1. Introduction
In recent years, human activities and the development of industry and agriculture have increased environmental pollution, leading to the contamination of soil and water with heavy metal ions [
1]. Water pollution has become a major environmental challenge [
2,
3,
4,
5], posing a threat to the health of humans and other organisms and offsetting the balance of aquatic ecosystems [
6,
7,
8,
9,
10]. Given that heavy metals are typically toxic, bioaccumulative, and persistent, many researchers have focused on eliminating heavy metal pollution with an emphasis on the removal of copper ions [
11,
12,
13]. Copper is one of the most important trace elements that influences metabolic processes. Thus, the guidelines of the World Health Organization and the US Environmental Protection Agency recommend limits of 2.0 mg/L and 1.3 mg/L, respectively, for copper in drinking water [
14,
15]. However, relatively higher concentrations of copper ions could lead to diseases in humans, such as cancer and Wilson’s disease, and affect the body’s immune and nervous systems [
16,
17,
18,
19,
20,
21]. Due to these potential negative implications of higher copper ion concentrations on humans, it is necessary to remove the excess potentially toxic copper ions from water and wastewater, improving the water quality.
Until now, different technologies have been developed for the treatment of heavy metal copper water pollution, such as chemical precipitation, ion exchange, membrane separation, the electrochemical method, electrostatic attraction, solvent extraction, reverse osmosis, flocculation, and adsorption [
22,
23,
24,
25,
26,
27,
28]. The adsorption method presents a prominent prospect in the removal of heavy metal due to its high removal efficiencies, low secondary pollution, low cost, and numerous potential adsorbents [
29,
30,
31,
32,
33,
34,
35]. The adsorbent material plays an important role in the adsorption efficiency of minerals (montmorillonite, kaolin, bentonite, and zeolite) [
36,
37,
38,
39], carbon adsorption materials (biochar, carbon nanotubes, and graphene) [
40], adsorption materials of metal compounds (MOFs) [
41], and gels (sodium alginate, chitosan, gelatin, starch, cellulose, pectin, agarose, and xanthan gum) [
42], etc. Among these materials, gel adsorption materials based on natural polymers are predominantly chosen due to their excellent biocompatibility, natural degradation, high stability, and ability to facilitate the easy formation of a 3-D network structure [
43]. The hydrogels’ outstanding 3-D network structure and substantial specific surface area significantly enhance the potentiality for interaction with heavy metal ions, thereby improving their efficiencies in removing such ions, leading to wide applications [
44,
45,
46]. However, this kind of hydrogel suffers from some drawbacks, such as chemical instability, less exposed active sites, and limited capability to adsorb heavy metal ions. The incorporation of certain carbon-based adsorbents with the hydrogel could increase the adsorption efficiency and enhance the mechanical characteristics.
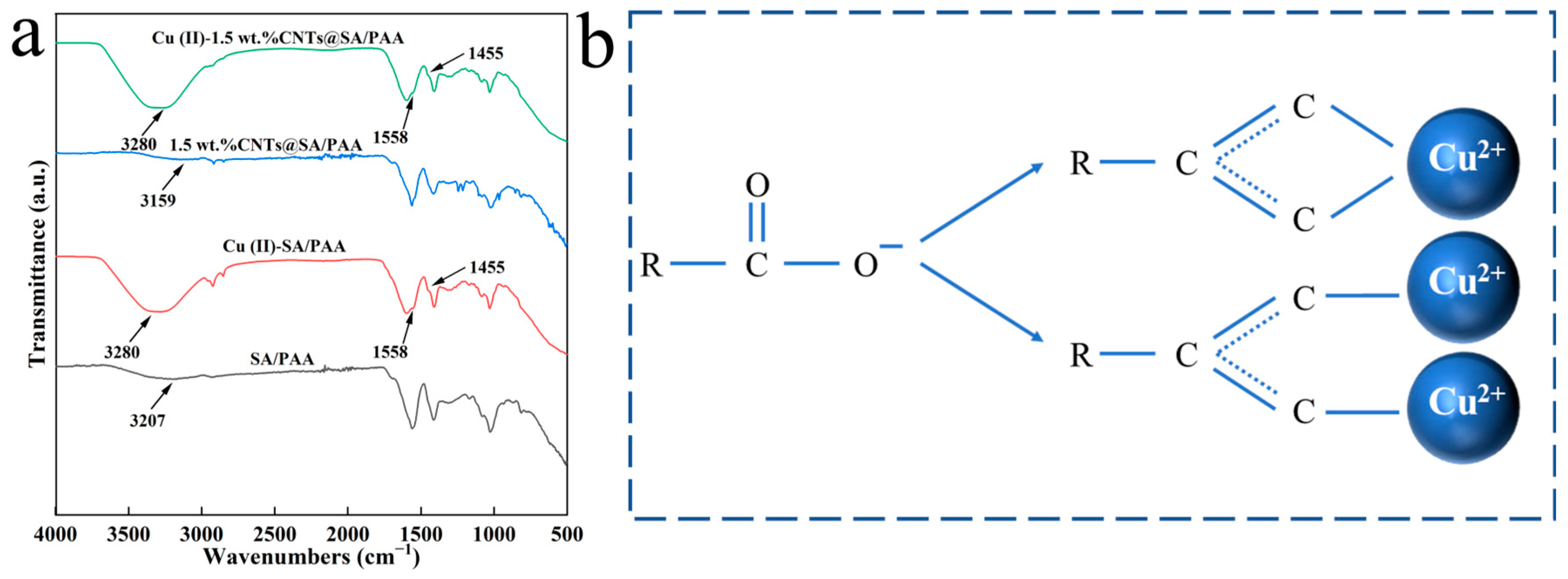
Sodium alginate (SA, C
6H
7NaO
6) is a natural hydrophilic anionic biopolymer extracted from brown algae kelp or sargassum. SA is a copolymer composed of β-
d-mannuronic acid (M unit) and α-
l-guluronic acid (G unit) linked by β-1,4-glycosidic bonds [
47]. The SA surface is rich in hydroxyl and carboxyl groups, typically forming rigid and compact gels when interacting with divalent alkaline earth metal ions, such as Ca
2+ and Ba
2+, via a salt bridge. This characteristic results in a robust binding capacity for heavy metal ions [
48]. However, the instability, weak mechanical strength, and low adsorption capacity of SA gel limit the polymer’s application [
49]. Hence, SA has been frequently combined with other adsorption materials to prepare composite hydrogels, enhancing the adsorption capacity for heavy metal ions. A combination of SA, magnetic chitosan, and polyethyleneimine was employed to prepare a composite gel that could effectively remove heavy metal ions and azo dyes from water [
50].
Polyacrylic acid (PAA) is a polymer formed by polymerization of an acrylic acid monomer (AA) [
51,
52,
53], with the characteristics of good water solubility, abundant carboxyl groups, and excellent water absorption capability. It is easy for PAA to form a 3-D network structure in aqueous solutions, which allows the release of anionic functional groups (–COO
−), enabling the chelation of positively charged heavy metal ions, thus removing heavy metal ions from aqueous solutions [
54,
55].
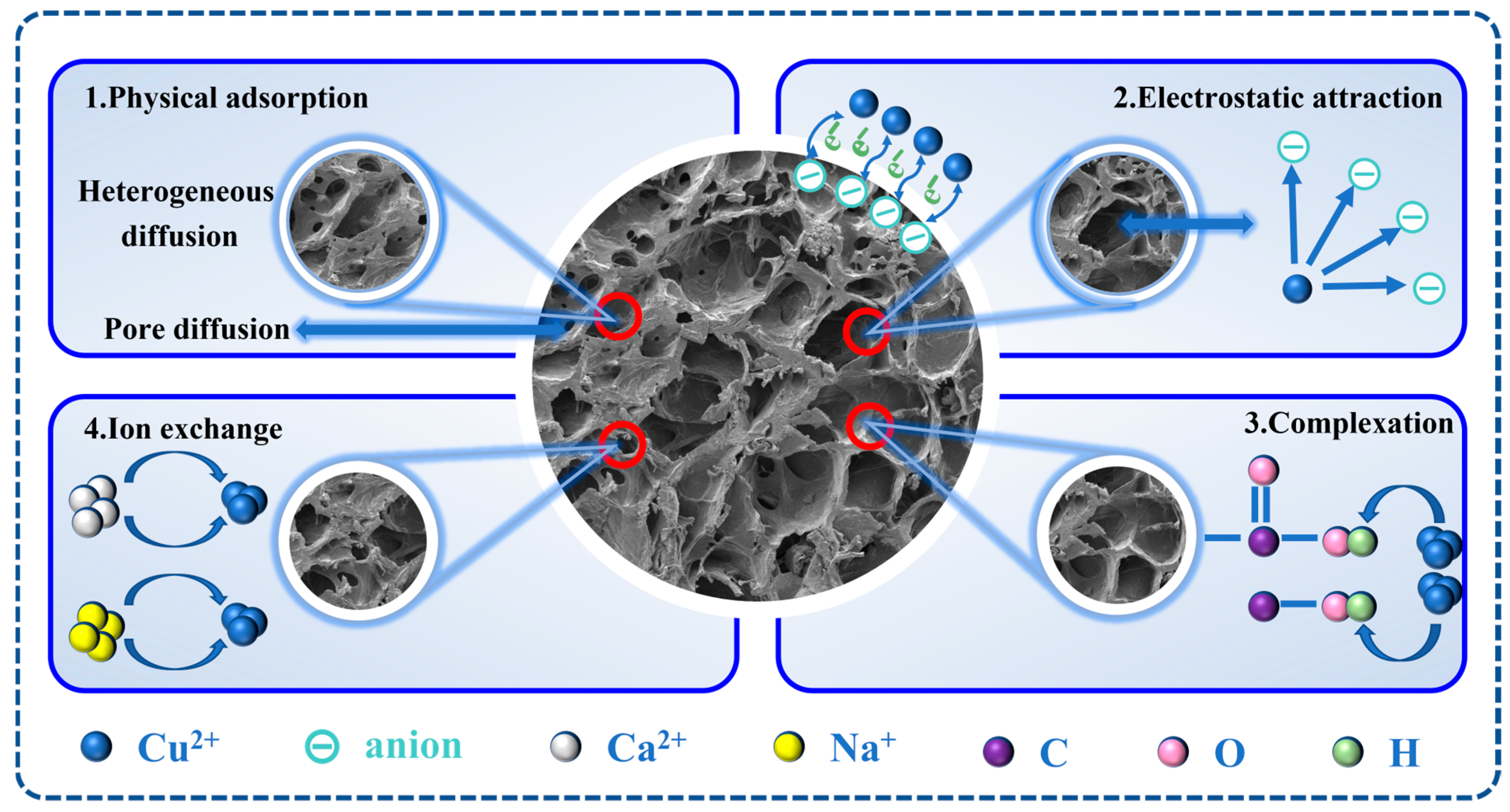
Carbon nanotubes (CNTs) are carbon adsorbents with unique characteristics of thermal stability, thermal conductivity, catalysis, and adsorption capability [
56]. There are single-walled (SWCNTs) and multi-walled (MWCNTs) nanotubes [
57]. Due to the nanoscale structures, CNTs have a very large surface area of active sites and thus could adsorb heavy metal ions to the surface through hydrogen bonding, electrostatic attraction, and van der Waals forces. Additionally, CNTs could be chemically bonded to heavy metal ions with carboxyl or hydroxyl groups, increasing the adsorption efficiency and capability. As for the applications to wastewater treatment, CNTs have shown excellent adsorption selectivity [
58]. However, the high costs and difficulties in recycling CNTs limit their applications in water and wastewater treatments, which could be solved via their integration into natural polymer networks [
59,
60].
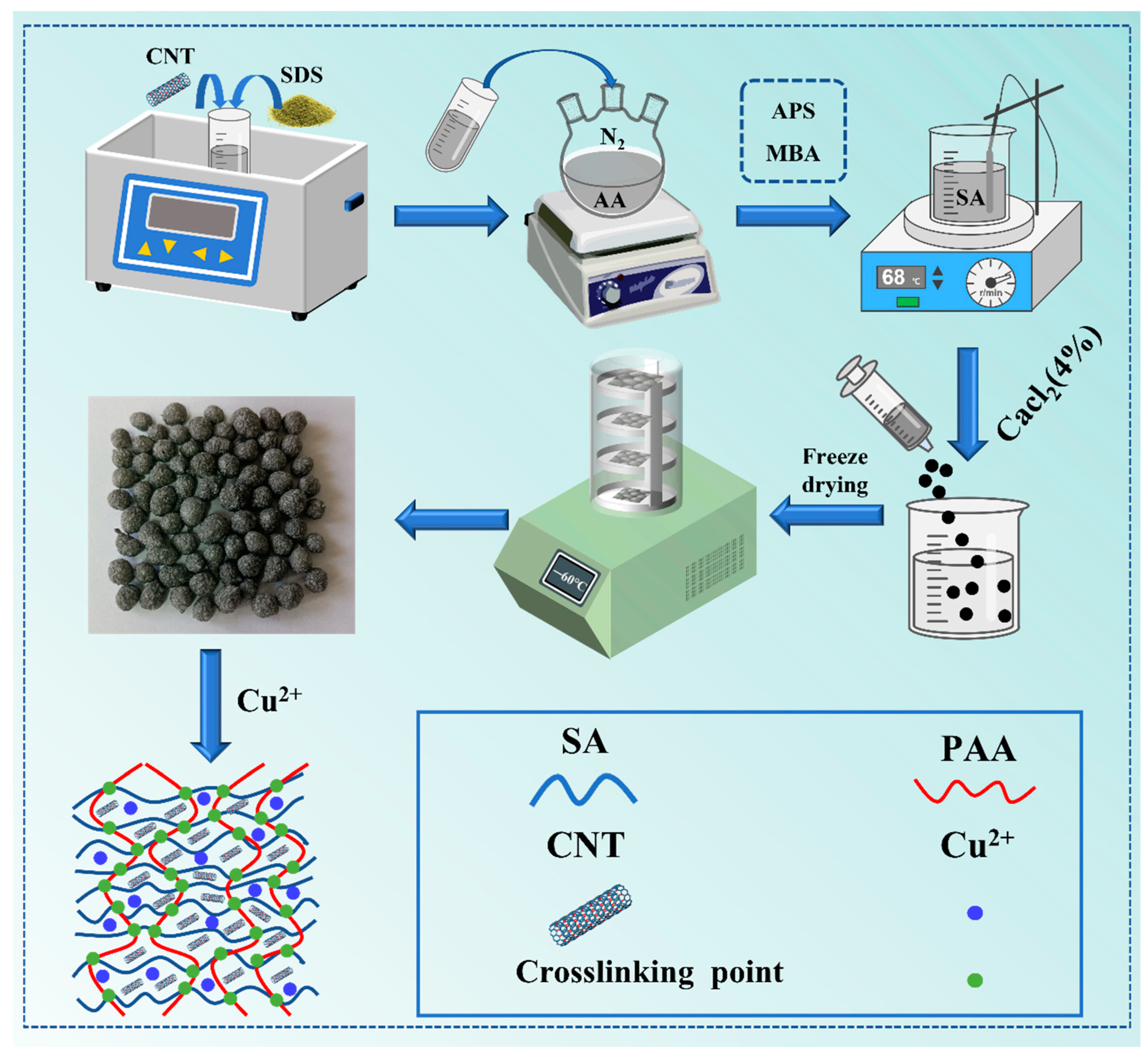
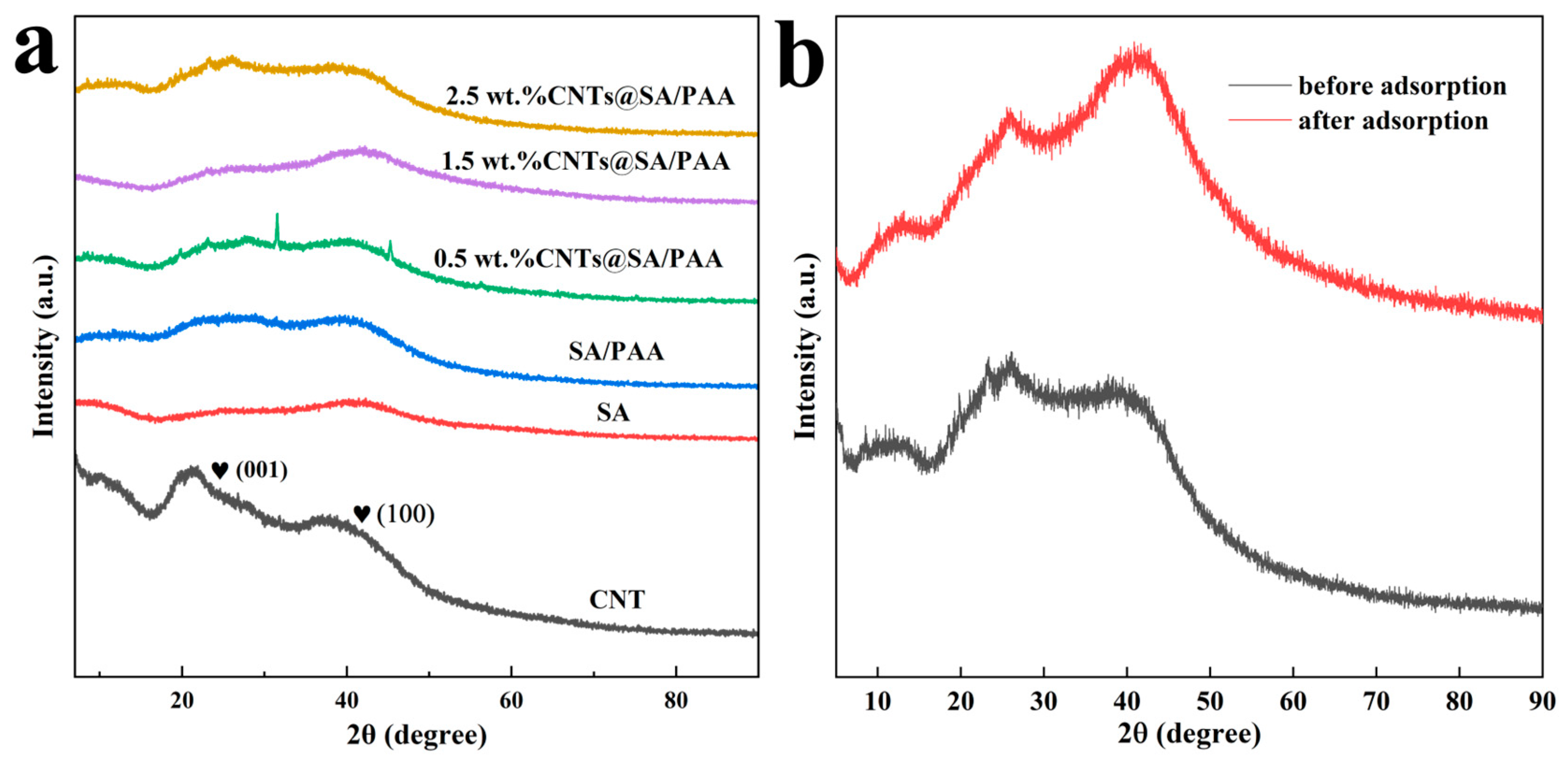
In this study, a novel composite hydrogel adsorbent composed of SA, PAA, and CNTs, CNTs@SA/PAA, was prepared with free radical polymerization, as shown in
Scheme 1, using
N,
N’-methylene bisacrylamide (MBA) and ammonium persulfate (APS) as the crosslinking agent and initiator, respectively. The resultant CNTs@SA/PAA hydrogel adsorbents were characterized with a scanning electron microscope–energy dispersive spectrometer (SEMEDS), X-ray diffraction (XRD), Fourier transform infrared spectrometer (FTIR), rheology and differential scanning calorimetry (DSC), etc. The adsorption capacities of the resultant hydrogels were investigated using the static adsorptions of heavy metal ions. The effects of different conditions on the adsorption capacity of the CNTs@SA/PAA hydrogels were investigated, including the pH of an aqueous solution, the adsorbent dosage, the contact time, and the initial Cu
2+ concentration. The adsorption kinetics, behavior, and capacity of the CNTs@SA/PAA hydrogels were analyzed with kinetic and isothermal models, and their adsorption mechanisms for the removal of copper ions are discussed extensively.
3. Materials and Methods
3.1. Chemicals and Reagents
SA, AA, CNT, and calcium chloride anhydrous (CaCl2) were obtained from Macklin Chemical Reagent Company (Shanghai, China). MBA and sodium dodecyl sulfate (SDS) were purchased from Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China). N,N,N,N-Tetramethylethylenediamine (TEMED), and copper sulfate pentahydrate (CuSO4·5H2O) were supplied by RON reagent Co., Ltd. (Shanghai, China). Sodium hydroxide (NaOH) and APS were purchased from Aladdin Co., Ltd. (Shanghai, China). All the chemical reagents were of analytical grade and used directly without further purification. Ultrapure water (UPW) was used in this study.
3.2. Preparation of SA/PAA and CNTs@SA/PAA Hydrogel
The CNT-doped SA/PAA composite hydrogel was prepared with free radical polymerization. Approximately 10 mg CNT and 1 mL SDS were dispersed in 20 mL UPW, then ultrasonicated (250 w, 40 KHz) for 30 min. The solution was poured into a 100 mL three-necked flask. After the additions of 1 g AA and 0.44 g NaOH, the CNTs solution was put in an oil bath at 68 °C for 10 min while purged with nitrogen. After 30 mg MBA was added to the solution, 10 mg APS was added to the mixture to produce free radicals under stirring at 200 rpm for 30 min. Then, 20 µL TEMED and 1 g SA were dispersed slowly into the solution. The mixture was then continuously stirred at 200 rpm for 5 h at room temperature. The solution was gradually dropped into a 400 mL 4% (w/v) CaCl2 aqueous solution, then set for 24 h for hardening. These composites were named 0.5 wt.%CNTs@SA/PAA, 1.5 wt.%CNTs@SA/PAA, and 2.5 wt.%CNTs@SA/PAA, according to the weight concentration of the CNTs. The preparation process of the SA/PAA hydrogel was the same as that of the CNTs@SA/PAA.
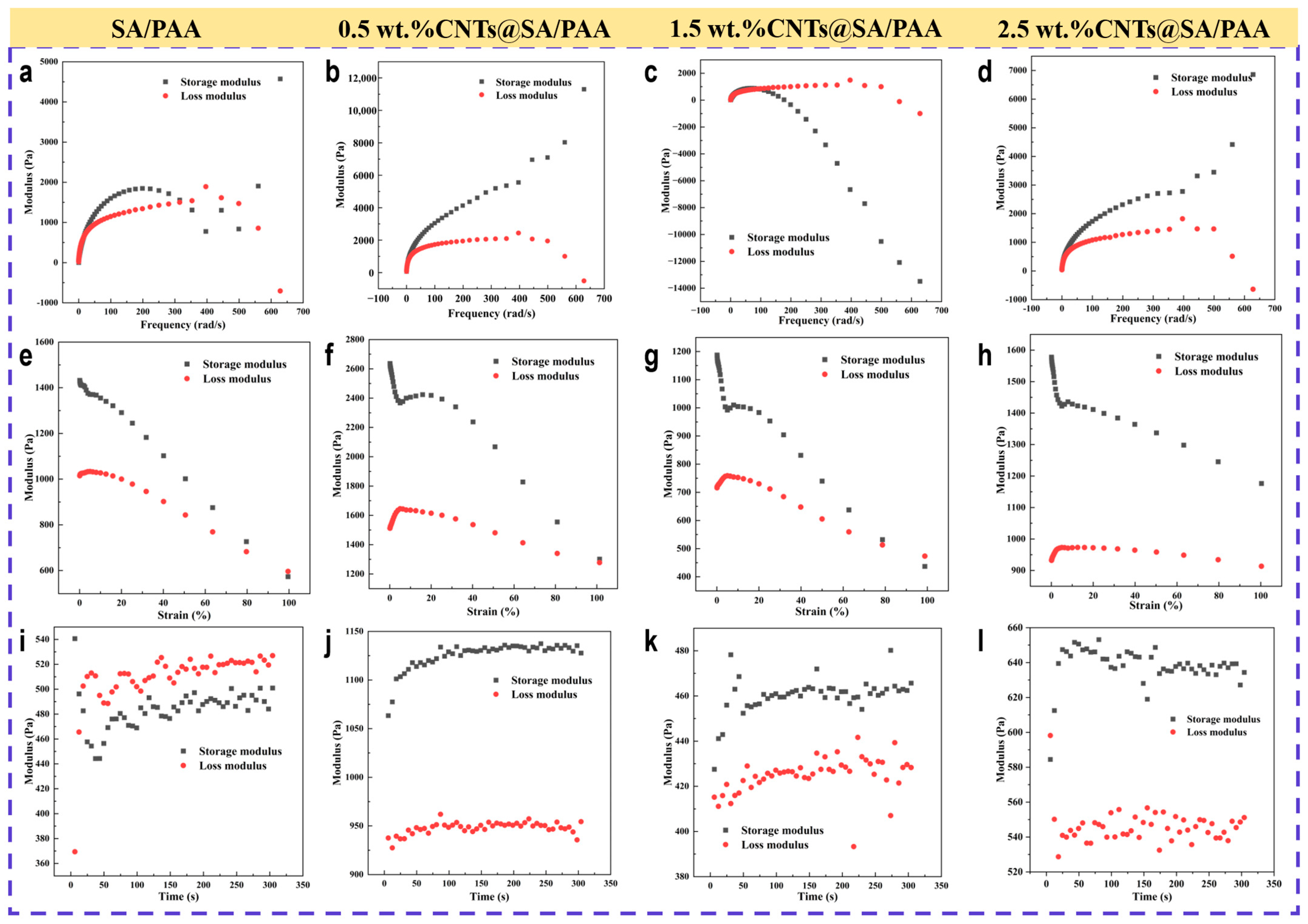
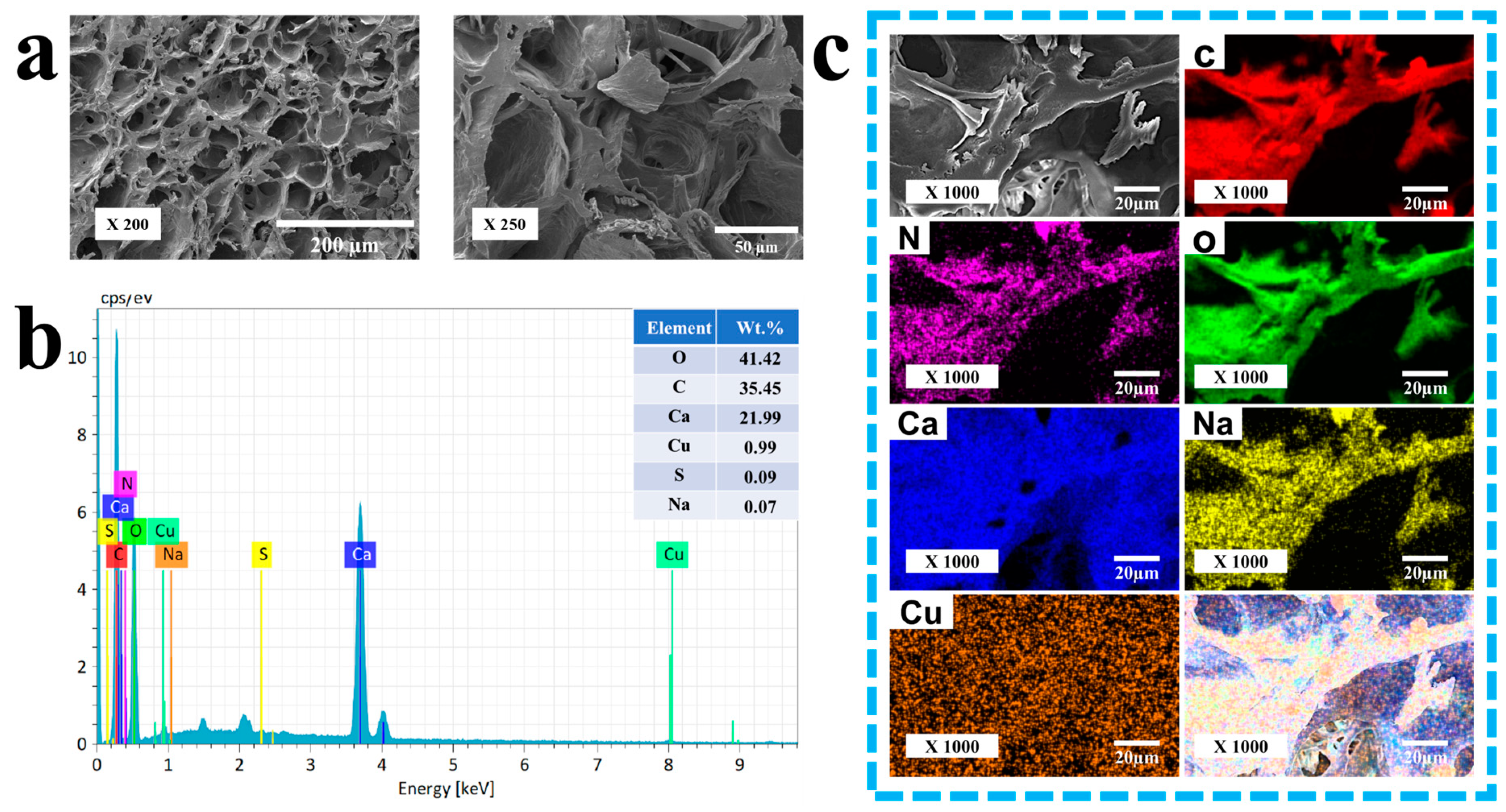
3.3. Characterization of Resultant Hydrogels
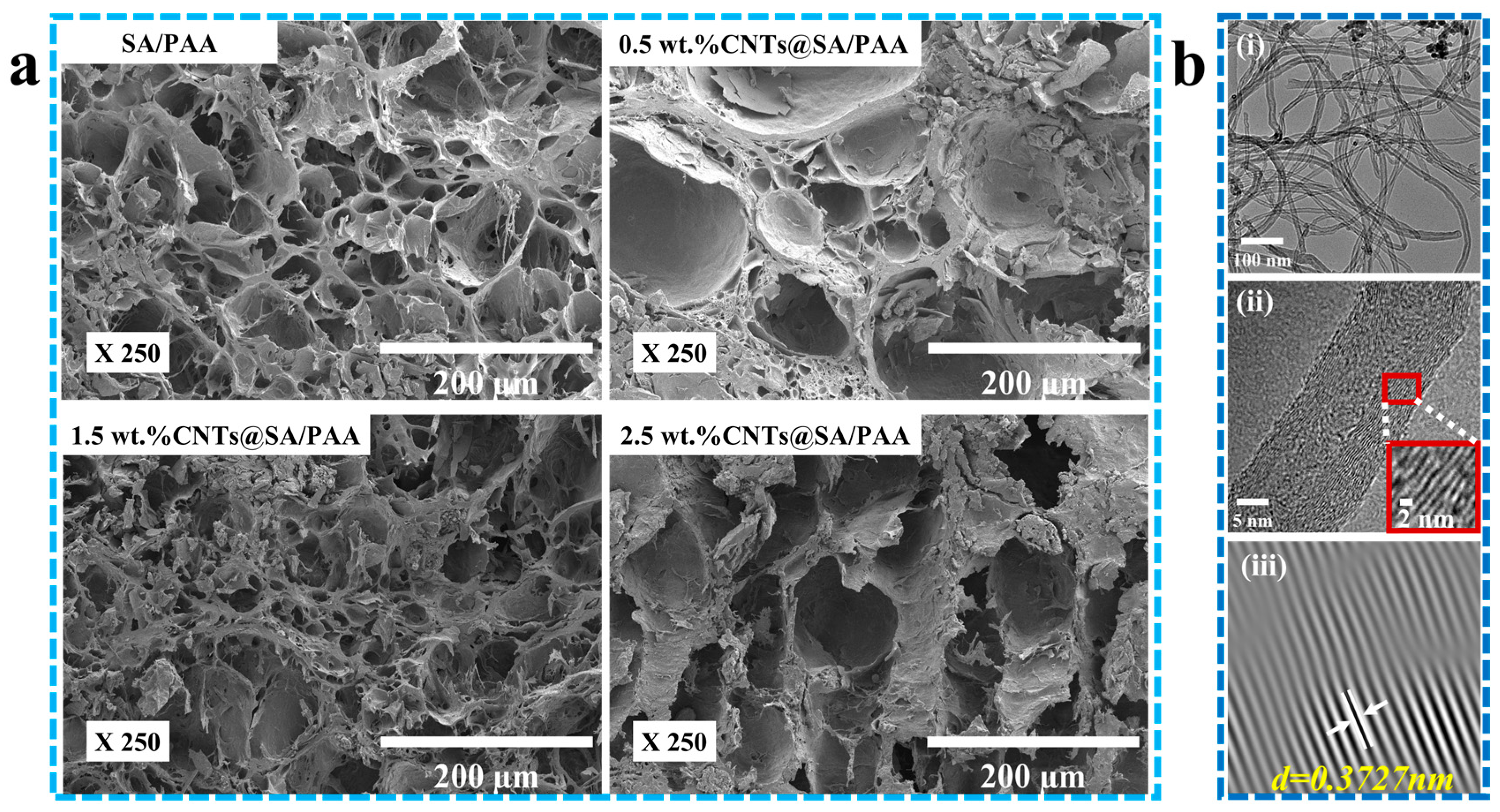
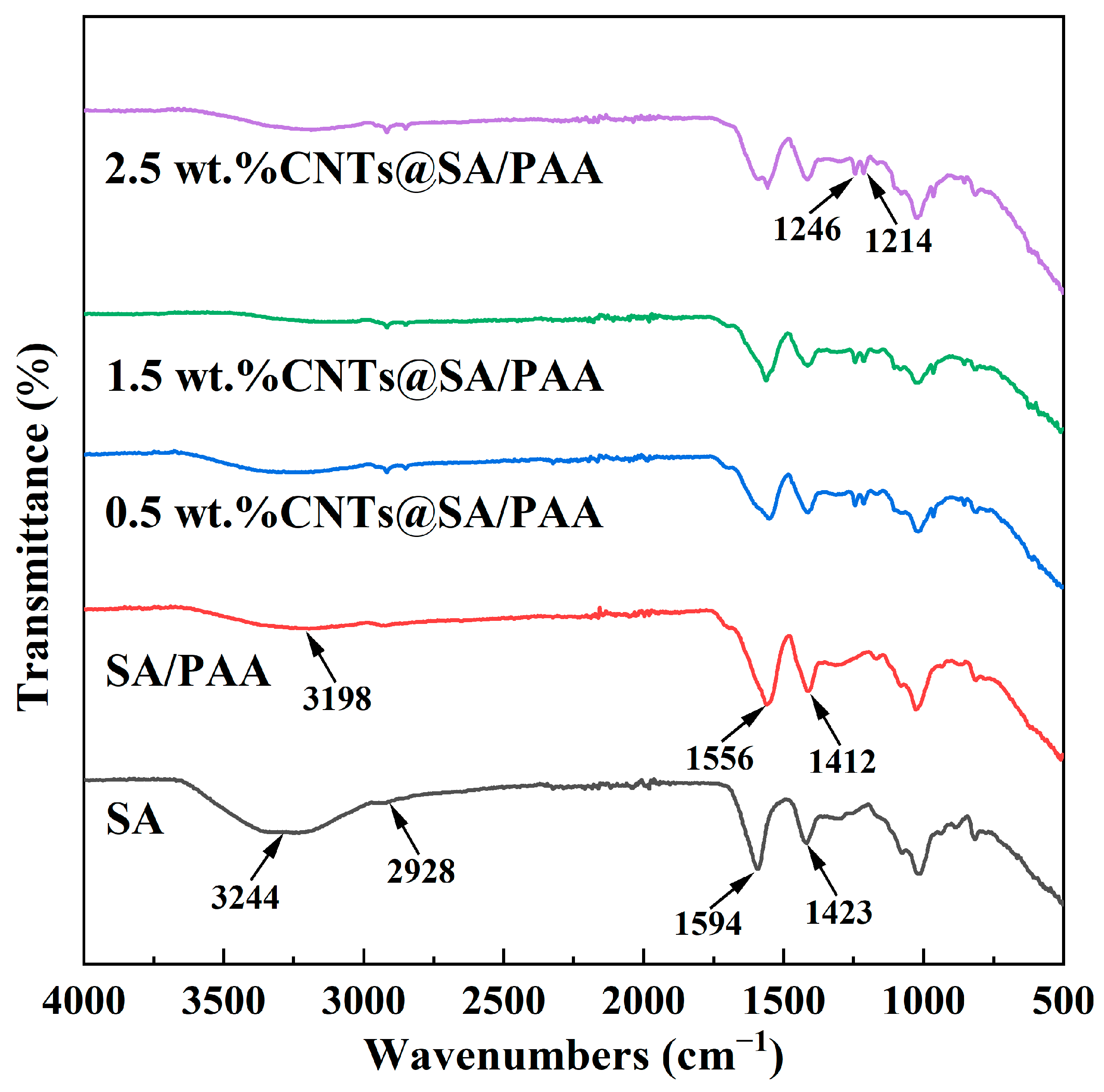
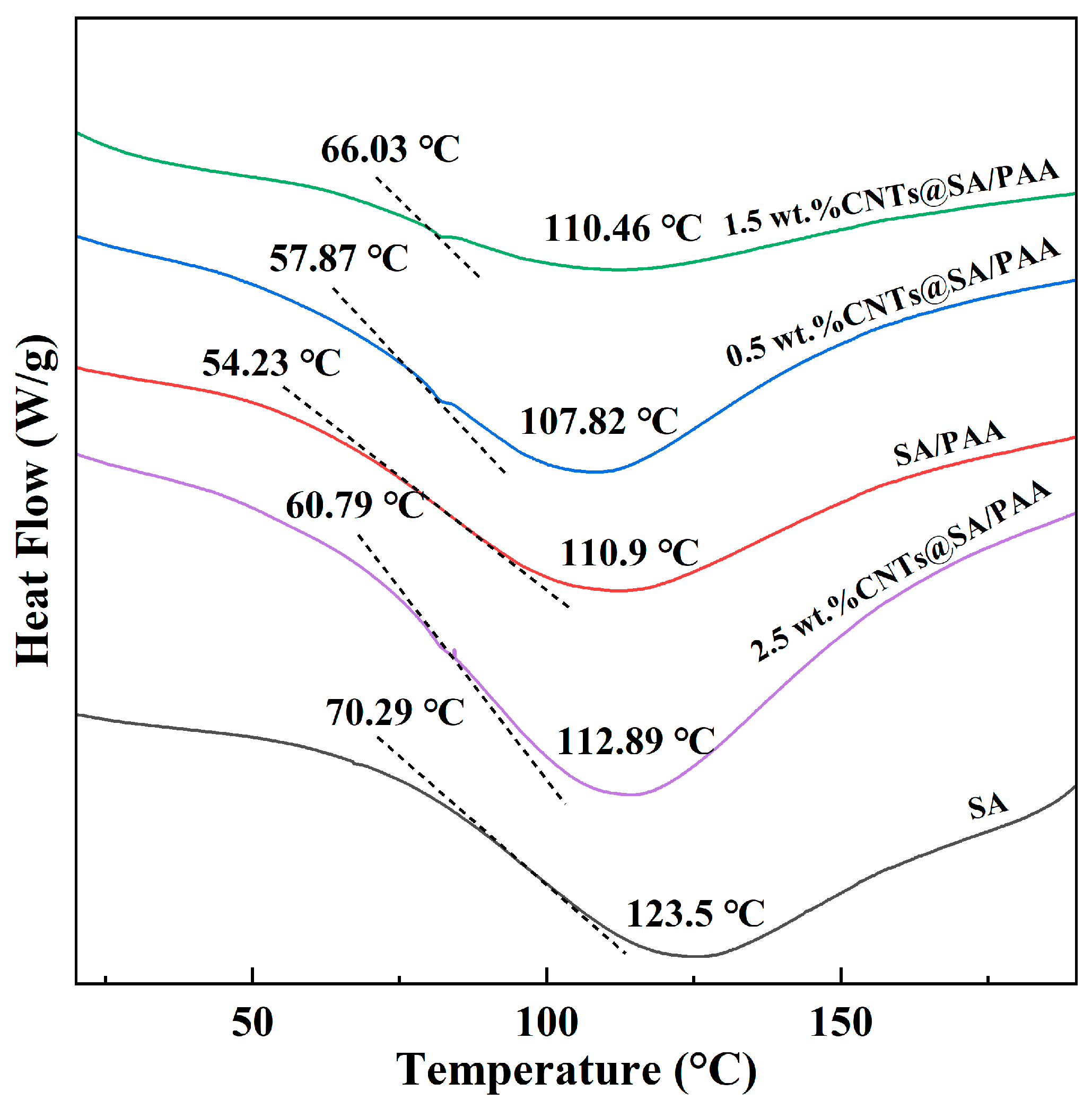
The 2-D morphologies were observed with a cold-field emission scanning electron microscope (S-4800, Hitachi, Tokyo, Japan). The chemical structures were characterized with an FT-IR spectrometer (Spectrum Two, PerkinElmer, Waltham, MA, USA). The X-ray diffraction patterns were characterized with an X-ray diffractometer (Smartlab 9 kW, Rigaku Corporation, Akishima, Japan). The structures were visualized with a transmission electron microscope (FEI Tecnai G2 F20 S-TWIN, FEI Company, Hillsboro, OR, USA).
3.4. Determination of Cu2+ Concentrations
The Cu2+ concentrations were determined with an atomic absorption spectrophotometer (AAS, TAS-990, Beijing Purkinje General Instrument Co., Ltd., Beijing, China).
3.5. Swelling Equilibria
The resultant hydrogels weighing 300 mg were soaked in 1000 mL UPW for 24 h. Then, the weights of the swollen hydrogels were measured. The swelling rate (%) could be calculated with Equation (1).
where
Wt and
Wd are the weights of the swollen and dry hydrogels, respectively.
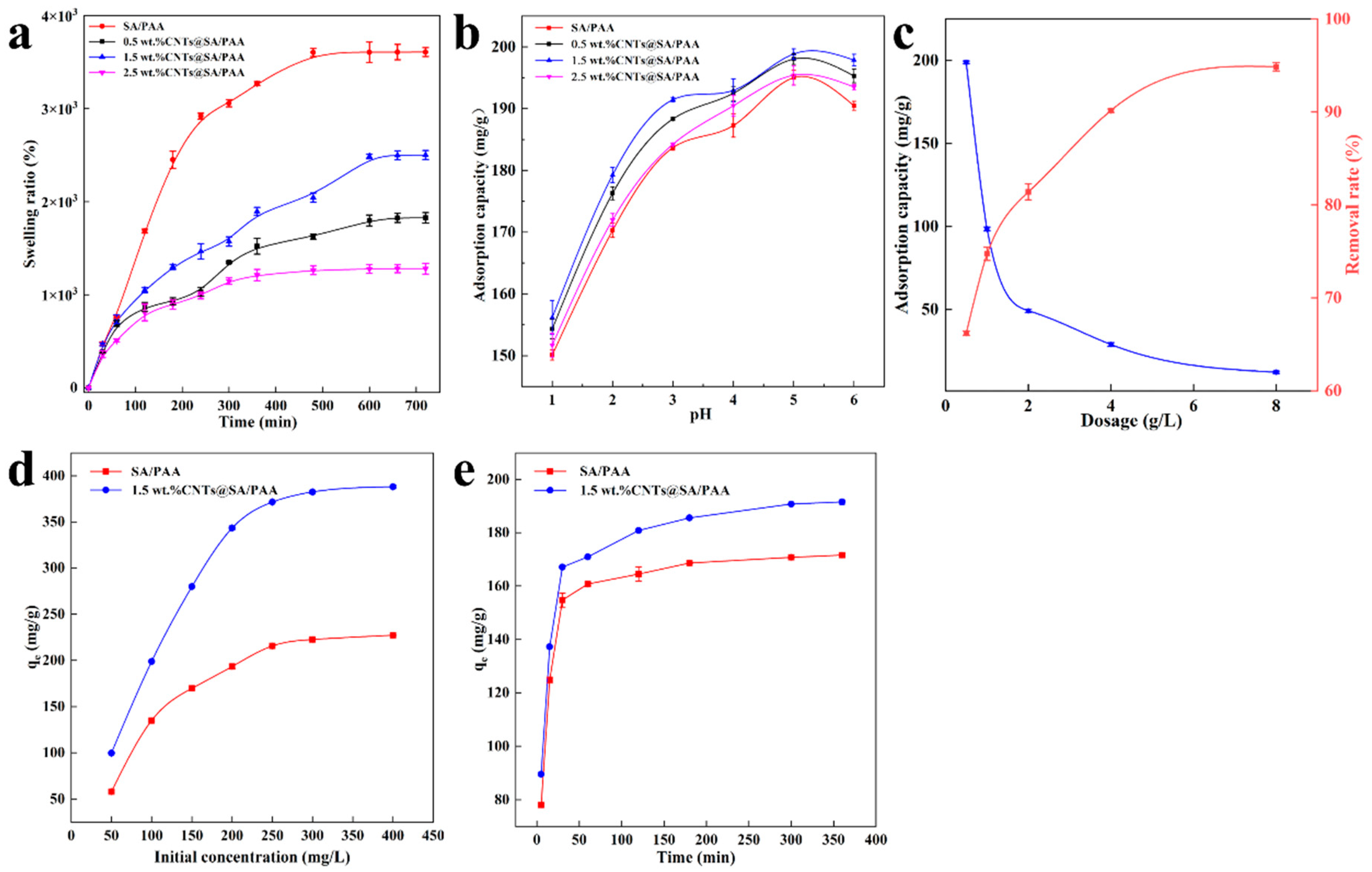
3.6. Batch Adsorption Experiments
The measurements of the Cu
2+ adsorbed were carried out with batch adsorption experiments, and the effect of the CNTs’ content doped on the adsorption capacity of the resultant hydrogel was investigated. CuSO
4·5H
2O was selected as the source of Cu
2+ ions. All the adsorption experiments were carried out at a speed of 200 rpm in a rotary oscillator with a 50 mL falcon tube at room temperature. The effect of the pH in the range of 1–6 on the adsorption capacity of the hydrogel was investigated. The adsorption kinetics were investigated under the following conditions: initial Cu
2+ concentration of 100 mg/L, adsorption time range of 0 to 360 min. The adsorption isotherms were investigated under the following conditions: Cu
2+ concentration in the range of 50 to 400 mg/L and an adsorption time of 24 h. According to the difference of ion concentrations in the solution before and after adsorption, the removal efficiency (
Re) and equilibrium adsorption (
qe) capacity of Cu
2+ were calculated as Equations (2) and (3).
where
C0 is the initial adsorbate concentration (mg/L) and
Ce is the adsorbate concentration (mg/L) at the liquid-phase equilibrium.
where
C0 (mg/L) and
Ce (mg/L) are the initial and equilibrium concentrations of Cu
2+, respectively;
V represents the volume of adsorbate (L); and
M is the weight of the adsorbent (g).
3.7. Mathematical Modeling
3.7.1. Adsorption Kinetics
Two kinetic models frequently used in the adsorption behaviors of hydrogels, the pseudo-first order (PFO) and pseudo-second order (PSO), were employed to evaluate the adsorption characteristics of the resultant CNTs@SA/PAA composite hydrogels. The linear kinetic models are represented by Equations (4) and (5), respectively.
where
k1 (1/min) and
k2 (g/(mg·min)) are the rate constant of the pseudo-first order and pseudo-second order of adsorptions, respectively.
qt (mg/g) is the adsorption capacity at time
t and
qe (mg/g) is the adsorption capacity at equilibrium and time
t.
The kinetics of the chemical adsorption mechanism were also investigated using the Elovich model, expressed as the following equation [
84]:
where
α (mg/(g·min)) is the initial adsorption rate and
β (g/mg) is the desorption constant relating to the surface coverage and the chemical adsorption activation energy.
The intraparticle diffusion model was employed to conduct further investigation, as this model could identify the reaction pathways, predicting the dominant or controlling steps of the adsorption rate [
85].
where
qt is the adsorption capacity of Cu
2+ ions (mg/g) at time
t,
kid is the intraparticle diffusion coefficient (mg/(g·min
0.5)), and
C is the boundary layer effect (mg/g). The value of
C is proportional to the thickness of the boundary layer; the larger the
C value, the greater the effect of the thickness of the diffusion boundary layer.
3.7.2. Adsorption Isotherms
The adsorption isotherm illustrates the adsorption equilibrium, indicating the proportion of the adsorption capacity to the equilibrium concentration or the pressure at a constant temperature. The isothermal adsorption line presents the equilibrium state of the adsorbate molecules between the solid and liquid phases [
86].
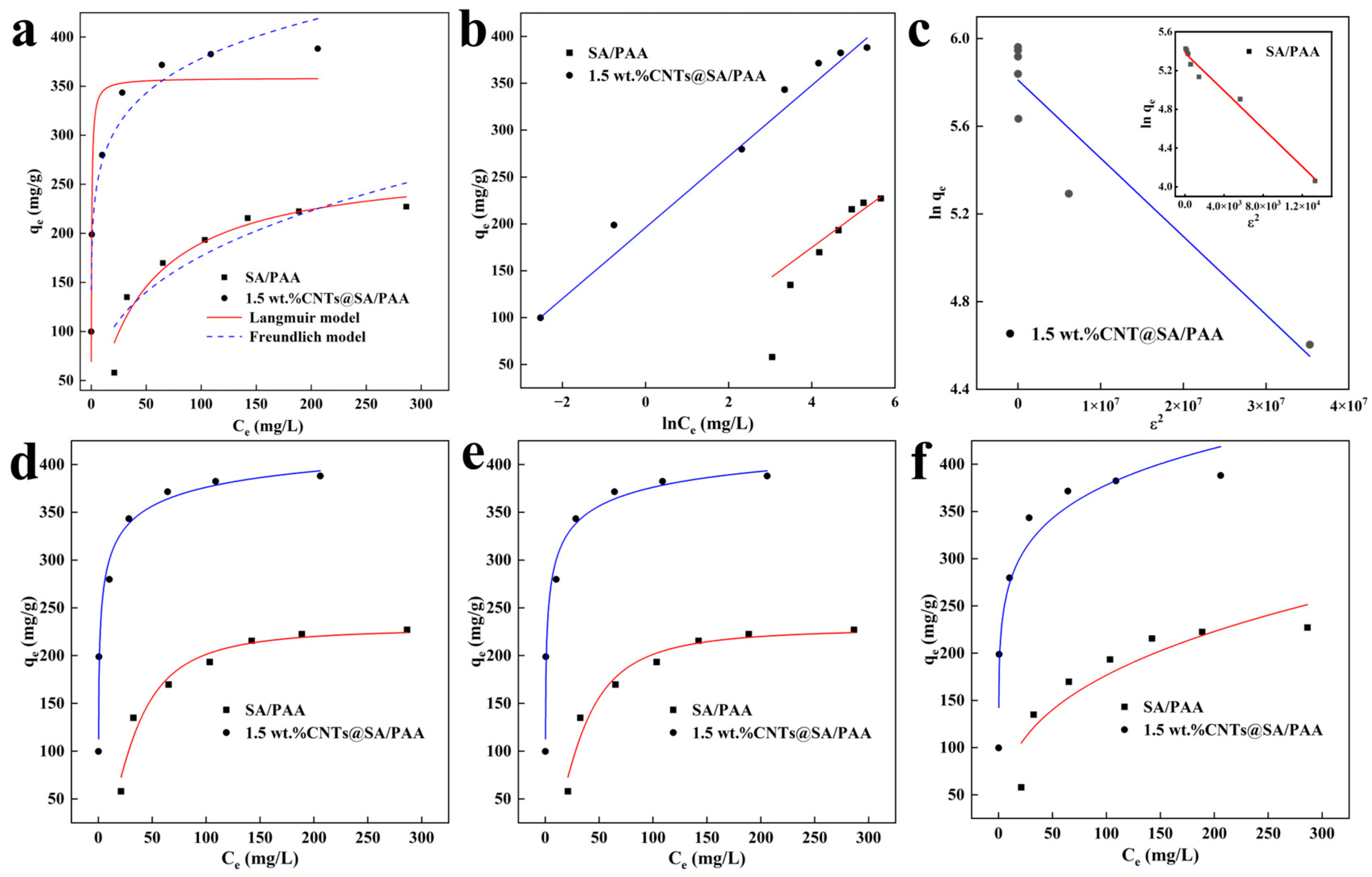
The adsorption isotherm could qualitatively analyze and determine the adsorption type and explain the interaction between the adsorbate and the surface selective sites of the adsorbent. The adsorption characteristics of the SA/PAA and CNTs@SA/PAA hydrogels for Cu2+ ions could be expressed by the adsorption isotherm, which plays an important role in inferring the adsorption mechanism and evaluating the adsorption capacity of adsorbents.
In this study, the Langmuir, Freundlich, Temkin, Dubinin–Radushkevich (D–R), Sips, Toth, and Khan isotherm models were employed to fit the experimental data and analyze the adsorption behaviors of the Cu
2+ by SA/PAA and CNTs@SA/PAA hydrogels. The derivation and establishment of the Langmuir isotherm model are based on the following three assumptions: (I) a single molecule layer formed on the surface of the adsorbent; (II) the interaction between the molecules adsorbed at adjacent positions could be ignored; (III) all the adsorption sites are the same and have equivalent energy, and the possibility of utilizing adsorption sites is the same. This model could be expressed with the following Equation (8) [
87].
where
Ce is the equilibrium concentration of the Cu
2+ solution (mg/L),
qe is the equilibrium adsorption capacity (mg/g),
qm (mg/g) is the maximum monolayer adsorption capacity, and
KL (L/mg) is the Langmuir equilibrium constant. The intercept and slope values of the curve are used to determine the
KL and
qm values, respectively.
The Langmuir isotherm reveals an important equilibrium parameter known as the separation factor (
RL). The
RL could be calculated with the Equation (9) [
88].
where
C0 (mg/L) is the initial concentration of the Cu
2+ ions.
RL is used to describe the experimental efficiency and the shape. The adsorption is linearly irreversible, as
RL is 1 and 0, respectively. The adsorption is unfavorable when
RL > 1, while it is favorable when
RL is in the range of 0 to 1 [
89].
Different from the Langmuir model, the Freundlich isotherm is based on the multilayer adsorption of metal ions on the heterogeneous surface of adsorbents, where the adsorption sites are not equivalent and the adsorption heat is uneven. This isotherm could be expressed with Equation (10) [
90].
where
qe is the equilibrium adsorption capacity (mg/g),
Ce (mg/L) is the equilibrium concentration of the Cu
2+ ions (mg/L),
KF is the empirical constant of the Freundlich isotherm, and
n is the empirical parameter related to the favorability of the adsorption process. The intercept and the slope value of
qe versus ln
Ce plot were used to calculate the
KF and
n values, respectively.
The Temkin isotherm was also used to calculate the adsorption heat change arising from the interaction between the adsorbent and the adsorbed substance. The Temkin equation is presented with Equation (11) [
91].
where
AT (L/g) is the Temkin isotherm binding constant,
b is the Temkin heat of adsorption coefficient (J/mol),
R is the universal gas parameter (8.314 J/mol/K), and
T is the absolute temperature (K), respectively.
The mathematical equivalence of the Dubinin–Radushkevich (D–R) isotherms provides additional information regarding the adsorption mechanism on heterogeneous surfaces with Equations (12)–(14) [
92].
where
qm (mg/g) is the adsorption capacity,
Kad is the energy of adsorption constant (mol
2/kJ
2), ε is the Dubinin–Radushkevich isotherm constant,
R is, and the universal gas parameter (8.314 J/mol/K), and
T is the absolute temperature (K), respectively. The average energy of the adsorption process (
E) could predict the adsorption mechanism.
The Sips isotherm is expressed by Equation (15) [
93].
where
qs is the adsorption capacity of the adsorbent,
KS is the equilibrium constant (1/mg), and
m is the Sips isotherm equation exponent. If
m converges to 1 or is equal to 1, the model tends towards the Langmuir equation; otherwise, if the
Ce or
Ks approach 0, the well-known Freundlich isotherm equation is reduced.
The Toth model is expressed by Equation (16) [
94].
where
qe is the adsorbed amount at equilibrium (mg/g),
Ce is the equilibrium concentration of the absorbate (mg/L),
qm is the maximum adsorption capacity according to the Toth model (mg/g),
aT is the Toth equilibrium constant, and
m is the exponent in the Toth model equation. If the value of 1/
m approaches 1, it is advisable to employ the Langmuir model. Otherwise, the Freundlich model is deemed more suitable.
The Khan model is expressed by Equation (17) [
94].
Here, aK serves as the model exponent and bK is a model constant. If aK approaches 1, the adsorption aligns well with the Langmuir model; otherwise, the Freundlich model is deemed to be the most appropriate.
3.8. Adsorption Thermodynamics
The Gibbs free energy could be calculated using the thermodynamic equilibrium constant
K0, which is defined as the following Equation (18) [
81].
where
as is the activity of adsorbed Cu
2+,
ae is the activity of Cu
2+ in the equilibrium state,
νs is the activity coefficient of adsorbed Cu
2+, and
νe is the activity coefficient of Cu
2+ in the equilibrium state.
As the Cu
2+ concentration in the solution decreases and approaches zero, the activity coefficient
ν is close to 1. Equation (18) could be written as the following Equation (19).
K0 could be obtained by plotting ln (qe/Ce) versus qe and extrapolating qe to zero. Its intercept gives the value of K0.
The change of the standard free energy of adsorption, ΔG° (kJ/mol), could be calculated according to the following Equation (20).
where
R is the general gas constant of 8.314 × 10
−3 kJ/K/mol and
T is the absolute temperature (K).
3.9. Statistical Analysis
The data were analyzed as the mean ± standard deviation (SD) of three determinations.