Coconut Shell Carbon Preparation for Rhodamine B Adsorption and Mechanism Study
Abstract
1. Introduction
2. Results and Discussion
2.1. PCSC Adsorption Ability
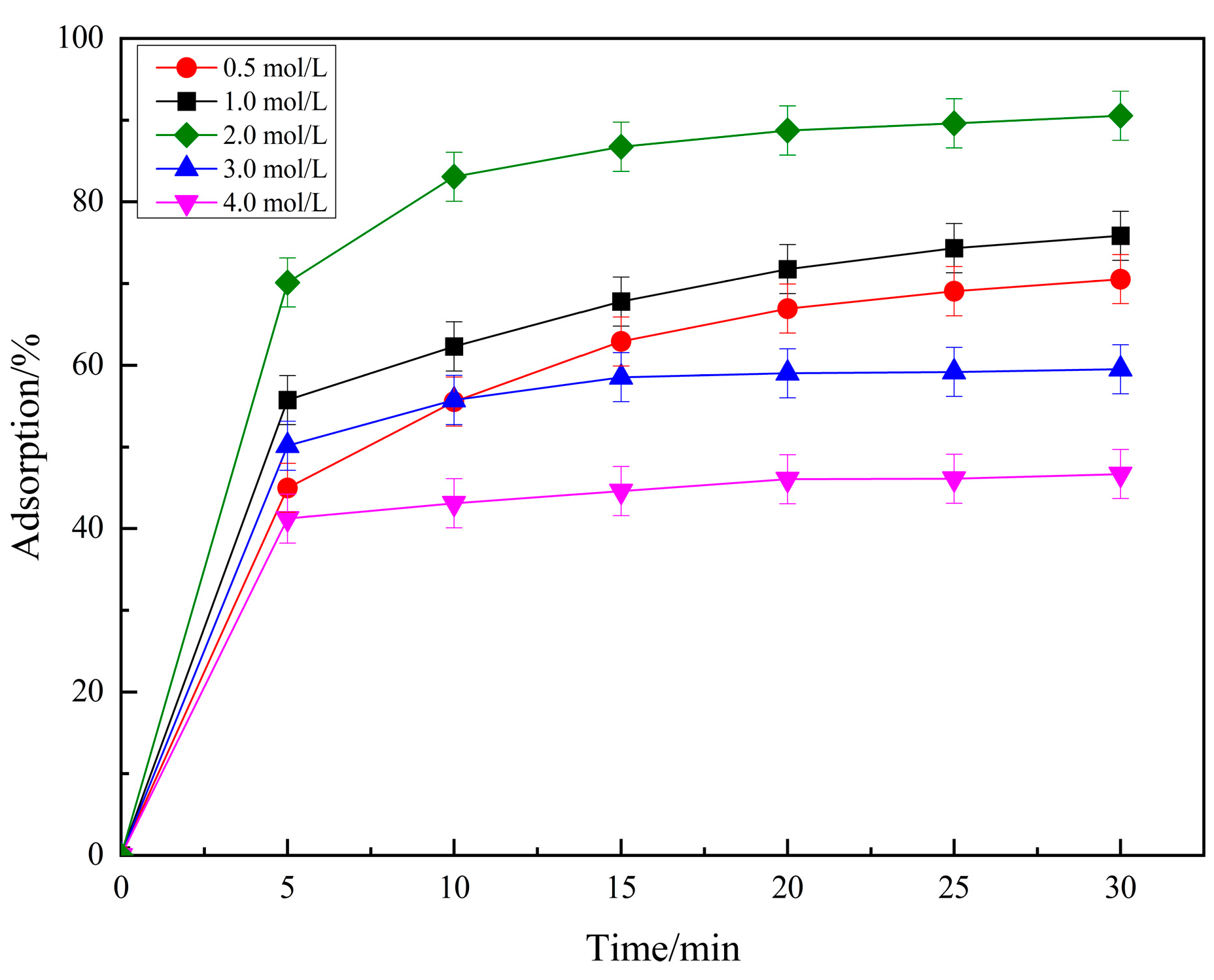
2.1.1. Effects of Treated Phosphoric Acid Concentrations Impregnation
2.1.2. Effect of PCSC Activation Temperatures by H3PO4 on RhB Adsorption
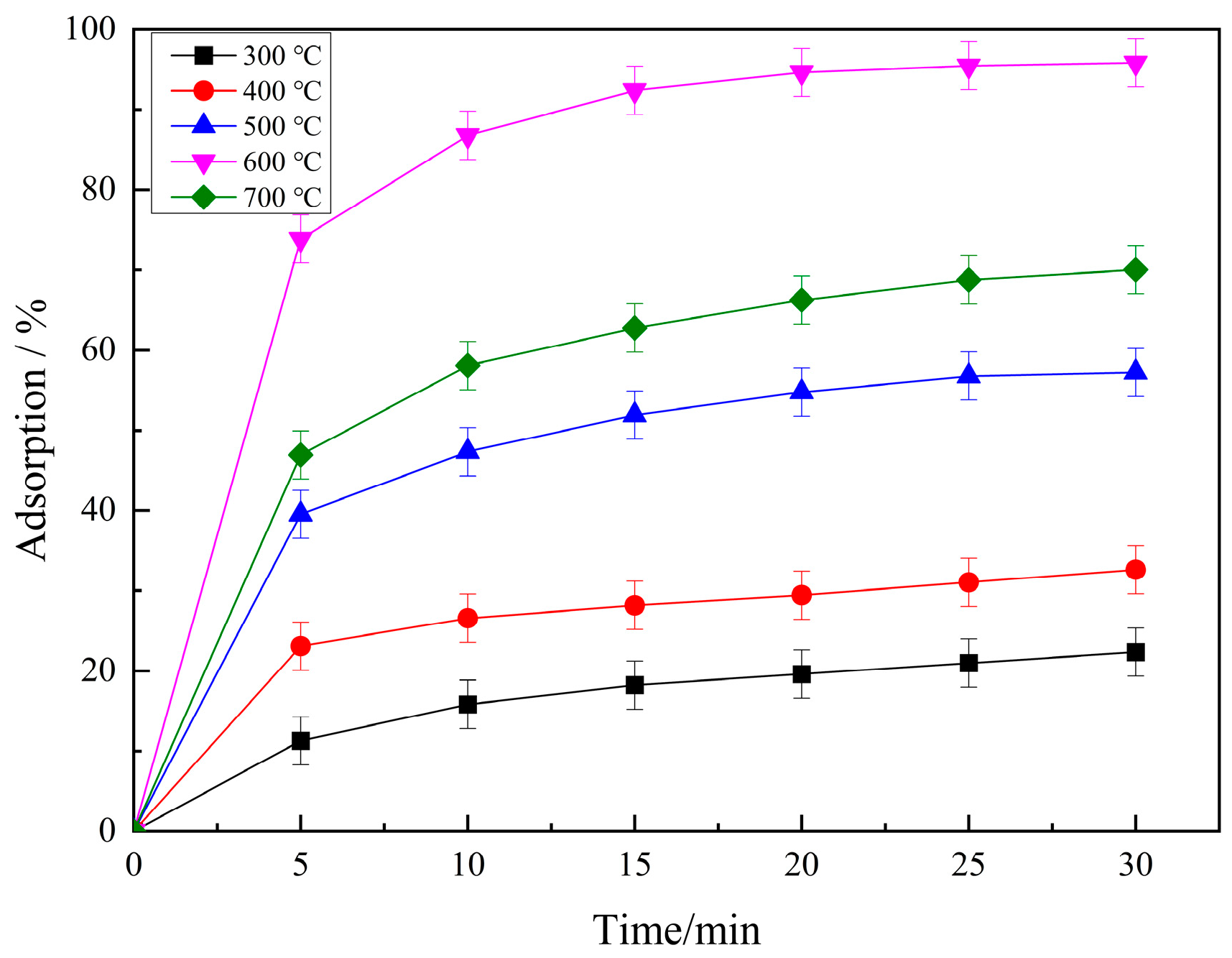
2.1.3. Effect of Calcination Temperature
2.1.4. The Effect of Different pH
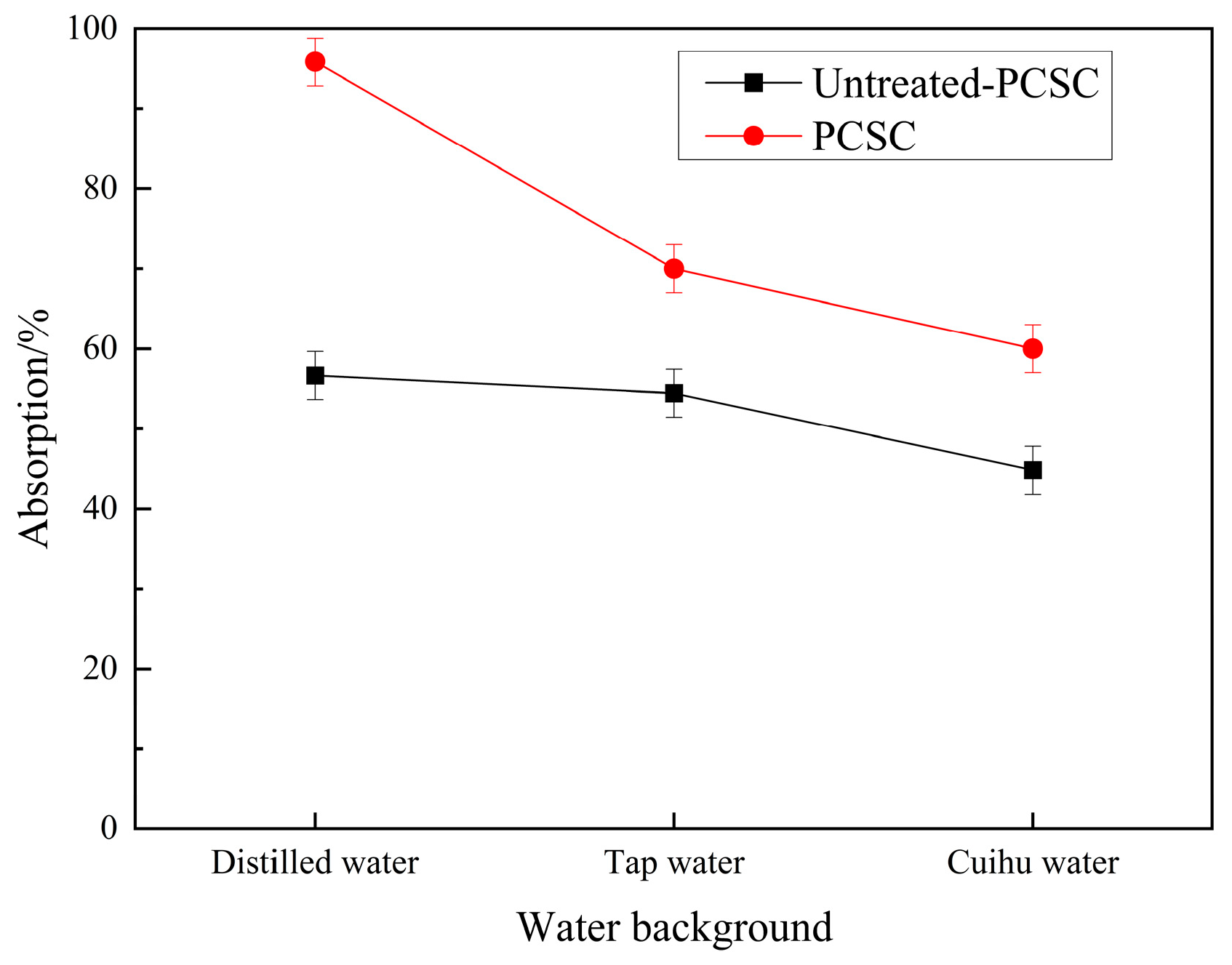
2.1.5. Influence of Water Source
2.1.6. The Degradation Effect of Different Pollutants
2.1.7. Cyclic Adsorption Performance of PCSC
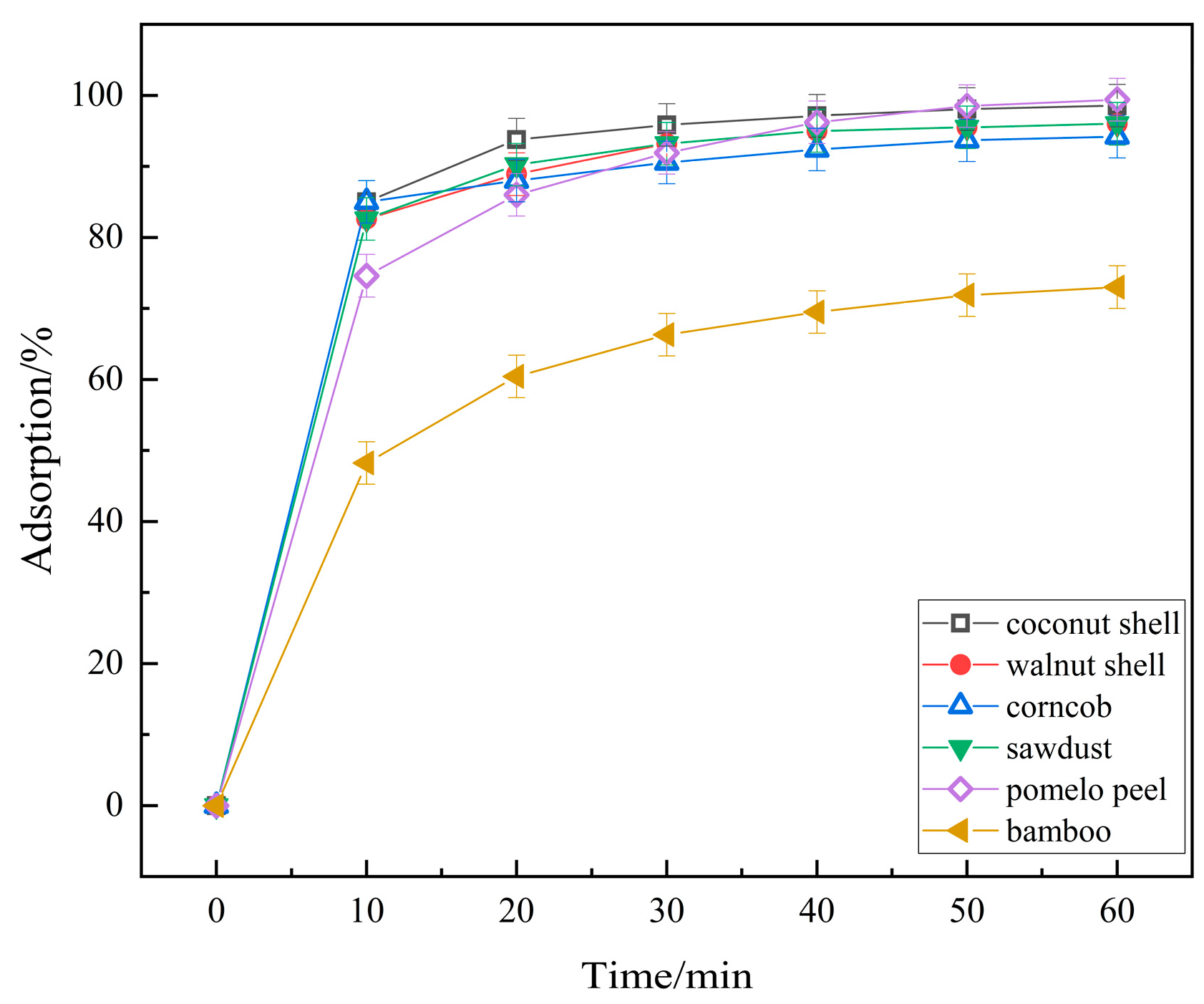
2.1.8. Effect of Biochar Species Activated by H3PO4 on RhB Adsorption Efficiency
2.1.9. Comparison with Other Adsorbents
2.1.10. Liquid UV Characterization of PCSC
2.2. PCSC Characterization
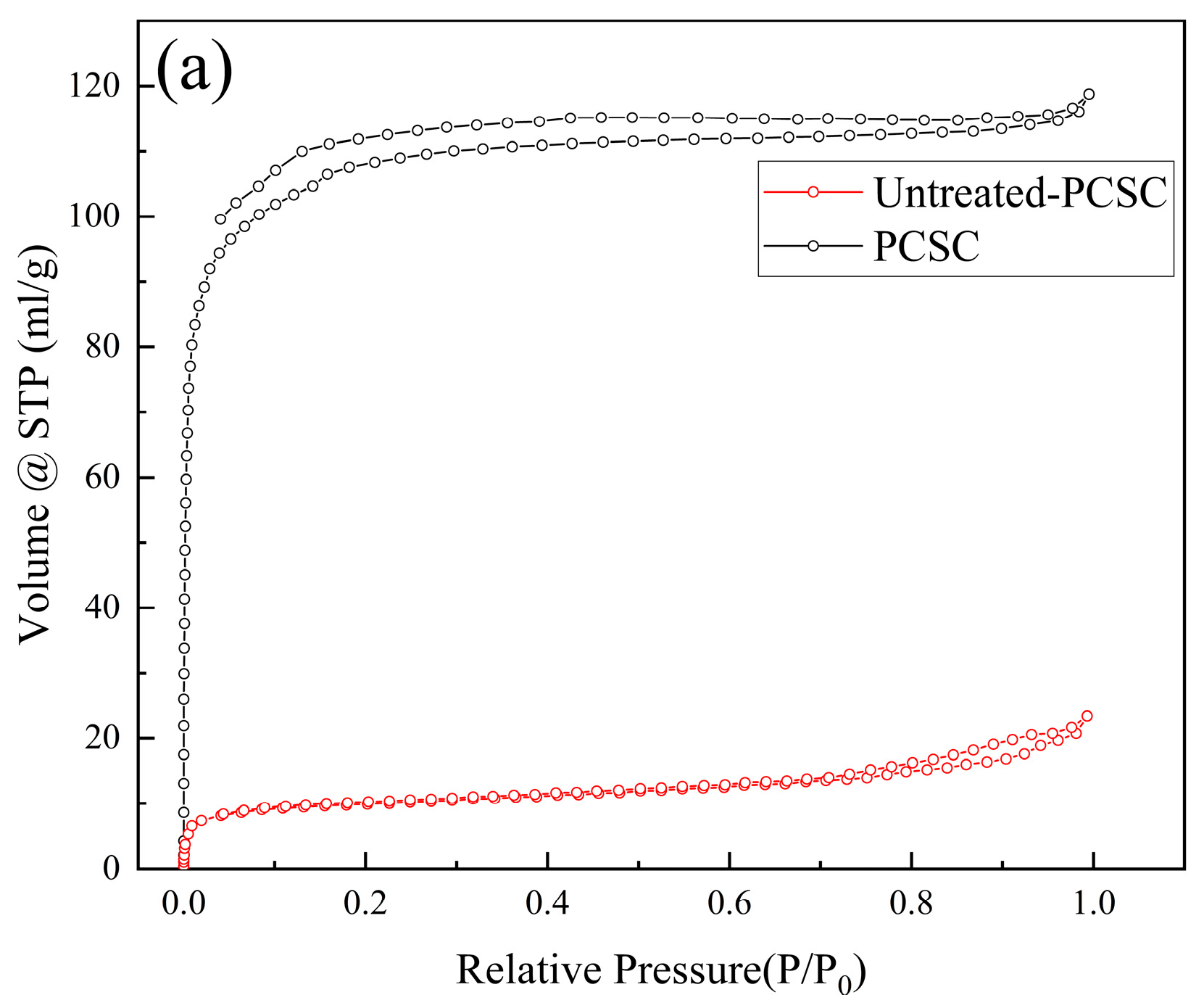
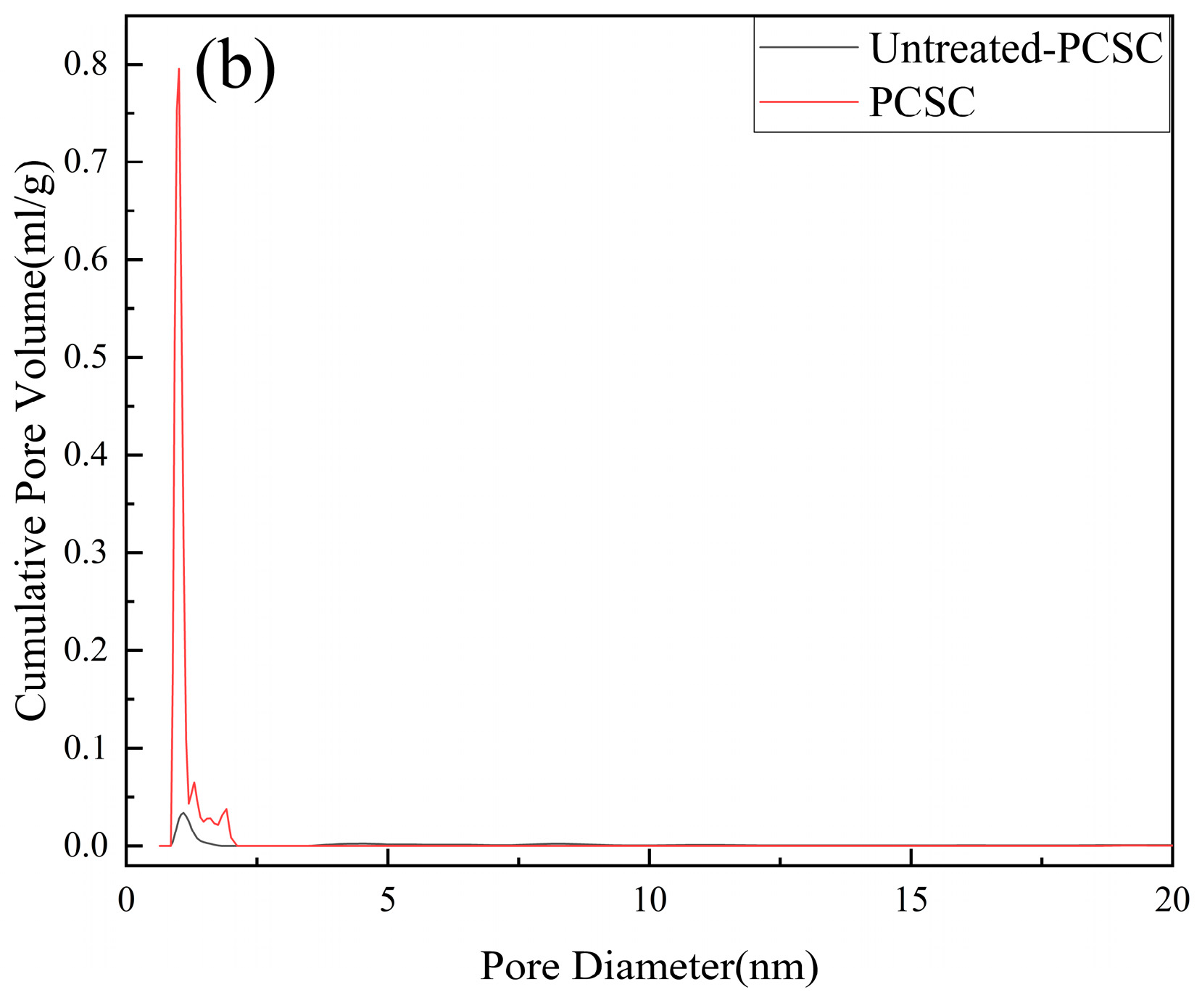
2.2.1. BET Surface Area and Pore Structure Analyses
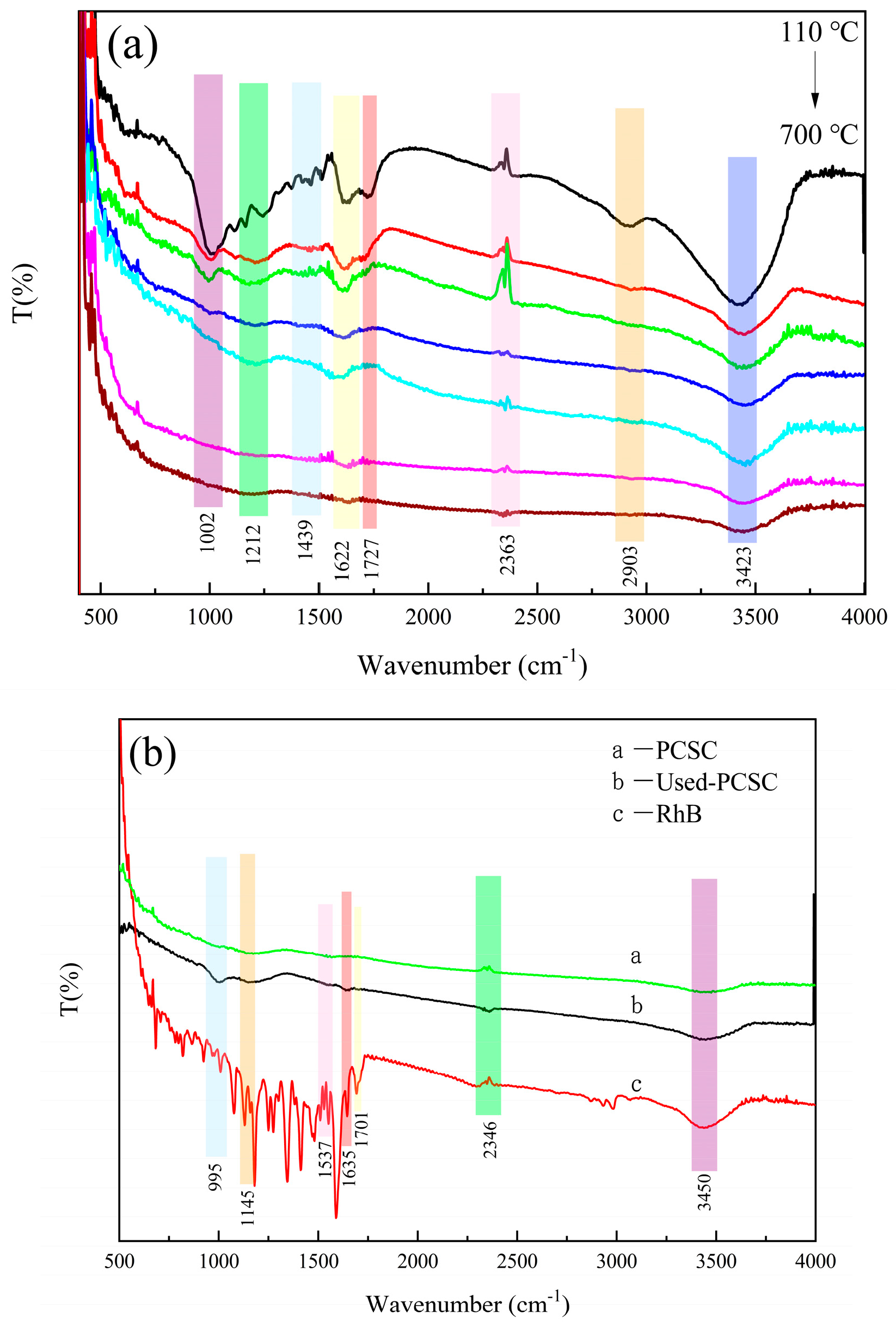
2.2.2. FTIR Characterization
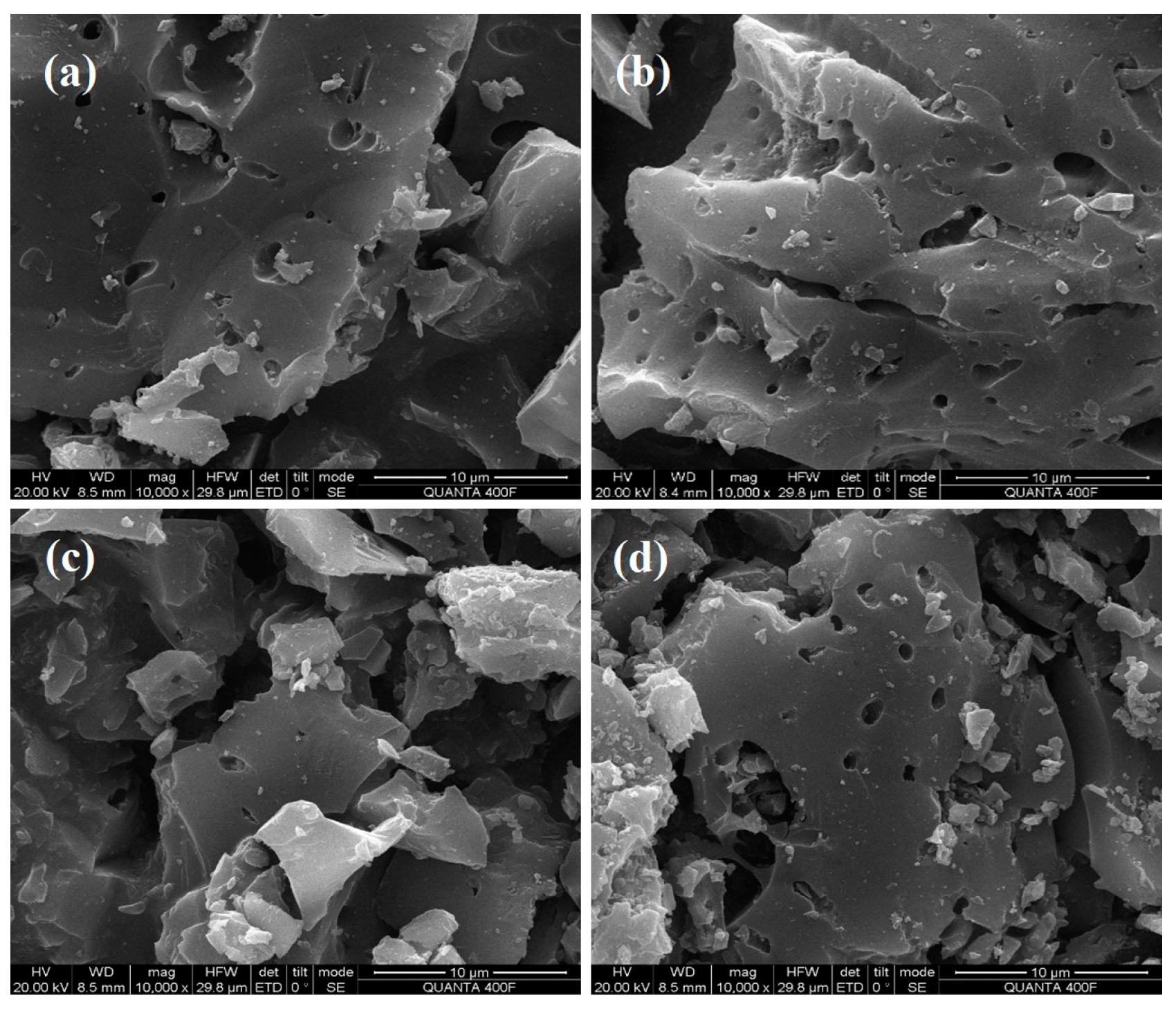
2.2.3. SEM and EDS Characterization
3. Materials and Methods
3.1. Materials and Reagents
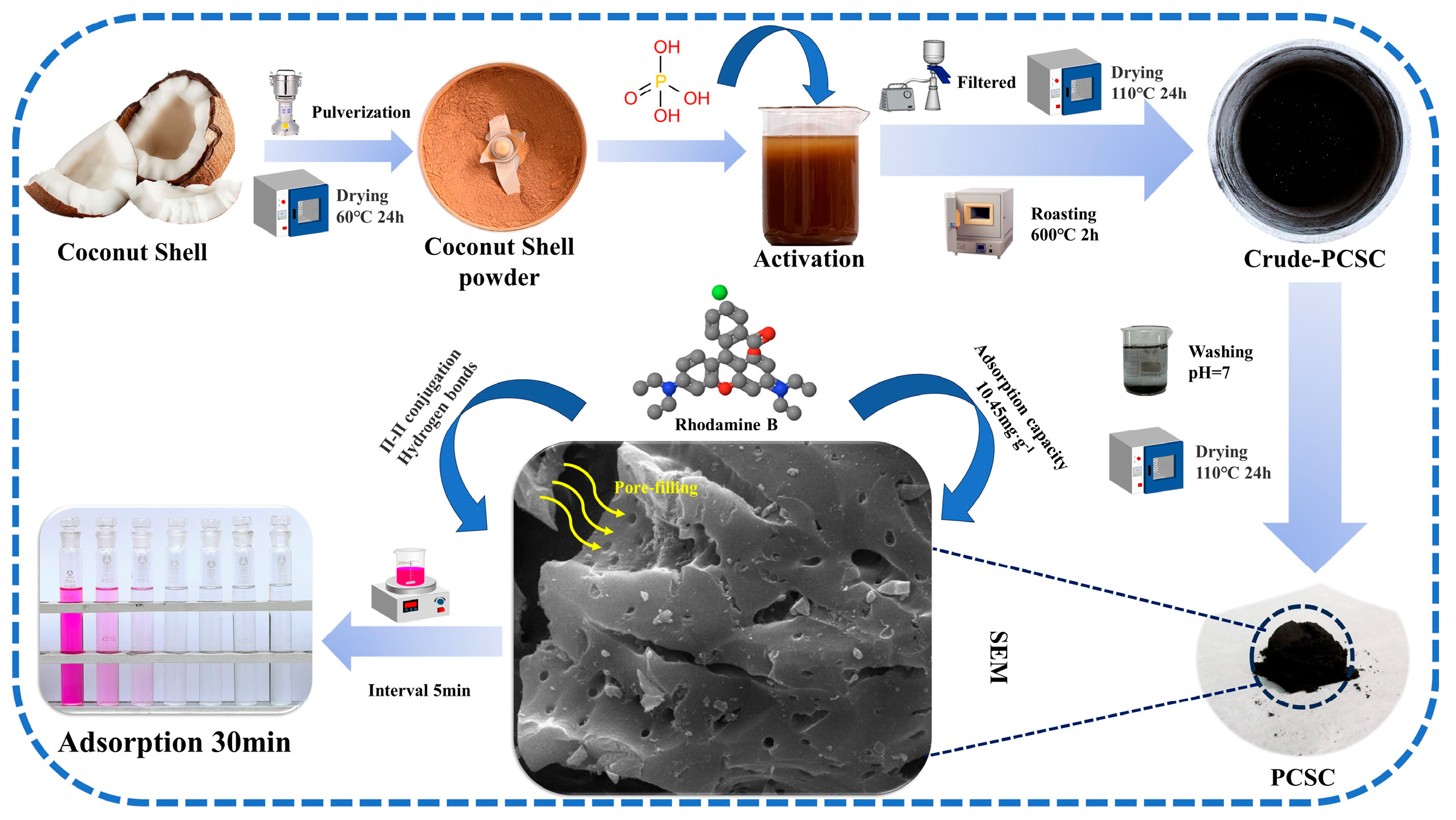
3.2. PSPC Sample Preparation
3.3. Adsorption Experiments
3.3.1. Adsorption Experiment
3.3.2. Study on the Influence Factors of RhB Adsorption over PSPC
3.3.3. Adsorption Ability Experiments
3.4. Characterization of PCSC
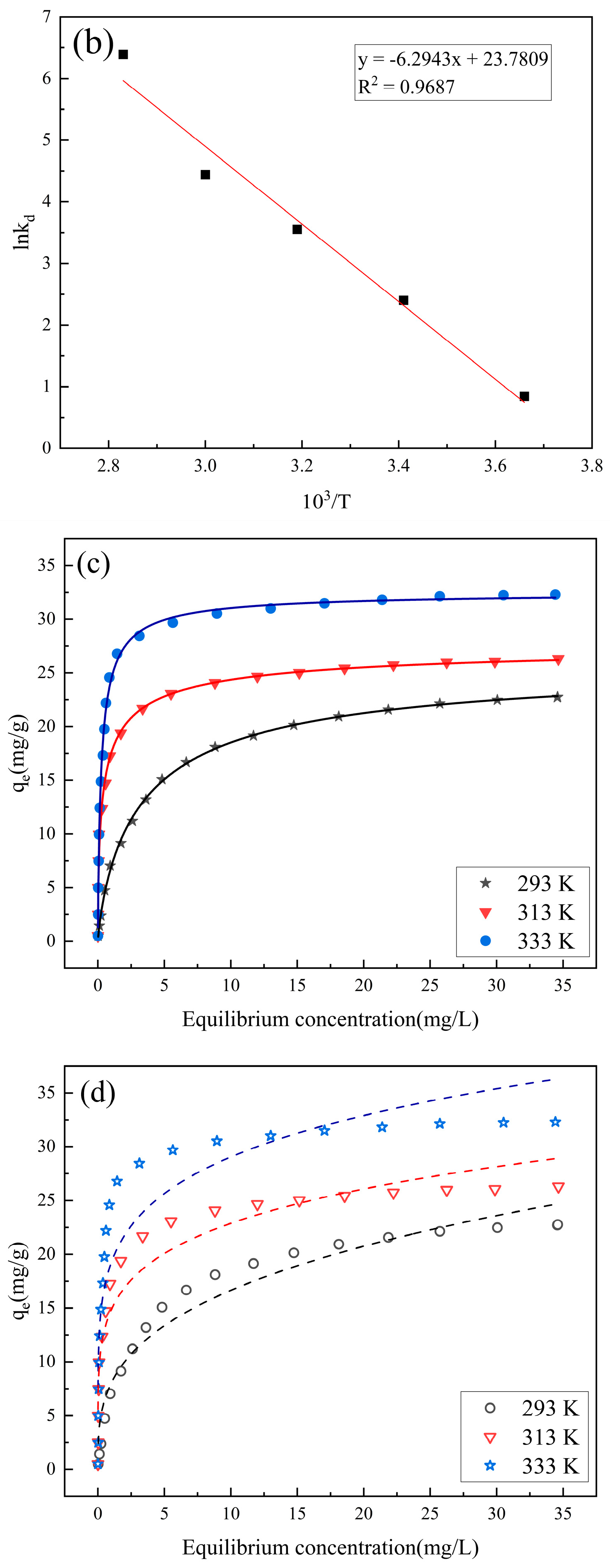
3.5. Kinetic Thermodynamic Analysis of RhB Adsorbed by PCSC
3.6. Thermodynamic Analysis of RhB Adsorbed by PCSC
3.7. Adsorption Isotherm
4. Kinetic, Thermodynamic Analysis and Mechanism of RhB Adsorbed on PCSC
4.1. Kinetic
4.2. Thermodynamic Analysis
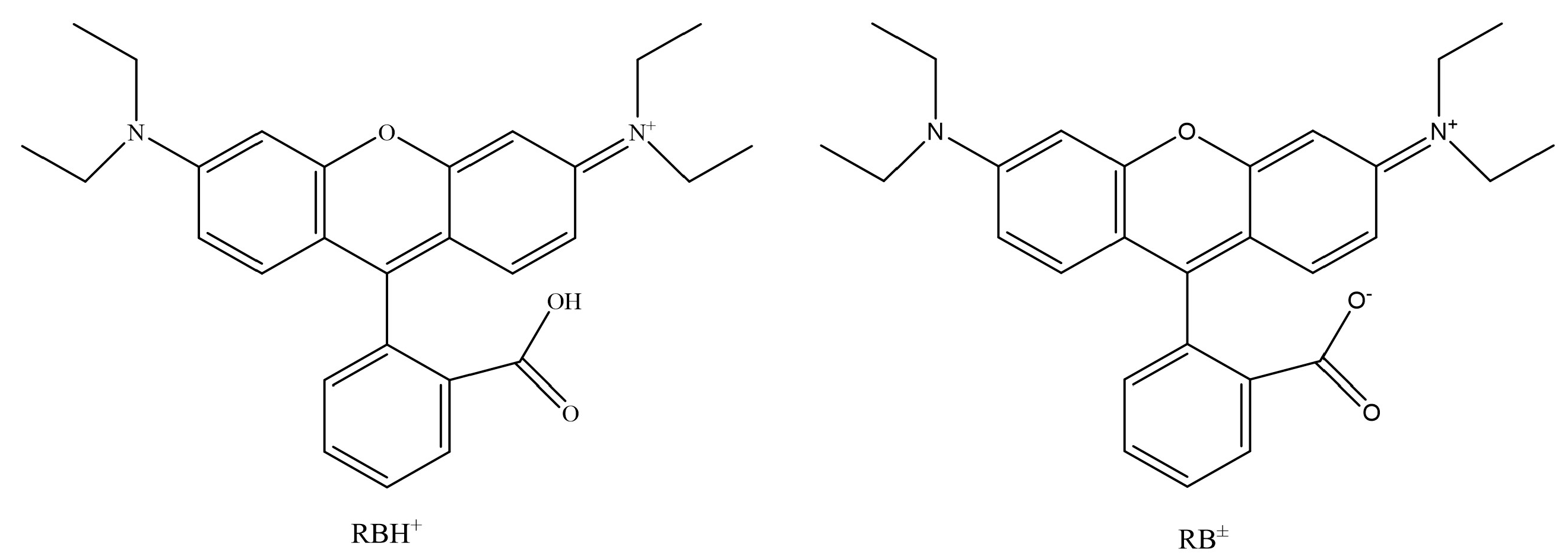
4.3. Adsorption Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Zhang, J.; Zhu, W.; Zhao, S.; Gao, Y.; Li, Y.; Ding, L.; Ding, H. Novel manganese and nitrogen co-doped biochar based on sodium bicarbonate activation for efficient removal of bisphenol A: Mechanism insight and role analysis of manganese and nitrogen by combination of characterizations, experiments and density functional theory calculations. Bioresour. Technol. 2024, 399, 130608. [Google Scholar]
- Malika, M.; Sonawane, S.S. Statistical modelling for the Ultrasonic photodegradation of Rhodamine B dye using aqueous based Bi-metal doped TiO2 supported montmorillonite hybrid nanofluid via RSM. Sustain. Energy Technol. Assess. 2021, 44, 100980. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.R.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef]
- Sachdeva, S.; Kumar, A. Preparation of nanoporous composite carbon membrane for separation of Rhodamine B dye. J. Membr. Sci. 2009, 329, 2–10. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- El-Desoky, H.S.; Ghoneim, M.M.; El-Sheikh, R.; Zidan, N.M. Oxidation of Levafix CA reactive azo-dyes in industrial wastewater of textile dyeing byelectro-generated Fenton’s reagent. J. Hazard. Mater. 2010, 175, 858–865. [Google Scholar] [CrossRef]
- Labanda, J.; Sabate, J.; Llorens, J. Modeling of the dynamic adsorption of an anionic dye through ion-exchange membrane adsorber. J. Membr. Sci. 2009, 340, 234–240. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo dye treatment with simultaneous electricity production in an anaerobic-aerobic sequential reactor andmicrobial fuel cell coupled system. Bioresour. Technol. 2010, 101, 4440–4445. [Google Scholar] [CrossRef]
- Kodali, J.; Talasila, S.; Arunraj, B.; Nagarathnam, R. Activated Coconut Charcoal as a Super Adsorbent for the Removal of Organophosphorous Pesticide Monocrotophos from Water. Case Stud. Chem. Environ. Eng. 2021, 3, 100099. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Chen, J.; Cheng, L.; Chang, J.; Sheng, W.; Hu, C.; Chao, S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Qu, K.; Huang, Z.; Liu, S.; Dong, M.; Guo, Z. Bimetallic metal-organic frameworks anchored corncob-derived porous carbon photocatalysts for synergistic degradation of organic pollutants. Chemosphere 2020, 259, 127389. [Google Scholar] [CrossRef] [PubMed]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Liquid phase adsorptions of Rhodamine B dye onto raw and chitosan supported mesoporous adsorbents: Isotherms and kinetics studies. Appl. Water Sci. 2017, 7, 2297–2307. [Google Scholar] [CrossRef]
- Li, Q.; Fu, F.; Yan, J.; Ding, S.; Pang, K.; Zhang, N.; Astruc, D.; Liu, X. Synthesis of N-doped porous biochar from chemical pollutant for efficient sulfadiazine degradation: Performance, mechanism and bio-toxicity assessment. Sep. Purif. Technol. 2025, 353, 128432. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, S.; Li, H.; Pan, D.; Rahul, R.P.; Guo, Z.; Ilwoo, S. Modification of coconut shell-based activated carbon and purification of wastewater. Adv. Compos. Hybrid Mater. 2021, 4, 65–73. [Google Scholar] [CrossRef]
- Prashanthakumar, T.K.M.; Ashok Kumara, S.K.; Suban, K. Sahoo. A quick removal of toxic phenolic compounds using porous carbon prepared from renewable biomass coconut spathe and exploration of new source for porous carbon materials. J. Environ. Chem. Eng. 2018, 6, 1434–1442. [Google Scholar]
- Karadag, D.; Turan, M.; Akgul, E.; Akgul, E.; Tok, S.; Faki, A. Adsorption equilibrium and kinetics of reactive Black 5 and Reactive Red 239 in aqueous solution onto surfactant-modified zeolite. J. Chem. Eng. Data 2007, 53, 322–323. [Google Scholar] [CrossRef]
- Ipeaiyeda, A.R.; Choudhary, M.I.; Ahmed, S. Ammonia and Ammonium Acetate Modifications and Characterisation of Activated Carbons from Palm Kernel Shell and Coconut Shell. Waste Biomass Valorization 2020, 11, 983–993. [Google Scholar] [CrossRef]
- Zhu, L.; Ren, X.; Yu, S. Use of Cetyltrimethylammonium Bromide-Bentonite To Remove Organic Contaminants of Varying Polar Character from Water. Environ. Sci. Technol. 1998, 32, 3374–3378. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, P.; Wang, H.; Xu, H.; Dang, L.; Liu, Z.; Lei, Z. Activation of graphene aerogel with phosphoric acid for enhanced electrocapacitive performance. Carbon 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Jagtoyen, M.; Derbyshire, F. Some considerations of the origins of porosity in carbons from chemically activated wood. Carbon 1993, 31, 1185–1192. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Shi, W.; He, J.; Feng, H.; Xu, Y.; Cui, F.; Wang, C. Carbon nanofiber matrix with embedded LaCO3OH synchronously captures phosphate and organic carbon to starve bacteria. J. Mater. Chem. A 2016, 4, 12799–12806. [Google Scholar] [CrossRef]
- Liu, X.; Zong, E.; Hu, W.; Song, P.; Wang, J.; Liu, Q.; Ma, Z.; Fu, S. Lignin-Derived Porous Carbon Loaded with La(OH)3 Nanorods for Highly Efficient Removal of Phosphate. ACS Sustain. Chem. Eng. 2019, 7, 758–768. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Lfebajo, A.O.; Nisar, N.; Ajayi, O.A. High-performance magnetic chicken bone-based biochar for efficient removal of rhodamine-B dye and tetracycline: Competitive sorption analysis. Water Sci. Technol. 2017, 76, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Li, B.; Ma, Z.; Xu, X.; Li, Y.; Fang, M.; Tao, C.; Sheng, H. Enhanced adsorption of Rhodamine B over Zoysia sinica Hance-based carbon activated by ammonium chloride and sodium hydroxide treatments. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126489. [Google Scholar]
- Luo, Y.; Li, D.; Chen, Y.; Sun, X.; Cao, Q.; Liu, Q. The performance of phosphoric acid in the preparation of activated carbon-containing phosphorus species from rice husk residue. J. Mater. Sci. 2019, 54, 5008–5021. [Google Scholar] [CrossRef]
- Yue, W.; Yu, Z.; Man, Y.; Zhang, X.; Li, J.; Liu, H.; Ma, X. Synthesis of natural oxygen-doped bamboo-derived hierarchical micro-mesoporous composite carbon materials using a green activation strategy. J. Energy Storage 2024, 90, 111871. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, T.; She, A.; Li, X.; Chen, W.; Ao, T.; Ni, F. One-step synthesis of iron and nitrogen co-doped porous biochar for efficient removal of tetracycline from water: Adsorption performance and fixed-bed column. J. Environ. Manag. 2024, 352, 119984. [Google Scholar] [CrossRef]
- Rao, M.M.; Ramesh, A.; Rao, G.C.; Seshaiah, K. Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls. J. Hazard. Mater. 2006, 129, 123–129. [Google Scholar]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Fouda, M. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Fu, J.; Wang, M.; Wang, X.; Han, R.; Xu, Q. Adsorption of methylene blue onto poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes: Kinetics, isotherm and thermodynamics analysis. J. Hazard. Mater. 2014, 273, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]


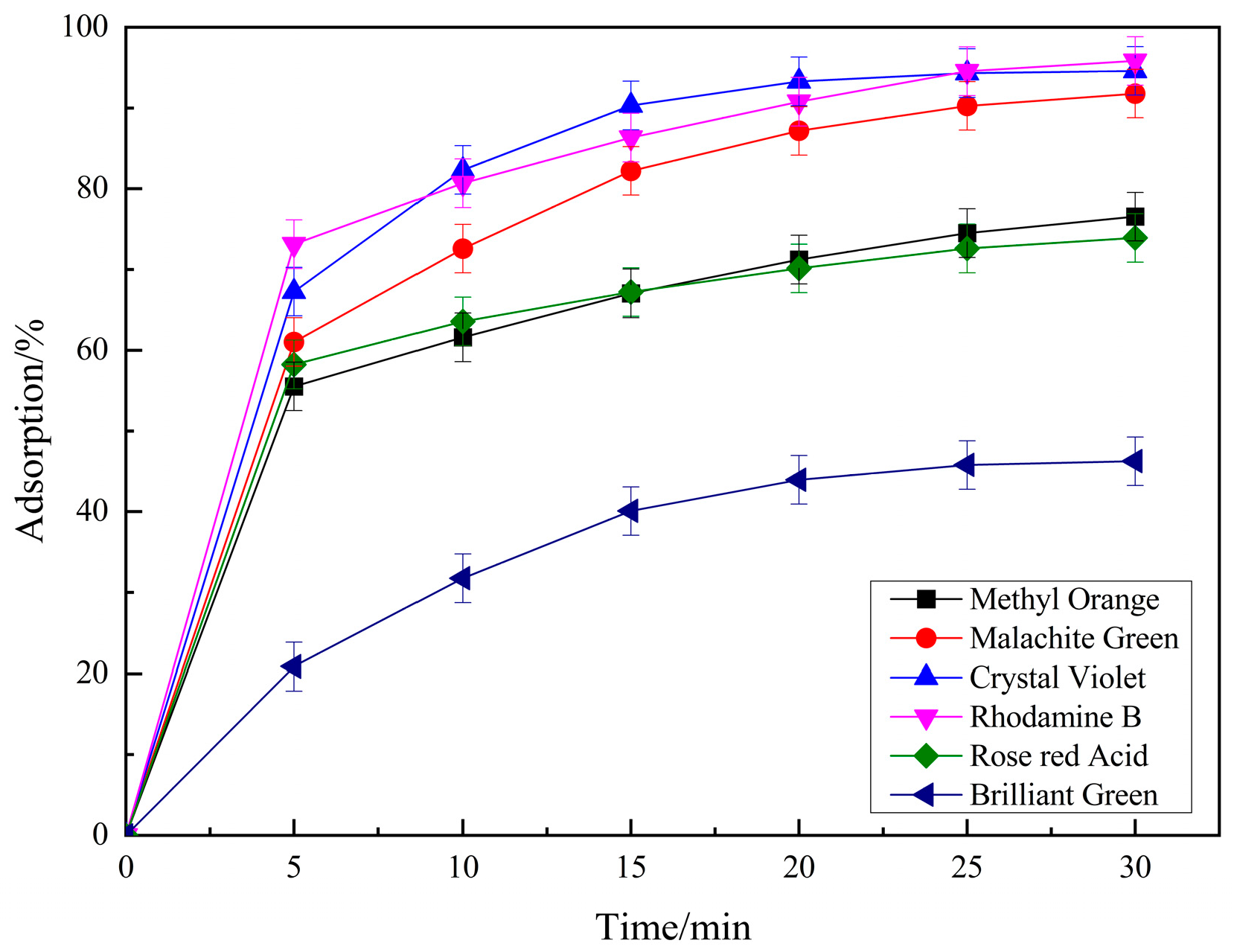





| Dye | Structure Fomula | Molecular Weight | Dye Type | Removal Efficiency/% |
|---|---|---|---|---|
| RhB | 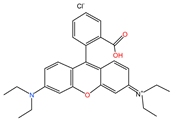 | 479.01 | Cationic dye | 95.84 |
| Methyl orange |  | 327.33 | Anionic type | 76.55 |
| Malachite green | 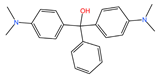 | 364.92 | Cationic dye | 91.79 |
| Bright Green | 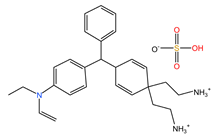 | 482.63 | Cationic dye | 46.26 |
| Rose Red Acid | 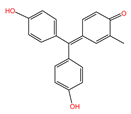 | 304.34 | Anionic type | 73.92 |
| Crystal Violet |  | 373.53 | Cationic dye | 94.59 |
| Adsorbent | pH | Adsorbent Dosage (g L−1) | qe (mg g−1) | Ref |
|---|---|---|---|---|
| LCNFS | 2–7 | / | 20.2 | Zhang et al., 2016 [22] |
| LPC@La(OH)3 | 3–7 | 1.25 | 31.9 | Liu et al., 2019 [23] |
| ML2-CGCS | 3–8 | 1.4 | 39.2 | Wang et al., 2016 [24] |
| CB | 10 | 5 | 96.5 | Oladipo, Akeem Adeyemi, 2017 [25] |
| PCSC | 6 | 2 | 32.6 | This work |
| Sample and Elements Content (wt.%) | C | N | O | P |
|---|---|---|---|---|
| PCSC(a) | 88.14 | 0 | 7.95 | 3.91 |
| Untreated-PCSC(b) | 94.19 | 0 | 5.46 | 0.36 |
| Used -PCSC(c) | 88.50 | 0 | 8.19 | 3.30 |
| Used-untreated PCSC(d) | 94.83 | 0 | 5.17 | 0 |
| Initial Concentration C0/(mg/L) | qe/(mg/g) | Quasi-First-Order Dynamics | Quasi-Second-Order Dynamics | Particle Diffusion Model | ||||
|---|---|---|---|---|---|---|---|---|
| K1 | R12 | K2 | R22 | Kp | C | R2 | ||
| 5 | 2.4277 | 0.1286 | 0.9959 | 0.4020 | 0.9998 | 0.0845 | 2.0118 | 0.6499 |
| 10 | 4.7832 | 0.1692 | 0.9609 | 0.1961 | 0.9996 | 0.3262 | 3.1746 | 0.8141 |
| 20 | 9.1329 | 0.0873 | 0.9701 | 0.0986 | 0.9992 | 0.8846 | 4.6505 | 0.9067 |
| 30 | 13.1936 | 0.0892 | 0.9446 | 0.0657 | 0.9987 | 1.5892 | 5.0805 | 0.9071 |
| 50 | 19.2052 | 0.1257 | 0.9655 | 0.0439 | 0.9891 | 2.5889 | 6.4994 | 0.7829 |
| C/(mg/L) | ΔHθ/(kJ/mol) | ΔSθJ/(mol·K) | ΔGθ/(kJ/mol) | Ea/(kJ/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 273 K | 293 K | 313 K | 333 K | 353 K | 273 K | 393 K | 313 K | 333 K | 353 K | |||
| 10 | 52.3312 | 197.9109 | −1.6507 | −5.8486 | −9.2411 | −12.2973 | −18.7505 | 3.7151 | 7.6451 | 11.0898 | 14.2325 | 20.7872 |
| Temperature T/(K) | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| qmax/(mg/g) | kL/(L/mg) | R2 | kF/(L/mg) | 1/n | R2 | |
| 293 | 26.3045 | 0.3570 | 0.9993 | 8.0156 | 0.3174 | 0.9537 |
| 313 | 28.1741 | 1.6468 | 0.9917 | 14.8143 | 0.1887 | 0.9193 |
| 333 | 32.5769 | 2.9828 | 0.9967 | 19.2044 | 0.1797 | 0.8569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Bian, Y.; Wang, R.; Zhou, S.; Wang, Z.; Wang, D.; Li, H. Coconut Shell Carbon Preparation for Rhodamine B Adsorption and Mechanism Study. Molecules 2024, 29, 4262. https://doi.org/10.3390/molecules29174262
Yu J, Bian Y, Wang R, Zhou S, Wang Z, Wang D, Li H. Coconut Shell Carbon Preparation for Rhodamine B Adsorption and Mechanism Study. Molecules. 2024; 29(17):4262. https://doi.org/10.3390/molecules29174262
Chicago/Turabian StyleYu, Jinrui, Yifan Bian, Rongfeng Wang, Shiping Zhou, Zhongying Wang, Dawei Wang, and Huijuan Li. 2024. "Coconut Shell Carbon Preparation for Rhodamine B Adsorption and Mechanism Study" Molecules 29, no. 17: 4262. https://doi.org/10.3390/molecules29174262
APA StyleYu, J., Bian, Y., Wang, R., Zhou, S., Wang, Z., Wang, D., & Li, H. (2024). Coconut Shell Carbon Preparation for Rhodamine B Adsorption and Mechanism Study. Molecules, 29(17), 4262. https://doi.org/10.3390/molecules29174262






