Three New Ent-Kaurane Diterpenes with Antibacterial Activity from Sigesbeckia orientalis
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Determination of Antimicrobial Activities
3.5. Assessment of Synergistic Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantosti, A.; Venditti, M. What is MRSA? Eur. Respir. J. 2009, 34, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus(VRE): A systematic review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; Tao, W.; You, H.; He, X.; He, Y. Treatment of MRSA infection: Where are we? Curr. Med. Chem. 2024, 31, 4425–4460. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Stitt, G.; Son, L.; Enioutina, E.Y. Probiotics and their bioproducts: A promising approach for targeting methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Microorganisms 2023, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhu, Y.; Zu, W.; Wang, H.; Bai, L.; Zhou, Z.; Zhao, Y.; Wang, Z.; Luo, X. Structure optimizing of flavonoids against both MRSA and VRE. Eur. J. Med. Chem. 2024, 271, 116401. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Shi, N.; Bai, L.; Jiang, Y.; Jiang, L.; Liu, T.; Wei, M.; Qin, M.; Luo, X. Anti-MRSA mechanism of spirostane saponin in Rohdea pachynema F.T.Wang & tang. J. Ethnopharmacol. 2024, 331, 118327. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. Med. Chem. Comm. 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Pai, L.; Patil, S.; Liu, S.; Wen, F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: Insights from reviews on antibiotic resistance. Front. Cell. Infect. Microbiol. 2023, 13, 1327069. [Google Scholar] [CrossRef]
- Wu, X.; Ma, G.; Chen, H.; Zhao, Z.; Zhu, Z.; Xiong, J.; Yang, G.; Hu, J. Antibacterial and antibiofilm efficacy of the preferred fractions and compounds from Euphorbia humifusa (herba euphorbiae humifusae) against Staphylococcus aureus. J. Ethnopharmacol. 2023, 306, 116177. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Onyedibe, K.I.; Sintim, H.O. Lipoteichoic acid biosynthesis inhibitors as potent inhibitors of S. Aureus and E. Faecalis growth and biofilm formation. Molecules 2020, 25, 2277. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, F.I.; Hussain, F.; Rahman, S.M.A.; Rahman, M.M. Antimicrobial diterpenes: Recent development from natural sources. Front. Pharmacol. 2022, 12, 820312. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Zhu, M.; Zhou, Z.; Hu, B.; Wei, M.; Zhao, Y.; Dai, Z.; Luo, X. A dual mechanism with H2S inhibition and membrane damage of morusin from Morus alba Linn. against MDR-MRSA. Bioorg. Med. Chem. 2024, 97, 117544. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.; Shi, Y.; Tao, R.; Huang, H.; Zhao, Y.; Luo, X. Curcusinol from the fruit of Carex baccans with antibacterial activity against multidrug-resistant strains. J. Ethnopharmacol. 2024, 318, 116892. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Z.; Chen, S.; Wang, H.; Xie, T.; Xu, X.; Xiang, M.; Chen, Y.; Luo, X. Phytochemical and anti-MRSA constituents of Zanthoxylum nitidum. Biomed. Pharmacother. 2022, 148, 112758. [Google Scholar] [CrossRef]
- Lin, L.; Ung, C.; Feng, Z.; Huang, L.; Hu, H. Naturally occurring diterpenoid dimers: Source, biosynthesis, chemistry and bioactivities. Planta Med. 2016, 82, 1309–1328. [Google Scholar] [CrossRef]
- Dan Thi Thuy, H.; Do Thi, T.; Duong Thi, D.; Duong Thi Hai, Y.; Nguyen Huy, H.; Ngo Anh, B.; Nguyen Thi, C.; Nguyen Xuan, N.; Phan Thi Thanh, H.; Bui Huu, T.; et al. Guaianolide sesquiterpenes and benzoate esters from the aerial parts of Siegesbeckia orientalis L. and their xanthine oxidase inhibitory activity. Phytochemistry 2021, 190, 112889. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.; Wang, D.; Hu, G.; Yan, T.; Jia, J.; Wang, A. Six novel lignanoids with complex structures from Sigesbeckia glabrescens Makino with their cytotoxic activities. Fitoterapia 2021, 148, 104799. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Z.; Chen, H.; Bao, T.; Gao, X.; Wang, A.; Jia, J. Diterpenoids from Sigesbeckia glabrescens with anti-inflammatory and AChE inhibitory activities. Phytochemistry 2023, 205, 113503. [Google Scholar] [CrossRef] [PubMed]
- Thi Viet Thanh, N.; Thanh Tung, D.; Van Duong, L.; Thi Minh, T.; Dinh Hoang, V.; Yang, S.Y.; Yen, P.H. Two new chlorinated sesquiterpenes from Sigesbeckia orientalis. Nat. Prod. Res. 2023, 37, 3677–3684. [Google Scholar] [CrossRef]
- Wang, D.; Dong, X.; Nie, Y.; Yang, W.; Li, C. A review on medical plants of genus Siegesbeckia: Phytochemical and pharmacological studies. Rec. Nat. Prod. 2022, 16, 515–537. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Y.; Li, K.; Li, Y.; Niu, F.; Zhou, S.; Wei, H.; Zhou, C. Herba Siegesbeckiae: A review on its traditional uses, chemical constituents, pharmacological activities and clinical studies. J. Ethnopharmacol. 2021, 275, 114117. [Google Scholar] [CrossRef]
- Xiang, Y.; Fan, C.; Yue, J. Novel sesquiterpenoids from Siegesbeckia orientalis. Helv. Chim. Acta 2005, 88, 160–170. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Q.; Lan, X.; Ge, N.; Tang, Z.; Fan, C. Effect of Siegesbeckia orientalis on cartilage damage in knee osteoarthritis rats by regulating sirt1/FOXO1 pathway. Chin. J. Immun. 2020, 36, 439–444. [Google Scholar] [CrossRef]
- Lee, K.; Jung, J.; Yang, G.; Ham, I.; Bu, Y.M.; Kim, H.; Choi, H.Y. Endothelium-independent vasorelaxation effects of Sigesbeckia glabrescens (Makino) Makino on isolated rat thoracic aorta. Phytother. Res. 2013, 27, 1308–1312. [Google Scholar] [CrossRef]
- Quan, P.; Jiao, B.; Shang, R.; Liu, C.; Fang, L. Alternative therapy of rheumatoid arthritis with a novel transdermal patch containing Siegesbeckiae herba extract. J. Ethnopharmacol. 2021, 265, 113294. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Cai, S.; Feng, R.; Lou, Z. Chemical constituents of Siegesbeckia glabrescens. Chin. Pharm. J. 1988, 33, 276–278. [Google Scholar] [CrossRef]
- Bohlmann, F.; Karl-Heinz, K.; Robinson, H.; King, R.M. Neue kauren-derivate und melampolide aus Smallanthus uvedalia. Phytochemistry 1980, 19, 107–110. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, C.; Wang, M.; Lu, J.; Hu, P.; Chen, J.; Li, X.; Chen, Y. Five new ent-kaurane diterpenes from Annona squamosa L. pericarps. Nat. Prod. Res. 2020, 34, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Miski, M.; Gage, D.A.; Norris, J.A.; Mabry, T.J. Terpenoids from Viguiera potosina. J. Nat. Prod. 1985, 48, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, H.; Gan, L.; Mo, J.; Feng, F.; Zhou, C. Constituents from the bark of Annona squamosa and their anti-tumor activity. China J. Chin. Mater. Med. 2012, 37, 2100–2104. [Google Scholar] [CrossRef]
- Wang, R.; Chen, W.; Shi, Y. Ent-kaurane and ent-pimarane diterpenoids from Siegesbeckia pubescens. J. Nat. Prod. 2010, 73, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Y.; Du, F.; Ou, Y.; Yuan, Y. Isolation and identification of two new diterpenoid from Rubus corchorifolius L.f. Acta Pharm. Sin. 2007, 42, 1155–1158. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, X.; Li, Y.; Shi, S.; Liu, J. ent-pimarane diterpenoids from Siegesbeckia orientalis and structure revision of a related compound. J. Nat. Prod. 2009, 72, 2005–2008. [Google Scholar] [CrossRef]
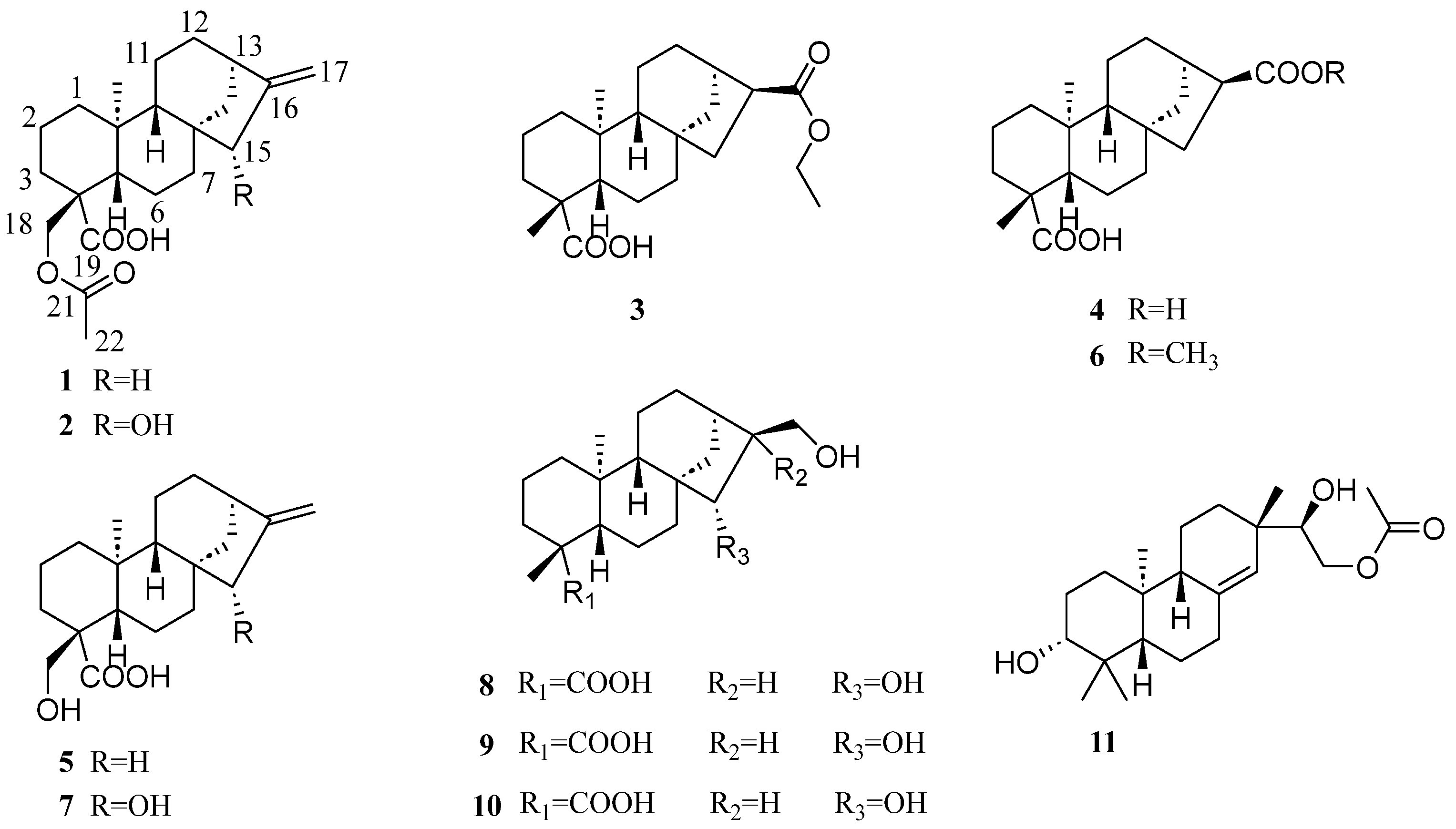
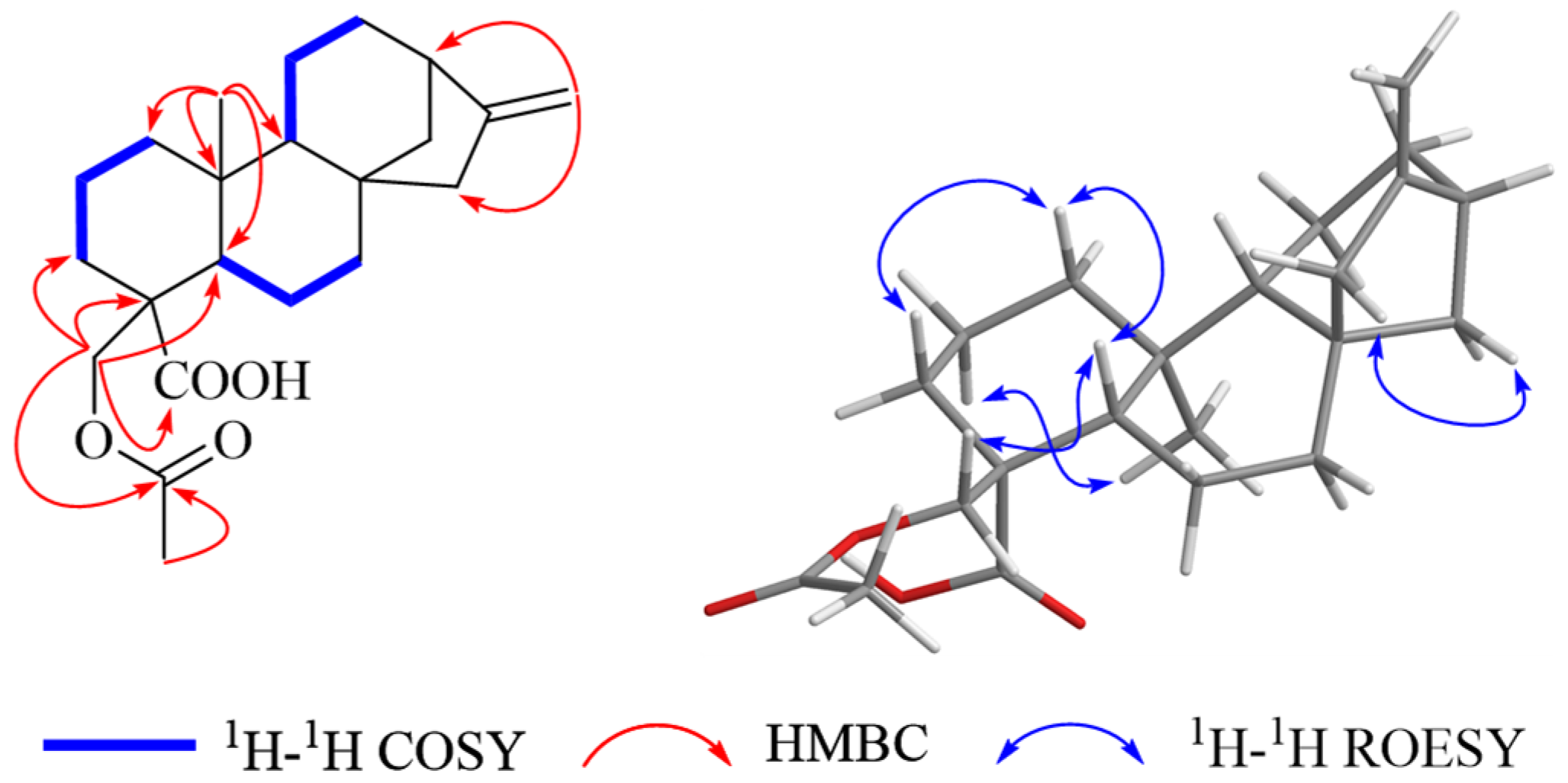
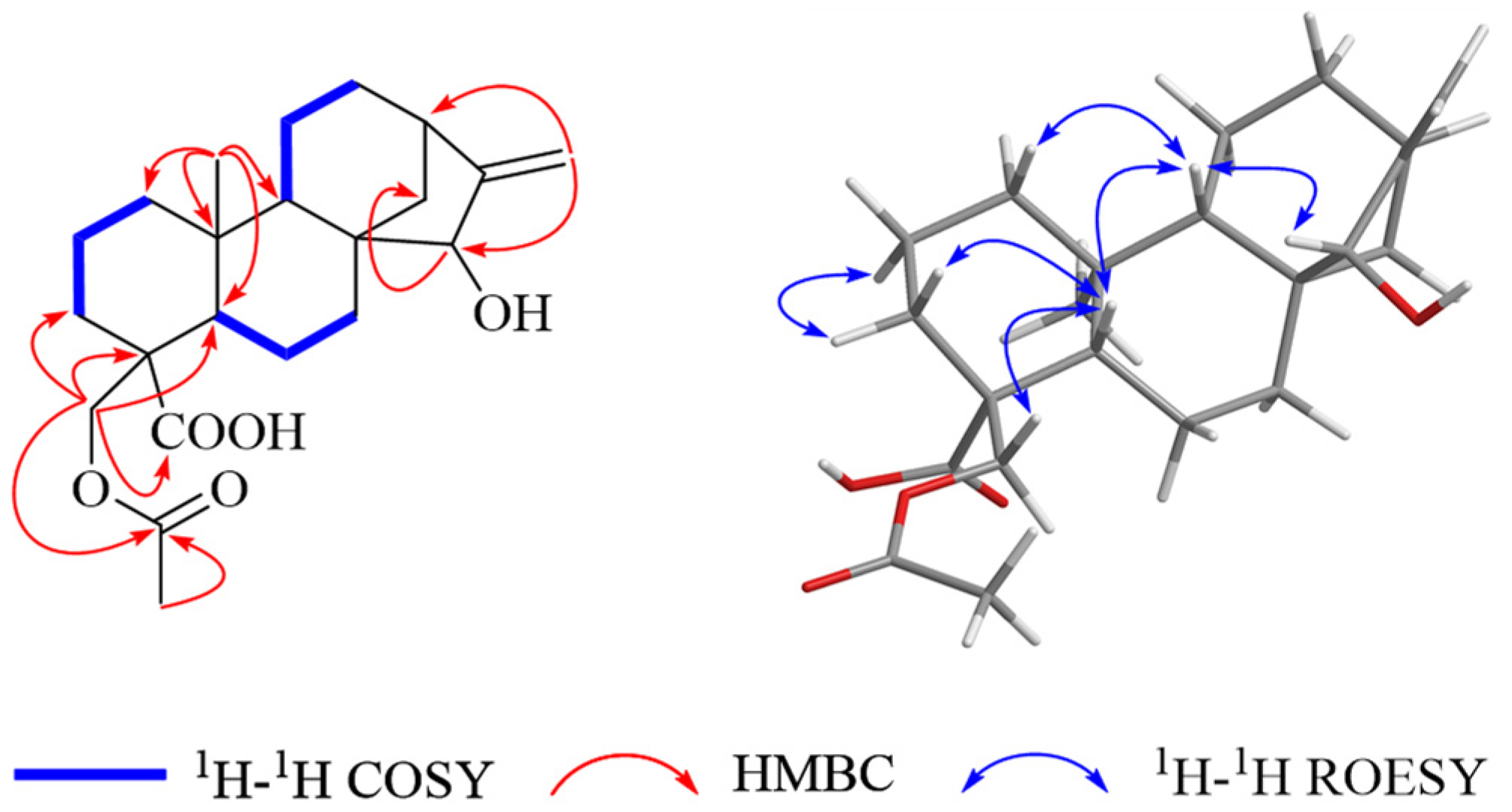
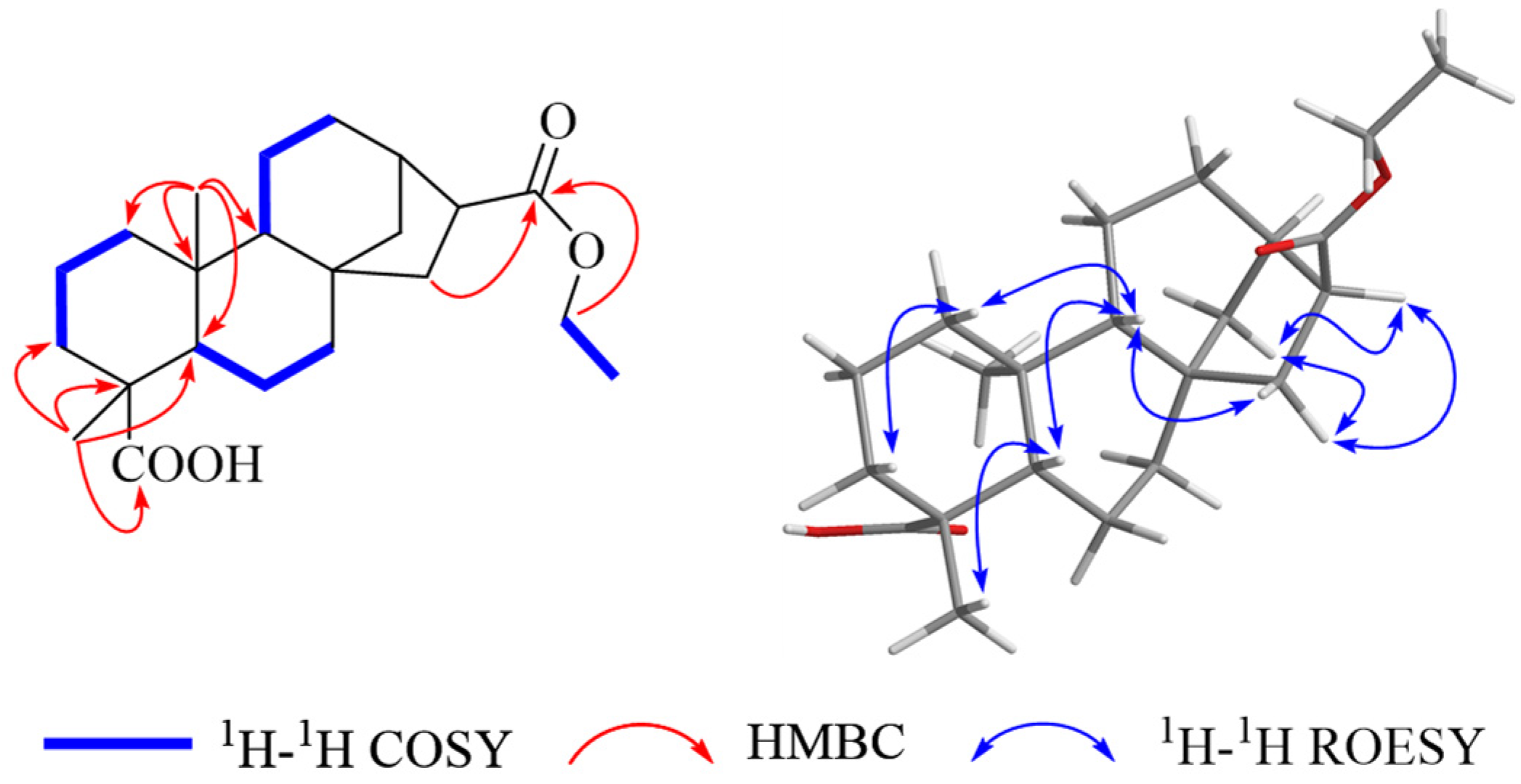
| 1 a | 2 b | 3 b | ||||
|---|---|---|---|---|---|---|
| No. | δC, Type | δH (Mult., J in Hz) | δC, Type | δH (Mult., J in Hz) | δC, Type | δH (Mult., J in Hz) |
| 1 | 40.7, t | Ha: 1.51, m | 39.7, t | Ha: 1.82, m | 40.7, t | Ha: 1.88, m |
| Hb: 1.43, m | Hb: 0.76, m | Hb: 0.83, m | ||||
| 2 | 18.4, t | 1.56, m | 17.9, t | Ha: 1.51, overlap | 18.7, t | Ha: 1.67, m |
| Hb: 1.33, m | Hb: 1.54, m | |||||
| 3 | 32.3, t | Ha: 2.31, m | 32.3, t | Ha: 1.51, overlap | 37.8, t | Ha: 2.17, m |
| Hb: 1.06, m | Hb: 1.36, m | Hb: 1.03, m | ||||
| 4 | 47.5, s | 46.7, s | 43.8, s | |||
| 5 | 52.3, d | 1.28, m | 51.0, d | 1.21, m | 56.9, d | 1.07, m |
| 6 | 21.8, t | 1.74, m | 20.8, t | Ha: 1.67, m | 22.4, t | 1.86, m |
| Hb: 1.59, overlap | ||||||
| 7 | 40.1, t | Ha: 1.91, m | 35.6, t | Ha: 1.59, overlap | 38.0, t | Ha: 1.87, m |
| Hb: 0.82, m | Hb: 1.27, overlap | Hb: 1.25, m | ||||
| 8 | 43.9, s | 47.0, s | 45.1, s | |||
| 9 | 55.1, d | 1.10, m | 53.0, d | 0.97, m | 55.1, d | 1.02, m |
| 10 | 39.4, s | 39.1, s | 39.6, s | |||
| 11 | 18.3, t | 1.82, m | 18.1, t | Ha: 1.84, overlap | 19.1, t | Ha: 1.89, m |
| Hb: 1.40, m | Hb: 1.45, m | |||||
| 12 | 33.0, t | Ha: 1.60, m | 31.9, t | Ha: 2.12, m | 31.1, t | 1.53, m |
| Hb: 1.48, m | Hb: 1.02, m | |||||
| 13 | 43.8, d | 2.64, m | 41.7, d | 2.63, m | 41.1, d | 2.47, m |
| 14 | 39.6, t | Ha: 1.95, m | 34.8, t | Ha: 1.59, overlap | 41.0, t | Ha: 1.59, m |
| Hb: 1.14, m | Hb: 1.27, overlap | Hb: 1.47, m | ||||
| 15 | 48.8, t | 2.05, overlap | 81.1, d | 3,61, s | 44.6, d | 1.67, m |
| 16 | 155.5, s | 159.6, s | 45.5, d | 2.62, m | ||
| 17 | 103.2, d | 4.79, d, J = 24.0 | 107.6, d | Ha: 5.06, s | 177.5, s | |
| Hb: 4.97, s | ||||||
| 18 | 72.1, t | Ha: 4.01, d, J = 10.5 | 71.3, t | Ha: 4.36, d, J = 10.3 | 28.9, q | 1.25, s |
| Hb: 3.49, d, J = 10.5 | Hb: 3.93, d, J = 10.3 | |||||
| 19 | 181.8, s | 175.9, s | 184.0, s | |||
| 20 | 15.5, q | 0.99, s | 15.5, q | 0.92, s | 15.5, q | 0.93, s |
| 21 | 171.0 s | 170.1 s | 60.3, t | 4.11, q | ||
| 22 | 20.8, q | 2.06, s | 20.6, q | 1.99, s | 14.3, q | 1.26, s |
| Compounds | MIC (μg·mL−1) | |
|---|---|---|
| MRSA | VRE | |
| 1 | 64 | 64 |
| 2 | 256 | 256 |
| 3 | 256 | 256 |
| 4 | 256 | 256 |
| 5 | 64 | 256 |
| 6 | 256 | 256 |
| 7 | 256 | 256 |
| 8 | 256 | 256 |
| 9 | 256 | 256 |
| 10 | 256 | 256 |
| 11 | 256 | 256 |
| Ampicillin (AMP) | 64 | 0.5 |
| Vancomycin (VAN) | 1 | 512 |
| Doxorubicin hydrochloride (DOX) | 16 | 128 |
| Strains | Agents | MIC in Combination (μg·mL−1) | FICI | |
|---|---|---|---|---|
| MRSA | 3 + DOX | 64 | 2 | 0.375 |
| 3 + DOX | 32 | 4 | 0.375 | |
| 3 + DOX | 32 | 2 | 0.25 | |
| 11 + DOX | 64 | 4 | 0.5 | |
| VRE | 3 + DOX | 64 | 32 | 0.5 |
| 3 + VAN | 16 | 256 | 0.3125 | |
| 5 + VAN | 16 | 64 | 0.3125 | |
| 11 + DOX | 64 | 8 | 0.3125 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.-S.; Wang, Z.-J.; Tian, B.; Zhu, Y.-Y.; Wei, M.-Z.; Zhao, Y.-L.; Luo, X.-D. Three New Ent-Kaurane Diterpenes with Antibacterial Activity from Sigesbeckia orientalis. Molecules 2024, 29, 4631. https://doi.org/10.3390/molecules29194631
Zhou Z-S, Wang Z-J, Tian B, Zhu Y-Y, Wei M-Z, Zhao Y-L, Luo X-D. Three New Ent-Kaurane Diterpenes with Antibacterial Activity from Sigesbeckia orientalis. Molecules. 2024; 29(19):4631. https://doi.org/10.3390/molecules29194631
Chicago/Turabian StyleZhou, Zhong-Shun, Zhao-Jie Wang, Bei Tian, Yan-Yan Zhu, Mei-Zhen Wei, Yun-Li Zhao, and Xiao-Dong Luo. 2024. "Three New Ent-Kaurane Diterpenes with Antibacterial Activity from Sigesbeckia orientalis" Molecules 29, no. 19: 4631. https://doi.org/10.3390/molecules29194631
APA StyleZhou, Z.-S., Wang, Z.-J., Tian, B., Zhu, Y.-Y., Wei, M.-Z., Zhao, Y.-L., & Luo, X.-D. (2024). Three New Ent-Kaurane Diterpenes with Antibacterial Activity from Sigesbeckia orientalis. Molecules, 29(19), 4631. https://doi.org/10.3390/molecules29194631







