Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Content
2.2. Antioxidant Activity (DPPH IC50)
2.3. Acetylcholinesterase Inhibitory Activity (AChE IC50)
2.4. UHPLC/MS Analysis of Polyphenolic Acids
2.5. HPTLC–DPPH Analysis
2.6. Statistical Correlation Analysis
2.6.1. Total Phenolic Content
2.6.2. Antioxidant Activity (DPPH IC50)
2.6.3. Acetylcholinesterase Inhibitory Activity (AChE IC50)
2.6.4. Correlation of TPC with DPPH IC50 and AChE IC50
2.6.5. Overview
2.7. Seasonal Variability Comparison
3. Discussion
3.1. Total Phenolic Content
3.2. Antioxidant Activity
3.3. Acetylcholinesterase Inhibitory Activity
3.4. Correlation of Polyphenol Content with Antioxidant and Anticholinesterase Activities
3.5. Importance of Seasonal Variations
3.6. Implications for Medicinal and Nutritional Use
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Sample Preparation
4.4. Total Phenolic Content
4.5. Antioxidant Assay
4.6. Acetylcholinesterase Inhibition Assay
4.7. UHPLC/MS Analysis
4.8. HPTLC–DPPH Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea. Vol. 3: Diapensiaceae to Myoporaceae, 1st ed.; Cambridge University Press: Cambridge, UK, 1972; p. 187. [Google Scholar]
- Săvulescu, T. (Ed.) Flora R.P.R., 1st ed.; Romanian Academy Publishing House: Bucharest, Romania, 1961; Volume VIII, pp. 108–109. (In Romanian) [Google Scholar]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Al Kamaly, O.; Alanazi, A.S.; Noman, O. Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches. Processes 2022, 10, 2422. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Shaffath Ali, S.; Vinod, K.R.; Reddy, S.; Banji, D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. Available online: https://www.food.actapol.net/volume12/issue1/abstract-6.html (accessed on 12 July 2024). [PubMed]
- Teja, Y.S.C.; Jyothika, L.S.; Vasavi, N.; Reddy, K.S.S.; Sudheer, A. Exploring the Therapeutic Potential of Rosemary: An In-depth Review of its Pharmacological Properties. Asian J. Adv. Med. Sci. 2024, 6, 19–31. Available online: https://journalmedicals.com/index.php/AJOAIMS/article/view/141 (accessed on 12 July 2024).
- Chatterjee, K.; Tamta, B.; Mukopadayay, S. A review on “pharmacological, phytochemical, and medicinal properties of Rosmarinus officinalis (Rosemary)”. Int. J. Health Sci. 2022, 6, 3491–3500. [Google Scholar] [CrossRef]
- Popescu, G.; Iancu, T.; Popescu, C.A.; Stanciu, S.M.; Luca, R.; Imbrea, F.; Radulov, I.; Sala, F.; Moatăr, M.M.; Camen, D.D. The influence of soil fertilization on the quality and extraction efficiency of rosemary essential oil (Rosmarinus officinalis L.). Rom. Biotechnol. Lett. 2020, 25, 1961–1968. [Google Scholar] [CrossRef]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Flauzino, L.G.B.; Souza, M.G.M.; Turatti, I.C.C.; Andrade e Silva, M.L.; Martins, C.H.G.; da Silva Filho, A.A.; Cunha, W.R. Antibacterial activity of the essential oil from Rosmarinus officinalis and its major components against oral pathogens. Z. Naturforsch. C J. Biosci. 2010, 65, 588–593. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Fernández, L.F.; Palomino, O.M.; Frutos, G. Effectiveness of Rosmarinus officinalis essential oil as antihypotensive agent in primary hypotensive patients and its influence on health-related quality of life. J. Ethnopharmacol. 2014, 151, 509–516. [Google Scholar] [CrossRef]
- Mersin, B.; Işcan, G.S. Rosmarinus officinalis L. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence, 1st ed.; Gürağaç Dereli, F.T., Ilhan, M., Belwal, T., Eds.; Springer: Cham, Switzerland, 2022; pp. 525–541. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis Leaves as a Natural Source of Bioactive Compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Quílez, M.; Ferreres, F.; López-Miranda, S.; Salazar, E.; Jordán, M.J. Seed Oil from Mediterranean Aromatic and Medicinal Plants of the Lamiaceae Family as a Source of Bioactive Components with Nutritional. Antioxidants 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Kakouri, E.; Nikola, O.; Kanakis, C.; Hatziagapiou, K.; Lambrou, G.I.; Trigas, P.; Kanaka-Gantenbein, C.; Tarantilis, P.A. Cytotoxic Effect of Rosmarinus officinalis Extract on Glioblastoma and Rhabdomyosarcoma Cell Lines. Molecules 2022, 27, 6348. [Google Scholar] [CrossRef] [PubMed]
- Luţă, E.A.; Biţă, A.; Moroşan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. Polyphenols and Biological Activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef]
- Lamponi, S.; Baratto, M.C.; Miraldi, E.; Baini, G.; Biagi, M. Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis. Plants 2021, 10, 97. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, Y.; Bai, X. A Green and Effective Polyethylene Glycols-Based Microwave-Assisted Extraction of Carnosic and Rosmarinic Acids from Rosmarinus officinalis Leaves. Foods 2023, 12, 1761. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, Y.; Wu, H. The Selective Separation of Carnosic Acid and Rosmarinic Acid by Solid-Phase Extraction and Liquid–Liquid Extraction: A Comparative Study. Molecules 2023, 28, 5493. [Google Scholar] [CrossRef]
- Danisman, B.; Cicek, B.; Yildirim, S.; Bolat, I.; Kantar, D.; Golokhvast, K.S.; Nikitovic, D.; Tsatsakis, A.; Taghizadehghalehjoughi, A. Carnosic Acid Ameliorates Indomethacin-Induced Gastric Ulceration in Rats by Alleviating Oxidative Stress and Inflammation. Biomedicines 2023, 11, 829. [Google Scholar] [CrossRef]
- Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Mirza, F.J.; Zahid, S.; Holsinger, R.M.D. Neuroprotective Effects of Carnosic Acid: Insight into Its Mechanisms of Action. Molecules 2023, 28, 2306. [Google Scholar] [CrossRef] [PubMed]
- Kallimanis, P.; Magiatis, P.; Panagiotopoulou, A.; Ioannidis, K.; Chinou, I. Extraction Optimization and Qualitative/Quantitative Determination of Bioactive Abietane-Type Diterpenes from Three Salvia Species (Common Sage, Greek Sage and Rosemary) by 1H-qNMR. Molecules 2024, 29, 625. [Google Scholar] [CrossRef] [PubMed]
- Vella, F.M.; Laratta, B. Rosemary Essential Oil Extraction and Residue Valorization by Means of Polyphenol Recovery. Biol. Life Sci. Forum 2023, 26, 8. [Google Scholar] [CrossRef]
- Hendel, N.; Sarri, D.; Sarri, M.; Napoli, E.; Palumbo Piccionello, A.; Ruberto, G. Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth. Int. J. Mol. Sci. 2024, 25, 7989. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Hcini, K.; Lozano-Pérez, A.A.; Luis Cenis, J.; Quílez, M.; José Jordán, M. Extraction and Encapsulation of Phenolic Compounds of Tunisian Rosemary (Rosmarinus officinalis L.) Extracts in Silk Fibroin Nanoparticles. Plants 2021, 10, 2312. [Google Scholar] [CrossRef]
- Lešnik, S.; Bren, U. Mechanistic Insights into Biological Activities of Polyphenolic Compounds from Rosemary Obtained by Inverse Molecular Docking. Foods 2022, 11, 67. [Google Scholar] [CrossRef]
- Guimarães, N.S.S.; Ramos, V.S.; Prado-Souza, L.F.L.; Lopes, R.M.; Arini, G.S.; Feitosa, L.G.P.; Silva, R.R.; Nantes, I.L.; Damasceno, D.C.; Lopes, N.P.; et al. Rosemary (Rosmarinus officinalis L.) Glycolic Extract Protects Liver Mitochondria from Oxidative Damage and Prevents Acetaminophen-Induced Hepatotoxicity. Antioxidants 2023, 12, 628. [Google Scholar] [CrossRef]
- Song, X.; Sui, X.; Jiang, L. Protection Function and Mechanism of Rosemary (Rosmarinus officinalis L.) Extract on the Thermal Oxidative Stability of Vegetable Oils. Foods 2023, 12, 2177. [Google Scholar] [CrossRef]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; Portella, R.L.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.M.; da Luz, S.C.A.; Boligon, A.A.; et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- Gonçalves, C.; Fernandes, D.; Silva, I.; Mateus, V. Potential Anti-Inflammatory Effect of Rosmarinus officinalis in Preclinical In Vivo Models of Inflammation. Molecules 2022, 27, 609. [Google Scholar] [CrossRef] [PubMed]
- Francolino, R.; Martino, M.; Caputo, L.; Amato, G.; Chianese, G.; Gargiulo, E.; Formisano, C.; Romano, B.; Ercolano, G.; Ianaro, A.; et al. Phytochemical Constituents and Biological Activity of Wild and Cultivated Rosmarinus officinalis Hydroalcoholic Extracts. Antioxidants 2023, 12, 1633. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.R.; Grando, J.; Brocardo, P.S.; Bettio, L.E.; Dalmarco, J.B.; Rial, D.; et al. Rosmarinus officinalis L. hydroalcoholic extract, similar to fluoxetine, reverses depressive-like behavior without altering learning deficit in olfactory bulbectomized mice. J. Ethnopharmacol. 2012, 143, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol are Potent Quorum Sensing Inhibitors of Staphylococcus aureus Virulence. Antibiotics 2020, 9, 149. [Google Scholar] [CrossRef]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.K.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Iobbi, V.; Parisi, V.; Bernabè, G.; De Tommasi, N.; Bisio, A.; Brun, P. Anti-Biofilm Activity of Carnosic Acid from Salvia rosmarinus against Methicillin-Resistant Staphylococcus aureus. Plants 2023, 12, 3679. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe-Barbu, I.; Marinas, I.C.; Avramescu, S.M.; Pecete, I.; Geanǎ, E.I.; Chifiriuc, M.C. Eco-Friendly Solution Based on Rosmarinus officinalis Hydro-Alcoholic Extract to Prevent Biodeterioration of Cultural Heritage Objects and Buildings. Int. J. Mol. Sci. 2022, 23, 11463. [Google Scholar] [CrossRef]
- Letsiou, S.; Pyrovolou, K.; Konteles, S.J.; Trapali, M.; Krisilia, S.; Kokla, V.; Apostolaki, A.; Founda, V.; Houhoula, D.; Batrinou, A. Exploring the Antifungal Activity of Various Natural Extracts in a Sustainable Saccharomyces cerevisiae Model Using Cell Viability, Spot Assay, and Turbidometric Microbial Assays. Appl. Sci. 2024, 14, 1899. [Google Scholar] [CrossRef]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R.; Schnitzler, P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef]
- Horváth, G.; Molnár, E.; Szabó, Z.; Kecskeméti, G.; Juhász, L.; Tallósy, S.P.; Nyári, J.; Bogdanov, A.; Somogyvári, F.; Endrész, V.; et al. Carnosic Acid Inhibits Herpes simplex Virus Replication by Suppressing Cellular ATP Synthesis. Int. J. Mol. Sci. 2024, 25, 4983. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, K.; Pettas, S.; Kanata, E.; Lioulia, E.; Thune, K.; Schmitz, M.; Tsamesidis, I.; Lymperaki, E.; Xanthopoulos, K.; Sklaviadis, T.; et al. Carnosic Acid and Carnosol Display Antioxidant and Anti-Prion Properties in In Vitro and Cell-Free Models of Prion Diseases. Antioxidants 2022, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Trudler, D.; Oh, C.K.; Lipton, S.A. Potential Therapeutic Use of the Rosemary Diterpene Carnosic Acid for Alzheimer’s Disease, Parkinson’s Disease, and Long-COVID through NRF2 Activation to Counteract the NLRP3 Inflammasome. Antioxidants 2022, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.W.E.; Yousef, M.; Coish, J.M.; Fajardo, V.A.; Tsiani, E.; MacNeil, A.J. Carnosic acid inhibits secretion of allergic inflammatory mediators in IgE-activated mast cells via direct regulation of Syk activation. J. Biol. Chem. 2023, 299, 102867. [Google Scholar] [CrossRef]
- Peno-Mazzarino, L.; Radionov, N.; Merino, M.; González, S.; Mullor, J.L.; Jones, J.; Caturla, N. Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study. Curr. Issues Mol. Biol. 2024, 46, 1530–1555. [Google Scholar] [CrossRef]
- Kayashima, T.; Matsubara, K. Antiangiogenic effect of carnosic acid and carnosol, neuroprotective compounds in rosemary leaves. Biosci. Biotechnol. Biochem. 2012, 76, 115–119. [Google Scholar] [CrossRef]
- Mirza, F.J.; Zahid, S.; Amber, S.; Sumera; Jabeen, H.; Asim, N.; Ali Shah, S.A. Multitargeted Molecular Docking and Dynamic Simulation Studies of Bioactive Compounds from Rosmarinus officinalis against Alzheimer’s Disease. Molecules 2022, 27, 7241. [Google Scholar] [CrossRef]
- Alrashdi, J.; Albasher, G.; Alanazi, M.M.; Al-Qahtani, W.S.; Alanezi, A.A.; Alasmari, F. Effects of Rosmarinus officinalis L. Extract on Neurobehavioral and Neurobiological Changes in Male Rats with Pentylenetetrazol-Induced Epilepsy. Toxics 2023, 11, 826. [Google Scholar] [CrossRef]
- Kosmopoulou, D.; Lafara, M.-P.; Adamantidi, T.; Ofrydopoulou, A.; Grabrucker, A.M.; Tsoupras, A. Neuroprotective Benefits of Rosmarinus officinalis and Its Bioactives against Alzheimer’s and Parkinson’s Diseases. Appl. Sci. 2024, 14, 6417. [Google Scholar] [CrossRef]
- Al-Tawarah, N.M.; Al-dmour, R.H.; Abu Hajleh, M.N.; Khleifat, K.M.; Alqaraleh, M.; Al-Saraireh, Y.M.; Jaradat, A.Q.; Al-Dujaili, E.A.S. Rosmarinus officinalis and Mentha piperita Oils Supplementation Enhances Memory in a Rat Model of Scopolamine-Induced Alzheimer’s Disease-like Condition. Nutrients 2023, 15, 1547. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.E.S.; Fetouh, F.A.; Albasha, M.O. Nephro-protective effects of curcumin, rosemary, and propolis against gentamicin induced toxicity in Guinea pigs: Morphological and biochemical study. Am. J. Clin. Exp. Med. 2014, 2, 28–35. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Moore, J.; Song, J.; Tsiani, E.L. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERK and AMPK. Life 2022, 12, 52. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Sze, N.S.K.; MacPherson, R.E.K.; Tsiani, E. Carnosic Acid against Lung Cancer: Induction of Autophagy and Activation of Sestrin-2/LKB1/AMPK Signalling. Int. J. Mol. Sci. 2024, 25, 1950. [Google Scholar] [CrossRef]
- Bouammali, H.; Zraibi, L.; Ziani, I.; Merzouki, M.; Bourassi, L.; Fraj, E.; Challioui, A.; Azzaoui, K.; Sabbahi, R.; Hammouti, B.; et al. Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study. Plants 2024, 13, 89. [Google Scholar] [CrossRef]
- Alzahri, R.Y.; Al-Ghamdi, F.A.; Al-Harbi, S.S. Immunological and Histological Studies of Different Concentrations of Rosmarinus officinalis and Thymus vulgaris Extracts on Thymus Gland of Chick Embryos. Toxics 2023, 11, 625. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Peng, C.H.; Chyau, C.C.; Lin, Y.C.; Wang, H.E.; Peng, R.Y. Low-density lipoprotein, collagen, and thrombin models reveal that Rosmarinus officinalis L. exhibits potent antiglycative effects. J. Agric. Food Chem. 2007, 55, 2884–2891. [Google Scholar] [CrossRef]
- Karthik, D.; Viswanathan, P.; Anuradha, C.V. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J. Cardiovasc. Pharmacol. 2011, 58, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Durán, R.E.; Medrano-Rodríguez, J.C.; Sánchez-Aguilar, M.; Soria-Castro, E.; Rubio-Ruíz, M.E.; Del Valle-Mondragón, L.; Sánchez-Mendoza, A.; Torres-Narvaéz, J.C.; Pastelín-Hernández, G.; Ibarra-Lara, L. Extracts of Crataegus oxyacantha and Rosmarinus officinalis Attenuate Ischemic Myocardial Damage by Decreasing Oxidative Stress and Regulating the Production of Cardiac Vasoactive Agents. Int. J. Mol. Sci. 2017, 18, 2412. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.; Bak, S.Y.; Yde, C.C.; Jensen, H.M.; Knudsen, T.A.; Bæch-Laursen, C.; Holst, J.J.; Laustsen, C.; Hedemann, M.S. Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet. Metabolites 2023, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Kabubii, Z.N.; Mbaria, J.M.; Mathiu, P.M.; Wanjohi, J.M.; Nyaboga, E.N. Diet Supplementation with Rosemary (Rosmarinus officinalis L.) Leaf Powder Exhibits an Antidiabetic Property in Streptozotocin-Induced Diabetic Male Wistar Rats. Diabetology 2024, 5, 12–25. [Google Scholar] [CrossRef]
- Boo, Y.C. Therapeutic Potential and Mechanisms of Rosmarinic Acid and the Extracts of Lamiaceae Plants for the Treatment of Fibrosis of Various Organs. Antioxidants 2024, 13, 146. [Google Scholar] [CrossRef]
- Del Baño, M.J.; Castillo, J.; Benavente-García, O.; Lorente, J.; Martín-Gil, R.; Acevedo, C.; Alcaraz, M. Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by gamma-rays. J. Agric. Food Chem. 2006, 54, 2064–2068. [Google Scholar] [CrossRef]
- Acharya, G.S.; Goyal, P.K. Role of Rosemary leaves extract against radiation-induced hematological and biochemical alterations in mice. Nucl. Technol. Radiat. Prot. 2008, 23, 72–78. [Google Scholar] [CrossRef]
- Zhaeintan, P.; Nickfarjam, A.; Shams, A.; Abdollahi-Dehkordi, S.; Hamzian, N. Radioprotective Effect of Rosmarinus officinalis L (Rosemary) Essential Oil on Apoptosis, Necrosis and Mitotic Death of Human Peripheral Lymphocytes (PBMCs). J. Biomed. Phys. Eng. 2022, 12, 245–256. [Google Scholar] [CrossRef]
- de Macedo, L.M.; Santos, É.M.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- Ventura-Martínez, R.; Rivero-Osorno, O.; Gómez, C.; González-Trujano, M.E. Spasmolytic activity of Rosmarinus officinalis L. involves calcium channels in the guinea pig ileum. J. Ethnopharmacol. 2011, 137, 1528–1532. [Google Scholar] [CrossRef]
- Sagorchev, P.; Lukanov, J.; Beer, A.M. Investigations into the specific effects of rosemary oil at the receptor level. Phytomedicine 2010, 17, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Zeraat Pisheh, F.; Falah, F.; Sanaei, F.; Vasiee, A.; Zanganeh, H.; Tabatabaee Yazdi, F.; Ibrahim, S.A. The Effect of Plasma-Activated Water Combined with Rosemary Extract (Rosmarinus officinalis L.) on the Physicochemical Properties of Frankfurter Sausage during Storage. Foods 2023, 12, 4022. [Google Scholar] [CrossRef] [PubMed]
- de Lima, A.F.; Leite, R.H.d.L.; Pereira, M.W.F.; Silva, M.R.L.; de Araújo, T.L.A.C.; de Lima Júnior, D.M.; Gomes, M.d.N.B.; Lima, P.d.O. Chitosan Coating with Rosemary Extract Increases Shelf Life and Reduces Water Losses from Beef. Foods 2024, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Raghuwanshi, R.; Masood, M.; Acharya, A.; Jain, S.K. Medicinal plants with acetylcholinesterase inhibitory activity. Rev. Neurosci. 2018, 29, 491–529. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Papakosta-Tsigkri, L.; Lymperopoulou, T.; Mamma, D.; Kekos, D.; Detsi, A. Conventional and Enzyme-Assisted Extraction of Rosemary Leaves (Rosmarinus officinalis L.): Toward a Greener Approach to High Added-Value Extracts. Appl. Sci. 2021, 11, 3724. [Google Scholar] [CrossRef]
- Dhouibi, N.; Manuguerra, S.; Arena, R.; Messina, C.M.; Santulli, A.; Kacem, S.; Dhaouadi, H.; Mahdhi, A. Impact of the Extraction Method on the Chemical Composition and Antioxidant Potency of Rosmarinus officinalis L. Extracts. Metabolites 2023, 13, 290. [Google Scholar] [CrossRef]
- Bencharif-Betina, S.; Benhamed, N.; Benabdallah, A.; Bendif, H.; Benslama, A.; Negro, C.; Plavan, G.; Abd-Elkader, O.H.; De Bellis, L. A Multi-Approach Study of Phytochemicals and Their Effects on Oxidative Stress and Enzymatic Activity of Essential Oil and Crude Extracts of Rosmarinus officinalis. Separations 2023, 10, 394. [Google Scholar] [CrossRef]
- Moliner, C.; López, V.; Barros, L.; Dias, M.I.; Ferreira, I.C.F.R.; Langa, E.; Gómez-Rincón, C. Rosemary Flowers as Edible Plant Foods: Phenolic Composition and Antioxidant Properties in Caenorhabditis elegans. Antioxidants 2020, 9, 811. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Kotsou, K.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Optimization of Four Different Rosemary Extraction Techniques Using Plackett–Burman Design and Comparison of Their Antioxidant Compounds. Int. J. Mol. Sci. 2024, 25, 7708. [Google Scholar] [CrossRef] [PubMed]
- Kamli, M.R.; Sharaf, A.A.M.; Sabir, J.S.M.; Rather, I.A. Phytochemical Screening of Rosmarinus officinalis L. as a Potential Anticholinesterase and Antioxidant–Medicinal Plant for Cognitive Decline Disorders. Plants 2022, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Chapter 15—Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants: Isolation, Purification and Extract Preparation, 2nd ed.; Watson, R.R., Ed.; Academic Press–Elsevier: London, UK, 2019; pp. 243–259. [Google Scholar] [CrossRef]
- Sharma, Y.; Fagan, J.; Schaefer, J. In vitro Screening for Acetylcholinesterase Inhibition and Antioxidant Potential in Different Extracts of Sage (Salvia officinalis L.) and Rosemary (Rosmarinus officinalis L.). J. Biol. Act. Prod. Nat. 2020, 10, 59–69. [Google Scholar] [CrossRef]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of UHPLC–ESI–QTOF–MS in Phytochemical Profiling of Sage (Salvia officinalis) and Rosemary (Rosmarinus officinalis). Planta Med. Int. Open 2020, 7, e133–e144. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant HPTLC–DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents upon Thermal Exposure. Foods 2021, 10, 357. [Google Scholar] [CrossRef]
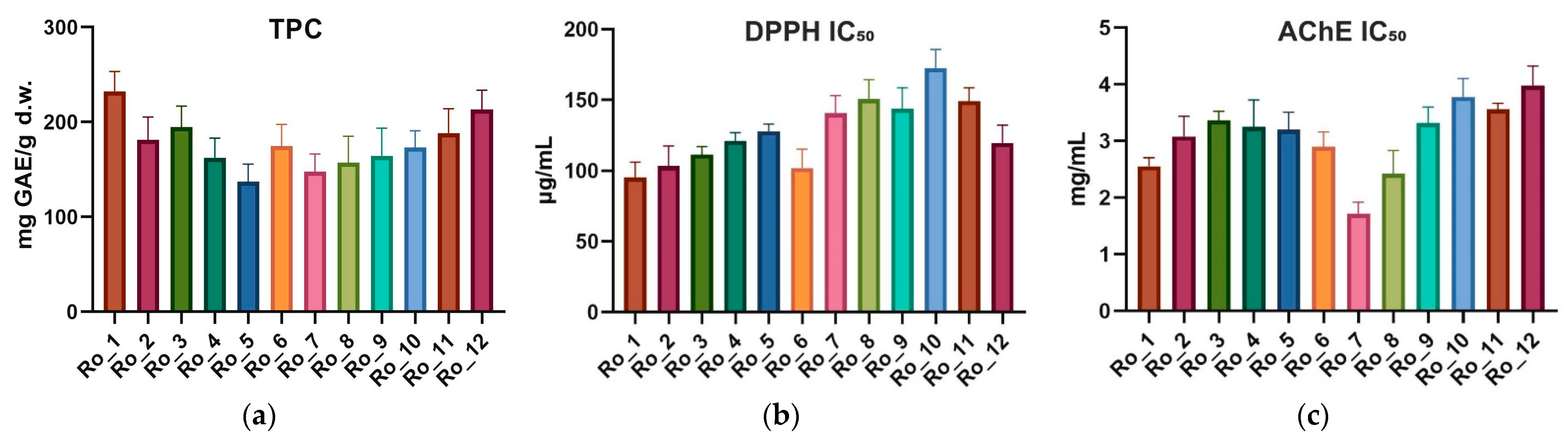
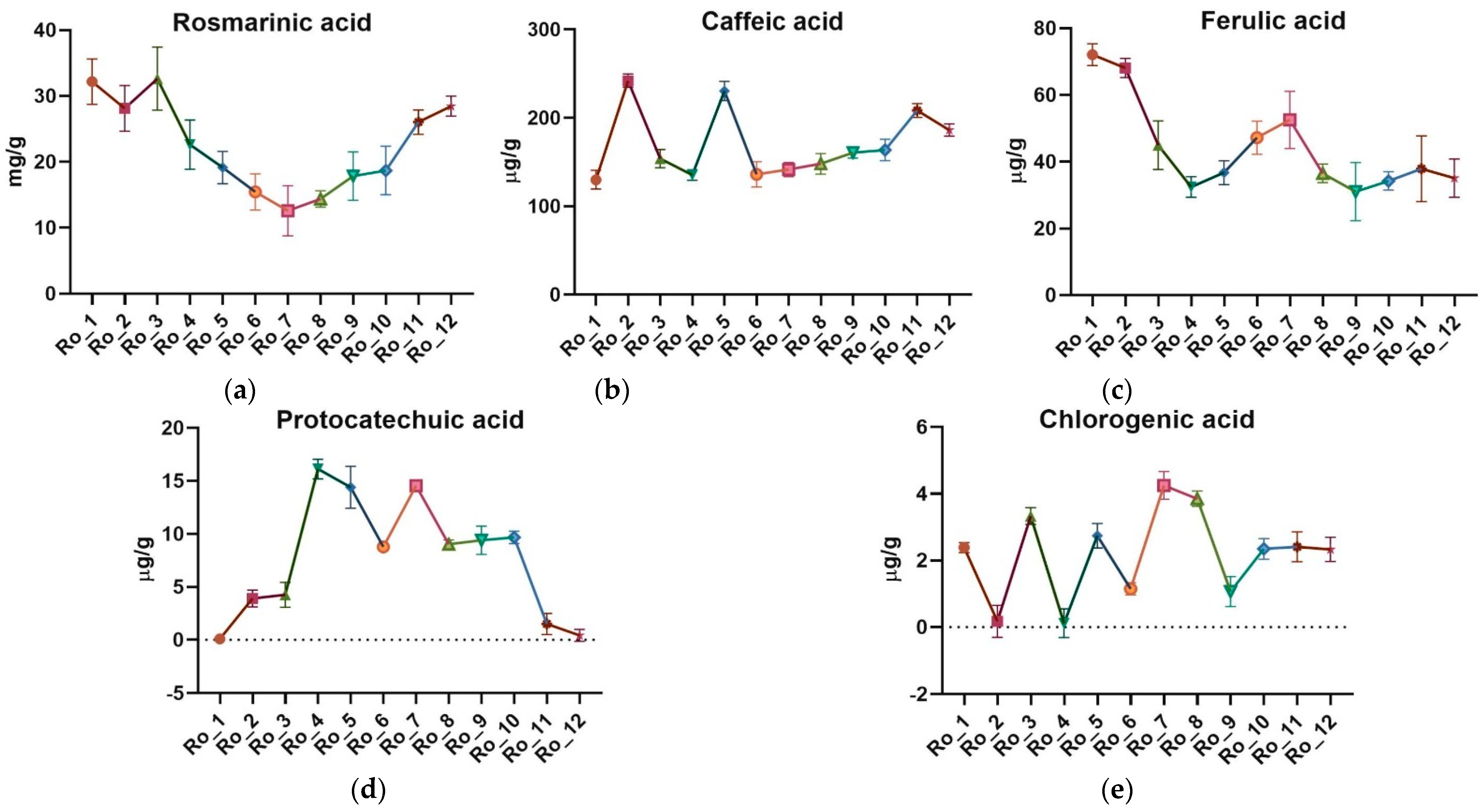
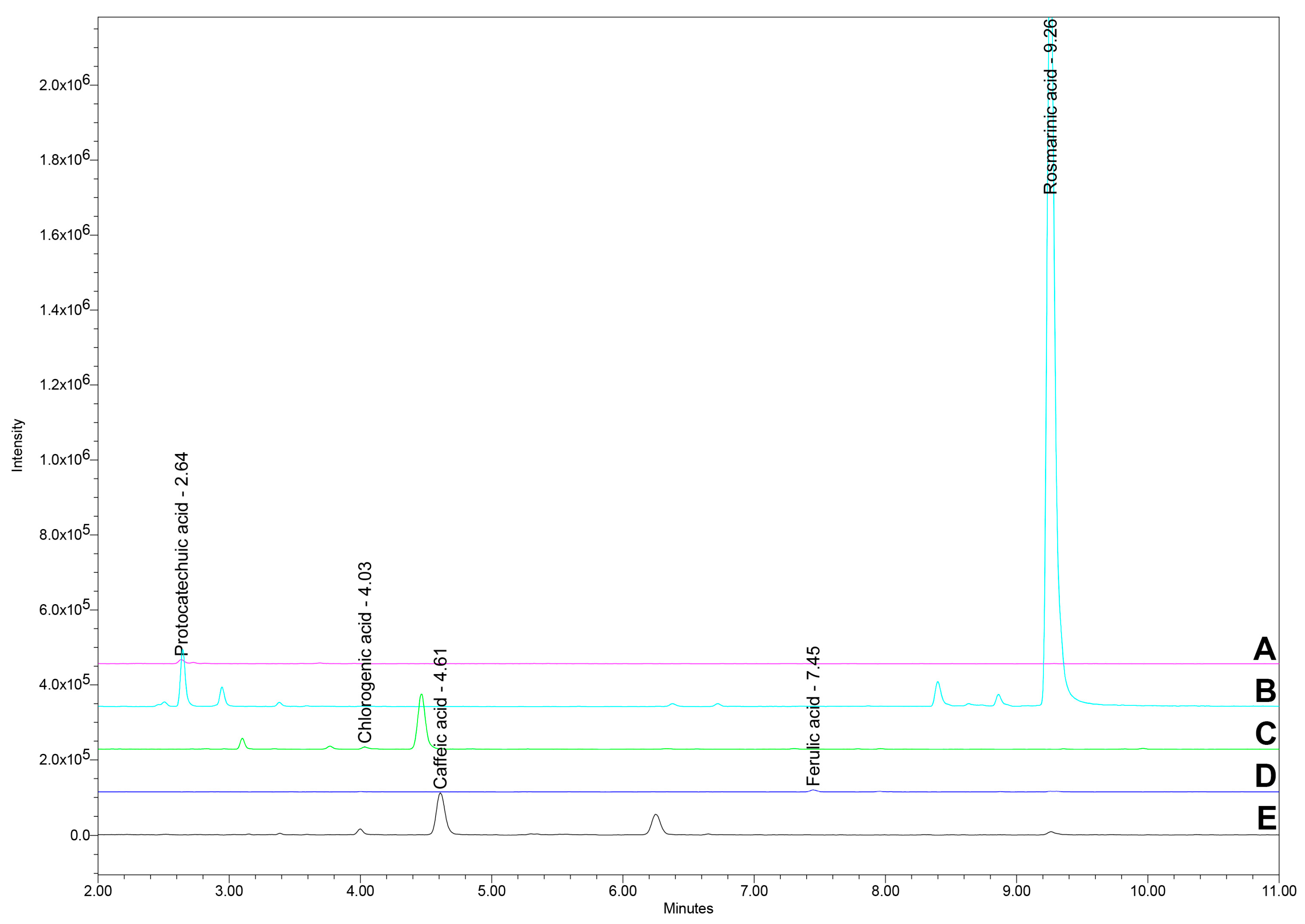
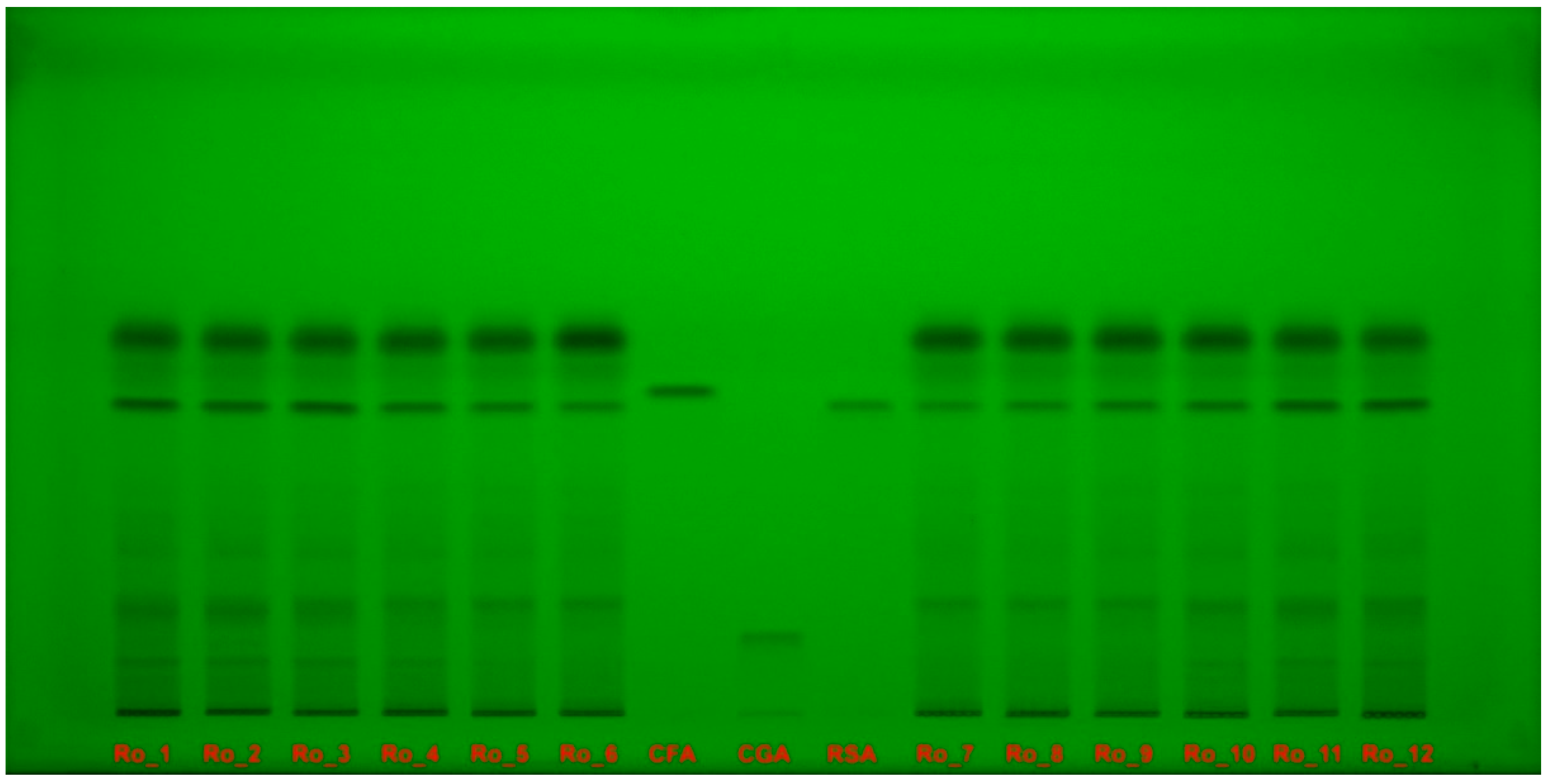
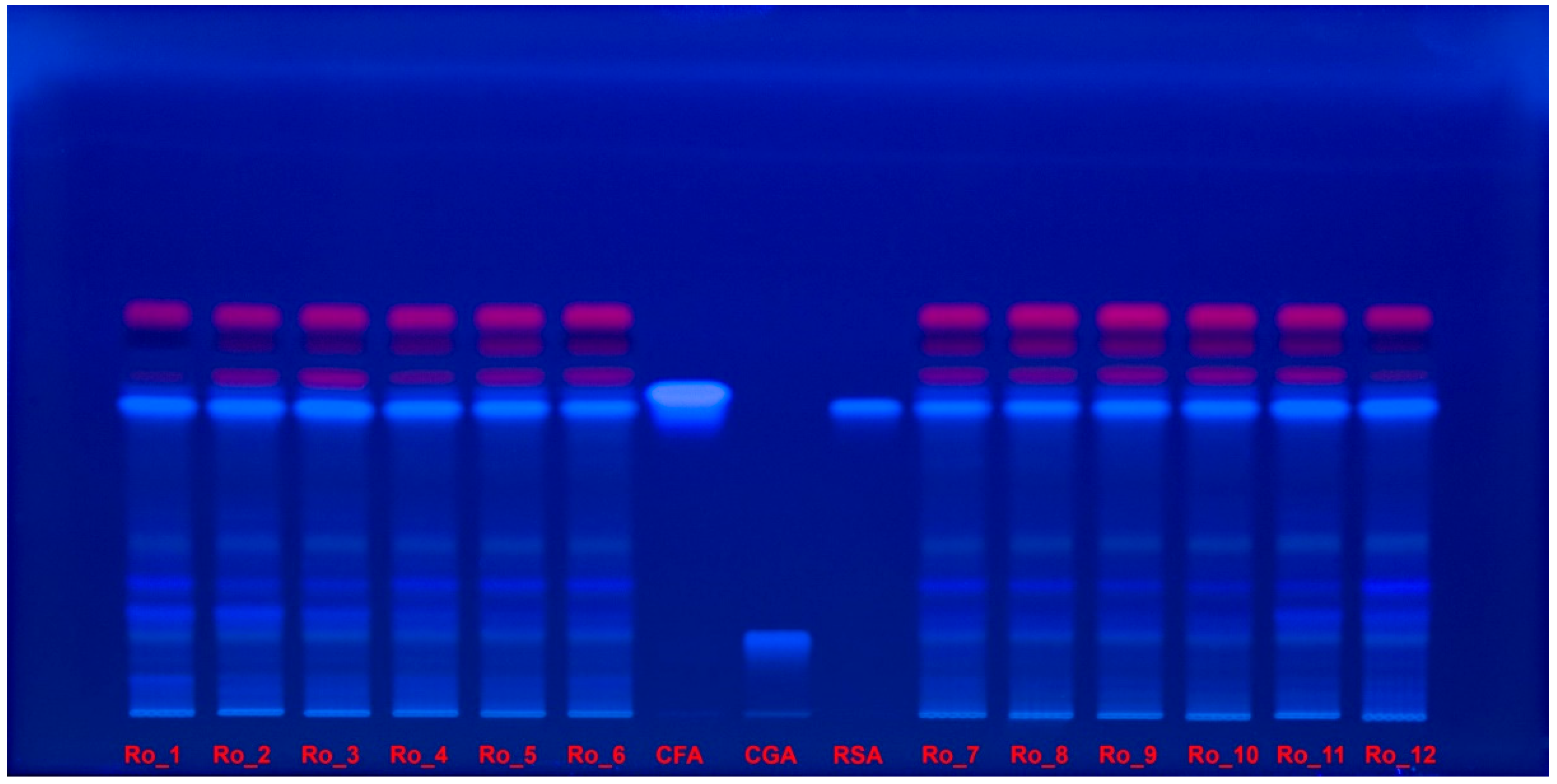
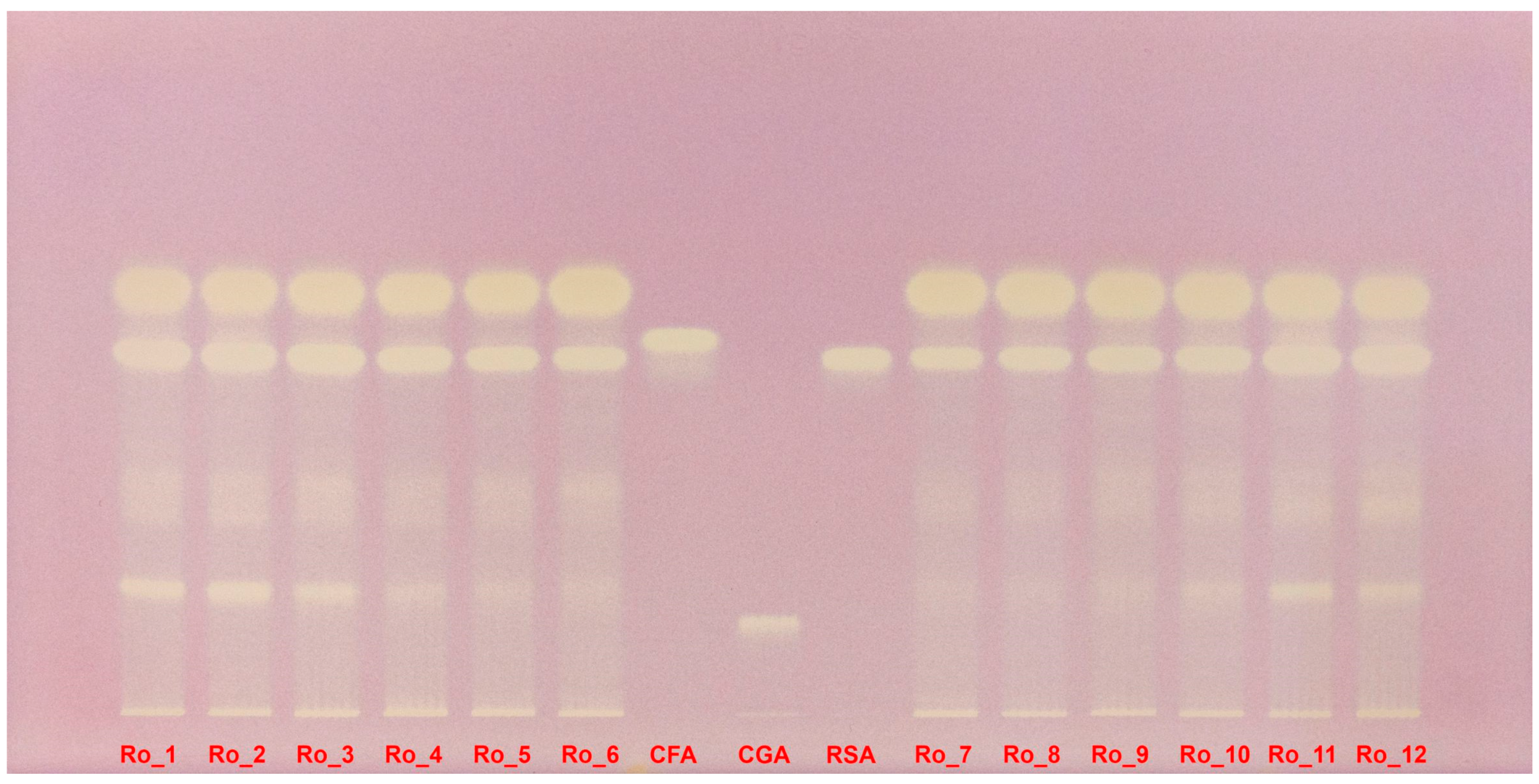
| Sample | TPC (mg GAE/g d.w.) | DPPH IC50 (μg/mL) | AChE IC50 (mg/mL) |
|---|---|---|---|
| Ro_1 (February 2022) | 232.3 ± 21.0 | 95.32 ± 10.68 | 2.558 ± 0.147 |
| Ro_2 (March 2022) | 181.0 ± 24.3 | 103.30 ± 14.26 | 3.083 ± 0.356 |
| Ro_3 (April 2022) | 194.8 ± 22.1 | 111.40 ± 5.71 | 3.370 ± 0.157 |
| Ro_4 (May 2022) | 162.3 ± 20.9 | 121.10 ± 5.87 | 3.250 ± 0.478 |
| Ro_5 (June 2022) | 137.2 ± 18.5 | 127.90 ± 5.20 | 3.200 ± 0.309 |
| Ro_6 (July 2022) | 174.8 ± 22.9 | 101.90 ± 13.33 | 2.900 ± 0.266 |
| Ro_7 (August 2022) | 147.6 ± 18.8 | 140.50 ± 12.78 | 1.716 ± 0.206 |
| Ro_8 (September 2022) | 157.2 ± 27.8 | 150.70 ± 13.70 | 2.425 ± 0.410 |
| Ro_9 (October 2022) | 164.4 ± 29.3 | 143.90 ± 14.79 | 3.320 ± 0.282 |
| Ro_10 (November 2022) | 173.1 ± 17.7 | 172.80 ± 12.99 | 3.780 ± 0.327 |
| Ro_11 (December 2022) | 188.4 ± 25.8 | 149.20 ± 9.61 | 3.560 ± 0.108 |
| Ro_12 (January 2023) | 213.0 ± 20.6 | 119.50 ± 12.81 | 3.980 ± 0.347 |
| Sample | Rosmarinic Acid (mg/g) | Caffeic Acid (μg/g) | Ferulic Acid (μg/g) | Protocatechuic Acid (μg/g) | Chlorogenic Acid (μg/g) |
|---|---|---|---|---|---|
| Ro_1 (February 2022) | 32.179 ± 3.448 | 129.960 ± 10.666 | 72.079 ± 4.907 | 0.100 ± 0.069 | 2.387 ± 0.150 |
| Ro_2 (March 2022) | 28.130 ± 3.468 | 241.906 ± 7.654 | 68.100 ± 3.419 | 3.904 ± 0.791 | 0.178 ± 0.479 |
| Ro_3 (April 2022) | 32.629 ± 4.775 | 153.950 ± 10.232 | 44.962 ± 3.957 | 4.251 ± 1.183 | 3.333 ± 0.251 |
| Ro_4 (May 2022) | 22.624 ± 3.727 | 135.378 ± 5.939 | 32.446 ± 1.157 | 16.126 ± 0.933 | 0.118 ± 0.043 |
| Ro_5 (June 2022) | 19.138 ± 2.438 | 230.384 ± 10.759 | 36.758 ± 2.131 | 14.400 ± 1.978 | 2.743 ± 0.365 |
| Ro_6 (July 2022) | 15.435 ± 2.748 | 136.149 ± 14.293 | 47.211 ± 1.481 | 8.783 ± 0.294 | 1.153 ± 0.184 |
| Ro_7 (August 2022) | 12.585 ± 3.791 | 141.502 ± 8.186 | 52.560 ± 2.185 | 14.554 ± 0.497 | 4.251 ± 0.416 |
| Ro_8 (September 2022) | 14.392 ± 1.241 | 148.004 ± 11.674 | 36.522 ± 1.475 | 9.028 ± 0.406 | 3.853 ± 0.228 |
| Ro_9 (October 2022) | 17.857 ± 3.667 | 160.610 ± 6.318 | 31.060 ± 2.272 | 9.407 ± 1.341 | 1.070 ± 0.446 |
| Ro_10 (November 2022) | 18.691 ± 3.683 | 163.689 ± 12.163 | 34.259 ± 2.657 | 9.678 ± 0.581 | 2.347 ± 0.312 |
| Ro_11 (December 2022) | 26.042 ± 1.842 | 208.323 ± 7.894 | 37.923 ± 1.257 | 1.502 ± 0.986 | 2.409 ± 0.447 |
| Ro_12 (January 2023) | 28.460 ± 1.516 | 186.245 ± 6.832 | 35.116 ± 3.770 | 0.435 ± 0.056 | 2.332 ± 0.362 |
| Phenolic Compound | TPC [mg GAE/g d.w.] | DPPH IC50 [μg/mL] | AChE IC50 [mg/mL] |
|---|---|---|---|
| Rosmarinic acid [mg/g] | 0.801 | −0.533 | 0.435 |
| Caffeic acid [μg/g] | −0.140 | 0.030 | 0.392 |
| Ferulic acid [μg/g] | 0.447 | −0.642 | −0.480 |
| Protocatechuic acid [μg/g] | −0.884 | 0.325 | −0.334 |
| Chlorogenic acid [μg/g] | −0.097 | 0.353 | −0.405 |
| TPC [mg GAE/g d.w.] | −0.469 | 0.293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejenaru, L.E.; Biţă, A.; Mogoşanu, G.D.; Segneanu, A.-E.; Radu, A.; Ciocîlteu, M.V.; Bejenaru, C. Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora. Molecules 2024, 29, 4438. https://doi.org/10.3390/molecules29184438
Bejenaru LE, Biţă A, Mogoşanu GD, Segneanu A-E, Radu A, Ciocîlteu MV, Bejenaru C. Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora. Molecules. 2024; 29(18):4438. https://doi.org/10.3390/molecules29184438
Chicago/Turabian StyleBejenaru, Ludovic Everard, Andrei Biţă, George Dan Mogoşanu, Adina-Elena Segneanu, Antonia Radu, Maria Viorica Ciocîlteu, and Cornelia Bejenaru. 2024. "Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora" Molecules 29, no. 18: 4438. https://doi.org/10.3390/molecules29184438
APA StyleBejenaru, L. E., Biţă, A., Mogoşanu, G. D., Segneanu, A.-E., Radu, A., Ciocîlteu, M. V., & Bejenaru, C. (2024). Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora. Molecules, 29(18), 4438. https://doi.org/10.3390/molecules29184438







