Abstract
Carotenoids are tetraterpene compounds acting as precursors to vitamin A, with functions that include protecting eyesight, enhancing immunity, promoting cell growth and differentiation, and providing antioxidative benefits. Lycopene, β-carotene, and astaxanthin are particularly critical for health and have diverse applications in food, health products, and medicine. However, natural carotenoids are encased within cell structures, necessitating mechanical methods to disrupt the cell wall for their extraction and purification—a process often influenced by environmental conditions. Thus, improving the efficiency of carotenoid extraction from natural resources is of great interest. This review delves into the research progress made on the extraction processes, structures, and biological functions of carotenoids, focusing on lycopene, β-carotene, and astaxanthin. Traditional extraction methods primarily involve organic solvent-assisted mechanical crushing. With deeper research and technological advancements, more environmentally friendly solvents, advanced machinery, and suitable methods are being employed to enhance the extraction and purification of carotenoids. These improvements have significantly increased extraction efficiency, reduced preparation time, and lowered production costs, laying the groundwork for new carotenoid product developments.
1. Introduction
Carotenoids, a class of natural pigments found in plants, impart vivid colors like orange, yellow, or red to some fruits and vegetables. They possess a wide range of physiological functions beneficial to humans, including anticancer [1] and antioxidative properties [2], the promotion of intestinal microecology [3], the alleviation of inflammatory respiratory disease symptoms [4], and a reduction in glaucoma risk [5]. These functions exhibit high bioavailability and research value. Consequently, carotenoid acquisition has become a prominent research topic. Due to safety concerns over chemically synthesized carotenoids and a preference for natural products, the focus has shifted to two main sources: natural extracts and biosynthesis [6]. While biosynthesis presents industrial production challenges due to low yields, natural extraction sources are diverse, including plants, algae, microorganisms, and food processing waste.
To date, over 700 carotenoids have been identified in nature, with lycopene, β-carotene, and astaxanthin being the most extensively studied due to their biological functions. Carotenoids are categorized into two groups based on their chemical structure: (1) carotenes such as lycopene, β-carotene, and α-carotene consist solely of carbon and hydrogen. (2) Xanthophylls, like zeaxanthin, meso-zeaxanthin, and lutein, serve as macular pigment carotenoids [7].
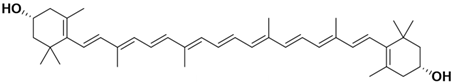
The molecular structure of carotenoids is highly sensitive, and it is particularly prone to photodegradation and breakdown under light exposure (especially ultraviolet (UV) exposure), high temperatures, and the presence of oxygen. Therefore, carotenoids are easily degraded when exposed to light, heat, and oxidizing agents. Careful handling is essential to prevent structural alterations that could render them inactive or unstable. The yield and purity of extracted carotenoids can differ based on the chosen extraction and purification techniques and the solvents used, as illustrated in Figure 1.
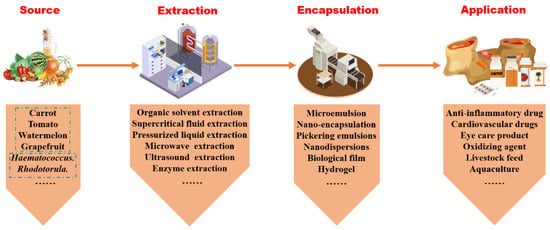
Figure 1.
The process of carotenoids from source to product.
Most carotenoids reside within cellular compartments. Conventional organic solvent extraction techniques and green solvents are solvents that are environmentally friendly during production and processing and are non-toxic, biodegradable, and usually non-flammable. These solvents have all struggled to efficiently penetrate the intracellular environment to directly contact carotenoids. Hence, physical methods such as microwave and ultrasonication are necessary to enhance the actual yield efficiency of carotenoid collection. The choice of extraction method and organic solvents impacts not only the efficiency of carotenoid extraction but also their biological activity. Post-extraction and purification, various packaging methods, including embedding, nano-encapsulation [8], microemulsion preparation [9], and edible membrane production [10], are employed to preserve the stability and bioactivity of carotenoids.
The burgeoning consumer preference for natural ingredients, coupled with the enhanced understanding of the health benefits conferred by carotenoids, is collectively propelling the market demand for functional carotenoid products. This demand is anticipated to reach a value of USD 1.45 billion in 2024. The global carotenoid market is forecast to expand at a compound annual growth rate (CAGR) of 5.4%, culminating in a market value of USD 2.45 billion by the close of 2034 [11]. Consequently, technological innovations in extraction processes and the formulation techniques are essential to cater to the expanding carotenoid market. The aim of this paper is to review the properties and roles of three specific carotenoids, lycopene, β-carotene, and astaxanthin, and summarize domestic and international research on carotenoid extraction methods. This will provide theoretical references for the extraction and purification of carotenoids.
2. Physicochemical Characteristics of Tetraterpenoids
Lycopene is the initial tetraterpenoid in the carotenoid metabolic pathway [12]. It is synthesized from geranylgeranyl diphosphate (GGPP), which is produced in plants, fungi, and bacteria through the mevalonate pathway (MVA) and the 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway. Figure 2 illustrates the conversion of GGPP into lycopene through a series of synthesis steps and dehydrogenation transitions involving IspA (FPP synthase), phytoene synthase, and phytoene desaturase [13]. Lycopene is subsequently converted into β-carotene by lycopene cyclase [12,14]. In fungi, β-carotene synthesis relies on key enzyme astaxanthin synthase (CrtS) and cytochrome P450 reductase (CrtR), which are both members of the cytochrome P450 protease family. Some bacteria, algae and protozoa, can further synthesize astaxanthin through β-carotene ketoacidase (CrtW) and β-carotene hydroxylase (CrtZ), whereas plants require carotenoid β-ring 4-dehydrogenase (CBFD) for this conversion [15,16,17].
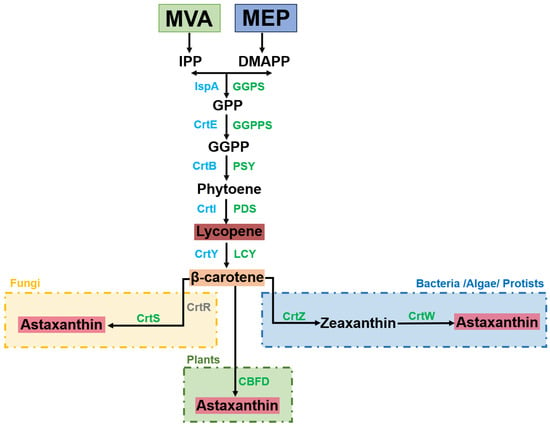
Figure 2.
Carotenoid biosynthetic pathway. Enzymes from IPP and DMAPP to β-carotene are shown in blue for bacteria and green for plants/algae as follows: the mevalonate pathway (MVA), the 2-C-methyl-d-erythritol-4-phosphate pathway (MEP), isopentenyl diphosphate (IPP), dimethylallyl diphosphate (DMAPP), farnesyl pyrophosphate synthase (IspA), geranyl pyrophosphate (GPP), geranylgeranyl diphosphate (GGPP), geranyl pyrophosphate synthase (GGPS), geranylgeranyl diphosphate synthase (CrtE/GGPPS), phytoene synthase (CrtB/PSY), phytoene desaturase (CrtI/PDS), lycopene-cyclase (CrtY/LCY), astaxanthin synthase (CrtS), the auxiliary cytochrome P450 reductase (CrtR), β-carotenoids ketoacidase (CrtW), β- Carotene hydroxylase (CrtZ), and carotenoids β-ring 4-dehydrogenase (CBFD).
2.1. Lycopene
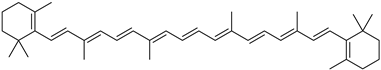
Lycopene is a tetraterpenoid pigment and a needle crystal [18] found in various plants, including tomatoes [19], citrus fruits [20], watermelons [21], carrots [22], and grapefruits [23]. It is insoluble in water but soluble in chloroform and benzene oil. Its molecular weight is 536.85 u(unit), and its chemical formula is C40H56. The molecular structure of lycopene comprises a straight-chain hydrocarbon with 11 conjugated double bonds and 2 non-conjugated double bonds, as shown in Table 1. Thus, lycopene is highly sensitive to light, and exposure can significantly accelerate its degradation. Additionally, lycopene is unstable at high temperatures and is prone to oxidation and isomerization. While it is relatively stable under aerobic conditions, oxidation reactions may occur in high-oxygen environments.

Table 1.
Molecular structure and biological activity of carotenoids.
In 1983, researchers demonstrated that lycopene has excellent antioxidant properties and free radical scavenging capabilities [24,52]. Subsequent research has revealed that lycopene also possesses anti-inflammatory [25], anticancer [26], and cardiovascular disease prevention effects [27], making it a valuable resource in medicine, biology, animal husbandry, and other fields. Leh et al. found that, due to the antioxidant effects of lycopene, taking lycopene supplements reduced oxidative stress in the pancreatic β-cells, which are responsible for the production of insulin, resulting in a 37% increase in insulin levels in diabetic patients [29]. Furthermore, oxidative stress was reduced in patients with type II diabetes. Lycopene has neuroprotective benefits and has been used successfully to treat Alzheimer’s disease [28]. It has also been shown to suppress the proliferation of gastric cancer cells without affecting normal gastric epithelial cells, making it a promising drug for the targeted therapy of gastric cancer [30].
Lycopene can also serve as a feed supplement in animal production. It has been shown to improve immunity, metabolism, and reproductive function in animals [53]. For example, the inclusion of 200 mg/kg of lycopene in pig feed resulted in a significantly improved muscle redness value (4.10 to 4.58), intramuscular fat content (3.26% to 3.98%), and crude protein content (22.93% to 25.30%) of pork [31].
2.2. β-Carotene
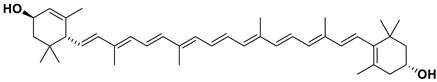
β-carotene is an important source of vitamin A for humans [54]. It enhances the absorption of vitamin A and is commonly found in plants, such as carrots and citrus fruits, which gives them an attractive orange or yellow hue. β-carotene is insoluble in water, propylene glycol, glycerol, acids, or alkalis. It is soluble in ether, petroleum ether, cyclohexane, and vegetable oils and is easily soluble in carbon disulfide, benzene, and chloroform [55].
As indicated in Table 1, β-carotene functions as a lipid radical scavenger and singlet oxygen quencher [56]. When contrasted with astaxanthin and lycopene, β-carotenoids demonstrate a notably lower sensitivity to light. Nonetheless, exposure to ultraviolet light can still hasten their degradation. Similar to other carotenoids, β-carotenoids are unstable at high temperatures and are prone to oxidation and isomerization. Under aerobic conditions, β-carotenoids tend to undergo oxidation reactions in high-oxygen environments.
In medicine, research has shown that the moderate supplement of β-carotene can reduce the risk of coronary heart disease [57] and certain types of cancer [58], boost the immune system [33] and prevent age-related macular degeneration [34]. Nimbalkar observed that the continuous treatment of rats with 20 mg/kg of β-carotene for 14 days reduced all indications of type II diabetes mellitus [32]. This research suggests that β-carotene improves glycometabolism and oxidative state in diabetic rats. However, the integration of β-carotene into various food systems is limited due to its low water solubility and sensitivity to breakdown under light, heat, and oxygen conditions [59]. Encapsulation technology has been employed to improve the stability of β-carotene, with various carriers used to encapsulate it and increase its stability. The highest β-carotene encapsulation efficiency achieved was 92% [60], allowing for its use in a wider variety of applications.
2.3. Astaxanthin
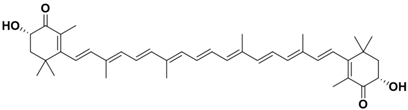
Astaxanthin exhibits antioxidant, antibacterial, and antiapoptotic properties and is also effective in scavenging free radicals [61]. Astaxanthin can be extracted from animal sources, while other carotenoids are less abundant in animals or humans to be useful for extraction. Unlike lycopene and β-carotene, which are composed solely of carbon and hydrogen, astaxanthin also contains oxygen-containing functional groups [62] (referred to Table 1). Its chemical formula is C40H52O4, and its molecular structure features a conjugated double-bond chain with an unsaturated ketone and a hydroxyl at the end of the chain. These functional groups can attract or donate unpaired electrons to free radicals, scavenging them and exhibiting antioxidant properties [35].
Astaxanthin, like other carotenoids, is particularly sensitive to light, heat, and oxygen. Astaxanthin is insoluble in water but soluble in fats, chloroform, acetone, benzene, and most other organic solvents. In 1949, astaxanthin was isolated in small quantities from marine crustacean shells, signifying its initial discovery [63]. According to Nair’s study, astaxanthin can inhibit NF-κB or JAK/STAT pathways, suppressing pro-inflammatory cytokine production [64]. Hirotaka et al. assessed the superoxide scavenging activity before and after astaxanthin supplementation, observing an increase in superoxide dismutase activity from approximately 18.2 U/mL to 19.9 U/mL over two weeks. Additionally, skin biopsies performed after UV irradiation with collagen hydrolysate supplemented with 2 mg/day of astaxanthin showed a significantly increased expression of type I procollagen in the astaxanthin group compared to the placebo group [36,37]. Liu et al. found that appropriate astaxanthin supplementation (4–12 mg/day) for three consecutive months significantly increased the production of type I procollagen in healthy individuals. Due to these physiological functions of astaxanthin, the appropriate supplementation of astaxanthin in the diet can prevent glaucoma (through antioxidant defense) [65], alleviate symptoms of associated vascular litis [66], and prevent cerebral hemorrhage (by inhibiting reactive oxygen species and activating antioxidant defenses) [67].
Astaxanthin is primarily found in Haematococcus pluvialis, Chlorella, Cladophora aegagropila, and Phaffia rhodozyma [68]. It has various applications, including in aquaculture [69] and the synthesis of antimicrobial drugs [70]. However, due to its scarcity and high cost, the extraction and purification of natural astaxanthin can be challenging, which limits its applicability.
2.4. Other Important Carotenoids
In addition to well-known carotenoids, such as lycopene, β-carotene, and astaxanthin, there are many other equally important carotenoids, such as lutein, zeaxanthin, and canthaxanthin. These carotenoids are also rich in nutritional value, possessing anti-inflammatory and antioxidant properties. For tabulated data, please refer to Table 1. Lutein and zeaxanthin are the only carotenoids that accumulate in the retina, particularly in the macula and are known as macular pigments. Their antioxidant properties are positively associated with brain and eye development and protection [41,49]. Additionally, lutein and zeaxanthin have neuroprotective properties and may be used to treat neurodegenerative diseases [43]. Canthaxanthin can improve animals’ resistance to hypoxic stress and promote their reproductive development by increasing the secretion of reproductive hormones in hens [46]. It can also protect ram sperm from oxidative stress [44].
However, the market demand for lutein, zeaxanthin, and canthaxanthin is limited compared to that for lycopene, β-carotene, and astaxanthin, which are more commonly used. Furthermore, it is worth noting that lutein, zeaxanthin, and canthaxanthin constitute a lower percentage of the plant or microbial content compared to the other three carotenoids. This is due to the challenges involved in extracting and isolating them, which often results in the extraction of carotenoids in their composite form. Consequently, there have been limited studies on the extraction of lutein, zeaxanthin, and canthaxanthin in isolation.
3. Extraction of Carotenoids
The characteristics of carotenoids require more careful consideration of solvent and extraction methods during the process of extraction and separation. This makes extracting significant quantities of carotenoids to fulfill human demand problematic, resulting in significant losses, particularly during the extraction process. Carotenoids are primarily extracted from three sources: plants, fungi, and bacteria, with a small portion derived from animals. Cell wall destruction is required prior to extraction as carotenoids are found largely in plant cytoplasm or within bacterial and fungal cells. The yield of extracted carotenoids has significantly increased over time due to technical developments and improvements in extraction processes.
Carotenoid extraction can be divided into two methods: extraction from natural resources and extraction after microbial fermentation or synthesis. The extraction of carotenoids from natural sources like carrots and tomato peels typically involves pretreatment steps, such as removing dirt and impurities, cutting, juicing, pureeing, and filtering, followed by mechanical and chemical extraction methods. The extracted carotenoids are then dried and quantitatively analyzed. Microbial biosynthesis requires the development of engineered strains capable of producing carotenoids or the screening and isolation of naturally carotenoid-producing strains. Following qualitative analysis, these strains are cultured in a fermenter to produce large quantities of carotenoids, which are then extracted using mechanical and chemical methods. The resulting product is then dried and subjected to quantitative analysis, as shown in Figure 3.
Figure 3.
Carotenoid extraction process.
In 1966, carotenoids and chlorophyll were extracted, isolated, and quantitatively measured from leaves and algae using thin-layer chromatography [71]. However, chromatography can only extract a limited number of carotenoids, which cannot be used as raw materials for further processing. By 1970, the microbial biosynthesis of carotenoids had been discovered along with extraction and purification from natural sources, but the output was still insufficient to meet manufacturing needs [72].
The core databases, PubMed, Web of Science, and Sciencedirect, were used to search for terms associated with carotenoid extraction, such as ‘carotenoid extraction’, ‘extraction of fat-soluble substances’, and ‘assisted extraction’, among others. The selection criteria included the following: (1) original experimental research articles and related review articles; (2) articles written in English; and (3) papers released between 2013 and 2023. During the screening phase, articles lacking sufficient data or information, such as important methodological details, results, or references, were eliminated. The article’s title and abstract served as the first selection criteria. Potentially appropriate full-text publications were then found and assessed for eventual inclusion in the research.
The methods for extraction can be categorized into several types, including organic solvent extraction, green solvent extraction, microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE), pressurized liquid-assisted extraction (PLE), pulsed electric field-assisted extraction (PEF), and enzyme-assisted extraction (EAE).
Figure 4 presents an analysis of the research articles searched, revealing that 40% of the literature used the solvent method for extracting carotenoids. The remaining 41% employed other assisted extractions such as EAE, SFE, and UAE. These four extraction methods used show promise for the development and use of carotenoid extraction. The processes require further investigation and improvement to increase extraction efficiency and reduce costs. The aim of this work is to provide a comprehensive overview of the research methodology and findings related to the industrial application of carotenoids.
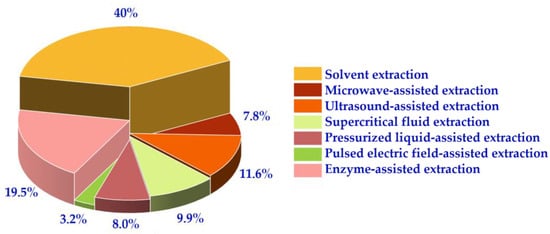
Figure 4.
The proportion of various extraction methods in the core database.
3.1. Solvent Extraction
One of the most common and traditional extraction methods is organic solvent extraction. This method involves dissolving carotenoids in organic solvents such as ethyl acetate, methanol, or ethanol. There are two general categories for the extraction process: solid–liquid extraction and liquid–liquid extraction. The nature of the extract determines which extraction agent is optimal for organic solvent extraction. Selecting a solvent with low solubility for contaminants and high solubility for carotenoids is crucial. This can be accomplished through methods such as Soxhlet extraction, crushing, and shock extraction. Additionally, liquid–liquid extraction can be employed to separate specific components from a solution by utilizing their distinct partition coefficients in two immiscible reagents, allowing them to transfer from one solvent phase to another and separate from other ingredients. Compared to solid–liquid extraction, liquid–liquid extraction is simpler to operate and facilitates automated high-throughput procedures. However, its efficacy is lower, necessitating multiple extractions to obtain highly pure compounds. In contrast, efficient solid–liquid extractions require specialized equipment, leading to higher costs and more complex procedural stages.
Table 2 indicates that Poojary et al. successfully recovered lycopene from tomato waste with a high purity level (98.3%) and recovery rate (94.7%) using a 1:3 (v/v) n-hexane/acetone concentration [19]. Additionally, carotenoids were extracted from the pericarp of wood betel seed using ethyl acetate, yielding 271 mg/100 g (dry weight) after 150 min [73].

Table 2.
Extraction methods of several typical carotenoids.
However, traditional organic solvent extraction presents several drawbacks, including lengthy extraction times, low efficiency, and environmental concerns. In 2012, Chemat et al. introduced the concept of ‘green extraction of natural products’, which involves the use of alternative solvents and renewable resources, along with six principles for the extraction of natural products. Since then, there has been an increasing focus on developing alternative, ecologically friendly solvents to replace conventional chemical solvents in the separation and extraction of bioactive components. Water, ionic liquids, deep eutectic solvents (DESs), and supercritical CO2 have become important members of the green solvent family [103]. Compared to traditional organic solvents, DESs represent a new class of green solvents with advantages such as easy production, high biocompatibility, excellent biodegradability, efficient solvation capability, strong thermal stability, and low volatility [104]. After blending eucalyptus oil with menthol for 20 min, the yield of extracted carotenoids reached approximately 359.3 mg/100 g (fresh weight) [74]. Ghany et al. fermented the exoskeletons of Penaeus japonicus and Penaeus semisulcatus with Saccharomyces cerevisiae, obtaining astaxanthin at a concentration of 8.5 mg/g after extraction with a 1:1 (v/v) hexane/acetone mixture [75].
The oleaginous yeast Rhodosporidium toruloides can convert low-cost carbon sources like cornstarch and tapioca starch into high-value carotenoids, which have diverse applications across industries, including medicine, food, livestock, and agriculture. However, R. toruloides also produces a significant amount of lipids during carotenoid synthesis, which increases the complexity and cost of isolating the metabolites. Most research focuses on two aspects as follows: enhancing the proportion of carotenoids in microbial metabolites through biosynthesis and improving extraction procedures to efficiently separate carotenoids from mixed metabolites. Liu et al. achieved a recovery rate of 78.7% for high-purity carotenoids from R. toruloides after saponification with an aqueous KOH solution, extraction with alcohol and hexane, thereby reducing the cost of extraction from oleaginous yeast [76].
FAEEs (fatty acid ethyl esters) also serves as green organic solvents that can be applied in carotenoid extraction. The FAEE method and ultrasonic-assisted extraction were employed to extract β-carotene and lycopene from tomato waste. The FAEE method alone achieved a β-carotene extraction concentration of 38.3 mg/100 g/15 min, which increased to 49.7 mg/100 g/15 min with the combined use of FAEE and UAE. For lycopene, the maximum concentration of 101.4 mg/100 g was reached in just 6 min using a combination of FAEE and UAE, compared to 15 min with FAEE alone [77]. Research has demonstrated that supramolecular solvents (SUPRAS) can extract up to 1 mg/g (dry weight) of total carotenoids from Scenedesmus sp. within five minutes [78]. In comparison to the traditional organic solvent extraction presented in Table 2, green solvents like SUPRAS and FAEE offer certain advantages in terms of extraction efficiency and speed.
Enhancing the recovery rate of solvent extraction and reusing the solvents can significantly reduce the environmental pollution caused by organic solvents while also decreasing the cost of extraction. The recovery of solvents can be achieved through separation technologies or by employing renewable solvents. These include ethanolic carboxylic acids mixtures and ethyl acetate [105] and the use of eutectic solvents such as menthol [106], ethyl acetate [107], ethyl lactate [108], and norflurane [109], which can also facilitate the recycling of solvents post-extraction.
Furthermore, due to the predominant cellular localization of carotenoids, extraction using only organic solvents proves inefficient. Therefore, various physical methods are being employed as adjuncts to enhance extraction efficiency.
3.2. Ultrasound-Assisted Extraction
Ultrasound enhances extraction by generating mechanical vibrations that produce strong shear forces and convert internal friction into heat. This raises temperatures, reducing the viscosity of incompressible fluids or increasing that of compressible ones, thereby rupturing plant cell walls and improving their dispersion in the solvent. This increases the contact area and mass transfer efficiency between the solvent and the target substance. Compared to other extraction procedures, ultrasonic extraction does not harm the structure or activity of carotenoids. It is compatible with most organic solvents, regardless of the polarity of the solution composition or molecular weight. This property can significantly reduce the extraction time of carotenoids, preserve their biological activity, and improve product quality [110]. Furthermore, research into the extraction of other antioxidant substances has revealed that UAE can significantly reduce the impact on the bioactivity of extracted compounds, owing to its short operational time and lower working temperature [111].
Table 2 shows that Luengo et al. used solvent-assisted pressure UAE with a hexane/ethanol mixture to increase the extraction of carotenoids from tomato waste. They were able to reduce the quantity of hexane to 25% without affecting the extraction rate [79]. Goula et al. employed ultrasonic extraction to extract carotenoids from pomegranate wastes and achieved a recovery rate of 93.8% (0.3255 mg of carotenoids/100 g of dry peels) [80]. Under the condition of 2:1 (v:v) ethanol/hexane, the maximum carotenoid extracted from Chlorella vulgaris and Microalgae Purpuricus porphyrinaceus was 6435.60 µg/g by UAE [81]. Gu et al. extracted carotenoids from Rhodobacter sphaeroides by UAE and achieved approximately 664 µg/g for 40 min [112]. UAE, combined with menthol and camphor (a combination of DES), increased the carotenoid extraction rate from 163.5 mg/100 g (fresh weight) to 653.5 mg/100 g (fresh weight) compared with DES alone [82]. Research by Elsa et al. found that the yield and purity of carotenoids extracted from Sechium edule using UAE were higher than those obtained through maceration and MAE [113]. Hemanta et al. optimized UAE for passion fruit peel using olive oil as a solvent, achieving an extraction rate of 91.4% compared to 86.9% with MAE under the same conditions, and when comparing the energy density of both methods, UAE proved superior [114]. The choice of solvent and the solvent-to-solid ratio in UAE were also significant factors affecting extraction efficiency and bioactivity [115]. H. Hadiyanto maximized the yield of β-carotene to 1.38 µg/mL by adjusting the solvent-to-solid ratio to 1:6 [116].
Researchers have started investigating the use of UAE for the direct extraction of carotenoids or incorporating a single organic solvent to mitigate the environmental impact and potential hazards associated with residual organic solvents under the framework of green environmental protection. They used response surface methods to optimize extraction conditions with 51% ethanol and recovered a total of 31.82 µg/g of carotenoids from carrot waste residue for 17 min at 32 °C. The study found that the sample contained 14.89 µg/g of β-carotene, 5.77 µg/g of lutein, and 2.65 µg/g of lycopene [83]. The researchers used ultrasound to break the cell structure of Rhodotorula glutinis in an aqueous medium, resulting in the formation of small droplets of carotenoids coated with phospholipid, creating a uniform microemulsion with an average particle size of 230 nm. The carotenoid recovery rate of R. glutinis was 82%, and the carotenoid content obtained was approximately 25 mg/L [84]. The use of UAE and MAE technology enabled the extraction of carotenoids (26.91–34.35 mg/100 g) from Hippophae rhamnoides (Sea buckthorn) pomace, using edible oils (corn and olive oils) as a green solvent instead of organic solvents [85].
3.3. Supercritical Fluid Extraction
The SFE method utilizes the distinctive characteristics of supercritical fluid to extract carotenoids. Supercritical fluid exhibits properties of both a gas and a liquid and in its supercritical state, its density and solving properties increase, enabling the efficient extraction of carotenoids. Other co-solvents can be added to further enhance the extraction process. Many critical fluids, such as carbon dioxide, ammonia, and water, are non-toxic and do not cause severe environmental pollution. Moreover, they can be easily recovered after use through simple processes of pressure reduction or temperature decrease, thereby minimizing environmental impact. Supercritical fluid extraction technology not only enhances extraction efficiency and stabilizes carotenoids [117] but also reduces the environmental hazards associated with toxic reagents [118].
Table 2 shows the extraction of lycopene from grapefruit using supercritical carbon dioxide with rice bran oil as a co-solvent, as demonstrated by Dhakane-Lad et al. The extraction efficiency of lycopene was 70.52% [86]. Sun used rapeseed oil as a co-solvent, resulting in a two-fold increase in the extraction rate of β-carotene and a four-fold increase in lutein extraction compared to the supercritical carbon dioxide method alone [87]. The CO2-SFE approach at 40 °C and a constant CO2 flow rate (6 mL/minute) was used to extract the total carotenoids from Rhodotorula spp., red yeast, resulting in an average extraction rate of about 68.0 µg/g yeast (dry weight) [88]. Popescu et al. used camelina oil as a modifier to extract 203.59 mg/100 g of carotenoids from tomatoes using SFE at 450 bar and 70 °C [89].
Under the conditions of 318.15 K and 20 MPa, with a mass fraction of 5% ethanol, the number of carotenoids extracted from microalgae was 25 g/kg. This is a significant increase compared to the 6 g/kg extracted by supercritical carbon dioxide alone [90]. Additionally, the recovery rate of β-carotene in Dunaliella salina by supercritical carbon dioxide extraction was 90% under conditions of 500 bar, 70 °C, and 10 wt% ethanol as a co-solvent, resulting in reduced solvent costs [91]. Similarly, Xie et al. used ethanol-optimized CO2-SFE to extract about 421 μg/g of astaxanthin from flaxseed seeds under response surface optimization conditions at 41.6 MPa, 36.6 °C, and a 42.0% ethanol concentration [92]. However, Romano et al. found that reducing the concentration of ethanol to 10% avoided a significant decrease in the extraction number of carotenoids from tomato waste when combined with CO2-SFE [119]. These studies suggest that optimizing conditions for cooperative-assisted extraction using various methods is critical for extracting carotenoids.
3.4. Enzyme-Assisted Extraction
The EAE method utilizes enzyme-specific catalysis to selectively extract target molecules. Carotenoids are present in plant and yeast cells, with cell walls consisting mainly of cellulose, hemicellulose, and other structurally dense components. Mechanical methods can be used to break down the cell wall in combination with biological enzymes to enzymatically degrade the plant or yeast cell wall and extract carotenoids from the cell. The EAE technique employs mild reaction conditions and maximizes product activity [120]. Currently, the enzymatic extraction of carotenoids primarily involves the use of enzymes to break down cell walls, facilitating the release of carotenoids after the cell walls are ruptured. The enzymes currently employed for carotenoid extraction include pectinases, cellulases, and hemicellulases, which are cell wall-degrading enzymes [121]. Research by Homa et al. has revealed that the concentration of pectinase enzyme affects both the extraction of β-carotene and the antioxidant activity of the extracted β-carotene [122].
As shown in Table 2, the combination of pectinase and cellulase enzymes with ethyl lactate, due to ethyl lactate as a solvent, more easily penetrates moist raw materials, aiding in the enzymatic hydrolysis of plant cell walls. This increased the carotenoid content by 127 mg/kg (dry weight) and lycopene by 89.4 mg/kg (dry weight). This method is 6–10 times more efficient than conventional extraction methods [93]. Barzana et al. achieved a 97% carotenoid recovery under optimal conditions by recovering carotenoids from 80 L of pulverized Tagetes erecta (marigold) using simultaneous enzyme treatment and solvent extraction [94]. Lysozyme can also be used to extract carotenoids from Rhodococcus sp., although the extraction rate was only around 19% (25.52–33.40 μg/g) [95]. The ‘Stela’ tomato, commonly used in the canning industry, has waste tomato skins containing a high concentration of carotenoids. Using a mixture of cellulase (100 U/g) and endoxylanase (400 U/g) at 50 °C, researchers obtained total carotenoids (55.15 mg/100 g dry weight), β-carotene (35.85 mg/100 g dry weight), and lycopene (15.44 mg/100 g dry weight) [96]. The extraction efficiency of β-carotene from pumpkin was 61.75% when using a cellulase/pectinase ratio of 0.97 w/w at 42 °C for 92 min at pH 4.8 [123]. By combining EAE with a green solvent, carotenoid yield can be increased. In one study, a green organic solvent, 1:2 (v/v) menthol/lactic acid, was used to extract carotenoids from sunflower waste using a multi-enzyme complex under optimal conditions, yielding 1449 mg/100 g [98]. Dehydrated tomato skins were treated with rice bran oil and 1.4% Viscozyme L, which was a blend of β-glucanases, pectinases, hemicellulases, and xylanases, and incubated at 52 °C for 92 min, yielding a lycopene concentration of 399.6 mg/100 g [99].
3.5. Other Extraction Methods
Researchers are exploring novel extraction technologies such as PLE, which combines organic solvents to extract β-carotene, achieving a recovery rate of approximately 80% at high temperature and pressure for 20 min [100]. Experiments by Kwang et al. have shown that high-temperature PLE reduces the formation of harmful chlorophyll derivatives [124].
PEF, as an innovative technology, induces cell membrane electroporation, enabling the efficient extraction of intracellular antioxidant chemicals like carotenoids (see Table 2). Studies have shown that PEF intervention can increase the recovery rate of carotenoids to 85–90% [125]. In a study by Martínez et al., carotenoids were extracted from mucilaginous R. glutinis at 267 µg/g (dry weight) with an extraction efficiency of 80% using ethanol-assisted PEF [101]. Similarly, Georgiopoulou et al. extracted carotenoids from Chlorella vulgaris using MAE at 60 °C and 300 watts for 14 min. The additional amount of carotene (lycopene, β-carotene, and astaxanthin) was 7.06 mg/g, while the total carotenoids extracted amounted to 24.88 mg/g [102].
PEF technology has been employed for the extraction of natural compounds. Pre-treating raw materials with PEF can enhance the extraction efficiency of lycopene in subsequent solutions by 27–37% [126]. Ana et al. combined PEF with Accelerated Solvent Extraction to improve the recovery rate of astaxanthin [127]. As a sustainable extraction method, PEF not only increases extraction efficiency (39%) but also reduces the percentage of hexane in a hexane/ethanol mixture, thereby lessening environmental pressure [128].
Recent reports indicate that combining PEF and PLE technologies can increase the extraction rate and antioxidant capacity of antioxidant compounds, including carotenoids, thus enhancing their quality [129]. The integration of various extraction methods has become a trend for improving extraction efficiency.
4. Conclusions
The study of carotenoids, including lycopene, β-carotene, and astaxanthin, has led to an increased demand for high yield and purity in their extraction. This has necessitated improved extraction methods and the use of more environmentally friendly solvents.
The traditional extraction of natural compounds often requires large volumes of organic solvents, which can cause significant environmental damage. The excessive use of organic solvents also can reduce the quality and safety of the final carotenoid products, thereby limiting their potential applications. The adoption of green solvents as an alternative to traditional organic solvents can mitigate environmental impacts and enhance product safety. However, current green solvent systems are not sufficiently developed for widespread industrial adoption, with limited selectivity. Identifying an effective green solvent requires extensive experimentation to determine its compatibility, whether it should be used in combination and the optimal solute-to-solvent ratio. These factors currently constrain broader industrial implementation. Looking ahead, there is anticipation for the standardization of green solvent applications and the emergence of novel, faster, and more user-friendly alternatives.
Carotenoids are located within cells and require external stimuli to be released. The use of biological enzymes to break down cell walls offers high selectivity, gentle conditions, and ecological benefits. Physical technologies, such as ultrasound, microwaves, and a pulsed electric field, aid in the separation and extraction of carotenoids. These methods not only increase the extraction rate but also reduce the environmental impact of solvents. However, physical approaches require sophisticated equipment and occupy significant space, which are considerations that must be taken into account during production.
However, current methods for extracting carotenoids make it difficult to isolate specific types, such as lycopene, β-carotene, and astaxanthin. Biological methods for synthesizing carotenoids are expensive and have low yields. To increase the number of target products in organisms, further in-depth study is required on the structure and properties of these common carotenoids, as well as the main limiting factors in their biosynthesis processes. Developing extraction procedures that are convenient, fast, environmentally friendly, and efficient is critical.
Author Contributions
Conceptualization, J.C., J.Y. (Jingyi Ye) and Y.J.; methodology, Y.J.; software, Y.J. and J.Y. (Jingyi Ye); validation, Y.J. and W.L.; formal analysis, J.Y. (Jingyi Ye), Y.J. and J.C.; resources, Y.H. (Yonghong Hu), W.Y., W.L., J.Z., J.J., J.C., M.Y., J.Y. (Jingwei Yang) and Y.Y.; writing—original draft preparation, Y.J.; writing—review and editing, J.C.; visualization, Y.J.; funding acquisition, Y.H. (Yonghong Hu), X.Z., Y.H. (Yadong Hu), W.Y., J.Z., J.J., J.Y. (Jingwei Yang) and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the the National Key Research and Development Program of China (Grant No. 2022YFD2101404), the Marine Science and Technology Innovation Project of Jiangsu Province (Grant Nos. JSZRHYKJ202210 and JSZRHYKJ202309), the Jiangsu Innovation Center of Marine Bioresource (Grant No. 2023YHTZZZ02), and the Jiangsu Funding Program for Excellent Postdoctoral Talent (Grant No. 2024ZB468).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Yadong Hu and Xinghu Zhou was employed by the company Jiangsu Coast Development Investment Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships.
Abbreviation
UV, ultraviolet; GPP, geranyl diphosphate; GGPP, geranylgeranyl diphosphate; MVA, the mevalonate pathway; MEP, the 2-C-methyl-d-erythritol-4-phosphate pathway; MAE, microwave-assisted extraction; UAE, ultrasound-assisted extraction; SFE, super-critical fluid extraction; PLE, pressurized liquid-assisted extraction; PEF, pulsed electric field-assisted extraction; EAE, enzyme assisted extraction; DES, deep eutectic solvent. FAEE, fatty acid ethyl esters; and SUPRAS, supramolecular solvents.
References
- Starska-Kowarska, K. Dietary Carotenoids in Head and Neck Cancer—Molecular and Clinical Implications. Nutrients 2022, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- García-Romera, M.-C.; Silva-Viguera, M.-C.; López-Izquierdo, I.; López-Muñoz, A.; Capote-Puente, R.; Gargallo-Martínez, B. Effect of macular pigment carotenoids on cognitive functions: A systematic review. Physiol. Behav. 2022, 254, 113891. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Al’Abri, I.S.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and Their Health Benefits as Derived via Their Interactions with Gut Microbiota. Adv. Nutr. 2023, 14, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Muni, M.; Mitra, S.; Emran, T.B.; Chandran, D.; Das, R.; Rauf, A.; Safi, S.Z.; Chidambaram, K.; Dhawan, M. Recent advances in respiratory diseases: Dietary carotenoids as choice of therapeutics. Biomed. Pharmacother. 2022, 155, 113786. [Google Scholar] [CrossRef]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients 2021, 13, 1949. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Rodríguez-Bernaldo de Quirós, A.; Costa, H.S. Analysis of carotenoids in vegetable and plasma samples: A review. J. Food Compos. Anal. 2006, 19, 97–111. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Li, Z.; McClements, D.J.; Xiao, H. Encapsulation of carotenoids in emulsion-based delivery systems: Enhancement of β-carotene water-dispersibility and chemical stability. Food Hydrocoll. 2017, 69, 49–55. [Google Scholar] [CrossRef]
- Pascual-Pineda, L.A.; Flores-Andrade, E.; Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Beristain, C.I.; Gutiérrez-López, G.F.; Azuara, E. Micropores and Their Relationship with Carotenoids Stability: A New Tool to Study Preservation of Solid Foods. Food Bioprocess. Technol. 2013, 7, 1160–1170. [Google Scholar] [CrossRef]
- Fact, M.R. Carotenoid Market Size and Share Industry Statistics—2034. 2023. Available online: https://www.factmr.com/report/1196/carotenoids-market (accessed on 20 March 2024).
- Wang, Y.H.; Zhang, R.R.; Yin, Y.; Tan, G.F.; Wang, G.L.; Liu, H.; Zhuang, J.; Zhang, J.; Zhuang, F.Y.; Xiong, A.S. Advances in engineering the production of the natural red pigment lycopene: A systematic review from a biotechnology perspective. J. Adv. Res. 2023, 46, 31–47. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Fu, J.; Yang, Q.; Feng, L. High-throughput screening of lycopene-overproducing mutants of Blakeslea trispora by combining ARTP mutation with microtiter plate cultivation and transcriptional changes revealed by RNA-seq. Biochem. Eng. J. 2020, 161, 107664. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, X.; Wang, H.; Jiang, Y.; Jiang, W.; Zhang, W.; Jiang, M.; Xin, F. Biosynthesis of astaxanthin by using industrial yeast. Biofuels Bioprod. Biorefin. 2022, 17, 602–615. [Google Scholar] [CrossRef]
- Fang, N.; Wang, C.; Liu, X.; Zhao, X.; Liu, Y.; Liu, X.; Du, Y.; Zhang, Z.; Zhang, H. De novo synthesis of astaxanthin: From organisms to genes. Trends Food Sci. Technol. 2019, 92, 162–171. [Google Scholar] [CrossRef]
- Li, L.; Tang, X.; Luo, Y.; Hu, X.; Ren, L. Accumulation and conversion of beta-carotene and astaxanthin induced by abiotic stresses in Schizochytrium sp. Bioprocess. Biosyst. Eng. 2022, 45, 911–920. [Google Scholar] [CrossRef]
- Shi, J.; Maguer, M.L. Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Passamonti, P. Optimization of extraction of high purity all-trans-lycopene from tomato pulp waste. Food Chem. 2015, 188, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Suwen, L.; Yin, Z.; Xiongjie, Z.; Kaijie, Z.; Qiang, X.; Xiuxin, D. Isolation and Functional Characterization of a Lycopene β-cyclase Gene Promoter from Citrus. Front. Plant Sci. 2016, 7, 1367. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Sharmin, T.; Ahmed, N.; Hossain, A.; Hosain, M.M.; Mondal, S.C.; Haque, M.R.; Almas, M.; Siddik, M.A.B. Extraction of bioactive compound from some fruits and vegetables (pomegranate peel, carrot and tomato). Am. J. Food Nutr. 2016, 4, 8–19. [Google Scholar] [CrossRef]
- Sadler, G.D.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Agarwal, A.; Shen, M.; Agarwal, S.; Rao, A.V. Lycopene Content of Tomato Products: Its Stability, Bioavailability and In Vivo Antioxidant Properties. J. Med. Food 2001, 4, 9–15. [Google Scholar] [CrossRef]
- Kang, J.; Li, Y.; Ma, Z.; Wang, Y.; Zhu, W.; Jiang, G. Protective effects of lycopene against zearalenone-induced reproductive toxicity in early pregnancy through anti-inflammatory, antioxidant and anti-apoptotic effects. Food Chem. Toxicol. 2023, 179, 113936. [Google Scholar] [CrossRef]
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene Treatment of Prostate Cancer Cell Lines Inhibits Adhesion and Migration Properties of the Cells. Int. J. Med. Sci. 2014, 11, 948–954. [Google Scholar] [CrossRef][Green Version]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases—A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 56, 1868–1879. [Google Scholar] [CrossRef]
- Kapoor, B.; Gulati, M.; Rani, P.; Kochhar, R.S.; Atanasov, A.G.; Gupta, R.; Sharma, D.; Kapoor, D. Lycopene: Sojourn from kitchen to an effective therapy in Alzheimer’s disease. BioFactors 2022, 49, 208–227. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, R.; Yang, M.; Liu, W.; Tong, Z. Lycopene suppresses gastric cancer cell growth without affecting normal gastric epithelial cells. J. Nutr. Biochem. 2023, 116, 109313. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef]
- Nimbalkar, V.; Joshi, U.; Shinde, S.; Pawar, G. In-vivo and in-vitro evaluation of therapeutic potential of β-Carotene in diabetes. J. Diabetes Metab. Disord. 2021, 20, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A. β-carotene and the immune response. Proc. Nutr. Soc. 1991, 50, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.; Yildiz, F. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with Dietary Astaxanthin Combined with Collagen Hydrolysate Improves Facial Elasticity and Decreases Matrix Metalloproteinase-1 and -12 Expression: A Comparative Study with Placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Hirotaka, H.; Kiyomi, A.; Takahashi, J.; Chikuda, M. The effect of astaxanthin on vascular endothelial growth factor (VEGF) levels and peroxidation reactions in the aqueous humor. J. Clin. Biochem. Nutr. 2016, 59, 10–15. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Zhou, L.; Zhang, J.; Chen, Z.; Xiao, J.; Cao, Y.; Xiao, H. Recent advances in health benefits and bioavailability of dietary astaxanthin and its isomers. Food Chem. 2023, 404, 134605. [Google Scholar] [CrossRef]
- Shi, H.; Deng, X.; Ji, X.; Liu, N.; Cai, H. Sources, dynamics in vivo, and application of astaxanthin and lutein in laying hens: A review. Anim. Nutr. 2023, 13, 324–333. [Google Scholar] [CrossRef]
- Ettefaghdoost, M.; Haghighi, H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish. Shellfish. Immunol. 2021, 115, 86–94. [Google Scholar] [CrossRef]
- Gazzolo, D.; Picone, S.; Gaiero, A.; Bellettato, M.; Montrone, G.; Riccobene, F.; Lista, G.; Pellegrini, G. Early Pediatric Benefit of Lutein for Maturing Eyes and Brain—An Overview. Nutrients 2021, 13, 3239. [Google Scholar] [CrossRef]
- Różanowska, M.B.; Czuba-Pelech, B.; Landrum, J.T.; Różanowski, B. Comparison of Antioxidant Properties of Dehydrolutein with Lutein and Zeaxanthin, and their Effects on Cultured Retinal Pigment Epithelial Cells. Antioxidants 2021, 10, 753. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, S.K.; Mehta, J.S.; Kumar, T.; Prakash, C.; Talluri, T.R.; Pal, R.S.; Kumar, A. Melatonin and canthaxanthin enhances sperm viability and protect ram spermatozoa from oxidative stress during liquid storage at 4 °C. Andrologia 2021, 54, 14304. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, S.; Wang, W.; Wu, M.; Yi, G.; Huang, X. Effects of dietary different canthaxanthin levels on growth performance, antioxidant capacity, biochemical and immune-physiological parameters of white shrimp (Litopenaeus vannamei). Aquaculture 2022, 556, 738276. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.; Liu, Y.; Zhuang, Y.; Yan, H.; Xiao, M.; Zhang, L.; An, L. Dietary Canthaxanthin Supplementation Promotes the Laying Rate and Follicular Development of Huaixiang Hens. Biology 2023, 12, 1375. [Google Scholar] [CrossRef]
- Al-thepyani, M.; Algarni, S.; Gashlan, H.; Elzubier, M.; Baz, L. Evaluation of the Anti-Obesity Effect of Zeaxanthin and Exercise in HFD-Induced Obese Rats. Nutrients 2022, 14, 4944. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Xie, J.; Liu, H.; Yin, W.; Ge, S.; Jin, Z.; Zheng, M.; Cai, D.; Liu, M.; Liu, J. Zeaxanthin remodels cytoplasmic lipid droplets via β3-adrenergic receptor signaling and enhances perilipin 5-mediated lipid droplet-mitochondrion interactions in adipocytes. Food Funct. 2022, 13, 8892–8906. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; Liu, H.; Jin, Z.; Guan, F.; Ge, S.; Yan, J.; Zheng, M.; Cai, D.; Liu, J. Zeaxanthin ameliorates obesity by activating the β3-adrenergic receptor to stimulate inguinal fat thermogenesis and modulating the gut microbiota. Food Funct. 2021, 12, 12734–12750. [Google Scholar] [CrossRef]
- Javor, T.; Bata, M.; Lovász, L.; Moron, F.; Nagy, L.; Patty, I.; Mózsik, G. Gastric cytoprotective effects of vitamin A and other carotenoids. Int. J. Tissue React. 1983, 5, 289–296. [Google Scholar] [PubMed]
- Chen, J.; Cao, X.; Huang, Z.; Chen, X.; Zou, T.; You, J. Research Progress on Lycopene in Swine and Poultry Nutrition: An Update. Animals 2023, 13, 883. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.-A.; Biesalski, H.K. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Treszczanowicz, T.; Treszczanowicz, A.J.; Kasprzycka-Guttman, T.; Kulesza, A. Solubility of β-carotene in binary solvents formed by some hydrocarbons with ketones. J. Chem. Eng. Data 2001, 46, 792–794. [Google Scholar] [CrossRef]
- Paiva, S.A.R.; Russell, R.M. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Kritchevsky, S.B. β-Carotene, carotenoids and the prevention of coronary heart disease. J. Nutr. 1999, 129, 5–8. [Google Scholar] [CrossRef]
- Kordiak, J.; Bielec, F.; Jabłoński, S.; Pastuszak-Lewandoska, D. Role of β-Carotene in Lung Cancer Primary Chemoprevention: A Systematic Review with Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 1361. [Google Scholar] [CrossRef]
- Stutz, H.; Bresgen, N.; Eckl, P.M. Analytical tools for the analysis of β-carotene and its degradation products. Free Radic. Res. 2015, 49, 650–680. [Google Scholar] [CrossRef]
- Zarif, B.; Shabbir, S.; Shahid, R.; Noor, T.; Imran, M. Proteosomes based on milk phospholipids and proteins to enhance the stability and bioaccessibility of β-carotene. Food Chem. 2023, 429, 136841. [Google Scholar] [CrossRef]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant effects of astaxanthin in various diseases—A review. J. Mol. Pathophysiol. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Mori, J.; Yokoyama, H.; Sawada, T.; Miyashita, Y.; Nagata, K. Anti-Oxidative Properties of Astaxanthin and Related Compounds. Mol. Cryst. Liq. Cryst. 2013, 580, 52–57. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Srisukh, S. Some Observations on Astaxanthin Distribution in Marine Crustacea. Biochem. J. 1949, 45, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Ahirwar, A.; Singh, S.; Lodhi, R.; Lodhi, A.; Rai, A.; Jadhav, D.A.; Harish; Varjani, S.; Singh, G.; et al. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Mar. Drugs 2023, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. Protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J. Pharmacol. Sci. 2013, 123, 209–218. [Google Scholar] [CrossRef]
- Sun, R.L.; Shang, J.C.; Han, R.H.; Xing, G.Q. Protective effect of astaxanthin on ANCA-associated vasculitis. Int. Immunopharmacol. 2024, 132, 111928. [Google Scholar] [CrossRef]
- Pan, L.; Zhou, Y.; Li, X.F.; Wan, Q.J.; Yu, L.H. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res. Bull. 2017, 130, 211–220. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Shpigel, T.; Harris, L.G.; Schuster, R.; Lewis, E.C.; Lewitus, D.Y. Astaxanthin-based polymers as new antimicrobial compounds. Polym. Chem. 2017, 8, 4182–4189. [Google Scholar] [CrossRef]
- Hager, A.; Meyer-Bertenrath, T. Extraction and quantitative determination of carotenoids and chlorophylls of leaves, algae and isolated chloroplasts with the aid of thin-layer chromatography. Planta 1966, 69, 198–217. [Google Scholar] [CrossRef]
- Hammond, R.K.; White, D.C. Carotenoid Formation by Staphylococcus aureus. J. Bacteriol. 1970, 103, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Optimisation of extraction conditions for recovering carotenoids and antioxidant capacity from Gac peel using response surface methodology. Int. J. Food Sci. Technol. 2017, 52, 972–980. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Frígola, A.; Blesa, J. Improving carotenoid extraction, stability, and antioxidant activity from Citrus sinensis peels using green solvents. Eur. Food Res. Technol. 2023, 249, 2349–2361. [Google Scholar] [CrossRef]
- Abd El-Ghany, M.N.; Hamdi, S.A.; Elbaz, R.M.; Aloufi, A.S.; El Sayed, R.R.; Ghonaim, G.M.; Farahat, M.G. Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste. Fermentation 2023, 9, 505. [Google Scholar] [CrossRef]
- Liu, Z.; van den Berg, C.; Weusthuis, R.A.; Dragone, G.; Mussatto, S.I. Strategies for an improved extraction and separation of lipids and carotenoids from oleaginous yeast. Sep. Purif. Technol. 2021, 257, 117946. [Google Scholar] [CrossRef]
- Diacon, A.; Călinescu, I.; Vinatoru, M.; Chipurici, P.; Vlaicu, A.; Boscornea, A.C.; Mason, T.J. Fatty Acid Ethyl Esters (FAEE): A New, Green and Renewable Solvent for the Extraction of Carotenoids from Tomato Waste Products. Molecules 2021, 26, 4388. [Google Scholar] [CrossRef]
- Lara-Abia, S.; Welti-Chanes, J.; Cano, M.P. Effect of Ultrasound-Assisted Extraction of Carotenoids from Papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils. Molecules 2022, 27, 638. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Vintila, A.C.N.; Vlaicu, A.; Radu, E.; Ciltea-Udrescu, M.; Enascuta, E.C.; Banu, I.; Oprescu, E.-E. Evaluation of ultrasound assisted extraction of bioactive compounds from microalgae. J. Food Meas. Charact. 2022, 16, 2518–2526. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Radojčić-Redovniković, I.; Blesa, J.; Esteve, M.J. Using novel hydrophobic deep eutectic solvents to improve a sustainable carotenoid extraction from orange peels. Food Biosci. 2023, 53, 102570. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Lu, Z.; Zhang, J.; Abid, M.; Murtaza, M.A.; Kieliszek, M.; Zhao, L. Ultrasound-Assisted Extraction of Carotenoids from Carrot Pomace and Their Optimization through Response Surface Methodology. Molecules 2021, 26, 6763. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Aguilar, D.E.; Álvarez, I.; Raso, J. Organic-solvent-free extraction of carotenoids from yeast Rhodotorula glutinis by application of ultrasound under pressure. Ultrason. Sonochem. 2020, 61, 104833. [Google Scholar] [CrossRef]
- Sharma, M.; Hussain, S.; Shalima, T.; Aav, R.; Bhat, R. Valorization of seabuckthorn pomace to obtain bioactive carotenoids: An innovative approach of using green extraction techniques (ultrasonic and microwave-assisted extractions) synergized with green solvents (edible oils). Ind. Crops Prod. 2022, 175, 114257. [Google Scholar] [CrossRef]
- Dhakane-Lad, J.; Kar, A. Supercritical CO2 extraction of lycopene from pink grapefruit (Citrus paradise Macfad) and its degradation studies during storage. Food Chem. 2021, 361, 130113. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Temelli, F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J. Supercrit. Fluid. 2006, 37, 397–408. [Google Scholar] [CrossRef]
- Larocca, V.; Martino, M.; Trupo, M.; Magarelli, R.A.; Spagnoletta, A.; Ambrico, A. Evaluation of carbon dioxide supercritical fluid extraction (CO2-SFE) on carotenoids recovery from red yeast cells. Biomass Convers. Biorefin. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Plesu, V.; Bildea, C.S. Carotenoids Recovery Enhancement by Supercritical CO2 Extraction from Tomato Using Seed Oils as Modifiers. Processes 2022, 10, 2656. [Google Scholar] [CrossRef]
- Tirado, D.F.; Calvo, L. The Hansen theory to choose the best cosolvent for supercritical CO2 extraction of β-carotene from Dunaliella salina. J. Supercrit. Fluid. 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Ludwig, K.; Rihko-Struckmann, L.; Brinitzer, G.; Unkelbach, G.; Sundmacher, K. β-Carotene extraction from Dunaliella salina by supercritical CO2. J. Appl. Phycol. 2021, 33, 1435–1445. [Google Scholar] [CrossRef]
- Xie, L.; Cahoon, E.; Zhang, Y.; Ciftci, O.N. Extraction of astaxanthin from engineered Camelina sativa seed using ethanol-modified supercritical carbon dioxide. J. Supercrit. Fluids. 2019, 143, 171–178. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Barzana, E.; Rubio, D.; Santamaria, R.I.; Garcia-Correa, O.; Garcia, F.; Ridaura Sanz, V.E.; López-Munguía, A. Enzyme-Mediated Solvent Extraction of Carotenoids from Marigold Flower (Tagetes erecta). J. Agric. Food. Chem. 2002, 50, 4491–4496. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, B.; Yang, J.; Chen, J.; Sun, Z. Identification of microbial carotenoids and isoprenoid quinones from Rhodococcus sp. B7740 and its stability in the presence of iron in model gastric conditions. Food Chem. 2018, 240, 204–211. [Google Scholar] [CrossRef]
- Prokopov, T.; Nikolova, M.; Dobrev, G.; Taneva, D.J.A.A. Enzyme-Assisted Extraction of Carotenoids from Bulgarian Tomato Peels. Acta Aliment. 2017, 46, 84–91. [Google Scholar] [CrossRef]
- Scarpitti, B.T.; Chitchumroonchokchai, C.; Clinton, S.K.; Schultz, Z.D. In vitro imaging of lycopene delivery to prostate cancer cells. Anal. Chem. 2022, 94, 5106–5112. [Google Scholar] [CrossRef]
- Ricarte, G.N.; Coelho, M.A.Z.; Marrucho, I.M.; Ribeiro, B.D. Enzyme-assisted extraction of carotenoids and phenolic compounds from sunflower wastes using green solvents. 3 Biotech 2020, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.N.; Nguyen, H.V.H. Optimization of enzyme-assisted lycopene extraction from tomato (Lycopersicon esculentum) peel using rice bran oil. J. Food Meas. Charact. 2023, 17, 5154–5162. [Google Scholar] [CrossRef]
- Mustafa, A.; Trevino, L.M.; Turner, C. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules 2012, 17, 1809–1818. [Google Scholar] [CrossRef]
- Martinez, J.M.; Schottroff, F.; Haas, K.; Fauster, T.; Sajfrtova, M.; Alvarez, I.; Raso, J.; Jaeger, H. Evaluation of pulsed electric fields technology for the improvement of subsequent carotenoid extraction from dried Rhodotorula glutinis yeast. Food Chem. 2020, 323, 126824. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Process Optimization of Microwave-Assisted Extraction of Chlorophyll, Carotenoid and Phenolic Compounds from Chlorella vulgaris and Comparison with Conventional and Supercritical Fluid Extraction. Appl. Sci. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.; Zhang, L.; Shao, P.; Wu, W.; Chen, Z.; Li, J.; Renard, C.M.G.C. An overview of carotenoid extractions using green solvents assisted by Z-isomerization. Trends Food Sci. Tech. 2022, 123, 145–160. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Farias, F.O.; Santos-Ebinuma, V.C.; Pereira, J.F.B.; Pessoa, A. Sustainable one-pot platform for the green recovery of carotenoids from Phaffia rhodozyma yeast and their use as natural additives in soap formulation. Environ. Technol. Innovation 2023, 29, 103029. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Radojčić-Redovniković, I.; Blesa, J.; Frígola, A.; Esteve, M.J.; López-Malo, D. Hydrophobic and Hydrophilic Deep Eutectic Solvents to Extract Carotenoids from Orange Peels and Obtain Green Extracts. In Proceedings of the 3rd International Electronic Conference on Foods: Food, Microbiome, and Health, Online, 1–15 October 2022; p. 28. [Google Scholar] [CrossRef]
- Duan, W.D.; Quan, K.J.; Huang, X.Y.; Gong, Y.; Xiao, S.; Liu, J.F.; Pei, D.; Di, D.L. Recovery and recycling of solvent of counter-current chromatography: The sample of isolation of zeaxanthin in the Lycium barbarum L. fruits. J. Sep. Sci. 2021, 44, 759–766. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Martins, M.; Pisani, L.P.; Ventura, S.P.M.; de Rosso, V.V. Insights on the use of alternative solvents and technologies to recover bio-based food pigments. Compr. Rev. Food Sci. Food Saf. 2020, 20, 787–818. [Google Scholar] [CrossRef]
- Colucci Cante, R.; Gallo, M.; Varriale, L.; Garella, I.; Nigro, R. Recovery of Carotenoids from Tomato Pomace Using a Hydrofluorocarbon Solvent in Sub-Critical Conditions. Appl. Sci. 2022, 12, 2822. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Sanjaya, Y.A.; Tola, P.S. Rahmawati R. Ultrasound-assisted Extraction as a Potential Method to Enhanced Extraction of Bioactive Compound. Nusant. Sci. Technol. Proc. 2022, 23, 191–198. [Google Scholar] [CrossRef]
- Gu, Z.; Deming, C.; Yongbin, H.; Zhigang, C.; Feirong, G. Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT Food Sci. Technol. 2008, 41, 1082–1088. [Google Scholar] [CrossRef]
- Vieira, E.F.; Souza, S.; Moreira, M.M.; Cruz, R.; Silva, A.B.D.; Casal, S.; Delerue-Matos, C. Valorization of Phenolic and Carotenoid Compounds of Sechium edule (Jacq. Swartz) Leaves: Comparison between Conventional, Ultrasound- and Microwave-Assisted Extraction Approaches. Molecules 2022, 27, 7193. [Google Scholar] [CrossRef] [PubMed]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Sanwal, N.; Mishra, S.; Sahu, J.K.; Naik, S.N. Effect of ultrasound-assisted extraction on efficiency, antioxidant activity, and physicochemical properties of sea buckthorn (Hippophae salicipholia) seed oil. LWT Food Sci. Technol. 2022, 153, 112386. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Marsya, M.A.; Fatkhiyatul, P. Improved yield of β-carotene from microalgae Spirulina platensis using ultrasound assisted extraction. J. Teknol. 2015, 77, 219–222. [Google Scholar] [CrossRef][Green Version]
- Moreira, R.C.; de Melo, R.P.F.; Martinez, J.; Marostica Junior, M.R.; Pastore, G.M.; Zorn, H.; Bicas, J.L. Supercritical CO2 as a Valuable Tool for Aroma Technology. J. Agric. Food Chem. 2023, 71, 9201–9212. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; Vaiano, V.; De Marco, I. Supercritical Carbon Dioxide-Based Processes in Photocatalytic Applications. Molecules 2021, 26, 2640. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Aiello, A.; Pizzolongo, F.; Rispoli, A.; De Luca, L.; Masi, P. Characterisation of oleoresins extracted from tomato waste by liquid and supercritical carbon dioxide. Int. J. Food Sci. Technol. 2020, 55, 3334–3342. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Maled, S.B.; Bhat, A.R.; Hegde, S.; Sivamani, Y.; Muthuraman, A.; Elayaperumal, S. Bioactive Extraction and Application in Food and Nutraceutical Industries; Sarkar, T., Pati, S., Eds.; Springer: New York, NY, USA, 2024; pp. 173–200. [Google Scholar]
- Shahram, H.; Dinani, S.T. Optimization of ultrasonic-assisted enzymatic extraction of β-carotene from orange processing waste. J. Food Process Eng. 2019, 42, e13042. [Google Scholar] [CrossRef]
- Sharma, A.; Sogi, D.S. Optimization of enzyme aided pigment extraction from pumpkin (Cucurbita maxima Duch) using response surface methodology. J. Food Meas. Charact. 2022, 16, 1184–1194. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.G.; Lee, D.U.; Pan, C.H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- De Aguiar Saldanha Pinheiro, A.C.; Marti-Quijal, F.J.; Barba, F.J.; Benitez-Gonzalez, A.M.; Melendez-Martinez, A.J.; Castagnini, J.M.; Tappi, S.; Rocculi, P. Pulsed Electric Fields (PEF) and Accelerated Solvent Extraction (ASE) for Valorization of Red (Aristeus antennatus) and Camarote (Melicertus kerathurus) Shrimp Side Streams: Antioxidant and HPLC Evaluation of the Carotenoid Astaxanthin Recovery. Antioxidants 2023, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Alvarez, I.; Raso, J. Improving carotenoid extraction from tomato waste by pulsed electric fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Berrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed electric fields (PEF), pressurized liquid extraction (PLE) and combined PEF + PLE process evaluation: Effects on Spirulina microstructure, biomolecules recovery and Triple TOF-LC-MS-MS polyphenol composition. Innov. Food Sci. Emerg. Technol. 2022, 77, 102989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).