Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. Removal of Levofloxacin Hydrochloride by Zn/Cr-MOFs/TiO2 Composites
2.3. Adsorption Equilibrium
2.4. Kinetic Modeling
2.5. Role of pH and Temperature
3. Experimental
3.1. Experimental Reagents and Instruments
3.2. Preparation of Zn/Cr-MOFs/TiO2 Composites
3.3. Study on Removal of Levofloxacin Hydrochloride by Zn/Cr-MOFs/TiO2 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset. Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Zhang, R.; Jiao, L.; Jiang, H.-L. Metal-organic framework derived porous materials for catalysis. Coord. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Swetha, S. Mixed Biosorbent of Agro Waste and Bacterial Biomass for the Separation of Pb(II) Ions from Water System. Chemosphere 2021, 277, 130236. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, J.-L.; Hao, H.; Li, X.; Lang, X. Polyimide-TiO2 Hybrid Photocatalysis: Visible Light-Promoted Selective Aerobic Oxidation of Amines. Chem. Eng. J. 2020, 379, 122399. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, J.-L.; Hao, H.; Li, X.; Lang, X. Selective Aerobic Oxidation of Sulfides by Cooperative Polyimide-TiO2 Photocatalysis and Triethylamine Catalysis. J. Colloid Interface Sci. 2020, 565, 614–622. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.-V.N. A Review on Cleaner Strategies for Extraction of Chitosan and Its Application in Toxic Pollutant Removal. Environ. Res. 2021, 196, 110996. [Google Scholar] [CrossRef]
- Sharma, G.; AlGarni, T.S.; Kumar, P.S.; Bhogal, S.; Kumar, A.; Sharma, S.; Naushad, M.; ALOthman, Z.A.; Stadler, F.J. Utilization of Ag2O–Al2O3–ZrO2 Decorated onto RGO as Adsorbent for the Removal of Congo Red from Aqueous Solution. Environ. Res. 2021, 197, 111179. [Google Scholar] [CrossRef]
- Ullah, S.; Al-Sehemi, A.G.; Mubashir, M.; Mukhtar, A.; Saqib, S.; Bustam, M.A.; Cheng, C.K.; Ibrahim, M.; Show, P.L. Adsorption Behavior of Mercury over Hydrated Lime: Experimental Investigation and Adsorption Process Characteristic Study. Chemosphere 2021, 271, 129504. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Bontempi, E.; Zafeiratos, S.; Jaén, J.J.D.; Fornasiero, P. Synthesis and Photocatalytic Application of Visible-Light Active β-Fe2O3/g-C3N4 Hybrid Nanocomposites. Appl. Catal. B Environ. 2016, 187, 171–180. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Chen, C.; Wang, X. Enhanced photocatalytic degradation of methylene blue under visible irradiation on graphene@TiO2 dyade structure. Appl. Catal. B Environ. 2012, 111–112, 303–308. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Crake, A.; Christoforidis, K.C.; Gregg, A.; Moss, B.; Kafizas, A.; Petit, C. The Effect of Materials Architecture in TiO2 /MOF Composites on CO2 Photoreduction and Charge Transfer. Small 2019, 15, 1805473. [Google Scholar] [CrossRef]
- Ramírez, D.J.; Alfonso Herrera, L.A.; Colorado-Peralta, R.; Rodríguez, R.P.; Camarillo Reyes, P.K.; Chiñas, L.E.; Sánchez, M.; Rivera, J.M. Highly Efficient Methyl Orange Adsorption by UV-012, a New Crystalline Coii MOF. CrystEngComm 2021, 23, 3537–3548. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, K.; Li, X.; Huang, L.; Liang, J.; Zheng, G.; Shan, G. Nickel-Metal-Organic Framework Nanobelt Based Composite Membranes for Efficient Sr2+ Removal from Aqueous Solution. Environ. Sci. Ecotechnol. 2020, 3, 100035. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Loganathan, M.; Senthil Kumar, P.; Vo, D.-V.N. Cobalt and Nickel Oxides Supported Activated Carbon as an Effective Photocatalysts for the Degradation Methylene Blue Dye from Aquatic Environment. Sustain. Chem. Pharm. 2021, 21, 100406. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, A.; Wu, J.; Zhao, J.; Yan, H. Adsorption Behaviors and Mechanisms of Methyl Orange on Heat-Treated Palygorskite Clays. Ind. Eng. Chem. Res. 2012, 51, 14026–14036. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Feng, X.; Yu, W.; Zhang, D.; Fu, J. Palygorskite-Cerium Oxide Filled Rubber Nanocomposites. Appl. Clay Sci. 2012, 67–68, 44–49. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Montini, T.; Fittipaldi, M.; Jaén, J.J.D.; Fornasiero, P. Photocatalytic Hydrogen Production by Boron Modified TiO2/Carbon Nitride Heterojunctions. ChemCatChem 2019, 11, 6408–6416. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, Y.; Chai, J.; Tian, J.; Jing, T.; Zhang, D.; Cheng, J.; Peng, H.; Liu, B.; Zheng, G. Highly Effective Photocatalytic Performance of {001}-TiO2/MoS2/RGO Hybrid Heterostructures for the Reduction of Rh B. RSC Adv. 2019, 9, 15033–15041. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. TiO2 structures doped with noble metals and/or graphene oxide to improve the photocatalytic degradation of dichloroacetic acid. Environ. Sci. Pollut. Res. 2017, 24, 12628–12637. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Shandilya, P.; Raizada, P.; Sudhaik, A.; Rahmani-Sani, A.; Bandegharaei, A.H. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 2020, 13, 3498–3520. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, Q.; Sun, W.-Y.; Yao, Z.-Y.; Zhu, W.; Li, T.; Wang, J. Assembling ultrafine TiO2 nanoparticles on UiO-66 octahedrons to promote selective photocatalytic conversion of CO2 to CH4 at a low concentration. Appl. Catal. B Environ. 2020, 270, 118856. [Google Scholar] [CrossRef]
- Man, Z.; Meng, Y.; Lin, X.; Dai, X.; Wang, L.; Liu, D. Assembling UiO-66@TiO2 nanocomposites for efficient photocatalytic degradation of dimethyl sulfide. Chem. Eng. J. 2022, 431, 133952. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, M.; Duan, W.; Liu, B. A dual-functional UiO-66/TiO2 composite for water treatment and CO2 capture. RSC Adv. 2017, 7, 16232–16237. [Google Scholar] [CrossRef]
- Yang, J.; Chang, X.; Wei, F.; Lv, Z.; Liu, H.; Li, Z.; Wu, W.; Qian, L. High performance photocatalyst TiO2@UiO-66 applied to degradation of methyl orange. Discov. Nano 2023, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Z.; Yang, Z.; Wang, J.; Xie, J.; Fu, M.; Hu, Y. TiO2@UiO-66 composites with efficient adsorption and photocatalytic oxidation of VOCs: Investigation of synergistic effects and reaction mechanism. ChemCatChem 2020, 13, 581–591. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, X.; Wang, C.C.; Fu, H.; Liu, Y.; Wang, P.; Zhao, C. S-TiO2/UiO-66-NH2 composite for boosted photocatalytic Cr(VI) reduction and bisphenol A degradation under LED visible light. J. Hazard Mater. 2020, 399, 123085. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Qin, J.; Yang, Z.; Fu, M. TiO2-UiO-66-NH2 nanocomposites as efficient photocatalysts for the oxidation of VOCs. Chem. Eng. J. 2020, 385, 123814. [Google Scholar] [CrossRef]
- Kaur, G.; Sud, D. Pillar-Layered Metal-Organic Framework decorated onto TiO2: An Efficient Photocatalyst for Mineralization of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). ChemistrySelect 2024, 9, e202303628. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, Z.; Shi, B.; Lu, C. Two-Dimensional Cu-Porphyrin Metal−Organic Framework Nanosheet-Supported Flaky TiO2 as an Efficient Visible-Light-Driven Photocatalyst for Dye Degradation and Cr(VI) Reduction. Langmuir 2023, 39, 15665–15675. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zheng, T.; Ren, Q.; Chen, H.; Peng, J.; Ma, Y.; Liu, Z.; Liang, Z.; Chen, D. Preparation of metal-organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater. Green Process. Synth. 2022, 11, 595–603. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Zhang, L.; Yu, J.; Li, Y. Synthesis of MgNiCo LDH hollow structure derived from ZIF-67 as superb adsorbent for Congo red. J. Colloid Interface Sci. 2022, 612, 598–607. [Google Scholar] [CrossRef]
- Wei, F.H.; Ren, Q.H.; Zhang, H.; Yang, L.L.; Chen, H.L.; Liang, Z.; Chen, D. Removal of tetracycline hydrochloride from wastewater by Zr/Fe-MOFs/GO composites. Rsc Adv. 2021, 11, 9977–9984. [Google Scholar] [CrossRef]
- Gu, Y.; Xie, D.; Wang, Y.; Qin, W.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Facile fabrication of composition-tunable Fe/Mg bimetal-organic frameworks for exceptional arsenate removal. Chem. Eng. J. 2019, 357, 579–588. [Google Scholar] [CrossRef]
- Wei, F.H.; Nie, M.; Ren, Q.H.; Yu, X.; Li, H.; Chen, H.L.; He, M.T.; Liang, Z.; Wang, S.Y.; Han, D.X. Preparation of bimetallic metal–organic frameworks for adsorbing doxycycline hydrochloride from wastewater. Appl. Organomet. Chem. 2023, 37, e7212. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, M.; Zhao, X.; Song, S.; Gao, Z. Ultra-high-capacity adsorption of rhodamine B in a carboxyl-functionalized metal-organic framework via surface adsorption. J. Chem. Eng. Data 2021, 66, 669–676. [Google Scholar] [CrossRef]
- Yılmaz, E.; Sert, E.; Atalay, F.S. Synthesis, characterization of a metal organic framework:MIL-53 (Fe) and adsorption mechanisms of methyl red onto MIL-53 (Fe). J. Taiwan Inst. Chem. Eng. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Hu, J.; Yu, H.; Dai, W.; Yan, X.; Hu, X.; Huang, H. Enhanced adsorptive removal of hazardous anionic dye “Congo red” by a Ni/Cu mixed-component metal–organic porous material. RSC Adv. 2014, 4, 35124. [Google Scholar] [CrossRef]
- Zhu, G.F.; Chen, L.T.; Cheng, G.H.; Zhao, J.; Yang, C.; Zhang, Y.Z.; Wang, X.; Fan, J. Efficient Removal of Levofloxacin Hydrochloride from Environment by UiO-66/CoSO4 Composites. Acta Chim. Sin. 2019, 77, 434–441. [Google Scholar] [CrossRef]
- Jin, J.H.; Yang, Z.H.; Xiong, W.P.; Zhou, Y.Y.; Xu, R.; Zhang, Y.R.; Cao, J.; Li, X. Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions. Sci. Total Environ. 2019, 650, 408. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Yuan, W.; Xue, Y. Co-modified MCM-41 as an effective adsorbent for levofloxacin removal from aqueous solution: Optimization of process parameters, isotherm, and thermodynamic studies. Environ. Sci. Pollut. Res. 2017, 24, 5238. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, W.; Shi, J. Enhanced levofloxacin removal from water using zirconium (IV) loaded corn bracts. Environ. Sci. Pollut. Res. 2017, 24, 10685. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhao, H.; Sun, H.; Xiao, K.; Wong, P.K. Enhanced adsorption and photocatalytic activities of ultrathin graphitic carbon nitride nanosheets: Kinetics and mechanism. Chem. Eng. J. 2020, 381, 122760. [Google Scholar] [CrossRef]
- Wei, F.H.; Xiao, L.; Ren, Q.H.; Wang, K.; Qin, L.; Chen, H.L.; Ma, Y.F.; Liang, Z. The application of Bimetallic metal-organic frameworks for antibiotics adsorption. J. Saudi Chem. Soc. 2022, 26, 101562. [Google Scholar] [CrossRef]
- Yoo, D.K.; Bhadra, B.N.; Jhung, S.H. Adsorptive removal of hazardous organics from water and fuel with functionalized metal-organic frameworks: Contribution of functional groups. J. Hazard. Mater. 2021, 403, 123655. [Google Scholar] [CrossRef]
- Wei, F.; Gong, J.; Ren, Q.; Yu, X.; Wang, Y.; Qin, L.; Chen, H.; Liang, Z. Reparation of Zn/Zr-MOFs by microwave-assisted ball milling and adsorption of lomefloxacin hydrochloride and levofloxacin hydrochloride in wastewater. Environ. Res. 2024, 252, 118941. [Google Scholar] [CrossRef]






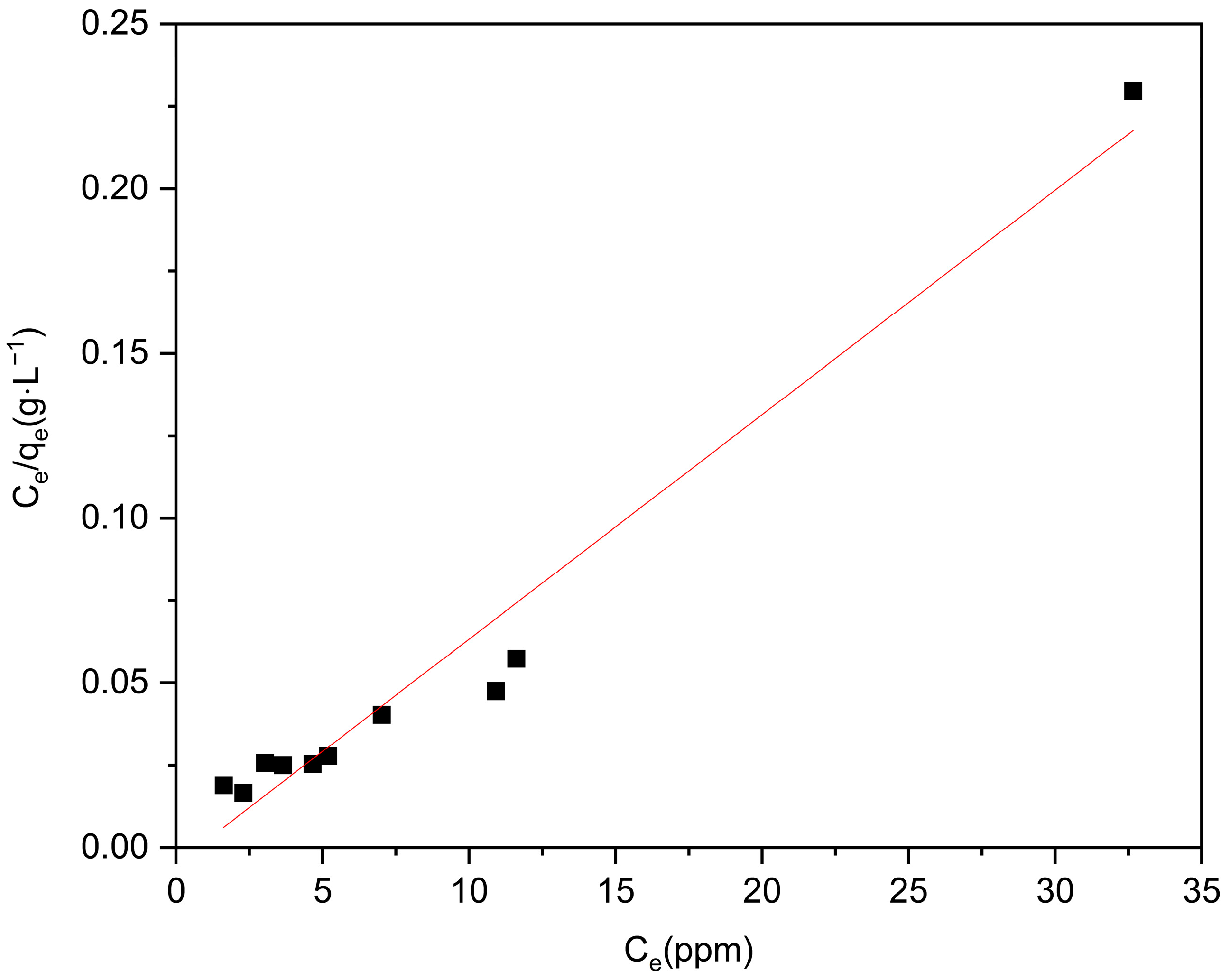






| Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||
|---|---|---|---|---|
| K | R2 | n | R2 | |
| MOFs/TiO2 | 0.00681 | 0.96188 | 5.3636 | 0.2426 |
| Concentration (mg·L−1, ≤±10%) | Mass (mg, <±1%) | Linear | Non-Linear | ||
|---|---|---|---|---|---|
| k2 (g·(mg·min)−1) | R2 | k2 (g·(mg·min)−1) | R2 | ||
| 20 | 20 | 0.00535 | 0.99916 | - | 0.99919 |
| 30 | 0.00721 | 0.99968 | - | 0.99964 | |
| 50 | 0.01198 | 0.99967 | - | 0.99971 | |
| 100 | 0.02744 | 0.99901 | - | 0.99993 | |
| 200 | 0.05838 | 0.99465 | - | 0.99522 | |
| 30 | 20 | 0.00427 | 0.99884 | - | 0.99894 |
| 30 | 0.00527 | 0.99921 | - | 0.9992 | |
| 50 | 0.00847 | 0.99982 | - | 0.9998 | |
| 100 | 0.01766 | 0.99914 | - | 0.99904 | |
| 200 | 0.04121 | 0.99708 | - | 0.99962 | |
| 50 | 20 | 0.00686 | 0.99401 | - | 0.99875 |
| 30 | 0.00551 | 0.99881 | - | 0.99871 | |
| 50 | 0.00683 | 0.99783 | - | 0.99894 | |
| 100 | 0.01192 | 0.99984 | - | 0.99996 | |
| 200 | 0.02643 | 0.99903 | - | 0.99985 | |
| T (K) | ΔG° (kJ/mol) | ΔH° (−Slope × R) (KJ/mol) | S° (Intercept × R) (J/mol/K) |
|---|---|---|---|
| 288 | −4.27 | −5.81 | 5.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, F.; Zhang, Q.; Ren, Q.; Chen, H.; Zhang, Y.; Liang, Z. Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal. Molecules 2024, 29, 4477. https://doi.org/10.3390/molecules29184477
Wei F, Zhang Q, Ren Q, Chen H, Zhang Y, Liang Z. Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal. Molecules. 2024; 29(18):4477. https://doi.org/10.3390/molecules29184477
Chicago/Turabian StyleWei, Fuhua, Qin Zhang, Qinhui Ren, Hongliang Chen, Yutao Zhang, and Zhao Liang. 2024. "Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal" Molecules 29, no. 18: 4477. https://doi.org/10.3390/molecules29184477
APA StyleWei, F., Zhang, Q., Ren, Q., Chen, H., Zhang, Y., & Liang, Z. (2024). Zn/Cr-MOFs/TiO2 Composites as Adsorbents for Levofloxacin Hydrochloride Removal. Molecules, 29(18), 4477. https://doi.org/10.3390/molecules29184477







