Fast Screening of Tyrosinase Inhibitors in Coreopsis tinctoria Nutt. by Ligand Fishing Based on Paper-Immobilized Tyrosinase
Abstract
1. Introduction
2. Results and Discussion
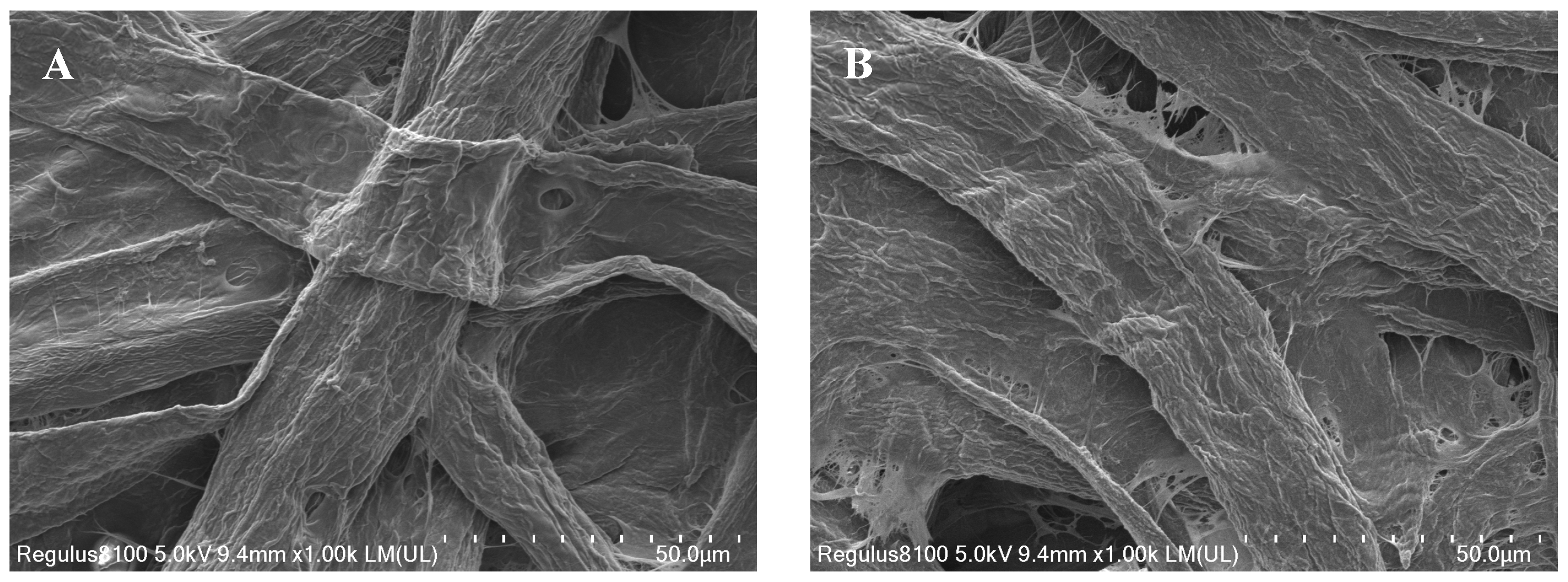
2.1. Characterization of CP@DA@TYR
2.2. Selection of the Buffer System
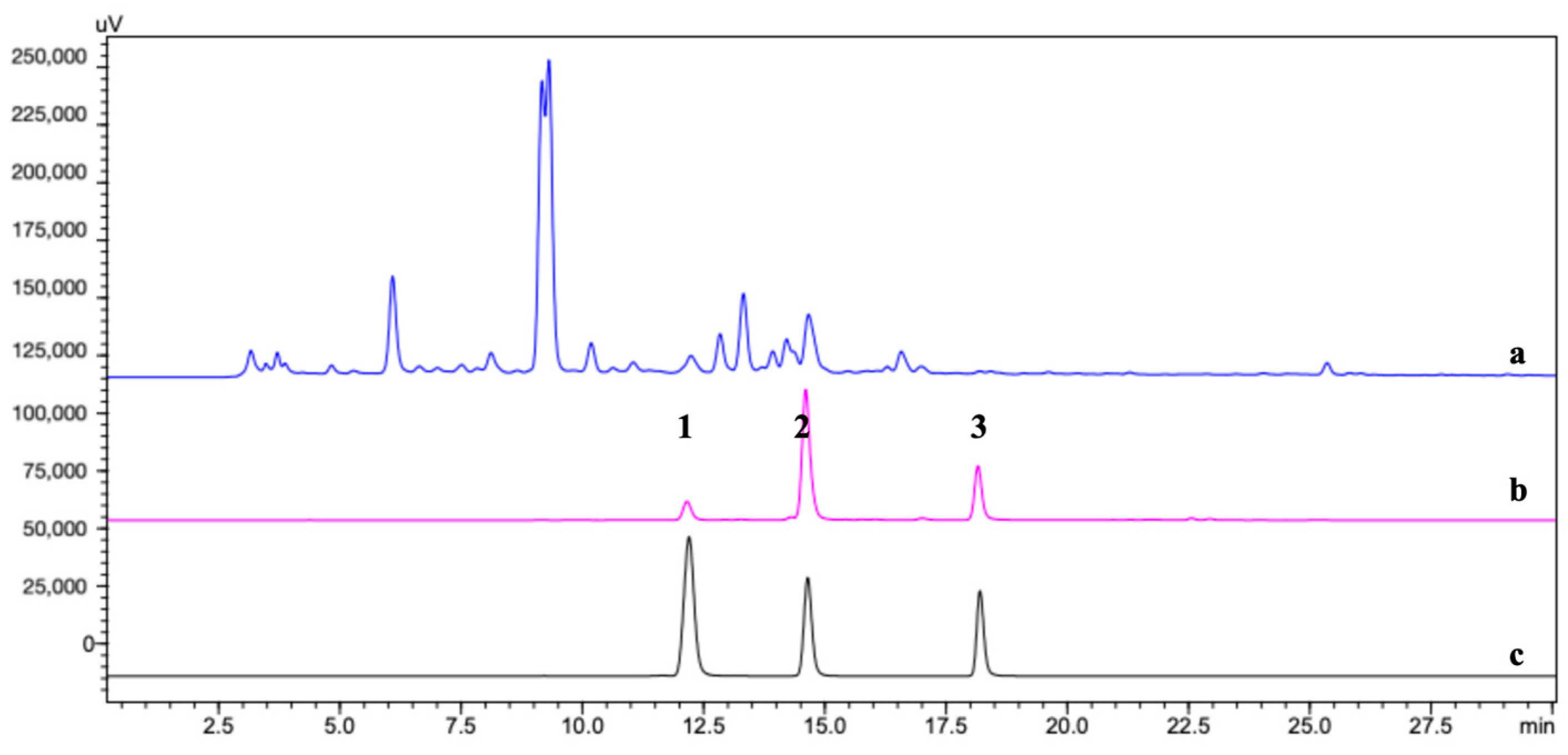
2.3. Identification of the TYR Ligands
2.4. Quantification of the TYR Ligands
2.5. TYR Inhibitory Activity
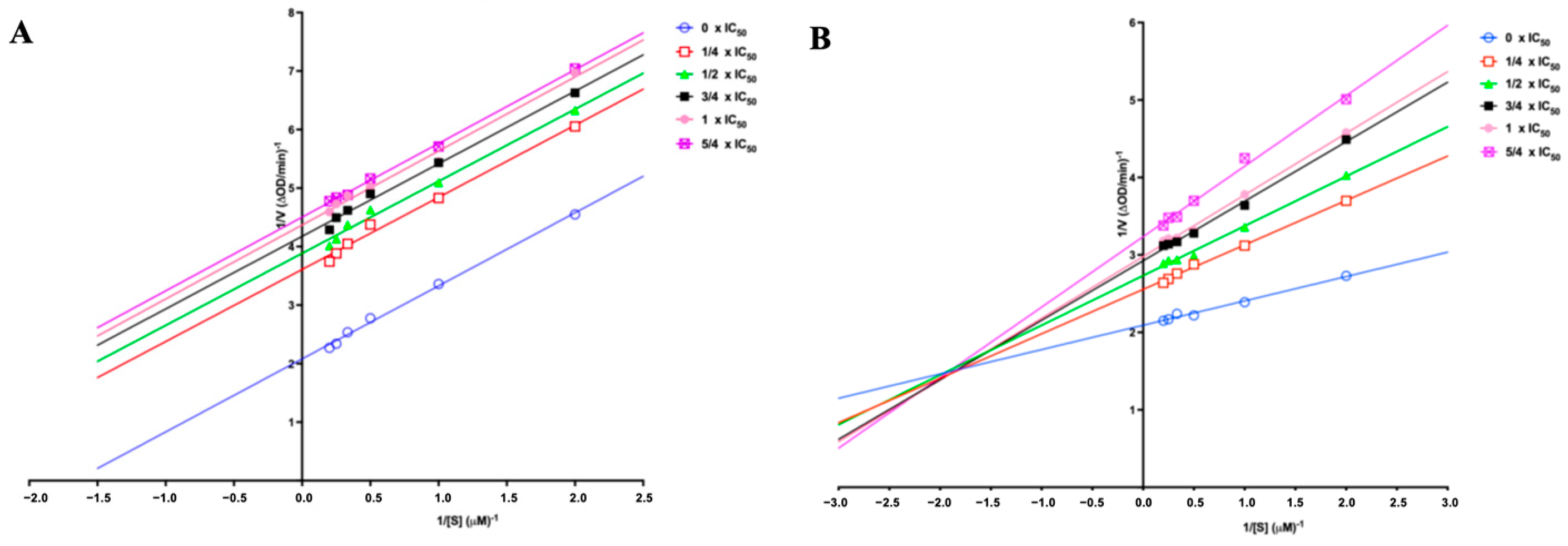
2.6. Enzyme Kinetic Study
2.7. Molecular Docking
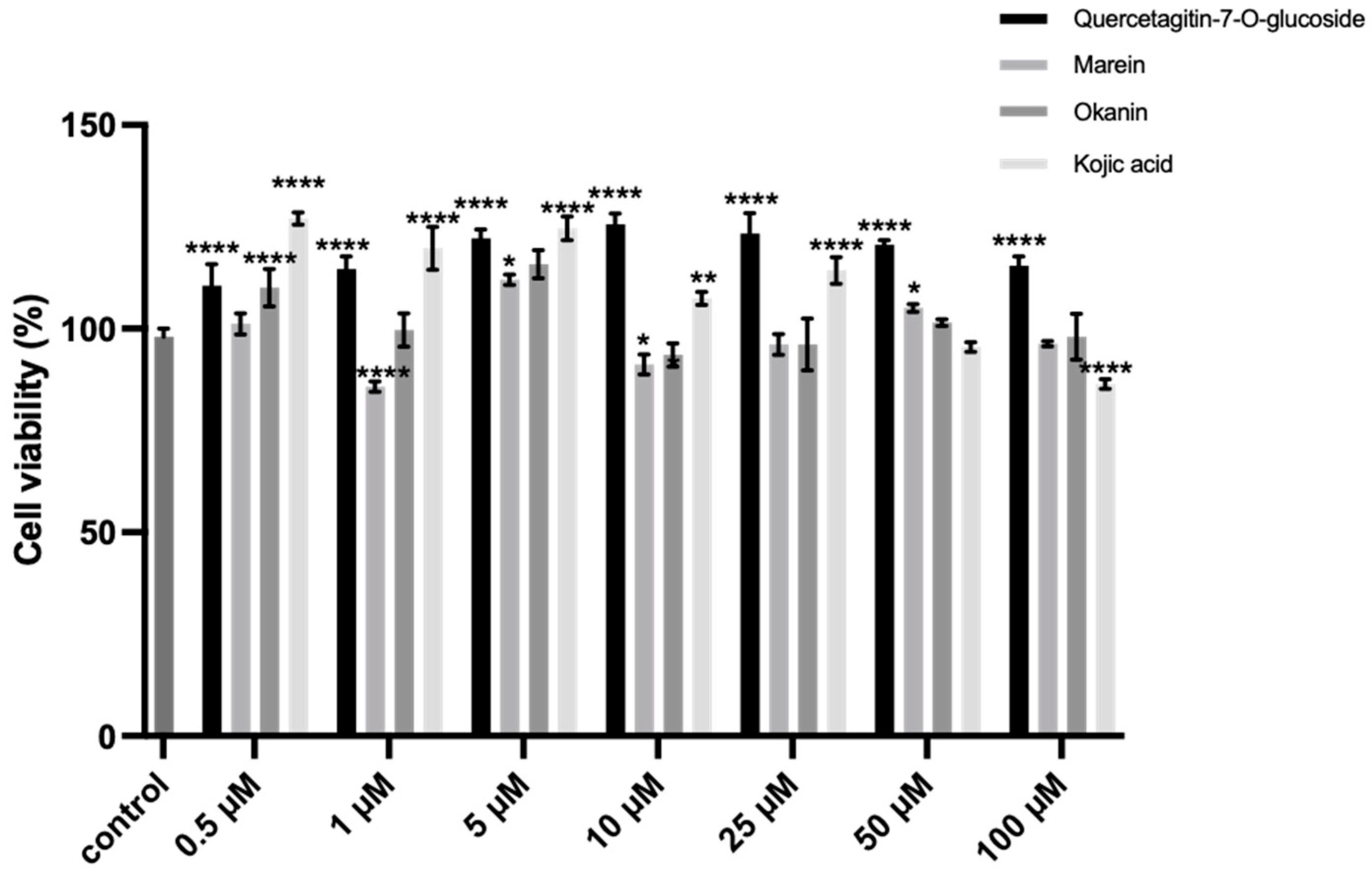
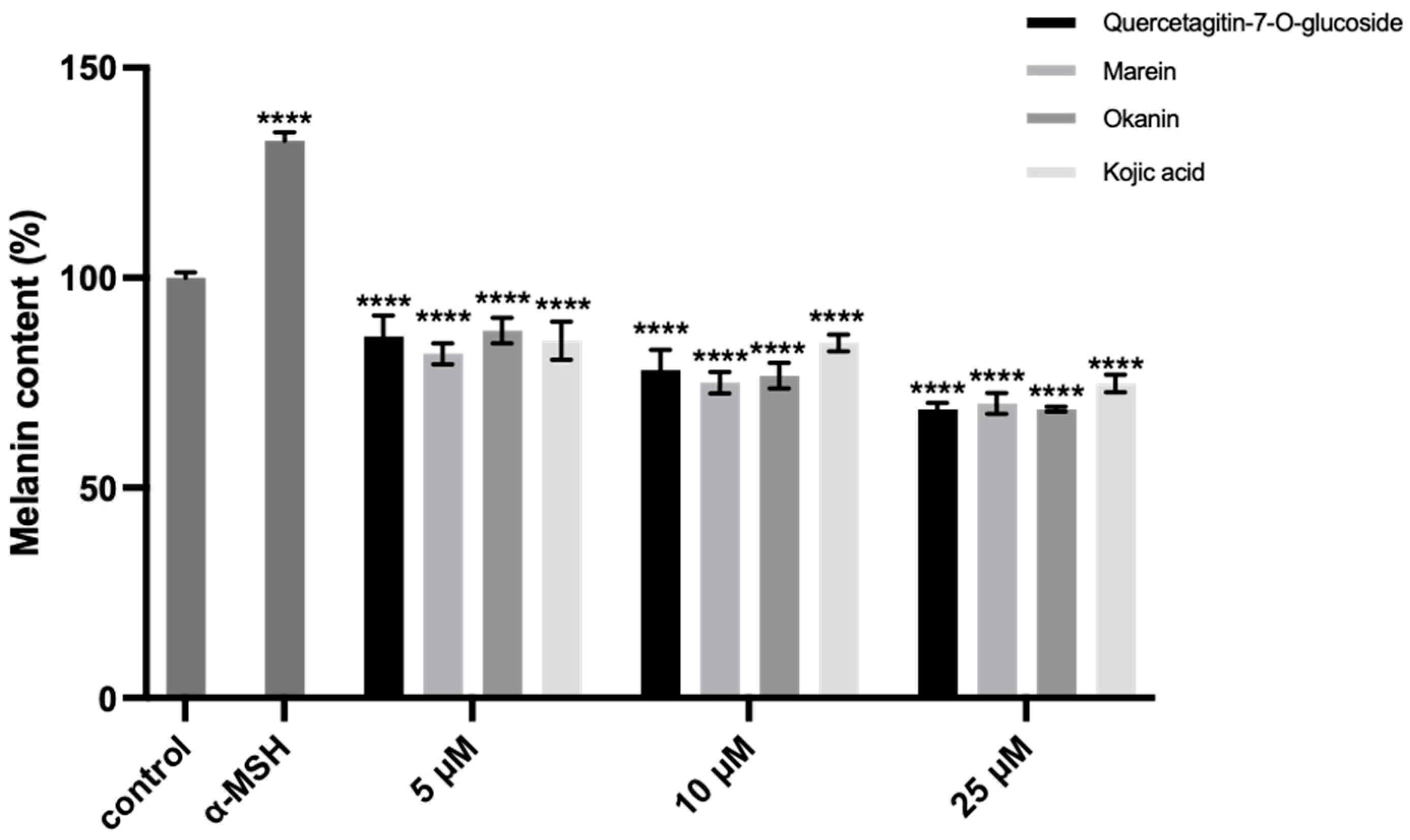
2.8. Inhibition of the Intracellular TYR Activity and Melanin Synthesis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Immobilization of TYR on Cellulose Chromatography Paper and Characterization
3.3. Ligand Fishing of the TYR Inhibitors
3.4. Selection of the Buffer System
3.5. Extraction and Isolation of the TYR Inhibitors
3.6. Structural Identification of the TYR Ligands by UPLC-MS
3.7. Quantification of the TYR Ligands
3.8. TYR Inhibition Assay
3.9. Enzymatic Kinetic Study
3.10. Molecular Docking
3.11. In Vitro TYR Inhibition Activity
3.11.1. Cell Culture
3.11.2. Cytotoxicity Assay
3.11.3. Intracellular TYR Inhibition Activity Assay
3.11.4. Quantification of Intracellular Melanin Content
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.L.; Jin, X.; Feng, Q.; Shi, X.P.; Tang, Z.W. Cultivation techniques of Kunlun snow chrysanthemum in the high-altitude areas of Linxia, Gansu. Agric. Eng. Technol. 2023, 43, 76–77. [Google Scholar]
- Li, Y.L.; Chen, X.M.; Xue, J.; Liu, J.Y.; Chen, X.H.; Wulasihan, M. Flavonoids furom Coreopsis tinctoria adjust lipid metabolism in hyperlipidemia animals by down-regulating adipose differentiation-related protein. Lipids Health Dis. 2014, 13, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, J.; Li, L.L.; Li, X.X.; Zhang, R.; Zhang, Y.J.; Mao, X.M. Coreopsis tinctoria Nutt. meliorates high glucose-induced renal fibrosis and inflammation via the TGF-β1/SMADS/AMPK/NF-κB pathways. BMC Complement Altern Med. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.M.; Zhang, W.S.; Li, S.M.; Ho, C.T. Chemical and nutraceutical properties of Coreopsis tinctoria. J. Funct. Foods 2015, 13, 11–20. [Google Scholar] [CrossRef]
- Guo, J.; Wang, A.; Yang, K.; Ding, H.; Hu, Y.; Yang, Y.; Huang, S.; Xu, J.; Liu, T.; Yang, H. Isolation, characterization and antimicrobial activities of polyacetylene glycosides from Coreopsis tinctoria Nutt. Phytochemistry 2017, 136, 65–69. [Google Scholar] [CrossRef]
- Shen, J.; Hu, M.Y.; Tan, W.; Ding, J.W.; Jiang, B.P.; Xu, L.; Hamulati, H.; He, C.N.; Sun, Y.H.; Xiao, P.G. Traditional uses, phytochemistry, pharmacology, and toxicology of Coreopsis tinctoria Nutt.: A review. J. Ethnopharmacol. 2021, 269, 113690. [Google Scholar] [CrossRef]
- Dajas, F.; Andrés, A.C.; Florencia, A.; Carolina, E.; Felicia, R.M. Neuroprotective actions of flavones and flavonols: Mechanisms and relationship to flavonoid structural features. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 30–35. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The roles of flavonols/flavonoids in neurodegeneration and neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Xia, Z.H.; Cao, P. Cosmetic Skin-Lightening Preparation Containing Active Ingredient from Chinese Medicine Coreopsis tinctoria. CN Patent 102961281, 22 April 2015. [Google Scholar]
- Wang, C.C.; Yan, S.Y.; Huang, R.; Feng, S.; Fu, B.S.; Weng, X.C.; Zhou, X. A turn-on fluorescent probe for detection of tyrosinase activity. J. Analyst. 2013, 138, 2825–2828. [Google Scholar] [CrossRef]
- Zhang, X.W.; Bian, G.L.; Kang, P.Y.; Cheng, X.J.; Yan, K.; Liu, Y.L.; Gao, Y.X.; Li, D.Q. Recent advance in the discovery of tyrosinase inhibitors from natural sources via separation methods. J. Enzyme Inhib. Med. Chem. 2021, 36, 2104–2117. [Google Scholar] [CrossRef]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef]
- Neto, C.F.G.; Nascimento, P.D.; Silveira, V.C.D.; Mattos, A.B.N.D.; Bertol, C.D. Natural sources of melanogenic inhibitors: A systematic review. Int. J. Cosmet. Sci. 2022, 44, 143–270. [Google Scholar] [CrossRef]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Rodriguez, E.L.; Poddar, S.; Iftekhar, S. Affinity chromatography: A review of trends and developments over the past 50 years. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1157, 122332. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.C.; Cao, J.; Ye, L.H. Recent advances on discovery of enzyme inhibitors from natural products using bioactivity screening. J. Sep. Sci. 2022, 45, 2766–2787. [Google Scholar] [CrossRef]
- Hu, Y.K.; Liu, Y.M.; Bai, X.L.; Ma, C.; Liao, X. Screening of monoamine oxidase B inhibitors from Fragaria nubicola by ligand fishing and their neuroprotective effects. J. Agric. Food Chem. 2023, 71, 512–521. [Google Scholar] [CrossRef]
- Li, Y.F.; Xu, J.; Chen, Y.; Mei, Z.N.; Xiao, Y.X. Screening of inhibitors of glycogen synthase kinase-3β from traditional Chinese medicines using enzyme-immobilized magnetic beads combined with high-performance liquid chromatography. J. Chromatogr. A 2015, 1425, 8–16. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.J.; Bai, X.L.; Liu, H.P.; Qi, X.W.; Liao, X. Fast screening of tyrosinase inhibitors from traditional Chinese medicinal plants by ligand fishing in combination with in situ fluorescent assay. Anal. Bioanal. Chem. 2022, 414, 2265–2273. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, L.; Bai, X.L.; Jiang, X.X.; Zhang, Y.; Fang, Q.; Zhang, Q.; Liao, X. Tyrosinase covalently immobilized on carboxyl functionalized magnetic nanoparticles for fishing of the enzyme’s ligands from Prunellae Spica. J. Sep. Sci. 2022, 45, 3635–3645. [Google Scholar] [CrossRef]
- Borzouee, F.; Varshosaz, J.; Cohan, R.A.; Norouzian, D.; Pirposhteh, R.T. A comparative analysis of different enzyme immobilization nanomaterials: Progress, constraints and recent trends. Curr. Med. Chem. 2021, 28, 3980–4003. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.; Trosien, S.; Avrutina, O.; Kolmar, H.; Biesalski, M. Covalent attachment of enzymes to paper fibers for paper-based analytical devices. Front. Chem. 2018, 6, 214. [Google Scholar] [CrossRef]
- Shi, X.Y.; Guo, Z.H.; Chen, J. Cellulose filter paper immobilized α-glucosidase and its application to screening inhibitors from traditional Chinese medicine. J. Sep. Sci. 2022, 45, 2724–2733. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, F.; Ma, Q.L.; Hao, L.L.; Liu, J.Y.; Li, Y.L.; Yang, S.L. Simultaneous determination of four flavonoids components in Coreopsis tinctoria by HPLC. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 49–52. [Google Scholar]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zeng, X.W.; Chen, H.Z.; Li, Z.M.; Zeng, W.F.; Mei, L.; Zhao, Y.L. Versatile polydopamine platforms: Synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.F.; Song, Y.Y.; Tang, N.; Cheng, P.G.; Xiang, J.; Du, W. Bioadhesive immobilize agarase on magnetic femriferous by polydopamine. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 93, 218–225. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Li, H.R.; Habasi, M.; Xie, L.Z.; Aisa, H.A. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Jin, M.; Lv, Y.; Pei, Z.; Pei, Y. A hypericin delivery system based on polydopamine coated cerium oxide nanorods for targeted photodynamic Ttherapy. Polymers 2019, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Güven, Z.B.; Saracoglu, I.; Nagatsu, A.; Yilmaz, M.A.; Basaran, A.A. Anti-tyrosinase and antimelanogenic effect of cinnamic acid derivatives from Prunus mahaleb L.: Phenolic composition, isolation, identification and inhibitory activity. J. Ethnopharmacol. 2023, 310, 116378. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ablat, A.; Li, M.-J.; Zhai, X.-R.; Wang, Y.; Bai, X.-L.; Shu, P.; Liao, X. Fast Screening of Tyrosinase Inhibitors in Coreopsis tinctoria Nutt. by Ligand Fishing Based on Paper-Immobilized Tyrosinase. Molecules 2024, 29, 4018. https://doi.org/10.3390/molecules29174018
Ablat A, Li M-J, Zhai X-R, Wang Y, Bai X-L, Shu P, Liao X. Fast Screening of Tyrosinase Inhibitors in Coreopsis tinctoria Nutt. by Ligand Fishing Based on Paper-Immobilized Tyrosinase. Molecules. 2024; 29(17):4018. https://doi.org/10.3390/molecules29174018
Chicago/Turabian StyleAblat, Ayzohra, Ming-Jie Li, Xiao-Rui Zhai, Yuan Wang, Xiao-Lin Bai, Peng Shu, and Xun Liao. 2024. "Fast Screening of Tyrosinase Inhibitors in Coreopsis tinctoria Nutt. by Ligand Fishing Based on Paper-Immobilized Tyrosinase" Molecules 29, no. 17: 4018. https://doi.org/10.3390/molecules29174018
APA StyleAblat, A., Li, M.-J., Zhai, X.-R., Wang, Y., Bai, X.-L., Shu, P., & Liao, X. (2024). Fast Screening of Tyrosinase Inhibitors in Coreopsis tinctoria Nutt. by Ligand Fishing Based on Paper-Immobilized Tyrosinase. Molecules, 29(17), 4018. https://doi.org/10.3390/molecules29174018





