An Integrated Strategy of UHPLC-ESI-MS/MS Combined with Bioactivity-Based Molecular Networking for Identification of Antitumoral Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann
Abstract
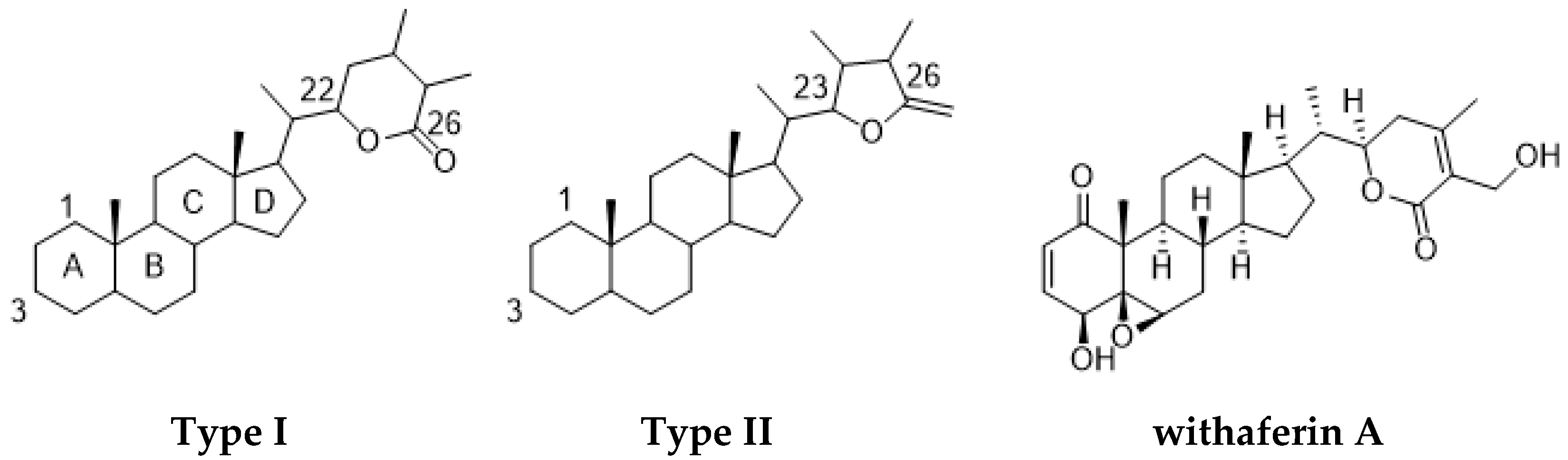
1. Introduction
2. Results and Discussion
2.1. Cytotoxic Activity of A. fasciculata Extracts and Partitions against Human Leukemia Cell Lines
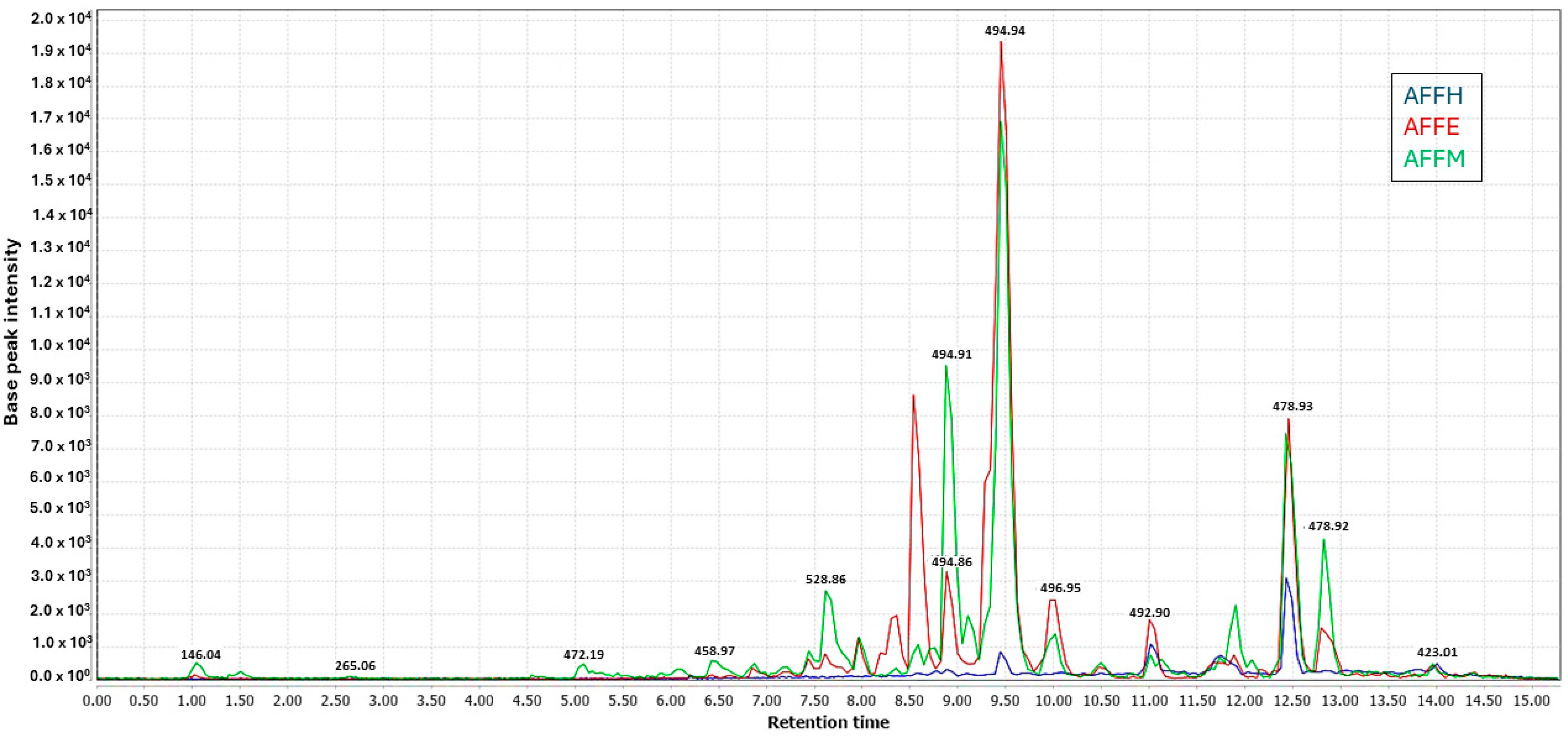
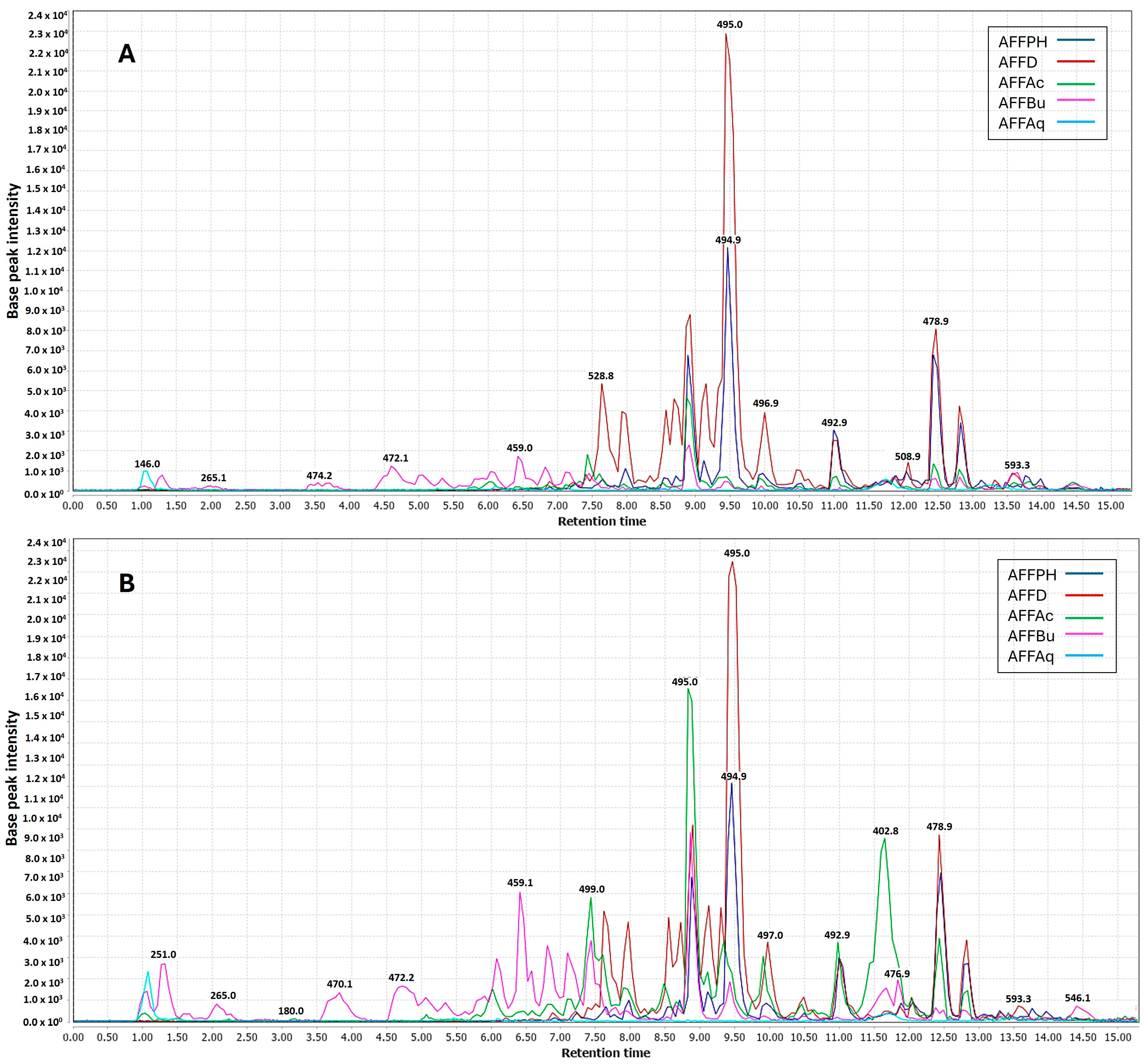
2.2. Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry Analysis
2.3. Chemometric Analyses Using PLS Regression Model
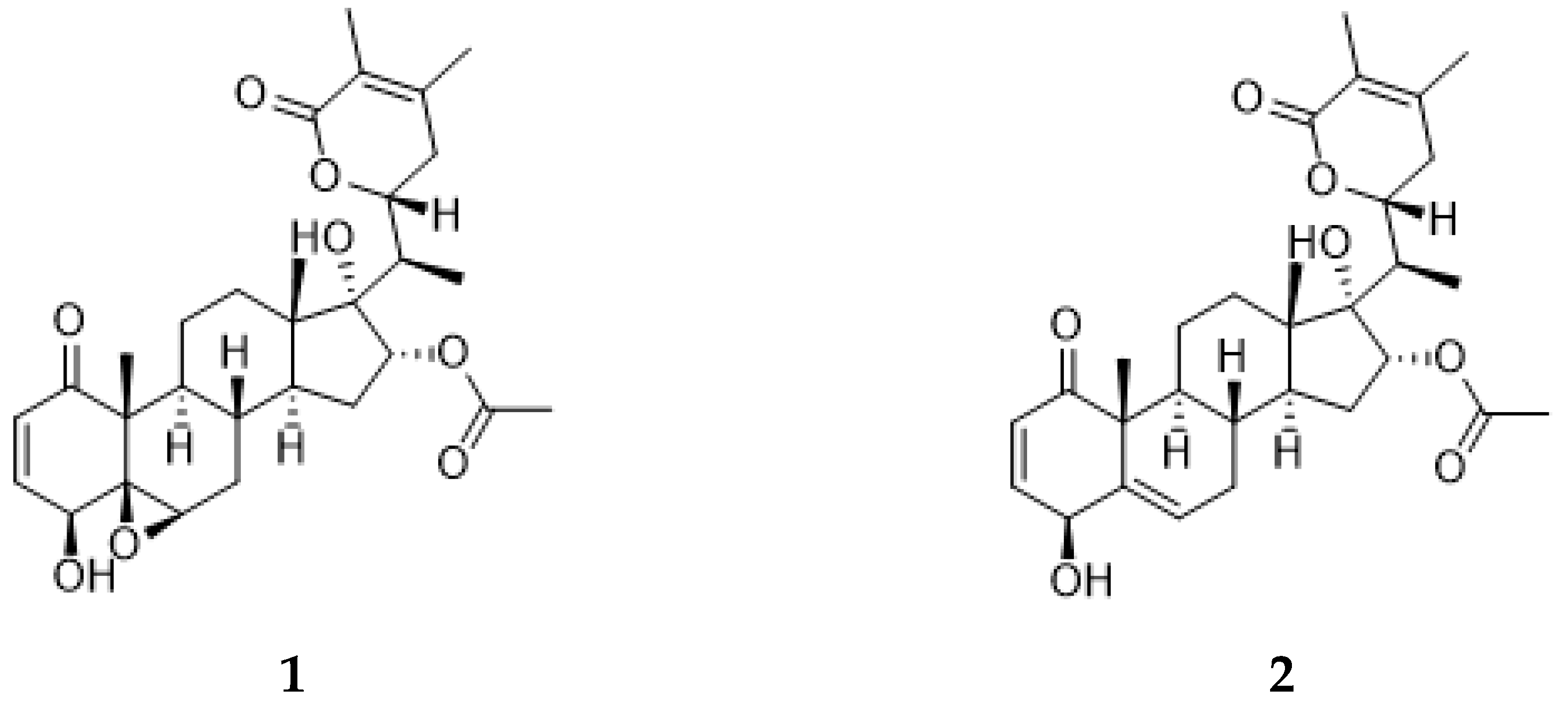
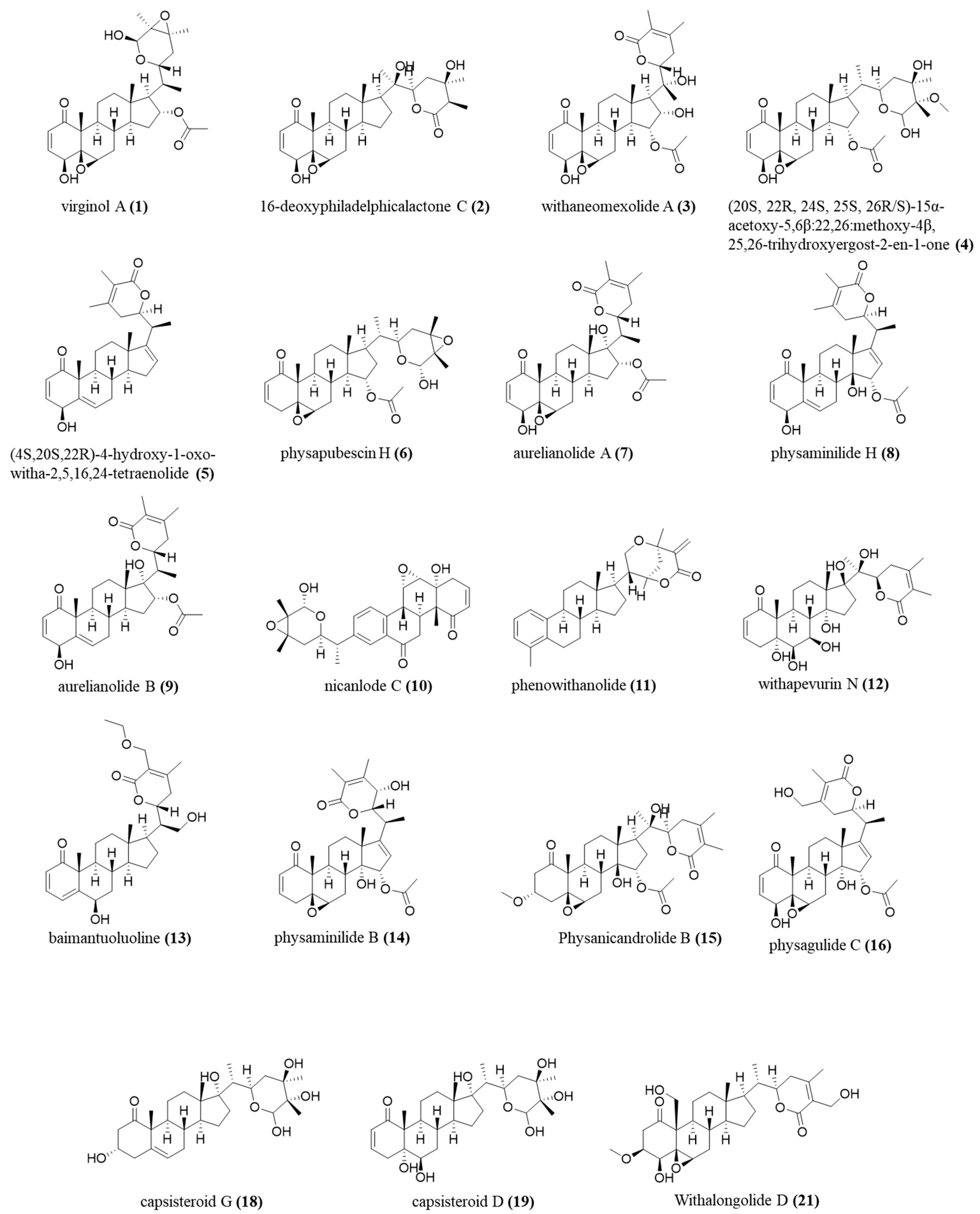
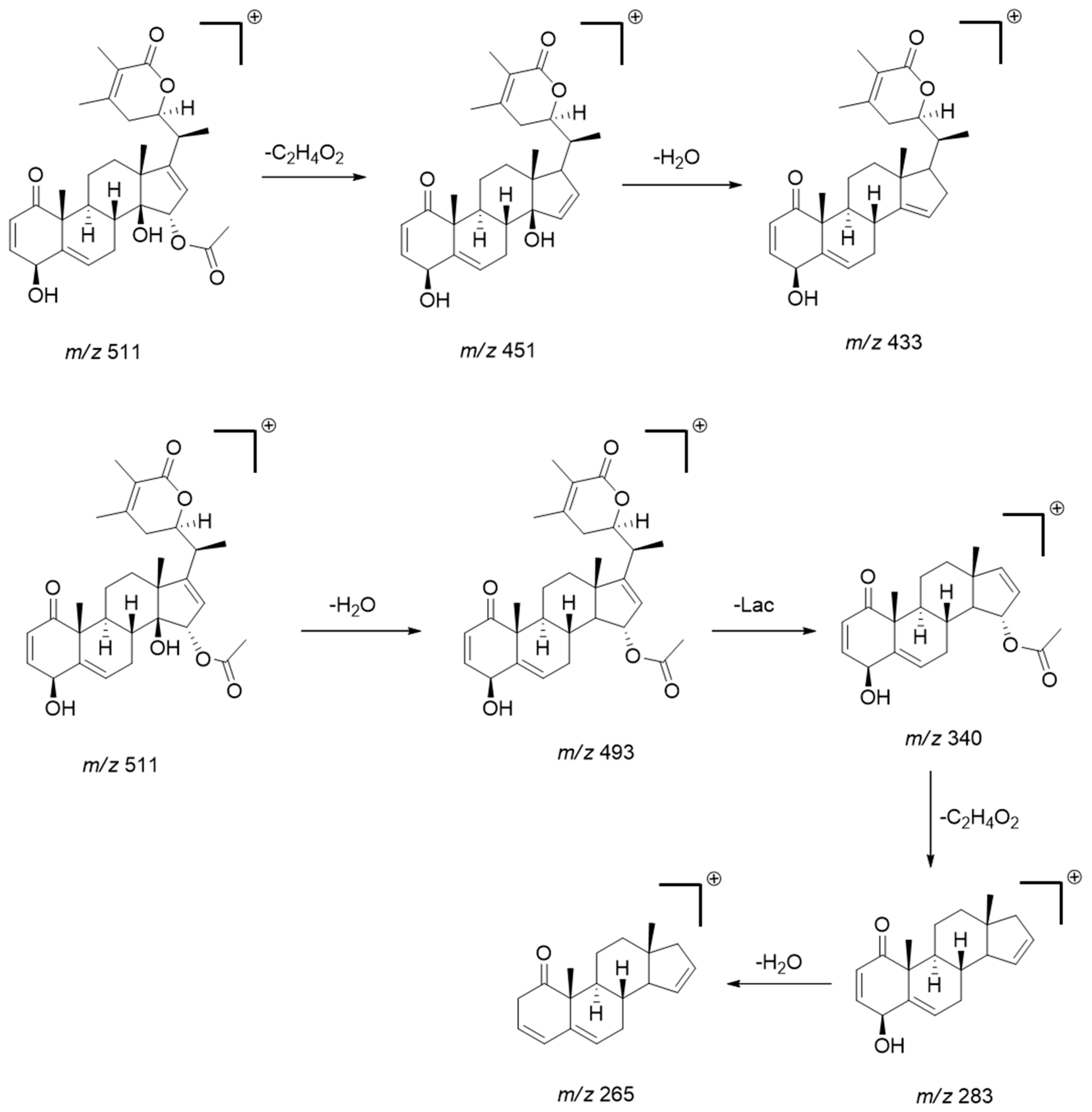
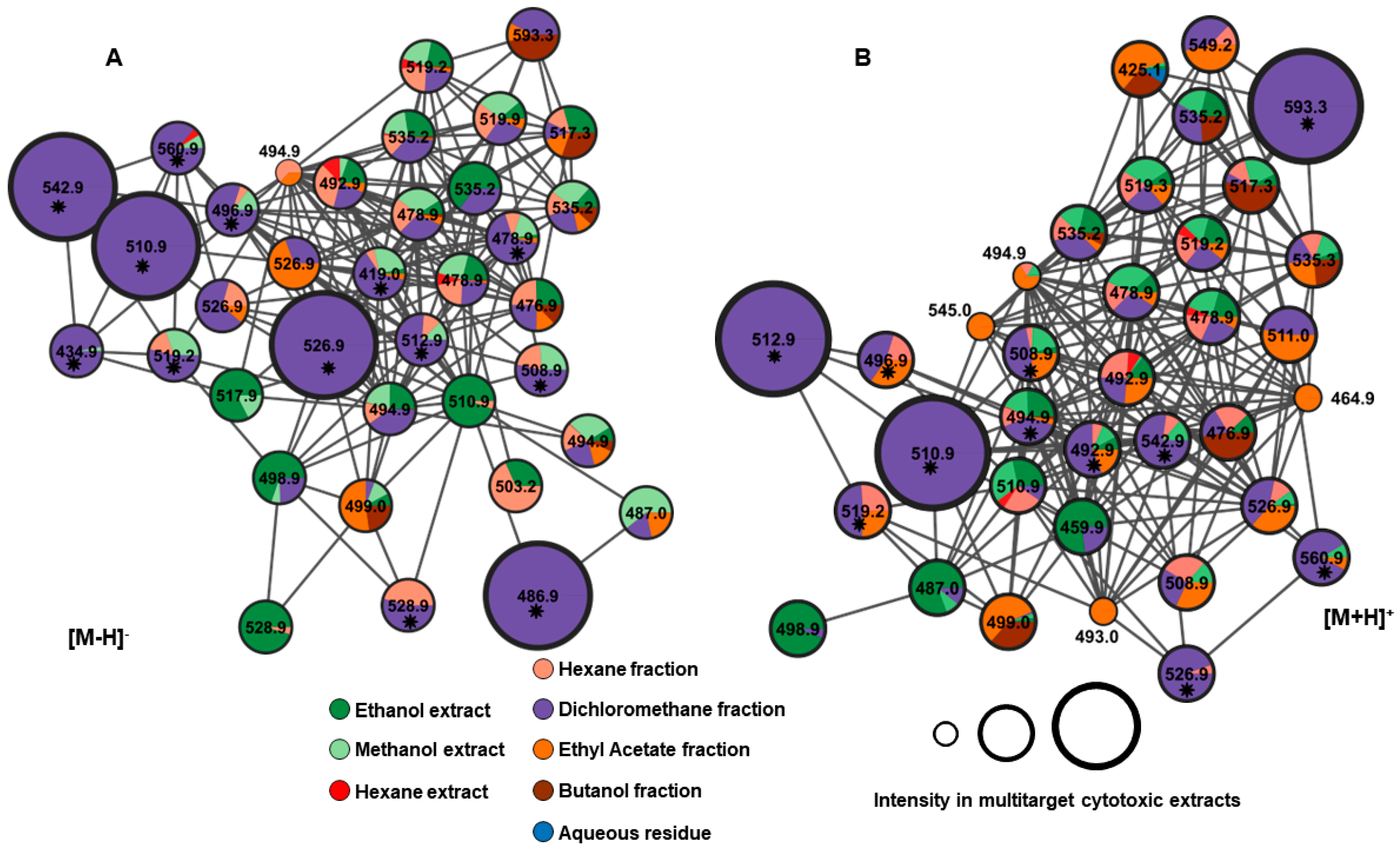
2.4. Annotated Compounds for the Predicted Ions in the Multitarget Model Using Molecular Networking Analyses
| Compound | Rt (min) | Molecular Formula | [M − H]− (m/z) | MS/MS (MS2) | Proposed/Annotated Compound | Reference |
|---|---|---|---|---|---|---|
| 1 | 7.2 | C30H42O8 | 528.9 | 511, 493, 469, 451, 433 | virginol A | [17] |
| 2 | 7.4 | C28H40O7 | 486.9 | 469, 451, 453 | 16-deoxyphiladelphicalactone C | [18] |
| 3 | 8.7 | C30H40O9 | 542.9 | 525, 510, 483, 465 | withaneomexolide A | [19] |
| 4 | 8.9 | C31H46O9 | 560.9 | 542, 529, 501, 489 | (20S,22R,24S,25S,26R/S)-15α-acetoxy-5,6β:22,26:diepoxy-24-methoxy-4β,25,26-trihydroxyergost-2-en-1-one | [20] |
| 5 | 9.1 | C28H36O4 | 434.9 | 417, 399, 391, 377 | (4S,20S,22R)-4-Hydroxy-1-oxo-witha-2,5,16,24-tetraenolide | [21] |
| 6 | 9.4 | C30H42O7 | 512.8 | 495, 435, 417 | physapubescin H | [22] |
| 7 | 10.6 | C30H40O8 | 526.9 | 509, 467, 449, 431, 421 | aurelianolide A | [6] |
| 8 | 11.2 | C30H38O7 | 508.9 | 449, 431, 413, 385 | physaminilide H | [23] |
| 9 | 11.5 | C30H40O7 | 510.9 | 492, 475, 463, 451 | aurelianolide B | [6] |
| 10 | 12.4 | C28H32O7 | 478.9 | 461, 419, 401 | nicanlode C | [24] |
| 11 | 12.4 | C28H36O3 | 419.0 | 401, 383, 373 | phenowithanolide | [25] |
| 12 | 12.4 | C28H40O9 | 519.2 | 501, 459, 441 | withaperuvin N | [26] |
| 13 | 12.5 | C30H42O6 | 496.9 | 479, 419 | baimantuoluoline R | [27] |
| Compound | Rt (min) | Molecular Formula | [M + H]+ (m/z) | MS/MS (MS2) | Proposed/Annotated Compound | Reference |
|---|---|---|---|---|---|---|
| 8 | 7.8 | C30H38O7 | 510.9 | 493, 451, 433, 340 | physaminilide H | [23] |
| 9 | 7.9 | C30H40O7 | 512.9 | 495, 453, 435, 417 | aurelianolide B | [6] |
| 14 | 8.5 | C30H38O8 | 526.9 | 509, 467, 449, 431 | physaminilide B | [28] |
| 15 | 8.7 | C31H44O9 | 560.9 | 543, 528, 517 | physanicandrolide B | [29] |
| 16 | 8.7 | C30H38O9 | 542.9 | 525, 510, 482, 464 | physagulide C | [30] |
| 17 | 9.4 | - | 494.9 | 477, 453, 435, 417 | not identified | |
| 18 | 11.0 | C28H44O7 | 492.9 | 475, 433, 415 | capsisteroid G | [31] |
| 19 | 11.1 | C28H44O8 | 508.9 | 491, 473, 449 | capsisteroid D | [32] |
| 20 | 12.4 | - | 496.9 | 479, 437, 419, 392 | not identified | |
| 21 | 12.8 | C29H42O8 | 519.2 | 501, 459, 441 | withalongolide D | [33] |
| 22 | 13.6 | - | 593.3 | 575, 565, 533, 505, 461, 433 | not identified |
3. Materials and Methods
3.1. Plant Material
3.2. A. fasciculata Sample Preparation (Extract and Partition)
3.3. Cytotoxicity Assays and Statistical Analysis
3.4. Liquid Chromatography–Mass Spectrometry Analysis
3.5. Data Processing and Chemometric Analysis
3.6. Molecular Networking and Annotation of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as sources of New Drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery based on natural products: From computational approaches to clinical studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Y.; Xu, Y.; Liu, Y.; Li, H.; Chen, L. Molecular targets and mechanisms of anti-cancer effects of withanolides. Chem. Biol. Interact. 2023, 384, 110698–110707. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cao, S.; Kang, N.; Qiu, F. Withanolides: Promising candidates for cancer therapy. Phytother. Res. 2024, 38, 1104–1158. [Google Scholar] [CrossRef]
- Rodrigues, I.M.C.; Knapp, S.; Stehmann, J.R. The nomenclatural re-establishment of Athenaea Sendtn. (Solanaceae) with a nomenclatural synopsis of the genus. Taxon 2019, 68, 840–848. [Google Scholar]
- Almeida-Lafetá, C.R.; Ferreira, J.P.M.; Emerenciano, P.V.; Kaplan, A.C.M. Withanolides from Aureliana fasciculata var. fasciculata. Helv. Chim. Acta. 2010, 93, 2478–2487. [Google Scholar] [CrossRef]
- Silva, G.W.D.S.; Marques, A.M.; Fontão, A.P.G.A.; Lima, S.C.M.; Kaplan, M.A.C.; Figueiredo, M.R.; Sampaio, A.L.F. Aurelianolides from Aureliana fasciculata var. fasciculata trigger apoptosis with caspase activation in human leukemia cells. Anticancer Res. 2023, 43, 1245–1253. [Google Scholar]
- Lima, S.C.M.; Pacheco, J.D.S.; Marques, A.M.; Veltri, E.R.P.; Almeida-Lafetá, R.C.; Figueiredo, M.R.; Kaplan, M.A.C.; Torres-Santos, E.C. Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata. Molecules 2018, 23, 3160. [Google Scholar] [CrossRef]
- Peres, R.B.; Fiuza, L.F.A.; Silva, P.B.D.; Batista, M.M.; Camillo, F.D.C.; Marques, A.M.; Brito, L.D.C.; Figueiredo, M.R.; Soeiro, M.N.C. In Vitro Phenotypic Activity and In Silico Analysis of Natural Products from Brazilian Biodiversity on Trypanosoma cruzi. Molecules 2021, 26, 5676. [Google Scholar] [CrossRef]
- Pilon, A.C.; Selegato, D.M.; Fernandes, R.P.; Bueno, P.C.P.; Pinho, D.R.; Neto, F.F.; Freire, R.T.; Castro-Gambo, I.; Bolzani, V.S.; Lopes, N.P. Metabolômica de plantas: Métodos e desafios. Quim. Nova. 2020, 43, 329–354. [Google Scholar] [CrossRef]
- Beccaria, M.; Cabooter, D. Current developments in LC-MS for pharmaceutical analysis. Analyst 2020, 145, 1129–1157. [Google Scholar] [CrossRef] [PubMed]
- Gurgul, A.; Youn, I.; Maldonado, A.; Wahid, F.; Che, C.-T.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Equisetum arvense L. And evaluation of cytotoxicity against human melanoma and ovarian cancer cells. Saudi J. Biol. Sci. 2022, 29, 103271–103279. [Google Scholar]
- Chibuye, B.; Singh, I.S.; Chimuka, L.; Maseka, K.K. Phytochemical profiling and bioactivity study of Adenia panduriformis in Zambia using UHPLC-MS/MS-MZmine3, GNPS, and METLIN Gen2. Sci. Afr. 2024, 24, 2151–2167. [Google Scholar] [CrossRef]
- Demarque, D.P.; Dusi, R.G.; Sousa, F.D.M.; Grossi, S.M.; Silvério, M.R.S.; Lopes, M.P.; Espindola, L.S. Mass spectrometry-based metabolomics approach in the isolation of bioactive natural products. Sci. Rep. 2020, 10, 1051–1060. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Pilon, A.C.; Vieira, N.C.; Amaral, J.G.; Monteiro, A.F.; Silva, R.R.D.; Spíndola, L.S.; Castro-Gamboa, I.; Lopes, N.P. Redes moleculares: Uma análise sobre anotações e descoberta de novos ativos. Quim. Nova 2021, 44, 1168–1179. [Google Scholar] [CrossRef]
- Maldonado, E.; Amador, S.; Martínez, M.; Pérez-Castorena, A.L. Virginols A-C, three new withanolides from Physalis virginiana. Steroids 2010, 75, 346–349. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Wijeratne, E.M.K.; Brooks, A.D.; Tewary, P.; Xuan, L.-J.; Wang, W.-Q.; Sayers, T.J.; Gunatilaka, A.A.L. Cytotoxic and other withanolides from aeroponically grown Physalis philadelphica. Phytochemistry 2018, 152, 174–181. [Google Scholar] [CrossRef]
- Cao, C.-M.; Wu, X.; Kindscher, K.; Xu, L.; Timmermann, B.N. Withanolides and Sucrose Esters from Physalis neomexicana. J. Nat. Prod. 2015, 78, 2488–2493. [Google Scholar] [CrossRef]
- Chen, L.-X.; Xia, G.-Y.; He, H.; Huang, J.; Qiu, F.; Zi, X.-L. New withanolides with TRAIL-sensitizing effect from Physalis pubescens L. RSC Adv. 2016, 6, 52925–52936. [Google Scholar] [CrossRef]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Vázquez, J.T.; Bazzocchi, I.L. Withanolides from Withania aristata and their cytotoxic activity. Steroids 2010, 75, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Li, Y.; Sun, J.; Wang, L.; Tang, X.; Lin, B.; Kang, N.; Huang, J.; Chen, L.; Qiu, F. Withanolides from the stems and leaves of Physalis pubescens and their cytotoxic activity. Steroids 2016, 115, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, B.; He, X.; Cao, S.; Ding, L.; Kang, N.; Chen, L.; Qiu, F. New cytotoxic withanolides from Physalis minima. Fitoterapia 2020, 146, 104728–104734. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-Y.; Zhao, G.-T.; Liu, J.-Q.; Khan, A.; Peng, X.-R.; Zhou, L.; Dong, J.-R.; Li, H.-Z.; Qiu, M.H. Withanolides from aerial parts of Nicandra physalodes. Phytochemistry 2017, 137, 148–155. [Google Scholar] [CrossRef]
- Bellila, A.; Tremblay, C.; Pichette, A.; Marzouk, B.; Mshvildadze, V.; Lavoie, S.; Legault, J. Cytotoxic activity of withanolides isolated from Tunisian datura Metel L. Phytochemistry 2011, 72, 2031–2036. [Google Scholar] [CrossRef]
- Fang, S.-T.; Liu, J.-K.; Li, B. Ten new withanolides from Physalis peruviana. Steroids 2012, 77, 36–44. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Sun, H.-P.; Wang, X.; Liu, Y.; Yang, B.-Y.; Kuang, H.-X. Immunosuppressive withanolides from the flower of Datura metel L. Fitoterapia 2020, 141, 101468–101475. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B.; Guang, C.; Jiang, B.; He, X.; Cao, S.; Ding, L.; Kang, N.; Chen, L.; Qiu, F. New withanolides from Physalis minima and their cytotoxicity against A375 human melanoma cells. RSC Adv. 2020, 38, 22819–22827. [Google Scholar] [CrossRef]
- Torres, F.R.; Pérez-Castorena, A.L.; Arredondo, L.; Toscano, R.A.; Nieto-Camacho, A.; Martínez, M.; Maldonado, E. Withanolides, and other constituents from Physalis nicandroides. J. Nat. Prod. 2019, 82, 2489–2500. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, W.-N.; Yang, L.; Zhang, C.; Lin, R.; Shan, S.M.; Zhu, M.-D.; Luo, J.-G.; Kong, L.-Y. Cytotoxic withanolides from Physalis angulata var. villosa and the apoptosis-inducing effect via ROS generation and the activation of MAPK in human osteosarcoma cells. RSC Adv. 2016, 6, 53089–53100. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chao, C.-H.; Ahmed, A.F.; Chen, Y.-Y.; Hwang, T.-L.; Liu, H.-Y.; Sheu, J.-H. Withanolides and 26-Hydroxylated derivatives with anti-inflammatory property from Solanum capsicoide. Bull. Chem. Soc. Jpn. 2019, 92, 336–343. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chen, Y.-Y.; Lin, Y.-C.; Huang, C.-Y.; Uvarani, C.; Hwang, T.-L.; Chiang, M.Y.; Liu, H.-Y.; Sheu, J.-H. Capsisteroids A–F, withanolides from the leaves of Solanum capsicoides. RSC Adv. 2015, 5, 88841–88847. [Google Scholar] [CrossRef]
- Zhang, H.; Samadi, A.K.; Gallagher, R.J.; Araja, J.J.; Tong, X.; Day, V.W.; Cohen, M.S.; Kindscher, K.; Gollapudi, R.; Timmermann, B.N. Cytotoxic withanolide constituents of Physalis longifolia. J. Nat. Prod. 2011, 74, 2532–2544. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Ali, A.; Ali, R.A.; Yousuf, S.; Rahman, A.-U.; Choudhary, M.I. Analysis and development of structure-fragmentation relationships in withanolides using an electrospray ionization quadropole time-of-flight tandem mass spectrometry hybrid instrument. Rapid Commun. Mass. Spectrom. 2011, 25, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quirós-Fallas, M.I.; Vargas-Huertas, F.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A. QTOF-ESI MS characterization and antioxidant activity of Physalis peruviana L. (Cape Gooseberry) husks and fruits from Costa Rica. Molecules 2022, 27, 4238. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.D.S.; Bellete, B.S.; Vieira, L.C.C.; Sampaio, O.M. Use of Molecular Networking for compound annotation in metabolomics. Rev. Virtual Quim. 2021, 14, 214–223. [Google Scholar] [CrossRef]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of sesquiterpene lactones: A Review of some potential success sases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Tewari, D.; Chander, V.; Dhyani, A.; Sahu, S.; Gupta, P.; Patni, P.; Kalick, L.S.; Bishayee, A. Withania somnifera (L.) Dunal: Phytochemistry, structure-activity relationship, and anticancer potential. Phytomedicine 2022, 98, 153949–153958. [Google Scholar] [CrossRef]
- Misakyan, M.F.F.; Wijeratne, E.M.K.; Issa, M.E.; Xu, Y.-M.; Monteillier, A.; Gunatilaka, A.A.L.; Cuendet, M. Structure–Activity relationships of withanolides as antiproliferative agents for multiple myeloma: Comparison of activity in 2D models and a 3D coculture model. J. Nat. Prod. 2021, 84, 2321–2335. [Google Scholar] [CrossRef]
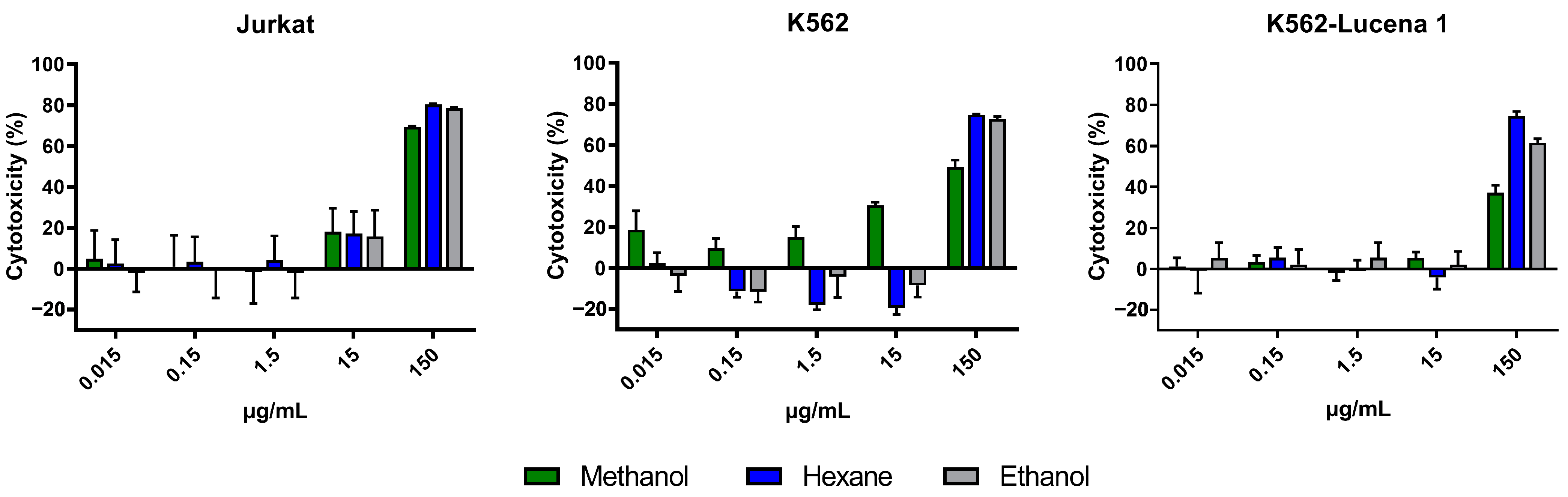
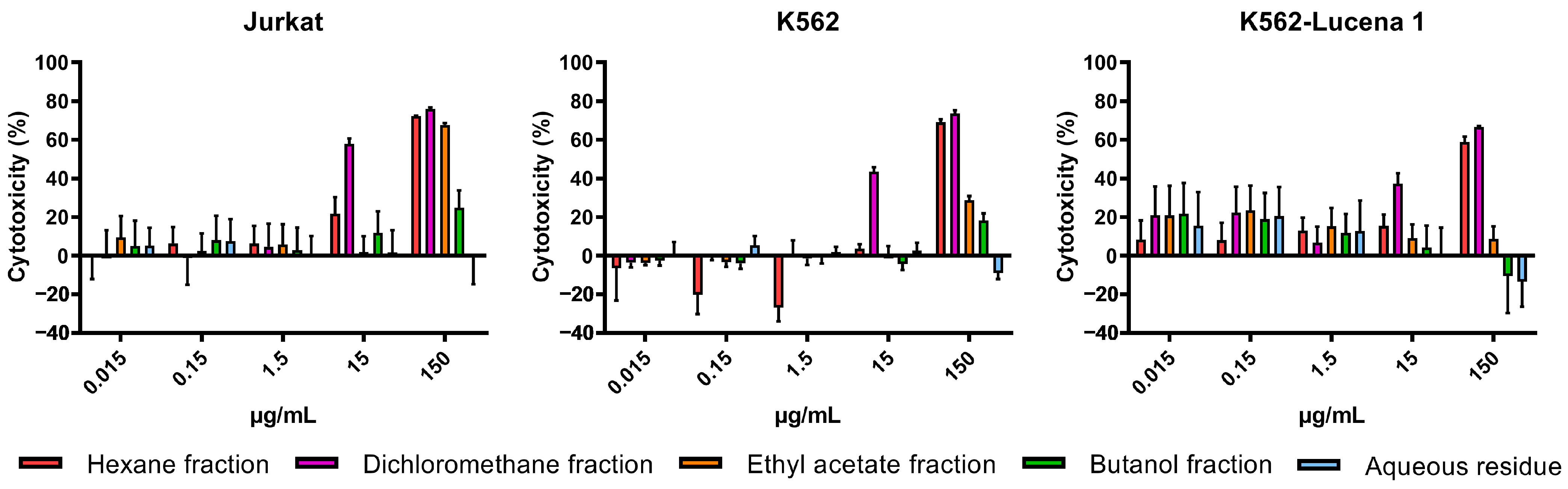


| Material | IC50 (µg/mL) | ||
|---|---|---|---|
| Jurkat | K562 | K562-Lucena 1 | |
| Methanolic extract (AFFM) | 67.70 | 108.00 | 255.20 |
| Hexanic extract (AFFH) | 50.08 | 104.30 | 84.81 |
| Ethanolic extract (AFFE) | 55.21 | 98.88 | 110.80 |
| Ethanolic extract hexane fraction (AFFPH) | 55.29 | 92.31 | 97.13 |
| Ethanolic extract dichloromethane fraction (AFFD) | 14.34 | 26.50 | 38.64 |
| Ethanolic extract ethyl acetate fraction (AFFAc) | 92.21 | 384.70 | >1000 |
| Ethanolic extract butanol fraction (AFFBu) | 418.4 | 716.5 | >1000 |
| Ethanolic extract aqueous residue (AFFAq) | >1000 | >1000 | >1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.M.; Brito, L.d.C.; Mendonça, S.C.; Gomes, B.A.; Camillo, F.d.C.; Silva, G.W.d.S.e.; Sampaio, A.L.F.; Leitão, S.G.; Figueiredo, M.R. An Integrated Strategy of UHPLC-ESI-MS/MS Combined with Bioactivity-Based Molecular Networking for Identification of Antitumoral Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann. Molecules 2024, 29, 4357. https://doi.org/10.3390/molecules29184357
Marques AM, Brito LdC, Mendonça SC, Gomes BA, Camillo FdC, Silva GWdSe, Sampaio ALF, Leitão SG, Figueiredo MR. An Integrated Strategy of UHPLC-ESI-MS/MS Combined with Bioactivity-Based Molecular Networking for Identification of Antitumoral Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann. Molecules. 2024; 29(18):4357. https://doi.org/10.3390/molecules29184357
Chicago/Turabian StyleMarques, André Mesquita, Lavinia de Carvalho Brito, Simony Carvalho Mendonça, Brendo Araujo Gomes, Flávia da Cunha Camillo, Gustavo Werneck de Souza e Silva, André Luiz Franco Sampaio, Suzana Guimarães Leitão, and Maria Raquel Figueiredo. 2024. "An Integrated Strategy of UHPLC-ESI-MS/MS Combined with Bioactivity-Based Molecular Networking for Identification of Antitumoral Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann" Molecules 29, no. 18: 4357. https://doi.org/10.3390/molecules29184357
APA StyleMarques, A. M., Brito, L. d. C., Mendonça, S. C., Gomes, B. A., Camillo, F. d. C., Silva, G. W. d. S. e., Sampaio, A. L. F., Leitão, S. G., & Figueiredo, M. R. (2024). An Integrated Strategy of UHPLC-ESI-MS/MS Combined with Bioactivity-Based Molecular Networking for Identification of Antitumoral Withanolides from Athenaea fasciculata (Vell.) I.M.C. Rodrigues & Stehmann. Molecules, 29(18), 4357. https://doi.org/10.3390/molecules29184357






