRef]
- Mross, S.; Pierrat, S.; Zimmermann, T.; Kraft, M. Microfluidic enzymatic biosensing systems: A review. Biosens Bioelectron. 2015, 70, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Liu, W.; Li, M.; Chen, C. Recent advances in the fabrication and application of nanomaterial based enzymatic microsystems in chemical and biological sciences. Anal. Chim. Acta 2019, 1067, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Calleri, E.; Temporini, C.; Colombo, R.; Tengattini, S.; Rinaldi, F.; Brusotti, G.; Furlanetto, S.; Gabriella Massolini, G. Analytical settings for in-flow biocatalytic reaction monitoring. TrAC Trend Anal. Chem. 2021, 143, 116348. [Google Scholar] [CrossRef]
- Cerdeira Ferreira, L.M.; da Costa, E.T.; do Lago, C.L.; Angnes, L. Miniaturized flow system based on enzyme modified PMMA microreactor for amperometric determination of glucose. Biosens. Bioelectron. 2013, 47, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Facchini Cerqueira, M.R.; Grasseschi, D.; Matos, R.C.; Angnes, L. A novel functionalisation process for glucose oxidase immobili-sation in poly(methyl methacrylate) microchannels in a flow system for amperometric determinations. Talanta 2014, 126, 20–26. [Google Scholar] [CrossRef]
- Al Lawati, H.A.J.; Hassanzadeh, J.; Bagheri, N. A handheld 3D-printed microchip for simple integration of the H2O2-producing enzymatic reactions with subsequent chemiluminescence detection: Application for sugars. Food Chem. 2022, 383, 132469. [Google Scholar] [CrossRef]
- Mousaabadi, K.Z.; Vandishi, Z.T.; Kermani, M.; Arab, N.; Ensafi, A.A. Recent developments toward microfluidic point-of-care diagnostic sensors for viral infections. TrAC Trend Anal. Chem. 2023, 169, 117361. [Google Scholar] [CrossRef]
- Valikhani, D.; Bolivar, J.M.; Viefhues, M.; McIlroy, D.N.; Vrouwe, E.X.; Nidetzky, B. A Spring in Performance: Silica Nanosprings Boost Enzyme Immobilization in Microfluidic Channels. ACS Appl. Mater. Interfaces 2017, 9, 34641–34649. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, H.; Jia, H.; Wei, P. A flow-through enzymatic microreactor immobilizing lipase based on layer-by-layer method for biosynthetic process: Catalyzing the transesterification of soybean oil for fatty acid methyl ester production. Proc. Biochem. 2017, 54, 73–80. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, H.; Jia, H.; Wei, P. Polydopamine-mediated preparation of an enzyme-immobilized microreactor for the rapid production of wax ester. RSC Adv. 2017, 7, 12283. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of Enzymes by Polymeric Materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Nagy, C.; Szabo, R.; Gaspar, A. Microfluidic Immobilized Enzymatic Reactors for Proteomic Analyses—Recent Developments and Trends (2017–2021). Micromachines 2022, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Sketa, B.; Galman, J.L.; Turner, N.J.; Znidarsic-Plazl, P. Immobilization of His6-tagged amine transaminases in microreactors using functionalized nonwoven nanofiber membranes. New Biotechnol. 2024, 83, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Dong, Y.; He, Q.; Chen, H.; Zhu, Z. Fabrication of a polystyrene microfluidic chip coupled to electrospray ionization mass spectrometry for protein analysis. J. Chromatogr. B 2015, 990, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kulsharova, G.; Dimov, N.; Marques, M.P.C.; Szita, N.; Baganz, F. Simplified immobilisation method for histidine-tagged enzymes in poly(methyl methacrylate) microfluidic devices. New Biotechnol. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Bataille, J.; Viodé, A.; Pereiro, I.; Lafleur, J.P.; Varenne, F.; Descroix, S.; Becher, F.; Kutter, J.P.; Roesch, C.; Poüs, C.; et al. On-a-chip tryptic digestion of transthyretin: A step toward an integrated microfluidic system for the follow-up of familial transthyretin amyloidosis. Analyst 2018, 143, 1077–1086. [Google Scholar] [CrossRef]
- Kecskemeti, A.; Bako, J.; Csarnovics, I.; Csosz, E.; Gaspar, A. Development of an enzymatic reactor applying spontaneously adsorbed trypsin on the surface of a PDMS microfluidic device. Anal. Bioanal. Chem. 2017, 409, 3573–3585. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Nedela, O.; Slepicka, P.; Švorcík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Primc, G.; Mozetic, M. Surface Modification of Polymers by Plasma Treatment for Appropriate Adhesion of Coatings. Materials 2024, 17, 1494. [Google Scholar] [CrossRef]
- Tsougeni, K.; Papageorgiou, D.; Tserepi, A.; Gogolides, E. “Smart” polymeric microfluidics fabricated by plasma processing: Controlled wetting, capillary filling and hydrophobic valving. Lab Chip 2010, 10, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Vourdas, N.; Cardinaud, C.; Tserepi, A.; Gogolides, E. Mechanisms of Oxygen Plasma Nanotexturing of Organic Polymer Surfaces: From Stable Super Hydrophilic to Super Hydrophobic Surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Petrou, P.S.; Tserepi, A.; Kakabakos, S.E.; Gogolides, E. Plasma Nanotextured PMMA Surfaces for Protein Arrays: Increased Protein Binding and Enhanced Detection Sensitivity. Langmuir 2010, 26, 13883–13891. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Petrou, P.S.; Awsiuk, K.; Marzec, M.M.; Ioannidis, N.; Petrouleas, V.; Tserepi, A.; Kakabakos, S.E.; Gogolides, E. Direct Covalent Biomolecule Immobilization on Plasma-Nanotextured Chemically Stable Substrates. ACS Appl. Mater. Inter. 2015, 7, 14670–14681. [Google Scholar] [CrossRef] [PubMed]
- Roman, H.E.; Cesura, F.; Maryam, R.; Levchenko, I.; de Alexander, K.; Riccardi, C. The fractal geometry of polymeric materials surfaces: Surface area and fractal length scales. Soft Matter 2024, 20, 3082. [Google Scholar] [CrossRef]
- Bourkoula, A.; Constantoudis, V.; Kontziampasis, D.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Gogolides, E. Roughness threshold for cell attachment and proliferation on plasma micro-nanotextured polymeric surfaces: The case of primary human skin fibroblasts and mouse immortalized 3T3 fibroblasts. J. Physics D Appl. Physics 2016, 49, 304002. [Google Scholar] [CrossRef]
- Kanioura, A.; Constantoudis, V.; Petrou, P.; Kletsas, D.; Tserepi, A.; Gogolides, E.; Chatzichristidi, M.; Kakabakos, S. Oxygen plasma micro-nanostructured PMMA plates and microfluidics for increased adhesion and proliferation of cancer versus normal cells: The role of surface roughness and disorder. Micro Nano Eng. 2020, 8, 100060. [Google Scholar] [CrossRef]
- Tsougeni, K.; Papadakis, G.; Gianneli, M.; Grammoustianou, A.; Constantoudis, V.; Dupuy, B.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Gizeli, E.; et al. Plasma nanotextured polymeric lab-on-a-chip for highly efficient bacteria capture and lysis. Lab Chip 2016, 16, 120–131. [Google Scholar] [CrossRef]
- Celebi, M.; Kaya, M.A.; Altikatoglu, M. Huseyin Yildirim Enzymatic Decolorization of Anthraquinone and Diazo Dyes Using Horseradish Peroxidase Enzyme Immobilized onto Various Polysulfone Supports. Appl. Biochem. Biotechnol. 2013, 171, 716–730. [Google Scholar] [CrossRef]
- Tudorache, M.; Mahalu, D.; Teodorescu, C.; Stan, R.; Bala, C.; Parvulescu, V.I. Biocatalytic microreactor incorporating HRP anchored on micro-/nano-lithographic patterns for flow oxidation of phenols. J. Molec. Catal. B Enzym. 2011, 69, 133–139. [Google Scholar] [CrossRef]
- Alemzadeh, I.; Nejati, S. Phenols removal by immobilized horseradish peroxidase. J. Hazard. Mater. 2009, 166, 1082–1086. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorvi, S.; Tsougeni, K.; Tserepi, A.; Kakabakos, S.; Petrou, P.; Gogolides, E. Enhanced Immobilization of Enzymes on Plasma Micro-Nanotextured Surfaces and Microfluidics: Application to HRP. Molecules 2024, 29, 4736. https://doi.org/10.3390/molecules29194736
Vorvi S, Tsougeni K, Tserepi A, Kakabakos S, Petrou P, Gogolides E. Enhanced Immobilization of Enzymes on Plasma Micro-Nanotextured Surfaces and Microfluidics: Application to HRP. Molecules. 2024; 29(19):4736. https://doi.org/10.3390/molecules29194736
Chicago/Turabian StyleVorvi, Stefania, Katerina Tsougeni, Angeliki Tserepi, Sotirios Kakabakos, Panagiota Petrou, and Evangelos Gogolides. 2024. "Enhanced Immobilization of Enzymes on Plasma Micro-Nanotextured Surfaces and Microfluidics: Application to HRP" Molecules 29, no. 19: 4736. https://doi.org/10.3390/molecules29194736
APA StyleVorvi, S., Tsougeni, K., Tserepi, A., Kakabakos, S., Petrou, P., & Gogolides, E. (2024). Enhanced Immobilization of Enzymes on Plasma Micro-Nanotextured Surfaces and Microfluidics: Application to HRP. Molecules, 29(19), 4736. https://doi.org/10.3390/molecules29194736









