Defense Molecules of the Invasive Plant Species Ageratum conyzoides
Abstract
1. Introduction
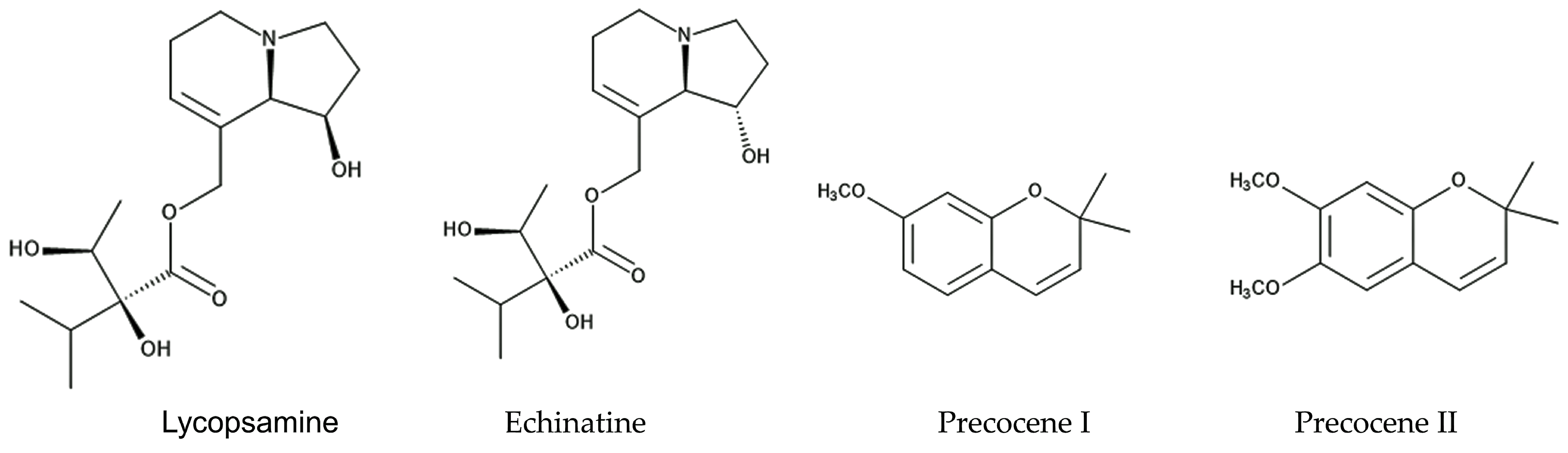
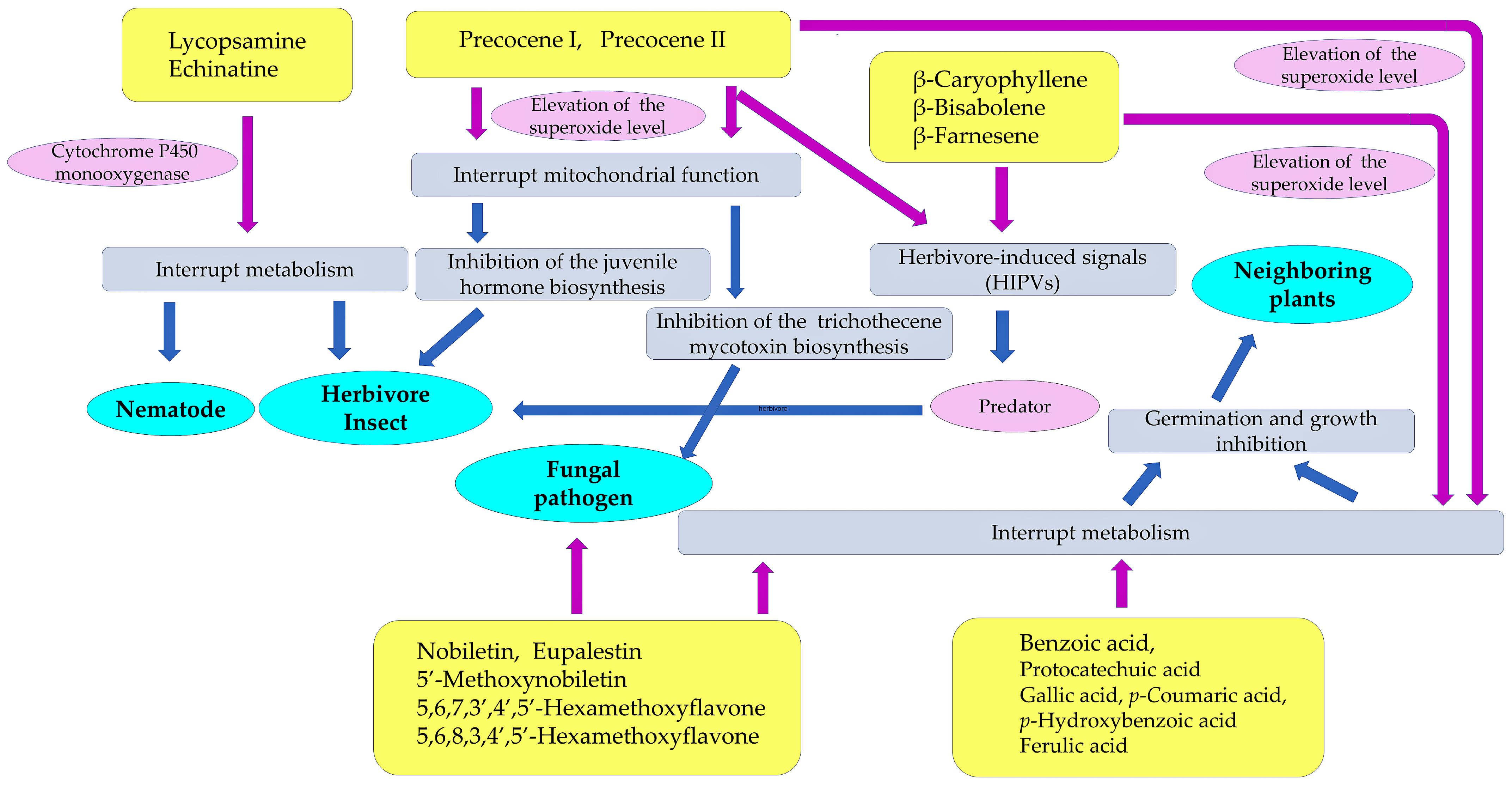
2. Defense Molecules against Herbivore Insects
3. Defense Molecules against Nematodes
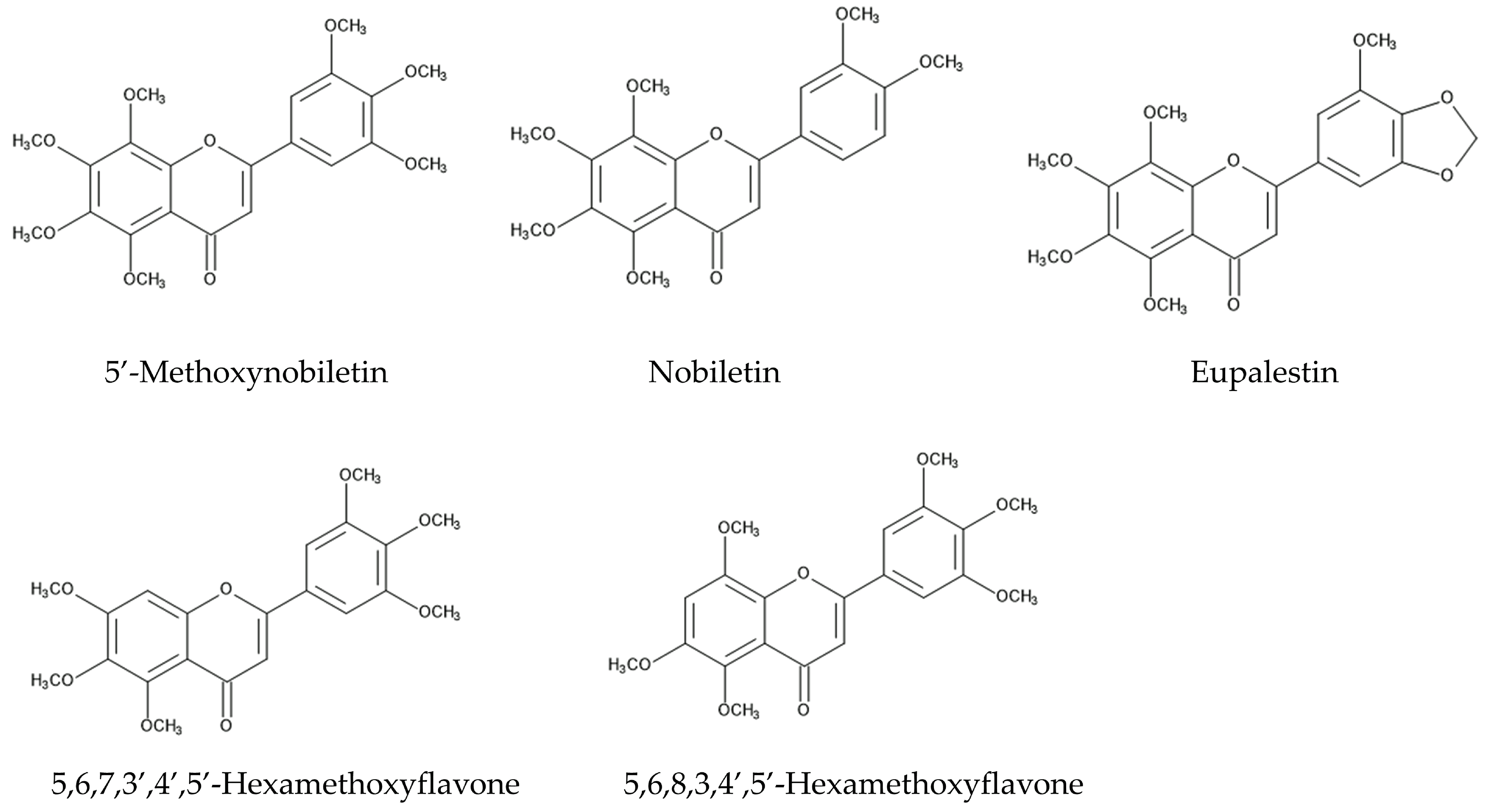
4. Defense Molecules against Fungal Pathogens
5. Inhibitors for Symbiosis
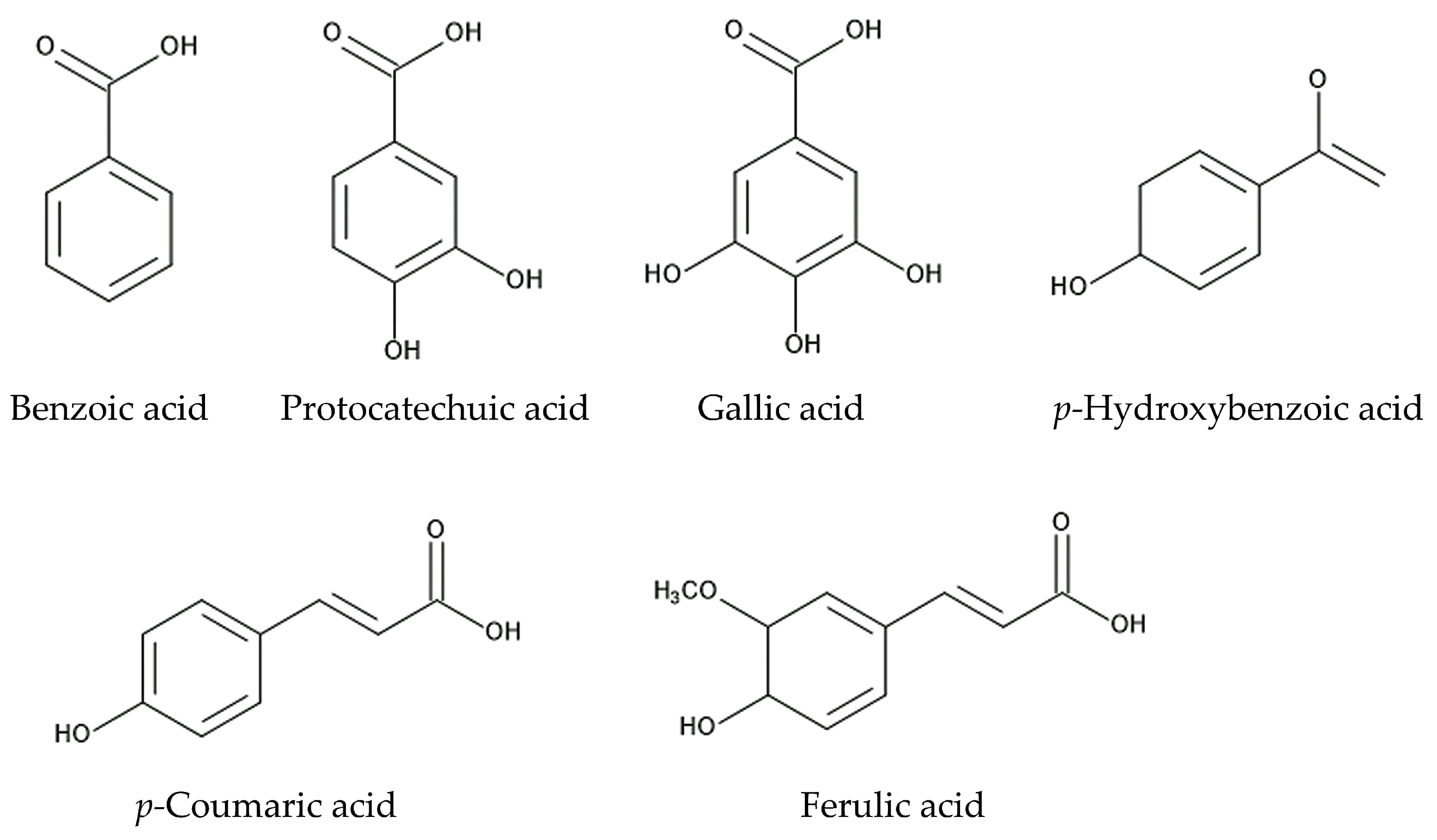
6. Defense Molecules against Neighboring Plants
7. Contributions of Defense Molecules to Invasive Traits of Ageratum conyzoides
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ming, L.C. Ageratum conyzoides: A tropical source of medicinal and agricultural products. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 469–473. [Google Scholar]
- Global Invasive Species Database. Species Profile: Ageratum conyzoides. Available online: https://www.iucngisd.org/gisd/species.php?sc=1493 (accessed on 12 August 2024).
- Royal Botanical Gardens, Kew, Ageratum conyzoides. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:7086-2 (accessed on 12 August 2024).
- CABI Copmpendium. Ageratum conyzoides (Billy Goat Weed). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.3572 (accessed on 12 August 2024).
- Kaur, A.; Kaur, S.; Singh, H.P.; Datta, A.; Chauhan, B.S.; Ullah, H.; Kohli, R.K.; Batish, D.R. Ecology, biology, environmental impacts, and management of an agro-environmental weed Ageratum conyzoides. Plants 2023, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Paraseth, P.; Banerjee, K. Goat weed (Ageratum conyzoides L.): A biological threat to plant diversity in Eastern Ghats of India. J. Biosci. 2024, 49, 72. [Google Scholar] [CrossRef]
- Manandhar, S.; Shrestha, B.B.; Lekhak, H.D. Weeds of paddy field at Kirtipur, Kathmandu. Sci. World 2007, 5, 100–106. [Google Scholar] [CrossRef]
- Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Role of root-mediated interactions in phytotoxic interference of Ageratum conyzoides with rice (Oryza sativa). Flora 2009, 204, 388–395. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Feasibility of vermicomposting for the management of terrestrial weed Ageratum conyzoides using earthworm species Eisenia fetida. Environ. Technol. Innov. 2020, 18, 100696. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Anwar, I.; Bukhari, H.A.; Nahid, N.; Rashid, K.; Amin, I.; Shaheen, S.; Hussain, K.; Mansoor, S. Association of cotton leaf curl Multan betasatellite and Ageratum conyzoides symptomless alphasatellite with tomato leaf curl New Delhi virus in Luffa cylindrica in Pakistan. Australas. Plant Pathol. 2020, 49, 25–29. [Google Scholar] [CrossRef]
- Sharman, M.; Thomas, J.E.; Tree, D.; Persley, D.M. Natural host range and thrips transmission of capsicum chlorosis virus in Australia. Australas. Plant Pathol. 2020, 49, 45–51. [Google Scholar] [CrossRef]
- Serfraz, S.; Amin, I.; Akhtar, K.P.; Mansoor, S. Recombination among Begomoviruses on Malvaceous plants leads to the evolution of okra enation leaf curl virus in Pakistan. J. Phytopathol. 2015, 163, 764–776. [Google Scholar] [CrossRef]
- Martins, D.D.S.; Ventura, J.A.; Paula, R.D.C.A.L.; Fornazier, M.J.; Rezende, J.A.M.; Culik, M.P.; Ferreira, P.S.F.; Peronti, A.L.B.G.; de Carvalho, R.C.Z.; Sousa-Silva, C.R. Aphid vectors of Papaya ringspot virus and their weed hosts in orchards in the major papaya producing and exporting region of Brazil. Crop Prot. 2016, 90, 191–196. [Google Scholar] [CrossRef]
- Kohli, R.K.; Batish, D.R.; Singh, H.P.; Dogra, K.S. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biol. Invasions 2006, 8, 1501–1510. [Google Scholar] [CrossRef]
- Dogra, K.S.; Kohli, R.K.; Sood, S.K.; Dobhal, P.K. Impact of Ageratum conyzoides L. on the diversity and composition of vegetation in the Shivalik hills of Himachal Pradesh (Northwestern Himalaya), India. Int. J. Biodivers. Conser. 2009, 1, 135–145. [Google Scholar]
- US Fish and Wildlife Service. 5-Year Review: Summary and Evaluation. In Lsodendrion longifolium (aupaka); US Fish and Wildlife Service: Falls Church, VA, USA, 2011. Available online: http://ecos.fws.gov/docs/five_year_review/doc3809.pdf (accessed on 12 August 2024).
- Thompson, J.D.; McNeilly, T.; Gray, A.J. Population variation in Spartina anglica C.E. Hubbard. I. Evidence from a common garden experiment. New Phytol. 1991, 117, 115–128. [Google Scholar] [CrossRef]
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Engin. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Warren, R.J.; Matt Candeias, M.; Labatore, A.; Olejniczak, M.; Yang, L. Multiple mechanisms in woodland plant species invasion. J. Plant Ecol. 2019, 12, 201–209. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977; pp. 1–609. [Google Scholar]
- Kaur, S.; Batish, D.R.; Kohli, R.K.; Singh, H.P. Ageratum conyzoides: An alien invasive weed in India. In Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent; Bhatt, J.R., Singh, J.S., Singh, S.P., Tripathi, R.S., Kohli, R.K., Eds.; CABI: Wallingford, UK, 2012; pp. 57–76. [Google Scholar]
- Horvitz, N.; Wang, R.; Wan, F.H.; Nathan, R. Pervasive human-mediated large-scale invasion: Analysis of spread patterns and their underlying mechanisms in 17 of China’s worst invasive plants. J. Ecol. 2017, 105, 85–94. [Google Scholar] [CrossRef]
- Marks, M.K.; Nwachuku, A.C. Seed-bank characteristics in a group of tropical weeds. Weed Res. 1986, 26, 151–157. [Google Scholar] [CrossRef]
- Sauerborn, J.; Koch, W.; Krage, J. On the influence of light, temperature, depth of burial and water stress on the germination of selected weed species. Z. Pflanzenkr. Pflanzenschutz Sonderh. 1988, 11, 47–53. [Google Scholar]
- PIER. Pacific Island Ecosystems at Risk, Ageratum conyzoides. Available online: http://www.hear.org/pier/species/ageratum_conyzoides.htm (accessed on 12 August 2024).
- PROTA. PROTA4U Web Database. Plant Resources of Tropical Africa. 2018. Available online: https://www.prota4u.org/database/ (accessed on 12 August 2024).
- Gupta, R.C. Meiotic abnormalities in Ageratum conyzoides from hot desert of India (Rajasthan). Chromosome Bot. 2015, 10, 67–74. [Google Scholar]
- do Rosário, C.J.R.M.; Lima, A.S.; de Mendonça, C.; Soares, I.S.; Júnior, E.B.A.; Gomes, M.N.; Costa-Junior, L.M.; Maia, J.G.S.; da Rocha, C.Q. Essential oil Ageratum conyzoides chemotypes and anti-tick activities. Vet. Parasitol. 2023, 319, 109942. [Google Scholar] [CrossRef] [PubMed]
- Dogra, K.S.; Kohli, R.K.; Sood, S.K. An assessment and impact of three invasive species in the Shivalik hills of Himachal Pradesh, India. Int. J. Biodiv. Conserv. 2009, 1, 4–10. [Google Scholar]
- Chaudhary, N.; Narayan, R. The advancing dominance of Ageratum conyzoides L. and Lantana camara L. in dry tropical peri-urban vegetation in India. Int. Res. J. Environ. Sci. 2013, 2, 88–95. [Google Scholar]
- Sengupta, R.; Dash, S.S. A comprehensive inventory of alien plants in the protected forest areas of Tripura and their ecological consequences. Nelumbo 2021, 63, 163–182. [Google Scholar] [CrossRef]
- Semy, K.; Singh, M.R. Changes in plant diversity and community attributes of coal mine affected forest in relation to a community reserve forest of Nagaland, Northeast India. Trop. Ecol. 2024, 65, 16–25. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1861–1867. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.L. Exotic plant invasions and the enemy release hypothesis. Trend Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants—A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Muller-Scharer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: Implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef]
- Ingrid, D.T.; Akwanjoh, S.R.; Yacouba, M. Insecticidal activity of Ageratum conyzoides (Asteraceae) aqueous extracts against the grasshopper Zonocerus variegatus (Orthoptera: Pyrgomorphidae). J. Agric. Ecol. Res. Int. 2020, 21, 29–36. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanco, M.C.; Barbosa, L.C.A.; Guedes, R.N.C.; Barros, E.C.; Campos, M.R. Compounds from Ageratum conyzoides: Isolation, structural elucidation and insecticidal activity. Pest Manag. Sci. 2007, 63, 615–621. [Google Scholar] [CrossRef]
- Raja, S.S.; Singh, A.; Rao, S. Effect of Ageratum conyzoides on Chilo partelus swinhoe (Lepidoptera: Pyralidae). J. Anim. Morphol. Physiol. 1987, 34, 35–37. [Google Scholar]
- Fagoonee, I.; Umrit, G. Antigonadotropic hormones from the goatweed, Ageratum conyzoides. Insect Sci. Appl. 1981, 4, 373–376. [Google Scholar]
- Suwaiba, H.; Barde, A.A.; Mao, P.S.; Aliyu, O.A. Larvicidal activity of Ageratum conyzoides L. extracts on Anopheles gambiae complex. GSC Biol. Pharm. Sci. 2018, 3, 1–5. [Google Scholar]
- Singh, P.J.; Prakash, B.; Dubey, N.K. Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J. Food Sci. Technol. 2014, 51, 2210–2215. [Google Scholar]
- Gbolade, A.A.; Onayade, O.A.; Ayinde, B.A. Insecticidal activity of Ageratum conyzoides L. volatile oil against Callosobruchus chinensis F in seed treatment and fumigation laboratory tests. Insect Sci. Appl. 1999, 19, 237. [Google Scholar]
- Wiedenfeld, H.; Roder, E. Pyrrozidine alkaloids form Ageratum conyzoides. Planta Med. 1991, 57, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Boppré, M. Lepidoptera and pyrrolizidine alkaloids. Exemplification of complexity in chemical ecology. J. Chem. Ecol. 1990, 16, 165–185. [Google Scholar] [CrossRef]
- Ober, D.; Hartmann, T. Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 14777–14782. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- van Dam, N.M.; Vuister, L.W.; Bergshoeff, C.; de Vos, H.; van der Meijden, E.D. The “Raison D’être” of pyrrolizidine alkaloids in Cynoglossum officinale: Deterrent effects against generalist herbivores. J. Chem. Ecol. 1995, 21, 507–523. [Google Scholar] [CrossRef]
- Joshi, J.; Vrieling, K. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005, 8, 704–714. [Google Scholar] [CrossRef]
- Gardner, D.R.; Thorne, M.S.; Molyneux, R.J.; Pfister, J.A.; Seawright, A.A. Pyrrolizidine alkaloids in Senecio madagascariensis from Australia and Hawaii and assessment of possible livestock poisoning. Biochem. Syst. Ecol. 2006, 34, 736–744. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Colegate, S.M.; Brown, A.W. Dehydropyrrolizidine alkaloid toxicity, cytotoxicity, and carcinogenicity. Toxins 2016, 8, 356. [Google Scholar] [CrossRef]
- Gordon, G.J.; Coleman, W.B.; Grisham, J.W. Induction of cytochrome P450 enzymes in the livers of rats treated with the pyrrolizidine alkaloid retrorsine. Exp. Mol. Pathol. 2000, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184. [Google Scholar] [CrossRef]
- Cheeke, P.R. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci. 1988, 66, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, J.; Burgueño-Tapia, E.; Vázquez, M.A.; Delgado, F. Pyrrolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Hans-Joachim Knölker, H.J., Ed.; Academic Press: Cambridge, UK, 2018; pp. 1–314. [Google Scholar]
- Macel, M. Attract and deter: A dual role for pyrrolizidine alkaloids in plant-insect interactions. Phytochem. Rev. 2011, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Livshultz, T.; Kaltenegger, E.; Straub, S.C.; Weitemier, K.; Hirsch, E.; Koval, K.; Mema, L.; Liston, A. Evolution of pyrrolizidine alkaloid biosynthesis in Apocynaceae: Revisiting the defense de-escalation hypothesis. New Phytol. 2018, 218, 762–773. [Google Scholar] [CrossRef]
- Bowers, W.S.; Ohta, T.; Cleere, J.S.; Marsella, P.A. Discovery of insect anti-juvenile hormones in plants. Science 1976, 193, 542–547. [Google Scholar] [CrossRef]
- Kong, C.H.; Hu, F.; Xu, X.H.; Zhang, M.X.; Liang, W.J. Volatile allelochemicals in the Ageratum conyzoides intercropped citrus orchard and their effects on mites Amblyseius newsami and Panonychus citri. J. Chem. Ecol. 2005, 31, 2193–2203. [Google Scholar] [CrossRef]
- Mathai, S.; Nair, V.S.K. Effects of precocene II on last instar larvae of Spodoptera mauritia (Lepidoptera: Noctuidae). Curr. Sci. 1983, 52, 376–377. [Google Scholar]
- Oi, C.A.; Ferreira, H.M.; da Silva, R.C.; Bienstman, A.; do Nascimento, F.S.; Wenseleers, T. Effects of juvenile hormone in fertility and fertility-signaling in workers of the common wasp Vespula vulgaris. PLoS ONE 2021, 16, e0250720. [Google Scholar] [CrossRef]
- Amsalem, E.; Teal, P.; Grozinger, C.M.; Hefetz, A. Precocene-I inhibits juvenile hormone biosynthesis, ovarian activation, aggression and alters sterility signal production in bumble bee (Bombus terrestris) workers. J. Exp. Biol. 2014, 217, 3178–3185. [Google Scholar] [CrossRef]
- Gupta, P.R.; Dogra, G.S. Bioactivity of Precocene II against the potato beetle Epilachna vigintioctopunctata. In Presented in National Symposium on Problems and Prospects of Botanical Pesticides in Integrated Pest Management; CTRI Press: Rajahmundry, India, 1990; pp. 21–22. [Google Scholar]
- Hammond, A.H.; Garle, M.J.; Fry, J.R. Mechanism of toxicity of precocene II in rat hepatocyte cultures. J. Biochem. Toxicol. 1995, 10, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.G.; Huang, M.D. Influence of citrus orchard ground cover plants on arthropod communities in China: A review. Agric. Ecosyst. Environ. 1994, 50, 29–37. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Aartsma, Y.; Bianchi, F.J.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Seid, A.; Fininsa, C.; Mekete, T.; Decraemer, W.; Wesemael, W.M. Tomato (Solanum lycopersicum) and root-knot nematodes (Meloidogyne spp.)—A century-old battle. Nematology 2015, 17, 995–1009. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- Lambert, K.; Bekal, S. Introduction to Plant-Parasitic Nematodes. The Plant Health Instructor. Available online: https://www.apsnet.org/edcenter/disandpath/nematode/intro/Pages/IntroNematodes.aspx (accessed on 12 August 2024).
- den Akker, S.E. Plant–nematode interactions. Curr. Opin. Plant Biol. 2021, 62, 102035. [Google Scholar]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Akhter, G.; Zafar, A.; Khan, W.; Jamshed, M. In vitro nemato-toxic potential of some leaf extracts on juvenile mortality of Meloidogyne incognita race-3. Arch. Phytopathol. Plant Prot. 2018, 51, 399–407. [Google Scholar] [CrossRef]
- Mamman, A. Nematicidal activity of Ageratum conyzoides leaf extract against root-knot nematode (Meloidogyne javanica) on eggplant in Jalingo, Nigeria. Niger. Dutse J. Pure Appl. Sci. 2023, 9, 120–128. [Google Scholar] [CrossRef]
- Pavaraj, M.; Karthikairaj, K.; Rajan, M.K. Effect of leaf extract of Ageratum conyzoides on the biochemical profile of blackgram Vigna mungo infected by root-knot nematode, Meloidogyne incognita. J. Biopest. 2010, 3, 313–316. [Google Scholar]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Ann. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defense responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Javed, S.; Basir, U. Antifungal activity of different extracts of Ageratum conyzoides for the management of Fusarium solani. Afr. J. Biotechnol. 2012, 11, 11022–11029. [Google Scholar]
- Hazirah, M.F.; Hamizah, O.; Natasya, W.A.W. Antifungal activity of Ageratum conyzoides extract against Fusarium oxysporum in Musa spp. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 012074. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Nguyen, T.Q.C.; Kanaori, K.; Binh, T.D.; Dao, X.H.T.; Vang, L.V.; Kamei, K. Antifungal activities of Ageratum conyzoides L. extract against rice pathogens Pyricularia oryzae Cavara and Rhizoctonia solani Kühn. Agriculture 2021, 11, 1169. [Google Scholar] [CrossRef]
- Kong, C.H.; Liang, W.J.; Hu, F.; Xu, X.H.; Wang, P.; Jiang, Y. Allelochemicals and their transformations in A. conyzoides intercropped citrus orchard soil. Plant Soil 2004, 264, 149–157. [Google Scholar] [CrossRef]
- Yaguchi, A.; Yoshinari, T.; Tsuyuki, R.; Takahashi, H.; Nakajima, T.; Sugita-Konishi, Y. Isolation and identification of precocenes and piperitone from essential oils as specific inhibitors of trichothecene production by Fusarium graminearum. J. Agric. Food. Chem. 2009, 57, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Chahal, R.; Nanda, A.; Akkol, E.K.; Sobarzo-Sánchez, E.; Arya, A.; Kaushik, D.; Dutt, R.; Bhardwaj, R.; Rahman, M.H.; Mittal, V. Ageratum conyzoides L. and its secondary metabolites in the management of different fungal pathogens. Molecules 2021, 26, 2933. [Google Scholar] [CrossRef] [PubMed]
- Nasmith, C.G.; Walkowiak, S.; Wang, L.; Leung, W.Y.Y.; Gong, Y.; Johnston, A.; Harris, L.J.; Guttman, D.S.; Subramaniamet, R. Tri6 is a global transcription regulator in the phytopathogen Fusarium graminearum. PLoS Pathog. 2011, 7, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Yoshinari, T.; Furukawa, T.; Jermnak, U.; Takagi, K.; Kimura, M.; Yamamoto, T.; Suzuki, M.; Nagasawa, H. Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci. Biotech. Biochem. 2016, 80, 43–54. [Google Scholar] [CrossRef]
- Furukawa, T.; Sakamoto, N.; Suzuki, M.; Kimura, M.; Nagasawa, H.; Sakuda, S. Precocene II, a trichothecene production inhibitor, binds to voltage-dependent anion channel and increases the superoxide level in mitochondria of Fusarium graminearum. PLoS ONE 2015, 10, e0135031. [Google Scholar] [CrossRef]
- Noriega, F.G. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? Int. Sch. Res. Notices 2014, 1, 967361. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K. Phytotoxicity of Ageratum conyzoides residues towards growth and nodulation of Cicer arietinum. Agric. Ecosyst. Environ. 2006, 113, 399–401. [Google Scholar] [CrossRef]
- Barrett, C.F.; Parker, M.A. Coexistence of Burkholderia, Cupriavidus and Rhizobium sp. nodule bacteria on two Mimosa species in Costa Rica. Appl. Environ. Microbiol. 2006, 72, 1198–1206. [Google Scholar] [CrossRef]
- Chen, W.M.; James, E.K.; Coenye, T.; Chou, J.H.; Barrios, E.; de Faria, S.M.; Ellio, N.; Sheu, S.Y.; Sprent, J.I.; Vandamme, P. Burkholderia mimosarum sp. nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. Int. J. Syst. Evol. Microbiol. 2006, 56, 1847–1851. [Google Scholar] [CrossRef]
- Elliott, G.N.; Chou, J.H.; Chen, W.M.; Bloemberg, G.V.; Bontemps, C.; Martínez-Romero, E.; Velázquez, E.; Young, J.P.W.; Sprent, J.I.; James, E.K. Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ. Microbiol. 2009, 11, 762–778. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 419–426. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 2023, 12, 3066. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Isolation and identification of allelochemicals and their activities and functions. J. Pesti. Sci. 2024, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and its management. Plants 2023, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants 2024, 13, 356. [Google Scholar] [CrossRef]
- Javaid, N.; Shah, M.H.; Khan, I.H.; Javaid, A.; Waleed, S.M. Herbicidal activity of Ageratum conyzoides against Parthenium. Pak. J. Weed Sci. Res. 2020, 26, 137–146. [Google Scholar] [CrossRef]
- Negi, B.; Bargali, S.S.; Bargali, K.; Khatri, K. Allelopathic interference of Ageratum conyzoides L. against rice varieties. Curr. Agric. Res. J. 2020, 8, 69–76. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Assessment of the allelopathic potential of Ageratum conyzoides. Biol. Plant. 2001, 44, 309–311. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Hasanuddin, H.; Sampietro, D.A.; Amist, N. Herbicidal effects of n-hexane, ethyl acetate and methanol extracts of billygoat weed (Ageratum conyzoides L.) leaves on Amaranthus spinosus L. growth. Allelopath. J. 2021, 54, 211–220. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Hasanuddin, H.; Syafruddin, S. Herbicidal effects of ethyl acetate extracts of billygoat weed (Ageratum conyzoides L.) on spiny amaranth (Amaranthus spinosus L.) growth. Agronomy 2021, 11, 1991. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichi, T.; Hong, N.H.; Khanh, T.D.; Min, C.I. Assessment of phytotoxic action of Ageratum conyzoides L. (Billy goat weed) on weeds. Crop Prot. 2004, 23, 915–922. [Google Scholar] [CrossRef]
- Batish, D.R.; Harminder, P.S.; Shalinder, K.; Kohli, R.K. Nature of interference potential of leaf debris of Ageratum conyzoides L. Plant Grow Regul. 2008, 57, 137–144. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. Phytotoxic interference of Ageratum conyzoides with wheat (Triticum aestivum). J. Agron. Crop Sci. 2003, 189, 341–346. [Google Scholar] [CrossRef]
- Akter, P.; Begum, R. Allelopathic effects of Ageratum conyzoides root exudates on germinability of selected crops: A comparative analysis. Middle East Res. J. Biol. Sci. 2024, 4, 22–29. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Xu, T.; Lu, Y. Allelopathic potential and chemical constituents of volatile oil from Ageratum conyzoides. Allelopath. J. 1999, 25, 2347–2356. [Google Scholar]
- Zheng, H.; Wei, N.; Wang, L.; He, P. Effects of Lantana camara leaf extract on the activity of superoxide dismutase and accumulation of H2O2 in water hyacinth leaf. J. Plant Physiol. Mol. Biol. 2006, 32, 189. [Google Scholar]
- Li, C.; Yang, X.; Tian, Y.; Yu, M.; Shi, S.; Qiao, B.; Zhao, C.; Mao, L. The effects of fig tree (Ficus carica L.) leaf aqueous extract on seed germination and seedling growth of three medicinal plants. Agronomy 2021, 11, 2564. [Google Scholar] [CrossRef]
- Mao, X.T.; Xu, R.X.; Gao, Y.; Li, H.Y.; Liu, J.S.; Yang, W.D. Allelopathy of Alexandrium pacificum on Thalassiosira pseudonana in laboratory cultures. Ecotoxicol. Environ. Saf. 2021, 215, 112123. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Fei, F.; Wenju, L.; Weng, P.; Yong, J. Allelopathic potential of Ageratum conyzoides at various growth stages in different habitats. Allelopath. J. 2004, 13, 233–240. [Google Scholar]
- Wang, R.; Peng, S.L.; Zeng, R.S.; Ding, L.W.; Xu, Z.F. Cloning, expression and wounding induction of β-caryophyllene synthase gene from Mikania micrantha HBK and allelopathic potential of β-caryophyllene. Allelopathy J. 2009, 24, 35–44. [Google Scholar]
- Richter, A.; Seidl-Adams, I.; Köllner, T.G.; Schaff, C.; Tumlinson, J.H.; Degenhardt, J. A small, differentially regulated family of farnesyl diphosphate synthases in maize (Zea mays) provides farnesyl diphosphate for the biosynthesis of herbivore-induced sesquiterpenes. Planta 2015, 241, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, S.; Kumari, B.D.R.; Moola, A.K.; Sathish, D.; Kumar, G.P.; Srimurali, S.; Rajendran, R.B. Enhanced production of β-caryophyllene by farnesyl diphosphate precursor-treated callus and hairy root cultures of Artemisia vulgaris L. Front. Plant Sci. 2021, 12, 634178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zong, C.; Bai, A.; Yuan, S.; Li, Y.; Yu, Z.; Tian, R.; Liu, T.; Hou, X.; Li, Y. Transcriptome sequencing and gas chromatography-mass spectrometry analyses provide insights into β-caryophyllene biosynthesis in Brassica campestris. Food Chem. Mol. Sci. 2022, 5, 100129. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Masi, M.; Cimmino, A.; Vilariño, S.; Evidente, A. Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramosa: Quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants 2021, 10, 543. [Google Scholar] [CrossRef]
- Okunade, A.L. Ageratum conyzoides L. (asteraceae). Fitoterapia 2002, 73, 1–16. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principals and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74. [Google Scholar]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mode of action of allelochemical action of phenolic compounds. In Chemistry and Mode of Action of Allelochemicals; Macías, F.A., Galindo, J.C.G., Molino, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2004; pp. 217–238. [Google Scholar]
- Šoln, K.; Koce, J.D. Allelopathic root inhibition and its mechanisms. Allelopath. J. 2021, 52, 181–198. [Google Scholar] [CrossRef]
- Paul, S.; Datta, B.K.; Ratnaparkhe, M.B.; Dholakia, B.B. Turning waste into beneficial resource: Implication of Ageratum conyzoides L. in sustainable agriculture, environment and biopharma sectors. Mol. Biotechnol. 2022, 64, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Sundufu, A.J.; Shoushan, H. Chemical composition of the essential oils of Ageratum conyzoides L. occurring in South China. Flavour Fragr. J. 2004, 19, 6–8. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K. Ageratum conyzoides L.: A review on its phytochemical and pharmacological profile. Int. J. Green Pharm. 2008, 2, 59–68. [Google Scholar] [CrossRef]
- Singh, S.B.; Devi, W.R.; Marina, A.; Devi, W.I.; Swapana, N.; Singh, C.B. Ethnobotany, phytochemistry and pharmacology of Ageratum conyzoides Linn (Asteraceae). J. Med. Plants Res. 2013, 7, 371–385. [Google Scholar]
- Rioba, N.B.; Stevenson, P.C. Ageratum conyzoides L. for the management of pests and diseases by small holder farmers. Ind. Crop Prod. 2017, 110, 22–29. [Google Scholar] [CrossRef]
- Yadav, N.; Ganie, S.A.; Singh, B.; Chhillar, A.K.; Yadav, S.S. Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phytother. Res. 2019, 33, 2163–2178. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H.; Kato, M. Defense Molecules of the Invasive Plant Species Ageratum conyzoides. Molecules 2024, 29, 4673. https://doi.org/10.3390/molecules29194673
Kato-Noguchi H, Kato M. Defense Molecules of the Invasive Plant Species Ageratum conyzoides. Molecules. 2024; 29(19):4673. https://doi.org/10.3390/molecules29194673
Chicago/Turabian StyleKato-Noguchi, Hisashi, and Midori Kato. 2024. "Defense Molecules of the Invasive Plant Species Ageratum conyzoides" Molecules 29, no. 19: 4673. https://doi.org/10.3390/molecules29194673
APA StyleKato-Noguchi, H., & Kato, M. (2024). Defense Molecules of the Invasive Plant Species Ageratum conyzoides. Molecules, 29(19), 4673. https://doi.org/10.3390/molecules29194673






