Investigation of Trimethylenemethane Cyclopentyl-Annulations as a Strategy to Obtain a Functionalized Angular Triquinane Skeleton †
Abstract
1. Introduction
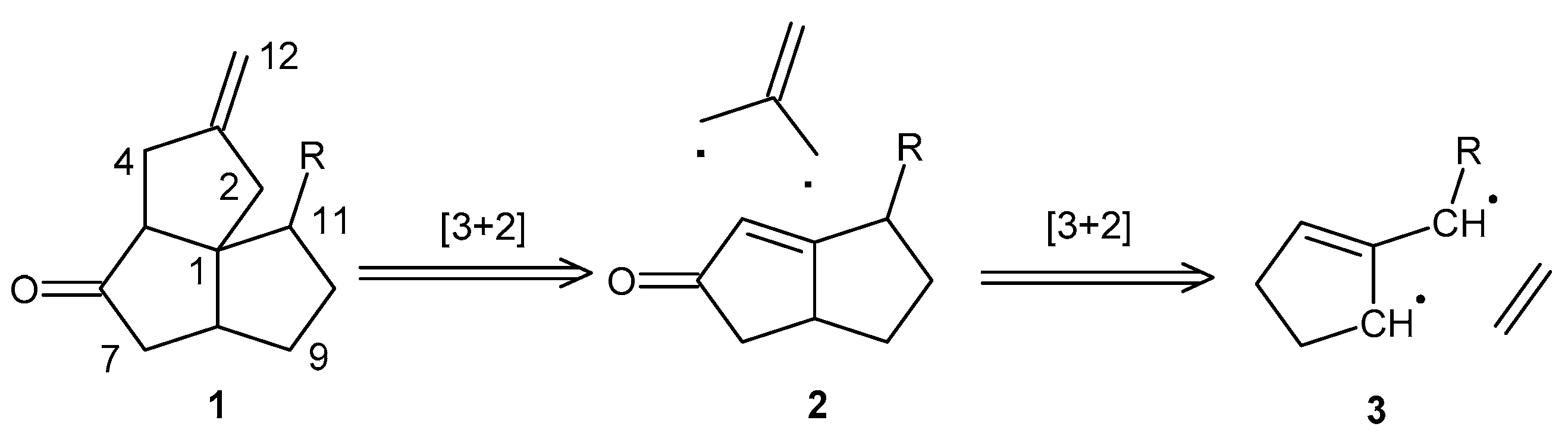
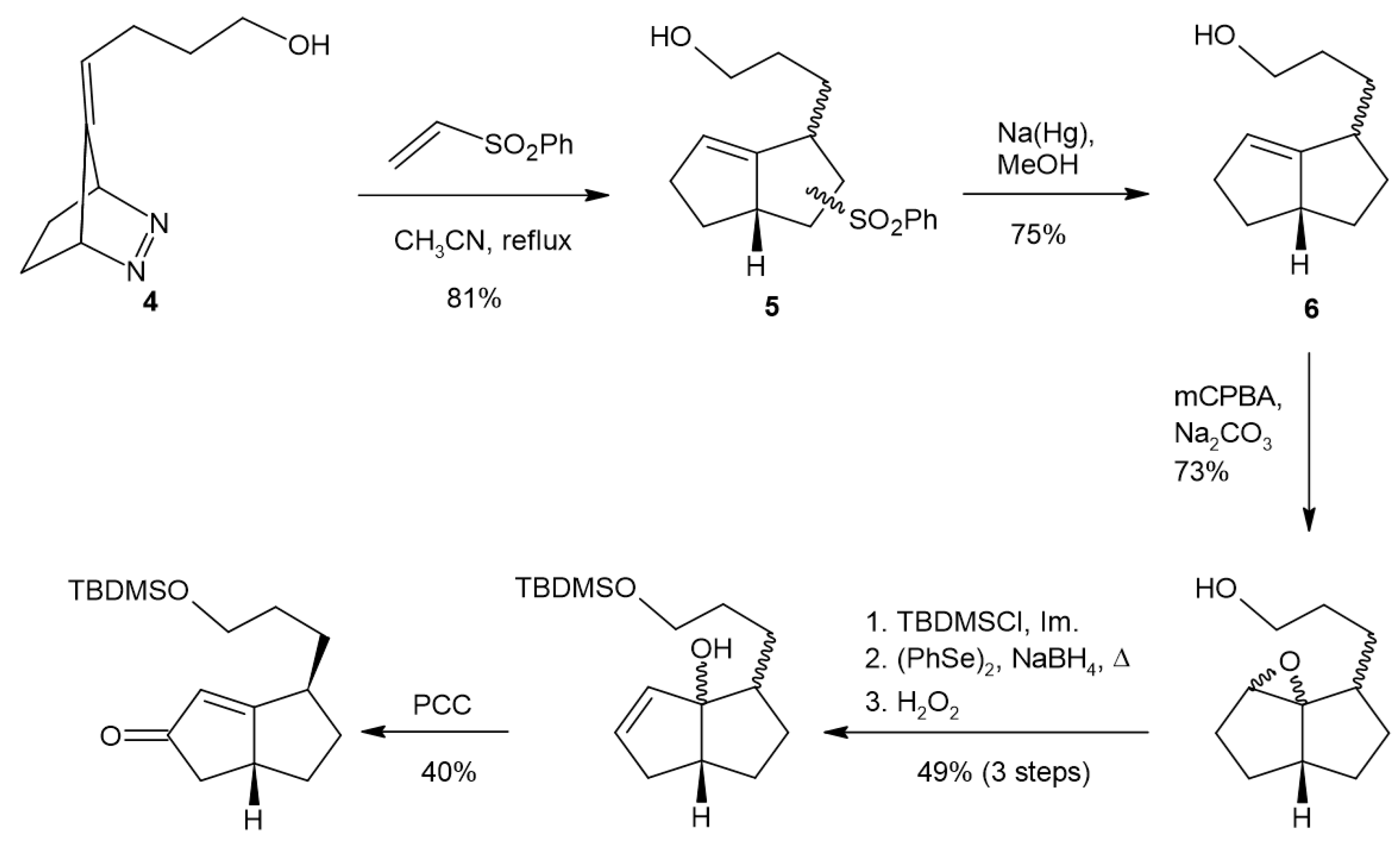
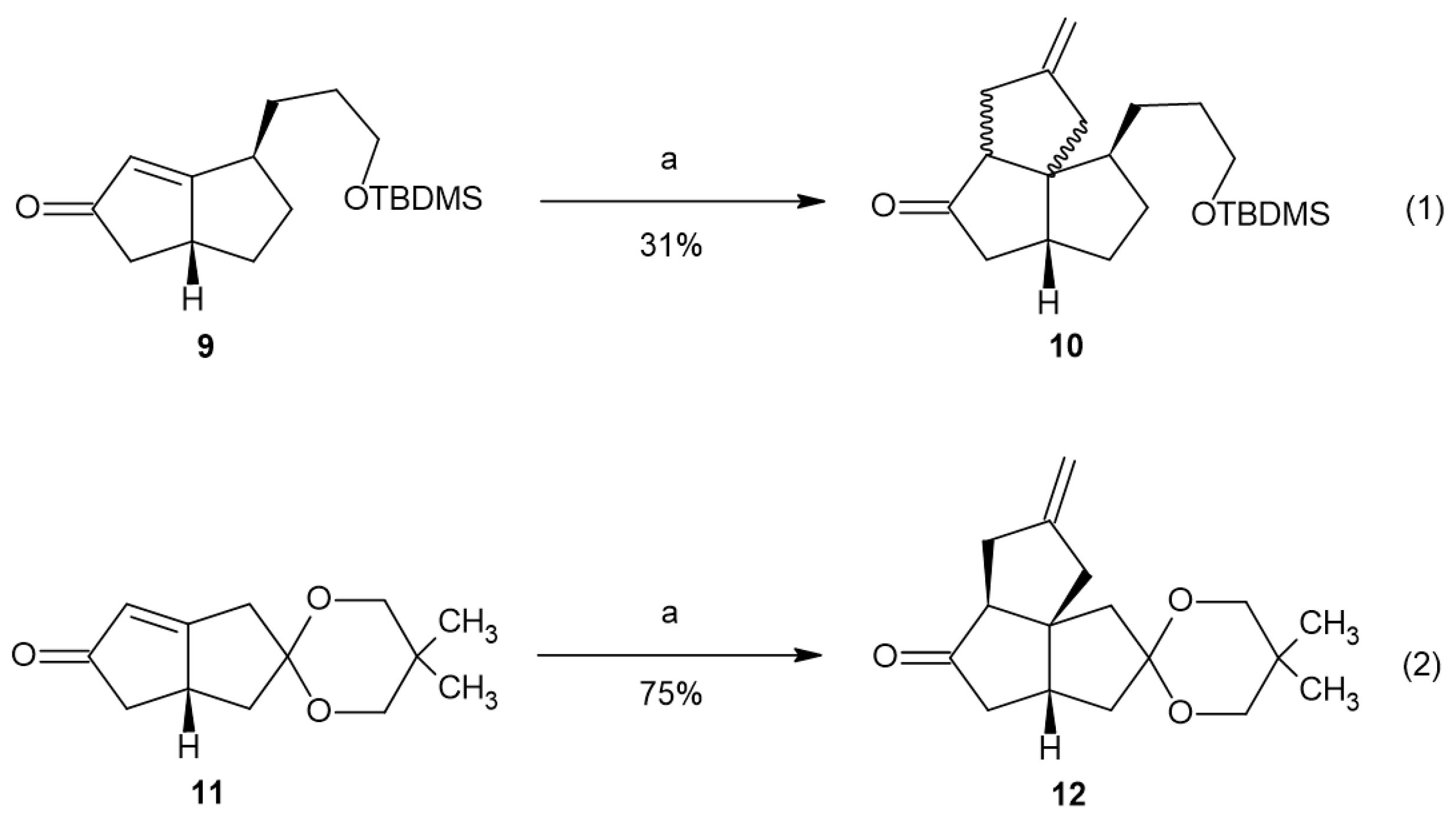
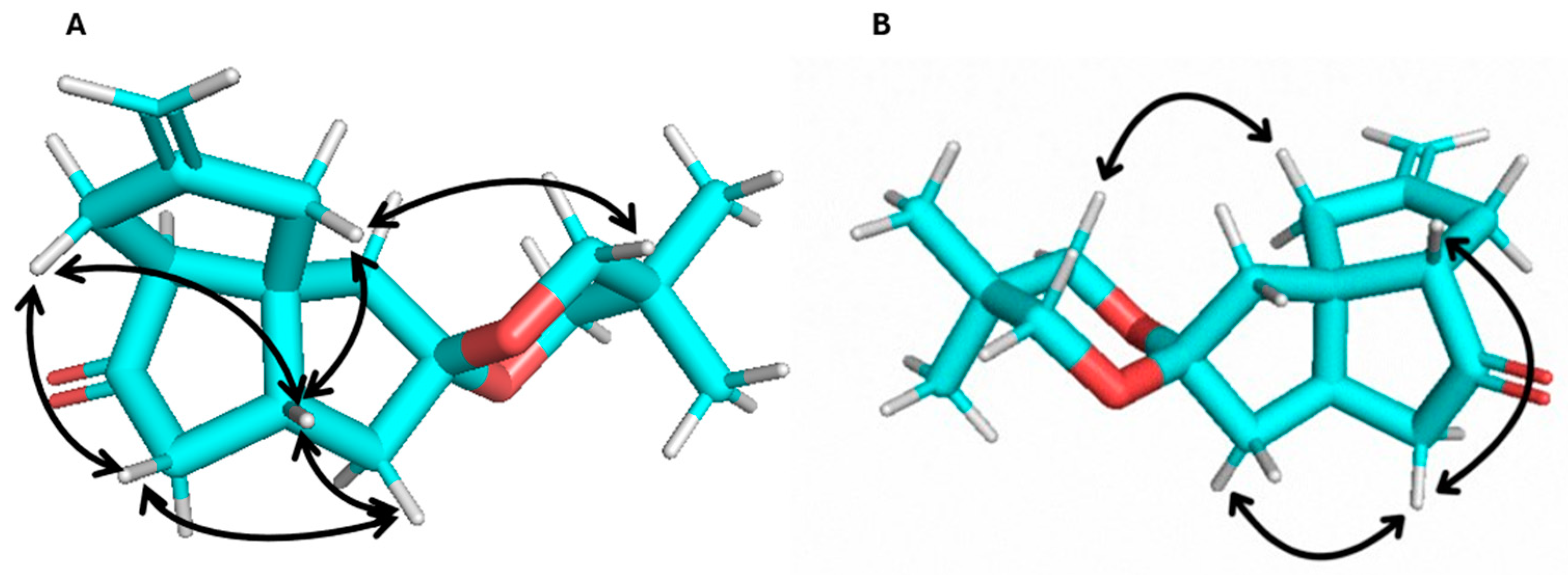
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Y.; Lan, H.-J.; Chen, L.-P. Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis. Molecules 2018, 23, 2095. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Srikrishna, A. Synthesis of Polyquinane Natural Products: An Update. Chem. Rev. 1997, 97, 671–719. [Google Scholar] [CrossRef] [PubMed]
- Hudlicky, T.; Price, J.D. Anionic Approaches to the Construction of Cyclopentanoids. Chem. Rev. 1989, 89, 1467–1486. [Google Scholar] [CrossRef]
- Jeon, H.; Winkler, J.D. Synthesis of Cyclohexane-Angularly-Fused Triquinanes. Synthesis 2021, 53, 475–488. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; Gutiérrez, C.; Cabrera, R.; Reina, M. Silphinene Derivatives: Their Effects and Modes of Action on Colorado Potato Beetle. J. Agric. Food Chem. 1997, 45, 946–950. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Chen, S.; Wu, W.; Sun, P. Isolation and Identification of Pentalenolactone Analogs from Steptomyces sp. NRRL S-4. Molecules 2021, 26, 7377. [Google Scholar] [CrossRef]
- Dowd, P. Trimethylenemethane. J. Am. Chem. Soc. 1966, 88, 2587–2589. [Google Scholar] [CrossRef]
- Corwin, L.R.; McDaniel, D.M.; Bushby, R.J.; Berson, J.A. Dimerization and cycloaddition reactions of a trimethylenemethane derivative, 2-isoprpylidenecyclopenta-1,3-diyl. Mechanistic separation of triplet and singlet reactions. J. Am. Chem. Soc. 1980, 102, 276–287. [Google Scholar] [CrossRef]
- Little, R.D. Diyl Trapping and Electroreductive Cyclization Reactions. Chem. Rev. 1996, 96, 93–114. [Google Scholar] [CrossRef]
- Hua, D.H. Asymmetric Total Synthesis of (+)-Pentalenene via Chiral Sulfinylallyl Anions. Hydrolytic Ring Closure of Enol Thioether Ketones. J. Am. Chem. Soc. 1986, 108, 3835–3837. [Google Scholar] [CrossRef]
- Piers, E.; Karunaratne, V. 4-Chloro-2-lithio-1-butene, a Novel Donor-Acceptor Conjunctive Reagent. J. Org. Chem. 1983, 48, 1774–1776. [Google Scholar] [CrossRef]
- Piers, E.; Karunaratne, V. Organotin-based reagents: 4-Chloro-2-lithio-1-butene and related sustances. Methylenecyclopentane annulations. Total synthesis of (±)-δ9(12)-capnellene. Tetrahedron 1989, 45, 1089–1104. [Google Scholar] [CrossRef]
- Dragojlovic, V. Synthesis of a Highly Functionalized Triquinane: Studies Towards a Total Synthesis of Subergorgic Acid and Its Analogues. Molecules 2000, 5, 674–698. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Olpp, T.; Baum, E.; Stiffle, T.; Knölker, H.-J. Organosilicon-Mediated Total Synthesis of the Triquinane Sesquiterpenes (±)-β-Isocomene and (±)-Isocomene. Org. Biomol. Chem. 2010, 8, 4562. [Google Scholar] [CrossRef] [PubMed]
- Danheiser, R.L.; Carini, D.J.; Basak, A. (Trimethylsilyl)cyclopentene Annulation: A Regiocontrolled Approach to the Synthesis of Five-Membered Rings. J. Am. Chem. Soc. 1981, 103, 1604–1606. [Google Scholar] [CrossRef]
- Xu, B.; Xun, W.; Su, S.; Zhai, H. Total Synthesis of (−)-Conidiogenone B, (−)-Conidiogenone, and (−)-Conidiogenol. Angew. Chem. 2020, 132, 16617–16621. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Jung, Y.; Yoon, Y.; Kim, B.G.; Kim, Y. Angularly Fused Triquinanes from Linear Substrates through Trimethylenemethane Diyl [2+3] Cycloaddition Reaction. Org. Lett. 2010, 12, 2672–2674. [Google Scholar] [CrossRef]
- Kang, T.; Kim, W.-Y.; Yoon, Y.; Kim, B.G.; Lee, H.-Y. Tandem Cycloaddition Reactions of Allenyl Diazo Compounds Forming Triquinanes via Trimethylenemethane Diyls. J. Am. Chem. Soc. 2011, 133, 18050–18053. [Google Scholar] [CrossRef]
- Kang, T.; Song, S.B.; Kim, W.-Y.; Kim, B.G.; Lee, H.-Y. Total Synthesi of (−)-Crinipellin A. J. Am. Chem. Soc. 2014, 136, 10274–10276. [Google Scholar] [CrossRef]
- Campopiano, O.; Little, R.D.; Petersen, J.L. Evidence for Hydrogen Atom Abstraction and Loss of Diylophile Stereochemistry in an Intramolecular 1,3-Diyl Trapping Reaction. J. Am. Chem. Soc. 1985, 107, 3721–3722. [Google Scholar] [CrossRef]
- Masjedizadeh, M.R.; Fite, C.; Little, R.D. Direct observation of intermediates involved in the intramolecular diyl trapping reaction. Tetrahedron Lett. 1990, 31, 1229. [Google Scholar] [CrossRef]
- Carr, R.V.C.; Paquette, L.A. An Ethylene and Terminal Olefin Equivalent in [4+2] π Cycloadditions. General Synthetic Application of Phenyl Vinyl Sulfone to the Construction of Functionalized Six-Membered Rings. J. Am. Chem. Soc. 1980, 102, 853–855. [Google Scholar] [CrossRef]
- Little, R.D.; Brown, L. Facile Construction of C10 Modified Prostaglandin Precursors. Diyl Trapping Reactions Using Phenyl Vinyl Sulfoxide and Phenyl Vinyl Sulfone. Tetrahedron Lett. 1980, 21, 2303–2304. [Google Scholar] [CrossRef]
- Little, R.D.; Myong, S.O. Oxidative desulfonylation. Phenyl vinyl sulfone as a ketene synthetic equivalent. Tetrahedron Lett. 1980, 21, 3339–3342. [Google Scholar] [CrossRef]
- Little, R.D.; Bukhari, A.; Venegas, M.G. A new route to linearly fused tricyclopentanoids. Diyl trapping reactions in organic synthesis. Tetrahedron Lett. 1979, 20, 305–308. [Google Scholar] [CrossRef]
- Venegas, M.G.; Little, R.D. Carbon-13 chemical shifts in tricyclo[6.3.0.03,7] undecanes (linearly fused tricyclopentanoids). Tetrahedron Lett. 1979, 20, 309–312. [Google Scholar] [CrossRef]
- Little, R.D.; Carroll, G.L. Elecrochemical Generation of the Azo Linkage. Synthesis of Bicyclic Azo Compounds, Precursors of 1,3-Diyls. J. Org. Chem. 1979, 44, 4720–4722. [Google Scholar] [CrossRef]
- Whitesell, J.K.; Matthews, R.S. Carbon-13 Chemical Shifts in Bicyclo[3.3.0]octanes. J. Org. Chem. 1977, 24, 3878–3882. [Google Scholar] [CrossRef]
- Van Hijfte, L.; Little, R.D.; Petersen, J.L.; Moeller, K.D. Intramolecular 1,3-Diyl Trapping Reactions. Total Synthesis of (±)-Hypnophilin and (±)-Coriolin. Formation of the Trans-Fused Bicyclo[3.3.0]octane Ring System. J. Org. Chem. 1987, 52, 4647–4661. [Google Scholar] [CrossRef]
- Kissel, C.L.; Rickborn, B. The Base-Induced Rearrangement of Epoxides. IV. Reaction of Cyclohexene Oxide wih Various Lithium Alkylamides. J. Org. Chem. 1972, 37, 2060–2063. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Lauer, R.F. A Mild Method for the Conversion of Epoxides to Allylic Alcohols. The First Organoselenium Reagent. J. Am. Chem. Soc. 1973, 95, 2697–2699. [Google Scholar] [CrossRef]
- Dauben, W.G.; Michno, D.M. Direct oxidation of tertiary allylic alcohols. A simple and effective method for alkylative carbonyl transposition. J. Org. Chem. 1977, 42, 682–685. [Google Scholar] [CrossRef]
- Trost, B.M.; Chan, D.M.T. Regiochemistry of the Cycloaddition of a Substituted Trimethylenemethanepalladium Complex. J. Am. Chem. Soc. 1981, 103, 5972–5974. [Google Scholar] [CrossRef]
- Trost, B.M.; Chan, D.M.T. Intramolecular Carbocyclic [3+2] Cycloaddition via Organopalladium Intermediates. J. Am. Chem. Soc. 1982, 104, 3733–3735. [Google Scholar] [CrossRef]
- Trost, B.M.; Chan, D.M.T. Palladium-Mediated Cycloaddition Approach to Cyclopentanoids. Introduction and Initial Studies. J. Am. Chem. Soc. 1983, 105, 2315–2325. [Google Scholar] [CrossRef]
- Trost, B.M.; Chan, D.M.T. Palladium-Mediated Cycloaddition Approach to Cyclopentanoids. Mechanistic Studies. J. Am. Chem. Soc. 1983, 105, 2326–2335. [Google Scholar] [CrossRef]
- Nanninga, T.N.; Trost, B.M. Palladium-Mediated Cycloaddition Approach to Loganin Aglucon. J. Am. Chem. Soc. 1985, 107, 1293–1299. [Google Scholar]
- Rowley, E.G.; Schore, N.E. The Pauson-Khand Reaction in Triquinane Synthesis: Approaches to Pentalenene, Pentalenic Acid, and Silphinene. J. Org. Chem. 1992, 57, 6853–6861. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webber, S.M.; Russu, W.A. Investigation of Trimethylenemethane Cyclopentyl-Annulations as a Strategy to Obtain a Functionalized Angular Triquinane Skeleton. Molecules 2024, 29, 5358. https://doi.org/10.3390/molecules29225358
Webber SM, Russu WA. Investigation of Trimethylenemethane Cyclopentyl-Annulations as a Strategy to Obtain a Functionalized Angular Triquinane Skeleton. Molecules. 2024; 29(22):5358. https://doi.org/10.3390/molecules29225358
Chicago/Turabian StyleWebber, S. Mason, and Wade A. Russu. 2024. "Investigation of Trimethylenemethane Cyclopentyl-Annulations as a Strategy to Obtain a Functionalized Angular Triquinane Skeleton" Molecules 29, no. 22: 5358. https://doi.org/10.3390/molecules29225358
APA StyleWebber, S. M., & Russu, W. A. (2024). Investigation of Trimethylenemethane Cyclopentyl-Annulations as a Strategy to Obtain a Functionalized Angular Triquinane Skeleton. Molecules, 29(22), 5358. https://doi.org/10.3390/molecules29225358





