Isothiocyanate-Based Microemulsions Loaded into Biocompatible Hydrogels as Innovative Biofumigants for Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
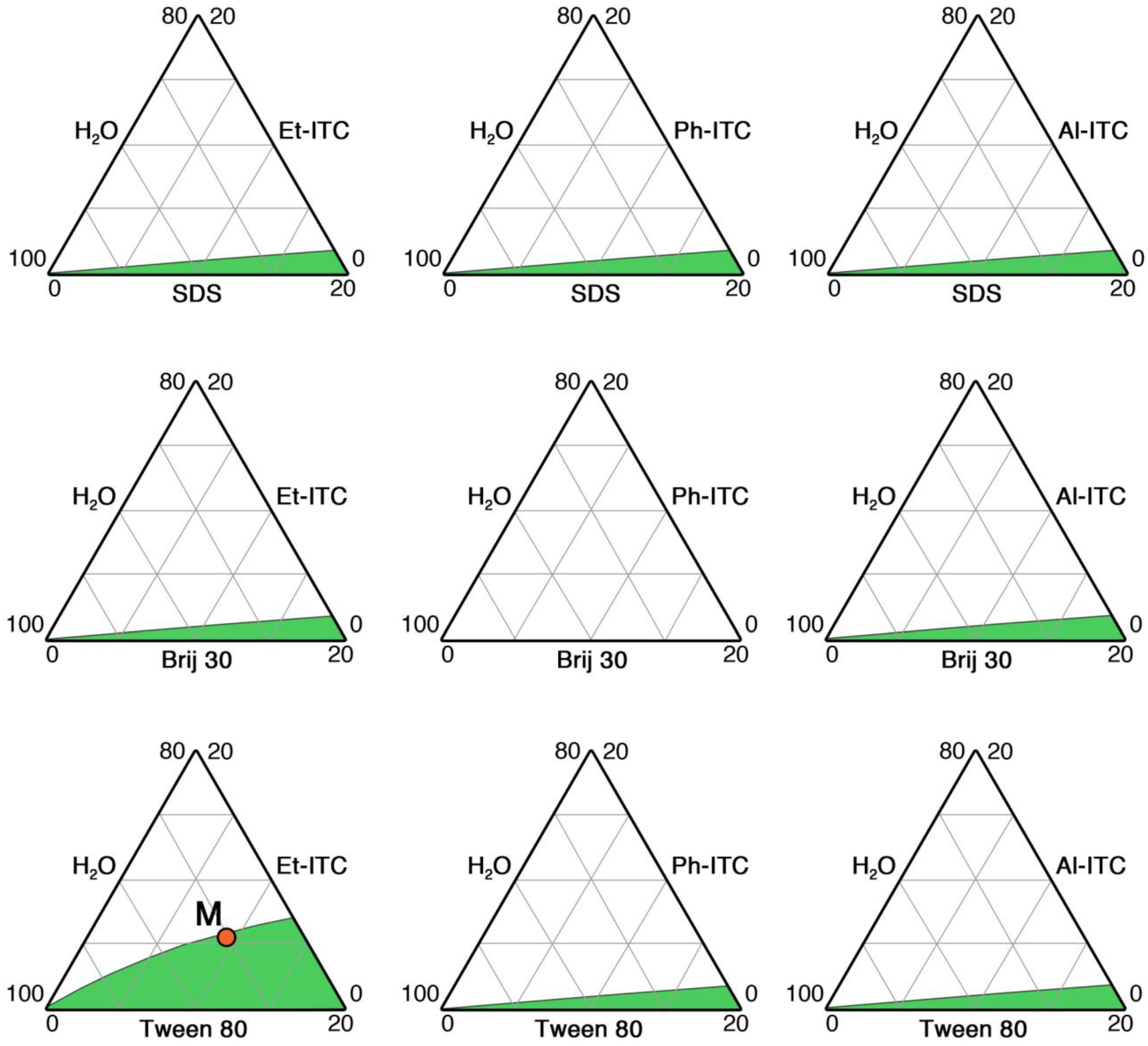
2.2. Microemulsions’ Phase Behavior
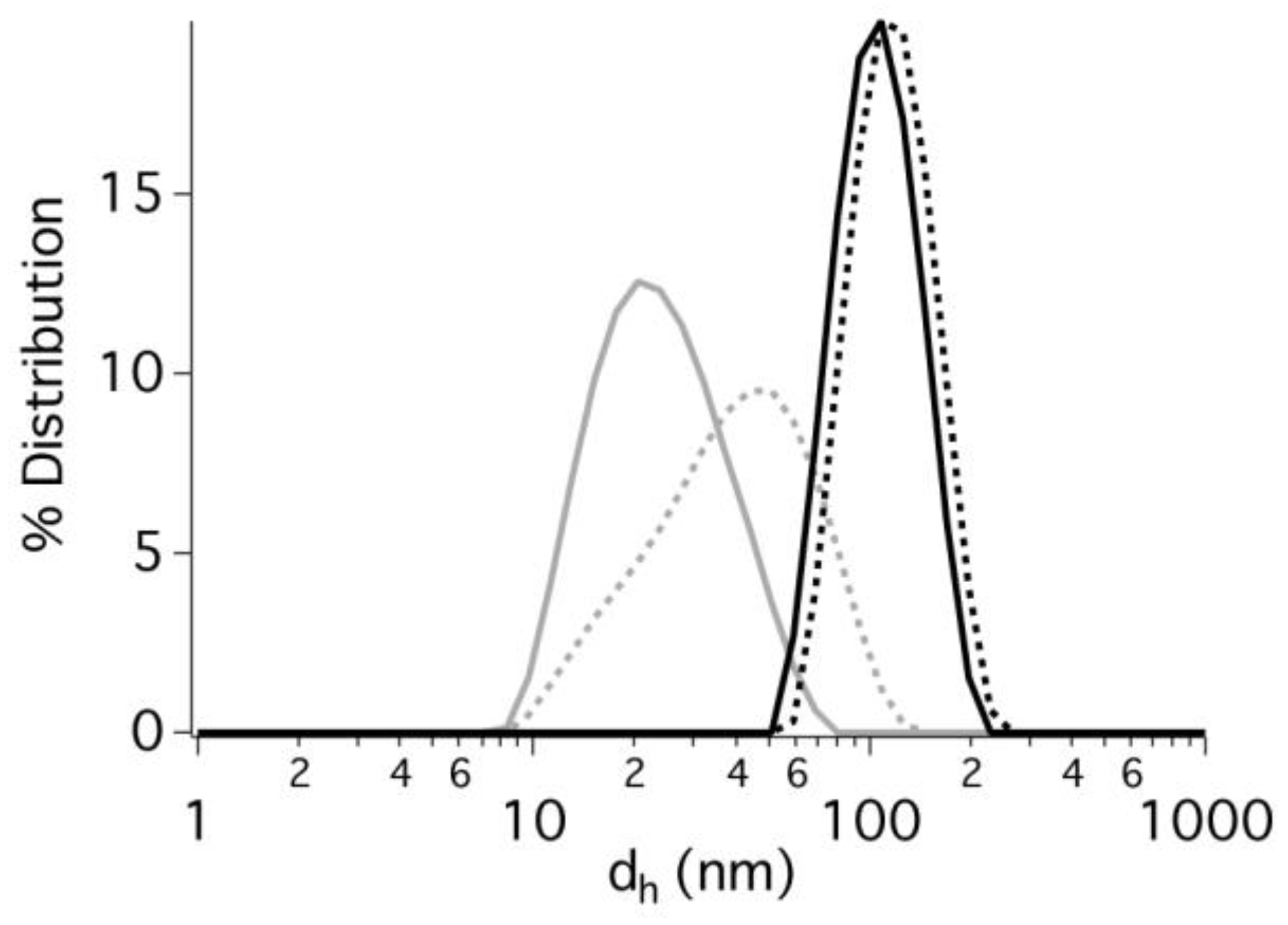
2.3. Dynamic Light Scattering
2.4. Hydrogels’ Synthesis
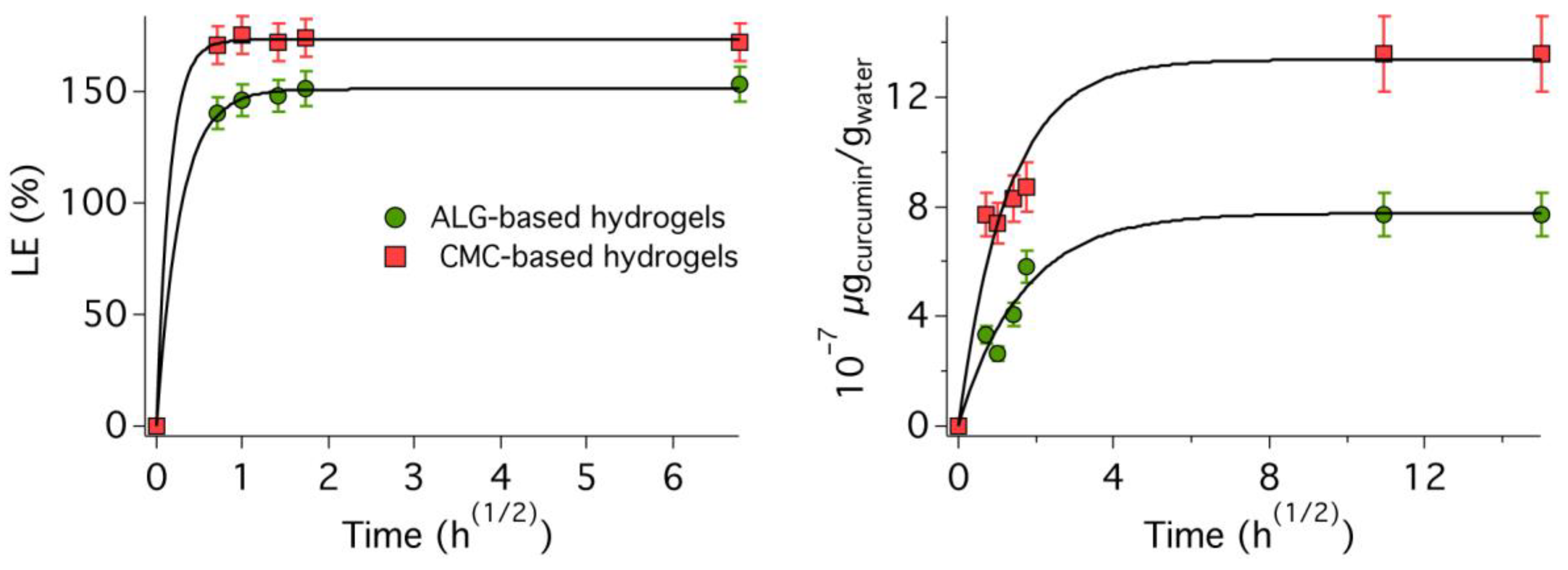
2.5. Loading Kinetics
2.6. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.7. Equilibrium Water Content (EWC) Determination
2.8. Differential Scanning Calorimetry (DSC) and Free Water Index (FWI)
2.9. Release Kinetics in Water
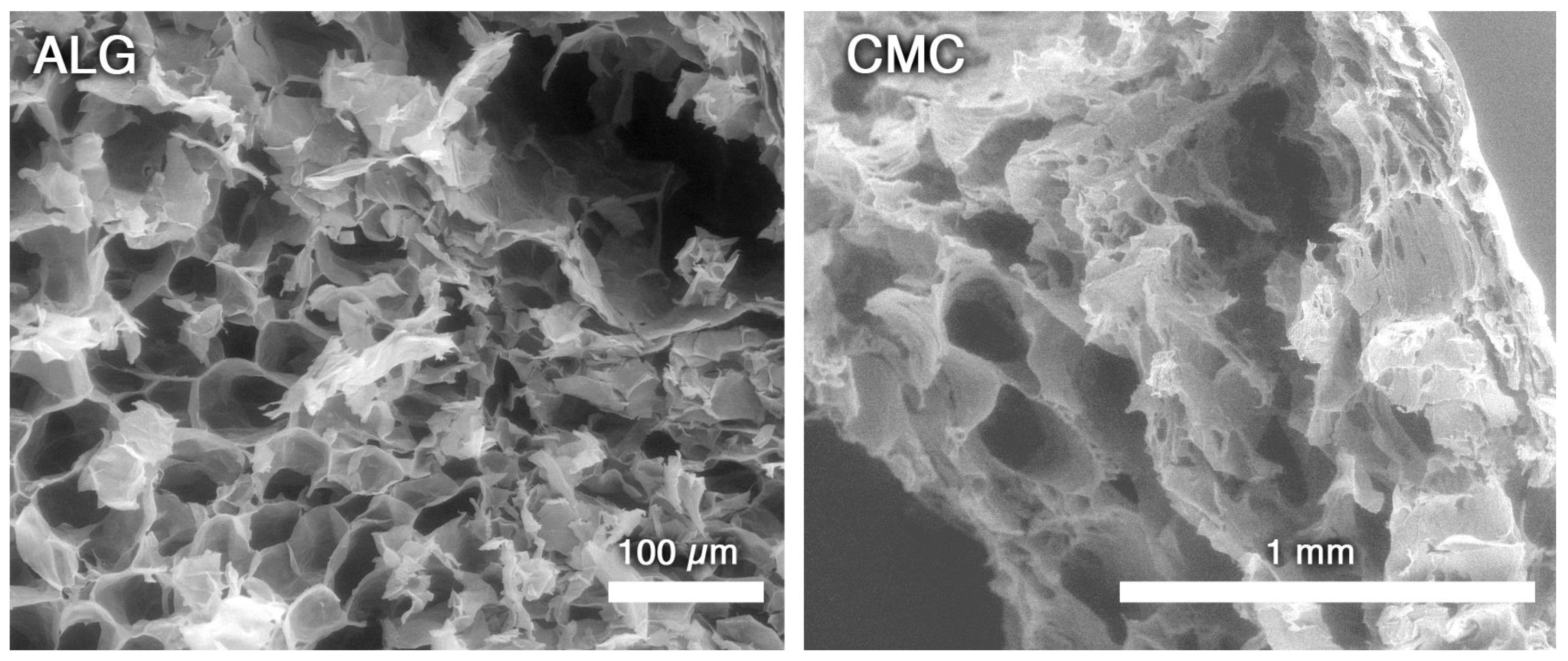
2.10. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Development and Characterization of ITCs-Based Microemulsions
3.2. Microemulsion-Loaded Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health—A Review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Toppari, J.; Larsen, J.C.; Christiansen, P.; Giwercman, A.; Grandjean, P.; Guillette, L.J.; Jégou, B.; Jensen, T.K.; Jouannet, P.; Keiding, N.; et al. Male Reproductive Health and Environmental Xenoestrogens. Environ. Health Perspect. 1996, 104, 741–803. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Leo, H.; Lawrence, R.S.; Polly, W. How Sustainable Agriculture Can Address the Environmental and Human Health Harms of Industrial Agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef]
- Devkota, P.; Norsworthy, J.K.; Rainey, R. Comparison of Allyl Isothiocyanate and Metam Sodium with Methyl Bromide for Weed Control in Polyethylene-Mulched Bell Pepper. Weed Technol. 2013, 27, 468–474. [Google Scholar] [CrossRef]
- Stephen, B.; Pruett Deborah, E.; Keil, L.P.M. Toxicology of Metam Sodium. J. Toxicol. Environ. Health Part B 2001, 4, 207–222. [Google Scholar] [CrossRef]
- Sederholm, M.R.; Schmitz, B.W.; Barberán, A.; Pepper, I.L. Effects of Metam Sodium Fumigation on the Abundance, Activity, and Diversity of Soil Bacterial Communities. Appl. Soil Ecol. 2018, 124, 27–33. [Google Scholar] [CrossRef]
- Zheng, W.; Yates, S.R.; Papiernik, S.K.; Nunez, J. Conversion of Metam Sodium and Emission of Fumigant from Soil Columns. Atmos. Environ. 2006, 40, 7046–7056. [Google Scholar] [CrossRef]
- Authority, E.F.S. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Metam. EFSA J. 2011, 9, 2334. [Google Scholar] [CrossRef][Green Version]
- Kirkegaard, J.A.; Sarwar, M. Biofumigation Potential of Brassicas: I. Variation in Glucosinolate Profiles of Diverse Field-Grown Brassicas. Plant Soil 1998, 201, 71–89. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation Potential of Brassicas: III. In Vitro Toxicity of Isothiocyanates to Soil-Borne Fungal Pathogens. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for Fighting Replant Disease—A Review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolate and Isothiocyanate Concentration in Soil Following Incorporation of Brassica Biofumigants. Soil Biol. Biochem. 2006, 38, 2255–2264. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and Biofumigation: Fate of Glucosinolates and Their Hydrolysis Products in Soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. CRC Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Pardini, A.; Tamasi, G.; De Rocco, F.; Bonechi, C.; Consumi, M.; Leone, G.; Magnani, A.; Rossi, C. Kinetics of Glucosinolate Hydrolysis by Myrosinase in Brassicaceae Tissues: A High-Performance Liquid Chromatography Approach. Food Chem. 2021, 355, 129634. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The Myrosinase-Glucosinolate System, Its Organisation and Biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of Glucosinolate-Derived Isothiocyanates on Fungi: A Comprehensive Review on Direct Effects, Mechanisms, Structure-Activity Relationship Data and Possible Agricultural Applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J.; McCaffrey, J.P.; Auld, D.L.; Williams, L. Allelochemicals Produced during Glucosinolate Degradation in Soil. J. Chem. Ecol. 1991, 17, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.A.S.; Heaney, R.K.; Fenwick, G.R.; Portas, C.A.M. Glucosinolates in Crop Plants. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 1996; pp. 99–215. ISBN 9780470650622. [Google Scholar]
- Mari, M.; Leoni, O.; Bernardi, R.; Neri, F.; Palmieri, S. Control of Brown Rot on Stonefruit by Synthetic and Glucosinolate-Derived Isothiocyanates. Postharvest Biol. Technol. 2008, 47, 61–67. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The Antibacterial Properties of Isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Leoni, O.; Manici, L.M. Biocidal Plant Dried Pellets for Biofumigation. Ind. Crops Prod. 2004, 20, 59–65. [Google Scholar] [CrossRef]
- Morris, E.K.; Fletcher, R.; Veresoglou, S.D. Effective Methods of Biofumigation: A Meta-Analysis. Plant Soil 2020, 446, 379–392. [Google Scholar] [CrossRef]
- De Nicola, G.R.; D’Avino, L.; Curto, G.; Malaguti, L.; Ugolini, L.; Cinti, S.; Patalano, G.; Lazzeri, L. A New Biobased Liquid Formulation with Biofumigant and Fertilising Properties for Drip Irrigation Distribution. Ind. Crops Prod. 2013, 42, 113–118. [Google Scholar] [CrossRef]
- Mun, A.; Simaan Yameen, H.; Edelbaum, G.; Seliktar, D. Alginate Hydrogel Beads Embedded with Drug-Bearing Polycaprolactone Microspheres for Sustained Release of Paclobutrazol. Sci. Rep. 2021, 11, 10877. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous Materials for Pesticide Formulation and Delivery in the Agricultural Sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan Nanoparticle Based Delivery Systems for Sustainable Agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Smith, A.; Kavanagh, K.; Devereux, M.; Colleran, J.; Breslin, C.; Richards, K.G.; McCann, M.; Rooney, A.D. Preparation and Antimicrobial Properties of Alginate and Serum Albumin/Glutaraldehyde Hydrogels Impregnated with Silver(I) Ions. Chemistry 2021, 3, 672–686. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of Plant Growth Promoting Rhizobacteria—Prospects and Potential in Agricultural Sector: A Review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing Cinnamon Essential Oil Activity by Nanoparticle Encapsulation to Control Seed Pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks Polysaccharide Hydrogels for Drug Delivery and Tissue Engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Mikula, K.; Skrzypczak, D.; Ligas, B.; Witek-Krowiak, A. Preparation of Hydrogel Composites Using Ca2+ and Cu2+ Ions as Crosslinking Agents. SN Appl. Sci. 2019, 1, 643. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Aguirre Calvo, T.R.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Alginate Beads Containing Lactase: Stability and Microstructure. Biomacromolecules 2017, 18, 1785–1792. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Gu, B.K.; Kim, C.-H. Ionically Crosslinked Alginate–Carboxymethyl Cellulose Beads for the Delivery of Protein Therapeutics. Appl. Surf. Sci. 2012, 262, 28–33. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O.A. Synthesis, Swelling and Adsorption Studies of a PH-Responsive Sodium Alginate–Poly(Acrylic Acid) Superabsorbent Hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite Modified Sodium Alginate Hydrogel Composite for Efficient Removal of Malachite Green Dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Song, R.; Shen, G.; Liu, Y.; Tang, F.; Chen, Q.; Sun, P. Preparation and Characterization of an Oil-in-Water Microemulsion of Thiamethoxam and Acetamiprid without Organic Solvent for Unmanned Aerial Vehicle Spraying. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125485. [Google Scholar] [CrossRef]
- Leng, P.; Zhang, Z.; Li, Q.; Zhang, Y.; Zhao, M.; Pan, G. Development of a Difenoconazole/Propiconazole Microemulsion and Its Antifungal Activities against Rhizoctonia Solani AG1-IA. Pharmazie 2012, 67, 534–541. [Google Scholar]
- Davis, E.L.; Meyers, D.M.; Dullum, C.J.; Feitelson, J.S. Nematicidal Activity of Fatty Acid Esters on Soybean Cyst and Root-Knot Nematodes. J. Nematol. 1997, 29, 677–684. [Google Scholar] [PubMed]
- Kohli, A.G.; Kierstead, P.H.; Venditto, V.J.; Walsh, C.L.; Szoka, F.C. Designer Lipids for Drug Delivery: From Heads to Tails. J. Control. Release 2014, 190, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Ben-Shabat, S.; Aponick, A.; Zimmermann, E.M.; Dahan, A. Lipids and Lipid-Processing Pathways in Drug Delivery and Therapeutics. Int. J. Mol. Sci. 2020, 21, 3248. [Google Scholar] [CrossRef] [PubMed]
- Ciani, L.; Ristori, S.; Bonechi, C.; Rossi, C.; Martini, G. Effect of the Preparation Procedure on the Structural Properties of Oligonucleotide/Cationic Liposome Complexes (Lipoplexes) Studied by Electron Spin Resonance and Zeta Potential. Biophys. Chem. 2007, 131, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Clemente, I.; Bonechi, C.; Rodolfi, L.; Bacia-Verloop, M.; Rossi, C.; Ristori, S. Lipids from Algal Biomass Provide New (Nonlamellar) Nanovectors with High Carrier Potentiality for Natural Antioxidants. Eur. J. Pharm. Biopharm. 2021, 158, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Clemente, I.; D’Aria, F.; Giancola, C.; Bonechi, C.; Slouf, M.; Pavlova, E.; Rossi, C.; Ristori, S. Structuring and De-Structuring of Nanovectors from Algal Lipids. Part 1: Physico-Chemical Characterization. Colloids Surf. B Biointerfaces 2022, 220, 112939. [Google Scholar] [CrossRef]
- Yi, G.; Yin, C.; Lao, Y.; Shi, Z.; He, X.; Wu, J.; Jiang, Y.; Gong, L. Antibacterial and Antitumor Activities of Chitosan/Polyvinyl Alcohol Films Containing Microemulsion of Papaya Seed Essential Oil. Mater. Today Commun. 2022, 31, 103475. [Google Scholar] [CrossRef]
- Uppal, S.; Aashima; Kumar, R.; Sareen, S.; Kaur, K.; Mehta, S.K. Biofabrication of Cerium Oxide Nanoparticles Using Emulsification for an Efficient Delivery of Benzyl Isothiocyanate. Appl. Surf. Sci. 2020, 510, 145011. [Google Scholar] [CrossRef]
- Kim, W.-T.; Chung, H.; Shin, I.-S.; Yam, K.L.; Chung, D. Characterization of Calcium Alginate and Chitosan-Treated Calcium Alginate Gel Beads Entrapping Allyl Isothiocyanate. Carbohydr. Polym. 2008, 71, 566–573. [Google Scholar] [CrossRef]
- Baglioni, M.; Sekine, F.H.; Ogura, T.; Chen, S.H.; Baglioni, P. Nanostructured Fluids for Polymeric Coatings Removal: Surfactants Affect the Polymer Glass Transition Temperature. J. Colloid Interface Sci. 2022, 606, 124–134. [Google Scholar] [CrossRef]
- Tripathi, S.; Brown, D.G. Effects of Linear Alkylbenzene Sulfonate on the Sorption of Brij 30 and Brij 35 onto Aquifer Sand. Environ. Sci. Technol. 2008, 42, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Bide, Y.; Fashapoyeh, M.A.; Shokrollahzadeh, S. Structural Investigation and Application of Tween 80-Choline Chloride Self-Assemblies as Osmotic Agent for Water Desalination. Sci. Rep. 2021, 11, 17068. [Google Scholar] [CrossRef]
- Clemente, I.; Baglioni, M.; Bonechi, C.; Bisozzi, F.; Rossi, C.; Tamasi, G. Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils. Polymers 2023, 15, 4455. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Skrodzka, M.; Sicińska, H.; Michalak, I.; Detyna, J. Influence of Cross-Linking Conditions on Drying Kinetics of Alginate Hydrogel. Gels 2023, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Tourne-Peteilh, C.; Begu, S.; Lerner, D.A.; Galarneau, A.; Lafont, U.; Devoisselle, J.-M. Sol–Gel One-Pot Synthesis in Soft Conditions of Mesoporous Silica Materials Ready for Drug Delivery System. J. Sol-Gel Sci. Technol. 2012, 61, 455–462. [Google Scholar] [CrossRef]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and Appraisal of Self-Assembled Valsartan-Loaded Amalgamated Pluronic F127/Tween 80 Polymeric Micelles: Boosted Cardioprotection via Regulation of Mhrt/Nrf2 and Trx1 Pathways in Cisplatin-Induced Cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.E.B.; Massadeh, S.; Alshwaimi, A.A.; Kittaneh, R.H.; Omer, M.E.; Ahmad, D.; Aodah, A.H.; Shakeel, F.; Halwani, M.; Alanazi, S.A.; et al. Tween 80-Based Self-Assembled Mixed Micelles Boost Valsartan Transdermal Delivery. Pharmaceuticals 2023, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Kaur, G.; Bhasin, K.K. Tween-Embedded Microemulsions—Physicochemical and Spectroscopic Analysis for Antitubercular Drugs. AAPS PharmSciTech 2010, 11, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zi, Y.; Shi, C.; Gong, H.; Zhang, H.; Wang, X.; Zhong, J. Tween Emulsifiers Improved Alginate-Based Dispersions and Ionic Crosslinked Milli-Sized Capsules. NPJ Sci. Food 2023, 7, 33. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Schott, M.; Schmid, M.; Müller, K. Effect of Presence and Concentration of Plasticizers, Vegetable Oils, and Surfactants on the Properties of Sodium-Alginate-Based Edible Coatings. Int. J. Mol. Sci. 2018, 19, 742. [Google Scholar] [CrossRef] [PubMed]
- Lateef, S.; Basim, M.; Mohammed, A. Effect of Different Variables on the Formulation of Sodium Alginate Beads. Al Mustansiriyah J. Pharm. Sci. 2024, 24, 117–126. [Google Scholar] [CrossRef]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Circular Dichroism Spectroscopic Studies Reveal PH Dependent Binding of Curcumin in the Minor Groove of Natural and Synthetic Nucleic Acids. Org. Biomol. Chem. 2004, 2, 2902–2910. [Google Scholar] [CrossRef]
- Caccavo, D. An Overview on the Mathematical Modeling of Hydrogels’ Behavior for Drug Delivery Systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef]
- Mulye, N.V.; Turco, S.J. A Simple Model Based on First Order Kinetics to Explain Release of Highly Water Soluble Drugs from Porous Dicalcium Phosphate Dihydrate Matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the Morphology of Hydrophilic Polymeric Matrices on the Diffusion and Release of Water Soluble Drugs. J. Memb. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mitra, S.; Johnson, M.; Mukhopadhyay, R. Dynamics in Anionic Micelles: Effect of Phenyl Ring. J. Phys. Chem. B 2013, 117, 6250–6255. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Mitra, S.; Verma, G.; Hassan, P.A.; Garcia Sakai, V.; Mukhopadhyay, R. Internal Dynamics in SDS Micelles: Neutron Scattering Study. J. Phys. Chem. B 2010, 114, 17049–17056. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mitra, S.; Garcia Sakai, V.; Hassan, P.A.; Peter Embs, J.; Mukhopadhyay, R. The Dynamical Landscape in CTAB Micelles. Soft Matter 2012, 8, 7151. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mitra, S.; Sakai, V.G.; Mukhopadhyay, R. Dynamical Features in Cationic Micelles of Varied Chain Length. J. Phys. Chem. B 2012, 116, 9007–9015. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Domingues, J.A.L.; Carretti, E.; Fratini, E.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Complex Fluids Confined into Semi-Interpenetrated Chemical Hydrogels for the Cleaning of Classic Art: A Rheological and SAXS Study. ACS Appl. Mater. Interfaces 2018, 10, 19162–19172. [Google Scholar] [CrossRef]
- Baglioni, M.; Mastrangelo, R.; Tempesti, P.; Ogura, T.; Baglioni, P. Cryogels Loaded with Nanostructured Fluids Studied by Ultra-Small-Angle X-Ray Scattering. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130857. [Google Scholar] [CrossRef]
- Sathisaran, I.; Balasubramanian, M. Physical Characterization of Chitosan/Gelatin-Alginate Composite Beads for Controlled Release of Urea. Heliyon 2020, 6, e05495. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Wang, Y.-S.; Zhao, J.-S.; Li, Z.-Y.; Chen, H.-H. A PH-Sensitive Curcumin Loaded Microemulsion-Filled Alginate and Porous Starch Composite Gels: Characterization, in Vitro Release Kinetics and Biological Activity. Int. J. Biol. Macromol. 2021, 182, 1863–1873. [Google Scholar] [CrossRef]
- Song, S.; Wang, Z.; Qian, Y.; Zhang, L.; Luo, E. The Release Rate of Curcumin from Calcium Alginate Beads Regulated by Food Emulsifiers. J. Agric. Food Chem. 2012, 60, 4388–4395. [Google Scholar] [CrossRef] [PubMed]
- Josef, E.; Zilberman, M.; Bianco-Peled, H. Composite Alginate Hydrogels: An Innovative Approach for the Controlled Release of Hydrophobic Drugs. Acta Biomater. 2010, 6, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Best, M.G.; Cunha-Reis, C.; Ganin, A.Y.; Sousa, A.; Johnston, J.; Oliveira, A.L.; Smith, D.G.E.; Yiu, H.H.P.; Cooper, I.R. Antimicrobial Properties of Gallium(III)- and Iron(III)-Loaded Polysaccharides Affecting the Growth of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, In Vitro. ACS Appl. Bio Mater. 2020, 3, 7589–7597. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Zhong, C.; Wang, H.-S.; Hu, X.-H.; Chu, L.-Q. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J. Mechanisms of Iron Loading and Toxicity. Am. J. Hematol. 2007, 82, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, I.; Polo-López, M.I.; Oller, I.; Fernández-Ibáñez, P. Bacteria and Fungi Inactivation Using Fe3+/Sunlight, H2O2/Sunlight and near Neutral Photo-Fenton: A Comparative Study. Appl. Catal. B Environ. 2012, 121–122, 20–29. [Google Scholar] [CrossRef]
- Elijošiutė, E.; Eicher-Lorka, O.; Griškonis, E.; Kuodis, Z.; Jankūnaitė, D.; Denafas, G. Spectroscopic and Structural Investigations of Iron(III) Isothiocyanates. A Comparative Theoretical and Experimental Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, P.G.; Somasekharappa, K.G. Structure and Antimicrobial Activity of Some New Diphenylpyraline Complexes of Iron(III) Chloride, Nitrate, Sulphate, and Thiocyanate. J. Inorg. Biochem. 1994, 55, 13–20. [Google Scholar] [CrossRef]
- Yadav, V.; Wen, L.; Rodriguez, R.J.; Siegler, M.A.; Goldberg, D.P. Nonheme Iron(III) Azide and Iron(III) Isothiocyanate Complexes: Radical Rebound Reactivity, Selectivity, and Catalysis. J. Am. Chem. Soc. 2022, 144, 20641–20652. [Google Scholar] [CrossRef]

| Sample | dh (nm) | Polydispersity Index (PI) |
|---|---|---|
| Microemulsion (freshly prepared) | 34 ± 14 | 0.2 ± 0.1 |
| Microemulsion (freshly prepared) + curcumin | 47 ± 4 | 0.4 ± 0.1 |
| Microemulsion (equilibrium) | 101 ± 1 | 0.01 ± 0.01 |
| Microemulsion (equilibrium) + curcumin | 112 ± 1 | 0.01 ± 0.01 |
| Sample | ΔHfus (exp) | EWC (%) | FWI (%) |
|---|---|---|---|
| ALG | 280 ± 14 | 95 ± 5 | 89 ± 6 |
| ALG (1:1) | 250 ± 13 | 92 ± 5 | 82 ± 6 |
| CMC | 260 ± 13 | 94 ± 5 | 84 ± 6 |
| CMC (1:1) | 260 ± 13 | 92 ± 5 | 85 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baglioni, M.; Clemente, I.; Tamasi, G.; Bisozzi, F.; Costantini, S.; Fattori, G.; Gentile, M.; Rossi, C. Isothiocyanate-Based Microemulsions Loaded into Biocompatible Hydrogels as Innovative Biofumigants for Agricultural Soils. Molecules 2024, 29, 3935. https://doi.org/10.3390/molecules29163935
Baglioni M, Clemente I, Tamasi G, Bisozzi F, Costantini S, Fattori G, Gentile M, Rossi C. Isothiocyanate-Based Microemulsions Loaded into Biocompatible Hydrogels as Innovative Biofumigants for Agricultural Soils. Molecules. 2024; 29(16):3935. https://doi.org/10.3390/molecules29163935
Chicago/Turabian StyleBaglioni, Michele, Ilaria Clemente, Gabriella Tamasi, Flavia Bisozzi, Sara Costantini, Giacomo Fattori, Mariangela Gentile, and Claudio Rossi. 2024. "Isothiocyanate-Based Microemulsions Loaded into Biocompatible Hydrogels as Innovative Biofumigants for Agricultural Soils" Molecules 29, no. 16: 3935. https://doi.org/10.3390/molecules29163935
APA StyleBaglioni, M., Clemente, I., Tamasi, G., Bisozzi, F., Costantini, S., Fattori, G., Gentile, M., & Rossi, C. (2024). Isothiocyanate-Based Microemulsions Loaded into Biocompatible Hydrogels as Innovative Biofumigants for Agricultural Soils. Molecules, 29(16), 3935. https://doi.org/10.3390/molecules29163935







