p38α Mitogen-Activated Protein Kinase—An Emerging Drug Target for the Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Overview of Mitogen-Activated Protein Kinase Signaling
3. Characteristics of p38α MAPK Signaling Cascade
4. Physiological Functions of p38α MAPK Signaling in Central Nervous System
5. Involvement of p38α MAPK in the Progression of Neuroinflammatory Process in AD
6. The Role of p38α MAPK Pathway in the Generation and Deposition of Aβ Plaques
7. p38α MAPK in the Phosphorylation of Tau Protein and the Formation of Neurofibrillary Tangles
8. Involvement of p38α MAPK in Oxidative Stress-Induced Damage in AD
9. Impact of p38 MAPK on Cholinergic Neurotransmission in the Brain
10. Available Treatment for AD
11. Drug Candidates for AD—The Past and the Future
11.1. Disease-Modifying Therapeutics
11.1.1. DMTs—Focus on Neuroinflammation
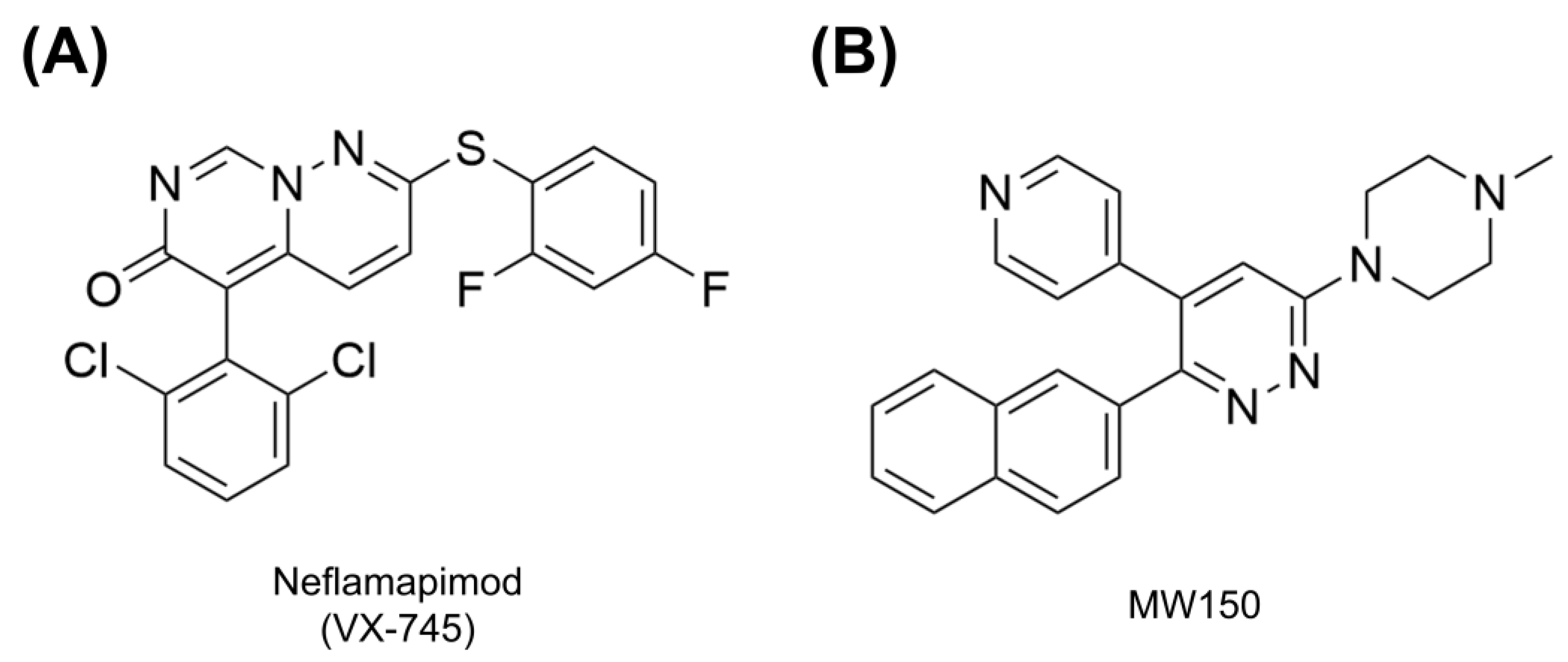
Neflamapimod
MW150
11.1.2. p38α MAPK Inhibitors—Safety Concerns
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ASK-1 | apoptosis signal-regulating kinase 1 |
| α7 nAChR | α7 nicotinic acetylcholine receptor |
| Aβ | amyloid β |
| BACE1 | β-site APP-cleaving enzyme (β-secretase) |
| BBB | Blood–brain barrier |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL5 | C-C motif chemokine ligand 5 |
| CNS | central nervous system |
| CXCL-1 | C-X-C motif chemokine ligand 1 |
| DMTs | disease-modifying therapeutics |
| GSK-3β | glycogen synthase kinase-3β |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| LTD | long-term depression |
| LTP | long-term potentiation |
| MAP2K | MAPK kinase |
| MAP3K | MAPK kinase anchor |
| MAPK | Mitogen-activated protein kinase |
| MKK3 | MAPK kinase 3 |
| MKK4 | MAPK kinase 4 |
| MKK6 | MAPK kinase 6 |
| NFTs | neurofibrillary tangles |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 inflammasome |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| OS | oxidative stress |
| PGE2 | prostaglandin E2 |
| pJNK | phospho c-Jun N-Terminal Kinase |
| RAGE | receptor for advanced glycation end product |
| ROS | reactive oxygen species |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor α |
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, N.-J. Recent Advances in the Inhibition of P38 MAPK as a Potential Strategy for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [PubMed]
- Doroszkiewicz, J.; Mroczko, P.; Kulczyńska-Przybik, A. Inflammation in the CNS: Understanding Various Aspects of the Pathogenesisof Alzheimer’s Disease. Curr. Alzheimer Res. 2022, 19, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s Disease: Current Evidence and Future Directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Rajesh, Y.; Kanneganti, T.-D. Innate Immune Cell Death in Neuroinflammation and Alzheimer’s Disease. Cells 2022, 11, 1885. [Google Scholar] [CrossRef]
- Cummings, J.L.; Osse, A.M.L.; Kinney, J.W. Alzheimer’s Disease: Novel Targets and Investigational Drugs for Disease Modification. Drugs 2023, 83, 1387–1408. [Google Scholar] [CrossRef]
- Colié, S.; Sarroca, S.; Palenzuela, R.; Garcia, I.; Matheu, A.; Corpas, R.; Dotti, C.G.; Esteban, J.A.; Sanfeliu, C.; Nebreda, A.R. Neuronal P38α Mediates Synaptic and Cognitive Dysfunction in an Alzheimer’s Mouse Model by Controlling β-Amyloid Production. Sci. Rep. 2017, 7, 45306. [Google Scholar] [CrossRef]
- Munoz, L.; Ammit, A.J. Targeting P38 MAPK Pathway for the Treatment of Alzheimer’s Disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. P38 MAP-Kinases Pathway Regulation, Function and Role in Human Diseases. Biochim. Biophys. Acta BBA Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Craig, E.A.; Stevens, M.V.; Vaillancourt, R.R.; Camenisch, T.D. MAP3Ks as Central Regulators of Cell Fate during Development. Dev. Dyn. 2008, 237, 3102–3114. [Google Scholar] [CrossRef]
- Krishna, M.; Narang, H. The Complexity of Mitogen-Activated Protein Kinases (MAPKs) Made Simple. Cell Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian Mitogen-Activated Protein Kinase Signal Transduction Pathways Activated by Stress and Inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Yong, H.-Y.; Koh, M.-S.; Moon, A. The P38 MAPK Inhibitors for the Treatment of Inflammatory Diseases and Cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple Sclerosis: Neuroimmune Crosstalk and Therapeutic Targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The Role of Inflammation in Epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.; Katzman, M.A. Examining the Role of Neuroinflammation in Major Depression. Psychiatry Res. 2015, 229, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Walss-Bass, C.; Bauer, M.E.; Teixeira, A.L. Revisiting Inflammation in Bipolar Disorder. Pharmacol. Biochem. Behav. 2019, 177, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, I.A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Brooks, D.J.; Edison, P. The Role of Neuroinflammation in Dementias. Curr. Neurol. Neurosci. Rep. 2015, 15, 17. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.-D.; Bibbs, L.; Ulevitch, R.J. A MAP Kinase Targeted by Endotoxin and Hyperosmolarity in Mammalian Cells. Science 1994, 265, 808–811. [Google Scholar] [CrossRef]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and Functions of P38 MAPK Signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef]
- Freshney, N.W.; Rawlinson, L.; Guesdon, F.; Jones, E.; Cowley, S.; Hsuan, J.; Saklatvala, J. Interleukin-1 Activates a Novel Protein Kinase Cascade That Results in the Phosphorylation of Hsp27. Cell 1994, 78, 1039–1049. [Google Scholar] [CrossRef]
- Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Land Vatter, S.W.; et al. A Protein Kinase Involved in the Regulation of Inflammatory Cytokine Biosynthesis. Nature 1994, 372, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.; Cohen, P.; Trigon, S.; Morange, M.; Alonso-Llamazares, A.; Zamanillo, D.; Hunt, T.; Nebreda, A.R. A Novel Kinase Cascade Triggered by Stress and Heat Shock That Stimulates MAPKAP Kinase-2 and Phosphorylation of the Small Heat Shock Proteins. Cell 1994, 78, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Raingeaud, J.; Gupta, S.; Rogers, J.S.; Dickens, M.; Han, J.; Ulevitch, R.J.; Davis, R.J. Pro-Inflammatory Cytokines and Environmental Stress Cause P38 Mitogen-Activated Protein Kinase Activation by Dual Phosphorylation on Tyrosine and Threonine. J. Biol. Chem. 1995, 270, 7420–7426. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.E.; Voigt, A.; Tollenaere, M.A.X.; Sahu, S.K.; Juretschke, T.; Kreim, N.; Mailand, N.; Choudhary, C.; Bekker-Jensen, S.; Akutsu, M.; et al. P38-MK2 Signaling Axis Regulates RNA Metabolism after UV-Light-Induced DNA Damage. Nat. Commun. 2018, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, C.S.; Koerner, T.J.; Jansen, M.M.; Hamilton, T.A. Characterization of Lipopolysaccharide-Induced Macrophage Gene Expression. J. Immunol. Baltim. Md 1950 1988, 140, 3640–3645. [Google Scholar] [CrossRef]
- Goedert, M.; Hasegawa, M.; Jakes, R.; Lawler, S.; Cuenda, A.; Cohen, P. Phosphorylation of Microtubule-associated Protein Tau by Stress-activated Protein Kinases. FEBS Lett. 1997, 409, 57–62. [Google Scholar] [CrossRef]
- Parker, C.G.; Hunt, J.; Diener, K.; McGinley, M.; Soriano, B.; Keesler, G.A.; Bray, J.; Yao, Z.; Wang, X.S.; Kohno, T.; et al. Identification of Stathmin as a Novel Substrate for P38 Delta. Biochem. Biophys. Res. Commun. 1998, 249, 791–796. [Google Scholar] [CrossRef]
- Kuma, Y.; Campbell, D.G.; Cuenda, A. Identification of Glycogen Synthase as a New Substrate for Stress-Activated Protein Kinase 2b/P38beta. Biochem. J. 2004, 379, 133–139. [Google Scholar] [CrossRef]
- Sabio, G.; Arthur, J.S.C.; Kuma, Y.; Peggie, M.; Carr, J.; Murray-Tait, V.; Centeno, F.; Goedert, M.; Morrice, N.A.; Cuenda, A. P38γ Regulates the Localisation of SAP97 in the Cytoskeleton by Modulating Its Interaction with GKAP. EMBO J. 2005, 24, 1134–1145. [Google Scholar] [CrossRef]
- Doza, Y.N.; Cueda, A.; Thomas, G.M.; Cohen, P.; Nebreda, A.R. Activation of the MAP Kinase Homologue RK Requires the Phosphorylation of Thr-180 and Tyr-182 and Both Residues Are Phosphorylated in Chemically Stressed KB Cells. FEBS Lett. 1995, 364, 223–228. [Google Scholar] [CrossRef]
- Alonso, G.; Ambrosino, C.; Jones, M.; Nebreda, A.R. Differential Activation of P38 Mitogen-Activated Protein Kinase Isoforms Depending on Signal Strength. J. Biol. Chem. 2000, 275, 40641–40648. [Google Scholar] [CrossRef] [PubMed]
- Brancho, D.; Tanaka, N.; Jaeschke, A.; Ventura, J.-J.; Kelkar, N.; Tanaka, Y.; Kyuuma, M.; Takeshita, T.; Flavell, R.A.; Davis, R.J. Mechanism of P38 MAP Kinase Activation in Vivo. Genes Dev. 2003, 17, 1969–1978. [Google Scholar] [CrossRef]
- Yasuda, S.; Sugiura, H.; Tanaka, H.; Takigami, S.; Yamagata, K. P38 MAP Kinase Inhibitors as Potential Therapeutic Drugs for Neural Diseases. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Gram, H.; Di Padova, F.; Huang, B.; New, L.; Ulevitch, R.J.; Luo, Y.; Han, J. MAPKK-Independent Activation of P38α Mediated by TAB1-Dependent Autophosphorylation of P38α. Science 2002, 295, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.M.; Mittelstadt, P.R.; Guszczynski, T.; Copeland, T.D.; Yamaguchi, H.; Appella, E.; Fornace, A.J.; Ashwell, J.D. Alternative P38 Activation Pathway Mediated by T Cell Receptor–Proximal Tyrosine Kinases. Nat. Immunol. 2005, 6, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Stefanoska, K.; Bertz, J.; Volkerling, A.M.; Van Der Hoven, J.; Ittner, L.M.; Ittner, A. Neuronal MAP Kinase P38α Inhibits C-Jun N-Terminal Kinase to Modulate Anxiety-Related Behaviour. Sci. Rep. 2018, 8, 14296. [Google Scholar] [CrossRef]
- Ittner, A.; Chua, S.W.; Bertz, J.; Volkerling, A.; Van Der Hoven, J.; Gladbach, A.; Przybyla, M.; Bi, M.; Van Hummel, A.; Stevens, C.H.; et al. Site-Specific Phosphorylation of Tau Inhibits Amyloid-β Toxicity in Alzheimer’s Mice. Science 2016, 354, 904–908. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Medina, I.; Krapivinsky, L.; Gapon, S.; Clapham, D.E. SynGAP-MUPP1-CaMKII Synaptic Complexes Regulate P38 MAP Kinase Activity and NMDA Receptor- Dependent Synaptic AMPA Receptor Potentiation. Neuron 2004, 43, 563–574. [Google Scholar] [CrossRef]
- Wittmack, E.K.; Rush, A.M.; Hudmon, A.; Waxman, S.G.; Dib-Hajj, S.D. Voltage-Gated Sodium Channel Na v 1.6 Is Modulated by P38 Mitogen-Activated Protein Kinase. J. Neurosci. 2005, 25, 6621–6630. [Google Scholar] [CrossRef]
- Yasuda, S.; Tanaka, H.; Sugiura, H.; Okamura, K.; Sakaguchi, T.; Tran, U.; Takemiya, T.; Mizoguchi, A.; Yagita, Y.; Sakurai, T.; et al. Activity-Induced Protocadherin Arcadlin Regulates Dendritic Spine Number by Triggering N-Cadherin Endocytosis via TAO2β and P38 MAP Kinases. Neuron 2007, 56, 456–471. [Google Scholar] [CrossRef]
- Ackerley, S.; Grierson, A.J.; Banner, S.; Perkinton, M.S.; Brownlees, J.; Byers, H.L.; Ward, M.; Thornhill, P.; Hussain, K.; Waby, J.S.; et al. P38α Stress-Activated Protein Kinase Phosphorylates Neurofilaments and Is Associated with Neurofilament Pathology in Amyotrophic Lateral Sclerosis. Mol. Cell Neurosci. 2004, 26, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Sama, R.R.K.; Fallini, C.; Gatto, R.; McKeon, J.E.; Song, Y.; Rotunno, M.S.; Penaranda, S.; Abdurakhmanov, I.; Landers, J.E.; Morfini, G.; et al. ALS-Linked FUS Exerts a Gain of Toxic Function Involving Aberrant P38 MAPK Activation. Sci. Rep. 2017, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Bachstetter, A.D.; Van Eldik, L.J. Inhibition of Neuronal P38α, but Not P38β MAPK, Provides Neuroprotection Against Three Different Neurotoxic Insults. J. Mol. Neurosci. 2015, 55, 509–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, G.; Bhat, N.R. P38α MAP Kinase Mediates Hypoxia-Induced Motor Neuron Cell Death: A Potential Target of Minocycline’s Neuroprotective Action. Neurochem. Res. 2007, 32, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Loy, B.; Apostolova, G.; Dorn, R.; McGuire, V.A.; Arthur, J.S.C.; Dechant, G. P38α and P38β Mitogen-Activated Protein Kinases Determine Cholinergic Transdifferentiation of Sympathetic Neurons. J. Neurosci. 2011, 31, 12059–12067. [Google Scholar] [CrossRef]
- Asih, P.R.; Prikas, E.; Stefanoska, K.; Tan, A.R.P.; Ahel, H.I.; Ittner, A. Functions of P38 MAP Kinases in the Central Nervous System. Front. Mol. Neurosci. 2020, 13, 570586. [Google Scholar] [CrossRef]
- Piao, C.S.; Che, Y.; Han, P.-L.; Lee, J.-K. Delayed and Differential Induction of P38 MAPK Isoforms in Microglia and Astrocytes in the Brain after Transient Global Ischemia. Mol. Brain Res. 2002, 107, 137–144. [Google Scholar] [CrossRef]
- Lo, U.; Selvaraj, V.; Plane, J.M.; Chechneva, O.V.; Otsu, K.; Deng, W. P38α (MAPK14) Critically Regulates the Immunological Response and the Production of Specific Cytokines and Chemokines in Astrocytes. Sci. Rep. 2014, 4, 7405. [Google Scholar] [CrossRef]
- Lee, Y.B.; Schrader, J.W.; Kim, S.U. P38 MAP KINASE REGULATES TNF-α PRODUCTION IN HUMAN ASTROCYTES AND MICROGLIA BY MULTIPLE MECHANISMS. Cytokine 2000, 12, 874–880. [Google Scholar] [CrossRef]
- Lee, J.; Shin, J.-S.; Park, J.Y.; Kwon, D.; Choi, S.-J.; Kim, S.-J.; Choi, I.-H. P38 Mitogen-activated Protein Kinase Modulates Expression of Tumor Necrosis Factor-related Apoptosis-inducing Ligand Induced by Interferon-γ in Fetal Brain Astrocytes. J. Neurosci. Res. 2003, 74, 884–890. [Google Scholar] [CrossRef]
- Nahirnyj, A.; Livne-Bar, I.; Guo, X.; Sivak, J.M. ROS Detoxification and Proinflammatory Cytokines Are Linked by P38 MAPK Signaling in a Model of Mature Astrocyte Activation. PLoS ONE 2013, 8, e83049. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Cuartero, M.I.; Palenzuela, R.; Draffin, J.E.; Konomi, A.; Serra, I.; Colié, S.; Castaño-Castaño, S.; Hasan, M.T.; Nebreda, Á.R.; et al. Astrocytic P38α MAPK Drives NMDA Receptor-Dependent Long-Term Depression and Modulates Long-Term Memory. Nat. Commun. 2019, 10, 2968. [Google Scholar] [CrossRef] [PubMed]
- Morganti, J.M.; Goulding, D.S.; Van Eldik, L.J. Deletion of P38α MAPK in Microglia Blunts Trauma-Induced Inflammatory Responses in Mice. J. Neuroinflammation 2019, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.-J.; Coley, W.; Cheng, Y.; Gallo, V. Mechanisms of Regulation of Oligodendrocyte Development by P38 Mitogen-Activated Protein Kinase. J. Neurosci. 2010, 30, 11011–11027. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.L.; Fang, J.; Kennedy, T.E.; Almazan, G.; Antel, J.P. Role of p38MAPK in S1P Receptor-Mediated Differentiation of Human Oligodendrocyte Progenitors: Effects of S1P Receptor Modulators on Human OPCs. Glia 2014, 62, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-H.; Biswas, S.; Selvaraj, V.; Liu, X.-B.; Sohn, J.; Jiang, P.; Chen, C.; Chmilewsky, F.; Marzban, H.; Horiuchi, M.; et al. The P38α Mitogen-Activated Protein Kinase Is a Key Regulator of Myelination and Remyelination in the CNS. Cell Death Dis. 2015, 6, e1748. [Google Scholar] [CrossRef]
- Marziali, L.N.; Hwang, Y.; Palmisano, M.; Cuenda, A.; Sim, F.J.; Gonzalez, A.; Volsko, C.; Dutta, R.; Trapp, B.D.; Wrabetz, L.; et al. P38γ MAPK Delays Myelination and Remyelination and Is Abundant in Multiple Sclerosis Lesions. Brain 2024, 147, 1871–1886. [Google Scholar] [CrossRef]
- Han, J.; Wu, J.; Silke, J. An Overview of Mammalian P38 Mitogen-Activated Protein Kinases, Central Regulators of Cell Stress and Receptor Signaling. F1000Research 2020, 9, 653. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, N.-R.; Gee, M.S.; Song, C.W.; Lee, S.J.; Lee, S.-K.; Lee, Y.; Kim, H.J.; Lee, J.K.; Inn, K.-S.; et al. Chemical Knockdown of Phosphorylated P38 Mitogen-Activated Protein Kinase (MAPK) as a Novel Approach for the Treatment of Alzheimer′s Disease. ACS Cent. Sci. 2023, 9, 417–426. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Xing, B.; De Almeida, L.; Dimayuga, E.R.; Watterson, D.M.; Van Eldik, L.J. Microglial P38α MAPK Is a Key Regulator of Proinflammatory Cytokine Up-Regulation Induced by Toll-like Receptor (TLR) Ligands or Beta-Amyloid (Aβ). J. Neuroinflammation 2011, 8, 79. [Google Scholar] [CrossRef]
- Yang, I.; Han, S.J.; Kaur, G.; Crane, C.; Parsa, A.T. The Role of Microglia in Central Nervous System Immunity and Glioma Immunology. J. Clin. Neurosci. 2010, 17, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial Activation and Chronic Neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Neuroinflammation for Alzheimer’s Disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Rowe, R.K.; Kaneko, M.; Goulding, D.; Lifshitz, J.; Van Eldik, L.J. The P38α MAPK Regulates Microglial Responsiveness to Diffuse Traumatic Brain Injury. J. Neurosci. 2013, 33, 6143–6153. [Google Scholar] [CrossRef]
- Xing, B.; Bachstetter, A.D.; Van Eldik, L.J. Microglial P38α MAPK Is Critical for LPS-Induced Neuron Degeneration, through a Mechanism Involving TNFα. Mol. Neurodegener. 2011, 6, 84. [Google Scholar] [CrossRef]
- Chen, J.; Mao, K.; Yu, H.; Wen, Y.; She, H.; Zhang, H.; Liu, L.; Li, M.; Li, W.; Zou, F. P38-TFEB Pathways Promote Microglia Activation through Inhibiting CMA-Mediated NLRP3 Degradation in Parkinson’s Disease. J. Neuroinflammation 2021, 18, 295. [Google Scholar] [CrossRef]
- He, Y.; She, H.; Zhang, T.; Xu, H.; Cheng, L.; Yepes, M.; Zhao, Y.; Mao, Z. P38 MAPK Inhibits Autophagy and Promotes Microglial Inflammatory Responses by Phosphorylating ULK1. J. Cell Biol. 2018, 217, 315–328. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Parpura, V.; Li, B.; Scuderi, C. Astrocytes: The Housekeepers and Guardians of the CNS. In Astrocytes in Psychiatric Disorders; Li, B., Parpura, V., Verkhratsky, A., Scuderi, C., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, Switzerland, 2021; Volume 26, pp. 21–53. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Revuelta, M.; Elicegui, A.; Scheuer, T.; Endesfelder, S.; Bührer, C.; Moreno-Cugnon, L.; Matheu, A.; Schmitz, T. In Vitro P38MAPK Inhibition in Aged Astrocytes Decreases Reactive Astrocytes, Inflammation and Increases Nutritive Capacity after Oxygen-Glucose Deprivation. Aging 2021, 13, 6346–6358. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and Versatility of P38 Kinase Signalling in Health and Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Schnöder, L.; Hao, W.; Qin, Y.; Liu, S.; Tomic, I.; Liu, X.; Fassbender, K.; Liu, Y. Deficiency of Neuronal P38α MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. J. Biol. Chem. 2016, 291, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.-Y. γ-Secretase in Alzheimer’s Disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R. When Good Kinases Go Rogue: GSK3, P38 MAPK and CDKs as Therapeutic Targets for Alzheimer’s and Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 5911. [Google Scholar] [CrossRef]
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of P38 MAPK in Synaptic Function and Dysfunction. Int. J. Mol. Sci. 2020, 21, 5624. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A.; Zheng, N.; Nael, R.; Robinson, K.A.; Nguyen, X.; Pye, Q.N.; Stewart, C.A.; Geddes, J.; Markesbery, W.R.; et al. P38 Kinase Is Activated in the Alzheimer’s Disease Brain. J. Neurochem. 1999, 72, 2053–2058. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, N.; Wang, C.; Qin, B.; Zhou, Y.; Xiao, M.; Chang, L.; Yan, L.-J.; Zhao, B. Role of RAGE in Alzheimer’s Disease. Cell Mol. Neurobiol. 2016, 36, 483–495. [Google Scholar] [CrossRef]
- Fang, F.; Yu, Q.; Arancio, O.; Chen, D.; Gore, S.S.; Yan, S.S.; Yan, S.F. RAGE Mediates Aβ Accumulation in a Mouse Model of Alzheimer’s Disease via Modulation of β- and γ-Secretase Activity. Hum. Mol. Genet. 2018, 27, 1002–1014. [Google Scholar] [CrossRef]
- Tenreiro, S.; Eckermann, K.; Outeiro, T.F. Protein Phosphorylation in Neurodegeneration: Friend or Foe? Front. Mol. Neurosci. 2014, 7, 42. [Google Scholar] [CrossRef]
- Niewiadomska, G.; Niewiadomski, W.; Steczkowska, M.; Gasiorowska, A. Tau Oligomers Neurotoxicity. Life 2021, 11, 28. [Google Scholar] [CrossRef]
- Zhu, X.; Rottkamp, C.A.; Boux, H.; Takeda, A.; Perry, G.; Smith, M.A. Activation of P38 Kinase Links Tau Phosphorylation, Oxidative Stress, and Cell Cycle-Related Events in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2000, 59, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Praticò, D. Glucose Deprivation Increases Tau Phosphorylation via P 38 Mitogen-activated Protein Kinase. Aging Cell 2015, 14, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Perea, J.R.; Bolós, M.; Cuadros, R.; García, E.; García-Escudero, V.; Hernández, F.; McManus, R.M.; Heneka, M.T.; Avila, J. P38 Inhibition Decreases Tau Toxicity in Microglia and Improves Their Phagocytic Function. Mol. Neurobiol. 2022, 59, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Maphis, N.; Jiang, S.; Xu, G.; Kokiko-Cochran, O.N.; Roy, S.M.; Van Eldik, L.J.; Watterson, D.M.; Lamb, B.T.; Bhaskar, K. Selective Suppression of the α Isoform of P38 MAPK Rescues Late-Stage Tau Pathology. Alzheimers Res. Ther. 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cel. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Butterfield, D.A. Brain Lipid Peroxidation and Alzheimer Disease: Synergy between the Butterfield and Mattson Laboratories. Ageing Res. Rev. 2020, 64, 101049. [Google Scholar] [CrossRef]
- Johri, A. Disentangling Mitochondria in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 11520. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Stefanova, N.A.; Kolosova, N.G. SkQ1 Suppresses the P38 MAPK Signaling Pathway Involved in Alzheimer’s Disease-Like Pathology in OXYS Rats. Antioxidants 2020, 9, 676. [Google Scholar] [CrossRef]
- Corre, I.; Paris, F.; Huot, J. The P38 Pathway, a Major Pleiotropic Cascade That Transduces Stress and Metastatic Signals in Endothelial Cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A Review: Inflammatory Process in Alzheimer’s Disease, Role of Cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.G.; Scali, C.; Prosperi, C.; Bellucci, A.; Vannucchi, M.G.; Rosi, S.; Pepeu, G.; Casamenti, F. β-Amyloid-Induced Inflammation and Cholinergic Hypofunction in the Rat Brain in Vivo: Involvement of the p38MAPK Pathway. Neurobiol. Dis. 2002, 11, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-G.; Lv, J.; Yang, W.-N.; Chang, K.-W.; Hu, X.-D.; Shi, L.-L.; Zhai, W.-Y.; Zong, H.-F.; Qian, Y.-H. The P38 Mitogen Activated Protein Kinase Regulates β-Amyloid Protein Internalization through the A7 Nicotinic Acetylcholine Receptor in Mouse Brain. Brain Res. Bull. 2018, 137, 41–52. [Google Scholar] [CrossRef]
- Dyer, O. Donanemab: FDA Experts Recommend Approval of Alzheimer’s Drug. BMJ 2024, 385, q1327. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Irizarry, M.C.; Lewcock, J.W.; Carrillo, M.C. Alzheimer’s Targeted Treatments: Focus on Amyloid and Inflammation. J. Neurosci. 2023, 43, 7894–7898. [Google Scholar] [CrossRef]
- Bateman, R.J.; Smith, J.; Donohue, M.C.; Delmar, P.; Abbas, R.; Salloway, S.; Wojtowicz, J.; Blennow, K.; Bittner, T.; Black, S.E.; et al. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1862–1876. [Google Scholar] [CrossRef]
- Hoy, S.M. Lecanemab: First Approval. Drugs 2023, 83, 359–365. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443, Erratum in Drugs 2021, 81, 1701. [Google Scholar] [CrossRef]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Batko, J.; Antosz, K.; Miśków, W.; Pszczołowska, M.; Walczak, K.; Leszek, J. Chaperones—A New Class of Potential Therapeutic Targets in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 3401. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.; Jain, P.; Jadhav, H.R. Glycogen Synthase Kinase 3 (GSK3): Its Role and Inhibitors. Curr. Top. Med. Chem. 2020, 20, 1522–1534. [Google Scholar] [CrossRef]
- Nygaard, H.B. Targeting Fyn Kinase in Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Gupta, V.; Gupta, V.; Chitranshi, N.; Wu, Y.; Amirkhani, A.; Meyfour, A.; Sheriff, S.; Shen, T.; Dhiman, K.; et al. Comparative Analysis of Aducanumab, Zagotenemab and Pioglitazone as Targeted Treatment Strategies for Alzheimer’s Disease. Aging Dis. 2021, 12, 1964. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.K.; Alexander, R.C.; Welsh-Bohmer, K.A.; Culp, M.; Chiang, C.; O’Neil, J.; Evans, R.M.; Harrigan, P.; Plassman, B.L.; Burke, J.R.; et al. Safety and Efficacy of Pioglitazone for the Delay of Cognitive Impairment in People at Risk of Alzheimer’s Disease (TOMMORROW): A Prognostic Biomarker Study and a Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2021, 20, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Mamun, A.A.; Barreto, G.E.; Rashid, M.; Perveen, A.; Ashraf, G.M. Pharmacological Approaches to Mitigate Neuroinflammation in Alzheimer’s Disease. Int. Immunopharmacol. 2020, 84, 106479. [Google Scholar] [CrossRef]
- Jiang, Y.; Alam, J.J.; Gomperts, S.N.; Maruff, P.; Lemstra, A.W.; Germann, U.A.; Stavrides, P.H.; Darji, S.; Malampati, S.; Peddy, J.; et al. Preclinical and Randomized Clinical Evaluation of the P38α Kinase Inhibitor Neflamapimod for Basal Forebrain Cholinergic Degeneration. Nat. Commun. 2022, 13, 5308. [Google Scholar] [CrossRef]
- Rutigliano, G.; Stazi, M.; Arancio, O.; Watterson, D.M.; Origlia, N. An Isoform-Selective P38α Mitogen-Activated Protein Kinase Inhibitor Rescues Early Entorhinal Cortex Dysfunctions in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2018, 70, 86–91. [Google Scholar] [CrossRef]
- Madkour, M.M.; Anbar, H.S.; El-Gamal, M.I. Current Status and Future Prospects of P38α/MAPK14 Kinase and Its Inhibitors. Eur. J. Med. Chem. 2021, 213, 113216. [Google Scholar] [CrossRef]
- Alam, J.J.; Krakovsky, M.; Germann, U.; Levy, A. Continuous Administration of a P38α Inhibitor during the Subacute Phase after Transient Ischemia-Induced Stroke in the Rat Promotes Dose-Dependent Functional Recovery Accompanied by Increase in Brain BDNF Protein Level. PLoS ONE 2020, 15, e0233073. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Zerin, F.; Menon, S.N.; Tithi, T.I.; Nguyen, K.P.; Vo, T.; Daniel, M.L.; Hafez, S.; Alam, M.A.; Hasan, R. Neflamapimod Induces Vasodilation in Resistance Mesenteric Arteries by Inhibiting P38 MAPKα and Downstream Hsp27 Phosphorylation. Sci. Rep. 2022, 12, 4905. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Mader, M. Brain Penetrant Kinase Inhibitors: Learning from Kinase Neuroscience Discovery. Bioorg. Med. Chem. Lett. 2018, 28, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Prins, N.; Lammertsma, A.; Yaqub, M.; Gouw, A.; Wink, A.M.; Chu, H.; Van Berckel, B.N.M.; Alam, J. An Exploratory Clinical Study of P38 α Kinase Inhibition in Alzheimer’s Disease. Ann. Clin. Transl. Neurol. 2018, 5, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; Harrison, J.E.; Chu, H.-M.; Blackburn, K.; Alam, J.J.; Scheltens, P. A Phase 2 Double-Blind Placebo-Controlled 24-Week Treatment Clinical Study of the P38 Alpha Kinase Inhibitor Neflamapimod in Mild Alzheimer’s Disease. Alzheimers Res. Ther. 2021, 13, 106. [Google Scholar] [CrossRef]
- Alam, J.J.; Maruff, P.; Doctrow, S.R.; Chu, H.-M.; Conway, J.; Gomperts, S.N.; Teunissen, C. Association of Plasma Phosphorylated Tau With the Response to Neflamapimod Treatment in Patients With Dementia With Lewy Bodies. Neurology 2023, 101, e1708–e1717. [Google Scholar] [CrossRef]
- Roy, S.M.; Minasov, G.; Arancio, O.; Chico, L.W.; Van Eldik, L.J.; Anderson, W.F.; Pelletier, J.C.; Watterson, D.M. A Selective and Brain Penetrant p38αMAPK Inhibitor Candidate for Neurologic and Neuropsychiatric Disorders That Attenuates Neuroinflammation and Cognitive Dysfunction. J. Med. Chem. 2019, 62, 5298–5311. [Google Scholar] [CrossRef]
- Roy, S.M.; Grum-Tokars, V.L.; Schavocky, J.P.; Saeed, F.; Staniszewski, A.; Teich, A.F.; Arancio, O.; Bachstetter, A.D.; Webster, S.J.; Van Eldik, L.J.; et al. Targeting Human Central Nervous System Protein Kinases: An Isoform Selective p38αMAPK Inhibitor That Attenuates Disease Progression in Alzheimer’s Disease Mouse Models. ACS Chem. Neurosci. 2015, 6, 666–680. [Google Scholar] [CrossRef]
- Zhou, Z.; Bachstetter, A.D.; Späni, C.B.; Roy, S.M.; Watterson, D.M.; Van Eldik, L.J. Retention of Normal Glia Function by an Isoform-Selective Protein Kinase Inhibitor Drug Candidate That Modulates Cytokine Production and Cognitive Outcomes. J. Neuroinflammation 2017, 14, 75. [Google Scholar] [CrossRef]
- Robson, M.J.; Quinlan, M.A.; Margolis, K.G.; Gajewski-Kurdziel, P.A.; Veenstra-VanderWeele, J.; Gershon, M.D.; Watterson, D.M.; Blakely, R.D. P38α MAPK Signaling Drives Pharmacologically Reversible Brain and Gastrointestinal Phenotypes in the SERT Ala56 Mouse. Proc. Natl. Acad. Sci. USA 2018, 115, E10245–E10254. [Google Scholar] [CrossRef]
- MW150|ALZFORUM. Available online: https://www.alzforum.org/therapeutics/mw150-0 (accessed on 8 September 2024).
- Yang, X.; Guo, A.-L.; Pang, Y.-P.; Cheng, X.-J.; Xu, T.; Li, X.-R.; Liu, J.; Zhang, Y.-Y.; Liu, Y. Astaxanthin Attenuates Environmental Tobacco Smoke-Induced Cognitive Deficits: A Critical Role of P38 MAPK. Mar. Drugs 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Wan, C.; Dong, D.; Wang, C.; Xi, Q.; Liu, Y.; Song, T. Astaxanthin Alleviates Inflammatory Pain by Regulating the P38 Mitogen-Activated Protein Kinase and Nuclear Factor-Erythroid Factor 2-Related Factor/Heme Oxygenase-1 Pathways in Mice. Food Funct. 2021, 12, 12381–12394. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, W.; Ye, T.; Sun, Y.; Chen, X.; Liu, X.; Shi, S.; Zhang, Y.; Qu, C.; Yang, B.; et al. Pinocembrin Alleviates Lipopolysaccharide-Induced Myocardial Injury and Cardiac Dysfunction in Rats by Inhibiting P38/JNK MAPK Pathway. Life Sci. 2021, 277, 119418. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Jiang, J.-G.; Zhu, W.; Ou-Yang, Q. Anti-Inflammatory Effect of Essential Oil from Citrus Aurantium L. Var. Amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Bengal, E.; Aviram, S.; Hayek, T. P38 MAPK in Glucose Metabolism of Skeletal Muscle: Beneficial or Harmful? Int. J. Mol. Sci. 2020, 21, 6480. [Google Scholar] [CrossRef]
- Dambach, D. Potential Adverse Effects Associated with Inhibition of P38α/β MAP Kinases. Curr. Top. Med. Chem. 2005, 5, 929–939. [Google Scholar] [CrossRef]
- Neflamapimod (VX-745)|Alzheimer’s News Today. Available online: https://alzheimersnewstoday.com/neflamapimod/ (accessed on 8 September 2024).
- Chen, Y.; Yu, Y. Tau and Neuroinflammation in Alzheimer’s Disease: Interplay Mechanisms and Clinical Translation. J. Neuroinflammation 2023, 20, 165. [Google Scholar] [CrossRef]
- Alzheimer’s Drug Discovery Foundation-p38α MAPK Inhibitors. Available online: https://www.alzdiscovery.org/uploads/cognitive_vitality_media/p38a-MAPK-inhibitors-Cognitive-Vitality-For-Researchers.pdf (accessed on 8 September 2024).
- Haller, V.; Nahidino, P.; Forster, M.; Laufer, S.A. An Updated Patent Review of P38 MAP Kinase Inhibitors (2014–2019). Expert Opin. Ther. Pat. 2020, 30, 453–466. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detka, J.; Płachtij, N.; Strzelec, M.; Manik, A.; Sałat, K. p38α Mitogen-Activated Protein Kinase—An Emerging Drug Target for the Treatment of Alzheimer’s Disease. Molecules 2024, 29, 4354. https://doi.org/10.3390/molecules29184354
Detka J, Płachtij N, Strzelec M, Manik A, Sałat K. p38α Mitogen-Activated Protein Kinase—An Emerging Drug Target for the Treatment of Alzheimer’s Disease. Molecules. 2024; 29(18):4354. https://doi.org/10.3390/molecules29184354
Chicago/Turabian StyleDetka, Jan, Natalia Płachtij, Martyna Strzelec, Aleksandra Manik, and Kinga Sałat. 2024. "p38α Mitogen-Activated Protein Kinase—An Emerging Drug Target for the Treatment of Alzheimer’s Disease" Molecules 29, no. 18: 4354. https://doi.org/10.3390/molecules29184354
APA StyleDetka, J., Płachtij, N., Strzelec, M., Manik, A., & Sałat, K. (2024). p38α Mitogen-Activated Protein Kinase—An Emerging Drug Target for the Treatment of Alzheimer’s Disease. Molecules, 29(18), 4354. https://doi.org/10.3390/molecules29184354






