Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells
Abstract
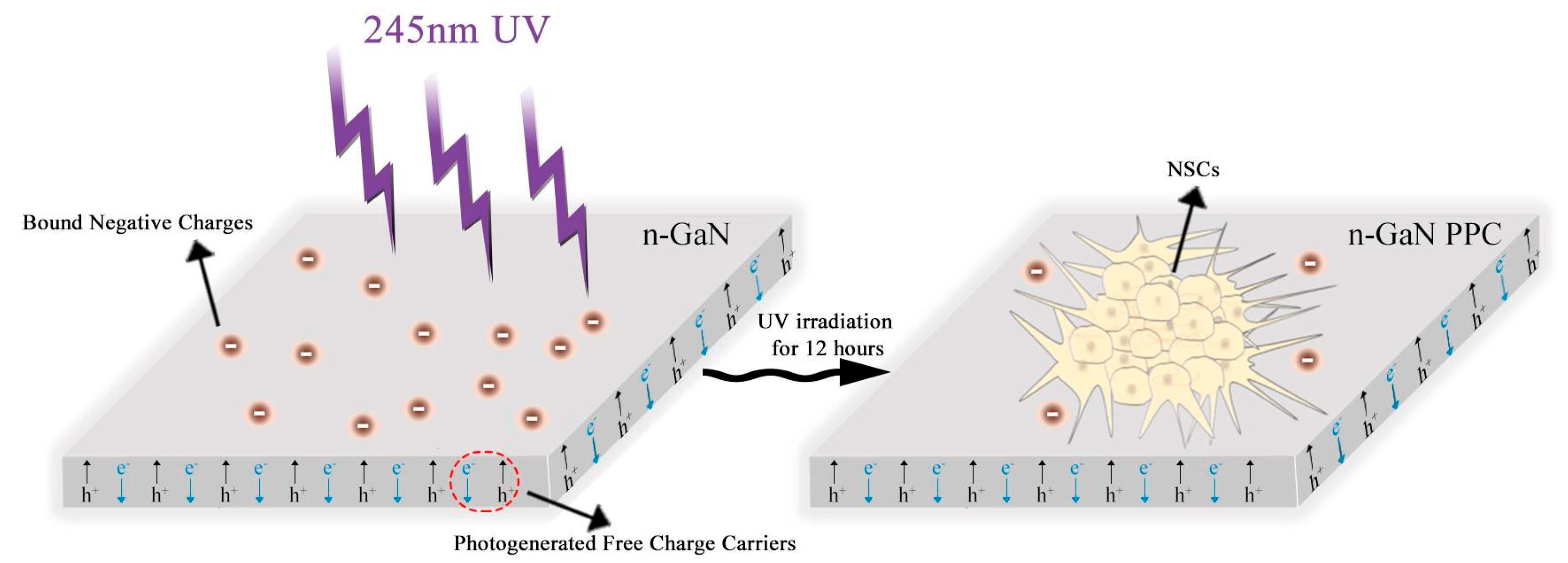
1. Introduction
2. Results and Discussion
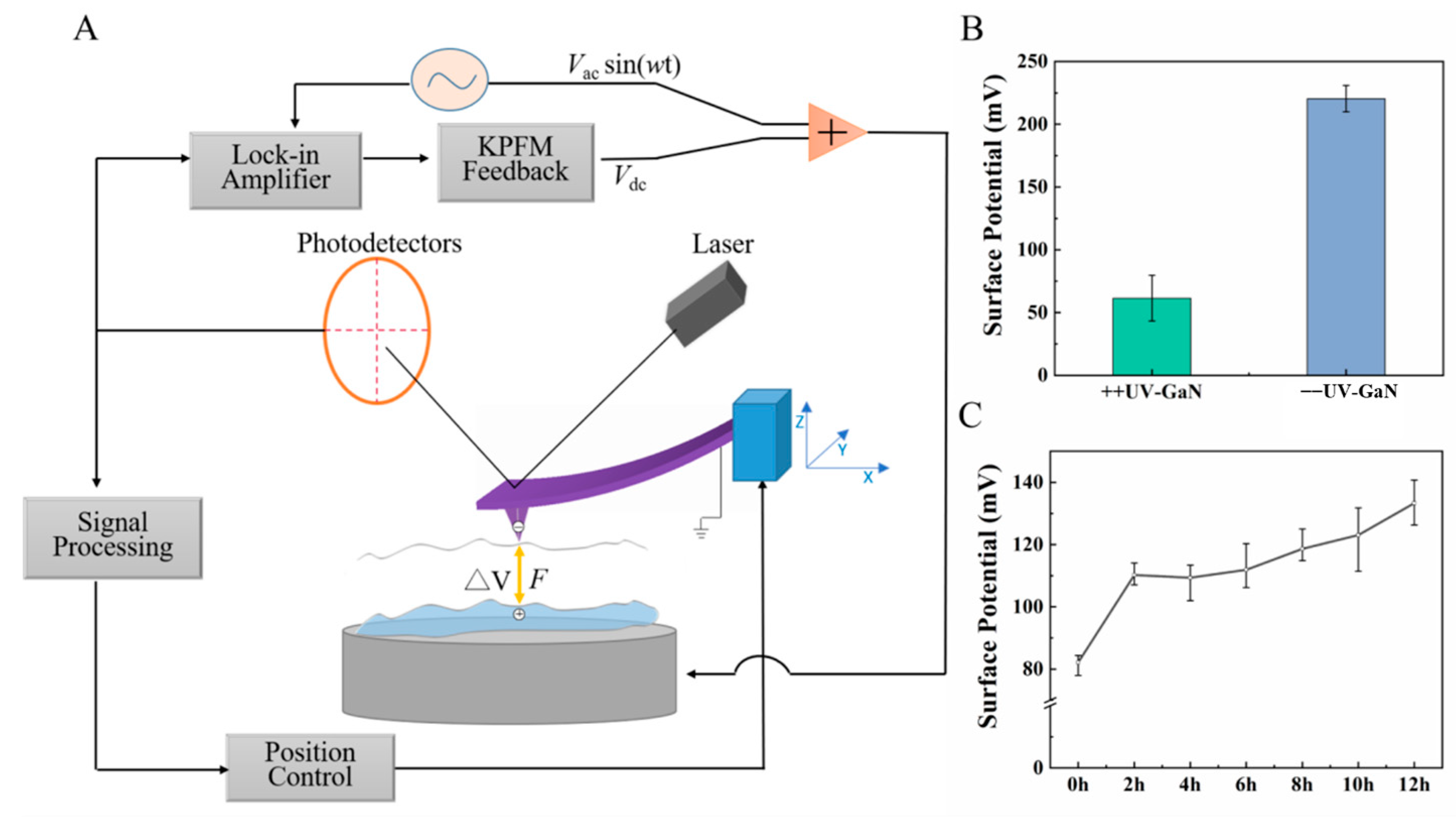
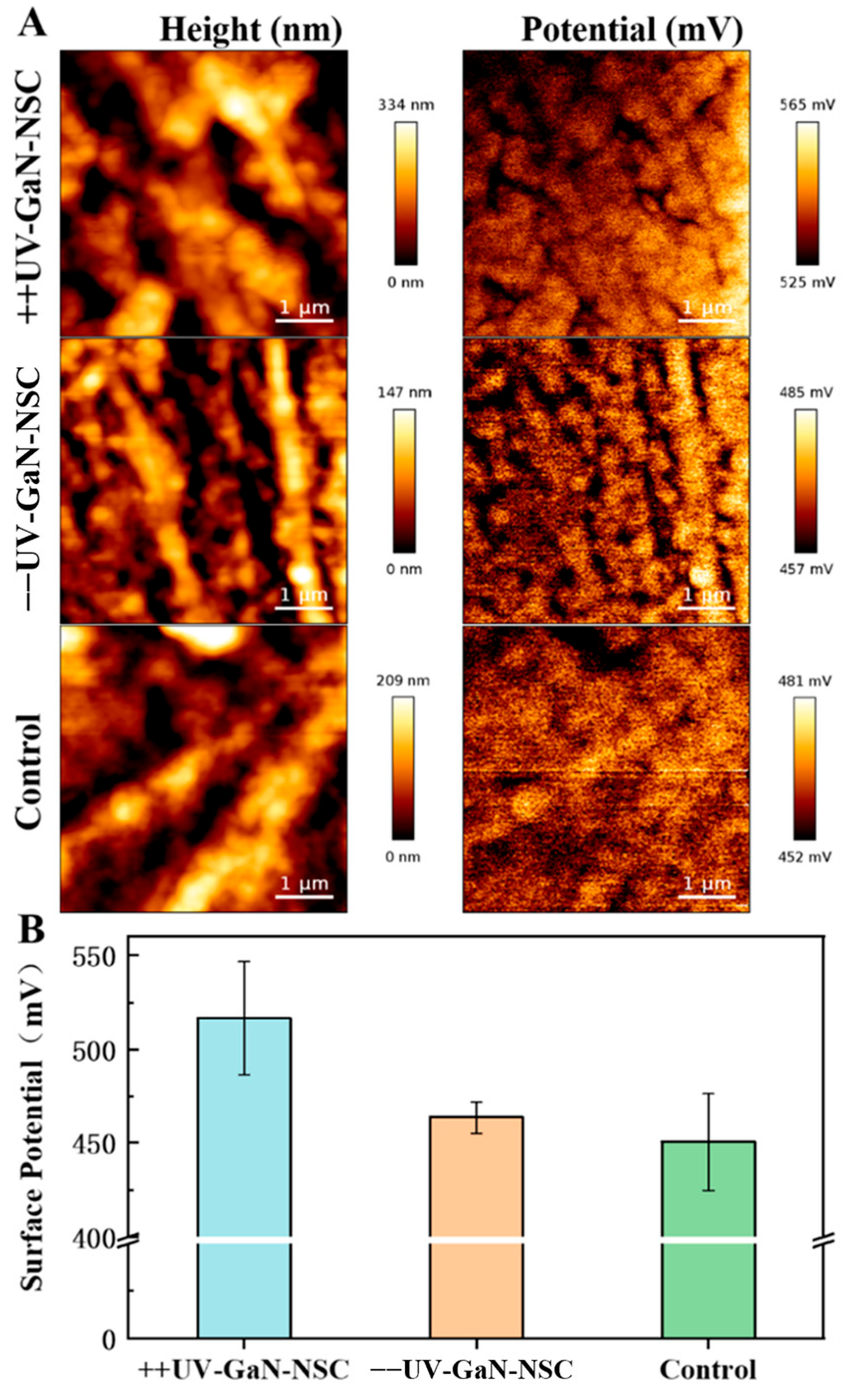
2.1. Surface Potential of GaN after UV Irradiation
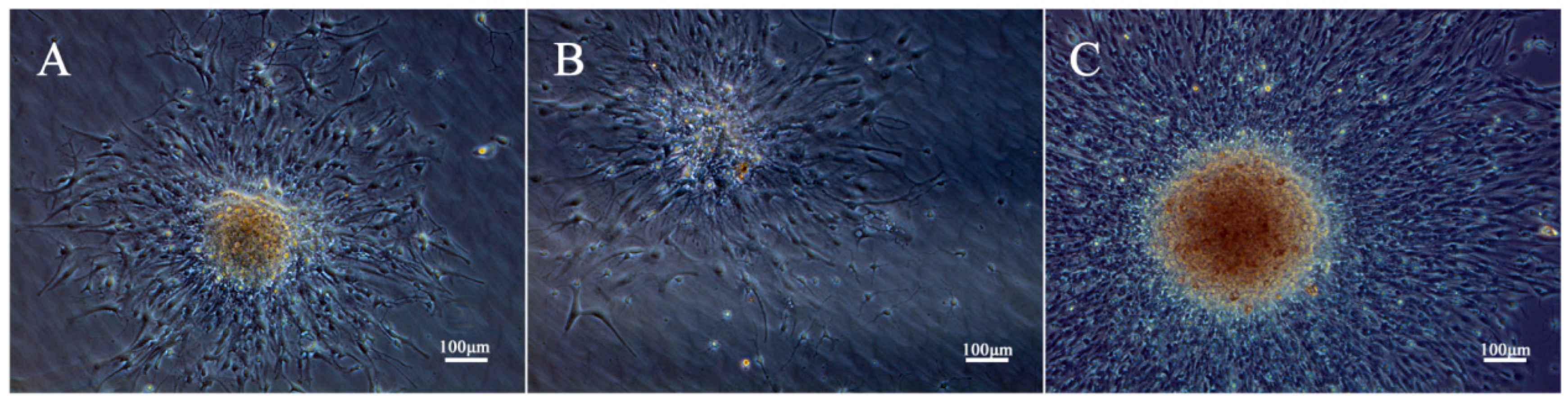
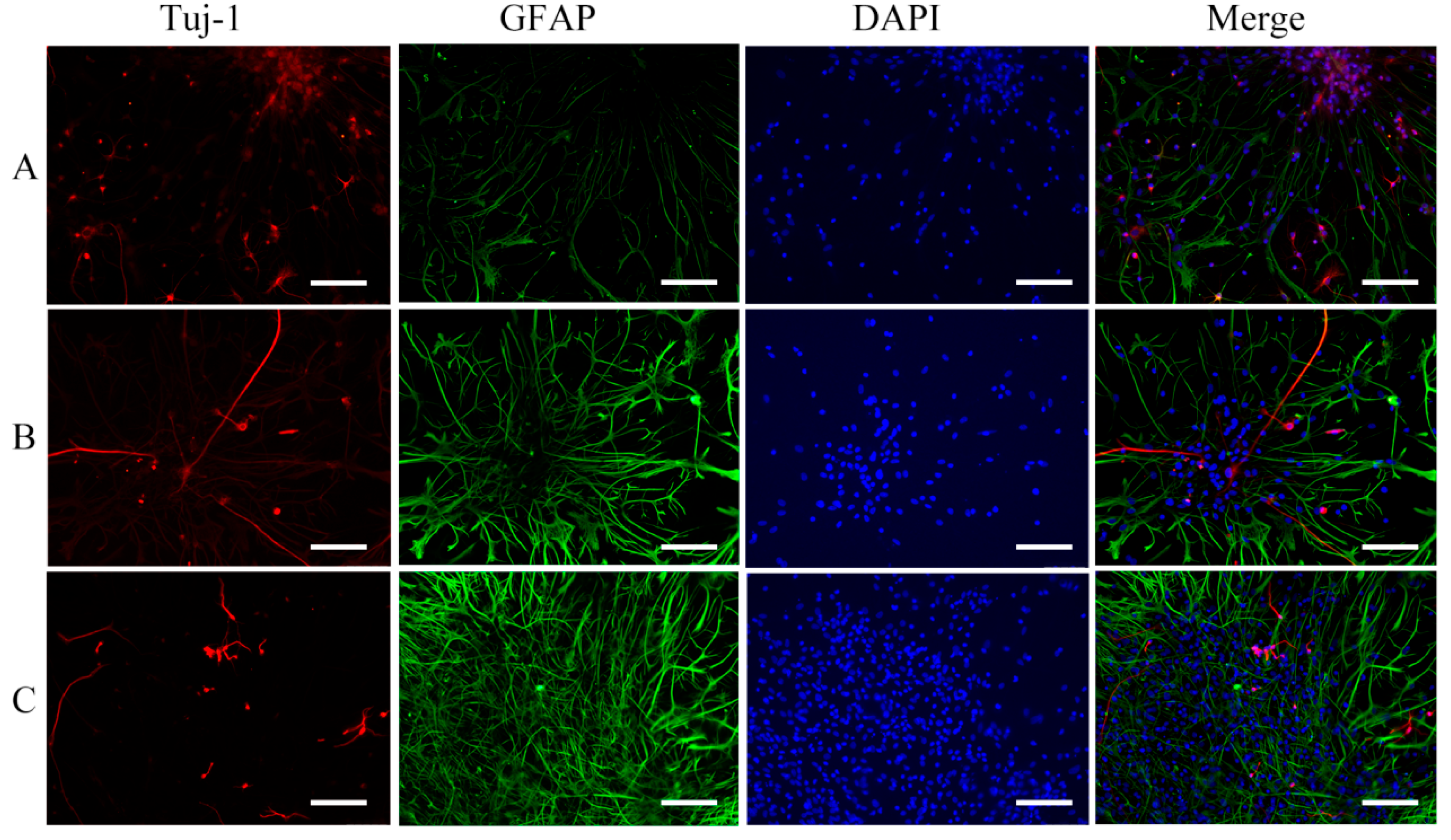
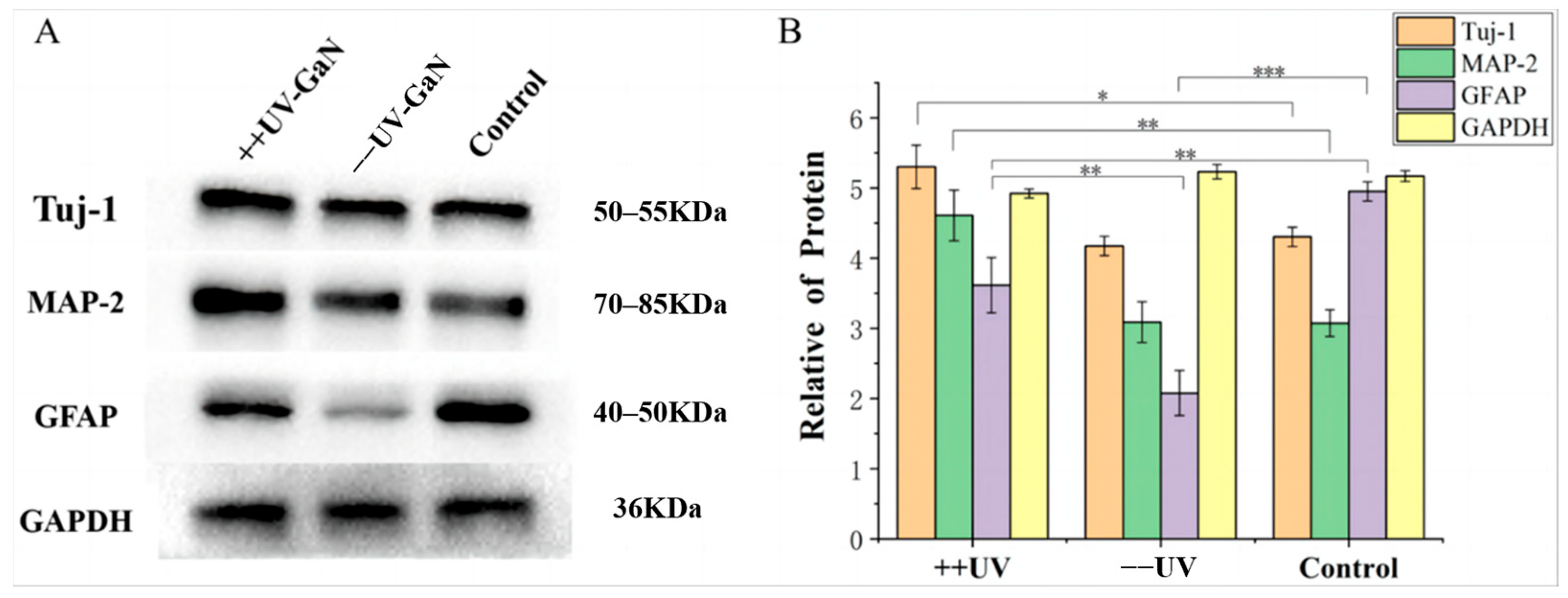
2.2. Effect of GaN on the Differentiation of NSCs
2.3. Characterization of the Surface Potential of NSCs on GaN with KPFM
3. Materials and Methods
3.1. Sample Preparation
3.2. Isolation and Culture of NSCs
3.3. Immunocytochemistry
3.4. Flow Cytometry Detection
3.5. Western Blot Analysis
3.6. KPFM Characterization
3.7. Experimental Animals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirste, R.; Rohrbaugh, N.; Bryan, I.; Bryan, Z.; Collazo, R.; Ivanisevic, A. Electronic biosensors based on III-nitride semiconductors. Annu. Rev. Anal. Chem. 2015, 8, 149–169. [Google Scholar] [CrossRef]
- Furqan, C.M.; Khan, M.U.; Awais, M.; Jiang, F.; Bae, J.; Hassan, A.; Kwok, H.-S. Humidity sensor based on Gallium Nitride for real time monitoring applications. Sci. Rep. 2021, 11, 11088. [Google Scholar] [CrossRef]
- Yadav, S.; Das, A.; Rewari, S. Dielectrically-modulated GANFET biosensor for label-free detection of DNA and avian influenza virus: Proposal and modeling. ECS J. Solid. State Sci. Technol. 2024, 13, 047001. [Google Scholar] [CrossRef]
- Thakur, R.R.; Saini, A.K.; Jain, A.K.; Taliyan, R.; Chaturvedi, N. Label-free GaN HEMT-based biosensing platform for interferon-γ detection. Mater. Sci. Semicond. Process. 2024, 178, 108416. [Google Scholar] [CrossRef]
- Louis, H.; Mbim, E.N.; Okon, G.A.; Edet, U.O.; Benjamin, I.; Ejiofor, E.U.; Manicum, A.-L.E. Systematic exo-endo encapsulation of hydroxyurea (HU) by Cu, Ag, and Au-doped gallium nitride nanotubes (GaNNT) for smart therapeutic delivery. Comput. Biol. Med. 2023, 161, 106934. [Google Scholar] [CrossRef] [PubMed]
- Poher, V.; Grossman, N.; Kennedy, G.; Nikolic, K.; Zhang, H.; Gong, Z.; Drakakis, E.; Gu, E.; Dawson, M.; French, P. Micro-LED arrays: A tool for two-dimensional neuron stimulation. J. Phys. D Appl. Phys. 2008, 41, 094014. [Google Scholar] [CrossRef]
- Hofstetter, M.; Howgate, J.; Schmid, M.; Schoell, S.; Sachsenhauser, M.; Adigüzel, D.; Stutzmann, M.; Sharp, I.D.; Thalhammer, S. In vitro bio-functionality of gallium nitride sensors for radiation biophysics. Biochem. Biophys. Res. Commun. 2012, 424, 348–353. [Google Scholar] [CrossRef]
- Steinhoff, G.; Baur, B.; Wrobel, G.; Ingebrandt, S.; Offenhäusser, A.; Dadgar, A.; Krost, A.; Stutzmann, M.; Eickhoff, M. Recording of cell action potentials with AlGaN/GaN field-effect transistors. Appl. Phys. Lett. 2005, 86, 033901. [Google Scholar] [CrossRef]
- Snyder, P.J.; Reddy, P.; Kirste, R.; LaJeunesse, D.R.; Collazo, R.; Ivanisevic, A. Variably doped nanostructured gallium nitride surfaces can serve as biointerfaces for neurotypic PC12 cells and alter their behavior. RSC Adv. 2018, 8, 36722–36730. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Hao, X.; Peng, Y.; Zheng, Y.; Liu, J.; Kang, Y.; Zhao, F.; Luo, Z.; Guo, J. A novel approach to enhance bone regeneration by controlling the polarity of GaN/AlGaN heterostructures. Adv. Funct. Mater. 2021, 31, 2007487. [Google Scholar] [CrossRef]
- Fan, L.; Xiao, C.; Guan, P.; Zou, Y.; Wen, H.; Liu, C.; Luo, Y.; Tan, G.; Wang, Q.; Li, Y. Extracellular matrix-based conductive interpenetrating network hydrogels with enhanced neurovascular regeneration properties for diabetic wounds repair. Adv. Healthc. Mater. 2022, 11, 2101556. [Google Scholar] [CrossRef]
- Young, T.-H.; Chen, C.-R. Assessment of GaN chips for culturing cerebellar granule neurons. Biomaterials 2006, 27, 3361–3367. [Google Scholar] [CrossRef]
- Chen, C.-R.; Young, T.-H. The effect of gallium nitride on long-term culture induced aging of neuritic function in cerebellar granule cells. Biomaterials 2008, 29, 1573–1582. [Google Scholar] [CrossRef]
- Snyder, P.J.; Reddy, P.; Kirste, R.; LaJeunesse, D.R.; Collazo, R.; Ivanisevic, A. Noninvasive stimulation of neurotypic cells using persistent photoconductivity of gallium nitride. ACS Omega 2018, 3, 615–621. [Google Scholar] [CrossRef]
- Iusupovskaia, E.; Gerasimenko, A.Y.; Selishchev, S.; Telyshev, D.; Markov, A. Organic semiconductors with p-i-n structure for optoelectronic neurostimulation. Biomed. Eng. 2024, 58, 143–146. [Google Scholar] [CrossRef]
- Gottlieb, P.A.; Barone, T.; Sachs, F.; Plunkett, R. Neurite outgrowth from PC12 cells is enhanced by an inhibitor of mechanical channels. Neurosci. Lett. 2010, 481, 115–119. [Google Scholar] [CrossRef][Green Version]
- Jewett, S.A.; Makowski, M.S.; Andrews, B.; Manfra, M.J.; Ivanisevic, A. Gallium nitride is biocompatible and non-toxic before and after functionalization with peptides. Acta Biomater. 2012, 8, 728–733. [Google Scholar] [CrossRef]
- Podolska, A.; Tham, S.; Hart, R.D.; Seeber, R.M.; Kocan, M.; Kocan, M.; Mishra, U.K.; Pfleger, K.D.; Parish, G.; Nener, B.D. Biocompatibility of semiconducting AlGaN/GaN material with living cells. Sens. Actuators B 2012, 169, 401–406. [Google Scholar] [CrossRef]
- Mishra, M.; Sharan, J.; Koul, V.; Kharbanda, O.P.; Kumar, A.; Sharma, A.; Hackett, T.A.; Sagar, R.; Kashyap, M.K.; Gupta, G. Surface functionalization of gallium nitride for biomedical implant applications. Appl. Surf. Sci. 2023, 612, 155858. [Google Scholar] [CrossRef]
- Foster, C.M.; Collazo, R.; Sitar, Z.; Ivanisevic, A. Aqueous stability of Ga-and N-polar gallium nitride. Langmuir 2013, 29, 216–220. [Google Scholar] [CrossRef]
- Snyder, P.J.; Kirste, R.; Collazo, R.; Ivanisevic, A. Persistent photoconductivity, nanoscale topography, and chemical functionalization can collectively influence the behavior of PC12 cells on wide bandgap semiconductor surfaces. Small 2017, 13, 1700481. [Google Scholar] [CrossRef]
- Thakur, R.R.; Sarathlal, K.; Mishra, S.; Taliyan, R.; Chaturvedi, N. Bio-interface analysis and detection of Aβ using GaN HEMT-based biosensor. J. Electrochem. Soc. 2024, 171, 037507. [Google Scholar] [CrossRef]
- Garg, M.; Tak, B.R.; Rao, V.R.; Singh, R. Giant UV photoresponse of GaN-based photodetectors by surface modification using phenol-functionalized porphyrin organic molecules. ACS Appl. Mater. Interfaces 2019, 11, 12017–12026. [Google Scholar] [CrossRef]
- Dash, S.; Shakeel, A.; Mohanty, S. Impact of Nanotechnology on the Realm of Stem Cells and Regenerative Medicine. ChemNanoMat 2022, 8, e202200177. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.-W.; Chen, W.-G.; Liu, J. Neuroregeneration and functional recovery after stroke: Advancing neural stem cell therapy toward clinical application. Neural Regen. Res. 2021, 16, 80–92. [Google Scholar]
- Moe, A.A.K.; Suryana, M.; Marcy, G.; Lim, S.K.; Ankam, S.; Goh, J.Z.W.; Jin, J.; Teo, B.K.K.; Law, J.B.K.; Low, H.Y. Microarray with micro-and nano-topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells. Small 2012, 8, 3050–3061. [Google Scholar] [CrossRef] [PubMed]
- Mattiassi, S.; Rizwan, M.; Grigsby, C.L.; Zaw, A.M.; Leong, K.W.; Yim, E.K. Enhanced efficiency of nonviral direct neuronal reprogramming on topographical patterns. Biomater. Sci. 2021, 9, 5175–5191. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Gruenewald, A.; Detsch, R.; Boccaccini, A.R.; Vogel, N. Cell interactions with size-controlled colloidal monolayers: Toward improved coatings in bone tissue engineering. Langmuir 2020, 36, 1793–1803. [Google Scholar] [CrossRef]
- Couvrette, L.J.; Walker, K.L.; Bui, T.V.; Pelling, A.E. Plant Cellulose as a Substrate for 3D Neural Stem Cell Culture. Bioengineering 2023, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wang, Y.; Xie, F.; Wang, S.; Xu, W.; Xu, J.; Sun, C. Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules 2022, 27, 8326. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Kothapalli, C.; Mahajan, G.; Farrell, K. Substrate stiffness induced mechanotransduction regulates temporal evolution of human fetal neural progenitor cell phenotype, differentiation, and biomechanics. Biomater. Sci. 2020, 8, 5452–5464. [Google Scholar] [CrossRef]
- Han, R.; Luo, L.; Wei, C.; Qiao, Y.; Xie, J.; Pan, X.; Xing, J. Stiffness-tunable biomaterials provide a good extracellular matrix environment for axon growth and regeneration. Neural Regener. Res. 2025, 20, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-R.; Li, Y.-C.; Young, T.-H. Gallium nitride induces neuronal differentiation markers in neural stem/precursor cells derived from rat cerebral cortex. Acta Biomater. 2009, 5, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, G.; Iemmolo, R.; La Cognata, V.; Zimbone, M.; La Via, F.; Fragalà, M.E.; Barcellona, M.L.; Pellitteri, R.; Cavallaro, S. Biocompatibility between silicon or silicon carbide surface and neural stem cells. Sci. Rep. 2019, 9, 11540. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A. Near infrared laser stimulation of human neural stem cells into neurons on graphene nanomesh semiconductors. Colloids Surf. B 2015, 126, 313–321. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Li, D.; Zhang, Y.; Zhang, S.; Kang, W.; Liu, C.; Le, W.; Wang, L.; Li, D. Halogen doped graphene quantum dots modulate TDP-43 phase separation and aggregation in the nucleus. Nat. Commun. 2024, 15, 2980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, J.; Zhang, Y.; Guo, H.; Zhang, S.; Song, J.; Liu, C.; Wang, L.; Li, D.; Dai, B. Graphene quantum dots modulate stress granule assembly and prevent abnormal phase transition of fused in sarcoma protein. ACS Nano 2023, 17, 10129–10141. [Google Scholar] [CrossRef]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-Based Nanocomposites for Neural Tissue Engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, M.; Gao, W.; Liu, F.; Duan, J.; Kong, Y.; Liu, D.; Liu, H. Neuron-like cell differentiation of hADSCs promoted by a copper sulfide nanostructure mediated plasmonic effect driven by near-infrared light. Nanoscale 2020, 12, 9833–9841. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Fang, Y.; Wang, L.; Li, P. Rational design of nitrogen-doped carbon dots for inhibiting β-amyloid aggregation. Molecules 2023, 28, 1451. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.C.; Vagaska, B.; Edgington, R.; Hébert, C.; Ferretti, P.; Bergonzo, P.; Jackman, R.B. Biocompatibility of nanostructured boron doped diamond for the attachment and proliferation of human neural stem cells. J. Neural Eng. 2015, 12, 066016. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Wang, X.; Xiao, H.; Wang, C.; Yang, C.; Yin, H.; Deng, Q.; Li, J.; Wang, Z.; Hou, X. Influence of electric field on persistent photoconductivity in unintentionally doped n-type GaN. Appl. Phys. Lett. 2011, 98, 102104. [Google Scholar] [CrossRef]
- Cho, S.-J.; Doğan, S.; Sabuktagin, S.; Reshchikov, M.A.; Johnstone, D.K.; Morkoç, H. Surface band bending in as-grown and plasma-treated n-type GaN films using surface potential electric force microscopy. Appl. Phys. Lett. 2004, 84, 3070–3072. [Google Scholar] [CrossRef]
- Imazeki, Y.; Sato, M.; Takeda, T.; Kobayashi, M.; Yamamoto, S.; Matsuda, I.; Yoshinobu, J.; Sugiyama, M.; Nakano, Y. Band bending of n-GaN under Ambient H2O vapor studied by x-ray photoelectron spectroscopy. J. Phys. Chem. C 2021, 125, 9011–9019. [Google Scholar] [CrossRef]
- Foussekis, M.; Baski, A.; Reshchikov, M. Comparison of surface photovoltage behavior for n-type versus p-type GaN. J. Vac. Sci. Technol. B 2011, 29, 041205. [Google Scholar] [CrossRef]
- Sinensky, A.K.; Belcher, A.M. Label-free and high-resolution protein/DNA nanoarray analysis using Kelvin probe force microscopy. Nat. Nanotechnol. 2007, 2, 653–659. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, W.; Xu, S.; Cheong, L.-Z.; Wang, D.; Shen, C. Direct study of the electrical properties of PC12 cells and hippocampal neurons by EFM and KPFM. Nanoscale Adv. 2019, 1, 537–545. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Du, X.; Zhou, S.; Li, J.; Feng, R.; Zhang, H.; Xu, Q.; Zhao, W.; Liu, Z.; Zhong, H. Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells. Molecules 2024, 29, 4439. https://doi.org/10.3390/molecules29184439
Meng Y, Du X, Zhou S, Li J, Feng R, Zhang H, Xu Q, Zhao W, Liu Z, Zhong H. Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells. Molecules. 2024; 29(18):4439. https://doi.org/10.3390/molecules29184439
Chicago/Turabian StyleMeng, Yu, Xiaowei Du, Shang Zhou, Jiangting Li, Rongrong Feng, Huaiwei Zhang, Qianhui Xu, Weidong Zhao, Zheng Liu, and Haijian Zhong. 2024. "Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells" Molecules 29, no. 18: 4439. https://doi.org/10.3390/molecules29184439
APA StyleMeng, Y., Du, X., Zhou, S., Li, J., Feng, R., Zhang, H., Xu, Q., Zhao, W., Liu, Z., & Zhong, H. (2024). Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells. Molecules, 29(18), 4439. https://doi.org/10.3390/molecules29184439






