Abstract
Carpesium macrocephalum, a species native to China, Korea, Japan, and Russia, has been used medicinally in the countries of its origin. Though mono- and sesquiterpenoids are known constituents of C. macrocephalum, the complete analysis of essential oils produced by the roots and aerial parts of the plant has not been published until now. The present study discloses considerable differences in the composition and cytotoxic activity of essential oils distilled from roots and shoots of C. macrocephalum. The GC-MS-FID analyses have led to the identification of 131 compounds in all, of which 114 were found in aerial parts and 110 in the roots of the plants. The essential oil distilled from shoots contained a mixture of nerol and thymol methyl ether (c. 26%), neryl isobutyrate (c. 12%) and linalool (c. 9%) as major constituents, whereas alantolactone (c. 29%), thymol methyl ether (c. 7%) and 2,5-dimethoxy-p-cymene (thymohydroquinone dimethyl ether, c. 7%) predominated in the essential oil obtained from the roots. The oils demonstrated weak antibacterial activity against Staphylococcus aureus and, at concentrations up to 2.08 mg/mL (oil from the aerial parts) and up to 3.38 mg/mL (oil from roots), were inactive against Gram-negative bacteria. The essential oil from the roots of the plant demonstrated strong but not selective cytotoxic activity.
1. Introduction
Carpesium macrocephalum Franch. & Sav. (synonym: Carpesium eximium C. Winkl., Inuleae-Inulinae, Asteraceae) is a perennial plant that inhabits deciduous or mixed forests in China, Japan, Korea, and Russia. The herb, ca. 1 m tall, has a flexuous crisp-pubescent stem and terminal capitula (25–35 mm in diameter), composed of yellow, tubular disc and marginal florets, which are surrounded by linear or lanceolate bracts [1]. Traditionally, in China and Korea, the herb has been used as an analgetic, antihemostatic, antipyretic, and vermifuge agent, as well as in order to suppress inflammatory conditions [2,3].
Phytochemical investigations of plants from the genus Carpesium led to the isolation of numerous biologically active compounds [2,4,5], mostly representatives of mono- and sesquiterpenoids. C. macrocephalum remains one of the less investigated medicinally used species of the genus. Konovalova and coworkers isolated sesquiterpene lactones: carabrone, telekin, ivalin, and carpesin from the aerial parts of C. eximium plants, collected in the south of the Maritime Territory (Russia) [6]. A new eudesmanolide from the aerial parts of C. macrocephalum and two eudesmanolide glucosides from the seeds of the plant were described by Yang et al. [7,8]. The dried whole plants of Korean origin yielded three guaianolides: 4β,10β-dihydroxy-1α(H),5α(H)-guai-11(13)-en-8α,12-olide, 4α,10α-dihydroxy-1β(H),5β(H)-guai-11(13)-en-8α,12-olide and 4β,10β-dihydroxy-5α(H)-guaia-1,11(13)-dien-8α,12-olide [9]. Detailed analysis of the methanol extract from seeds of C. macrocephalum, except for the two eudesmanolide glucosides described earlier, revealed the presence of eight eudesmanolides, carabrone, carabrol, one coumarin (scopoletin), 3,5,7,4′-tetrahydroxydihydroflavonol, β-sitosterol and daucosterol [10]. From the dried aerial parts of the plants collected in Gansu province (China), two new eudesmanolides together with telekin, 11α,13-dihydrotelekin, 2α,5α-dihydroxy-11α(H)-eudesma-4(15)-en-12,8β-olide, ivalin, 11α,13-dihydroivalin, carabrone, carabrol, scopoletin, β-sitosterol, and daucosterol were isolated [11]. The whole C. macrocephalum plants, harvested in Korea, provided five sesquiterpene lactones (carabron, carabrol, tomentosin, ivalin, 4H-tomentosin) and three other low-molecular-weight terpenoids (vomifoliol, loliolide, citrusin C) [12]. Five monoterpenoid thymol derivatives, including (Z)-10-isobutyryloxy-9-chloro-8,9-dihydrothymol, and three new sesquiterpene lactone dimers (carpedilactones E-G) were isolated from the whole plants of Chinese origin [13,14]. In addition to the previously identified compounds, a new xanthanolide (4-(2-methylbutyryl)-4H-tomentosin), α-costic acid, 5α-epoxyalantolactone, and 4α,5α-epoxy-10α,14H-1-epi-inuviscolide were found in a methanol extract from C. macrocephalum. Some of the described sesquiterpene lactones inhibited Candida albicans biofilm formation or the yeast-to-hyphae transition in the fungal cells [15].
A hydroalcoholic extract from C. macrocephalum demonstrated inhibitory activity towards histamine release from rat mast cells and against nitric oxide (NO) production by the activated RAW 264.7 murine macrophage cell line [16]. A methanol extract from the Korean plants inhibited lipopolysaccharide (LPS)-induced NO and PGE2 (prostaglandin E2) production in murine macrophages by suppression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) activity due to suppression of mRNA and protein expression [3]. Moreover, the extract attenuated pro-inflammatory cytokines production inhibited protein kinase B (Akt) and the nuclear transcription factor NF-κB activation, as well as expression of signal transducer and activator of transcription proteins (STATs) and diminished vascular permeability in mice. The suppression of the NO production and other anti-inflammatory effects of the extract may be explained, at least in part, by the presence of sesquiterpene lactones [17].
Essential oils (EOs) produced by plants are aromatic mixtures of volatile, low-molecular-weight compounds of a hydrophobic nature. Terpenoids and hydrocarbons are their principal components. Apart from their use in perfumery, pharmaceutical, cosmetic, and food industries (food aromatization), EOs play a significant role in the ecological relationships of plants within their environment (allelopathy, deterrence, attraction of pollinators). Antimicrobial activities of EOs, as well as their effects on the human nervous system, are well documented [18,19,20,21]. Their cytostatic and anti-inflammatory effects have been also described [22,23,24]. Numerous mono- and sesquiterpenoids isolated from Carpesium spp., including those found in C. macrocephalum, demonstrated antibacterial and cytotoxic activities against different microbial and cancer cell lines [2]. As the EOs are mixtures of low-molecular-weight, volatile plant metabolites (including mono- and sesquiterpenoids), it may be expected that the EOs distilled from C. macrocephalum may have antibacterial or cytotoxic effects too. Our previous studies on compositions and activity of the EOs from closely taxonomically related species of the Inuleae-Inulinae: Telekia speciosa (Schreb.) Baumg., Carpesium divaricatum Sieb. & Zucc., and Carpesium cernuum L. disclosed the presence of numerous monoterpenoid thymol derivatives in the examined oils (especially in the EOs from the plant roots) [25,26,27]. The EOs distilled from T. speciosa and C. cernuum demonstrated antibacterial activity against both Gram-positive and Gram-negative bacteria and cytotoxicity against several human melanoma cell lines in vitro. The aim of the present study was to reveal the chemical composition of the previously unanalyzed EOs from roots and aerial parts of C. macrocephalum and to assess their antibacterial and cytotoxic effects.
2. Results
2.1. Chemical Composition of C. macrocephalum Essential Oils
The aerial parts of C. macrocephalum contained 0.08% (w/w) of a yellowish, fragrant essential oil (APEO). The 114 compounds that were identified as components of the APEO (all listed in Table 1) accounted for 96% of the oil. The primary constituents of the APEO were a mixture (9:1) of nerol and thymol methyl ether (32 and 33; c. 26%), neryl isobutyrate (76; c. 12%) and linalool (18; c. 9%). Thymohydroqinone dimethyl ether (2,5-dimethoxy-p-cymene; 58) together with other thymol derivatives like thymol methyl ether (33), 6-methoxythymyl isobutyrate (109), 40, 70, 112, 113, 122, 123, and 134, and sesquiterpene lactones of eudesmane type (alantolactone, 121; isolantolactone, 125 and dihydroisoalantolactone, 120) constituted c. 12.9% and c. 1% of the APEO, respectively. Five components of the APEO (94, 97, 98, 108, and 118) remained unidentified.

Table 1.
Chemical composition of Carpesium macrocephalum essential oils distilled from aerial parts (APEO) and roots (REO) of the plants cultivated in the open field.
The distillation yield for the EO from roots of C. macrocephalum (REO) was 0.13% (w/w). Alantolactone (121) was the most abundant constituent of the REO (c. 29.3%). The remaining eudesmanolides (isoalantolactone, 125; alloalantolactone, 126; isoalantodiene, 128) and thymol derivatives (33, 40, 58, 70, 71, 109, 112, 113, 122, 123, 131, 132, and 134) accounted for c. 5.2% and c. 20.7% of REO, respectively. The 110 identified components of REO made up c. 96.7% of the oil. Structures of four constituents (94, 98, 108, and 129) were not resolved.
2.2. Antibacterial and Cytotoxic Activities of C. macrocephalum Essential Oils
At the concentration range tested (1.02–2080 μg/mL, APEO; 1.65–3380 μg/mL, REO), both APEO and REO were inactive against Gram-negative bacteria (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853) and modestly inhibited growth of methicillin-resistant (ATCC 43300) and methicillin-susceptible (ATCC 29213) strains of Staphylococcus aureus (APEO, MIC = 2.08 mg/mL; REO, MIC = 3.38 mg/mL). The results of the antibacterial activity assessment and reference values, estimated for thymol and gentamycin sulfate, are summarized in Table 2.

Table 2.
Antibacterial activities of essential oils from aerial parts (APEO) and roots (REO) of Carpesium macrocephalum.
As shown in Figure 1, REO demonstrated much higher cytotoxic activity towards the investigated human cell lines in vitro than APEO. Viabilities of melanoma cells (A375, C32, and SK-MEL-28), dermal keratinocytes (HaCaT), and fibroblasts were assessed after the treatment with APEO and REO for 24 h and 48 h. APEO (Figure 1A) was cytotoxic in all tested cell lines at a concentration of 50 nL/mL and above. Its IC50 values, after 24 h of the treatment, ranged from 48.4 nL/mL in A375 cells to 80.4 nL/mL in fibroblasts. Extension of the exposure time to 48 h resulted in a further decrease in the cell viability (from 10% in SK-MEL-28 cells to 27% in HaCaT keratinocytes). REO (Figure 1B) was cytotoxic in all tested cell lines at a concentration of 6.2 nL/mL and above. Its IC50 values, after 24 h of the treatment, were from 5.3 times (C32 cells) to 12.7 times (A375 cells) lower when compared with those for APEO and ranged from 3.8 nL/mL in A375 cells to 10.5 nL/mL in C32 cells. Similarly to the effects observed in the cells exposed to APEO, prolonged time of incubation (48 h) with REO resulted in a moderate reduction in cell viability (from 12% in SK-MEL-28 cells and HaCaT keratinocytes to 29% in C32 cells).
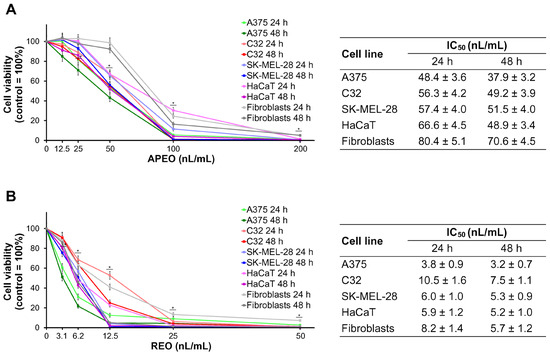
Figure 1.
Cell viability after the treatment of human melanoma and normal skin cell lines with the essential oils from Carpesium macrocephalum for 24 h and 48 h: APEO (A) and REO (B). Data are reported as mean ± SD from three independent experiments. * p < 0.05 compared to control group.
Analysis of apoptosis in melanoma cells (Figure 2A) treated with APEO at a concentration of 100 nL/mL showed a significant increase in the percentage of apoptotic cells: 59.3% in the C32 cell line, 91.6% in SK-MEL-28 cells and 98.7% in A375 cells. The REO exerted a much stronger effect and, at a concentration of 12 nL/mL, caused an increase in the percentages of apoptotic cells in line C32 (68.1%), in A375 cells (95.7%), and in SK-MEL-28 cells (97.7%). The levels of the key mediators of apoptosis in melanoma cells treated with APEO and REO for 48 h were evaluated by western blotting. As shown in Figure 2B, treatment of the cells with both EOs resulted in the downregulation of initiator caspase zymogen levels and their cleavage to active forms. Expression of (pro)caspase-8 in the cell line C32 was undetectable. The level of antiapoptotic Bcl-2 family member Mcl-1 gradually decreased with the increasing concentrations of APEO and REO. These changes were accompanied by the reduction in the levels of zymogens of executioner caspase-3 and caspase-7 and with the processing of poly(ADP-ribose) polymerase (PARP) into its cleavage product (cPARP).
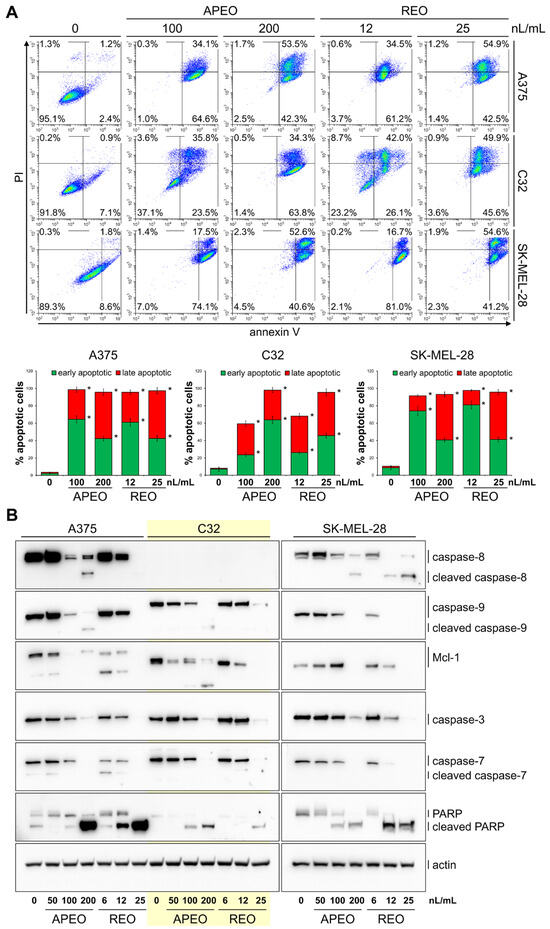
Figure 2.
Apoptosis of melanoma cells after treatment with APEO and REO for 48 h (A). Western blot analysis of caspase-8, caspase-9, Mcl-1, caspase-3, caspase-7, and PARP in melanoma cells after the treatment with APEO and REO for 48 h. Actin served as a control for protein loading (B). Data are presented as mean ± SD from three independent experiments. * p < 0.05 compared to control group.
3. Discussion
The genus Carpesium, according to the current taxonomic viewpoint based on the results of DNA sequencing, is closely related to Telekia speciosa (Schreb.) Baumg. and the genus Inula in its current form (including I. helenium L.), the group of large herbs with radiate capitula and resin canals in the stem [28,29]. The composition and antimicrobial activity of EOs from roots of I. helenium and different organs of T. speciosa have been repeatedly studied [30,31,32,33]. Even recently, a paper on the cytotoxic activity of the EO obtained from flowers of T. speciosa and the antibacterial activities of the EOs accumulated by leaves, roots, and flowers of the plant has been published [27]. Much less is known about the composition and biological activity of EOs from Carpesium spp. Most data concern C. abrotanoides, the species that has been used as a TCM (traditional Chinese medicine) for a long time in China. The data on the composition of essential oils from C. abrotanoides L., however, are not congruent. Kameoka et al. [34], from a “commercial herb sample” of C. abrotanoides, distilled the EO that contained β-bisabolene as the dominant constituent (c. 24.7%). The EOs obtained from the whole plants of C. abrotanoides by Wang et al. [35] contained eudesma-5,11(13)-dien-8,12-olide (alantolactone or its stereoisomer; c. 21.9%) and caryophyllene oxide (c. 13.0%), previously undetected by Kameoka et al., as major components. β-Bisabolene made up about 7% of the EO. Haris et al. [36] found caryophyllene (c. 24.3%), trans-nerolidol (c. 12.0%), geranyl isobutyrate (c. 10.6%), and δ-cadinene (c. 8.8%) in the EO from fresh aerial parts of C. abrotanoides. The EO investigated by Wang et al. [35] demonstrated moderate activity against human hepatocellular carcinoma (Hep G2) cells in vitro (IC50 = 22.59 ± 6.51 μg/mL after 48 h treatment) by inducing apoptosis via the mitochondrial pathway. The EO obtained from fresh aerial parts of C. abrotanoides showed some effectiveness against Aedes aegypti, a vector of the dengue virus [36].
α-Pinene, the major constituent in the EOs from aerial parts of C. divaricatum and C. cernuum (c. 40.2% and 34.7%, respectively) [25,26], was not detected in the EOs distilled from C. macrocephalum. Nerol and neryl isobutyrate that dominated in the C. macrocephalum APEO occurred as major constituents in the EO from aerial parts of C. divaricatum (c. 3.7% and 3.2%) and were present in smaller quantities in the EOs from C. cernuum. Thymohydroquinone dimethyl ether was found in the APEO from C. macrocephalum (c. 7.9%) and in the EOs from aerial parts of C. cernuum and C. divaricatum (c. 11.6% and 2.1%, respectively). Linalool, which constituted c. 9.3% of C. macrocephalum APEO, made up c. 4.3% and 2.1% of the essential oils from aerial parts of C. cernuum and C. divaricatum, respectively. Alantolactone/eudesma-5,11(13)-dien-8,12-olide was the only sesquiterpene lactone of eudesmane type that was identified in EOs from aerial parts of C. cernuum and C. divaricatum. Unlike in the EO from whole plants of C. abrotanoides [35], alantolactone was a minor constituent of C. cernuum and C. divaricatum EOs (c. 0.1%). C. macrocephalum APEO contained at least three eudesmanolides (alantolactone, isoalantolactone, and dihydroisoalantolactone), albeit in small quantities (c. 0.1–0.7%).
In contrast to the roots of C. cernuum and C. divaricatum, the roots of C. macrocephalum contained an EO that was rich in alantolactone (c. 29.3%). The other eudesmanolides identified in the REO were alloalantolactone (c. 4.4%), isoalantolactone (c. 0.7%), and isoalantodiene (c. 0.1%). High contents of eudesmanolides are characteristic of the EOs from roots of T. speciosa (c. 46.2–83.4% of isoalantolactone and c. 0.2–2.6% of alantolactone) [27,32,33] and I. helenium (alantolactone, c. 42.3–65.8% and isoalantolactone, c. 25.5–37.3%) [30]. Thymohydroquinone dimethyl ether that constituted c. 54.8% of the EO from C. cernuum roots made up c. 2.7% of the EO from roots of C. divaricatum and c. 6.6% of C. macrocephalum REO. The most abundant (c. 29.2%) constituent of the EO from roots of C. divaricatum, 10-isobutyryloxy-8,9-epoxythymyl isobutyrate, was identified in the EOs from roots of T. speciosa (c. 1.4–2.9%), C. cernuum (c. 5.1%) and C. macrocephalum REO (c. 2.2%) [25,26,27,32,33]. The remaining major constituents of REO were as follows: thymol methyl ether (c. 7.1%), isoshyobunone (c. 5.6%), modheph-2-ene (c. 4.6%) and caryophyllene epoxide (c. 4.0%). Thymol methyl ether was earlier identified as a substantial component of C. cernuum root EOs (c. 8.4%).
Bourrel et al. [37] studied the antimicrobial activity of EOs from roots of I. helenium originating from Central Europe. MIC values determined against E. coli (from 2000 μg/mL to >4000 μg/mL), S. aureus (62.5–2000 μg/mL), P. aeruginosa (≥4000 μg/mL), and Candida albicans (62.5–2000 μg/mL) varied depending on the assay method used. Boatto et al. [38] confirmed the activity of the I. helenium EOs towards S. aureus and Streptococcus pyogenes and the low effectiveness of the EO towards Gram-negative bacteria and Streptococcus faecalis. The EO from I. helenium examined by Deriu and coworkers [39] was active against Enterococcus faecalis ATCC 24912 (MIC = 2.9 mg/mL), S. aureus ATCC 29213 (MIC = 0.6 mg/mL), E. coli ATCC 25922 (MIC = 14.8 mg/mL), P. aeruginosa (MIC = 14.8 mg/mL) and C. albicans (MIC: 0.009–0.07 mg/mL). Antistaphylococcal activity of the I. helenium EO was further investigated by Stojanović-Radić et al. [40] using S. aureus ATCC 6538 strain (MIC: 0.01 μL/mL = 13.00 μg/mL). It was shown that the EO increased the bacterial membrane permeability, leading to cell autolysis. The effects on the cell-membrane function may also be engaged in the anticandidal activity of I. helenium root EO (MIC: 0.009–0.312 μg/mL) [41]. EOs from roots and aerial parts of T. speciosa collected in Bosnia and Herzegovina demonstrated antimicrobial activity against S. aureus (MIC: 1.1–15.0 mg/mL), P. aeruginosa (MIC: 4.0–11.0 mg/mL), E. coli ATCC 35210 (MIC: 1.0–7.0 mg/mL) and C. albicans (MIC: 1.0–15.0 mg/mL). The EOs from roots were more active than those distilled from the aerial parts of the plant [33]. The EOs obtained from different organs of T. speciosa plants of French origin, cultivated in the Garden of Medicinal Plants, Maj Institute of Pharmacology PAS were active against S. aureus ATCC 29213 (MIC: 7.8–31.3 μL/mL) and E.coli ATCC 25922 (MIC: 7.8–62.5 μL/mL). The EOs from T. speciosa flowers and leaves were more effective against the tested pathogens than the EO from the plant roots [27]. EOs from roots and aerial parts of C. cernuum were tested against Gram-positive (S. aureus ATCC 29213, E. faecalis ATCC 29212) and Gram-negative (E. coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, P. aeruginosa ATCC 27853, Serratia marcescens ATCC 13880 and Acinetobacter baumanii ATCC 19606) bacteria. The most susceptible of the tested microorganisms was A. baumanii (MIC = 11.7 μL/mL) the most resistant turned out to be S. marcescens (MIC ≥ 250 μL/mL). Up to the concentrations of 2.08 mg/mL (APEO) and 3.38 mg/mL (REO), the EOs from C. macrocephalum did not inhibit the growth of E. coli ATCC 25922 and P. aeruginosa ATCC 27853 strains. It was expected, taking into consideration the MIC values determined for the EOs from I. helenium and T. speciosa. Both MRSA-susceptible (ATCC 29213) and MRSA-resistant (ATCC 43300) strains of S. aureus were inhibited by the maximum concentrations of EOs used in the assay. The proper assessment of the antimicrobial activity of C. macrocephalum EOs should be conducted using higher concentrations of APEO and REO, which could not be performed in the current study due to the limited availability of the EOs.
Cytotoxic activities of the APEO and REO were assessed using a panel of human skin cell lines, including fibroblasts, keratinocytes (HaCaT), and melanoma cells (A375, C32, and SK-MEL-28). The APEO exerted a moderate cytotoxic effect on the melanoma cell lines (IC50: 37.9–51.5 nL/mL, after 48 h treatment), but its toxicity against keratinocytes was at the same level (IC50 = 48.9 nL/mL). The cytotoxic effect of APEO was less pronounced in human fibroblasts (IC50 = 70.6 nL/mL), suggesting some selectivity of action. The activity of REO was stronger but not selective. The IC50 values determined for both normal (HaCaT, fibroblasts) and melanoma cell lines, after 48 h treatment with REO, ranged from 3.2 nL/mL (A375 cells) to 7.5 nL/mL (C32 cells). Alantolactone, the dominant component of REO in micromolar concentrations, demonstrated cytotoxic activity against a variety of cancer lines in vitro, including mouse and human melanoma cells [42,43,44]. The sesquiterpene lactone significantly inhibited the proliferative, migratory, and invasive capacity of A375 melanoma cells by inhibiting the Wnt/β-catenin signaling pathway [43]. Moreover, in A375 cells as well as in the triple-negative breast cancer cells MDA-MB-231, alantolactone exerts its antiproliferative and apoptosis-inducing effect by the inhibition of signal transducer and activator of transcription 3 (STAT3) signaling pathway. In contrast to REO, the cytotoxic activity of alantolactone seems to be selective [44]. The compound undoubtedly contributed to the cytotoxic activity of REO, but its effect was modified by the other ingredients of the oil. The EOs distilled from roots and aerial parts of C. cernuum, tested against the same cell lines, were less active (IC50: 71.7–107.2 nL/mL). Moreover, their cytotoxic effect was not selective, and the fibroblast cell line was relatively strongly affected (IC50: 75.7–83.0 nL/mL) [26]. The EO from flowers of T. speciosa displayed cytotoxic activity comparable to that of REO (IC50: 5.1–17.1 μg/mL, 48 h treatment; cells: A375, C32, HaCaT, and fibroblasts). The highest IC50 value was determined for fibroblasts, suggesting some selectivity towards melanoma cells. Cisplatin applied as a reference drug, demonstrated high cytotoxicity against melanoma cells (A375, C32) and keratinocytes (IC50: 2.8–3.7 μg/mL) and was significantly less toxic against fibroblasts (IC50 > 25 μg/mL) [27].
The results of the apoptosis assay revealed a dose-dependent increase in the percentage of apoptotic cells in the melanoma cell lines treated either with APEO (50, 100, and 200 nL/mL) or with REO (6, 12, and 25 nL/mL), especially marked in A375 and SK-MEL-28 cells. The apoptosis-inducing activity of APEO and REO towards melanoma cells was further supported by the results of Western blotting. In the treated cells, initiator caspase-8 and caspase-9 and executioner caspase-3 and caspase-7 zymogen levels were dose-dependently reduced, except for the C32 cells, where the procaspase-8 was undetectable. The reduction of zymogen levels was accompanied by their cleavage to active forms. Concomitantly, the level of antiapoptotic Mcl-1 protein decreased in the cells treated with the EOs. Previously investigated EO from whole plants of C. abrotanoides [35] and EO from T. speciosa flowers [27] reduced the viability of human cancer cells in vitro, at least in part, via induction of the apoptotic process.
4. Materials and Methods
4.1. General Experimental Procedures
GC-MS-FID analyses of essential oils were performed using a Trace GC Ultra Gas Chromatograph coupled with a DSQII mass spectrometer (Thermo Electron, Waltham, MA, USA). Simultaneous GC-FID and GC-MS analyses were performed with an MS-FID splitter (SGE Analytical Science, Ringwood, VIC, Australia). The mass range was set at 33–550 amu, ion source-heating: 200 °C; ionization energy: 70 eV. One microliter of essential oil solution (80% v/v), diluted in pentane:diethyl ether, was injected in a split mode at the split ratios (50:1). Chromatograph operating conditions were as follows: capillary column Rtx-1 MS (60 m × 0.25 mm i.d., film thickness 0.25 μm), and temperature program: 50 °C (3 min)—300 °C (30 min) at 4 °C/min. Injector and detector temperatures were 280 °C and 300 °C, respectively. Helium was used as a carrier gas (constant pressure: 300 kPa). The relative composition of each essential oil sample was calculated from GC peak areas according to total peak normalization.
4.2. Plant Material
Seeds of Carpesium macrocephalum Franch. & Sav. were supplied by the Perm State University Botanic Garden in Perm (Russia). The seeds were collected in 2016 from plants growing outdoors in the Botanic Garden (58°00′ N; 56°19′ W) and were sown at the end of March 2020 into multipots with garden soil. In the stage of several mature leaves, the plants were transferred to the pots with a substrate composed of garden soil, peat, and sand. Plants were grown in a glasshouse of the Garden of Medicinal Plants, Maj Institute of Pharmacology PAS in Krakow, under controlled conditions (temperatures by day 18–38 °C; by night 12–18 °C), without any chemical treatment. In the third week of May, the plants were transferred into the open field. Aerial parts and roots of the plants were collected at the second year of growth, at the beginning of the flowering period (July), and dried under shade at room temperature. The voucher specimen (6/22) was deposited in the collection kept at the Garden of Medicinal Plants, Maj Institute of Pharmacology PAS, Kraków, Poland. The dry plant material was stored no longer than six months before analysis.
4.3. Isolation of Essential Oil
EOs from the dried aerial parts (leaves, stalks, flowers; 2.7 kg) or roots (0.7 kg) of C. macrocephalum were obtained by hydrodistillation in a Clevenger-type apparatus. Each process lasted 5 h, and a portion of 100–550 g of the dry plant material was used once. The EO from aerial parts (APEO, translucent colorless liquid) and roots (REO, yellowish liquid) were dried over anhydrous magnesium sulfate and stored at 4 °C in the dark until tested and analyzed.
4.4. Identification of Essential Oil Constituents
Volatiles from the essential oils were identified based on their MS spectra and their comparison with those from mass spectra libraries: NIST 2012, Wiley Registry of Mass Spectral Data 8th edition and MassFinder 4.1, along with the relative retention indices (RI) on DB-1 column (available from MassFinder 4.1) and on HP-5ms column (available from NIST 2012 or [45]). The identification of isoalantodiene was supported by literature data [46,47,48].
4.5. Antibacterial Activity of Carpesium macrocephalum Essential Oils
4.5.1. Bacterial Strains and Culture Conditions
Four reference strains were used in the current study: Staphylococcus aureus ATCC 29213 (methicillin-susceptible S. aureus—MSSA), S. aureus ATCC 43300 (methicillin-resistant S. aureus—MRSA), Escherichia coli ATCC 8739, and Pseudomonas aeruginosa ATCC 27853. The bacteria were cultured on Columbia agar with 5% sheep blood (bioMeriéux, Warsaw, Poland) and incubated for 18 h at 37 °C in an aerobic atmosphere prior to each experiment.
4.5.2. Determination of the Minimum Inhibitory Concentrations (MICs) and the Effectiveness of the Tested C. macrocephalum EOs against the Reference Bacterial Strains
The MICs of the essential oils (APEO and REO) against the selected Gram-positive (S. aureus) and Gram-negative (E. coli, P. aeruginosa) bacteria were determined by the serial microdilution method in Mueller-Hinton broth (MHB; Sigma-Aldrich, Darmstadt, Germany) according to the Clinical and Laboratory Standards Institute recommendations (CLSI, 2012) [49]. Thymol (Sigma-Aldrich, Darmstadt, Germany) and gentamycin sulfate (solution for injections; Krka, Novo Mesto, Slovenia) were used as positive controls. Thymol, a monoterpenoid being found in numerous essential oils and having well-documented antimicrobial activity [50] was chosen as a reference in the previously described experiments [26,27]. The antibacterial activity of this compound against Staphylococcus aureus ATCC 43300 was better than those of β-lactam antibiotics tested in the same experiment [51]. Briefly, 50 µL of the appropriate concentration of EOs or the compound of known antibacterial activity (thymol, gentamicin sulfate) was added to a 96-well microplate. Concentrations of APEO in a range from 1.02 µg/mL to 2080 µg/mL and REO in a range from 1.65 µg/mL to 3380 µg/mL were prepared by dissolving the substances in Tween 80 (1.0%, v/v) (Sigma-Aldrich, Darmstadt, Germany) and diluting by MHB. The solutions of thymol (from 625 µg/mL to 1.22 µg/mL) were prepared by dissolving the compound in dimethyl sulfoxide (2.0%, v/v) (DMSO, Loba Chemie, Mumbai, India) and diluting it using MHB. In turn, concentrations of gentamycin sulfate, in a range from 0.31 µg/mL to 625 µg/mL, were prepared by diluting the antibiotic solution using MHB. In the next step, 50 µL of a bacterial suspension at 106 CFU/mL was transferred to each well of the microplate. After an 18 h incubation at 37 °C, MICs for individual substances were determined by adding 20 µL resazurin solution (0.02%, w/v; Sigma-Aldrich, Darmstadt, Germany) to the microwells. The color change from dark blue to pink after a 3 h incubation at 37 °C indicated the presence of live bacteria. The first well in which the blue color persisted determined the MIC value. The bacteria suspension with 1.0% (v/v) Tween 80 or 2.0% (w/v) DMSO was regarded as a negative control. All tests were run in duplicate.
4.6. Cytotoxic Activity of Essential Oils from Carpesium macrocephalum
4.6.1. Cell Lines and Culture Conditions
Human melanoma cell lines A375, C32, and SK-MEL-28 and dermal fibroblasts CCD25Sk were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Skin keratinocytes HaCaT were obtained from AddexBio (San Diego, CA, USA). Melanoma cells A375 and C32, fibroblasts, and keratinocytes were cultured in Dulbecco’s Modified Eagle’s Medium, while SK-MEL-28 cells were maintained in Eagle’s Minimum Essential Medium. Both growth media were supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The media and supplements were purchased from Thermo Fischer Scientific (Waltham, MA, USA). Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
4.6.2. Cell-Viability Assay
The examined cells were seeded in 96-well plates at a density of 1 × 104 cells per well and allowed to adhere for 24 h. APEO and REO were solubilized in dimethyl sulfoxide (DMSO) at a ratio of 1:10 (v/v) and mixed with the culture medium to obtain final concentrations of 12.5, 25, 50, 100, and 200 nL/mL (APEO) or 3.1, 6.2, 12.5, 25, and 50 nL/mL (REO). The cells were then treated either with the solutions of EOs or with the culture medium containing DMSO (0.2%, control for the APEO-treated group; 0.05%, control for the REO-treated group). The viability of the cells was evaluated after 24 h and 48 h of the treatment by adding 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution to each well and subsequent incubation of the cells at 37 °C for 2 h. Then, the cells were lysed in DMSO supplemented with 1% (v/v) Sorensen’s glycine buffer. The absorbance was measured at 570 nm. The half-maximal inhibitory concentration values (IC50) were calculated using GraphPad Prism 7 software.
4.6.3. Apoptosis Assay
Cells were seeded at a density of 1 × 105 cells per well in 6-well plates and allowed to adhere for 24 h. Then, cells were treated with the respective concentrations of APEO and REO for 48 h. Control cells were treated with 0.2% DMSO. Floating and adherent cells were collected and assayed using a Dead Cell Apoptosis Kit with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) for flow cytometry (#V13242, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. Briefly, cells were resuspended in 100 μL annexin-binding buffer containing 5 μL annexin V-FITC conjugate solution and 1 μg/mL PI followed by 15 min incubation at room temperature. Subsequently, 400 μL of the annexin-binding buffer was added, and the cells were quantified with a DxFLEX flow cytometer (Beckman Coulter, Brea, CA, USA).
4.6.4. Western Immunoblot
Cells were treated with the respective concentration of APEO, REO, or with 0.2% DMSO (control) for 48 h, then adherent and floating cells were harvested and sonicated. The Lowry assay was performed to quantify protein content in the obtained homogenates. Proteins (20–40 μg) were resolved on 7.5%, 10%, or 12% SDS-PAGE gels using the Mini-Protean Tetra system (Bio-Rad, Hercules, CA, USA). Proteins were transferred to nitrocellulose membranes (Bio-Rad) using the Mini Trans-Blot Cell wet blotting system (Bio-Rad). Membranes were blocked with 5% skim milk for 1 h at room temperature and probed overnight at 4 °C with primary antibodies. The following antibodies purchased from Cell Signaling Technology (Danvers, MA, USA) were used: caspase-8 (#9746, 1:1000), caspase-9 (#9508, 1:1000), Mcl-1 (#5453, 1:1000), caspase-3 (#9662, 1:1000), caspase-7 (#12827, 1:1000), PARP (#9542, 1:1000). Antibody against actin (#A2066, 1:2000) was obtained from Sigma-Aldrich (Saint Louis, MO, USA). After extensive washes, a secondary antibody solution in 5% skim milk (anti-mouse IgG-HRP, Sigma-Aldrich, #A9044, 1:5000 or anti-rabbit IgG-HRP, Sigma-Aldrich, #A9169, 1:5000) was added for 1 h at room temperature. Membranes were incubated with ECL-HRP substrate (GE Healthcare, Chicago, IL, USA), and the signal was detected using the Alliance Q9 Advanced imaging system (Uvitec, Cambridge, UK).
4.6.5. Statistical Analysis
Data were analyzed in GraphPad Prism 7 software using a one-way ANOVA followed by Tukey’s test and reported as mean ± standard deviation. Values of p < 0.05 were considered as statistically significant.
5. Conclusions
The EOs distilled from roots and aerial parts of C. macrocephalum share some similarities in composition with the EOs found in taxonomically related species. The high content of eudesmanolides in REO reflects the relationships with Telekia and Inula spp. and the results of the previous phytochemical studies on the plant. Moreover, the presence of eudesmanolides in REO seems to be responsible for the high cytotoxic activity of the oil. Its cytotoxic effect on keratinocytes and human skin fibroblasts should be taken into consideration before the external application of the plant extract as a component of medicines or cosmetics. EOs from I. helenium (rich in eudesmanolides) demonstrated good anticandidal and antistaphylococcal activity. The antimicrobial activity of REO and APEO should be further investigated using a wider concentration range and including additional bacterial strains as well as yeast-like fungi.
Author Contributions
Conceptualization, A.S. and A.W.-B.; methodology, A.W.-B. (chemical analysis), P.K. (antimicrobial activity) and Ł.S. (cytotoxic activity); investigation, A.W.-B., P.K. and Ł.S.; resources, A.S., J.M., A.W.-B., P.K. and Ł.S.; data curation, A.S., A.W.-B., P.K. and Ł.S.; writing—original draft preparation, A.S., A.W.-B., J.M., P.K. and Ł.S.; writing—review and editing, A.S.; visualization, J.M. and Ł.S.; supervision, A.S. and A.W.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data that support the findings of this study are available from the authors, [A.W.-B., Ł.S., P.K., A.S.], upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- eFloras. Missouri Botanical Garden; St. Louis, MO & Harvard University Herbaria: Cambridge, MA, USA, 2008; Available online: http://www.efloras.org (accessed on 30 July 2024).
- Zhang, J.-P.; Wang, G.-W.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharm. 2015, 163, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Kim, W.-J.; Jiang, J.; Shim, D.-W.; Oh, N.-H.; Kim, T.-J.; Kang, T.-B.; Lee, K.-H. Carpesium macrocephalum attenuates lipopolysaccharide-induced inflammation in macrophages by regulating the NF-κB/IκB-α, Akt, and STAT signaling pathways. Am. J. Chin. Med. 2013, 41, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Butala, S.; Suvarna, V.; Mallya, R.; Khan, T. An insight into cytotoxic activity of flavonoids and sesquiterpenoids from selected plants of Asteraceae species. Chem. Biol. Drug Des. 2021, 98, 1116–1130. [Google Scholar] [CrossRef]
- Shi, N.-N.; Hou, C.-C.; Liu, Y.; Li, K.-Y.; Mi, S.-D.; Tong, B.-L.; Zhang, M.-L. Chemical constituents of plants from the genus Carpesium. Heterocycl. Comm. 2022, 28, 95–123. [Google Scholar] [CrossRef]
- Konovalova, O.A.; Rybalko, K.S.; Kabanov, V.S. Sesquiterpene lactones from Carpesium eximium. Chem. Nat. Compd. 1972, 8, 705–707. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, C.S.; Han, Y.F.; Jia, Z.-J. A new eudesmanolide from Carpesium macrocephalum. Chin. Chem. Lett. 2002, 13, 855–856. [Google Scholar]
- Yang, C.; Yuan, C.S.; Han, Y.F.; Jia, Z.-J. Sesquiterpene lactone glycosides from Carpesium macrocephalum. Chin. Chem. Lett. 2002, 13, 247–248. [Google Scholar]
- Kim, M.-R.; Kim, C.-S.; Hwang, K.-H.; Park, I.-Y.; Hong, S.-S.; Son, J.-K.; Moon, D.-C. Isolation and structures of guaianolides from Carpesium macrocephalum. J. Nat. Prod. 2002, 65, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shi, Y.-P.; Jia, Z.-J. Sesquiterpene lactone glycosides, eudesmanolides, and other constituents from Carpesium macrocephalum. Planta Med. 2002, 68, 626–630. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Jia, Z.-J. Sesquiterpene lactones and other constituents from the aerial parts of Carpesium macrocephalum. Aust. J. Chem. 2003, 56, 621–624. [Google Scholar] [CrossRef]
- Kim, M.-R.; Lee, S.-K.; Kim, C.-S.; Kim, K.-S.; Moon, D.-C. Phytochemical constituents of Carpesium macrocephalum Fr. et. Sav. Arch. Pharm. Res. 2004, 27, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L. Monoterpenoids from whole plants of Carpesium macrocephalum. Chin. Tradit. Herb. Drugs 2015, 46, 2985–2988. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Xu, X.-K.; Ye, J.; Yang, Y.-X.; Gao, S.; Li, H.-L.; Zhang, W.-D. Three new sesquiterpene lactone dimers from Carpesium macrocephalum. Fitoterapia 2016, 110, 72–76. [Google Scholar] [CrossRef]
- Xie, C.; Sun, L.; Meng, L.; Wang, M.; Xu, J.; Bartlam, M.; Guo, Y. Sesquiterpenes from Carpesium macrocephalum inhibit Candida albicans biofilm formation and dimorphism. Bioorg. Med. Chem. Lett. 2015, 25, 5409–5411. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Yao, X.; Ishii, R.; Kitanaka, S. Inhibitory activity of Chinese herbal medicines toward histamine release from mast cells and nitric oxide production by macrophage-like cell line, RAW 264.7. J. Nat. Med. 2006, 60, 73–77. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, H.J.; Lee, D.Y.; Jung, H.; Kim, M.-R.; Moon, D.-C.; Kim, K.I.; Lee, M.-S.; Ryu, J.-H. Carabrol suppresses LPS-induced nitric oxide synthase expression by inactivation of p38 and JNK via inhibition of I-κBα degradation in RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2010, 391, 1400–1404. [Google Scholar] [CrossRef]
- Kalemba, D.; Matla, M.; Smętek, A. Antimicrobial Activities of Essential Oils. In Dietary Phytochemicals and Microbes, 1st ed.; Patra, A.K., Ed.; Science + Business Media: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2012; Chapter 5; pp. 157–183. [Google Scholar] [CrossRef]
- Aponso, M.; Hearn, M.T.W.; Patti, A.F.; Bennett, L.E. Relaxation effects of essential oils are explained by their interactions with human brain neurotransmitter receptors and electroencephalography rhythms. ACS Chem. Neurosci. 2022, 13, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation aromatherapy via brain-targeted nasal delivery: Natural volatiles or essential oils on mood disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The effects of essential oils on the nervous system: A scoping review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.-H.; Park, M.-J.; Kim, J.-W.; Yang, J.; Yoo, Y.-M.; Jeung, E.-B. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Malarz, J.; Stojakowska, A. Composition of essential oils from roots and aerial parts of Carpesium divaricatum, a traditional herbal medicine and wild edible plant from south-east Asia, grown in Poland. Molecules 2019, 24, 4418. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Malarz, J.; Szoka, Ł.; Kwiatkowski, P.; Stojakowska, A. Composition of essential oils from roots and aerial parts of Carpesium cernuum and their antibacterial and cytotoxic activities. Molecules 2021, 26, 1883. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Szoka, Ł.; Kwiatkowski, P.; Meena, S.N.; Stojakowska, A. Bioprospecting of the Telekia speciosa: Uncovering the composition and biological properties of its essential oils. Appl. Sci. 2023, 13, 5674. [Google Scholar] [CrossRef]
- Englund, M.; Pornpongrungrueng, P.; Geustafsson, M.H.G.; Anderberg, A.A. Phylogenetic relationships and generic delimitation in Inuleae subtribe Inulinae (Asteraceae) based on ITS and cpDNA sequence data. Cladistics 2009, 25, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Larruscain, D.; Santos-Vicente, M.; Anderberg, A.A.; Rico, E.; Martínez-Ortega, M.M. Phylogeny of the Inula group (Asteraceae: Inuleae): Evidence from nuclear and plastid genomes and a recircumscription of Pentanema. Taxon 2018, 67, 149–164. [Google Scholar] [CrossRef]
- Lunz, K.; Stappen, I. Back to the roots—An overview of the chemical composition and bioactivity of selected root-essential oils. Molecules 2021, 26, 3155. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.; Blagojević, P.; Palić, R.; Zlatković, B. Volatiles of Telekia speciosa (Schreb.) Baumg. (Asteraceae) from Serbia. J. Essent. Oil Res. 2010, 22, 250–254. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Stojakowska, A.; Kalemba, D. Chemical composition of essential oils from a multiple shoot culture of Telekia speciosa and different plant organs. Nat. Prod. Commun. 2012, 7, 625–628. [Google Scholar] [CrossRef]
- Cilović Kozarević, E.; Šarić-Kundalić, B.; Ibišević, M.; Horozić, E.; Glamočlija, J.; Soković, M.; Arsenijević, J.; Maksimović, Z. GC/MS analysis and antimicrobial activity of essential oils of Telekia speciosa (Schreb.) Baumg. Lek. Sirovine 2021, 41, 35–40. [Google Scholar] [CrossRef]
- Kameoka, H.; Sagara, K.; Miyazawa, M. Components of essential oils of Kakushitsu (Daucus carota L. and Carpesium abrotanoides L.). Nippon. Nōgeikagaku Kaishi 1989, 63, 185–188. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, L.; Lin, L.; Zhang, R.; Du, Y.; Chen, H.; Huang, M.; Guo, K.; Yang, X. Essential oil from Carpesium abrotanoides L. induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018, 38, 1045–1053. [Google Scholar] [CrossRef]
- Haris, A.; Azeem, M.; Binyameen, M. Mosquito repellent potential of Carpesium abrotanoides essential oil and its main components against a dengue vector, Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 801–809. [Google Scholar] [CrossRef]
- Bourrel, C.; Vilarem, G.; Perineau, F. Chemical analysis, bacteriostatic and fungistatic properties of the essential oil of elecampane (Inula helenium L.). J. Essent. Oil Res. 1993, 5, 411–417. [Google Scholar] [CrossRef]
- Boatto, G.; Pintore, G.; Palomba, M.; de Simone, F.; Ramundo, E.; Iodice, C. Composition and antibacterial activity of Inula helenium and Rosmarinus officinalis essential oils. Fitoterapia 1994, 65, 279–280. [Google Scholar]
- Deriu, A.; Zanetti, S.; Sechi, L.A.; Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E. Antimicrobial activity of Inula helenium L. essential oil against Gram-positive and Gram-negative bacteria and Candida spp. Int. J. Antimicrob. Agents 2008, 31, 588–590. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Blagojević, P.; Denić, M.; Miltojević, A.; Rajković, J.; Mihajilov-Krstev, T. Antistaphylococcal activity of Inula helenium L. root essential oil: Eudesmane sesquiterpene lactones induce cell membrane damage. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1015–1025. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Dimitrijević, M.; Genčić, M.; Pejčić, M.; Radulović, N. Anticandidal activity of Inula helenium root essential oil: Synergistic potential, anti-virulence efficacy and mechanism of action. Ind. Crops Prod. 2020, 149, 112373. [Google Scholar] [CrossRef]
- Konishi, T.; Shimada, Y.; Nagao, T.; Okabe, H.; Konoshima, T. Antiproliferative sesquiterpene lactones from the roots of Inula helenium. Biol. Pharm. Bull. 2002, 25, 1370–1372. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Chen, Y.; Zou, D.; Pu, Y.; Wei, M.; Huang, Y.; Li, Y.; Huang, Q.; Chen, J. Alantolactone inhibits melanoma cell culture viability and migration and promotes apoptosis by inhibiting Wnt/β-catenin signaling. Anticancer Agents Med. Chem. 2023, 23, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, Q.; Dai, X.; Wen, X.; Luo, X.; Duan, Y.; Yang, Z.; Dai, Q. Alantolactone enhances the sensitivity of melanoma to MAPK pathway inhibitors by targeting inhibition of STAT3 activation and down-regulating stem cell markers. Cancer Cell Int. 2024, 24, 191. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Kalsi, P.S.; Goyal, R.; Talwar, K.K.; Chhabra, B.R. Stereostructures of two biologically active sesquiterpene lactones from Inula racemosa. Phytochemistry 1989, 28, 2093–2096. [Google Scholar] [CrossRef]
- Perez, A.L.C.; Nava, L.M.; Romo de Vivar, A. Absinthifolide, a sesquiterpene glycoside from Bahia absinthifolia var. absinthifolia. Phytochemistry 1987, 26, 765–767. [Google Scholar] [CrossRef]
- Klochkov, S.G.; Afanaseva, S.V.; Pushin, A.N. Acidic isomerization of alantolactone derivatives. Chem. Nat. Compd. 2006, 42, 400–406. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial activity of selected essential oil compounds alone and in combination with β-lactam antibiotics against MRSA strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).