Red Grape By-Products from the Demarcated Douro Region: Chemical Analysis, Antioxidant Potential and Antimicrobial Activity against Food-Borne Pathogens
Abstract
1. Introduction
2. Results
2.1. Phenolic Compounds in Hydroethanolic Extracts
2.2. Antioxidant Activity
2.3. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Plant Material and Samples Preparation
4.3. Extraction of Phenolic Compounds
4.4. HPLC-DAD Analysis of Phenolic Compounds
4.5. Antioxidant Activity and Biological Assays
4.5.1. DPPH• Scavenging
4.5.2. Superoxide Anion Radical Scavenging
4.5.3. Nitric Oxide Radical Scavenging
4.5.4. Data Processing and Statistical Analysis
4.5.5. Antibacterial Activity against Food-Borne Pathogens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Enes Dapkevicius, M.D.L.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. An Overview on Sustainability in the Wine Production Chain. Beverages 2021, 7, 15. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Bugaian, L.; Diaconu, C. Quantifying the sustainability of the wine sector through life cycle assessment (LCA). Econ. Contemp. 2022, 7, 63–69. [Google Scholar]
- Guerrini, A.; Burlini, I.; Huerta Lorenzo, B.; Grandini, A.; Vertuani, S.; Tacchini, M.; Sacchetti, G. Antioxidant and Antimicrobial Extracts Obtained from Agricultural By-Products: Strategies for a Sustainable Recovery and Future Perspectives. Food Bioprod. Process. 2020, 124, 397–407. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative Properties of Phenolic Compounds and Their Effect on Oxidative Stress Induced by Severe Physical Exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Pourreza, N. Phenolic Compounds as Potential Antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149–150. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, X.; Zhang, J. Antioxidant Activity In Vitro Guided Screening and Identification of Flavonoids Antioxidants in the Extract from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Anal. Chem. 2021, 2021, 7195125. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Moselhy, M.; Abd-Elhafez, K.; El-Kholany, E.; Gohar, M.; Nasr, N. Antimicrobial, Antioxidant and Anticancer Properties of Globe Artichoke and Grape by-Products as a Source of the Bio-Active Phenolic Compounds. Egypt. J. Chem. 2023, 65, 609–624. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic Compounds Classification and Their Distribution in Winemaking By-Products; Springer: Berlin/Heidelberg, Germany, 2023; Volume 249, ISBN 0123456789. [Google Scholar]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery Byproducts as Precious Resource of Natural Antimicrobials: In Vitro Antibacterial and Antibiofilm Activity of Grape Pomace Extracts against Foodborne Pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Decendit, A.; Mamani-Matsuda, M.; Aumont, V.; Waffo-Teguo, P.; Moynet, D.; Boniface, K.; Richard, E.; Krisa, S.; Rambert, J.; Mérillon, J.M.; et al. Malvidin-3-O-β Glucoside, Major Grape Anthocyanin, Inhibits Human Macrophage-Derived Inflammatory Mediators and Decreases Clinical Scores in Arthritic Rats. Biochem. Pharmacol. 2013, 86, 1461–1467. [Google Scholar] [CrossRef]
- Liu, Y.; Pukala, T.L.; Musgrave, I.F.; Williams, D.M.; Dehle, F.C.; Carver, J.A. Gallic Acid is the Major Component of Grape Seed Extract That Inhibits Amyloid Fibril Formation. Bioorganic Med. Chem. Lett. 2013, 23, 6336–6340. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Disease: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Reis, G.M.; Faccin, H.; Viana, C.; da Rosa, M.B.; de Carvalho, L.M. Vitis vinifera L. Cv Pinot Noir Pomace and Lees as Potential Sources of Bioactive Compounds. Int. J. Food Sci. Nutr. 2016, 67, 789–796. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Spizzirri, U.G.; Crupi, P.; Ioele, G.; Gorelli, B.; Clodoveo, M.L.; Saponara, S.; Aiello, F. Wine Lees as Source of Antioxidant Molecules: Green Extraction Procedure and Biological Activity. Antioxidants 2023, 12, 622. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Trad, M.; Le Bourvellec, C.; Ben Hamda, H.; Renard, C.M.G.C.; Harbi, M. Flavan-3-Ols and Procyanidins in Grape Seeds: Biodiversity and Relationships among Wild and Cultivated Vines. Euphytica 2017, 213, 242. [Google Scholar] [CrossRef]
- Kolb, C.A.; Kopecký, J.; Riederer, M.; Pfündel, E.E. UV Screening by Phenolics in Berries of Grapevine (Vitis vinifera). Funct. Plant Biol. 2003, 30, 1177–1186. [Google Scholar] [CrossRef]
- Mihanović, M.; Restek-Petrović, B.; Bodor, D.; Molnar, S.; Orešković, A.; Presečki, P. Suicidality and Side Effects of Antidepressants and Antipsychotics. Psychiatr. Danub. 2010, 22, 79–84. [Google Scholar]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Gougoulias, N.; Mashev, N. Evaluation of Polyphenols Antioxidant Activity of Grape Seeds (V. vinifera). Oxid. Commun. 2008, 31, 88–97. [Google Scholar]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128032701. [Google Scholar]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry by-Products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Pozzo, L.; Grande, T.; Raffaelli, A.; Longo, V.; Weidner, S.; Amarowicz, R.; Karamać, M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules 2023, 28, 4924. [Google Scholar] [CrossRef]
- Abdel-Khalek, H.H.; Mattar, Z.A. Biological Activities of Egyptian Grape and Mulberry By-Products and Their Potential Use as Natural Sources of Food Additives and Nutraceuticals Foods. J. Food Meas. Charact. 2022, 16, 1559–1571. [Google Scholar] [CrossRef]
- de Andrade, R.B.; Machado, B.A.S.; Barreto, G.d.A.; Nascimento, R.Q.; Corrêa, L.C.; Leal, I.L.; Tavares, P.P.L.G.; Ferreira, E.d.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Anti-Microbial Bioactive Compounds. Biology 2021, 10, 1262. [Google Scholar] [CrossRef]
- Javanmard, M. Antimicrobial Effects of Grape and Pomegranate Waste Extracts against Two Foodborne Pathogens. J. Food Biosci. Technol. 2020, 10, 39–48. [Google Scholar]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Gonçalves, L.A.; Lorenzo, J.M.; Trindade, M.A. Fruit and Agro-Industrial Waste Extracts as Potential Antimicrobials in Meat Products: A Brief Review. Foods 2021, 10, 1469. [Google Scholar] [CrossRef]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A.M. Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Deligeorgis, S.G. Agroindustrial By-Products and Animal Products: A Great Alternative for Improving Food-Quality Characteristics and Preserving Human Health; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128114421. [Google Scholar]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and Grape By-Products and Their Active Compounds: Are They a Valuable Source for Food Applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernandes, F.; Pinto-Carnide, O.; Valentão, P.; Falco, V.; Martín, J.P.; Ortiz, J.M.; Arroyo-García, R.; Andrade, P.B.; Castro, I. Identification of Vitis vinifera L. Grape Berry Skin Color Mutants and Polyphenolic Profile. Food Chem. 2016, 194, 117–127. [Google Scholar] [CrossRef]
- Dopico-García, M.S.; Fique, A.; Guerra, L.; Afonso, J.M.; Pereira, O.; Valentão, P.; Andrade, P.B.; Seabra, R.M. Principal Components of Phenolics to Characterize Red Vinho Verde Grapes: Anthocyanins or Non-Coloured Compounds? Talanta 2008, 75, 1190–1202. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, Á.; Andrade, P.B. In Vitro Multifunctionality of Phlorotannin Extracts from Edible Fucus Species on Targets Underpinning Neurodegeneration. Food Chem. 2020, 333, 127456. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to Control Hyperglycemia and Diabetes-Related Vascular Complications. J. Appl. Phycol. 2019, 31, 3143–3152. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jim, C.; Rodr, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentão, P. Anti-inflammatory effects of 5α, 8α-epidioxycholest-6-en-3β-ol, a steroidal endoperoxide isolated from Aplysia depilans, based on bioguided fractionation and NMR analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Dapkevicius, M.d.L.E.; Azevedo, M.; Cordeiro, R.; Pereira, J.E.; Valentão, P.; Falco, V.; Igrejas, G.; Caniça, M.; et al. Unveiling Antibiotic Resistance, Clonal Diversity, and Biofilm Formation in E. coli Isolated from Healthy Swine in Portugal. Pathogens 2024, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Tavares, T.; López, M.; Rojo-Bezares, B.; Pereira, J.E.; Falco, V.; Valentão, P.; Igrejas, G.; Sáenz, Y.; et al. Rabbits as a Reservoir of Multidrug-Resistant Escherichia coli: Clonal Lineages and Public Health Impact. Antibiotics 2024, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, V.; Gomes, J.P.; Coelho, A.; Batista, R.; Saraiva, C.; Esteves, A.; Martins, Â.; Contente, D.; Diaz-Formoso, L.; et al. Listeria Monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights. Antibiotics 2024, 13, 447. [Google Scholar] [CrossRef]
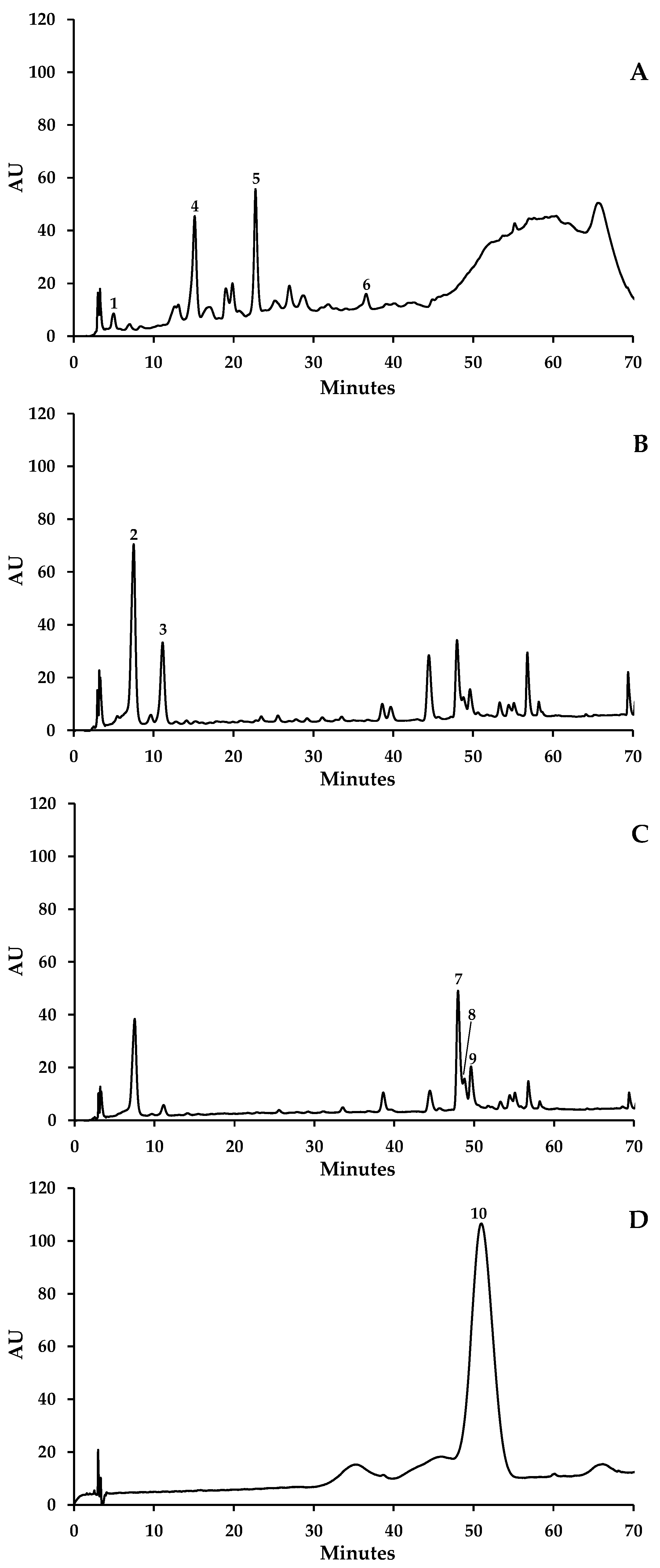

| Phenolic Compounds | Retention Time (min) | Seeds | Skins | Stems | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Periquita | Gamay | Donzelinho Tinto | Periquita | Gamay | Donzelinho Tinto | Periquita | Gamay | Donzelinho Tinto | ||
| Phenolic acids | ||||||||||
| Hydroxybenzoic acid | ||||||||||
| Gallic acid (1) | 5.35 | 0.24 (0.00) | 0.31 (0.00) | 0.30 (0.00) | - | - | - | - | - | - |
| Hydroxycinnamic acid | ||||||||||
| Caftaric acid (2) | 9.17 | - | - | - | - | - | - | 1.21 (0.15) | 0.56 (0.01) | 0.75 (0.04) |
| Coutaric acid (3) | 12.52 | - | - | - | - | - | - | 0.28 (0.00) | nq | 0.21 (0.01) |
| ∑ | 0.24 (0.00) | 0.31 (0.00) | 0.30 (0.00) | - | - | - | 1.49 (0.15) | 0.56 (0.01) | 0.96 (0.05) | |
| Flavonoids | ||||||||||
| Flavan-3-ols | ||||||||||
| Catechin (4) | 16.43 | 3.82 (0.15) | 8.96 (0.05) | 4.08 (0.03) | - | - | - | 2.36 (0.02) | 2.98 (0.11) | 6.74 (0.05) |
| Epicatechin (5) | 23.69 | 2.95 (0.04) | 3.96 (0.03) | 0.66 (0.05) | - | - | - | nq | 1.48 (0.04) | - |
| Epicatechin-3-O-gallate (6) | 37.86 | nq | nq | nq | - | - | - | - | - | - |
| ∑ | 6.77 (0.19) | 12.92 (0.07) | 4.74 (0.08) | - | - | - | 2.36 (0.02) | 4.46 (0.15) | 6.74 (0.05) | |
| Flavonols | ||||||||||
| Quercetin-3-O-galactoside (7) + Quercetin-3-O-rutinoside (8) * | 49.65 | - | - | - | 1.72 (0.01) | 0.93 (0.01) | 5.93 (0.10) | 0.25 (0.01) | 0.47 (0.01) | 1.68 (0.04) |
| Quercetin-3-O-glucoside (9) | 51.11 | - | - | - | 1.37 (0.01) | 0.95 (0.02) | 2.16 (0.04) | 0.67 (0.02) | 0.56 (0.00) | - |
| Kaempferol-3-O-glucoside (11) | 55.41 | - | - | - | 7.58 (0.02) | 3.37 (0.04) | 7.89 (0.16) | - | - | - |
| ∑ | - | - | - | 10.67 (0.04) | 5.25 (0.07) | 15.98 (0.30) | 0.92 (0.03) | 0.98 (0.01) | 1.68 (0.04) | |
| Anthocyanins | ||||||||||
| Malvidin-3-O-glucoside (10) | 51.61 | - | - | - | 39.13 (1.33) | 42.59 (0.72) | 41.28 (0.12) | 0.83 (0.03) | 0.68 (0.01) | nq |
| Total | 7.01 (0.19) | 13.23 (0.07) | 5.04 (0.08) | 49.80 (1.37) | 47.84 (0.79) | 57.26 (0.42) | 5.60 (0.23) | 6.68 (0.18) | 9.38 (0.14) | |
| Varieties | Extracts | DPPH Scavenging |
|---|---|---|
| Periquita | Seeds | 0.43 ± 0.01 |
| Gamay | 0.23 ± 0.01 | |
| Donzelinho Tinto | 0.36 ± 0.01 | |
| Periquita | Skins | 0.72 ± 0.02 |
| Gamay | 0.90 ± 0.01 | |
| Donzelinho Tinto | 0.98 ± 0.01 | |
| Periquita | Stems | 0.50 ± 0.05 |
| Gamay | 0.50 ± 0.02 | |
| Donzelinho Tinto | 0.41 ± 0.01 |
| Bacteria Collection | MIC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Seeds | Stems | Skins | |||||||
| Periquita | Gamay | Donzelinho Tinto | Periquita | Gamay | Donzelinho Tinto | Periquita | Gamay | Donzelinho Tinto | |
| Gram-negative | |||||||||
| E. coli from pigs | |||||||||
| S1 | 10 | 10 | 10 | 25 | 10 | 50 | - | - | - |
| S2 | - | - | - | - | - | - | - | - | - |
| S3 | - | - | - | - | - | - | - | - | - |
| S4 | - | - | - | - | - | - | - | - | - |
| S17 | - | - | - | - | - | - | - | - | - |
| S18 | 50 | 25 | - | - | - | - | - | - | - |
| S21 | - | - | - | - | - | - | - | - | - |
| S25 | - | - | - | - | - | - | - | - | - |
| S31 | - | - | - | - | - | - | - | - | - |
| S33 | - | - | - | - | - | - | - | - | - |
| S34 | - | - | - | - | - | 75 | - | - | - |
| S40 | - | - | - | - | - | - | - | - | - |
| S42 | - | - | - | - | - | - | - | - | - |
| E. coli from rabbits | |||||||||
| C1 | - | - | - | - | - | - | - | - | - |
| C3 | - | - | - | - | - | - | - | - | - |
| C5 | - | - | - | - | - | - | - | - | - |
| C9 | - | - | - | - | - | - | - | - | - |
| C18 | - | - | - | - | - | - | - | - | - |
| C24 | - | - | - | - | - | - | - | - | - |
| C30 | - | - | - | - | - | - | - | - | - |
| C31 | - | - | - | - | - | - | - | - | - |
| C33 | - | - | - | - | - | - | - | - | - |
| C34 | - | - | - | - | - | - | - | - | - |
| C36 | - | - | - | - | - | - | - | - | - |
| C40 | - | - | - | - | - | - | - | - | - |
| C48 | - | - | - | - | - | - | - | - | - |
| Gram-positive | |||||||||
| L. monocytogenes from food products and associated environments | |||||||||
| L1 | 10 | 10 | 10 | 25 | 10 | - | - | - | - |
| L2 | - | - | - | 10 | 25 | - | - | - | - |
| L3 | 10 | 10 | 10 | 25 | 25 | 50 | 75 | - | 75 |
| L4 | 10 | 25 | 25 | 25 | - | 10 | 75 | - | 75 |
| L6 | 10 | 10 | 10 | 25 | 50 | 50 | 75 | - | 75 |
| L7 | 10 | 10 | 10 | 10 | 25 | 10 | 75 | - | 75 |
| L8 | 10 | 10 | 10 | 10 | 10 | - | 75 | - | 75 |
| L10 | 10 | 10 | 10 | 25 | - | - | - | - | - |
| L11 | 10 | 10 | 10 | 25 | 25 | 10 | - | - | - |
| L12 | 10 | 10 | 10 | 25 | 50 | - | - | - | - |
| L13 | 10 | 10 | 25 | 25 | 25 | - | 75 | - | 75 |
| L14 | 25 | 10 | 25 | 10 | - | 100 | - | - | - |
| L15 | 25 | 10 | 10 | 50 | 50 | - | - | - | - |
| Compound | Regression Equation (mg/mL) | r2 | Linearity (mg/mL) |
|---|---|---|---|
| Gallic acid | y = 4.20 × 104x − 226.34 | 0.997 | 0.011–0.178 |
| Caffeic acid | y = 9.20 × 104x − 1370.40 | 0.998 | 0.066–1.060 |
| p-Coumaric acid | y = 1.20 × 105x − 286.89 | 0.998 | 0.008–0.567 |
| Catechin | y = 1.12 × 104x − 172.76 | 0.998 | 0.050–1.270 |
| Epicatechin | y = 1.20 × 105x + 85.90 | 0.986 | 0.018–1.140 |
| Epigallocatechin-3-O-gallate | y = 2.90 × 104x + 1218.40 | 0.993 | 0.063–1,000 |
| Quercetin-3-O-rutinoside | y = 2.90 × 104x + 39.21 | 0.997 | 0.016–0.194 |
| Quercetin-3-O-glucoside | y = 4.80 × 104x − 126.34 | 0.995 | 0.005–0.380 |
| Kaempferol-3-O-glucoside | y = 3.70 × 103x − 14.74 | 0.997 | 0.060–0.730 |
| Malvidin-3-O-glucoside | y = 1.00 × 104x + 127.23 | 0.992 | 0.062–1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Martins, R.; Silva, V.; Fernandes, F.; Carvalho, R.; Aires, A.; Igrejas, G.; Falco, V.; Valentão, P.; Poeta, P. Red Grape By-Products from the Demarcated Douro Region: Chemical Analysis, Antioxidant Potential and Antimicrobial Activity against Food-Borne Pathogens. Molecules 2024, 29, 4708. https://doi.org/10.3390/molecules29194708
Silva A, Martins R, Silva V, Fernandes F, Carvalho R, Aires A, Igrejas G, Falco V, Valentão P, Poeta P. Red Grape By-Products from the Demarcated Douro Region: Chemical Analysis, Antioxidant Potential and Antimicrobial Activity against Food-Borne Pathogens. Molecules. 2024; 29(19):4708. https://doi.org/10.3390/molecules29194708
Chicago/Turabian StyleSilva, Adriana, Raquel Martins, Vanessa Silva, Fátima Fernandes, Rosa Carvalho, Alfredo Aires, Gilberto Igrejas, Virgílio Falco, Patrícia Valentão, and Patrícia Poeta. 2024. "Red Grape By-Products from the Demarcated Douro Region: Chemical Analysis, Antioxidant Potential and Antimicrobial Activity against Food-Borne Pathogens" Molecules 29, no. 19: 4708. https://doi.org/10.3390/molecules29194708
APA StyleSilva, A., Martins, R., Silva, V., Fernandes, F., Carvalho, R., Aires, A., Igrejas, G., Falco, V., Valentão, P., & Poeta, P. (2024). Red Grape By-Products from the Demarcated Douro Region: Chemical Analysis, Antioxidant Potential and Antimicrobial Activity against Food-Borne Pathogens. Molecules, 29(19), 4708. https://doi.org/10.3390/molecules29194708













