Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing
Abstract
1. Introduction
2. Results and Discussion
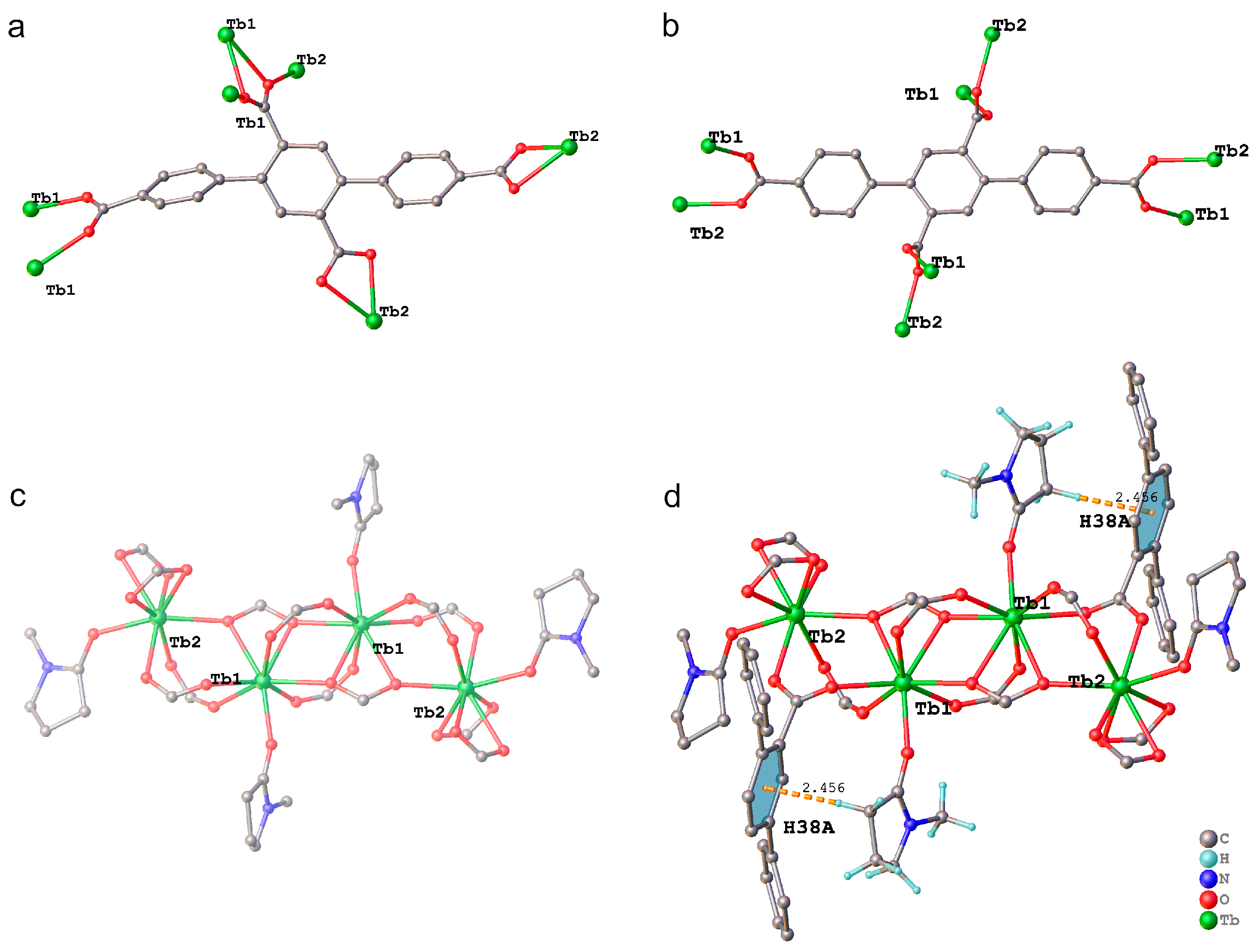
2.1. Structural Properties of TbL and EuxTb1−xL
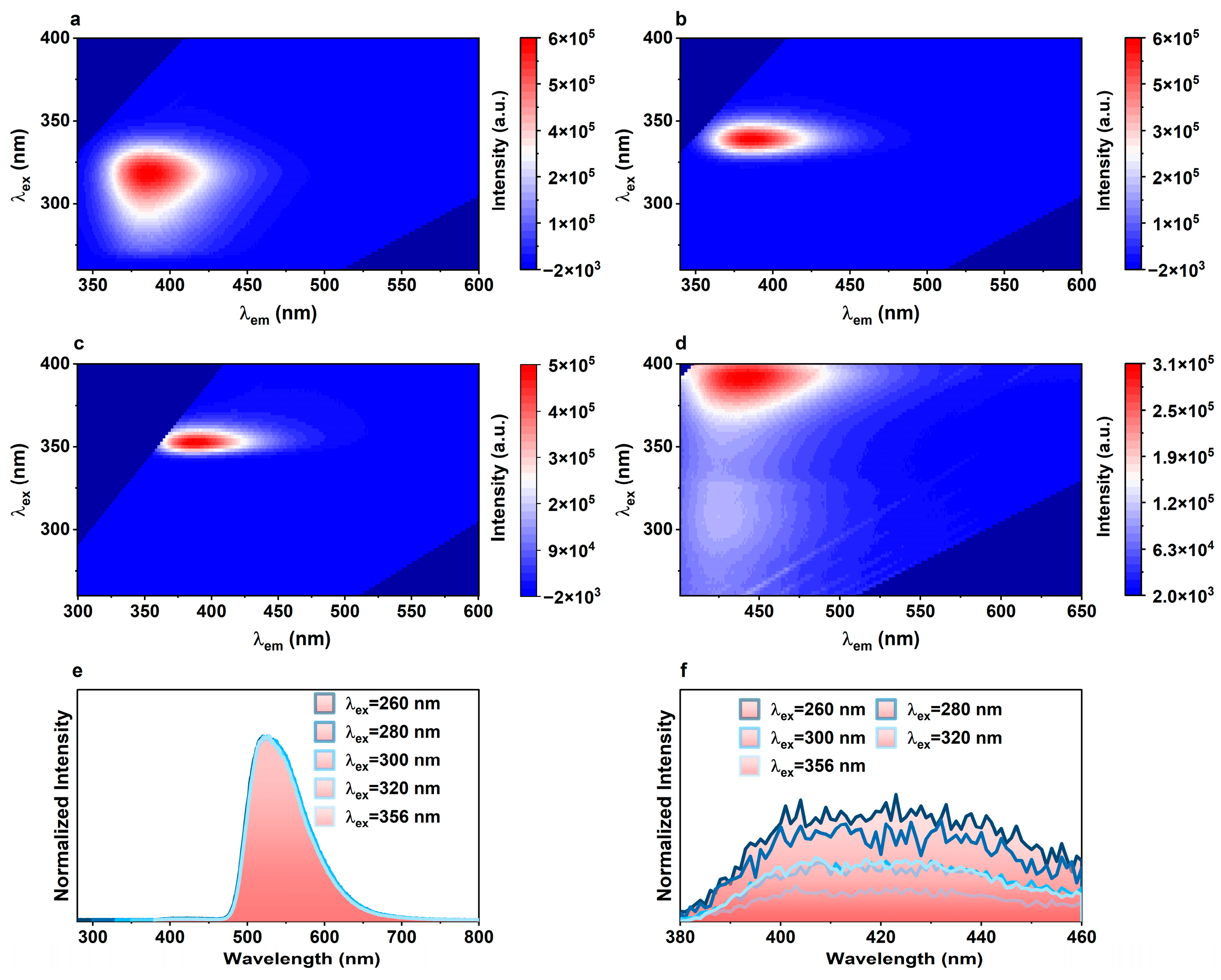
2.2. Photoluminescence Properties of TbL and EuxTb1−xL
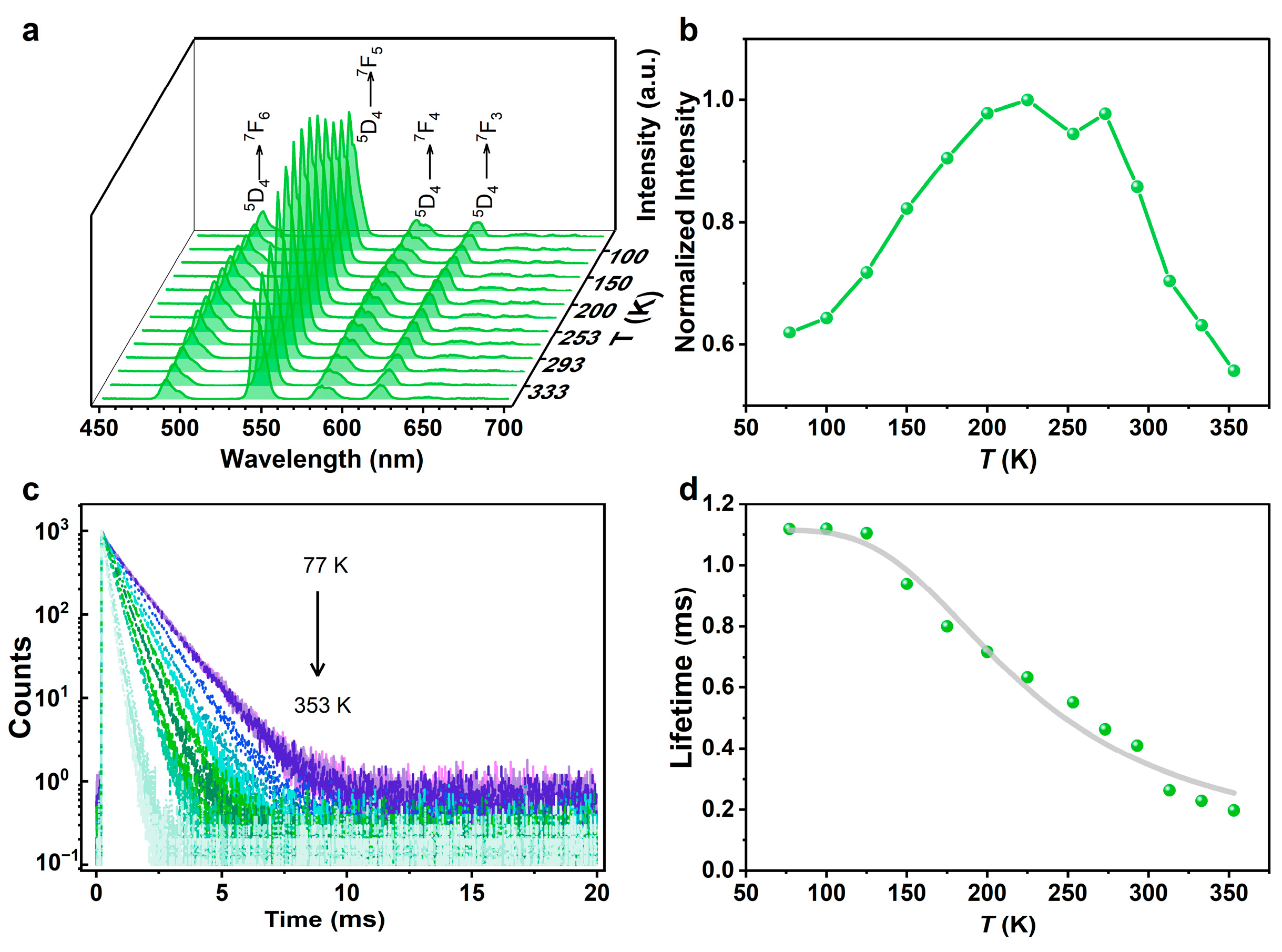
2.3. Temperature-Dependent Photoluminescence Properties of TbL and EuxTb1−xL
2.4. Energy Transfer
3. Materials and Methods
3.1. Materials and Characterization
3.2. Preparation of [Tb2L1.5(NMP)2]n (TbL)
3.3. Preparation of EuxTb1−xL (x = 0.0001, 0.0005, and 0.001)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geitenbeek, R.G.; Nieuwelink, A.; Jacobs, T.S.; Salzmann, B.B.V.; Goetze, J.; Meijerink, A.; Weckhuysen, B.M. In Situ Luminescence Thermometry To Locally Measure Temperature Gradients during Catalytic Reactions. ACS Catal. 2018, 8, 2397–2401. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rijckaert, H.; Maegawa, Y.; Inagaki, S.; Van Der Voort, P.; Kaczmarek, A.M. Luminescent Nanorattles Based on Bipyridine Periodic Mesoporous Organosilicas for Simultaneous Thermometry and Catalysis. Chem. Mater. 2022, 34, 3770–3780. [Google Scholar] [CrossRef]
- Guo, H.; Raj, J.; Wang, Z.; Zhang, T.; Wang, K.; Lin, L.; Hou, W.; Zhang, J.; Wu, M.; Wu, J.; et al. Synergistic Effects of Amine Functional Groups and Enriched-Atomic-Iron Sites in Carbon Dots for Industrial-Current–Density CO2 Electroreduction. Small 2024, 20, 2311132. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, L.; Huang, K.; Li, Y.; Hou, W.; Liao, H.; Lian, C.; Yang, S.; Wu, D.; Lei, Z.; et al. Nitrogen-Rich Carbon Dot-Mediated N→π* Electronic Transition in Carbon Nitride for Superior Photocatalytic Hydrogen Peroxide Production. Adv. Funct. Mater. 2024, 2402650. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, Z.; Miao, X.; Wang, K.; Yao, F.; Gao, Z.; Mi, B.; Yi, Y.; Yang, G.; Qian, Y. Fabrication of Flexible High-Temperature Film Thermometers and Heat-Resistant OLEDs Using Novel Hot Exciton Organic Fluorophores. Adv. Funct. Mater. 2022, 32, 2205697. [Google Scholar] [CrossRef]
- Maturi, F.E.; Brites, C.D.S.; Silva, R.R.; Nigoghossian, K.; Wilson, D.; Ferreira, R.A.S.; Ribeiro, S.J.L.; Carlos, L.D. Sustainable Smart Tags with Two-Step Verification for Anticounterfeiting Triggered by the Photothermal Response of Upconverting Nanoparticles. Adv. Photon. Res. 2022, 3, 2100227. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, W.; Huang, K.; Guo, H.; Wang, Z.; Lian, C.; Zhang, J.; Wu, D.; Lei, Z.; Liu, Z.; et al. Engineering Built-In Electric Field Microenvironment of CQDs/g-C3N4 Heterojunction for Efficient Photocatalytic CO2 Reduction. Adv. Sci. 2024, 11, 2403607. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Li, D.; Zhang, Y.; Zhang, S.; Kang, W.; Liu, C.; Le, W.; Wang, L.; Li, D.; et al. Halogen Doped Graphene Quantum Dots Modulate TDP-43 Phase Separation and Aggregation in the Nucleus. Nat. Commun. 2024, 15, 2980. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lu, Y.; Lei, Z.; Bao, H.; Zhang, M.; Wang, Z.; Guan, C.; Tang, B.; Liu, Z.; Wang, L. Machine learning-guided realization of full-color high-quantum-yield carbon quantum dots. Nat. Commun. 2024, 15, 4843. [Google Scholar] [CrossRef]
- Zheng, M.; Li, L.; Tian, D.; Zhang, Z.; Zhou, W.; He, M. Tailoring Dye Emissions within Metal-Organic Frameworks for Tunable Luminescence and Ratiometric Temperature Sensing. ACS Appl. Mater. Interfaces 2023, 15, 23479–23488. [Google Scholar] [CrossRef]
- McLaurin, E.J.; Bradshaw, L.R.; Gamelin, D.R. Dual-Emitting Nanoscale Temperature Sensors. Chem. Mater. 2013, 25, 1283–1292. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. ISBN 978-0-444-63699-7. [Google Scholar]
- Dramićanin, M.D. Trends in Luminescence Thermometry. J. Appl. Phys. 2020, 128, 040902. [Google Scholar] [CrossRef]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and Challenges for Fluorescence Nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.F.C.B.; Carneiro Neto, A.N.; Carlos, L.D.; André, P.S.; Ferreira, R.A.S. Chapter 324—Lanthanides for the New Generation of Optical Sensing and Internet of Things. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Including Actinides; Elsevier: Amsterdam, The Netherlands, 2022; Volume 61, pp. 31–128. [Google Scholar]
- Chen, D.; Haldar, R.; Wöll, C. Stacking Lanthanide-MOF Thin Films to Yield Highly Sensitive Optical Thermometers. ACS Appl. Mater. Interfaces 2023, 15, 19665–19671. [Google Scholar] [CrossRef] [PubMed]
- Hang, M.; Cheng, Y.; Wang, Y.; Li, H.; Zheng, M.; He, M.; Chen, Q.; Zhang, Z. Rational synthesis of isomorphic rare earth metal-organic framework materials for simultaneous adsorption and photocatalytic degradation of organic dyes in water. CrystEngComm 2022, 24, 552–559. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Cheng, Y.; Zheng, M.; Qian, J.; He, M.; Chen, Q.; Zhang, Z. Synthesis of rare earth MOF/CZS materials derived from aromatic tetracarboxylic acids and photocatalytic hydrogen production. Int. J. Hydrog. Energy 2024, 65, 225–235. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A Luminescent Mixed-Lanthanide Metal–Organic Framework Thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, B.; Lei, F. Postsynthetic Lanthanide Functionalization of Nanosized Metal–Organic Frameworks for Highly Sensitive Ratiometric Luminescent Thermometry. Chem. Commun. 2014, 50, 15235–15238. [Google Scholar] [CrossRef]
- Wang, Z.; Ananias, D.; Carné-Sánchez, A.; Brites, C.D.S.; Imaz, I.; Maspoch, D.; Rocha, J.; Carlos, L.D. Lanthanide-Organic Framework Nanothermometers Prepared by Spray-Drying. Adv. Funct. Mater. 2015, 25, 2824–2830. [Google Scholar] [CrossRef]
- Ananias, D.; Brites, C.D.S.; Carlos, L.D.; Rocha, J. Cryogenic Nanothermometer Based on the MIL-103(Tb,Eu) Metal–Organic Framework. Eur. J. Inorg. Chem. 2016, 2016, 1967–1971. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, D.; Zhang, L.; Jiang, K.; Qian, G. Cryogenic Luminescent Tb/Eu-MOF Thermometer Based on a Fluorine-Modified Tetracarboxylate Ligand. Inorg. Chem. 2018, 57, 12596–12602. [Google Scholar] [CrossRef]
- Ramalho, J.F.C.B.; Correia, S.F.H.; Fu, L.; António, L.L.F.; Brites, C.D.S.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. Luminescence Thermometry on the Route of the Mobile-Based Internet of Things (IoT): How Smart QR Codes Make It Real. Adv. Sci. 2019, 6, 1900950. [Google Scholar] [CrossRef]
- Feng, T.; Ye, Y.; Liu, X.; Cui, H.; Li, Z.; Zhang, Y.; Liang, B.; Li, H.; Chen, B. A Robust Mixed-Lanthanide PolyMOF Membrane for Ratiometric Temperature Sensing. Angew. Chem. Int. Ed. 2020, 59, 21752–21757. [Google Scholar] [CrossRef]
- Carneiro Neto, A.N.; Mamontova, E.; Botas, A.M.P.; Brites, C.D.S.; Ferreira, R.A.S.; Rouquette, J.; Guari, Y.; Larionova, J.; Long, J.; Carlos, L.D. Rationalizing the Thermal Response of Dual-Center Molecular Thermometers: The Example of an Eu/Tb Coordination Complex. Adv. Opt. Mater. 2022, 10, 2101870. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Lin, L.; Long, L.; Zheng, L. Doped Luminescent Lanthanide Coordination Polymers Exhibiting Both White-Light Emission and Thermal Sensitivity. Inorg. Chem. 2021, 60, 6986–6990. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, D.; Jiang, K.; Zhang, L.; Li, C.; Qian, G. Isostructural Tb3+/Eu3+ Co-Doped Metal–Organic Framework Based on Pyridine-Containing Dicarboxylate Ligands for Ratiometric Luminescence Temperature Sensing. Inorg. Chem. 2019, 58, 2637–2644. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Lu, Y.; Wang, S.; Zhao, D.; Li, C. Isostructural Single- And Dual-Lanthanide Metal–Organic Frameworks Based On Substituent-Group-Modifying Tetracarboxylate Ligands for Ratiometric Temperature Sensing. Inorg. Chem. 2021, 60, 4133–4143. [Google Scholar] [CrossRef]
- Miao, W.; Liu, B.; Li, H.; Zheng, S.; Jiao, H.; Xu, L. Fluorescent Eu3+/Tb3+ Metal–Organic Frameworks for Ratiometric Temperature Sensing Regulated by Ligand Energy. Inorg. Chem. 2022, 61, 14322–14332. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, M.; Xu, Q.; Long, L.; Zheng, L. A Doped Lanthanide-Based Coordination Polymer Exhibiting High Relative Sensitivity to Ratiometric Luminescent Thermometers at 440 K. Inorg. Chem. 2023, 62, 18009–18013. [Google Scholar] [CrossRef]
- Xia, T.; Cao, W.; Guan, L.; Zhang, J.; Jiang, F.; Yu, L.; Wan, Y. Three Isostructural Hexanuclear Lanthanide–Organic Frameworks for Sensitive Luminescence Temperature Sensing over a Wide Range. Dalton Trans. 2022, 51, 5426–5433. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Yang, X.; Kou, Y.; Chen, W.; Liu, W. Construction of a Series of Ln-MOF Luminescent Sensors Based on a Functional “V” Shaped Ligand. Dalton Trans. 2022, 51, 12549–12557. [Google Scholar] [CrossRef]
- Kanzariya, D.B.; Chaudhary, M.Y.; Pal, T.K. Engineering of Metal–Organic Frameworks (MOFs) for Thermometry. Dalton Trans. 2023, 52, 7383–7404. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yan, X.; Ji, Z.; Liu, T.; Cao, R. Fabrication of Lanthanide-Functionalized Hydrogen-Bonded Organic Framework Films for Ratiometric Temperature Sensing by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2020, 12, 29854–29860. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Y.; Zhou, X.; Brites, C.D.S.; Ananias, D.; Lin, Z.; Paz, F.A.A.; Rocha, J.; Huang, W.; Carlos, L.D. Visible-Light Excited Luminescent Thermometer Based on Single Lanthanide Organic Frameworks. Adv. Funct. Mater. 2016, 26, 8677–8684. [Google Scholar] [CrossRef]
- Shen, X.; Yan, B. Polymer Hybrid Thin Films Based on Rare Earth Ion-Functionalized MOF: Photoluminescence Tuning and Sensing as a Thermometer. Dalton Trans. 2015, 44, 1875–1881. [Google Scholar] [CrossRef]
- Cui, Y.; Song, R.; Yu, J.; Liu, M.; Wang, Z.; Wu, C.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Dual-Emitting MOF⊃Dye Composite for Ratiometric Temperature Sensing. Adv. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, T.; Shi, J.; Gao, S.; Cao, R. Dual-Emitting UiO-66(Zr&Eu) Metal–Organic Framework Films for Ratiometric Temperature Sensing. ACS Appl. Mater. Interfaces 2018, 10, 20854–20861. [Google Scholar]
- Ananias, D.; Firmino, A.D.G.; Mendes, R.F.; Paz, F.A.A.; Nolasco, M.; Carlos, L.D.; Rocha, J. Excimer Formation in a Terbium Metal–Organic Framework Assists Luminescence Thermometry. Chem. Mater. 2017, 29, 9547–9554. [Google Scholar] [CrossRef]
- Li, L.; Cheng, J.; Liu, Z.; Song, L.; You, Y.; Zhou, X.; Huang, W. Ratiometric Luminescent Sensor of Picric Acid Based on the Dual-Emission Mixed-Lanthanide Coordination Polymer. ACS Appl. Mater. Interfaces 2018, 10, 44109–44115. [Google Scholar] [CrossRef]


| Items | A1 | A2 | T0 | R2 |
|---|---|---|---|---|
| Eu0.0001Tb0.9999L | 6.70 | 1.20 | 278.00 | 0.9990 |
| Eu0.005Tb0.999 L | 6.80 | 0.42 | 240.00 | 0.9993 |
| Eu0.001Tb0.999L | 6.40 | 0.10 | 183.00 | 0.9997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Cheng, S.; Zhang, Z.; He, M.; Qian, J.; Li, L. Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing. Molecules 2024, 29, 3914. https://doi.org/10.3390/molecules29163914
Tang H, Cheng S, Zhang Z, He M, Qian J, Li L. Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing. Molecules. 2024; 29(16):3914. https://doi.org/10.3390/molecules29163914
Chicago/Turabian StyleTang, Hui, Siyuan Cheng, Zhihui Zhang, Mingyang He, Junfeng Qian, and Liang Li. 2024. "Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing" Molecules 29, no. 16: 3914. https://doi.org/10.3390/molecules29163914
APA StyleTang, H., Cheng, S., Zhang, Z., He, M., Qian, J., & Li, L. (2024). Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing. Molecules, 29(16), 3914. https://doi.org/10.3390/molecules29163914







