Hybrid Hydroxyapatite–Metal Complex Materials Derived from Amino Acids and Nucleobases
Abstract
1. Introduction
- -
- The biodecoration of HAp with carbohydrates has been achieved by directly and covalently bonding nanostructured apatite granules to various polysaccharides, like cellulose [136,137], chitosan [138], pectine [139], carrageenan [140], alginate [141], hyaluronic acid [142,143], and, very recently, acemannan mucopolysaccharide [144]. Additionally, monosaccharides such as D-glucose, D-galactose, and L-fructose are also utilized in this context [145,146].
- -
- The interaction of HAp with different carboxylic acids has been extensively analyzed based on the affinity of Ca2+ ions for carboxylate groups, such as those present in aliphatic (propionic, malonic, glutaric, adipic, maleic, fumaric…), aromatic, polycarboxylic, and lactic/glycolic acid derivatives or even more complex carboxylate-containing organic compounds [147,148,149,150,151]. Studies have also included fatty acids and lipids, including stearic acid, ricinoleic acid, linoleic acid, and oleic acid, among others [152,153,154,155]. In fact, the presence of lipids promotes the precipitation of HAp [156], given, at least in part, that phospholipids trigger the in vivo transformation of OCP into HAp [157]. These phase transitions seem to be crucial in the early stages of bone biosynthesis, probably boosted by the formation of calcium–phospholipid complexes [158].
- -
2. Functionalization of HAp with Amino Acids, Peptides, Proteins, and Metal Complex Hybrids
2.1. HAp and Amino Acids
2.2. HAp and Peptides
2.3. HAp and Proteins
- -
- Type I forms 90% of organic bone mass and is a major protein constituent in various tissues, such as tendons, ligaments, the cornea, or the skin.
- -
- Type II is found in elastic cartilage, providing resilience and support to joints.
- -
- Type III is present in muscles, arteries, and organs, offering structural support and elasticity.
- -
- Type IV is located in skin layers, contributing to basement membranes and tissue organization.
- -
- Type V is found in the cornea of the eye, some skin layers, hair, and placental tissue, contributing towards tissue stability and function.
2.4. HAp–Peptide–Metal Complex Hybrids
3. Functionalization of HAp with Nucleobases, Nucleotides, Nucleic Acids, and Nucleic Acid–Metal Complex Hybrids
3.1. HAp and Nucleobases
3.2. HAp and Nucleotides
3.3. HAp and Nucleic Acids
3.4. HAp–Nucleic Acid–Metal Complex Hybrids
- Natural counterions: Common cellular ions like K⁺, Mg2⁺, and Na⁺ act to neutralize the charges of polyanionic nucleic acids.
- Folding and stabilization: These are essential for the proper folding of nucleic acids and for the stabilization of many RNA structures, as well as for the catalytic function of ribozymes, maintaining DNA structures such as Gua-quartets in telomeres and in Holliday junctions, and for the cross-shape structures formed during genetic recombination.
- Exogenous ions and mimicry: Both physiological and non-physiological (exogenous) metal ions can mimic natural ions, affecting nucleic acid stability and charge neutralization and potentially causing DNA condensation or mutations.
- Damage by Reactive Oxygen Species (ROS): Redox-active metal ions can cause damage to nucleic acids by breaking DNA strands. These ions can be essential (e.g., Cu⁺, Fe2⁺), both in certain disease states and in therapeutic and DNA sequencing applications.
- Phosphodiester reactions: Metal ions are involved in the formation and degradation of nucleic acid phosphodiester bonds. They can provide the OH⁻ nucleophile, polarize P-O bonds, or stabilize transition states or leaving groups.
4. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Le Flem, G. The phosphates of the World and the world of phosphates. In Advances in Inorganic Phosphate Materials Advances in Inorganic Phosphate Materials: Ceramic Transactions; Belharouak, I., Pol, V.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 233, pp. 1–13. [Google Scholar]
- Yakubovich, O.; Khasanova, N.; Antipov, E. Mineral-inspired materials: Synthetic phosphate analogues for battery applications. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, D. Phosphate Rock. An Industry in Transition; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Ameen, S.; Akhtar, M.S. An overview of phosphate mineral and electrochemical detection of phosphate for environmental remediation. In Functional Phosphate Materials and Their Applications [Internet]; Ameen, S., Akhtar, M.S., Shin, H.-S., Eds.; Intech Open Limited: London, UK, 2023. [Google Scholar]
- Huminicki, D.M.C.; Hawthorne, F.C. The crystal chemistry of the phosphate minerals. Rev. Mineral. Geochem. 2002, 48, 123–253. [Google Scholar] [CrossRef]
- Tzavellas, A.-N.; Katrilaka, C.; Karipidou, N.; Kanari, M.; Pitou, M.; Koliakos, G.; Cheva, A.; Choli-Papadopoulou, T.; Aggeli, A.; Tsiridis, E. The “forgotten” hydroxyapatite crystals in regenerative bone tissue engineering: A critical review. Crystals 2024, 14, 448. [Google Scholar] [CrossRef]
- Tung, M.S. Calcium phosphates: Structure, composition, solubility, and stability. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Springer: New York, NY, USA, 1998; pp. 1–19. [Google Scholar]
- Leigh, G.J. Principles of Chemical Nomenclature: A Guide to IUPAC Recommendations, 2011 ed.; RSC Publishing: London, UK, 2011. [Google Scholar]
- Dickens, B.; Prince, E.; Schroeder, L.W.; Brown, W.E. Ca(H2PO4)2, a crystal structure containing unusual hydrogen bonding. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1973, B29, 2057–2070. [Google Scholar] [CrossRef]
- MacLennan, G.; Beevers, C.A. The crystal structure of monocalcium phosphate monohydrate, Ca(H2PO4)2.H2O. Acta Crystallogr. 1956, 9, 187–190. [Google Scholar] [CrossRef]
- McLennan, G.; Beevers, C.A. The crystal structure of dicalcium phosphate CaHPO4. Acta Crystallogr. 1955, 8, 579–583. [Google Scholar] [CrossRef]
- Beevers, C.A. The crystal structure of dicalcium phosphate dihydrate, CaHPO4.2H2O. Acta Crystallogr. 1958, 11, 273–277. [Google Scholar] [CrossRef]
- Brown, W.E. Octacalcium phosphate and hydroxyapatite: Crystal structure of octacalcium phosphate. Nature 1962, 196, 1048–1050. [Google Scholar] [CrossRef]
- Mathew, M.; Brown, W.E.; Schroeder, L.W.; Dickens, B. Crystal structure of octacalcium bis(hydrogenphosphate) tetrakis(phosphate)pentahydrate, Ca8(HPO4)2(PO4)4·5H2O. J. Crystallogr. Spectrosc. Res. 1988, 18, 235–250. [Google Scholar] [CrossRef]
- Mathew, M.; Schroeder, L.W.; Dickens, B.; Brown, W.E. The crystal structure of α-Ca3(PO4)2. Acta Cryst. 1977, B33, 1325–1333. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly springs hydroxyapatite. Acta Cryst. 1969, B25, 1534–1543. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Ikoma, T.; Tanaka, J. Single crystal growth and structure analysis of monoclinic hydroxyapatite. Key Eng. Mater. 2001, 192–195, 287–290. [Google Scholar] [CrossRef]
- Henning, P.A.; Landa-Cánovas, A.R.; Larsson, A.K.; Lidin, S. Elucidation of the crystal structure of oxyapatite by high-resolution electron microscopy. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 170–176. [Google Scholar] [CrossRef]
- Dickens, B.; Brown, W.E.; Kruger, G.J.; Stewart, J.M. Ca4(PO4)2O Tetracalcium diphosphate monoxide. Crystal structure and relationships to Ca5(PO4)3OH and K3Na(SO4)2. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1973, B29, 2046–2056. [Google Scholar] [CrossRef]
- Mandel, N.S. The crystal structure of calcium pyrophosphate dihydrate. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1975, B31, 1730–1734. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Bulina, N.V.; Avakyan, L.A.; Makarova, S.V.; Orehov, I.B.; Bystrov, V.S. Structural features of oxyapatite. Minerals 2023, 13, 102. [Google Scholar] [CrossRef]
- Drouet, C. A comprehensive guide to experimental and predicted thermodynamic properties of phosphate apatite minerals in view of applicative purposes. J. Chem. Thermodyn. 2015, 81, 143–159. [Google Scholar] [CrossRef]
- Somavilla, A.; Caner, L.; Bortoluzzi, E.C.; Santanna, M.A.; dos Santos, D.R. P-legacy effect of soluble fertilizer added with limestone and phosphate rock on grassland soil in subtropical climate region. Soil Tillage Res. 2021, 211, 105021. [Google Scholar] [CrossRef]
- Foley, B.; Greiner, M.; McGlynn, G.; Schmahl, W.W. Anatomical variation of human bone bioapatite crystallography. Crystals 2020, 10, 859. [Google Scholar] [CrossRef]
- Kendall, C.; Eriksen, A.M.H.; Kontopoulos, I.; Collins, M.J.; Turner-Walker, G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 491, 21–37. [Google Scholar] [CrossRef]
- Derocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical gradients in human enamel crystallites. Nature 2020, 583, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, B.; Tang, R. Biomineralization: Biomimetic synthesis of materials and bomimetic regulation of organisms. Chin. J. Chem. 2021, 39, 2071–2082. [Google Scholar] [CrossRef]
- Barinov, S.M.; Rau, J.V.; Cesaro, S.N.; Ďurišin, J.; Fadeeva, I.V.; Ferro, D.; Medvecky, L.; Trionfetti, G. Carbonate release from carbonated hydroxyapatite in the wide temperature rage. J. Mater. Sci. Mater. Med. 2006, 17, 597–604. [Google Scholar] [CrossRef]
- Lu, T.; Yan, S.; Shi, H.; Ye, J. Synthesis, characterization, in vitro cytological responses, and in vivo bone regeneration effects of low-crystalline nanocarbonated hydroxyapatite. ACS Biomater. Sci. Eng. 2023, 9, 918–931. [Google Scholar] [CrossRef]
- The presence of a third polymorph, with triclinic crystal structure, has been reported in apatites. See: Baikie, T.; Mercier, P.H. J.; Elcombe, M.M.; Kim, J.Y.; Le Page, Y.; Mitchell, L.D.; White, T.J.; Whitfield, P.S. Triclinic apatites. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 251–256.
- Yan, Y.; Fang, Y.; Li, J.; Yang, Y.; Chen, F.; Wu, S.; Hooper, T.; Jaiswal, A.; White, T. Transformation of amorphous calcium phosphate to monoclinic nano-hydroxylapatite. CrystEngComm 2022, 24, 7034–7038. [Google Scholar] [CrossRef]
- Haverty, D.; Tofail, S.A.M.; Stanton, K.T.; McMonagle, J.B. Structure and stability of hydroxyapatite: Density functional calculation and Rietveld analysis. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 094103. [Google Scholar] [CrossRef]
- Uskoković, V. The role of hydroxyl channel in defining selected physicochemical peculiarities exhibited by hydroxyapatite. RSC Adv. 2015, 5, 36614–36633. [Google Scholar] [CrossRef]
- Pérez-Solis, R.; Gervacio-Arciniega, J.J.; Joseph, B.; Mendoza, M.E.; Moreno, A. Synthesis and characterization of a monoclinic crystalline phase of hydroxyapatite by synchrotron X-ray powder diffraction and piezoresponse force microscopy. Crystals 2018, 8, 458. [Google Scholar] [CrossRef]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Cryst. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bag, S.; Pal, S.; Mukherjee, A.K. Structural and microstructural characterization of bioapatites and synthetic hydroxyapatite using X-ray powder diffraction and Fourier transform infrared techniques. J. Apply. Cryst. 2006, 39, 385–390. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Rial, R.; Gonzalez-Durruthy, M.; Liu, Z.; Ruso, J.M. Advanced materials based on nanosized hydroxyapatite. Molecules 2021, 26, 3190. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite nanoparticles in drug delivery: Physicochemistry and applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Salvachúa-De la Fuente, M.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Gil-Muñoz, R.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Amorphous vs. nanocrystalline calcium phosphate as efficient nanocarriers of elicitors in vineyards. CrystEngComm 2023, 25, 2372–2378. [Google Scholar] [CrossRef]
- Diez-Escudero, A. High-aspect-ratio nanostructured hydroxyapatite: Towards new functionalities for a classical material. Chem. Sci. 2024, 15, 55–76. [Google Scholar] [CrossRef]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, R.; Yao, J.; Wang, D.; Ke, J. Morphology control of hydroxyapatite through hydrothermal process. J. Alloys Comp. 2008, 457, 555–559. [Google Scholar] [CrossRef]
- Karpikhin, A.E.; Fedotov, A.Y.; Komlev, V.S.; Barinov, S.M.; Sirotinkin, V.P.; Gordeev, A.S.; Shamrai, V.F. Structure of hydroxyapatite powders prepared through dicalcium phosphate dihydrate hydrolysis. Inorg. Mater. 2016, 52, 170–175. [Google Scholar] [CrossRef]
- Szterner, P.; Biernat, M. Effect of reaction time, heating and stirring rate on the morphology of HAp obtained by hydrothermal synthesis. J. Therm. Anal. Calorim. 2022, 147, 13059–13071. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef]
- Alam, M.K.; Hossain, M.S.; Kawsar, M.; Bahadur, N.M.; Ahmed, S. Synthesis of nano-hydroxyapatite using emulsion, pyrolysis, combustion, and sonochemical methods and biogenic sources: A review. RSC Adv. 2024, 14, 3548–3559. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Kesarwani, U.; Basu, B.; Dubey, A.K. 1- and 2- dimensional (1 D/2 D) hydroxyapatite nanocrystals: A deep insight into synthesis strategies and multidimensional applications. Appl. Mater. Today 2024, 36, 1020622. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Nikolaev, A.L.; Kharisov, B.I.; Dorozhkin, S.V.; López, I.; Méndez, Y.P.; de la Fuente, I.G. Enzymatic synthesis of calcium phosphates: A review. Nano-Struct. Nano-Objects 2024, 39, 101214. [Google Scholar] [CrossRef]
- Kareem, Z.; Eyiler, E. Synthesis of hydroxyapatite from eggshells via wet chemical precipitation: A review. RSC Adv. 2024, 14, 21439–21452. [Google Scholar] [CrossRef] [PubMed]
- Hoeher, A.J.; Mergelsberg, S.T.; Borkiewicz, O.J.; Michel, F.M. Impacts of initial Ca/P on amorphous calcium phosphate. Cryst. Growth Des. 2021, 21, 3736–3745. [Google Scholar] [CrossRef]
- Szterner, P.; Biernat, M. The synthesis of hydroxyapatite by hydrothermal process with calcium lactate pentahydrate: The effect of reagent concentrations, pH, temperature, and pressure. Bioinorg. Chem. Appl. 2022, 147, 13059–13071. [Google Scholar] [CrossRef] [PubMed]
- Li-Yun, C.; Chuan-bo, Z.; Jian-feng, H. Influence of temperature, [Ca2+], Ca/P ratio and ultrasonic power on the crystallinity and morphology of hydroxyapatite nanoparticles prepared with a novel ultrasonic precipitation method. Mater. Lett. 2005, 59, 1902–1906. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. Bioactive and biostable hyaluronic acid-pullulan dermal hydrogels incorporated with biomimetic hydroxyapatite spheres. Mater. Sci. Eng. C 2020, 112, 110906. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.K.; Giang, L.T.; Huy, T.Q.; Viet, P.H.; Migliaresi, C. Surfactant assisted size control of hydroxyapatite nanorods for bone tissue engineering. Colloids Surf. B 2014, 116, 666–673. [Google Scholar] [CrossRef]
- Kuki, Y.; Morinaga, K.; Uemura, N.; Okamura, T.; Hontsu, S.; Hashimoto, Y.; Baba, S. Biocompatibility of dental implants coated with hydroxyapatite using pulsed Er:YAG laser deposition. Dent. Mater. J. 2024, 43, 269–275. [Google Scholar] [CrossRef]
- Lu, B.Q.; Zhu, Y.J.; Chen, F. Highly flexible and nonflammable inorganic hydroxyapatite paper. Chem. Eur. J. 2014, 20, 1242–1246. [Google Scholar] [CrossRef]
- Zhang, H.; Darvell, B.W. Morphology and structural characteristics of hydroxyapatite whiskers: Effect of the initial Ca concentration, Ca/P ratio and pH. Acta Biomater. 2011, 7, 2960–2968. [Google Scholar] [CrossRef]
- Rodríguez Lugo, V.; Castaño, V.M.; Rubio-Rosas, E. Biomimetic growth of hydroxylapatite on SiO2-PMMA hybrid coatings. Mater. Lett. 2016, 184, 265–268. [Google Scholar] [CrossRef]
- An, L.; Li, W.; Xu, Y.; Zeng, D.; Cheng, Y.; Wang, G. Controlled additive-free hydrothermal synthesis and characterization of uniform hydroxyapatite nanobelts. Ceram. Int. 2016, 42, 3104–3112. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Mendoza-Anaya, D.; Sánchez-Campos, D.; Fernandez-García, M.E.; Salinas-Rodríguez, E.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V. The pH effect on the growth of hexagonal and monoclinic hydroxyapatite synthesized by the hydrothermal method. J. Nanomater. 2020, 2020, 5912592. [Google Scholar] [CrossRef]
- Awan, A.A.; Liaqat, U.; Hussain, Z. The effect of pH on the morphological transformation of nanocrystalline hydroxyapatite during wet chemical synthesis. J. Korean Chem. Soc. 2023, 60, 1010–1027. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Chuprunov, K.; Yudin, A.; Lysov, D.; Kolesnikov, E.; Kuznetsov, D.; Leybo, D.; Ilinykh, I.; Godymchuk, A. The pH level influence on hydroxyapatite phase composition synthesized with hydrothermal method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 731, 012023. [Google Scholar] [CrossRef]
- Suchanek, K.; Bartkowiak, A.; Perzanowski, M.; Marszalek, M. From monetite plate to hydroxyapatite nanofibers by monoethanolamine assisted hydrothermal approach. Sci. Rep. 2018, 8, 15408. [Google Scholar] [CrossRef]
- Reynaud, C.; Thomas, C.; Casale, S.; Nowak, S.; Costentin, G. Development of a thermodynamic approach to assist the control of the precipitation of hydroxyapatites and associated calcium phosphates in open systems. CrystEngComm 2021, 23, 4857–4870d. [Google Scholar] [CrossRef]
- Reynaud, C.; Thomas, C.; Costentin, G. On the comprehensive precipitation of hydroxyapatites unraveled by a combined kinetic-thermodynamic approach. Inorg. Chem. 2022, 61, 3296–3308. [Google Scholar] [CrossRef]
- Scalera, F.; Gervaso, F.; Sanosh, K.P.; Sannino, A.; Licciulli, A. Influence of the calcination temperature on morphological and mechanical properties of highly porous hydroxyapatite scaffolds. Ceram. Int. 2013, 39, 4839–4846. [Google Scholar] [CrossRef]
- Ptacek, P. Apatites and Their Synthetic Analogues. Synthesis, Structure, Properties and Applications; Intech Open Limited: London, UK, 2016. [Google Scholar]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; Klein Gunnewiek, R.; Grosfeld, E.C.; De Vries, R.B.M.; Habibović, P.; Jansen, J.A.; Van Den Beucken, J.J.J.P. Bioinorganic supplementation of calcium phosphate-based bone substitutes to improve: In vivo performance: A systematic review and meta-analysis of animal studies. Biomater. Sci. 2020, 8, 4792–4809. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Vakhnovetsky, J.; Vakhnovetsky, A.; Morgano, S.M. Functional role of inorganic trace elements in dentin apatite tissue. Part III: Se, F., Ag, and B. J. Trace Elem. Med. Biol. 2022, 72, 126990. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.G.; Surdu, V.A.; Paduraru, A.V.; Andronescu, E. Current development in biomaterials-hydroxyapatite and bioglass for applications in biomedical field: A review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.E.; Vasile, O.R.; Andronescu, E.; Ficai, A. Latest research of doped hydroxyapatite for bone tissue engineering. Int. J. Mol. Sci. 2023, 24, 13157. [Google Scholar] [CrossRef]
- Junqiang, Q.; Chao, G.; Dongyang, N.; Haotian, W.; Bing, X.; Guohua, X. Characteristics and application of bone repair materials of metal ion doped hydroxyapatite. Chin. J. Tissue Eng. Res. 2023, 27, 3415–3422. [Google Scholar]
- Anenburg, M.; Panikorovskii, T.L.; Jennings, E.S.; Shendrik, R.Y.; Antonov, A.A.; Gavrilenko, V. An apatite-group praseodymium carbonate fluoroxybritholite: Hydrothermal synthesis, crystal structure, and implications for natural and synthetic britholites. Inorg. Chem. 2024, 63, 11788–11801. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Donskaya, N.O.; Valeev, D.V.; Fomin, A.S.; Murzakhanov, F.F.; Leonov, A.V.; Konovalov, A.A.; Antonova, O.S.; Shoppert, A.A.; Kudryavtsev, E.A.; et al. Mesoporous molybdate-substituted hydroxyapatite nanopowders obtained via a hydrothermal route. Ceram. Int. 2024, 50, 17404–1741829. [Google Scholar] [CrossRef]
- Li, W.P.; Ma, D.S.; Higginbotham, C.; Hoffman, T.; Cutler, C.S.; Jurisson, S.S. Development of an in vitro model for assessing the in vivo stability of lanthanide chelates. Nucl. Med. Biol. 2001, 28, 145–154. [Google Scholar] [CrossRef]
- Makris, G.; Tseligka, E.D.; Pirmettis, I.; Papadopoulos, M.S.; Vizirianakis, I.S.; Papagiannopoulou, D. Development and pharmacological evaluation of new bone-targeted 99mTc-radiolabeled bisphosphonates. Mol. Pharm. 2016, 13, 2301–2317. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E.; Klouras, N. Inhibition of hydroxyapatite crystal growth by substituted titanocenes. Langmuir 2000, 16, 6745–6749. [Google Scholar] [CrossRef]
- Li, X.; Coffer, J.L.; Chen, Y.; Pinizzotto, R.F.; Newey, J.; Canham, L.T. Transition metal complex-doped hydroxyapatite layers on porous silicon. J. Am. Chem. Soc. 1998, 120, 11706–11709. [Google Scholar] [CrossRef]
- Burgos, A.E.; Belchior, J.C.; Sinisterra, R.D. Controlled release of rhodium(II) carboxylates and their association complexes with cyclodextrins from hydroxyapatite matrix. Biomaterials 2002, 23, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Vitha, T.; Kubíček, V.; Hermann, P.; Kolar, Z.I.; Wolterbeek, H.T.; Peters, J.A.; Lukeš, I. Complexes of DOTA—Bisphosphonate conjugates: Probes for determination of adsorption capacity and affinity constants of hydroxyapatite. Langmuir 2008, 24, 1952–1958. [Google Scholar] [CrossRef]
- Çalişkan, F.; Tuna, M.; Akça, S.G. Investigation on effects of Schiff base complex to hydroxyapatite bioceramics. Acta Phys. Pol. A 2015, 127, 1393–1396. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, C.; Zhou, Y.; Luo, J.; Li, J. Universal and biocompatible hydroxyapatite coating induced by phytic acid-metal complex multilayer. Colloids Surf. B Biointerfaces 2018, 169, 478–485. [Google Scholar] [CrossRef]
- Rangel Resgala, L.C.; Scardini Santana, H.; Mendoça Portela, B.; Souza Zanovello, M.V.; Santos da Costa, C.; Santamaria Niño, O.M.; Endlich Bicudo, N.; Zipinotti dos Santos, D.; Lang Podratz, P.; Da Cunha, M.; et al. Effects of tributyltin (TBT) on rat bone and mineral metabolism. Cell. Physiol. Biochem. 2019, 52, 1166–1177. [Google Scholar]
- Riedl, C.A.; Rosner, A.; Harringer, S.; Salomon, P.; Hejl, M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Water-soluble trithiolato-bridged dinuclear ruthenium(II) and osmium(II) arene complexes with bisphosphonate functionalized ligands as anticancer organometallics. J. Inorg. Biochem. 2021, 225, 111618. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Ruan, D.; Xu, H.; Wang, F.; Lei, W.; Xia, M. Efficient removal of heavy metal ions by diethylenetriaminepenta (methylene phosphonic) acid-doped hydroxyapatite. Sci. Total Environ. 2022, 849, 157557. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Pasquini, D.; Mattoso, L.H.C. Enhanced antibacterial activity of wound dressings based on alginate/hydroxyapatite modified with copper and naproxen. J. Mater. Res. 2024, 39, 762–773. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Mendes, R.F.; Figueira, F.; Gaspar, V.M.; Mano, J.F.; Braga, S.S.; Rocha, J.; Almeida Paz, F.A. Bone tissue disorders: Healing through coordination chemistry. Chem. A Eur. J. 2020, 26, 15416–15437. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, D. Osteocompatible zinc-copper substituted hydroxyapatite reinforced biocomposites for bone tissue regeneration. J. Pharm. Innov. 2024, 19, 26. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, D.; Bao, W.; Ding, R.; Shen, Z.; Huang, W.; Lu, Y.; Zhang, P.; Sun, Y.; Chen, H.; et al. Polydopamine-functionalized strontium alginate/hydroxyapatite composite microhydrogel loaded with vascular endothelial growth factor promotes bone formation and angiogenesis. ACS Appl. Mater. Interfaces 2024, 16, 4462–4477. [Google Scholar] [CrossRef] [PubMed]
- Bin Jumah, M.N.; Al Othman, S.I.; Alomari, A.A.; Allam, A.A.; Abukhadra, M.R. Potentiality of zinc phosphate@hydroxyapatite/β-cyclodextrin composites for carrying cisplatin: Loading, release and cytotoxicity. New J. Chem. 2024, 48, 11243–11258. [Google Scholar] [CrossRef]
- Nudelman, F.; Sommerdijk, N.J.M. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef]
- Cartwright, J.H.E.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef]
- Oggerin, M.; Rodriguez, N.; Del Moral, C.; Amils, R. Fungal jarosite biomineralization in Río Tinto. Res. Microbiol. 2014, 165, 719–725. [Google Scholar] [CrossRef]
- Ping, H.; Xie, H.; Wan, Y.; Zhang, Z.; Zhang, J.; Xiang, M.; Xie, J.; Wang, H.; Wang, W.; Fu, Z. Confinement controlled mineralization of calcium carbonate within collagen fibrils. J. Mater. Chem. B 2016, 4, 880–886. [Google Scholar] [CrossRef]
- Faivre, D.; Godec, T.U. From bacteria to mollusks: The principles underlying the biomineralization of iron oxide materials. Angew. Chem. Int. Ed. 2015, 54, 4728–4747. [Google Scholar] [CrossRef]
- Jones, S.R.; Wilson, T.D.; Brown, M.E.; Rahn-Lee, L.; Yu, Y.; Fredriksen, L.L.; Ozyamak, E.; Komeili, A.; Chang, M.C.Y. Genetic and biochemical investigations of the role of MamP in redox control of iron biomineralization in Magnetospirillum magneticum. Proc. Natl. Acad. Sci. USA 2015, 112, 3904–39092. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.U.P.A.; Porter, S.M.; Sun, C.-Y.; Xiao, S.; Gibson, B.M.; Shenkar, N.; Knoll, A.H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wu, X.; Dong, Y.; Shao, R.; Chen, X.; Zhou, P.; Xu, F. In vivo behavior of bioactive glass-based composites in animal models for bone regeneration. Biomater. Sci. 2021, 9, 1924–1944. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.S.; Almeida Paz, F.A.; Braga, S.S. Bisphosphonates, old friends of bones and new trends in clinics. J. Med. Chem. 2021, 64, 1260–1282. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of nanoparticles in bone tissue engineering; A review on the molecular mechanisms driving osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Chen, F.; Ma, X.; Yu, Y.; Liu, C. Calcium phosphate bone cements: Their development and clinical applications. In Developments and Applications of Calcium Phosphate Bone Cements; Springer Series in Biomaterials Science and, Engineering; Liu, C., He, H., Eds.; Springer: Singapore, 2018; Volume 9, pp. 1–39. [Google Scholar]
- Thirumalai, P. Hydroxyapatite. Advances in Composite Materials, Biomedical Applications and Its Technological Facets; Thirumalai, P., Ed.; IntechOpen: Zagreb, Croatia, 2018. [Google Scholar]
- Campodoni, E.; Montanari, M.; Artusi, C.; Basi, G.; Furlani, F.; Montesi, M.; Pansseri, S.; Sanri, M.; Tampieri, A. Calcium-based biomineralization: A smart approach for the design of novel multifunctional hybrid materials. J. Compos. Sci. 2021, 5, 278. [Google Scholar] [CrossRef]
- Boccaccio, A. Design of Materials for Bone Tissue Scaffolds; Boccaccio, A., Ed.; MDPI: Basel, Switzerland, 2022. [Google Scholar]
- Bazin, D.; Chevallier, P.; Matzen, G.; Jungers, P.; Daudon, M. Heavy elements in urinary stones. Urol. Res. 2007, 35, 179–184. [Google Scholar] [CrossRef]
- Louvet, L.; Bazin, D.; Büchel, J.; Steppan, S. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS ONE 2015, 10, e0115342. [Google Scholar] [CrossRef]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef]
- Omelon, S.; Ariganello, M.; Bonucci, E.; Grynpas, M.; Nanci, A. A review of phosphate mineral nucleation in biology and geobiology. Calcif. Tissue Int. 2013, 93, 382–396. [Google Scholar] [CrossRef]
- Schmidt, F.N.; Zimmermann, E.A.; Campbell, G.M.; Sroga, G.E.; Püschel, K.; Amling, M.; Tang, S.Y.; Vashishth, D.; Busse, B. Assessment of collagen quality associated with non-enzymatic cross-links in human bone using Fourier-transform infrared imaging. Bone 2017, 97, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Moraveji, M.; Nezafati, N.; Pazouki, M.; Hesaraki, S. Bioorthogonal surface modified α-TCP-based bone filler for enhancement of apatite formation and bioactivity. Ceram. Int. 2019, 45, 5981–5986. [Google Scholar] [CrossRef]
- Paramasivan, M.; Sampath Kumar, T.S.; Kanniyappan, H.; Muthuvijayan, V.; Chandra, T.S. Microbial biomineralization of hydroxyapatite nanocrystals using Bacillus tequilensis. Ceram. Int. 2023, 49, 5621–5629. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Tourkova, I.L.; Nelson, D.J.; Dobrowolski, S.F.; Schlesinger, P.H. Epithelial-like transport of mineral distinguishes bone formation from other connective tissues. J. Cell. Biochem. 2023, 124, 1889–1899. [Google Scholar] [CrossRef]
- Robin, M.; Von Euw, S.; Renaudin, G.; Gomes, S.; Krafft, J.M.; Nassif, N.; Azaïs, T.; Costentin, G. Insights into OCP identification and quantification in the context of apatite biomineralization. CrystEngComm 2020, 22, 2728–2742. [Google Scholar] [CrossRef]
- Feng, X. Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [PubMed]
- Schwarcz, H.P. The ultrastructure of bone as revealed in electron microscopy of ion-milled sections. Semin. Cell Dev. Biol. 2015, 46, 44–50. [Google Scholar] [CrossRef]
- Pang, S.; Schwarcz, H.P.; Jasiuk, I. Interfacial bonding between mineral platelets in bone and its effect on mechanical properties of bone. J. Mech. Behav. Biomed. Mater. 2021, 113, 104132. [Google Scholar] [CrossRef]
- Hong, M.H.; Lee, J.H.; Jung, H.S.; Shin, H.; Shin, H. Biomineralization of bone tissue: Calcium phosphate-based inorganics in collagen fibrillar organic matrices. Biomater. Res. 2022, 26, 42. [Google Scholar] [CrossRef]
- Godberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. 2011, 3, 711. [Google Scholar]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-based biomimetically mineralized composite materials in human hard tissue repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Jagadeeshanayaka, N.; Awasthi, S.; Jambagi, S.C.; Srivastava, C. Bioactive surface modifications through thermally sprayed hydroxyapatite composite coatings: A review of selective reinforcements. Biomater. Sci. 2022, 10, 2484–2523. [Google Scholar] [CrossRef]
- Fukumoto, S.; Nakamura, T.; Yamada, A.; Arakaki, M.; Saito, K.; Xu, J.; Fukumoto, E.; Yamada, Y. New insights into the functions of enamel matrices in calcified tissues. Jpn. Dent. Sci. Rev. 2014, 50, 47–54. [Google Scholar] [CrossRef]
- Lama-Odría, M.C.; Valle, L.J.; Puiggalí, J. Hydroxyapatite biobased materials for treatment and diagnosis of cancer. Int. J. Mol. Sci. 2022, 23, 11352. [Google Scholar] [CrossRef]
- Salama, A. Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kitamura, T.; Morita, Y.; Mizuno, M.; Yubuta, K.; Teshima, K. Growth of dispersed hydroxyapatite crystals highly intertwined with TEMPO-oxidized cellulose nanofiber. CrystEngComm 2020, 22, 4933–4941. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Fox, J.T. Synthesis and appraisal of a hydroxyapatite/pectin hybrid material for zinc removal from water. RSC Adv. 2019, 9, 21095–21105. [Google Scholar] [CrossRef]
- Alinavaz, S.; Mahdavinia, G.R.; Jafari, H.; Hazrati, M.; Akbari, A. Hydroxyapatite (HA)-based hybrid bionanocomposite hydrogels: Ciprofloxacin delivery, release kinetics and antibacterial activity. J. Mol. Struct. 2021, 1225, 129095. [Google Scholar] [CrossRef]
- Szurkowska, K.; Zgadzaj, A.; Kuras, M.; Kolmas, J. Novel hybrid material based on Mg2+ and SiO44- co-substituted nano-hydroxyapatite, alginate and chondroitin sulphate for potential use in biomaterials engineering. Ceram. Int. 2018, 44, 18551–18559. [Google Scholar] [CrossRef]
- Xiong, H.; Du, S.; Ni, J.; Zhou, J.; Yao, J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials 2016, 94, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Mu, Z.; Yu, Y.; Zhang, L.; Hu, J. Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery. RSC Adv. 2016, 6, 101790–101799. [Google Scholar] [CrossRef]
- Negi, D.; Bhavya, K.; Pal, D.; Singh, Y. Acemannan coated, cobalt-doped biphasic calcium phosphate nanoparticles for immunomodulation regulated bone regeneration. Biomater. Sci. 2024, 12, 3672–3685. [Google Scholar] [CrossRef]
- Russo, L.; Landi, E.; Tampieri, A.; Natalello, A.; Doglia, S.M.; Gabrielli, L.; Cipolla, L.; Nicotra, F. Sugar-decorated hydroxyapatite: An inorganic material bioactivated with carbohydrates. Carbohydr. Res. 2011, 346, 1564–1568. [Google Scholar] [CrossRef]
- Sandria, M.; Natalellob, A.; Binib, D.; Gabriellib, L.; Cipolla, L.; Nicotra, F. Sweet and salted: Sugars meet hydroxyapatite. Synlett 2011, 13, 1845–1848. [Google Scholar]
- Skinner, J.C.; Presser, H.J.; Scott, R.P.; Wilson, A.D. Adhesion of carboxylate cements to hydroxyapatite. I. The effect of the structure of aliphatic carboxylates on their uptake by hydroxyapatite. Biomaterials 1986, 7, 438–440. [Google Scholar] [CrossRef]
- Scott, R.P.; Jackson, A.M.; Wilson, A.D. Adhesion of carboxylate cements to hydroxyapatite. II. Adsorption of aromatic carboxylates. Biomaterials 1990, 11, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Scott, R.P.; Jackson, A.M.; Wilson, A.D. Adhesion of carboxylate cements to hydroxyapatite. III. Adsorption of poly(alkenoic acids). Biomaterials 1990, 11, 379–384. [Google Scholar] [CrossRef]
- Tenhuisen, K.S.; Brown, P.W.; Reed, C.S.; Allcock, H.R. Low temperature synthesis of a self-assembling composite: Hydroxyapatite-poly[bis(sodium carboxylatophenoxy)phosphazene]. J. Mater. Sci. Mater. Med. 1996, 7, 673–682. [Google Scholar] [CrossRef]
- Soheilmoghaddam, M.; Padmanabhan, H.; Cooper-White, J.J. Biomimetic cues from poly(lactic- co -glycolic acid)/hydroxyapatite nano-fibrous scaffolds drive osteogenic commitment in human mesenchymal stem cells in the absence of osteogenic factor supplements. Biomater. Sci. 2020, 8, 5677–5689. [Google Scholar] [CrossRef]
- Placente, D.; Benedini, L.A.; Baldini, M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Multi-drug delivery system based on lipid membrane mimetic coated nano-hydroxyapatite formulations. Int. J. Pharm. 2018, 548, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Lett, J.A.; Sundareswari, M.; Ravichandran, K.; Latha, M.B.; Sagadevan, S.; Bin Johan, M.R. Tailoring the morphological features of sol-gel synthesized mesoporous hydroxyapatite using fatty acids as an organic modifier. RSC Adv. 2019, 9, 6228–6240. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Yao, S.; Wang, W.; Qi, L.; Wang, Y.; Zhang, H. Hydroxyapatite nanomaterials with tailored length regulated by different fatty acids. Micro Nano Lett. 2021, 16, 649–655. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, J.N.; Xiao, G.Y.; Huang, S.Y.; Xu, W.L.; Yan, W.X.; Lu, Y.P. Investigation of various fatty acid surfactants on the microstructure of flexible hydroxyapatite nanofibers. CrystEngComm 2021, 23, 7049–7055. [Google Scholar] [CrossRef]
- Odutuga, A.A.; Prout, R.E.S.; Hoare, R.J. Hydroxyapatite precipitation in vitro by lipids extracted from mammalian hard and soft tissues. Arch. Oral Biol. 1975, 20, 311–316. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Eanes, E.D. Effect of phospholipids on the transformation of amorphous calcium phosphate to hydroxyapatite in vitro. Calcif. Tissue Res. 1975, 19, 197–210. [Google Scholar] [CrossRef]
- Boskey, A.L.; Posner, A.S. The role of synthetic and bone extracted Ca-phospholipid-PO4 complexes in hydroxyapatite formation. Calcif. Tissue Res. 1977, 23, 251–258. [Google Scholar] [CrossRef]
- Verma, G.; Shetake, N.G.; Pandrekar, S.; Pandey, B.; Hassan, P.; Priyadarsini, K. Development of surface functionalized hydroxyapatite nanoparticles for enhanced specificity towards tumor cells. Eur. J. Pharm. Sci. 2020, 144, 105206. [Google Scholar] [CrossRef]
- Baeza, A.; Izquierdo-Barba, I.; Vallet-Regí, M. Biotinylation of silicon-doped hydroxyapatite: A new approach to protein fixation for bone tissue regeneration. Acta Biomater. 2010, 6, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Web of Science. Available online: https://www.webofscience.com/wos/ (accessed on 11 July 2024).
- Cartier, P. Les constituants mineraux des tissus calcifies. 1.La structure minerale de los, de la dentine et du cement. Bull. Soc. Chim. Biol. 1948, 30, 65–73. [Google Scholar]
- Uneyama, H.; Kobayashi, H.; Tonouchi, N. New Functions and potential applications of amino acids. In Amino Acid Fermentation; Yokota, A., Ikeda, M., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Tokyo, Japan, 2016; Volume 159. [Google Scholar]
- Flores, E.; Martinez, E.; Rodriguez, L.E.; Weber, J.M.; Khodayari, A.; Vandervelde, D.G.; Barge, L.M. Effects of amino acids on phosphate adsorption onto iron (oxy)hydroxide minerals under early earth conditions. ACS Earth Space Chem. 2021, 5, 1048–1057. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050. [Google Scholar] [CrossRef] [PubMed]
- Fleet, M.E. Infrared spectra of carbonate apatites: ν2-region bands. Biomaterials 2009, 30, 1473–1481. [Google Scholar] [CrossRef]
- Pham Minh, D.; Tran, N.D.; Nzihou, A.; Sharrock, P. Carbonate-containing apatite (CAP) synthesis under moderate conditions starting from calcium carbonate and orthophosphoric acid. Mater. Sci. Eng. C 2013, 33, 2971–2980. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Transformable carbonate apatite chains as a novel type of bone graft. Adv. Healthc. Mater. 2024, 13, e2303245. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Giardino, R.; Bigi, A. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials 2006, 27, 4428–4433. [Google Scholar] [CrossRef]
- Indira, J.; Malathi, K.S. Comparison of template mediated ultrasonic and microwave irradiation method on the synthesis of hydroxyapatite nanoparticles for biomedical applications. Mater. Today Proc. 2021, 51, 1765–1769. [Google Scholar] [CrossRef]
- Jahromi, M.T.; Cerruti, M. Amino acid/ion aggregate formation and their role in hydroxyapatite precipitation. Cryst. Growth Des. 2015, 15, 1096–1104. [Google Scholar] [CrossRef]
- Moussa, S.B.; Bachouâ, H.; Gruselle, M.; Beaunier, P.; Flambard, A.; Badraoui, B. Hybrid organic-inorganic materials based on hydroxyapatite structure. J. Solid State Chem. 2017, 248, 171–177. [Google Scholar] [CrossRef]
- Krukowski, S.; Lysenko, N.; Kolodziejski, W. Synthesis and characterization of nanocrystalline composites containing calcium hydroxyapatite and glycine. J. Solid State Chem. 2018, 264, 59–67. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Y.; Cai, Y.; Yao, J. Effect of amino acids on the crystal growth of hydroxyapatite. Mater. Lett. 2012, 68, 361–363. [Google Scholar] [CrossRef]
- Bigi, E.A.; Boanini, M.; Gazzano, M.A.; Kordecki, K. Rubini. Microstructural investigation of hydroxyapatite-polyelectrolyte composites. J. Mater. Chem. 2004, 14, 274–279. [Google Scholar] [CrossRef]
- Jack, K.S.; Vizcarra, T.G.; Trau, M. Characterization and surface properties of amino acid-modified, carbonate-containing hydroxyapatite particles. Langmuir 2007, 23, 12233–12242. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-McQuire, R.; Chane-Ching, J.Y.; Vignaud, E.; Lebuglec, A.; Manna, S. Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 2004, 14, 2277–2281. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. The effect of acidic amino acids on hydroxyapatite crystallization. J. Cryst. Growth 2000, 217, 410–415. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Hydroxyapatite crystallization in the presence of serine, tyrosine and hydroxyproline amino acids with polar side groups. J. Cryst. Growth 2000, 216, 443–449. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. The crystallization of hydroxyapatite in the presence of lysine. J. Colloid Int. Sci. 2000, 231, 207–212. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Inhibition of hydroxyapatite formation in aqueous solutions by amino acids with hydrophobic side groups. Langmuir 2000, 16, 6739–6744. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Hydroxyapatite crystallization in the presence of amino acids with uncharged polar side groups: glycine, cysteine, cystine, and glutamine. Langmuir 2001, 17, 1074–1079. [Google Scholar] [CrossRef]
- Spanos, N.; Klepetsanis, P.G.; Koutsoukos, P.G. Model studies on the interaction of amino acids with biominerals: The effect of L-serine at the hydroxyapatite-water interface. J. Colloid Interface Sci. 2001, 236, 260–265. [Google Scholar] [CrossRef]
- Dalas, E.; Malkaj, P.; Vasileiou, Z.; Kanellopoulou, D.G. The effect of Leucine on the crystal growth of calcium phosphate. J. Mater. Sci. Mater. Med. 2008, 19, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Hoff, S.E.; Liu, J.; Heinz, H. Binding mechanism and binding free energy of amino acids and citrate to hydroxyapatite surfaces as a function of crystallographic facet, pH, and electrolytes. J. Colloid Interface Sci. 2022, 605, 685–700. [Google Scholar] [CrossRef]
- Kresak, M.; Moreno, E.C.; Zahradnik, R.T.; Hay, D.I. Adsorption of amino acids onto hydroxyapatite. J. Colloid Interface Sci. 1977, 59, 283–292. [Google Scholar] [CrossRef]
- Sharma, R.; Pandey, R.R.; Gupta, A.A.; Kar, S.; Dhayal, M. In situ amino acid functionalization and microstructure formation of hydroxyapatite nanoparticles synthesized at different pH by precipitation route. Mater. Chem. Phys. 2012, 133, 718–725. [Google Scholar] [CrossRef]
- Ratnatilaka Na Bhuket, P.; Li, Y.; Yu, S.M. From collagen mimetics to collagen hybridization and back. Acc. Chem. Res. 2024, 57, 1649–1657. [Google Scholar] [CrossRef]
- Saurav, S.; Sharma, P.; Kumar, A.; Tabassum, Z.; Girdhar, M.; Mamidi, N.; Mohan, A. Harnessing natural polymers for nano-scaffolds in bone tissue engineering: A comprehensive overview of bone disease treatment. Curr. Issues Mol. Biol. 2024, 46, 585–611. [Google Scholar] [CrossRef]
- Kawasaki, A.; Kawano, K.; Terada, Y.; Hirayasu, R. Interaction of hydroxyapatite with amino acids. J. Jpn. Prostodonthic Soc. 1989, 33, 522–527. [Google Scholar] [CrossRef] [PubMed]
- El Shafei, G.M.S.; Moussa, N.A. Adsorption of some essential amino acids on hydroxyapatite. J. Colloid Interface Sci. 2001, 238, 160–166. [Google Scholar] [CrossRef]
- Chauhan, N.; Singh, Y. L-histidine controls the hydroxyapatite mineralization with plate-like morphology: Effect of concentration and media. Mater. Sci. Eng. C 2021, 120, 111669. [Google Scholar] [CrossRef] [PubMed]
- Gorbunoff, M.J.; Timasheff, S.N. The interaction of proteins with hydroxyapatite. III. Mechanism. Anal. Biochem. 1984, 136, 440–445. [Google Scholar] [CrossRef]
- El Rhilassi, A.; Mourabet, M.; Bennani-Ziatni, M.; El Hamri, R.; Taitai, A. Interaction of some essential amino acids with synthesized poorly crystalline hydroxyapatite. J. Saudi Chem. Soc. 2016, 20, S632–S640. [Google Scholar] [CrossRef]
- El Rhilassi, A.; Oukkass, O.; Bennani-Ziatni, M. Isotherms, kinetics, and thermodynamics of methionine adsorption onto poorly crystalline hydroxyapatite with different Ca/P ratios. Curr. Chem. Lett. 2023, 12, 781–798. [Google Scholar] [CrossRef]
- Gorbunoff, M.J. The interaction of proteins with hydroxyapatite. I. Role of protein charge and structure. Anal. Biochem. 1984, 136, 425–432. [Google Scholar] [CrossRef]
- Wassell, D.T.H.; Hall, R.C.; Embery, G. Adsorption of bovine serum albumin onto hydroxyapatite. Biomaterials 1995, 16, 697–702. [Google Scholar] [CrossRef]
- Gorbunoff, M.J. The interaction of proteins with hydroxyapatite. II. Role of acidic and basic groups. Anal. Biochem. 1984, 136, 433–439. [Google Scholar] [CrossRef]
- Kollath, V.O.; den Broeck, F.V.; Fehér, K.; Martins, J.C.; Luyten, J.; Traina, K.; Mullens, S.; Cloots, R. A modular approach to study protein adsorption on surface modified hydroxyapatite. Chem. Eur. J. 2015, 21, 10497–10505. [Google Scholar] [CrossRef]
- Victor, S.P.; Sharma, C.P. Tryptophan complexed hydroxyapatite nanoparticles for immunoglobulin adsorption. J. Mater. Sci. Mater. Med. 2011, 22, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.H.; Matsumoto, T.; Ishihara, S.; Nakahira, A.; Okazaki, M.; Sohmura, T. Apatite containing aspartic acid for selective protein loading. J. Dent. Res. 2010, 89, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Holden, D.T.; Morato, N.M.; Cooks, R.G. Aqueous microdroplets enable abiotic synthesis and chain extension of unique peptide isomers from free amino acids. Proc. Natl. Acad. Sci. USA 2022, 119, e2212642119. [Google Scholar] [CrossRef]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.C. Chemopreventive role of food-derived proteins and peptides: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2358–2376. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Kang, Q. Neuromodulation of bone: Role of different peptides and their interactions (review). Mol. Med. Rep. 2021, 23, 320. [Google Scholar] [CrossRef]
- Pérez, J.J. Exploiting knowledge on structure-activity relationships for designing peptidomimetics of endogenous peptides. Biomedicines 2021, 9, 651–669. [Google Scholar] [CrossRef]
- Kastin, A.J. (Ed.) Handbook of Biological Active Peptides; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.J. Versatile types of organic/inorganic nanohybrids: From strategic design to biomedical applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Zhang, G.; Su, R.; Qi, W. Biomimetic mineralization based on self-assembling peptides. Chem. Soc. Rev. 2023, 52, 1549–1590. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Al-Kattan, A.; Choimet, M.; Tourrette, A.; Santran, V.; Dexpert-Ghys, J.; Pipy, B.; Brouillet, F.; Tourbin, M. Biomimetic apatite-based functional nanoparticles as promising newcomers in nanomedicine: Overview of 10 years of initiatory research. J. Gen. Pract. Med. Diagn. 2015, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodríguez, G.B.; Patrício, T.; Esposti, L.D.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10, eaan6205. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Carella, F.; Degli Esposti, L.; Adamiano, A.; Catalucci, D.; Modica, J.; Bragonzi, A.; Vitali, A.; Torelli, R.; Sanguinetti, M.; et al. Biocompatible antimicrobial colistin loaded calcium phosphate nanoparticles for the counteraction of biofilm formation in cystic fibrosis related infections. J. Inorg. Biochem. 2022, 230, 111751. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, L.A.; Beebe, T.P.; Schneider, J.P. Hydroxyapatite surface-induced peptide folding. J. Am. Chem. Soc. 2007, 129, 5281–5287. [Google Scholar] [CrossRef]
- Fujisawa, R.; Mizuno, M.; Nodasaka, Y.; Kuboki, Y. Attachment of osteoblastic cells to hydroxyapatite crystals by a synthetic peptide (Glu7-Pro-Arg-Gly-Asp-Thr) containing two functional sequences of bone sialoprotein. Matrix Biol. 1997, 16, 21–28. [Google Scholar] [CrossRef]
- Ling, C.; Zhao, W.; Wang, Z.; Chen, J.; Ustriyana, P.; Gao, M.; Sahai, N. Structure-activity relationships of hydroxyapatite-binding peptides. Langmuir 2020, 36, 2493–2742. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage-novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Phage display. Immunotechnology 1995, 1, 87–94. [Google Scholar] [CrossRef]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Addison, W.N.; Miller, S.J.; Ramaswamy, J.; Mansouri, A.; Kohn, D.H.; McKee, M.D. Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences. Biomaterials 2010, 31, 9422–9430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, Z.; Cui, Q.; Sahai, N. Predicting the structure-activity relationship of hydroxyapatite-binding peptides by enhanced-sampling molecular simulation. Langmuir 2016, 32, 7009–7022. [Google Scholar] [CrossRef]
- Do Nascimento, M.; Almeida, A.R.S.; Hirata, M.C.; Elzubair, A.; da Rocha, D.N.; da Silva, M.H.P. Biomineralization of calcium phosphates functionalized with hydroxyapatite-binding peptide. J. Mech. Behav. Biomed. Mater. 2023, 146, 106082. [Google Scholar] [CrossRef]
- Guérin, M.; Lebrun, A.; Kuhn, L.; Azaïs, T.; Laurent, G.; Marsan, O.; Drouet, C.; Subra, G. One-pot synthesis of bioinspired peptide-decorated apatite nanoparticles for nanomedicine. Small 2024, 20, e2306358. [Google Scholar] [CrossRef]
- Carpino, L.A.; Han, G.Y. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J. Am. Chem. Soc. 1970, 92, 5748–5749. [Google Scholar] [CrossRef]
- DeGrado, W.F.; Lear, J.D. Induction of peptide conformation at apolar water interfaces. 1. A study with model peptides of defined hydrophobic periodicity. J. Am. Chem. Soc. 1985, 107, 7684–7689. [Google Scholar]
- Xia, J.; Wang, W.; Jin, X.; Zhao, J.; Chen, J.; Li, N.; Xiao, S.; Lin, D.; Song, Z. Effects of chain lengths and backbone chirality on the bone-targeting ability of poly(glutamic acid)s. Biomater. Sci. 2024, 12, 3896–3904. [Google Scholar] [CrossRef]
- Bernardi, G.; Cook, W.H. Separation and characterization of the two high density lipoproteins of egg yolk, α- and β-lipovitellin. BBA Biochim. Biophys. Acta 1960, 44, 96–105. [Google Scholar] [CrossRef]
- Bernardi, G.; Kawasaki, T. Chromatography of polypeptides and proteins on hydroxyapatite columns. BBA Protein Struct. 1968, 160, 301–310. [Google Scholar] [CrossRef]
- Kimura, R.; Noda, D.; Liu, Z.; Shi, W.; Akutsu, R.; Tagaya, M. Biological surface layer formation on bioceramic particles for protein adsorption. Biomimetics 2024, 9, 347. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Hashizume, M. Utilization of proteins and peptides to create organic-hydroxyapatite hybrids. Protein Pept. Lett. 2018, 25, 25–33. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Vekilov, P.G. Principles of crystal nucleation and growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Nishimoto, S.K.; Araki, N.; Robinson, F.D.; Waite, J.H. Discovery of bone gamma-carboxyglutamic acid protein in mineralized scales. The abundance and structure of Lepomis macrochirus bone gamma-carboxyglutamic acid protein. J. Biol. Chem. 1992, 267, 11600–11605. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Cui, P.; Shao, T.; Liu, W.; Li, M.; Yu, M.; Zhao, W.; Song, Y.; Ding, Y.; Liu, J. Advanced review on type II collagen and peptide: Preparation, functional activities and food industry application. Crit. Rev. Food Sci. Nutr. 2023, 2023., 1–18. [Google Scholar] [CrossRef]
- McCormick, R.J. The flexibility of the collagen compartment of muscle. Meat Sci. 1994, 36, 79–91. [Google Scholar] [CrossRef]
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in normal skin and in pathological processes. N. Am. J. Med. Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef]
- Massoudi, D.; Malecaze, F.; Galiacy, S.D. Collagens and proteoglycans of the cornea: Importance in transparency and visual disorders. Cell Tissue Res. 2016, 363, 337–349. [Google Scholar] [CrossRef]
- Boskey, A.L. Hydroxyapatite formation in a dynamic collagen gel system: Effects of type I collagen, lipids, and proteoglycans. J. Phys. Chem. 1989, 93, 1628–1633. [Google Scholar] [CrossRef]
- Bradt, J.H.; Mertig, M.; Teresiak, A.; Pompe, W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem. Mater. 1999, 11, 2694–2701. [Google Scholar] [CrossRef]
- Qu, H.; Xia, Z.; Knecht, D.A.; Wei, M. Synthesis of dense collagen/apatite composites using a biomimetic method. J. Am. Ceram. Soc. 2008, 91, 3211–3215. [Google Scholar] [CrossRef]
- Rhee, S.H.; Lee, J.D.; Tanaka, J. Nucleation of hydroxyapatite crystal through chemical interaction with collagen. J. Am. Ceram. Soc. 2000, 83, 2890–2892. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Ji, C.; Ma, J.; He, B. The impact of hydroxyapatite crystal structures and protein interactions on bone’s mechanical properties. Sci. Rep. 2024, 14, 9786. [Google Scholar] [CrossRef]
- Tan, X.; Xue, Z.; Zhu, H.; Wang, X.; Xu, D. How charged amino acids regulate nucleation of biomimetic hydroxyapatite nanoparticles on the surface of collagen mimetic peptides: Molecular dynamics and free energy investigations. Cryst. Growth Des. 2020, 20, 4561–4572. [Google Scholar] [CrossRef]
- Li, X.; Chang, J. Preparation of bone-like apatite-collagen nanocomposites by a biomimetic process with phosphorylated collagen. J. Biomed. Mater. Res. Part A 2008, 85, 293–300. [Google Scholar] [CrossRef]
- Li, D.; He, J.; Cheng, W.; Wu, Y.; Hu, Z.; Tian, H.; Huang, Y. Redox-responsive nanoreservoirs based on collagen end-capped mesoporous hydroxyapatite nanoparticles for targeted drug delivery. J. Mater. Chem. B 2014, 2, 6089–6096. [Google Scholar] [CrossRef]
- Bolander, M.E.; Young, M.F.; Fisher, L.W.; Yamada, Y.; Termine, J.D. Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid). Proc. Natl. Acad. Sci. USA 1988, 85, 2919–2923. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef]
- Robin, M.; Tovani, C.B.; Krafft, J.-M.; Costentin, G.; Azaïs, T.; Nassif, N. The concentration of bone-related organic additives drives the pathway of apatite formation. Cryst. Growth Des. 2021, 21, 3994–4004. [Google Scholar] [CrossRef]
- Bromley, K.M.; Kiss, A.S.; Lokappa, S.B.; Lakshminarayanan, R.; Fan, D.; Ndao, M.; Evans, J.S.; Moradian-Oldak, J. Dissecting amelogenin protein nanospheres: Characterization of metastable oligomers. J. Biol. Chem. 2011, 286, 34643–34653. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, Z.; Ackerman, L.; Zhang, Y.; Bonde, J.; Li, W.; Cheng, Y.; Habelitz, S. Protein nanoribbons template enamel mineralization. Proc. Natl. Acad. Sci. USA 2020, 117, 19201–19208. [Google Scholar] [CrossRef]
- Tao, J.; Shin, Y.; Jayasinha, R.; Buchko, G.W.; Burton, S.D.; Dohnalkova, A.C.; Wang, Z.; Shaw, W.J.; Tarasevich, B.J. The energetic basis for hydroxyapatite mineralization by amelogenin variants provides insights into the origin of amelogenesis imperfecta. Proc. Natl. Acad. Sci. USA 2019, 116, 13867–13872. [Google Scholar] [CrossRef] [PubMed]
- Belcarz, A.; Ginalska, G.; Zalewska, J.; Rzeski, W.; Ślósarczyk, A.; Kowalczuk, D.; Godlewski, P.; Niedźwiadek, J. Covalent coating of hydroxyapatite by keratin stabilizes gentamicin release. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89B, 102–113. [Google Scholar] [CrossRef]
- Matsumoto, R.; Yoshii, T.; Egawa, S.; Hashimoto, M.; Hirai, T.; Inose, H.; Oh, Y.; Fujita, K.; Okawa, A.; Sotome, S. Local suppression effect of paclitaxel-impregnated hydroxyapatite/collagen on breast cancer bone metastasis in a rat model. Spine Surg. Relat. Res. 2022, 6, 294–302. [Google Scholar] [CrossRef]
- Li, G.; Tang, D.; Wang, D.; Xu, C.; Liu, D. Effective chemotherapy of lung cancer using bovine serum albumin-coated hydroxyapatite nanoparticles. Med. Sci. Monit. 2020, 26, e919716. [Google Scholar] [CrossRef] [PubMed]
- Iung, J.; Doyen, A.; Remondetto, G.; Pouliot, Y.; Brisson, G. The affinity of milk fat globule membrane fragments and buttermilk proteins to hydroxyapatite. J. Dairy Sci. 2024, 107, 4235–4247. [Google Scholar]
- Qin, D.; Wang, N.; You, X.G.; Zhang, A.D.; Chen, X.G.; Liu, Y. Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: Ongoing research and perspectives. Biomater. Sci. 2022, 10, 318–353. [Google Scholar] [CrossRef]
- Schickle, K.; Zurlinden, K.; Bergmann, C.; Lindner, M.; Kirsten, A.; Laub, M.; Telle, R.; Jennissen, H.; Fischer, H. Synthesis of novel tricalcium phosphate-bioactive glass composite and functionalization with rhBMP-2. J. Mater. Sci.: Mater. Med. 2011, 22, 763–771. [Google Scholar] [CrossRef]
- Cuylear, D.L.; Elghazali, N.A.; Kapila, S.D.; Desai, T.A. Calcium phosphate delivery systems for regeneration and biomineralization of mineralized tissues of the craniofacial complex. Mol. Pharm. 2023, 20, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Duarte, R. Mechanistic insights into the spontaneous induction of bone formation. Biomater. Adv. 2024, 158, 213795. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wang, Z.; Zhou, X.; Hu, B.; Li, C.; Yang, P. Protein-based bioactive coatings: From nanoarchitectonics to applications. Chem. Soc. Rev. 2024, 53, 1514–1551. [Google Scholar] [CrossRef] [PubMed]
- Yuca, E.; Karatas, A.Y.; Seker, U.O.S.; Gungormus, M.; Dinler-Doganay, G.; Sarikaya, M.; Tamerler, C. In vitro labeling of hydroxyapatite minerals by an engineered protein. Biotechnol. Bioeng. 2011, 108, 1021–1030. [Google Scholar] [CrossRef]
- Fernane, F.; Mecherri, M.O.; Sharrock, P.; Fiallo, M.; Sipos, R. Hydroxyapatite interactions with copper complexes. Mater. Sci. Eng. C 2010, 30, 1060–1064. [Google Scholar] [CrossRef]
- Mou, P.; Peng, H.; Zhou, L.; Li, L.; Li, H.; Huang, Q. A novel composite scaffold of Cu-doped nano calcium-deficient hydroxyapatite/multi-(amino acid) copolymer for bone tissue regeneration. Int. J. Nanomed. 2019, 14, 3331–3343. [Google Scholar] [CrossRef]
- Honda, Y.; Anada, T.; Morimoto, S.; Suzuki, O. Labile Zn ions on octacalcium phosphate-derived Zn-containing hydroxyapatite surfaces. Appl. Surf. Sci. 2013, 273, 343–348. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Nanostructured samarium doped fluorapatites and their catalytic activity towards synthesis of 1,2,4-triazoles. Molecules 2016, 21, 1281. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Efficient synthetic route for thio-triazole derivatives catalyzed by iron doped fluorapatite. Res. Chem. Intermed. 2017, 43, 1793–1811. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. A study on behavioral influence of glutamic acid and histidine on morphology and fluorescence activity of cadmium-doped fluorapatite. J. Alloys Compd. 2017, 690, 817–824. [Google Scholar] [CrossRef]
- Chrissanthopoulos, A.; Klouras, N.; Ntala, C.; Sevastos, D.; Dalas, E. Inhibition of hydroxyapatite formation in the presence of titanocene–aminoacid complexes: An experimental and computational study. J. Mater. Sci. Mater. Med. 2015, 26, 15. [Google Scholar] [CrossRef] [PubMed]
- Huan, W.; Xing, M.; Cheng, C.; Li, J. Facile fabrication of magnetic metal-organic framework nanofibers for specific capture of phosphorylated peptides. ACS Sustain. Chem. Eng. 2019, 7, 2245–2254. [Google Scholar] [CrossRef]
- Farinas, C.S.; Reis, P.C.; Ferraz, H.C.; Salim, V.M.M.; Alves, T.L.M. Adsorption of myoglobin onto hydroxyapatite modified with metal ions. Adsorpt. Sci. Technol. 2007, 25, 717–727. [Google Scholar] [CrossRef]
- Kalidas, S.; Sumathi, S. Copper substituted hydroxyapatite reinforced gelatin/polyvinyl alcohol/silk fibre-based scaffold for bone tissue engineering application. Mater. Chem. Phys. 2024, 320, 129410. [Google Scholar] [CrossRef]
- Dowd, T.L.; Rosen, J.F.; Gundberg, C.M.; Gupta, R.K. The displacement of calcium from osteocalcin at submicromolar concentrations of free lead. BBA Mol. Basis Dis. 1994, 1226, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Vukomanović, M.; Logar, M.; Škapin, S.D.; Suvorov, D. Hydroxyapatite/gold/arginine: Designing the structure to create antibacterial activity. J. Mater. Chem. B 2014, 2, 1557–1564. [Google Scholar] [CrossRef]
- Cao, B.; Yang, M.; Mao, C. Phage as a genetically modifiable supramacromolecule in chemistry, materials and medicine. Acc. Chem. Res. 2016, 49, 1111–1120. [Google Scholar] [CrossRef]
- Rautaray, D.; Mandal, S.; Sastry, M. Synthesis of hydroxyapatite crystals using amino acid-capped gold nanoparticles as a scaffold. Langmuir 2005, 21, 5185–5191. [Google Scholar] [CrossRef]
- Meisel, C.L.; Bainbridge, P.; Mitsouras, D.; Wong, J.Y. Targeted nanoparticle binding to hydroxyapatite in a high serum environment for early detection of heart disease. ACS Appl. Nano Mater. 2018, 1, 4927–4939. [Google Scholar] [CrossRef]
- Zhang, O.L.; Niu, J.Y.; Yin, I.X.; Yu, O.Y.; Mei, M.L.; Chu, C.H. Bioactive materials for caries management: A literature review. Dent. J. 2023, 11, 59. [Google Scholar] [CrossRef]
- Pedersen, S.Ø.; Støchkel, K.; Byskov, C.S.; Baggesen, L.M.; Nielsen, S.B. Gas-phase spectroscopy of protonated adenine, adenosine 5´-monophosphate and monohydrated ions. Phys. Chem. Chem. Phys. 2013, 15, 19748–19752. [Google Scholar] [CrossRef] [PubMed]
- Levene, P.A.; Bass, L.W.; Simms, H.S. The ionization of pyrimidines in relation to the structure of pyrimidine nucleosides. J. Biol. Chem. 1926, 70, 229–241. [Google Scholar] [CrossRef]
- Wierzchowski, K.L.; Litonska, E.; Shugar, D. Infrared and ultraviolet studies on the tautomeric equilibria in aqueous medium between monoanionic species of uracil, thymine, 5-fluorouracil, and other 2,4-diketopyrimidines. J. Am. Chem. Soc. 1965, 87, 4621–4629. [Google Scholar] [CrossRef]
- Sigel, H. Acid-base properties of purine residues and the effect of metal ions: Quantification of rare nucleobase tautomers. Pure Appl. Chem. 2004, 76, 1869–1886. [Google Scholar] [CrossRef][Green Version]
- Lippert, B. Alterations of nucleobase pKa values upon metal coordination: Origins and consequences. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 54, pp. 385–447. [Google Scholar]
- Roy, B.; Depaix, A.; Perigaud, C.; Peyrottes, S. Recent trends in nucleotide synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef]
- Mucha, A.; Knobloch, B.; Jeżowska-Bojczuk, M.; Kozłowski, H.; Sigel, R.K.O. Comparison of the acid–base properties of ribose and 2′-deoxyribose nucleotides. Chem. Eur. J. 2008, 14, 6663–6671. [Google Scholar] [CrossRef]
- Sigel, H.; Massoud, S.S.; Nicolas, A.C. Comparison of the extent of macrochelate formation in complexes of divalent metal ions with guanosine (GMP2-), inosine (IMP2-), and adenosine 5′-monophosphate (AMP2-). The crucial role of N-7 basicity in metal ion-nucleic base recognition. J. Am. Chem. Soc. 1994, 116, 2958–2971. [Google Scholar] [CrossRef]
- Sigel, H.; Lippert, B. The effects of N7-coordinated cis-diammine-platinum(II) on the acid-base properties of guanine derivatives. Pure Appl. Chem. 1998, 70, 845–854. [Google Scholar] [CrossRef]
- Song, B.; Feldmann, G.; Bastian, M.; Lippert, B.; Sigel, H. Acid-base and metal ion-binding properties of 2′-deoxycytidine 5′-monophosphate (dCMP2-) alone and coordinated cis-diammine-platinum(II). Formation of mixed metal ion nucleotide complexes. Inorg. Chim. Acta 1995, 235, 99–109. [Google Scholar] [CrossRef]
- Massoud, S.S.; Sigel, H. Metal ion coordinating properties of pyrimidine-nucleoside 5′-monophosphates (CMP, UMP, TMP) and of simple phosphate monoesters, including D-ribose 5-monophosphate. Establishment of relations between complex stability and phosphate basicity. Inorg. Chem. 1988, 27, 1447–1453. [Google Scholar] [CrossRef]
- Bianchi, E.M.; Griesser, R.; Sigel, H. Influence of decreasing solvent polarity (1,4-dioxane/water mixtures) on the acid-base and copper(II)-binding properties of guanosine 5′-diphosphate. Helv. Chim. Acta 2005, 88, 406–425. [Google Scholar] [CrossRef]
- Bianchi, E.M.; Sajadi, S.A.A.; Song, B.; Sigel, H. Stabilities and isomeric equilibria in aqueous solution of monomeric metal ion complexes of adenosine 5′-diphosphate (ADP3-) in comparison with those of adenosine 5′-monophosphate (AMP2-). Chem. Eur. J. 2003, 9, 881–892. [Google Scholar] [CrossRef]
- Sajadi, S.A.A.; Song, B.; Gregáň, F.; Sigel, H. Acid-base and metal ion-coordinating properties of pyrimidine-nucleoside 5′-diphosphates (CDP, UDP, dTDP) and of several simple diphosphate monoesters. Establishment of relations between complex stability and diphosphate basicity. Inorg. Chem. 1999, 38, 439–448. [Google Scholar] [CrossRef]
- Sigel, H.; Bianchi, E.M.; Corfû, N.A.; Kinjo, Y.; Tribolet, R.; Martin, R.B. Acid–base properties of the 5′-triphosphates of guanosine and inosine (GTP4- and ITP4-) and of several related nucleobase derivatives. J. Chem. Soc. Perkin Trans. 2 2001, 4, 507–511. [Google Scholar] [CrossRef]
- Sigel, H.; Tribolet, R.; Malini-Balakrishnan, R.; Martin, R.B. Comparison of the stabilities of monomeric metal ion complexes formed with adenosine 5′-triphosphate (ATP) and pyrimidine-nucleoside 5′-triphosphates (CTP, UTP, TTP) and evaluation of the isomeric equilibria in the complexes of ATP and CTP. Inorg. Chem. 1987, 26, 2149. [Google Scholar] [CrossRef]
- Krane, S.M.; Glimcher, M.J. Transphosphorylation from nucleoside di- and triphosphates by apatite crystals. J. Biol. Chem. 1962, 9, 2991–2998. [Google Scholar] [CrossRef]
- Taves, D.R.; Reedy, R.C. A structural basis for transphosphorylation of nucleotides with hydroxyapatite. Calc. Tiss. Res. 1969, 3, 284–292. [Google Scholar] [CrossRef]
- Orriss, I.R.; Arnett, T.R.; Russell, G.G. Pyrophosphate: A key inhibitor of mineralization. Curr. Opin. Biotechnol. 2016, 28, 57–68. [Google Scholar]
- Benedetti, M.; Antonucci, D.; De Castro, F.; Girelli, C.R.; Lelli, M.; Roveri, N.; Fanizzi, F.P. Metalated nucleotide chemisorption on hydroxyapatite. J. Inorg. Biochem. 2015, 153, 279–283. [Google Scholar] [CrossRef]
- Watson, J.D.; Crick, F.H.C. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef]
- Bernardi, G. Chromatography of nucleic acids on hydroxyapatite. Nature 1965, 206, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, G. Chromatography of nucleic acids on hydroxyapatite. III. Chromatography of RNA and polyribonucleotides. Biochim. Biophys. Acta 1969, 174, 449–457. [Google Scholar] [CrossRef]
- Bastia, D.; Chiang, K.S.; Swift, H.; Siersma, P. Heterogeneity, complexity, and repetition of the chloroplast DNA of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1971, 68, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Wittelsberger, S.C.; Hansen, J.N. Hydroxyapatite chromatography of short single-stranded DNA. BBA Sect. Nucleic Acids Protein Synth. 1979, 565, 125–130. [Google Scholar] [CrossRef]
- Andrews-Pfannkoch, C.; Fadrosh, D.W.; Thorpe, J.; Williamson, S.J. Hydroxyapatite-mediated separation of double-stranded DNA, single-stranded DNA, and RNA genomes from natural viral assemblages. Appl. Environ. Microbiol. 2010, 76, 5039–5045. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.L.; van der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Xing, Z.; Chen, S.; Liu, Z.; Yang, X.; Zhu, X.; Zhang, X. Harnessing the power of hydroxyapatite nanoparticles for gene therapy. Appl. Mater. Today 2024, 39, 102317. [Google Scholar] [CrossRef]
- Ridi, F.; Meazzini, I.; Castroflorio, B.; Bonini, M.; Berti, D.; Baglioni, P. Functional calcium phosphate composites in nanomedicine. Adv. Colloid Interface Sci. 2017, 244, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Adsorption of DNA on biomimetic apatites: Toward the understanding of the role of bone and tooth mineral on the preservation of ancient DNA. Appl. Surf. Sci. 2014, 292, 867–875. [Google Scholar] [CrossRef]
- Oyane, A.; Yazaki, Y.; Araki, H.; Sogo, Y.; Ito, A.; Yamazaki, A.; Tsurushima, H. Fabrication of a DNA-lipid-apatite composite layer for efficient and area-specific gene transfer. J. Mater. Sci. Mater. Med. 2012, 23, 1011–1019. [Google Scholar] [CrossRef]
- Yazaki, Y.; Oyane, A.; Tsurushima, H.; Araki, H.; Sogo, Y.; Ito, A.; Yamazaki, A. Coprecipitation of DNA-lipid complexes with apatite and comparison with superficial adsorption for gene transfer applications. J. Biomater. Appl. 2014, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ito, A.; Li, X.; Sogo, Y.; Hirose, M.; Oyane, A.; Tsurushima, H. DNA-lipid-apatite composite layers enhance gene expression of mesenchymal stem cells. Mater. Sci. Eng. C 2013, 33, 512–518. [Google Scholar] [CrossRef]
- Gibbs, D.; Lohrmann, R.; Orgel, L.E. Template-directed synthesis and selective adsorption of oligoadenylates on hydroxyapatite. J. Mol. Evol. 1980, 15, 347–354. [Google Scholar] [CrossRef]
- Martinson, H.G. The basis of fractionation of single-stranded nucleic acids on hydroxylapatite. Biochemistry 1973, 12, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Fadrosh, D.W.; Andrews-Pfannkoch, C.; Williamson, S.J. Separation of single-stranded DNA, double-stranded DNA and RNA from an environmental viral community using hydroxyapatite chromatography. J. Vis. Exp. 2011, 55, e3146. [Google Scholar] [CrossRef]
- Shlaferman, J.; Paige, A.; Meserve, K.; Miech, J.A.; Gerdon, A.E. Selected DNA aptamers influence kinetics and morphology in calcium phosphate mineralization. ACS Biomater. Sci. Eng. 2019, 5, 3228–3236. [Google Scholar] [CrossRef]
- Wu, Y.X.; Kwon, Y.J. Aptamers: The ‘‘evolution” of SELEX. Methods 2016, 106, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Florek, J.; Colon, S.; Gerdon, A.E. Selected DNA aptamers as hydroxyapatite affinity reagents. Anal. Chim. Acta 2020, 1110, 115–121. [Google Scholar] [CrossRef]
- Clarke, M.J.; Taube, H. Pentaammineruthenium-guanine complexes. J. Am. Chem. Soc. 1974, 96, 5413–5419. [Google Scholar] [CrossRef]
- Chu, G.Y.H.; Mansy, S.; Duncan, R.E.; Tobias, R.S. Heavy metal nucleotide interactions. 11. Stereochemical and electronic effects in the electrophilic attack of cis- and trans-diammineplatinum(II) on 5′-guanosine monophosphate and polyguanylate in aqueous solution. J. Am. Chem. Soc. 1978, 100, 593. [Google Scholar] [CrossRef]
- Sigel, H. Komplexbildung von nucleinbasen mit Cu2+. Eur. J. Biochem. 1968, 3, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Sigel, H. Zur katalatischen und peroxidatischen aktivität von Cu2+-komplexen. Angew. Chem. 1969, 81, 161–171. [Google Scholar] [CrossRef]
- Sigel, H. Nucleic base-metal ion interactions. Acidity of the N(1) or N(3) proton in binary and ternary complexes of Mn2+, Ni2+, and Zn2+ with the 5′-triphosphates of inosine, guanosine, uridine, and thymidine. J. Am. Chem. Soc. 1975, 97, 3209–3214. [Google Scholar] [CrossRef]
- Caradonna, J.P.; Lippard, S.J. Synthesis and characterization of [d(ApGpGpCpCpT)]2 and its adduct with the anticancer drug cis-diamminedichloroplatinum(II). Inorg. Chem. 1988, 27, 1454–1466. [Google Scholar] [CrossRef]
- Garderen, C.J.; Altona, C.; Reedijk, J. Conformational changes in a single- and double-stranded nonanucleotide upon complexation of a monofunctional platinum compound as studied by proton NMR, phosphorus-31 NMR and CD methods. Inorg. Chem. 1990, 29, 1481–1487. [Google Scholar] [CrossRef]
- Liu, Y.; Pacifico, C.; Natile, G.; Sletten, E. Antitumor trans platinum complexes can form cross-links with adjacent purine groups. Angew. Chem. Int. Ed. 2001, 40, 1226–1228. [Google Scholar] [CrossRef]
- Meshkini, A.; Sistanipour, E.; Oveisi, H.; Asoodeh, A. Induction of osteogenesis in bone tumour cells by purine-conjugated zinc-hydroxyapatite. Bioinspired Biomim. Nanobiomater. 2021, 10, 16–27. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, M.; Heng, C.; Huang, Q.; Mao, L.; Huang, H.; Hui, J.; Deng, F.; Zhang, X.; Wei, Y. Surface polyPEGylation of Eu 3+ doped luminescent hydroxyapatite nanorods through the combination of ligand exchange and metal free surface initiated atom transfer radical polymerization. Appl. Surf. Sci. 2017, 399, 499–505. [Google Scholar] [CrossRef]
- Uskoković, V. When 1 + 1 > 2: Nanostructured composites for hard tissue engineering applications. Mater. Sci. Eng. C 2015, 57, 434–451. [Google Scholar] [CrossRef]
- Chang, Q.; Li, K.K.; Hu, S.L.; Dong, Y.G.; Yang, J.L. Hydroxyapatite supported N-doped carbon quantum dots for visible-light photocatalysis. Mater. Lett. 2016, 175, 44–47. [Google Scholar] [CrossRef]
- Piccirillo, C.; Castro, P.M.L. Calcium hydroxyapatite-based photocatalysts for environment remediation: Characteristics, performances and future perspectives. J. Environ. Manag. 2017, 193, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.P.; Fontes, A.; Santos, B.S.; Pereira, G.A.L.; Pereira, G. Recent advances in hydroxyapatite/polymer/silver nanoparticles scaffolds with antimicrobial activity for bone regeneration. Mater. Lett. 2023, 338, 134027. [Google Scholar] [CrossRef]
- Inam, H.; Sprio, S.; Tavoni, M.; Abbas, Z.; Pupilli, F.; Tampieri, A. Magnetic hydroxyapatite nanoparticles in regenerative medicine and nanomedicine. Int. J. Mol. Sci. 2024, 25, 2809. [Google Scholar] [CrossRef] [PubMed]
- Farazin, A.; Mahjoubi, S. Dual-functional hydroxyapatite scaffolds for bone regeneration and precision drug delivery. J. Mech. Behav. Biomed. Mater. 2024, 157, 106661. [Google Scholar] [CrossRef]
- Gong, Y.; Gan, Y.; Wang, P.; Gong, C.; Han, B.; Li, P.; Liu, E.; Yu, Z.; Sheng, L.; Wang, X. Injectable foam-like scaffolds release glucose oxidase-integrated metal–organic framework hybrids for diabetic bone defects. Appl. Mater. Today 2024, 38, 102190. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhang, H.; Cui, M.; Lin, X.; Huang, S.; Tang, Z.; Hou, Y.; Iqbal, M.Z.; Kong, X. ROS-responsive chlorin e6 and silk fibroin loaded ultrathin magnetic hydroxyapatite nanorods for T1-magnetic resonance imaging guided photodynamic therapy in vitro. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 656, 130513. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Chen, L.; Li, W. A ternary doped single matrix material with dual functions of bone repair and multimodal tracking for applications in orthopedics and dentistry. J. Mater. Chem. B 2018, 6, 6047–6056. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.Y.; Lin, A.Y.M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- He, B.; Zhao, J.; Ou, Y.; Jiang, D. Biofunctionalized peptide nanofiber-based composite scaffolds for bone regeneration. Mater. Sci. Eng. C 2018, 90, 728–738. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, J.; Łukaszewska, I.; Pielichowski, K. POSS and SSQ materials in dental applications: Recent advances and future outlooks. Int. J. Mol. Sci. 2023, 24, 4493. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.W.; Li, S.P.; Han, Y.C.; Yuan, L.; Yin, M.Z. Internalization of hydroxyapatite nanoparticles in liver cancer cells. J. Mater. Sci. Mater. Med. 2008, 19, 1091–1095. [Google Scholar] [CrossRef]
- Xu, D.; Wan, Y.; Li, Z.; Wang, C.; Zou, Q.; Du, C.; Wang, Y. Tailorable hierarchical structures of biomimetic hydroxyapatite micro/nano particles promoting endocytosis and osteogenic differentiation of stem cells. Biomater. Sci. 2020, 8, 3286–3300. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, X.; Liu, Z.; Chen, H.; Wu, H.; Yang, X.; Zhu, X.; Ma, J.; Dong, H. The morphology of hydroxyapatite nanoparticles regulates cargo recognition in clathrin-mediated endocytosis. Front. Mol. Biosci. 2021, 8, 627015. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, E.; García-Tojal, J.; Santana, J.; Jorge-Villar, S.E.; Teira, L.; Muñiz, J.; Ibañez, J.J. Geochemical and spectroscopic approach to the characterization of earliest cremated human bones from the Levant (PPNB of Kharaysin, Jordan). J. Archaeol. Sci. Rep. 2020, 30, 102211. [Google Scholar] [CrossRef]
- Gilbert, W. The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; De La Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Drouet, C.; Aufray, M.; Rollin-Martinet, S.; Vandecandelaère, N.; Grossin, D.; Rossignol, F.; Champion, E.; Navrotsky, A.; Rey, C. Nanocrystalline apatites: The fundamental role of water. Am. Mineral. 2018, 103, 550–564. [Google Scholar] [CrossRef]
- Carter, O.W.L.; Xu, Y.; Sadler, P.J. Minerals in biology and medicine. RSC Adv. 2021, 11, 1939–1951. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Zhang, Y.; Liu, Y.; Liu, S.; Liu, X.; Luo, E. Metal ions: The unfading stars of bone regeneration-from bone metabolism regulation to biomaterial applications. Biomater. Sci. 2023, 11, 7268–7295. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Wu, C.; Chang, J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [CrossRef] [PubMed]
- Ellingham, S.T.D.; Thompson, T.J.U.; Islam, M.; Taylor, G. Estimating temperature exposure of burnt bone—A methodological review. Sci. Justice 2015, 55, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Maia, M.T.; Luz, É.P.C.G.; Andrade, F.K.; Rosa, M.d.F.; Borges, M.d.F.; Arcanjo, M.R.A.; Vieira, R.S. Advances in Bacterial cellulose/strontium apatite composites for bone applications. Polym. Rev. 2021, 61, 736–764. [Google Scholar] [CrossRef]
- Munir, M.U.; Salman, S.; Ihsan, A.; Elsaman, T. Synthesis, characterization, functionalization and bio-applications of hydroxyapatite nanomaterials: An overview. Int. J. Nanomed. 2022, 17, 1903–1925. [Google Scholar] [CrossRef]
- Deng, Z.; Jia, Z.; Li, L. Biomineralized materials as model systems for structural composites: Intracrystalline structural features and their strengthening and toughening mechanisms. Adv. Sci. 2022, 9, 2103524. [Google Scholar] [CrossRef]
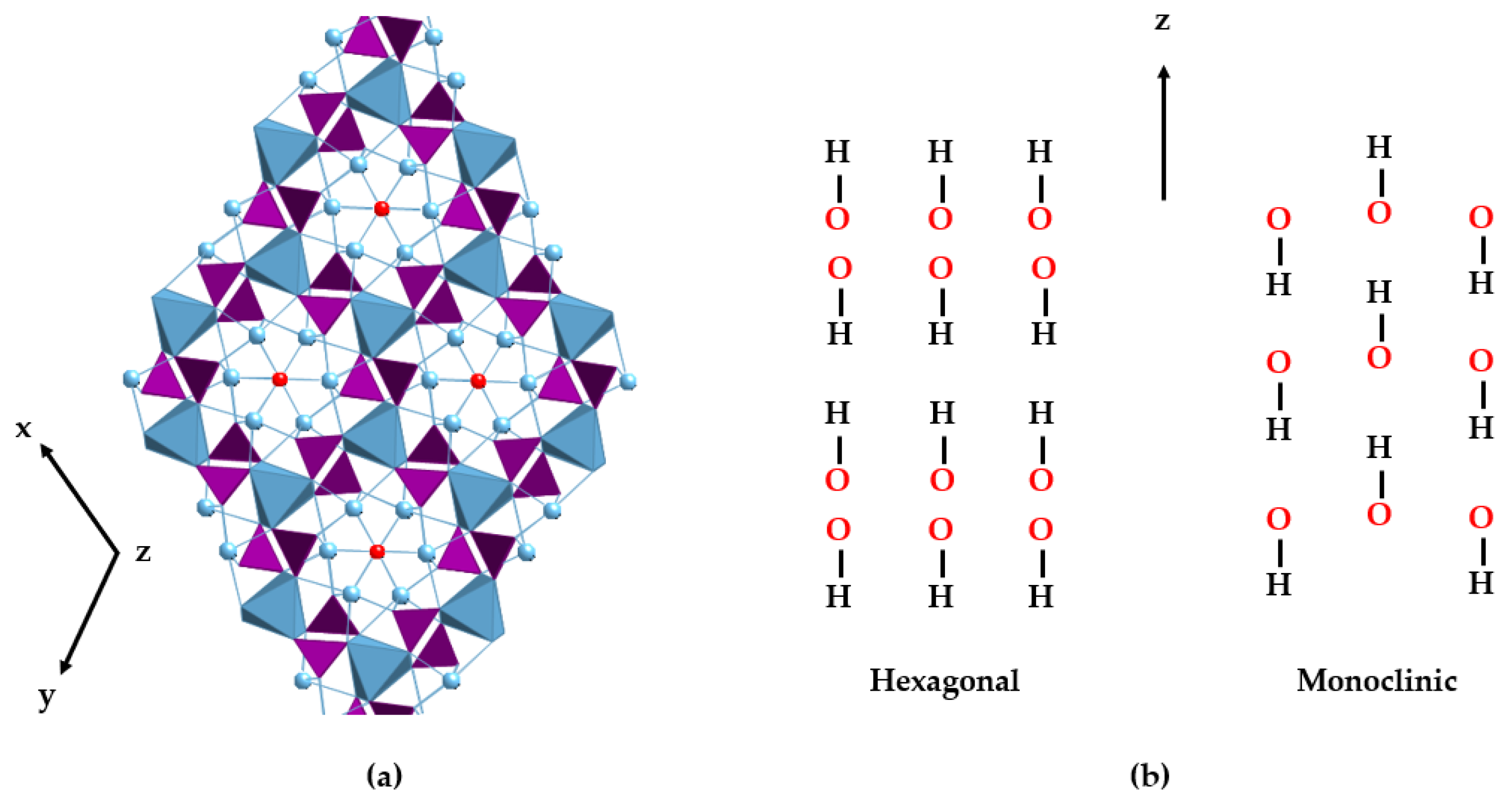
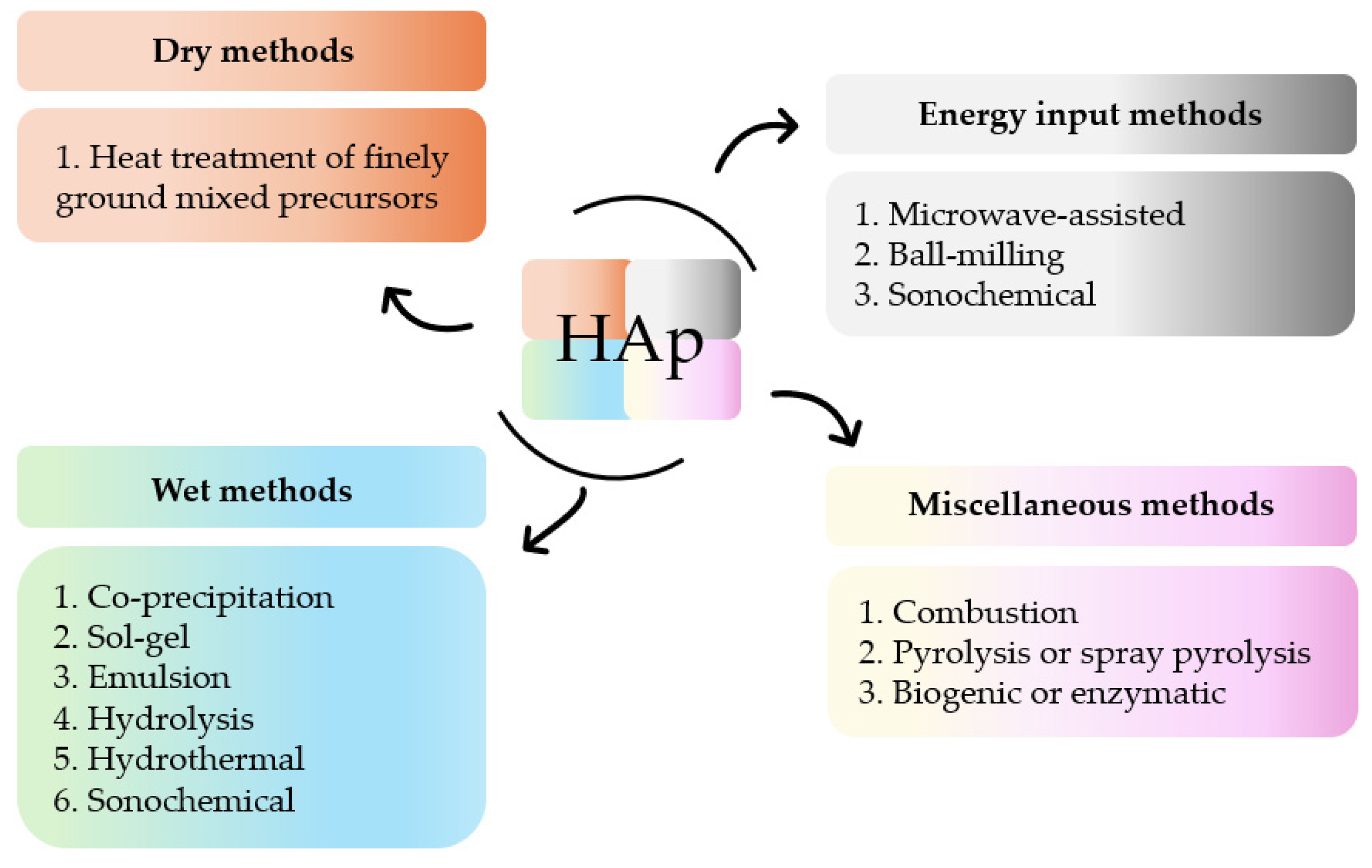

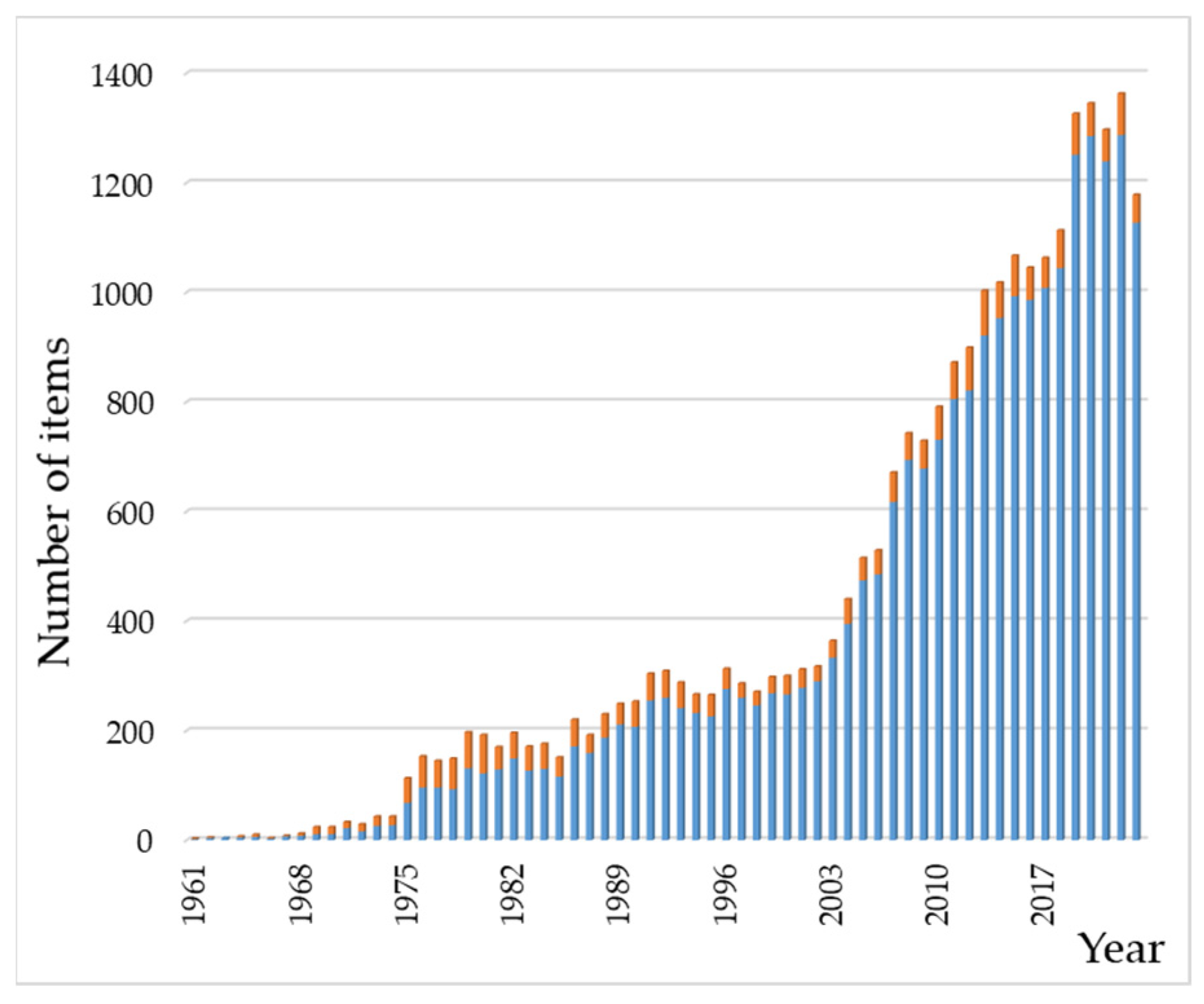
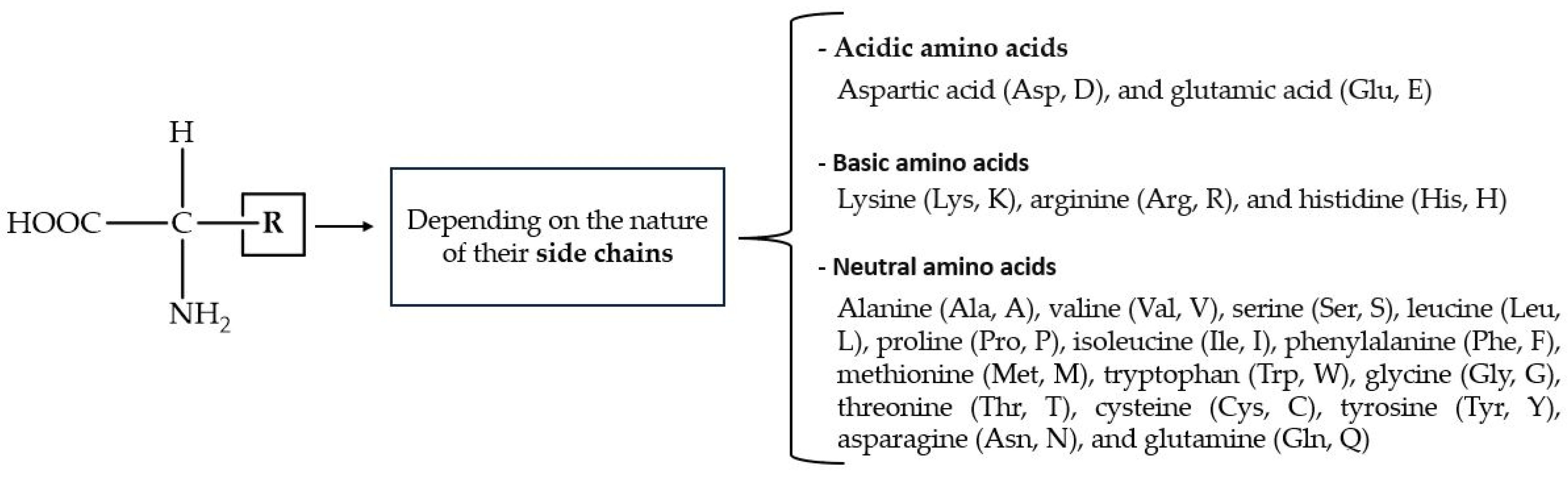
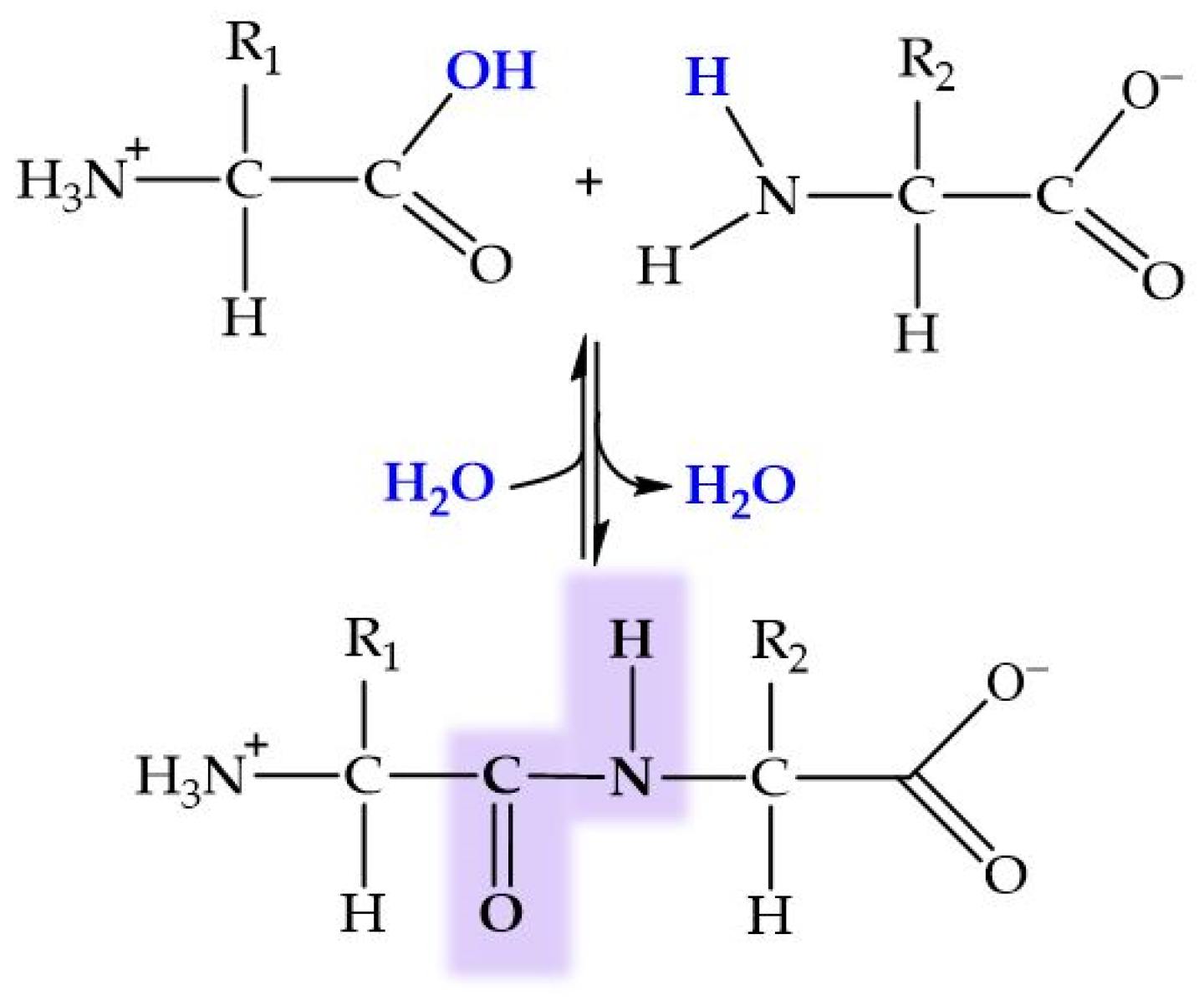
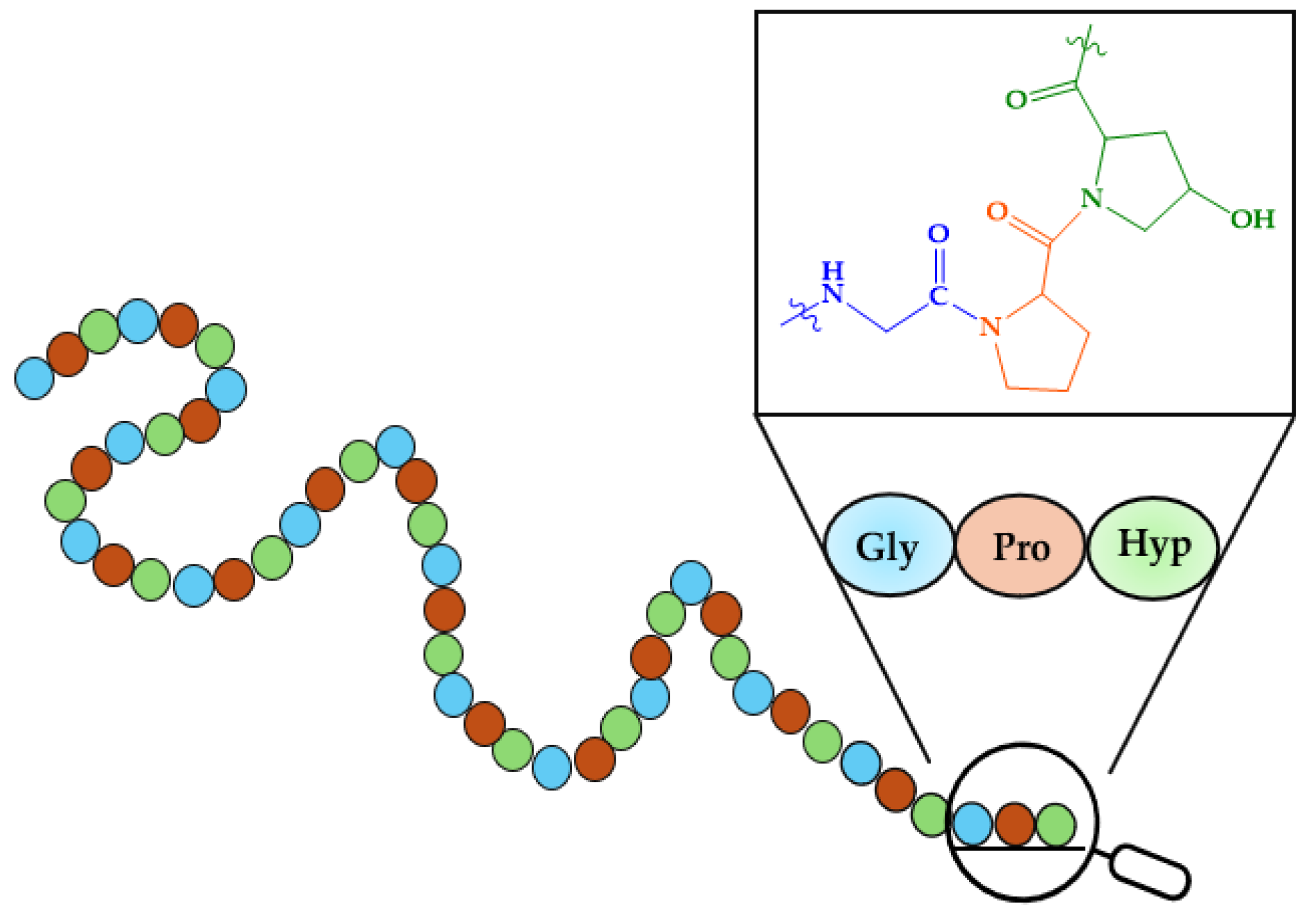
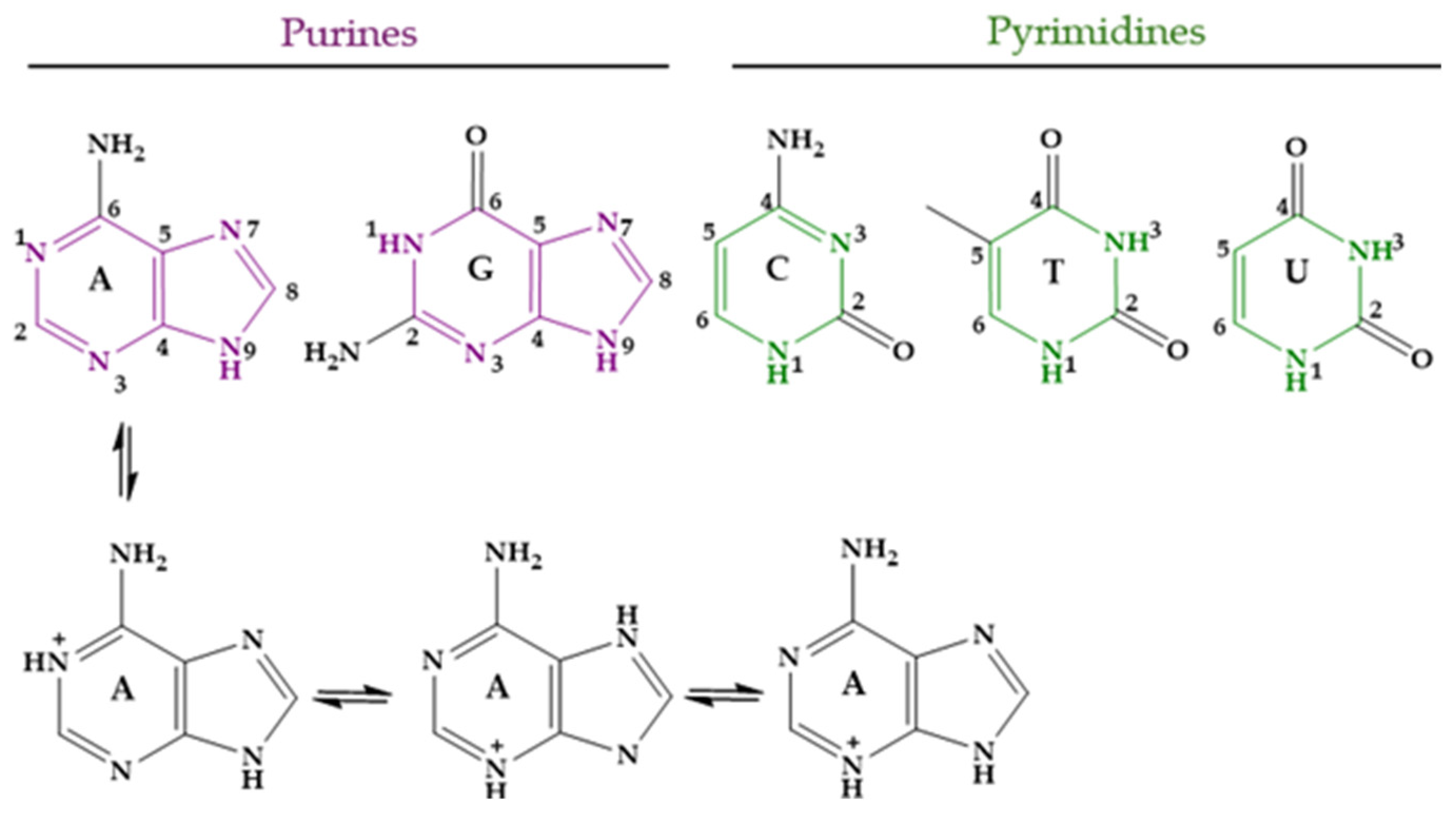
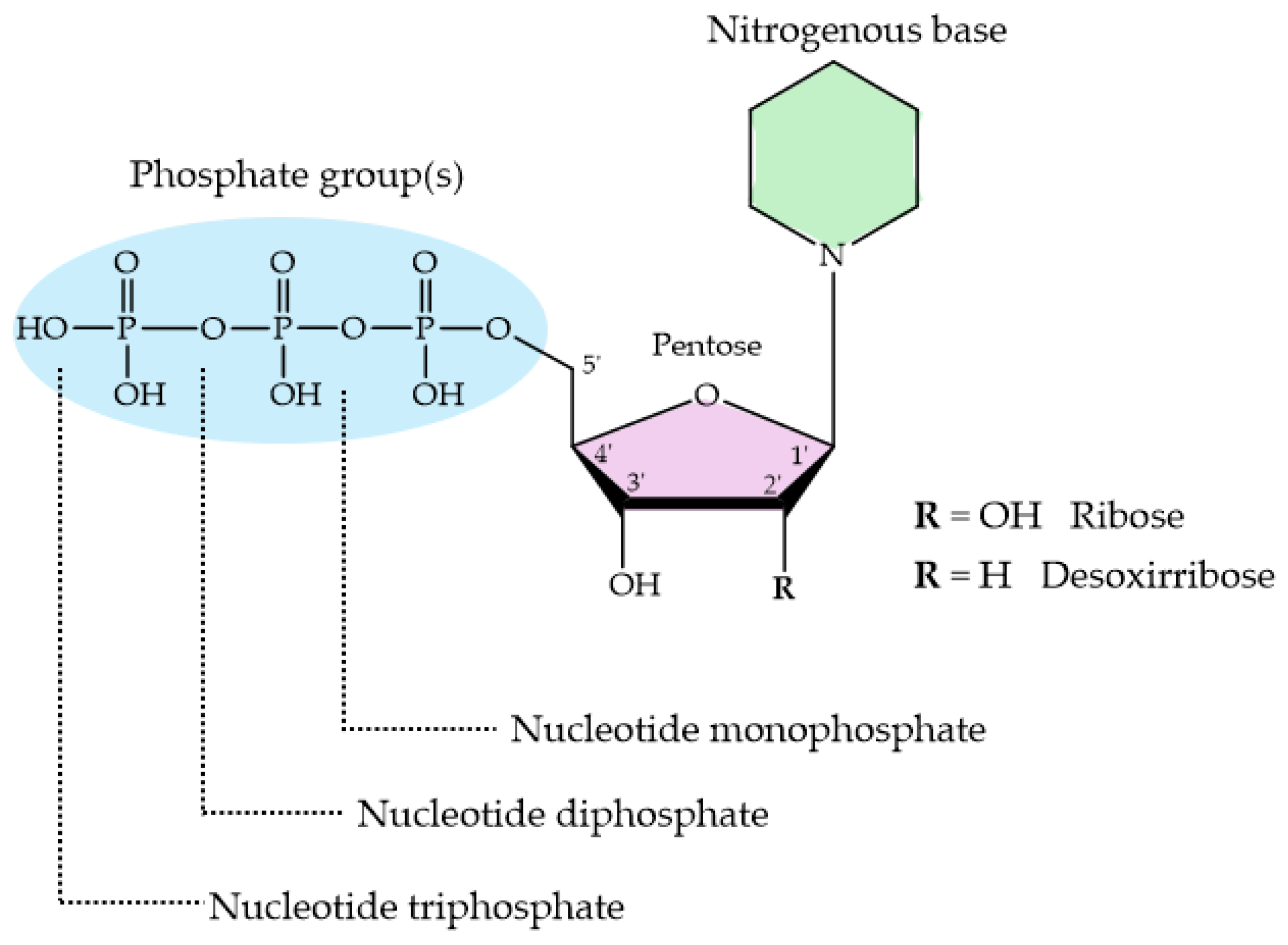
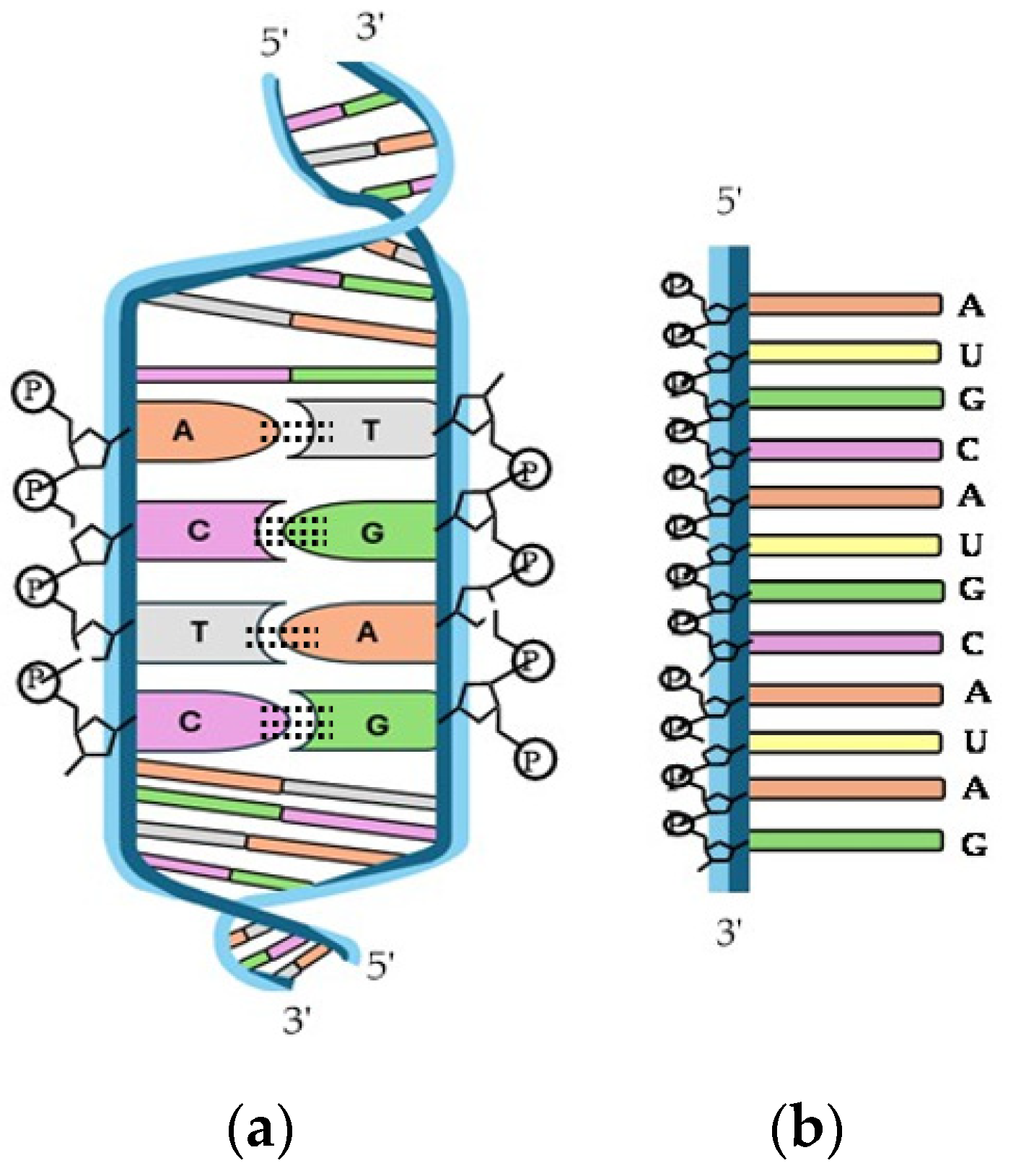
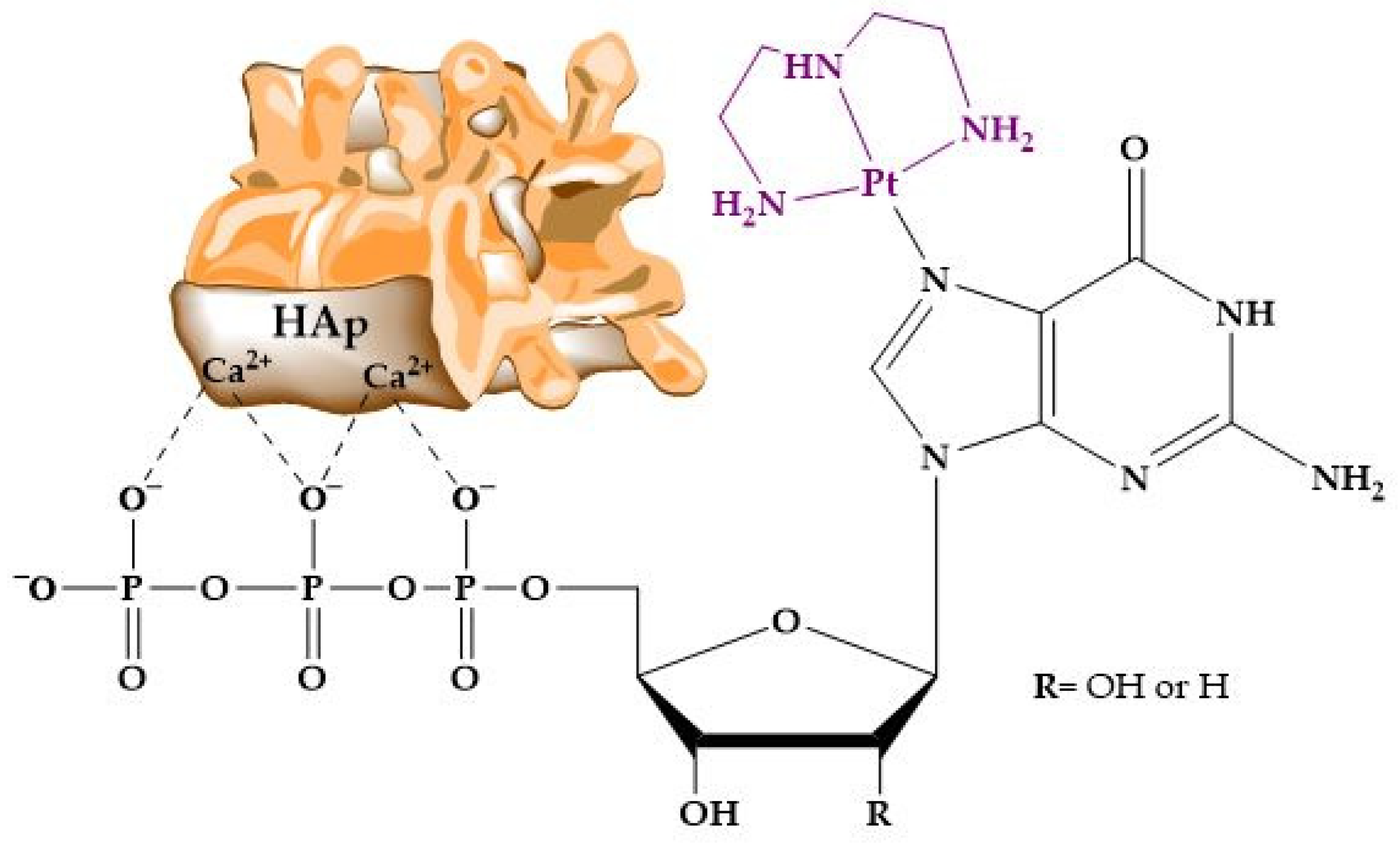
| Name (Abbreviation) Formula | Ca/P Ratio | System (Space Group) Cell Parameters (Å, ⁰) | Refs. |
|---|---|---|---|
| Monocalcium phosphate anhydrous (MCPA) Ca(H2PO4)2 | 0.50 | Triclinic (P1) a = 7.5577(5), b = 8.2531(6), c = 5.5504(3) α = 109.87(1), β = 93.68(1), γ = 109.15(1) | [9] |
| Monocalcium phosphate monohydrate (MCPM) Ca(H2PO4)2∙H2O | 0.50 | Triclinic (P1) a = 5.6261(5), b = 11.889(2), c = 6.4731(8) α = 98.633(6), β = 118.262(6), γ = 83.344(6) | [10] |
| Dicalcium phosphate anhydrous (DCPA, monetite) CaHPO4 | 1.00 | ) a = 6.90(1), b = 6.65(1), c = 7.00(1) α = 96.35(2), β = 103.90(2), γ = 88.73(2) | [11] |
| Dicalcium phosphate dihydrate (DCPD, brushite) CaHPO4∙2H2O | 1.00 | Monoclinic (Ia) a = 5.812(2), b = 15.180(3), c = 6.239(2) β = 116.42(2) | [12] |
| Amorphous calcium phosphates (ACPs) 1 CaxHy(PO4)z∙nH2O (n = 3.0–4.5) 2 | 1.20–2.20 3 | ||
| Octacalcium phosphate (OCP) Ca8H2(PO4)6∙5H2O | 1.33 | ) a = 19.692(4), b = 9.523(2), c = 6.835(2) α = 90.15(2), β = 92.54(2), γ = 108.65(2) | [13,14] |
| α-tricalcium phosphate (α-TCP) α-Ca3(PO4)2 | 1.50 | Monoclinic (P21/a) a = 12.887(2), b = 27.280(4), c = 15.219(2) β = 126.20(1) | [15] |
| β-tricalcium phosphate (β-TCP, synthetic whitlockite) β-Ca3(PO4)2 | 1.50 | Rhombohedral (R3c) (hexagonal setting) a = b = 10.439(1), c = 37.375(6) | [16] |
| Hydroxyapatite (HAp) Ca10(OH)2(PO4)6 | 1.67 | Hexagonal (P63/m) a = b = 9.424(4), c = 6.879(4) | [17] |
| Hydroxyapatite (HApM) M-Ca10(OH)2(PO4)6 | 1.67 | Monoclinic (P21/b) a = 9.419(3), b = 18.848(6), c = 6.884(2) β = 119.98(2) | [18] |
| Oxyapatite (OAp, voelckerite) 4 Ca10O(PO4)6 | 1.67 | ) a = b = 9.432, c = 6.881 | [19] |
| Tetracalcium phosphate (TTCP, hilgenstockite) Ca4O(PO4)2 | 2.00 | Monoclinic (P21) a = 7.023(1), b = 11.986(4), c = 9.473(2) β = 90.90(1) | [20] |
| Dicalcium diphosphate dihydrate (DCDD) Ca2(P2O7)∙2H2O | 1.00 | ) a = 7.365(4), b = 8.287(4), c = 6.691(4) α = 102.96(1), β = 72.73(1), γ = 95.01(1) | [21] |
| Peptides | Net Charge | Kaff/L·mol−1 |
|---|---|---|
| Asp | −1 | 4166 |
| Glu | −1 | 3021 |
| Ala | 0 | 286 |
| Phe | 0 | 2439 |
| Pro | 0 | 574 |
| Met | 0 | 621 |
| Gly | 0 | 1714 |
| Cys | 0 | 664 |
| Gln | 0 | 670 |
| Ser | 0 | 901 |
| Leu | 0 | 2026 |
| Tyr | 0 | 3030 |
| Lys | +1 | 877 |
| Peptides | Net Charge | Kaff/L·mol−1 |
|---|---|---|
| VTK | +1 | 2534 |
| VTK_s | +1 | 2082 |
| VTK_7E | 0 | 1777 |
| pVTK | −3 | 6194 |
| Base | Atom | pKa |
|---|---|---|
| Uracil | N3 | 9.63 |
| Thymine | N3 | 10.30 |
| Guanine | N1 | 9.56 |
| Guanine | N7 | 3.11 |
| Cytosine | N3 | 4.60 |
| Adenine | N1 | 4.10 |
| Refs. | Acid NXP/dNXP | pKa for N1H+ or N7H+ NXP/dNXP (4a) | pKa for PO2(OH)− NXP/dNXP (5a) | pKa for N1H or N3H NXP/dNXP (6a) |
|---|---|---|---|---|
| [290,291] | H2(GMP)±/H2(dGMP)± | 2.48 ± 0.04/2.69 ± 0.03 | 6.25 ± 0.02/6.29 ± 0.01 | 9.49 ± 0.02/9.56 ± 0.02 |
| [289,290] | H2(AMP)±/H2(dAMP)± | 3.84 ± 0.02/3.97 ± 0.02 | 6.21 ± 0.01/6.27 ± 0.04 | |
| [292,293] | H2(CMP)±/H2(dCMP)± | 4.33 ± 0.04/4.46 ± 0.01 | 6.19 ± 0.02/6.24 ± 0.01 | |
| [293] | H(UMP)−/H(dTMP) | 6.15 ± 0.01/6.36 ± 0.01 | 9.45 ± 0.02/9.90 ± 0.03 | |
| [289,294] | H2(GDP)−/H2(dGDP)− | 2.67 ± 0.02/2.91 ± 0.07 | 6.38 ± 0.01/6.46 ± 0.03 | 9.56 ± 0.03/9.64 ± 0.04 |
| [289,295] | H2(ADP)−/H2(dADP)− | 3.92 ± 0.02/4.00 ± 0.03 | 6.40 ± 0.01/6.45 ± 0.01 | |
| [296] | H3(CDP)± | 6.39 ± 0.02/ | ||
| [296] | H2(UDP)−/H2(dTDP) | 6.38 ± 0.02/6.44 ± 0.01 | 9.47 ± 0.02/9.93 ± 0.02 | |
| [289,297] | H2(GTP)2−/H2(dGTP)2− | 2.94 ± 0.02/3.16 ± 0.05 | 6.50 ± 0.02/6.64 ± 0.02 | 9.57 ± 0.02/9.66 ± 0.04 |
| [289,297] | H2(ATP)2−/H2(dATP)2− | 4.00 ± 0.01/4.14 ± 0.02 | 6.47 ± 0.01/6.62 ± 0.03 | |
| [297,298] | H2(CTP)2− | 4.55 ± 0.02 | 6.55 ± 0.02 | |
| [297,298] | H2(UTP)2−/H2(dTTP)2− | 6.45 ± 0.01/6.52 ± 0.02 | 9.57 ± 0.02/10.08 ± 0.05 |
| Aptamer | Sequence | kf (min−1) | Kaff (M−1) | ΔG (Kcal∙mol−1) |
|---|---|---|---|---|
| 1 | CAGGGCGCTACGGTATGTGTTGGGTCTGGCGTAGGGCTGGC | 12 ± 2 × 105 | 3 ± 1 × 106 | −7.37 |
| 2 | GAGCGCGCTACGGTATGTGTTGCGTGTGGCGTAGCGGTGCG | 6 ± 1 × 105 | 7 ± 4 × 106 | −8.54 |
| 3 | CAGCGCCCTACGCTATGTCTTGCGTCTCGCCTAGCGCTCGC | 2 ± 2 × 105 | 7 ± 4 × 106 | −7.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Pérez, A.; Martínez-Alonso, M.; García-Tojal, J. Hybrid Hydroxyapatite–Metal Complex Materials Derived from Amino Acids and Nucleobases. Molecules 2024, 29, 4479. https://doi.org/10.3390/molecules29184479
Jiménez-Pérez A, Martínez-Alonso M, García-Tojal J. Hybrid Hydroxyapatite–Metal Complex Materials Derived from Amino Acids and Nucleobases. Molecules. 2024; 29(18):4479. https://doi.org/10.3390/molecules29184479
Chicago/Turabian StyleJiménez-Pérez, Alondra, Marta Martínez-Alonso, and Javier García-Tojal. 2024. "Hybrid Hydroxyapatite–Metal Complex Materials Derived from Amino Acids and Nucleobases" Molecules 29, no. 18: 4479. https://doi.org/10.3390/molecules29184479
APA StyleJiménez-Pérez, A., Martínez-Alonso, M., & García-Tojal, J. (2024). Hybrid Hydroxyapatite–Metal Complex Materials Derived from Amino Acids and Nucleobases. Molecules, 29(18), 4479. https://doi.org/10.3390/molecules29184479








